Abstract
Aim
This study explored the development and comorbidity of allergic diseases by analysing the relationship between allergic manifestations in infancy and at the age of 8.
Methods
We included 5654 children born in Sweden in 2003 in a longitudinal study. Parents answered postal questionnaires when the children were six months and one, four‐and‐a‐half and eight years of age.
Results
The response rate at eight years was 4051 (71.6%), and we analysed 3382 children with complete data. The number of manifestations in infancy increased the risk of allergic disease at eight years of age: 72% of children with one early manifestation were symptom free at 8, compared to 45% with two or more manifestations. Similar manifestations occurred in infancy and at the age of 8, for example recurrent wheeze increased the risk of doctor‐diagnosed asthma by an adjusted odds ratio of 6.5. Eczema and food allergy independently increased the risk of all four allergic manifestations at eight years.
Conclusion
Allergic disease at the age of 8 was related to the number of allergic manifestations in infancy. Manifestations were similar at both ages, suggesting an allergic march with the coexistence of disease patterns rather than the progressive development of one disease.
Keywords: Allergic march, Allergic rhinitis, Asthma, Eczema, Food allergy
Abbreviations
- ALSPAC
Avon Longitudinal Study of Parents and Childhood
- BAMSE
Children, allergy, milieu, Stockholm, epidemiology (translation)
- ISAAC
International Study of Asthma and Allergies in Childhood
- MAAS
Manchester Asthma and Allergy Study
Key notes.
This study explored the development and comorbidity of allergic diseases by analysing the relationship between allergic manifestations in infancy and at the age of 8.
Allergic disease at the age of 8 was related to the number of allergic manifestations in infancy, and similar manifestations were associated at both ages.
The findings suggest the existence of related patterns of allergic disease rather than the progressive development of one underlying disease.
Introduction
A natural course of allergic disease during childhood has been proposed, with a progression from eczema and food allergy in early childhood to asthma and allergic rhinitis at school age 1, 2. The overall concept of this allergic march has been supported by cross‐sectional and longitudinal studies 2, 3. However, many individual children do not follow the classical allergic march 4, 5. For this reason, different patterns of allergic morbidity and the coexistence of the different manifestations, rather than progressive development, have been suggested 4, 5, 6. In order to improve the understanding of eczema, allergic rhinitis and asthma, it is important to increase our knowledge of the development and comorbidity of allergic diseases 7, 8.
In this prospective, longitudinal birth cohort study of more than 4000 children, data on asthma and allergic manifestations were collected from infancy until eight years of age. We had previously analysed how early factors affected the risk of allergic diseases and asthma up to school age 9, 10, 11, 12, 13, 14.
The main aim of this study was to understand the relationship between allergic manifestations in infancy and at the age of 8. We also wanted to understand comorbidities of different allergic diseases and describe the onset and prevalence of asthma and allergic diseases from infancy to eight years of age.
Methods
Participants and procedures
Data were obtained from a longitudinal cohort study of children born in the region of western Sweden in 2003. The random sample comprised 8176 families, 50% of the birth cohort, and 5654 families who responded when their child was six months old entered the study. The procedures are illustrated in a flowchart in Figure S1.
After written informed consent, the parents answered questionnaires at six months and one, four‐and‐a‐half and eight years of age. The questionnaires were based on the Swedish version of the International Study of Asthma and Allergies in Childhood (ISAAC) and the Swedish BAMSE study, which can be translated from Swedish as ‘children, allergy, milieu, Stockholm, epidemiology’. Details of the questionnaires and response rates have previously been published 9, 10, 11, 12, 13, 14.
As reported earlier, the material was largely representative of the population 11. Analyses relating to responders and nonresponders when the children were eight years of age showed a higher prevalence of preterm birth, parents with a low educational level and maternal smoking during pregnancy among the nonresponders 9.
Information on current allergy symptoms and reported diagnosis was collected at one, four‐and‐a‐half and eight years of age. The questions relating to the onset of different allergic symptoms and asthma were asked at eight years of age. The questions on allergic diagnoses, symptoms and onset are summarised in Table S1.
At four‐and‐a‐half and eight years of age, the parents were asked whether their children had shown reactions to inhaled allergens and, or, food allergens during an allergy test, such as a skin prick test or blood test. The inhaled allergens included dog, cat, horse, rabbit, house dust mites, birch, timothy, mugwort and moulds. The food allergens included at four‐and‐a‐half and eight years were eggs, milk, fish, wheat, peanuts, soy and peas. Hazelnuts, almonds and other nuts were added to the questionnaire at eight years of age.
Definitions
The questions on allergic diagnoses, symptoms and onset are summarised in Table S1.
Doctor‐diagnosed asthma, doctor‐diagnosed allergic rhinitis and doctor‐diagnosed eczema were defined as a parentally reported diagnosis and either medication and, or, symptoms during the last 12 months. However, at one year of age, doctor‐diagnosed eczema was defined as reported eczema.
Doctor‐diagnosed food allergy at one year of age was defined as a parentally reported diagnosis during the first year. At four‐and‐a‐half and eight years of age, a doctor‐diagnosed food allergy was defined as fulfilling the following four criteria: (1) parentally reported diagnosis, (2) current food allergy, (3) a positive allergy test to a specific food allergen and (4) reported symptoms for the same allergen. In addition, a positive allergy test for peanuts, or reported symptoms for almonds and other nuts at four‐and‐a‐half years of age, together with criteria (1) and (2) was also regarded as a doctor‐diagnosed food allergy at four‐and‐a‐half years of age.
Recurrent wheeze was defined as three or more episodes of wheezing during the last 12 months.
Atopic asthma at eight years of age was defined as parentally reported doctor‐diagnosed asthma and reported allergic sensitisation and, or, current doctor‐diagnosed food allergy, allergic rhinitis or eczema.
Nonatopic asthma at eight years of age was defined as parentally reported doctor‐diagnosed asthma and not having reported allergic sensitisation or doctor‐diagnosed food allergy, allergic rhinitis or eczema.
Statistical analyses
In the statistical analysis, the chi‐square test and binary logistic regression were used. Odds ratios (OR) were estimated with 95% confidence intervals (95% CI). A p‐value of <0.05 was considered statistically significant. For analyses comparing the mean values, an independent sample t‐test was used. SPSS Statistics version 20.0 (IBM Corp., Armonk, NY, USA) was used for the statistical calculations. The chi‐square for mean trends was calculated using Epi Info 6.0.4d (Centers for Disease Control and Prevention, Atlanta, GA, USA).
In the logistic regression analyses, allergic manifestations in infancy were analysed with regard to the different outcomes seen at eight years of age, namely any allergic disease and each disease separately. The allergic manifestations considered in infancy were recurrent wheeze, eczema or food allergy or combinations of these. At eight years of age, doctor‐diagnosed asthma, allergic rhinitis, eczema and, or, food allergy were considered. In addition, the outcome of comorbidity at eight years of age was analysed in relation to comorbidity at one and four years of age, with and without reported allergic sensitisation. Comorbidity at eight years of age was having any combinations of doctor‐diagnosed asthma, allergic rhinitis, eczema and, or, food allergy. In the logistic regression analyses, adjustments were made for gender and atopic heredity.
The assumption of missing data was that it was missing at random and that it did not significantly alter the main results of the study. This assumption was tested by analysing the prevalence of allergic manifestations in children with complete data at all follow‐up ages compared with children with any available data.
Results
Study population
The response rate at eight years of age was 4051/5044 (80.3%) of the questionnaires distributed, which was equivalent to 71.6% of the 5654 families who entered the study. Only the 3382 children with complete data on all questions relating to allergic diseases at one, four‐and‐a‐half and eight years of age were included in the present study. This is illustrated in a flowchart in Figure S1.
The results presented in Table S2 support the assumption that data were missing at random, by showing a similar prevalence of allergic manifestations among children with any information and in complete cases. In addition, the representativeness of the study subjects in relation to the total number of responders at eight years of age was analysed. The parents of the study subjects had a somewhat higher education level, a slightly lower prevalence of smoking during pregnancy and a slightly lower prevalence of breastfeeding for less than four months (p < 0.05). The populations did not differ statistically in terms of the mother's medication during pregnancy, gender, atopic heredity, gestational age, mode of delivery, neonatal antibiotic treatment or early allergic manifestations.
Allergic manifestations in infancy – allergic disease at eight years of age
Children with allergic manifestations in infancy, for example either a recurrent wheeze, eczema or food allergy, had a higher prevalence of allergic disease at eight years of age, namely either doctor‐diagnosed asthma, allergic rhinitis, eczema or food allergy. An increased risk of any allergic disease at eight years of age was seen with increasing numbers of allergic manifestations in infancy (Fig. 1, Table 1).
Figure 1.
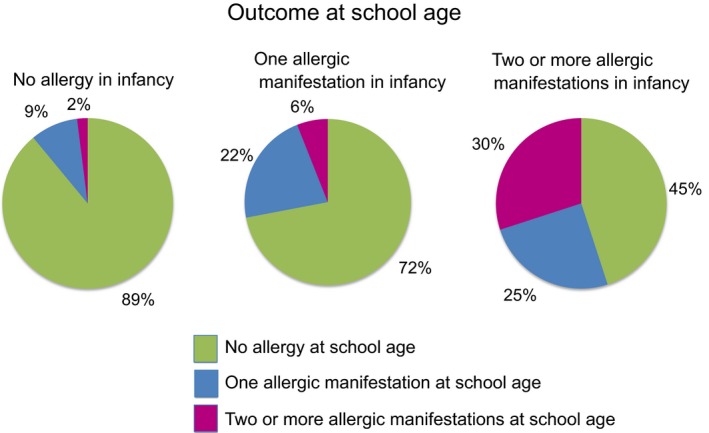
Prevalence of allergic disease at eight years of age (doctor‐diagnosed asthma, rhinitis, eczema or food allergy) among children with one or two or more allergic manifestations in infancy (recurrent wheeze, eczema or food allergy), compared with no manifestation.
Table 1.
The risk of any allergic disease at eight years of age (i.e. having either doctor‐diagnosed asthma, allergic rhinitis, eczema or food allergy) in relation to increasing numbers of allergic manifestations in infancy (recurrent wheeze, eczema and/or food allergy, all combinations) analysed using multivariate logistic regression analysis, adjusting for gender and age. Adjusted odds ratios are shown with a 95% confidence interval
| Any allergic disease at eight years, aOR (95% CI) | |
|---|---|
| No manifestation | Ref. |
| One manifestation | 3.1 (2.5–3.8) |
| Two or more manifestations | 9.2 (6.7–13.2) |
In addition, the risk of comorbidity at eight years of age, namely having two or more allergic manifestations – asthma, allergic rhinitis, eczema or food allergy or all combinations – was high among children who also had comorbidity in infancy – recurrent wheeze, eczema and, or, food allergy or all combinations – with an adjusted OR (aOR) of 22.1 and 95% CI of 13.7–35.7, adjusted for atopic heredity and gender. No interaction was seen between parental allergic disease and own allergic disease in infancy (p = 0.834) when it came to the risk of allergic disease at eight years of age, such as having either doctor‐diagnosed asthma, allergic rhinitis, eczema or food allergy.
Table 2 shows the prevalence of any allergic disease seen at eight years of age, and each allergic manifestation separately, among children affected by different early manifestations. The associations between the three early manifestations and each outcome at eight years of age, adjusted for atopic heredity and gender, are shown in Figure S2 and listed in Table 2.
Table 2.
The prevalence of any allergic disease and the separate allergic manifestations, that is doctor‐diagnosed asthma, rhinitis, eczema and food allergy, seen at school age among children affected by the different early manifestations, that is recurrent wheeze, eczema and food allergy. The corresponding prevalence among those unaffected in infancy is shown in brackets. The associations between the three early manifestations and each outcome at school age were analysed using multivariate logistic regression analysis, adjusting for gender and age. Adjusted odds ratios, aORs, are shown with a 95% confidence interval
| Any allergic disease eight years | aOR (95% CI) | |
|---|---|---|
| Recurrent wheeze one year | 32 (15) | 2.4 (1.7–3.4) |
| Eczema one year | 33 (12) | 2.8 (2.3–3.4) |
| Food allergy one year | 52 (14) | 4.1 (2.9–5.7) |
| DD asthma eight years | aOR (95% CI) | |
|---|---|---|
| Recurrent wheeze one year | 25 (4) | 6.5 (4.4–9.7) |
| Eczema one year | 10 (4) | 1.7 (1.2–2.4) |
| Food allergy one year | 19 (5) | 3.7 (2.3–5.8) |
| DD rhinitis eight years | aOR (95% CI) | |
|---|---|---|
| Recurrent wheeze one year | 6 (5) | 0.9 (0.5–1.7) |
| Eczema one year | 12 (4) | 2.7 (1.9–3.7) |
| Food allergy one year | 21 (5) | 3.4 (2.2–5.3) |
| DD eczema eight years | aOR (95% CI) | |
|---|---|---|
| Recurrent wheeze one year | 8 (8) | 0.9 (0.5–1.8) |
| Eczema one year | 19 (5) | 3.8 (2.9–5.0) |
| Food allergy one year | 27 (7) | 2.9 (2.0–4.3) |
| DD food allergy eight years | aOR (95% CI) | |
|---|---|---|
| Recurrent wheeze one year | 4 (3) | 1.1 (0.5–2.5) |
| Eczema one year | 8 (2) | 2.6 (1.6–4.1) |
| Food allergy one year | 26 (2) | 11.9 (7.4–19.1) |
Of the children with recurrent wheeze in infancy who had doctor‐diagnosed allergic disease at eight years of age, 76% reported asthma, 31% eczema, 21% allergic rhinitis and 13% food allergy. The corresponding figures for food allergy in infancy were 39% asthma, 52% eczema, 40% allergic rhinitis and 48% food allergy. For eczema in infancy, the figures were 31% asthma, 58% eczema, 37% allergic rhinitis and 23% food allergy.
Asthma at eight years of age – previous or current allergic manifestations
Among the children with asthma at eight years of age, 37.6% had no previous or current allergic manifestation, but 24.9% had current doctor‐diagnosed allergic rhinitis, 18.8% had current doctor‐diagnosed eczema and 14.4% had current doctor‐diagnosed food allergy. The corresponding figures among children without asthma at eight years of age were 67.2%, 4.3%, 7.0% and 2.3% (p < 0.001 for all).
The prevalence of asthma at eight years of age increased with the number of allergic manifestations during infancy (p for trend < 0.001). With no allergic manifestation in infancy, 3.1% had asthma at eight years of age, 9.8% had asthma with one manifestation, 22.3% had asthma with two manifestations and 66.7% had asthma with three manifestations. Moreover, an increased risk of asthma at eight years of age was seen with increasing numbers of allergic manifestations in infancy. With one manifestation, the aOR was 3.0 (2.1–4.1); with two manifestations, the aOR was 6.9 (4.3–11.3); and with three manifestations, the aOR was 51.9 (12.6–213.8), compared with symptom‐free subjects, adjusted for atopic heredity and gender. The confidence intervals did not overlap.
Onset and prevalence of allergic manifestations
The overall prevalence of the different allergic manifestations from infancy to eight years of age is illustrated in Figure 2. The onset of allergic disease at eight years of age is illustrated in Figure S3. For doctor‐diagnosed eczema, 47.8% had onset during the first year, with the mean age of onset being two years and three months (median 1.5, SD 2.3). For doctor‐diagnosed food allergy, 46.7% had onset during the first year, with the mean age of onset being two years and two months (median 1.5, SD 2.2). For doctor‐diagnosed asthma, 44.8% had onset before the age of two years, with the mean age of onset being two years and seven months of age (median 2.0, SD 2.2). For doctor‐diagnosed allergic rhinitis, 7.9% had onset before the age of two years and 68.5% between four‐and‐a‐half and eight years of age, with the mean age of onset being four years and nine months (median 5.0, SD 2.0). The onset of allergic rhinitis was significantly later than the age of onset of other manifestations (p = 0.001).
Figure 2.
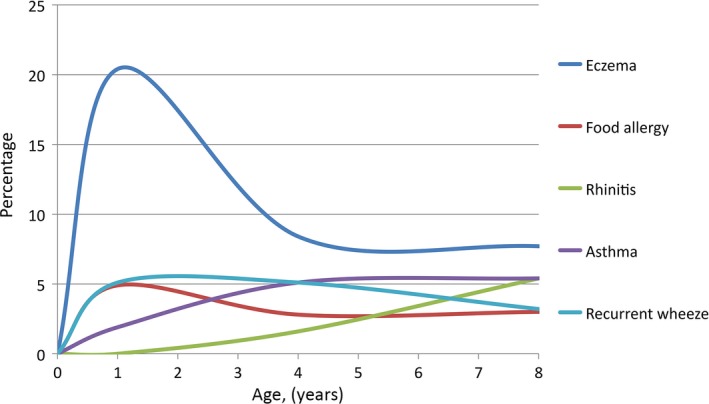
Population‐based prevalence of allergic diseases from infancy to school age, presented cross‐sectionally at each follow‐up. For exact figures, see Table 3.
Atopic asthma had a significantly later onset than nonatopic asthma, with means of three years of age (median 2.3, SD 2.2) and two years and two months of age (median 1.5, SD 2.0), respectively (p = 0.019).
The prevalence and cumulative prevalence of different allergic manifestations from infancy to eight years of age are shown in Table 3.
Table 3.
Prevalences and cumulative prevalences of allergic manifestations at one, four‐and‐a‐half and eight years of age (n = 3382)
| One year | Four‐and‐a‐half years | Eight years | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Prevalence | ||||||
| Eczema | 690 | 20.4 | 284 | 8.4 | 259 | 7.7 |
| Food allergy | 165 | 4.9 | 94 | 2.8 | 101 | 3.0 |
| Recurrent wheeze | 173 | 5.1 | 174 | 5.1 | 108 | 3.2 |
| Asthma | 65 | 1.9 | 173 | 5.1 | 181 | 5.4 |
| Rhinitis | 53 | 1.6 | 183 | 5.4 | ||
| Cumulative prevalence | ||||||
| Eczema | 690 | 20.4 | 819 | 24.2 | 896 | 26.5 |
| Symptoms of eczema | 690 | 20.4 | 1005 | 29.7 | 1213 | 35.9 |
| Food allergy | 165 | 4.9 | 210 | 6.2 | 248 | 7.3 |
| Symptoms from food | 648 | 19.2 | 1015 | 30 | 1271 | 37.6 |
| Recurrent wheeze | 173 | 5.1 | 308 | 9.1 | 372 | 11.0 |
| Any wheeze | 670 | 19.8 | 1276 | 37.7 | 1337 | 39.5 |
| Asthma | 65 | 1.9 | 201 | 5.9 | 277 | 8.2 |
| Rhinitis | 53 | 1.6 | 200 | 5.9 | ||
| Symptoms of rhinitis | 218 | 6.4 | 466 | 13.8 | ||
Comorbidity
The prevalence and comorbidity of eczema, food allergy, recurrent wheeze/asthma and allergic rhinitis at the age of one, four‐and‐a‐half and eight years of age are shown in Table S3. The progress of different manifestations from infancy to four‐and‐a‐half and eight years of age, respectively, is shown in Table 4.
Table 4.
Progress of different manifestations from infancy to four‐and‐a‐half and eight years of age
| One year of age | Four‐and‐a‐half years of age | % (n) | Eight years of age | % (n) |
|---|---|---|---|---|
|
Eczema only n = 552 = 100% |
Symptom‐free | 73 (402) | Symptom‐free | 73 (402) |
| Eczema only | 16 (87) | Eczema only | 12 (66) | |
| Eczema + other | 4 (22) | Eczema + other | 3.5 (20) | |
| Other, no eczema | 7 (41) | Other, no eczema | 11.5 (64) | |
|
Food allergy (FA) only n = 57 = 100% |
Symptom‐free | 74 (42) | Symptom‐free | 68 (42) |
| FA only | 12 (7) | FA only | 7 (4) | |
| FA + other | 7 (4) | FA + other | 7 (4) | |
| Other, no FA | 7 (4) | Other, no FA | 17.5 (10) | |
|
Recurrent wheeze (RW) only n = 126 = 100% |
Symptom‐free | 76 (96) | Symptom‐free | 70 (88) |
| RW only | 18.5 (23) | Asthma only | 22 (28) | |
| RW + other | 2.5 (3) | Asthma+ other | 3 (4) | |
| Other, no RW | 3 (4) | Other, no Asthma | 5 (6) | |
|
Food allergy (FA) and eczema n = 95 = 100% |
Symptom‐free | 41 (39) | Symptom‐free | 36 (34) |
| FA + eczema only | 13 (12) | FA + eczema only | 5 (5) | |
| FA or eczema only | 30 (29) | FA or eczema only | 18 (17) | |
| FA or eczema + other | 8.5 (8) | FA or eczema + other | 28.5 (27) | |
| Other, no FA or eczema | 7.5 (7) | Other, no FA or eczema | 12.5 (12) | |
|
Recurrent wheeze (RW) and eczema n = 34 = 100% |
Symptom‐free | 47 (16) | Symptom‐free | 71 (24) |
| RW + eczema only | 0 (0) | Asthma + eczema only | 3 (1) | |
| RW or eczema only | 41 (14) | Asthma or eczema only | 12 (4) | |
| RW or eczema + other | 9 (3) | Asthma or eczema + other | 9 (3) | |
| Other, no RW or eczema | 3 (1) | Other, no asthma or eczema | 6 (2) | |
|
Recurrent wheeze (RW) and Food allergy (FA) n = 4 = 100% |
Symptom‐free | 100 (4) | Symptom‐free | 100 (4) |
| RW + FA only | 0 (0) | Asthma + FA only | 0 (0) | |
| RW or FA only | 0 (0) | Asthma or FA only | 0 (0) | |
| RW or FA + other | 0 (0) | Asthma or FA + other | 0 (0) | |
| Other, no RW or FA | 0 (0) | Other, no asthma or FA | 0 (0) | |
|
Recurrent wheeze (RW) and Food allergy (FA) and eczema n = 9 = 100% |
Symptom‐free | 0 (0) | Symptom‐free | 22 (2) |
| RW+ FA + eczema only | 11 (1) | Asthma + FA + eczema only | 22 (2) | |
| Either, no rhinitis | 78 (7) | Either, no rhinitis | 33 (3) | |
| Either + rhinitis | 11 (1) | Either + rhinitis | 22 (2) | |
| Only rhinitis | 0 (0) | Only rhinitis | 0 (0) | |
|
Symptom‐free n = 2505 = 100% |
Symptom‐free | 90.5 (2271) | Symptom‐free | 89 (2236) |
| One manifestation | 9 (221) | One manifestation | 9 (228) | |
| Two manifestations | 0.5 (12) | Two manifestations | 1.5 (34) | |
| ≥3 manifestations | 0 (1) | ≥3 manifestations | 0.5 (7) |
Allergic disease was reported in 26% of the children at one year of age, but only among 15% at four‐and‐a‐half years of age and 16% at eight years of age (p for trend < 0.001). However, among those with allergic disease, comorbidity – having two or more allergic manifestations – was more common at eight years of age (p for trend = 0.002) At the age of one year, 16% of children with allergic disease had comorbidity, compared to 15% at four‐and‐a‐half years of age and 23% at eight years of age. Current allergic sensitisation, with a reported positive allergy test, was associated with comorbidity at four‐and‐a‐half years of age and at eight years of age (p < 0.001). At four‐and‐a‐half years of age, 76% of those with comorbidity reported allergic sensitisation and the figure was 85% at eight years of age. In addition, allergic sensitisation at four‐and‐a‐half years of age was associated with a comorbidity of 26% at eight years of age (p < 0.001). However, comorbidity at four‐and‐a‐half years of age increased the risk of comorbidity at eight years of age, regardless of reported allergic sensitisation at four‐and‐a‐half years of age: without allergic sensitisation, the OR was 47.2 (20.3–109.8), and with allergic sensitisation, the OR was 91.4 (52.0–160.5). No data on allergic sensitisation were available at one year of age. However, a reported doctor's diagnosis of food allergy at the age of one year was associated with comorbidity (p < 0.001). At the age of one year, comorbidity was seen in 69% of those with food allergy in infancy; at four‐and‐a‐half years of age, it was seen in 18%; and at eight years of age, the figure was 26%. A reported doctor's diagnosis of food allergy at the age of one year was also associated with reported allergic sensitisation (p < 0.001). At four‐and‐a‐half years of age, 69% of those with food allergy in infancy reported allergic sensitisation, and at eight years of age, the figure was 73%. In addition, comorbidity was significantly more common among children with atopic heredity at all ages (p < 0.001). However, no significant difference was seen between boys and girls at one and four‐and‐a‐half years of age, but at eight years of age comorbidity was significantly more common among males (p = 0.012).
Discussion
The main finding of this prospective, longitudinal birth cohort study was the strong link between allergic disease during infancy, for example having either recurrent wheeze, eczema or food allergy, and an increased risk of allergic disease at eight years of age, namely having either asthma, allergic rhinitis, eczema or food allergy. The impact was also increased if there was more than one early manifestation. In addition, we found different patterns of association between allergic manifestations over time. Manifestations in infancy were connected to similar manifestations at eight years of age. Recurrent wheeze only increased the risk of doctor‐diagnosed asthma and not of the other allergic manifestations at eight years of age, while eczema and food allergy in infancy independently increased the risk of all four allergic manifestations at eight years of age.
The cross‐sectional overall pattern of allergic manifestations at the population level in our study was in accordance with the proposed allergic march supported by previous studies 2, 3, 5, 15. The concept of the allergic march describes the variation in allergic manifestations over time and suggests a progression of symptoms from early eczema and food allergy, to asthma and allergic rhinitis with increasing age 1, 2, 3.
However, several studies have suggested that, even though they are associated, the different combinations of allergic manifestations seen over time suggest the coexistence rather than the progressive development of the same underlying disease 4, 5, 6. Children with early eczema who develop asthma and allergic rhinitis might represent one specific phenotype of eczema, characterised by eczema plus either wheezing or a specific pattern of sensitisation 6. In a study based on the Avon Longitudinal Study of Parents and Childhood (ALSPAC) and the Manchester Asthma and Allergy Study (MAAS) studies, Belgrave et al. 5 used latent class analysis to investigate the developmental profiles of eczema, wheeze and allergic rhinitis and found that only a small proportion of the children followed the profile resembling the ideal allergic march. Instead, eight different distinct disease profiles were described and associated with different sensitisation patterns, arguing in favour of different underlying mechanisms and questioning a causal relationship between eczema, wheeze and allergic rhinitis 5. However, the coexistence of allergic manifestations in the same child has been shown to be more common than expected by chance alone, and therefore, the allergic manifestations seem to share causal mechanisms 16.
Many agree that the occurrence of early allergic manifestations is associated with subsequent allergic disease 1, 7, 17. An increased risk of both subsequent asthma and allergic rhinitis has been described following eczema in early life 9, 10, 18, 19, 20. The findings of these studies, and of our study, support the allergic march on a population level, even though the concept is not the strongest factor at the individual level of children with allergic disease. In our study, the risk of asthma and other allergic disease at the age of eight years increased with an increasing number of allergic manifestations in infancy. Among the children with two or more of any allergic manifestations in infancy, more than half had any allergic disease at eight years of age. In addition, comorbidity increased from infancy to eight years of age as a consequence of the increasing prevalence of asthma and allergic rhinitis. Furthermore, the more allergic manifestations a child had in infancy, the greater the risk of allergic disease and comorbidity at eight years of age. Comorbidity at preschool age has been shown to increase the risk of comorbidity regardless of immunoglobulin E sensitisation, and this was also confirmed in our study 16. In addition, we found comorbidity to be more common among children with atopic heredity, in line with the results of the BAMSE study 8. These associations suggest the progression of allergic disease over time, not only at a population level but also at an individual level, with a poorer prognosis in subjects with early disease.
However, we found that manifestations in infancy tended to be associated with similar manifestations at eight years of age. In subjects with eczema in infancy, only about a third had any allergic disease at eight years of age, but 60% of these still had eczema. This pattern was especially pronounced for recurrent wheeze, where a third of the subjects had any allergic disease at the age of eight years, and of these, 76% had doctor‐diagnosed asthma. Interestingly, 52% of those with food allergy in infancy were still affected by any allergic disease at eight years of age and about half of those had eczema and, or, food allergy, while 40% had allergic rhinitis and, or, asthma. In fact, recurrent wheeze was only an independent risk factor for doctor‐diagnosed asthma, while early eczema and food allergy independently increased the risk of all the different manifestations at eight years of age.
Overall, early food allergy appeared to be the strongest predictor of subsequent allergic disease. In addition, the occurrence of only one allergic manifestation, such as recurrent wheeze, eczema or food allergy, during infancy, was associated with a good prognosis, where over 70% were symptom free at eight years of age. This supports the notion of different progressive patterns of allergic disease. However, the development of allergic diseases is complex and the phenotype and progression of allergic disease at individual levels may vary, depending on individual predisposition, susceptibility and exposure to different environmental and life events.
The prevalence of allergic manifestations at different time points in our study was largely in line with previous studies 8, 15, 20, 21, 22, 23. However, the levels varied to some degree between studies, most probably because of different definitions. We used definitions that included a reported doctor's diagnosis, explaining, for example, the lower prevalence of eczema and asthma (wheeze) compared with that of the MAAS and ALSPAC studies 5.
Reported doctor‐diagnosed asthma increased with older age, while recurrent wheeze decreased, pinpointing the disparity in asthma definitions and the heterogeneous course of wheezing in early life. A similar pattern was seen in the ALSPAC and MAAS studies 5.
We found that the onset of allergic rhinitis was clearly later than that of the other allergic manifestations, as it tripled in prevalence from four‐and‐a‐half to eight years of age, which is in line with the results of the BAMSE, MAAS and ALSPAC studies 5, 8. This increase in prevalence can be explained by the coincidence of sensitisation to airborne allergens seen during school age 22.
Strengths and limitations
The strengths of the study were the longitudinal design, the large size of the birth cohort and the high response rate. In addition, the definitions used for allergic disease were based on a parentally reported doctor's diagnosis, medication and, or, symptoms.
The limitations of the study are those inherent in questionnaire‐based studies, for example that there can always be some uncertainty regarding the validity of answers. To improve the validity of answers, we used questions based on well‐known, validated questionnaires.
However, no objective data on sensitisation or doctor's diagnosis were available. Thus, the reported data were based on parental recollections of a doctor's diagnosis and of the results of their children's blood tests or skin prick tests. The lack of objective data may have affected the validity of the definitions of allergic disease, for example that of allergic asthma and a food allergy diagnosis. Other studies have reported a discrepancy between the reported prevalence of food allergy and the prevalence following objective evaluations with allergy tests and, or, provocation 24. This has to be kept in mind before generalising the results of this study.
As reported earlier, our cohort appeared to be largely representative of the population 11. However, as is often seen in questionnaire studies, responders tend to be more health conscious and well educated than nonresponders 9. This was also evident in this study population.
Conclusion
In conclusion, allergic disease at eight years of age was strongly related to the presence of recurrent wheeze and allergic manifestations in infancy. Manifestations in infancy tended to be associated with similar manifestations at eight years of age, suggesting the coexistence of patterns of allergic disease rather than the progressive development of one underlying disease.
Funding
The study was supported by the Research Foundation of the Swedish Asthma and Allergy Association, the Queen Silvia Children's Hospital Research Foundation, the Health and Medical Care Committee of the Regional Executive Board, Västra Götaland Region, and the Swedish Government under the ALF agreement between the Government and the County Councils concerning economic support of medical research.
Conflicts of interest
The authors declare that they have no conflict of interests.
Ethical approval
The study was approved by the ethics committee at the University of Gothenburg, Sweden. All parents provided written, informed consent.
Supporting information
Figure S1 Flowchart showing number of study subjects from inclusion to the follow‐up at eight years of age.
Figure S2 The associations between the three early manifestations (recurrent wheeze, eczema or food allergy) and each outcome at eight years of age (doctor‐diagnosed asthma, rhinitis, eczema or food allergy) analysed using multivariate logistic regression analysis, adjusting for gender and age.
Figure S3 Age of onset of allergic diseases.
Table S1 Questions on allergic symptoms and diagnoses at one, four‐and‐a‐half and eight years of age.
Table S2 Children included in the present analyses (complete data on all questions, n = 3382) versus all available data on responders at eight years of age (n = 4051).
Table S3 Prevalence and comorbidity of eczema, food allergy and recurrent wheeze at one year of age (n = 3382).
References
- 1. Ker J, Hartert TV. The atopic march: what's the evidence? Ann Allergy Asthma Immunol 2009; 103: 282–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zheng T, Yu J, Oh MH, Zhu Z. The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. Allergy Asthma Immunol Res 2011; 3: 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Spergel JM. From atopic dermatitis to asthma: the atopic march. Ann Allergy Asthma Immunol 2010; 105: 99–106. [DOI] [PubMed] [Google Scholar]
- 4. Ziyab AH, Karmaus W, Zhang H, Holloway JW, Steck SE, Ewart S, et al. Allergic sensitization and filaggrin variants predispose to the comorbidity of eczema, asthma, and rhinitis: results from the Isle of Wight birth cohort. Clin Exp Allergy 2014; 44: 1170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Belgrave DC, Granell R, Simpson A, Guiver J, Bishop C, Buchan I, et al. Developmental profiles of eczema, wheeze, and rhinitis: two population‐based birth cohort studies. PLoS Med 2014; 11: e1001748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. lIli S, von Mutius E, Lau S, Nickel R, Grüber C, Niggemann B, et al. The natural course of atopic dermatitis from birth to age 7 years and the association with asthma. J Allergy Clin Immunol 2004; 113: 925–31. [DOI] [PubMed] [Google Scholar]
- 7. Sly PD, Boner AL, Björkstén B, Bush A, Custovic A, Eigenmann PA, et al. Early identification of atopy in the prediction of persistent asthma in children. Lancet 2008; 372: 1100–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ballardini N, Kull I, Lind T, Hallner E, Almqvist C, Östblom E, et al. Development and comorbidity of eczema, asthma and rhinitis to age 12: data from the BAMSE birth cohort. Allergy 2012; 67: 537–44. [DOI] [PubMed] [Google Scholar]
- 9. Goksör E, Alm B, Pettersson R, Möllborg P, Erdes L, Åberg N, et al. Early fish introduction and neonatal antibiotics affect the risk of asthma into school age. Pediatr Allergy Immunol 2013; 24: 339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alm B, Goksör E, Pettersson R, Möllborg P, Erdes L, Åberg N, et al. Antibiotics in the first week of life is a risk factor for allergic rhinitis at school age. Pediatr Allergy Immunol 2014; 25: 468–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alm B, Erdes L, Möllborg P, Pettersson R, Norvenius SG, Åberg N, et al. Neonatal antibiotic treatment is a risk factor for early wheezing. Pediatrics 2008; 121: 697–702. [DOI] [PubMed] [Google Scholar]
- 12. Alm B, Åberg N, Erdes L, Möllborg P, Pettersson R, Norvenius SG, et al. Early introduction of fish decreases the risk of eczema in infants. Arch Dis Child 2009; 94: 11–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alm B, Goksör E, Thengilsdottir H, Pettersson R, Möllborg P, Norvenius G, et al. Early protective and risk factors for allergic rhinitis at age 4½ years. Pediatr Allergy Immunol 2011; 22: 398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goksör E, Alm B, Thengilsdottir H, Pettersson R, Åberg N, Wennergren G. Preschool wheeze – impact of early fish introduction and neonatal antibiotics. Acta Paediatr 2011; 100: 1561–6. [DOI] [PubMed] [Google Scholar]
- 15. Liu AH, Jaramillo R, Sicherer SH, Wood RA, Bock SA, Burks AW, et al. National prevalence and risk factors for food allergy and relationship to asthma: results from the National Health and Nutrition Examination Survey 2005–2006. J Allergy Clin Immunol 2010; 126: 798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pinart M, Benet M, Annesi‐Maesano I, von Berg A, Berdel D, Carlsen KC, et al. Comorbidity of eczema, rhinitis, and asthma in IgE‐sensitised and non‐IgE‐sensitised children in MeDALL: a population‐based cohort study. Lancet Respir Med 2014; 2: 131–40. [DOI] [PubMed] [Google Scholar]
- 17. Almqvist C, Li Q, Britton WJ, Kemp AS, Xuan W, Tovey ER, et al. Early predictors for developing allergic disease and asthma: examining separate steps in the ‘allergic march’. Clin Exp Allergy 2007; 37: 1296–302. [DOI] [PubMed] [Google Scholar]
- 18. Saunes M, Øien T, Dotterud C, Romundstad PR, Storrø O, Holmen TL, et al. Early eczema and the risk of childhood asthma: a prospective, population‐based study. BMC Pediatr 2012; 12: 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gustafsson D, Sjöberg O, Foucard T. Development of allergies and asthma in infants and young children with atopic dermatitis‐a prospective follow‐up to 7 years of age. Allergy 2000; 55: 240–5. [DOI] [PubMed] [Google Scholar]
- 20. von Kobyletzki LB, Bornehag CG, Hasselgren M, Larsson M, Lindström CB, Svensson Å. Eczema in early childhood is strongly associated with the development of asthma and rhinitis in a prospective cohort. BMC Dermatol 2012; 12: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Andersson M, Bjerg A, Forsberg B, Lundbäck B, Rönmark E. The clinical expression of asthma in schoolchildren has changed between 1996 and 2006. Pediatr Allergy Immunol 2010; 21: 859–66. [DOI] [PubMed] [Google Scholar]
- 22. Notenboom ML, Mommers M, Jansen EH, Penders J, Thijs C. Maternal fatty acid status in pregnancy and childhood atopic manifestations: KOALA Birth Cohort Study. Clin Exp Allergy 2011; 58: 1053–8. [DOI] [PubMed] [Google Scholar]
- 23. Nissen SP, Kjaer HF, Høst A, Nielsen J, Halken S. The natural course of sensitization and allergic diseases from childhood to adulthood. Pediatr Allergy Immunol 2013; 24: 549–55. [DOI] [PubMed] [Google Scholar]
- 24. Burks AW, Tang M, Sicherer S, Muraro A, Eigenmann PA, Ebisawa M, et al. ICON: food allergy. J Allergy Clin Immunol 2012; 129: 906–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Flowchart showing number of study subjects from inclusion to the follow‐up at eight years of age.
Figure S2 The associations between the three early manifestations (recurrent wheeze, eczema or food allergy) and each outcome at eight years of age (doctor‐diagnosed asthma, rhinitis, eczema or food allergy) analysed using multivariate logistic regression analysis, adjusting for gender and age.
Figure S3 Age of onset of allergic diseases.
Table S1 Questions on allergic symptoms and diagnoses at one, four‐and‐a‐half and eight years of age.
Table S2 Children included in the present analyses (complete data on all questions, n = 3382) versus all available data on responders at eight years of age (n = 4051).
Table S3 Prevalence and comorbidity of eczema, food allergy and recurrent wheeze at one year of age (n = 3382).


