Abstract
Objective
Arhalofenate is a novel antiinflammatory uricosuric agent. The objective of this study was to evaluate its antiflare activity in patients with gout.
Methods
This was a 12‐week, randomized, double‐blind, controlled phase IIb study. Eligible patients had had ≥3 flares of gout during the previous year, had discontinued urate‐lowering therapy and colchicine, and had a serum uric acid (UA) level of 7.5–12 mg/dl. Patients were randomly assigned at a 2:2:2:2:1 ratio to receive 600 mg arhalofenate, 800 mg arhalofenate, 300 mg allopurinol, 300 mg allopurinol plus 0.6 mg colchicine, or placebo once a day. The primary outcome measure was the flare incidence (number of flares divided by time of exposure). The serum UA level was a secondary outcome measure.
Results
A total of 239 gout patients were randomized and took at least 1 dose of study medication. The primary outcome measure comparing flare incidence between 800 mg arhalofenate and 300 mg allopurinol was achieved, with a 46% decrease in the 800 mg arhalofenate group (0.66 versus 1.24; P = 0.0056). Treatment with 800 mg arhalofenate was also significantly better than placebo (P = 0.049) and not significantly different from treatment with 300 mg allopurinol plus 0.6 mg colchicine (P = 0.091). Mean changes in serum UA level were −12.5% with 600 mg arhalofenate and −16.5% with 800 mg arhalofenate (P = 0.001 and P = 0.0001, respectively, versus −0.9% with placebo). There were no meaningful differences in adverse events (AEs) between groups, and there were no serious AEs related to arhalofenate. Urinary calculus occurred in 1 patient receiving 300 mg allopurinol. No abnormal serum creatinine values >1.5‐fold the baseline value were observed in the arhalofenate‐treated groups.
Conclusion
Arhalofenate at a dosage of 800 mg decreased gout flares significantly compared to allopurinol at a dosage of 300 mg. Arhalofenate was well tolerated and appeared safe. Arhalofenate is the first urate‐lowering antiflare therapy.
Gout is the most common cause of inflammatory arthritis 1. It is manifested by extremely painful joint attacks, also called flares, which have a major impact on quality of life 1, 2. Gout results from the deposition of urate crystals in joints. Chronic hyperuricemia, the biochemical signature of the disease, leads to the deposition of urate crystals in articular structures, and the disruption of these crystals is believed to trigger flares 3. Beyond its articular consequences, the hyperuricemia of gout is independently associated with multiple morbidities, including hypertension, increased cardiovascular risks, and progressive kidney disease 4, 5. Currently available urate‐lowering therapies, while effective for decreasing serum uric acid (UA) levels, dramatically increase the chance of experiencing a flare attack, probably by disrupting intraarticular urate crystals 6, 7, 8. This paradoxical effect may be associated with gout patients’ difficulty in understanding and adhering to their treatment.
Gout treatment guidelines recommend colchicine and nonsteroidal antiinflammatory drugs (NSAIDs) for the prevention of flares, notably when initiating urate‐lowering therapy 9. However, colchicine is poorly tolerated, and its prescription is limited in time because of associated toxicities such as azoospermia, bone marrow suppression, neuropathy, and myopathy 10. Colchicine is also associated with numerous potential drug–drug interactions 10, a significant concern in polymedicated gout patients 11. Therefore, a new treatment that both prevents gout flares and lowers serum UA levels would address a major unmet need of gout patients.
Arhalofenate is a uricosuric drug which lowers serum UA by blocking its reabsorption by the proximal tubules of the kidney 12. Arhalofenate activity is mediated by inhibition of URAT1. Additionally, arhalofenate has been suggested to exert potent antiinflammatory activity. In a mouse model of urate crystal–induced inflammation, arhalofenate suppressed the local release of proinflammatory cytokines and, most notably, the release of interleukin‐1β (IL‐1β), a key inducer of gout flare 13. In this model, arhalofenate also prevented the influx of neutrophils at the site of inflammation 13. In vitro studies in isolated murine macrophages have also documented that this cell, which plays a key role in the pathophysiology of a gout attack, is the site of action of arhalofenate 13.
In previous phase II studies in gout patients, it was suggested that in addition to decreasing serum UA levels, arhalofenate could suppress gout flares 14. These phase II studies, however, were conducted on a background of colchicine prophylaxis 14, and arhalofenate antiflare activity could not be definitively proven. Thus, the primary objective of the current study was to demonstrate the antiflare activity of arhalofenate in gout patients in the absence of colchicine prophylaxis.
PATIENTS AND METHODS
The study was conducted in accordance with the Declaration of Helsinki and the Principles of Good Clinical Practice. The study protocol was approved by relevant health authorities in the US, Canada, and the Republic of Georgia and by institutional review boards/ethics committees at each study center. All patients provided written informed consent. A list of participating investigators in the Arhalofenate Flare Study is provided in Appendix A.
Study design
This was a 12‐week, multicenter, randomized, double‐blind, active‐ and placebo‐controlled, parallel‐group phase IIb study. Patients underwent a screening visit within 3 weeks of randomization. Randomization was stratified by serum UA level (<9.5 mg/dl or ≥9.5 mg/dl) and the presence or absence of tophi. Randomized patients entered a double‐blind treatment phase and began receiving treatment on study day 1 with subsequent study visits at the end of weeks 2, 4, 8, and 12. There was a safety follow‐up visit 2 weeks after the last dose of study drugs.
Patient selection
Patients ages 18–75 years diagnosed as having gout according to the American College of Rheumatology criteria 15 and who had experienced ≥3 flares during the 12 months before screening were eligible. At screening, patients had a serum UA level of 7.5–12 mg/dl and had not received any urate‐lowering therapy or colchicine for at least 2 weeks. Patients with an estimated creatinine clearance of <60 ml/minute/1.73 m2, a fractional excretion of urate >10%, or a history of kidney stones were excluded. Liver function test results and creatine kinase levels had to be ≤3× the upper limit of normal (ULN). Patients with secondary hyperuricemia or xanthinuria were excluded. Blood pressure had to be controlled, and findings of electrocardiography (EKG) had to be within normal limits. Patients with a body mass index of >42 kg/m2 or with a medical condition that could interfere with the conduct of the study were excluded.
Patients receiving medication known to affect serum UA levels were required to be receiving a stable dose for at least 2 weeks and to continue to receive the same dose during the study. Concomitant use of potent cytochrome 3A4 inhibitors, cytotoxic drugs, or anticoagulants was prohibited, as was long‐term treatment with NSAIDs or systemic corticosteroids. Women of reproductive potential had to use accepted forms of contraception.
Study treatments and treatment assignment
Study drugs were administered once daily for 12 weeks. There were 5 treatment groups: 600 mg arhalofenate, 800 mg arhalofenate, 300 mg allopurinol, 300 mg allopurinol plus 0.6 mg colchicine, and placebo. Eligible patients were randomly assigned at a 2:2:2:2:1 ratio to the 5 treatment groups; the placebo group had half the number of patients in the other groups.
Study assessments
Each patient was provided with a handheld electronic diary to be used once a day to report flare episodes. Patients were trained during screening by certified study site personnel. The diary first asked patients if they had a joint pain, if this pain was typical of a flare, and whether it was present at rest. If pain was present, the patient was asked additional questions about the nature of this potential gout attack and its treatment. Beginning with the first dose of study drug and for the 12 weeks of the study, the patient reported flare(s) via the electronic diary. Electronic diary data were reviewed with site personnel at weeks 2, 4, 8, and 12. Site personnel also checked patients’ compliance with the electronic diary in an ongoing manner. The electronic diary was also used to fill out a patient's quality‐of‐life questionnaire (the Health Assessment Questionnaire II) 16.
Safety assessments included a complete physical examination, 12‐lead EKG, evaluation of vital signs, and height and weight. Laboratory tests included standard chemistry and hematology panels, estimated creatinine clearance, and the fractional excretion of UA (UA clearance divided by creatinine clearance). During study treatment, investigators, study staff, monitoring personnel, and the sponsor were blinded with regard to the results of testing for serum UA level and fractional excretion of UA.
Study outcome measures
The primary outcome measure of efficacy was the flare incidence from baseline to week 12. The flare incidence was calculated as the number of flares in a treatment arm divided by the total period of time with available electronic diary data. A flare was defined as an episode of patient‐reported acute articular or bursal pain that occurred at rest and was typical of past gout attacks. In addition, both of the following criteria had to be met: the intensity of pain at rest had to be ≥4 on an 11‐point numerical rating scale, and the pain had to be determined by the patient and/or the investigator to require antiinflammatory/analgesic treatment. Finally, at least 2 of 3 possible joint symptoms (swelling, warmth, or tenderness) had to be present, and at least 1 of the following had to be present: rapid onset of pain, decreased range of joint motion, or joint redness. All these were reported by the patient in response to simple questions appearing in the electronic diary (e.g., “was the pain of rapid onset?”). Patients had the opportunity to assess their understanding of each question during the training session.
Secondary efficacy outcome measures included the percent reduction in serum UA level and the proportion of patients with a serum UA level of <6 mg/dl. Other efficacy outcome measures such as quality of life were considered exploratory.
Safety outcome measures were serious adverse events (SAEs), discontinuation from the study for reasons of safety, treatment‐emergent adverse events (TEAEs), and changes in laboratory parameter values. Arhalofenate acid trough levels were determined.
Statistical analysis
A sample size of 50 patients per treatment group provided 80% power to detect a treatment group difference in flare incidence of ∼50% between the 800 mg arhalofenate group and the 300 mg allopurinol group, based on a 2‐sided, 2‐sample test at the 5% level of significance. A placebo group of 25 patients also provided 80% power to detect a 50% difference in serum UA level between the 800 mg arhalofenate group and the placebo group, based on a 2‐sided, 2‐sample test at the 5% level of significance.
The primary efficacy analysis included all randomized patients who took at least 1 dose of study drug and had at least 1 postbaseline day recorded with the electronic diary (modified intent‐to‐treat [ITT] group). Missing electronic diary data were not imputed.
A Poisson regression model was used to analyze flare incidence between treatment groups 17. The model included treatment groups and the randomization stratification criteria as factors. Incidence rate ratios with 95% confidence intervals (95% CIs) and P values for treatment group differences were calculated. Quantitative outcome measures were assessed with analysis of covariance models with the same factors and with the baseline value of the corresponding end point included as covariate. The least squares mean (LSM) ± SEM, 95% CI, and P value were calculated. The time to onset of first flare was estimated using a Kaplan‐Meier method, and treatment groups were compared using the log rank test. For serum UA level, any data available were used with a last observation carried forward imputation. The safety analysis included all randomized patients who took at least 1 dose of study drug. Summary statistics were used.
RESULTS
Overall, 248 patients were randomized for the study. A total of 239 took at least 1 dose of study medication and were included in the modified ITT and safety populations. Thirty‐two patients (13.4%) were withdrawn from the study. The most common reason was a TEAE (8 patients, 3.3%) (Figure 1). Baseline characteristics were comparable among treatment groups (Table 1). The mean ± SD age of the patients was 52.0 ± 10.2 years; most patients were men (95.8%), and 70.7% were white. The mean ± SD serum UA level at baseline was 9.1 ± 1.5 mg/dl, and 20.1% of the patients had tophi.
Figure 1.
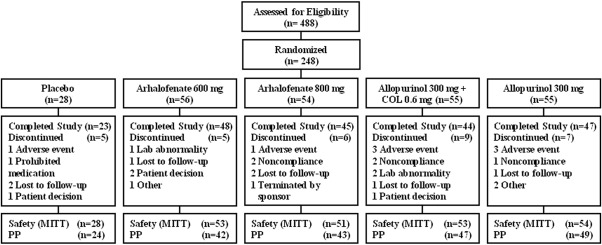
Disposition of the patients in the study. COL = colchicine; MITT = modified intent‐to‐treat; PP = per protocol.
Table 1.
Baseline demographic and clinical characteristics of the safety population (n = 239)a
| Arhalofenate | Allopurinol | Placebo | |||
|---|---|---|---|---|---|
| 600 mg | 800 mg | 300 mg plus colchicine | 300 mg | ||
| (n = 53) | (n = 51) | (n = 53) | (n = 54) | (n = 28) | |
| Age, years | 53.0 ± 11.8 | 52.5 ± 9.8 | 50.2 ± 10.7 | 53.4 ± 8.6 | 49.6 ± 10.0 |
| Men, no. (%) | 51 (96.2) | 47 (92.2) | 53 (100) | 52 (96.3) | 26 (92.9) |
| Ethnicity, no. (%) | |||||
| White | 38 (71.7) | 32 (62.7) | 40 (75.5) | 38 (70.4) | 21 (75.0) |
| Black | 12 (22.6) | 12 (23.5) | 7 (13.2) | 9 (16.7) | 7 (25.0) |
| Asian | 2 (3.8) | 6 (11.8) | 1 (1.9) | 4 (7.4) | 0 |
| Other | 1 (1.9) | 1 (2.0) | 5 (9.5) | 3 (5.6) | 0 |
| Weight, kg | 98.7 ± 18.2 | 100.7 ± 16.3 | 102.3 ± 16.1 | 98.0 ± 19.5 | 105.1 ± 20.0 |
| BMI, kg/m2 | 31.4 ± 4.9 | 32.3 ± 4.8 | 32.7 ± 4.6 | 31.5 ± 4.9 | 33.3 ± 5.6 |
| Serum urate, mg/dl | 9.0 ± 1.6 | 9.3 ± 1.4 | 9.2 ± 1.6 | 9.0 ± 1.4 | 9.1 ± 1.4 |
| Gout flares in last 12 months | 5.0 ± 2.6 | 5.3 ± 3.8 | 4.7 ± 2.8 | 4.6 ± 2.4 | 4.7 ± 2.6 |
| Patients with tophi, no. (%) | 11 (20.8) | 10 (19.6) | 11 (20.8) | 10 (18.5) | 6 (21.4) |
Except where indicated otherwise, values are the mean ± SD. BMI = body mass index.
Efficacy
The primary outcome measure of the study was achieved. The flare incidence was 0.66 in the 800 mg arhalofenate group and 1.24 in the 300 mg allopurinol group, a decrease of 46% (P = 0.0056) (Figure 2).
Figure 2.
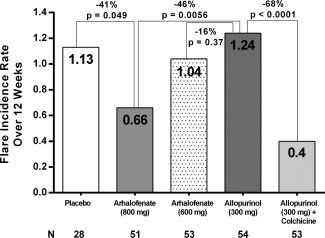
Flare incidence in the 4 treatment arms and in the placebo arm over 12 weeks.
The 800 mg arhalofenate treatment group also had a 41% decrease in flare incidence compared to the placebo group (0.66 versus 1.13; P = 0.049). The flare incidence in the 300 mg allopurinol plus 0.6 mg colchicine group was 0.40, significantly lower than that in the 300 mg allopurinol group (P < 0.0001) but not significantly different from that in the 800 mg arhalofenate group (P = 0.091). There was a 16% decrease in flare incidence in the 600 mg arhalofenate group compared to the 300 mg allopurinol group (P = 0.37) (Figure 2).
For patients who experienced a flare, the median duration of flare was 2.0 days (range 1.0–6.0 days) in the 800 mg arhalofenate group and 1.5 days (range 1.0–18.0 days) in the 300 mg allopurinol group (P not significant). The median duration of flares was 2.4 days (range 1.0–7.0 days) in the placebo group, 2.4 days (range 1.0–28.0 days) in the 600 mg arhalofenate group, and 2.0 days (range 1.0–3.5 days) in the 300 mg allopurinol plus 0.6 mg colchicine group. The median time to onset of first flare in patients experiencing flares was 13 days in the 800 mg arhalofenate group and 10 days in the 300 mg allopurinol group (P = 0.033) (see Supplementary Figure 1, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39684/abstract). It was 31 days in the placebo group, 14 days in the 600 mg arhalofenate group, and 5 days in the 300 mg allopurinol plus 0.6 mg colchicine group. No patients experienced a new flare after 2 months of treatment in either the 800 mg arhalofenate group or the 300 mg allopurinol plus 0.6 mg colchicine group (Supplementary Figure 1). Sixty‐five percent of patients in the 800 mg arhalofenate group experienced no flares during treatment, compared with 39% of patients in the 300 mg allopurinol group (Figure 3). Also, patients in the 800 mg arhalofenate group tended to have fewer flares than did patients in the 300 mg allopurinol group (Figure 3).
Figure 3.
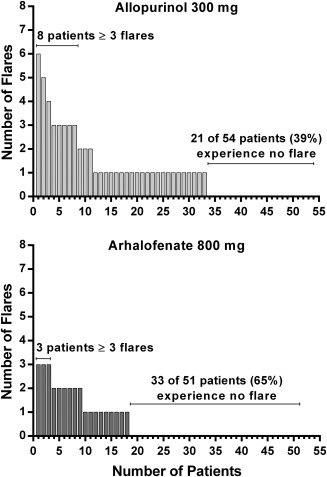
Number of gout flares per individual patient in the 300 mg allopurinol group and in the 800 mg arhalofenate group.
The mean change in serum UA level from baseline to week 12 was −12.5% in the 600 mg arhalofenate group and −16.5% in the 800 mg arhalofenate group, compared with −0.9% in the placebo group (LSM differences P = 0.001 and P = 0.0001, respectively) (Table 2). The mean change in serum UA level from baseline to week 12 was −28.8% in the 300 mg allopurinol group and −24.9% in the 300 mg allopurinol plus 0.6 mg colchicine group. Compared with patients who received placebo, greater proportions of patients treated with 600 mg arhalofenate and 800 mg arhalofenate achieved serum UA levels of <6 mg/dl at week 12 (13.2% and 11.8%, respectively, versus none in the placebo group; both P < 0.001).
Table 2.
Reduction in serum UA level from baseline and number of patients achieving a serum UA level of <6 mg/dl at 12 weeks
| Arhalofenate | Allopurinol | ||||
|---|---|---|---|---|---|
| 600 mg | 800 mg | 300 mg plus colchicine | 300 mg | Placebo | |
| (n = 53) | (n = 51) | (n = 53) | (n = 54) | (n = 28) | |
| Change in serum UA level from baseline, mean ± SD % | −12.5 ± 16.0 | −16.5 ± 15.0 | −24.9 ± 19.7 | −28.8 ± 20.3 | −0.9 ± 14.8 |
| P a | 0.001 | 0.0001 | – | – | – |
| Patients with serum UA level of <6 mg/dl, no. (%) | 7 (13.2) | 6 (11.8) | 18 (34.0) | 26 (48.1) | 0 |
| P a | <0.001 | <0.001 | – | – | – |
Versus placebo group by analysis of covariance, with baseline serum uric acid (UA) level and stratification as covariates.
Safety and tolerability
No patients died during the study. Five patients had an SAE: 3 in the 300 mg allopurinol group (procedural pain after hernia repair, increase in blood creatinine concentration, urinary calculus), 1 in the 300 mg allopurinol plus 0.6 mg colchicine group (peripheral neuropathy), and 1 in the 600 mg arhalofenate group (angioedema). The urinary calculus was considered possibly related to the study drug. The angioedema was considered possibly related to the concomitant use of lisinopril and probably not related to arhalofenate. The angioedema resolved despite continuation of arhalofenate. The other SAEs were considered unrelated to the study drugs.
Nine patients were withdrawn from the study because of TEAEs (Table 3). One patient in the placebo group discontinued because of blurred vision, fatigue, headache, nausea, and vomiting. One patient in the 800 mg arhalofenate group discontinued because of abdominal pain, headache, and a rash. Four patients in the 300 mg allopurinol plus 0.6 mg colchicine group discontinued: 1 because of an abnormal liver function test result, 1 because of a drug hypersensitivity, 1 because of both abdominal pain and hypersomnia, and 1 because of a peripheral neuropathy. Three patients in the 300 mg allopurinol group discontinued: 1 because of eosinophilia of up to 2.4 × 103/μl (20% of white blood cells) and elevated gamma glutamyl transferase, 1 because of diarrhea and vomiting, and 1 because of urinary calculus. No patients in the 600 mg arhalofenate group discontinued because of a TEAE. Of the TEAEs that led to study discontinuation, 1 was considered both serious and severe (urinary calculus in a patient in the 300 mg allopurinol group) and 1 was considered serious (peripheral neuropathy in a patient in the 300 mg allopurinol plus 0.6 mg colchicine group).
Table 3.
Incidence of TEAEs occurring in at least 5% of patientsa
| Arhalofenate | Allopurinol | ||||
|---|---|---|---|---|---|
| 600 mg | 800 mg | 300 mg plus colchicine | 300 mg | Placebo | |
| TEAE | (n = 53) | (n = 51) | (n = 53) | (n = 54) | (n = 28) |
| Any TEAE | 24 (45.3) | 21 (41.2) | 24 (45.3) | 22 (40.7) | 17 (60.7) |
| Serious AE | 1 (1.9) | 0 | 1 (1.9) | 3 (5.6) | 0 |
| TEAE causing discontinuation | 0 | 1 (2.0) | 4 (7.5) | 3 (5.6) | 1 (3.6) |
| CK level increased | 3 (5.7) | 2 (3.9) | 3 (5.7) | 3 (5.6) | 0 |
| Arthralgia | 3 (5.7) | 1 (2.0) | 1 (1.9) | 0 | 1 (3.6) |
| Back pain | 1 (1.9) | 1 (2.0) | 0 | 3 (5.6) | 0 |
| Upper respiratory tract infection | 3 (5.7) | 2 (3.9) | 2 (3.8) | 0 | 2 (7.1) |
| Headache | 3 (5.7) | 2 (3.9) | 0 | 2 (3.7) | 1 (3.6) |
| Hypertension | 1 (1.9) | 2 (3.9) | 2 (3.8) | 1 (1.9) | 2 (7.1) |
| Overdose | 3 (5.7) | 0 | 0 | 0 | 1 (3.6) |
Values are the number (%) of patients. TEAEs = treatment‐emergent adverse events; CK = creatine kinase.
Overall, 108 of 239 patients (45.2%) experienced a TEAE (Table 3). The incidence of TEAEs was similar among active treatment groups. The most common TEAE was increased creatine kinase in 11 patients (4.6%), with no differences between active treatment groups. Most TEAEs were of mild or moderate intensity. Three severe TEAEs were also serious: angioedema, peripheral neuropathy, and urinary calculus.
Elevated liver function test results ≥3× the ULN included 1 elevation of aspartate aminotransferase (AST)/alanine aminotransferase (ALT) in the 800 mg arhalofenate group, 1 elevation of ALT in the 300 mg allopurinol plus 0.6 mg colchicine group, and 1 elevation of AST/ALT in the 300 mg allopurinol group. Overall, the mean estimated creatinine clearance at baseline was 97.3 ml/minute/1.73 m2, with no imbalance between groups. At week 12 overall, the mean estimated creatinine clearance was 100.3 ml/minute/1.73 m2, with no relevant differences within or between groups. Estimated creatinine clearance did not decrease in either arhalofenate group. No renal TEAEs and no abnormal serum creatinine values >1.5‐fold the baseline value were observed in the arhalofenate‐treated groups. Overall, the mean ± SD fractional excretion of UA at baseline was 4.0 ± 1.8%, with no imbalance between groups. At week 12, the increases from baseline were 1% in the 600 mg arhalofenate group and 0.9% in the 800 mg arhalofenate group. No patients receiving arhalofenate had a fractional excretion of urate greater than normal. At week 12, the fractional excretion of UA decreased in the 300 mg allopurinol plus 0.6 mg colchicine group and in the 300 mg allopurinol group (both −0.4%). No clinically meaningful changes in urinary pH were observed.
DISCUSSION
This 12‐week, double‐blind, randomized, active‐ and placebo‐controlled study was primarily designed to demonstrate and evaluate the antiflare activity of arhalofenate, a new uricosuric agent 12. Arhalofenate has also been suggested to exert a unique antiinflammatory activity by inhibiting the local intraarticular production of IL‐1β triggered by monosodium urate monohydrate crystals 13 that is responsible for gout flare attacks. Previous phase II studies, conducted on a background of colchicine prophylaxis, indicated that arhalofenate could have antiflare activities 14. The present study was the first to test the hypothesis that arhalofenate could have antiflare activity in the absence of background colchicine.
In this study, gout patients recorded signs, symptoms, and treatment of painful joint episodes using an electronic diary. An accepted definition of gout flares, consistent with experts’ recommendations 18, was used to collect and analyze flare data. This technology allowed a precise evaluation of each flare episode and avoided the recall biases that occur when flares are recorded only at predefined intervals or through other means, such as telephone interviews, which could be open to subjectivity.
Two hundred thirty‐nine gout patients who had had at least 3 flare episodes during the previous year were randomized and treated in this study. The primary efficacy outcome measure was met, as demonstrated by the finding that arhalofenate at a daily dose of 800 mg reduced the incidence of flare compared to allopurinol at a daily dose of 300 mg, in both a statistically and clinically significant manner (a 46% incidence reduction). Also, more patients in the 800 mg arhalofenate group experienced no flares during treatment than did patients in the 300 mg allopurinol group, and patients in the 800 mg arhalofenate group tended to have fewer flares than did patients in the 300 mg allopurinol group.
In this study, the known antiflare activity of colchicine 19 was confirmed, further validating the electronic diary technology and the definition of flare that was used. The flare incidence was also both clinically and statistically decreased in the 300 mg allopurinol plus 0.6 mg colchicine group compared with the 300 mg allopurinol group. There was also no significant difference in flare incidence between the 800 mg arhalofenate group and the 300 mg allopurinol plus 0.6 mg colchicine group, although the study was not powered to test this comparison. Thus, the clinical utility of 800 mg arhalofenate to prevent gout attacks will have to be judged in the context of the respective risk:benefit ratio of each flare treatment. While colchicine is an effective antiflare therapy 19, it does not reduce serum UA, it can be poorly tolerated with diarrhea or abdominal cramps, and it can potentially induce rare yet severe reactions such as myelosuppression and neuromuscular toxicity 10. In addition, colchicine has multiple potential interactions with commonly prescribed drugs, and the dose needs to be adjusted in patients with renal impairment 10.
The present study also confirmed that arhalofenate decreases serum UA levels through its uricosuric activity 12. However, compared to the arhalofenate‐treated groups, both allopurinol‐treated groups achieved greater reductions in serum UA levels. Thus, both the antiflare and uricosuric activities of arhalofenate will be better suited for a combination regimen with a xanthine oxidase inhibitor.
Overall, the incidence of TEAEs was similar across active treatment groups. The increased incidence of creatine kinase elevation in gout patients, irrespective of treatment received, has been recognized 20. The documented SAEs, such as drug hypersensitivity reactions and a peripheral neuropathy, were already known to be associated with allopurinol and colchicine 6, 8, 10, 19. However, arhalofenate was well tolerated and appeared safe. Notably, no significant impacts on hepatic or renal functions were associated with arhalofenate.
Clinical studies completed in patients with gout thus far indicate that arhalofenate, when used in combination with febuxostat, demonstrates potent serum UA–lowering activities. In a recent study 14, all patients receiving the combination of arhalofenate and febuxostat achieved a serum UA level below the recommended target goal of 6 mg/dl 8, and most achieved a serum UA level of <5 mg/dl, a target goal recommended for gout patients with tophi 8. Furthermore, and consistent with these results, the absence of pharmacodynamic interaction between arhalofenate and febuxostat has been demonstrated in a preliminary study 21.
Multiple studies have shown that a minority of gout patients receive effective treatment, and many of them continue to experience recurrent flare attacks and further joint damage 22, 23. Available urate‐lowering therapies have not improved the problem of the mismanagement of gout. One of the key issues associated with urate‐lowering therapies is that they make flares worse when treatment is initiated 6, 7, 24, 25, and this paradox is difficult for patients to understand. Consequently, gout treatment guidelines recommend the use of colchicine, systemic corticosteroids, or NSAIDs for prevention and treatment of acute gout flares 9. While colchicine and NSAIDs are effective for treating gout flares, they are poorly tolerated or contraindicated in the majority of patients 4, 11. In one analysis of the safety of gout therapy, >90% of patients had at least 1 contraindication to NSAIDs and systemic steroids, and >30% had at least 1 strong contraindication to colchicine 11. Using the antiflare activity and serum UA–lowering properties of arhalofenate in combination with febuxostat could potentially help more gout patients to reach their serum UA target goal without triggering urate‐lowering therapy–induced flares.
One limitation of this phase II study is that it covered only a 12‐week treatment period and enrolled a limited number of patients. The results will have to be confirmed in a larger group of patients for a longer period of time using a gout treatment regimen that combines arhalofenate with febuxostat. This will be the objective of the arhalofenate phase III program.
In conclusion, this phase II study demonstrated that in gout patients the antiinflammatory properties of arhalofenate, a uricosuric agent, can be translated in the clinic to prevent acute gout flare attacks. Arhalofenate was well tolerated and appeared safe. Further studies will evaluate the antiflare and serum UA–lowering activities of arhalofenate when combined with the xanthine oxidase inhibitor febuxostat.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Boudes had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Steinberg, Martin, McWherter, Boudes.
Acquisition of data
Poiley, Steinberg, Choi, Martin, Boudes.
Analysis and interpretation of data
Poiley, Steinberg, Davis, Martin, McWherter, Boudes.
ROLE OF THE STUDY SPONSOR
CymaBay Therapeutics was involved in the study design and in the collection, analysis, and interpretation of the data, the writing of the manuscript, and the decision to submit the manuscript for publication. Publication of this article was not contingent upon approval by CymaBay Therapeutics.
Supporting information
Supplementary Figure 1. Kaplan‐Meier plot of time from baseline to onset of first flare during the treatment period (modified intent‐to‐treat set).
ACKNOWLEDGMENTS
The authors would like to acknowledge Richard S. Perry for preparing the first draft of the manuscript and Yunbin Zhang of INC Research for performing the statistical analysis.
ARHALOFENATE FLARE STUDY PARTICIPATING INVESTIGATORS
The following investigators participated in the arhalofenate flare study: in the US, Drs. H. Bays (Louisville, KY), D. Bolshoun (Denver, CO), E. Bolster (Summerville, SC), D. Cheung (Long Beach, CA), C. Cone (Missoula, MT), R. DeGarmo (Greer, SC), J. Earl (Hickory, NC), I. El Asmar (Los Angeles, CA), D. Felber (Omaha, NE), D. Fitz‐Patrick (Honolulu, HI), A. Gaffo (Birmingham, AL), G. Gottschlich (Cincinnati, OH), J. Greenwald (New York, NY), C. Griffin (Oklahoma City, OK), M. Guice (Los Angeles, CA), W. Harper (Raleigh, NC), J. Hill (DeLand, FL), C. Huffman (Tampa Bay, FL), R. Huling Jr. (Olive Branch, MS), F. Johnson (Owensboro, KY), S. Kafka (Clarksburg, WV), J. Keane (Tucson, AZ), W. Kirby (Birmingham, AL), J. Kirstein (West Jordan, UT), E. Kolettis (Clearwater, FL), G. Lefebvre (St. Petersburg, FL), A. Mabaquiao (El Cajon, CA), K. Maynard (Brownsburg, IN), H. McIlwain (Tampa, FL), R. McNeill (Salisbury, NC), D. Mehta (Elizabethtown, KY), R. Montgomery (Greensboro, NC), C. Morgan (Washington, DC), J. Poiley (Orlando, FL), S. Powell (Bristol, TN), K. Radbill (Orlando, FL), M. Reschak (St. Louis, MO), R. Surowitz (Jupiter, FL), J. Tarro (Portland, OR), M. Turner (Meridian, ID), F. Velazquez (Houston, TX), L. Watkins (Little Rock, AR), J. White (Kingsport, TN), H. Williams (Birmingham, AL), J. Wilson (Winston‐Salem, NC), F. Zaidi (New Port Richey, FL); in Canada, Drs. S. Henein (Newmarket, ON), A. Toma (Toronto, ON); in Georgia, Drs. T. Burtchuladze (Tbilisi), E. Kartvelishvili (Tbilisi), L. Kilasonia (Tbilisi), L. Lagvilava (Tbilisi), L. Shalamberidze (Tbilisi), N. Tsiskarishvili (Tbilisi).
ClinicalTrials.gov identifier: NCT02063997.
Supported by CymaBay Therapeutics.
Drs. Steinberg, Choi, Martin, McWherter, and Boudes own stock or stock options in CymaBay Therapeutics; Dr. McWherter also is listed as an inventor on CymaBay Therapeutics arhalofenate patents. Dr. Davis received consulting fees from CymaBay Therapeutics for work on the current study (less than $10,000).
REFERENCES
- 1. Wertheimer A, Morlock R, Becker MA. A revised estimate of the burden of illness of gout. Curr Ther Res Clin Exp 2013;75:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Edwards NL, Sundy JS, Forsythe A, Blume S, Pan F, Becker MA. Work productivity loss due to flares in patients with chronic gout refractory to conventional therapy. J Med Econ 2011;14:10–5. [DOI] [PubMed] [Google Scholar]
- 3. Brook RA, Forsythe A, Smeeding JE, Edwards NL. Chronic gout: epidemiology, disease progression, treatment and disease burden. Curr Med Res Opin 2010;26:2813–21. [DOI] [PubMed] [Google Scholar]
- 4. Stamp LK. Safety profile of anti‐gout agents: an update. Curr Opin Rheumatol 2014;26:162–8. [DOI] [PubMed] [Google Scholar]
- 5. Smith E, Hoy D, Cross M, Merriman TR, Vos T, Buchbinder R, et al. The global burden of gout: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis 2014;73:1470–6. [DOI] [PubMed] [Google Scholar]
- 6. Becker MA, Schumacher HR Jr, Wortmann RL, MacDonald PA, Eustace D, Palo WA, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med 2005;353:2450–61. [DOI] [PubMed] [Google Scholar]
- 7. Schumacher HR Jr, Becker MA, Wortmann RL, MacDonald PA, Hunt B, Streit J, et al. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28‐week, phase III, randomized, double‐blind, parallel‐group trial. Arthritis Rheum 2008;59:1540–8. [DOI] [PubMed] [Google Scholar]
- 8. Khanna D, Fitzgerald JD, Khanna PP, Bae S, Singh MK, Neogi T. 2012 American College of Rheumatology guidelines for management of gout. Part 1: Systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken) 2012;64:1431–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khanna D, Khanna PP, Fitzgerald JD, Singh MK, Bae S, Neogi T. 2012 American College of Rheumatology guidelines for management of gout. Part 2: Therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken) 2012;64:1447–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Colcrys [package insert]. Deerfield (IL): Takeda Pharmaceuticals America; 2012. [Google Scholar]
- 11. Keenan RT, O'Brien WR, Lee KH, Crittenden DB, Fisher MC, Goldfarb DS, et al. Prevalence of contraindications and prescription of pharmacologic therapies for gout. Am J Med 2011;124:155–63. [DOI] [PubMed] [Google Scholar]
- 12. Lavan BE, McWherter C, Choi YJ. Arhalofenate, a novel uricosuric agent, is an inhibitor of human uric acid transporters [abstract]. Ann Rheum Dis 2013;71 Suppl 3:450–1. [Google Scholar]
- 13. Choi YJ, Larroca V, Lucman A, Vicena V, Abarca N, Rantz T, et al. Arhalofenate is a novel dual‐acting agent with uricosuric and anti‐inflammatory properties [abstract]. Arthritis Rheum 2012;64 Suppl:S697. [Google Scholar]
- 14. Steinberg A, Vince B, Choi YJ, Martin R, McWerther C, Boudes P. A study to evaluate the pharmacodynamics, pharmacokinetics and safety of arhalofenate in combination with febuxostat when treating hyperuricemia associated with gout [abstract]. Ann Rheum Dis 2015;74 Suppl 2:543–4. [DOI] [PubMed] [Google Scholar]
- 15. Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yu TF. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum 1977;20:895–900. [DOI] [PubMed] [Google Scholar]
- 16. Wolfe F, Michaud K, Pincus T. Development and validation of the Health Assessment Questionnaire II: a revised version of the Health Assessment Questionnaire. Arthritis Rheum 2004;50:3296–305. [DOI] [PubMed] [Google Scholar]
- 17. Stokes ME, Davis CS, Koch GG. Poisson regression and related loglinear models In: Categorical data analysis using SAS. 3rd ed. Cary (NC): SAS Institute; 2012. p 367–413. [Google Scholar]
- 18. Gaffo AL, Schumacher HR, Saag KG, Taylor WJ, Dinnela J, Outman R, et al. Developing a provisional definition of flare in patients with established gout. Arthritis Rheum 2012;64:1508–17. [DOI] [PubMed] [Google Scholar]
- 19. Borstad GC, Bryant LR, Abel MP, Scroggie DA, Harris MD, Alloway JA. Colchicine for prophylaxis of acute flares when initiating allopurinol for chronic gouty arthritis. J Rheumatol 2004;31:2429–32. [PubMed] [Google Scholar]
- 20. Choi YJ, Steinberg A, Boudes P. AB0929. Elevation of creatine kinase in gout subjects: a systematic evaluation [abstract]. Ann Rheum Dis 2015;74 Suppl 2:1210–1. [Google Scholar]
- 21. Steinberg A, Choi YJ, Martin R, McWherter C, Boudes P. A study to evaluate the pharmacodynamics, pharmacokinetics and safety of arhalofenate in combination with febuxostat when treating hyperuricemia associated with gout [abstract]. Arthritis Rheumatol 2015;67 Suppl 10 URL: http://acrabstracts.org/abstract/a-study-to-evaluate-the-pharmacodynamics-pharmacokinetics-and-safety-of-arhalofenate-in-combination-with-febuxostat-when-treating-hyperuricemia-associated-with-gout/. [DOI] [PubMed] [Google Scholar]
- 22. Neogi T, Hunter DJ, Chaisson CE, Allensworth‐Davies D, Zhang Y. Frequency and predictors of inappropriate management of recurrent gout attack in a longitudinal study. J Rheumatol 2006;33:104–9. [PubMed] [Google Scholar]
- 23. Kuo CF, Grainge MJ, Mallen C, Zhang W, Doherty M. Rising burden of gout in the UK but continuing suboptimal management: a nationwide population study. Ann Rheum Dis 2015;74:661–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Becker MA, Schumacher HR Jr, Wortmann RL, MacDonald PA, Palo WA, Eustace D, et al. Febuxostat, a novel nonpurine selective inhibitor of xanthine oxidase: a twenty‐eight–day, multicenter, phase II, randomized, double‐blind, placebo‐controlled, dose‐response clinical trial examining safety and efficacy in patients with gout. Arthritis Rheum 2005;52:916–23. [DOI] [PubMed] [Google Scholar]
- 25. Saag K, Fitz‐Patrick D, Kopicko J, Fung M, Bhakta N, Adler S, et al. Lesinurad, a selective uric acid reabsorption inhibitor, in combination with allopurinol: results from a phase III study in gout patients having an inadequate response to standard of care (CLEAR 1) [abstract]. Ann Rheum Dis 2015;74 Suppl 2:540. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Kaplan‐Meier plot of time from baseline to onset of first flare during the treatment period (modified intent‐to‐treat set).


