Abstract
Objective
To explore development of a screening test for rheumatoid arthritis (RA) patients most likely to develop radiographic damage in the next year. The test is a simple, objective measurement of elevated dermal temperature over an inflamed joint in this observational, prospective cohort study.
Methods
Seropositive RA patients were sequentially enrolled into cohorts with hot or cool joints, as determined by a dermal thermometer. Patients naive to biologic therapy were maintained on a stable dosage of methotrexate (20–25 mg/week). The hot‐joint cohort had a joint skin temperature greater than their body temperature on vital signs. Hand/wrist radiographs obtained at baseline and 1 year later were read and scored using modified Sharp/van der Heijde scores (SHS) by a single reader without sequence order or identifiers.
Results
Each cohort consisted of 104 patients enrolled into observation between 2009 and 2014. Patients in the cohort with hot joints had a mean ± SD joint temperature of 1.06 ± 0.69°F above central body temperature and a nearly 4‐fold higher risk of new radiographic damage than those with cool joints (SHS score 8.7 ± 6.2 versus 2.5 ± 1.4; P < 0.001). Sensitivity and specificity for joint temperature to predict radiographic damage in the next year were 92% and 78%, respectively, in the hot‐joint cohort. As expected, this cohort at baseline was younger, had more recent onset RA, and had higher Westergren erythrocyte sedimentation rate levels than the cool‐joint cohort (P < 0.001 for each).
Conclusion
Dermal joint temperature may become a screening test to quickly and accurately identify individual RA patients at high risk for radiographic damage and those who may benefit most from biologic therapy.
Introduction
There is great debate over which rheumatoid arthritis (RA) patients to treat with the expensive, effective therapies currently available 1. When determining the therapy that is most appropriate for a particular patient, clinicians often rely heavily on multiple assessment tools to calculate future disease progression, which can be time‐consuming, and in the utilization of multiple laboratory or ultrasound/computed tomography/magnetic resonance imaging (MRI) techniques, raise evaluation costs markedly 2.
Box 1. Significance & Innovations.
This study efficiently identified rheumatoid arthritis patients at high risk for radiographic damage and assessed them 1 year later.
The objective measurement of dermal temperature was used. This tool requires minimal time (less than 1 minute), is low‐cost, and uses standard office equipment (dermal thermometer).
Currently, there are several prognostic factors in the literature predicting ongoing joint damage and radiographic deformity in RA patients. These include early disease, age at disease onset, seropositive status (i.e., positive rheumatoid factor [RF] or cyclic citrullinated peptide [CCP] positive), and previous evidence of joint damage on radiograph 3. However, despite having lower sensitivity and specificity for predicting serious disease, there is a trend toward utilizing laboratory tests and scales to interpret inflammation (e.g., Westergren erythrocyte sedimentation rate [ESR] and C‐reactive protein [CRP] level) or pain (visual analog scale), as well as numerous functional assessment tools (i.e., the Health Assessment Questionnaire [HAQ] and Routine Assessment of Patient Index Data [RAPID3]) 4. Newer approaches like the multibiomarker disease activity test and ultrasound or MRI measurement of joints are quite costly 5, 6, 7. Thus, there is a need for a simple, cost‐effective tool for determining which patients will develop progressive, destructive RA. The primary objective of this observational trial was to evaluate an assessment tool (dermal temperature) that could be used easily from Uganda to Uruguay, and not involve subjective measurement, literacy, high cost, or a lot of time.
Joints that are warm to the touch have long been associated with the presence of gout, sepsis, or arthritis. Every major medical textbook notes that inflamed joints are hot to the touch on physical examination. Typically this warmth is assessed subjectively, as a sign of disease. In this small series, we quantified dermal temperature at an inflamed joint in order to rapidly and easily identify RA patients who were most likely to develop progressive, destructive disease in the next year.
Patients and methods
Patients
We evaluated stage I–III RA patients >18 years of age who visited an outpatient rheumatology clinic (Desert Medical Advances, Palm Desert, California) between 2009 and 2014. This 1‐year parallel‐group observational study enrolled seropositive (RF‐positive and/or CCP‐positive) patients who met the 1987 American College of Rheumatology (ACR) revised criteria and the 2011 European League Against Rheumatism criteria for RA 8, 9. Patients stable for 3 months or more on methotrexate (20–25 mg/week) and folate supplements (1 mg/day) were eligible for the observational study and were permitted to use corticosteroid therapy (up to 5 mg/day of prednisone) and nonsteroidal antiinflammatory drugs. Patients were excluded from entering a cohort if they used another biologic therapy or disease‐modifying antirheumatic drug (DMARD) before or during the year of observation. If a subject was excluded during the year, another patient was enrolled as a replacement. Separately, a small group of healthy volunteers without RA (n = 25) were evaluated as a matter of reference, in order to determine the usual drop in skin temperature from the central forehead body temperature to the left wrist joint temperature in the general population. This study was conducted in compliance with the Declaration of Helsinki, International Conference on Harmonisation Guidelines for Good Clinical Practice, and with local regulations, with approval from the Western Institutional Review Board.
Protocol design
Patients with stage I–III RA were asked to participate in an observational study to assess RA disease over the subsequent year by radiograph of the hands/wrists. Eligible patients were taking stable doses of methotrexate (and folate) for ≥12 weeks, with no prior use of biologic therapy or other DMARDs. The methotrexate was continued for the observation year. No future therapy was prohibited, but if no biologic agent or other DMARD therapy was initiated, then the 1‐year followup hand/wrist radiograph was obtained. RA patients were sequentially enrolled into the hot‐joint cohort if the joint dermal temperature exceeded the central forehead body temperature obtained during vital signs (n = 104). Those patients with joint temperature lower than central forehead body temperature were sequentially enrolled as the cool‐joint cohort (n = 104) (Figure 1).
Figure 1.
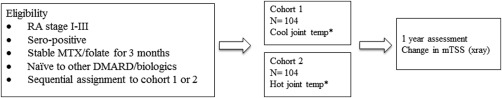
Protocol design. Cool joint defined as less than central temperature recorded on vital signs; hot joint defined as higher than or equal to central temperature recorded on vital signs.
*Dermal temperature over an inflamed rheumatoid arthritis (RA) joint. MTX = methotrexate; DMARD = disease‐modifying antirheumatic drug; mTSS = modified total Sharp/van der Heijde score.
Assessments
At baseline, medical history and Westergren ESR and CRP levels were obtained, along with a physical examination and hand/wrist radiographs. The physical examination included assessment of central dermal forehead temperature and a single joint temperature. The forehead/temporal body temperature has been found to be more reproducible and accurate than when temperature is taken by an oral, ear, or rectal thermometer 10, 11. A digital dermal thermometer (Exergen TemporalScanner TAT‐2000C; accuracy as per manufacturer, 0.3°F) was placed on the forehead to record central body temperature and then on the most painful joint (according to the patient) to record dermal joint temperature. The left wrist was measured as the default joint, unless the patient stated that another joint was more painful. In more than 80% of the patients, the left wrist dermal temperature was recorded; in other patients, the chosen joint was the knee (n = 12), elbow (n = 3), a single metacarpophalangeal joint (n = 2), and shoulder (n = 1). A healthy group of volunteers (n = 25) had dermal temperatures recorded in order to assess the average difference between central temperature and that of the distal wrist in the general population. Hand/wrist radiographs were repeated in the RA patients after 1 year. No laboratory tests or questionnaires were administered at 1 year, since the objective of the study was to determine if a simple measurement at baseline could predict radiographic damage. In December 2013, radiographs were read without sequence order or identifiers and were scored using a modified Sharp/van der Heijde score (SHS) by a single reader (MG) 12. The minimum meaningful change in SHS scores was ≥5 13.
Statistical analysis
The study was designed as exploratory, observational, nonrandomized, and single‐center, and involved no investigational therapy or experimental invasive procedure. This exploratory study was sized by expert opinion and based on the sample sizes used in numerous radiographic RA studies 6, 12, 13, 14. The baseline temperature discrimination threshold (0.1°F) defines 2 sets of patients, the hot‐joint (≥0.1°F) and cool‐joint (<0.1°F) predictor sets. Statistical power was also explored as a proposed screening test, expressed as the difference between anticipated estimates of the positive predictive value and the false omission rate. This difference was assumed to be 20%, to be tested 2‐sided for statistical significance (α = 0.05). A sample size of 104 evaluable patients per predictor set was conservatively expected to yield 81.5% power.
Baseline data were descriptively summarized. The baseline comparisons between predictor sets were based on Wilcoxon's rank sum test coupled with estimated Hodges‐Lehmann empirical distribution shifts and nonparametric confidence intervals (CIs) for the true shift.
Test performance estimates include Cohen's simple kappa coefficient of agreement and Clopper‐Pearson CIs. Logistic regression modeled the statistical robustness of the proposed test to potentially confounding baseline covariates.
The SHS score change from baseline to 1‐year followup was utilized to determine whether a patient had new, destructive joint damage (SHS ≥5) or not (SHS <5) 13. Radiographic analyses are shown as cumulative probability plots of SHS scores and box graphs.
Results
Baseline
In total, 208 RA patients were evaluated. One RA cohort had hot joints (n = 104), with a mean ± SD joint temperature exceeding central body temperature by 1.1 ± 0.69°F (see Supplementary Table 1, available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.22813/abstract). The other RA cohort had cool joints (n = 104), with a mean joint temperature of − 4.3 ± 2.7°F below central body temperature. A healthy group of volunteers without arthritis (n = 25) averaged − 6.3 ± 2.3°F below central body temperature at the wrist. This drop in temperature between the recorded vital sign temperature and the peripheral wrist in the healthy population gives context to the temperature recorded at the wrist in RA patients.
There were baseline differences in the 2 cohorts; the hot‐joint group had active inflammatory disease at baseline. As expected, these patients were younger (49.6 ± 14.1 versus 62.2 ± 14.2 years; P = 0.001), had less time since diagnosis (5.1 ± 2.3 versus 8.3 ± 3.3 years; P < 0.001), and had higher Westergren ESR levels (54.3 ± 19.8 versus 31.4 ± 11.6 mm/hour; P = 0.001) than patients in the cool‐joint cohort at baseline (see Supplementary Table 1, available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.22813/abstract). Notably, Westergren ESR rates in the hot‐ and cool‐joint cohorts had substantial overlap due to high variability (54 ± 20 versus 31 ± 12). The main correlation between elevated dermal temperature over an inflamed joint and subsequent radiographic progression did not change with logistic sensitivity regression analysis for potential confounders (data not shown). Westergren ESR levels, age, and time since diagnosis did not negate the results. There was no difference between the hot‐ or cool‐joint cohorts in the number of female patients (83%, both cohorts) or in the mean baseline CRP levels (∼2 mg/dl, both cohorts).
Change after 1 year
It took 9 months to identify 104 RA patients with cool joints and 42 months to identify 104 RA patients with hot joints. Therefore, at a single office visit, RA patients taking methotrexate were 5 times more likely to have cool joints than hot joints. The SHS score change after 1 year was statistically significantly greater among patients with hot joints (8.7 ± 6.2; 95% CI 6.6–10.8) than among patients with cool joints (2.5 ± 1.4; 95% CI 2.0–3.0; P < 0.001) (Figure 2). Figure 3 shows that the significance in joint destruction is not attributed to a few outliers, but is an inexorable increase of bone damage along with inflammation. In the hot‐joint cohort, 77 of 104 patients (74%) had clear radiographic evidence of new joint damage (SHS ≥5) at 1‐year followup. The remaining 27 (26%) with hot joints did not have progressive, destructive disease (SHS <5) (see Supplementary Table 2, available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.22813/abstract).
Figure 2.
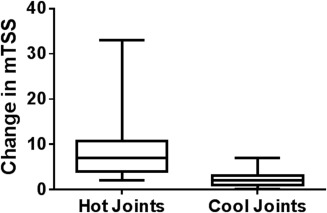
Change in modified total Sharp/van der Heijde score (mTSS) of hand/wrist radiograph at 1 year in patients with hot joints versus cool joints. Minimum meaningful change in mTSS is ≥5. P < 0.001 hot‐joint cohort versus cool‐joint cohort.
Figure 3.
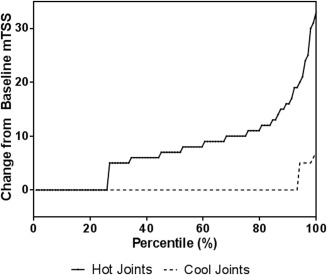
Cumulative probability of hot joints to predict progressive, erosive disease as measured by modified total Sharp/van der Heijde score (mTSS) at 1 year. Minimum meaningful change for mTSS = 5.
In the cool‐joint cohort, 97 of 104 patients (93%) did not have new joint damage on radiograph (SHS <5), whereas the 7 remaining patients (7%) did have bone or cartilage damage. The sensitivity and specificity of using joint temperature to predict the development of joint damage were 92% and 78%, respectively.
Discussion
Determining elevated skin temperature through touch has been an enduring diagnostic maneuver used for centuries worldwide. In this small series, touch was measured objectively and quantified by a simple infrared dermal thermometer to evaluate the risk for progressive, destructive radiographic disease in RA patients. The left wrist was the default joint measured for dermal temperature in the majority of RA patients, but as RA is a systemic disease, multiple joints were warm. A single hot joint would not be consistent with RA, but more likely indicative of a septic joint. The measurement of heat over a joint must be evaluated in context (a single joint versus multiple joints). In this study, the observed subjects had all been diagnosed with RA, and a single hot‐joint temperature was recorded for simplicity of clinical assessment.
Our findings suggest that dermal temperature can quickly and accurately identify individual, specific RA patients at high risk for further destructive change. Patients with hot joints had a nearly 4‐fold higher risk of new radiographic damage than those with cool joints, and more than 70% of patients with hot joints had clear radiographic evidence of new destructive radiographic joint damage 1 year later. Joint temperature had high sensitivity and specificity for predicting new damage in the next year.
It has been reported that Westergren ESR and CRP levels are poorly correlated with radiographic progression in RA patients 14, 15, 16. Because increases in these serum markers are systemic reactions to any form of inflammation (from, e.g., fatty liver, periodontitis, diabetes mellitus, etc.), it is possible that these markers may not be detecting inflammation at a joint per se, but an unrelated comorbid condition 17. For diseases like RA, in which we can measure inflammation directly with a thermometer, there may be no need for serum markers of poor specificity, large variability, and additional time and cost.
Investigators have struggled to find a screening method to predict destructive change in individual, specific RA patients. It is important to detect moderate‐to‐severe RA as soon as possible and to prescribe therapy. Radiographs are now widely accepted as the gold standard to define severe disease for an individual patient, but long‐term followup is costly and evaluation for an individual patient takes years 18. Attempts to quantify RA activity evolved into commonly used scales like the ACR 20% improvement criteria, the Disease Activity Score using ESR or CRP, the HAQ, and the RAPID3. All involve time‐consuming questionnaires, clinical assessment, and scoring. This study was a proof‐of‐concept design, not to compare multiple scoring systems evaluating RA but to determine if joint skin temperature identifying individuals with active inflammation had prognostic significance. The correlation of dermal temperature over an inflamed joint with radiographic progression may be causal through the pathology of RA cytokines. Our findings suggest that joint temperature could be used either as a single measurement of RA or as an objective measure within a multifaceted approach.
Numerous reports have documented poor patient acceptance of expensive RA therapies and poor adherence to therapy 19. Assessment of joint temperature may help to identify patients with progressive, destructive RA who would benefit most from expensive, effective biologic therapy 2. Any patient can understand the concept that their joints “have a fever.” Using a simple clinical tool to identify patients (e.g., a hot joint versus a complex assessment tool) may also positively impact patient understanding, acceptance, and adherence, and, unlike other tools, can quickly and easily be used to ascertain joint temperature as part of standard vital signs in less than 1 minute, with no added cost. This information would be available to the physician at the start of the clinic visit.
Future studies are needed to evaluate the prognostic value of joint temperature and the potential for individualized identification of disease risk. This small pilot study set a cutoff of any joint temperature higher than the central body temperature. A future study might define a hot‐joint cohort as RA patients with a joint that measures over a set point, such as 98°F. Such a cutoff would make assessment very simple and would maintain the specificity and sensitivity of the model. Alternatively, receiver operating characteristic curves using a variety of cutoff points would strengthen the hypothesis that local inflammation indicates active bone loss. This may advance the concept of individualized medicine. Ultimately, assessment of joint temperature provides objective data that may help determine which patients may benefit most from biologic therapy.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. Greenwald had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Greenwald, Ball.
Acquisition of data
Greenwald, Ball.
Analysis and interpretation of data
Greenwald, Ball, Guerrettaz, Paulus.
Supporting information
Supplementary Table 1. Characteristics of Patients at Baseline
Supplementary Table 2. Performance of Proposed Screening Test1
ACKNOWLEDGMENTS
The authors gratefully acknowledge Armando Garsd, PhD, for statistical analysis and support.
REFERENCES
- 1. Moreland LW, O'Dell JR, Paulus HE, Curtis JR, Bathon JM, St.Clair EW, et al. A randomized comparative effectiveness study of oral triple therapy versus etanercept plus methotrexate in early aggressive rheumatoid arthritis: the Treatment of Early Aggressive Rheumatoid Arthritis trial. Arthritis Rheum 2012;64:2824–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease‐modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64:625–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med 2011;365:2205–19. [DOI] [PubMed] [Google Scholar]
- 4. Pincus T, Swearingen CJ, Bergman M, Yazici Y. RAPID 3 (Routine Assessment of Patient Index Data 3), a rheumatoid arthritis index without formal joint counts for routine care: proposed severity categories compared to disease activity score and clinical disease activity index categories. J Rheumatol 2008;35:2136–47. [DOI] [PubMed] [Google Scholar]
- 5. Curtis JR, van der Helm‐van Mil AH, Knevel R, Huizinga TW, Haney DJ, Shen Y, et al. Validation of a novel multibiomarker test to assess rheumatoid arthritis disease activity. Arthritis Care Res (Hoboken) 2012;64:1794–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fukae J, Isobe M, Kitano A, Henmi M, Sakamoto F, Narita A, et al. Radiographic prognosis of finger joint damage predicted by early alteration in synovial vascularity in patients with rheumatoid arthritis: potential utility of power Doppler sonography in clinical practice. Arthritis Care Res (Hoboken) 2011;63:1247–53. [DOI] [PubMed] [Google Scholar]
- 7. American College of Rheumatology Rheumatoid Arthritis Clinical Trials Task Force Imaging Group and Outcome Measures in Rheumatology Magnetic Resonance Imaging Inflammatory Arthritis Working Group . The utility of magnetic resonance imaging for assessing structural damage in randomized controlled trials in rheumatoid arthritis. Arthritis Rheum 2013;65:2513–23. [DOI] [PubMed] [Google Scholar]
- 8. Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- 9. Felson DT, Smolen JS, Wells G, Zhang B, van Tuyl LH, Funovits J, et al. American College of Rheumatology/European League Against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Ann Rheum Dis 2011;70:404–13. [DOI] [PubMed] [Google Scholar]
- 10. Al‐Mukhaizeem F, Allen U, Komar L, Naser B, Roy L, Stephens D, et al. Comparison of temporal artery, rectal and esophageal core temperatures in children: results of a pilot study. Paediatr Child Health 2004;9:461–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lawson L, Bridges EJ, Ballou I, Eraker R, Greco S, Shively J, et al. Accuracy and precision of noninvasive temperature measurement in adult intensive care. Am J Crit Care 2007;16:485–96. [PubMed] [Google Scholar]
- 12. Van der Heijde DM. How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol 2000;27:261–3. [PubMed] [Google Scholar]
- 13. Lassere M, Boers M, van der Heijde D, Boonen A, Edmonds J, Saudan A, et al. Smallest detectable difference in radiological progression. J Rheumatol 1999;26:731–9. [PubMed] [Google Scholar]
- 14. Bruynesteyn K, van der Heijde DM, Boers M, Lassere M, Boonen A, Edmonds J, et al. Minimal clinically important difference in radiological progression of joint damage over 1 year in rheumatoid arthritis: preliminary results of a validation study with clinical experts. J Rheumatol 2001;28:904–10. [PubMed] [Google Scholar]
- 15. Christensen A, Tarp S, Furst D, Dossing A, Amris K, Bliddel H, et al. Most trial eligibility criteria and patient based characteristics do not modify treatment effect: a meta‐epidemiological study of 62 trials and 19,923 rheumatoid arthritis patients. PLoS One 2015;10:1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Connolly M, Mullan RH, McCormick J, Matthews C, Sullivan O, Kennedy A, et al. Acute‐phase serum amyloid A regulates tumor necrosis factor α and matrix turnover and predicts disease progression in patients with inflammatory arthritis before and after biologic therapy. Arthritis Rheum 2012;64:1035–45. [DOI] [PubMed] [Google Scholar]
- 17. Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C‐reactive protein. N Engl J Med 2008;359:2195–207. [DOI] [PubMed] [Google Scholar]
- 18. Drossaers‐Bakker KW, de Buck M, van Zeben D, Zwinderman AH, Breedveld FC, Hazes JM. Long‐term course and outcome of functional capacity in rheumatoid arthritis: the effect of disease activity and radiologic damage over time. Arthritis Rheum 1999;42:1854–60. [DOI] [PubMed] [Google Scholar]
- 19. Wolfe F, Michaud K. Resistance of rheumatoid arthritis patients to changing therapy: discordance between disease activity and patients’ treatment choices. Arthritis Rheum 2007;56:2135–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Characteristics of Patients at Baseline
Supplementary Table 2. Performance of Proposed Screening Test1


