Summary
The biosynthesis of cell surface‐located galactofuranose (Galf)‐containing glycostructures such as galactomannan, N‐glycans and O‐glycans in filamentous fungi is important to secure the integrity of the cell wall. UgmA encodes an UDP‐galactopyranose mutase, which is essential for the formation of Galf. Consequently, the ΔugmA mutant lacks Galf‐containing molecules. Our previous work in Aspergillus niger work suggested that loss of function of ugmA results in activation of the cell wall integrity (CWI) pathway which is characterized by increased expression of the agsA gene, encoding an α‐glucan synthase. In this study, the transcriptional response of the ΔugmA mutant was further linked to the CWI pathway by showing the induced and constitutive phosphorylation of the CWI‐MAP kinase in the ΔugmA mutant. To identify genes involved in cell wall remodelling in response to the absence of galactofuranose biosynthesis, a genome‐wide expression analysis was performed using RNAseq. Over 400 genes were higher expressed in the ΔugmA mutant compared to the wild‐type. These include genes that encode enzymes involved in chitin (gfaB, gnsA, chsA) and α‐glucan synthesis (agsA), and in β‐glucan remodelling (bgxA, gelF and dfgC), and also include several glycosylphosphatidylinositol (GPI)‐anchored cell wall protein‐encoding genes. In silico analysis of the 1‐kb promoter regions of the up‐regulated genes in the ΔugmA mutant indicated overrepresentation of genes with RlmA, MsnA, PacC and SteA‐binding sites. The importance of these transcription factors for survival of the ΔugmA mutant was analysed by constructing the respective double mutants. The ΔugmA/ΔrlmA and ΔugmA/ΔmsnA double mutants showed strong synthetic growth defects, indicating the importance of these transcription factors to maintain cell wall integrity in the absence of Galf biosynthesis.
Introduction
The fungal cell wall is essential to prevent lysis during growth and development. The cell wall of filamentous fungi is composed of several different carbohydrate polymers (chitin, β‐1,3‐glucan, β‐1,3/1,4‐glucan, α‐glucan and galactomannan) and glycoproteins (galactomannoproteins) (Guest and Momany, 2000; Gastebois et al., 2009; Free, 2013). In Aspergilli, β‐1,3‐glucan together with chitin form the cell wall scaffold to which other components (galactomannan and α‐glucans) bind and are retained (Fontaine et al., 2000; Dichtl et al., 2015). The β‐1,3‐glucan also acts as a scaffold to cell wall proteins as the A. niger, cell wall protein A (CwpA) and at least six other cell wall proteins have been shown to be covalently linked to the β‐glucan part of the cell wall (Damveld et al., 2005b).
Attacks by compounds that inhibit cell wall synthesis or by cell wall‐degrading enzymes secreted by competing microorganisms or plants represent a serious threat for fungal survival. Fungi have developed several surviving strategies to cope with these threats. The best known strategy of cell wall reinforcement in response to cell wall stress is to activate the so‐called cell wall integrity (CWI) pathway. This pathway is studied in Saccharomyces cerevisiae in most detail, but is conserved in several other species, including Aspergilli (Levin, 2011; Valiante et al., 2015). The CWI signalling pathway is composed of cell wall stress sensors, which generate a signal that activates a Rho‐Pkc1‐MAP kinase module, resulting in the activation of the Rlm1 and Swi4/6 transcription factors. These transcription factors are required for modulating gene expression in response to cell wall stress (see for review: Levin, 2011). In A. niger the requirement of RlmA for the induction of the α‐glucan synthase A gene (agsA) in response to Calcofluor white has been established (Damveld et al., 2005a, 2005b, 2005c). Deletion of RlmA renders A. niger sensitive to Calcofluor white, and to a lesser extent also to other cell wall disturbing agents such as caspofungin (CA), aureobasidinA (AbaA), FK506 or fenpropimorph (FP), indicating that other remodelling mechanisms might exist (Fiedler et al., 2014). In the same study, transcription factor binding site enrichment analysis of differentially expressed genes in response to CA, AbaA, FK506 and FP was performed, which identified possible involvement of both RlmA and MsnA (homolog of Msn2/4 in S. cerevisiae) transcription factors in counteracting CA‐induced cell wall stress. Indeed, deletion of the MsnA transcription factor, like deletion of the RlmA transcription factor, led to increased susceptibility towards CA (Fiedler et al., 2014). MsnA has been shown to be required for coping with several stress responses, including osmotic and oxidative stress (Han and Prade, 2002; Bose et al., 2005; Hong et al., 2013).
The observation that agsA expression is induced in response to cell wall stress was previously used to set up a mutant screen for cell wall mutants (Damveld et al., 2008). The rationale of this screen was that mutants with impaired cell wall strength, e.g. because of a mutation in a gene that encodes an enzyme involved in cell wall biosynthesis, would activate agsA expression. To isolate mutants showing constitutive activation of the agsA gene, the acetamidase reporter gene was cloned behind the agsA promoter, and mutants able to grow on acetamide were isolated. Using this screen, we identified the ΔugmA mutant, which lacks Galf in its cell wall (Damveld et al., 2008; Park et al., 2014). The ugmA gene encodes a UDP‐galactopyranose mutase, which converts UDP‐galactopyranose to UDP‐galactofuranose (UDP‐Galf). UDP‐Galf is subsequently used for the synthesis of Galf‐containing glycoconjugates such as cell wall galactomannan, N‐ and O‐glycans, and glycolipids (Tefsen et al., 2012).
In this study, we aimed at obtaining a broader view of understanding cell wall remodelling in the absence of Galf‐glycoconjugate biosynthesis. We therefore used the ΔugmA mutant to perform a genome‐wide expression analysis using RNA‐seq to identify differentially expressed genes. As expected, among the genes induced in the ΔugmA mutant, the agsA gene was included, as well as several other genes whose function could be directly linked to cell wall biosynthesis. A transcription factor binding site enrichment analysis of genes up‐regulated in the ΔugmA mutant strain revealed several transcription factor binding sites to be overrepresented, including binding sites for the RlmA, MsnA, PacC and SteA transcription factors. The ΔugmA/ΔrlmA and ΔugmA/ΔmsnA double mutants showed severe synthetic growth defects, indicating that RlmA and MsnA play an important role in survival of the ΔugmA mutant. By aligning the new results obtained in this study to previous cell wall stress related transcriptomic studies in A. niger, a better understanding of the transcriptional response of the CWI signalling response is obtained which serves as a solid basis for comparing both differences and similarities in relation to CWI signalling in filamentous fungi.
Results
Constitutive phosphorylation of CWI‐MAPK in the ΔugmA mutant of A. niger
The ΔugmA mutant of A. niger was isolated in a screen for cell wall mutants by selecting for mutants with induced expression of agsA. Targeted deletion of the ugmA gene confirmed its importance for CWI (Damveld et al., 2008). In subsequent studies, we showed that the ΔugmA mutant does not contain Galf‐containing glycostructures in the cell wall (galactomannan) or the medium (galactomannoproteins) (Park et al., 2014; Park et al., 2015). Because the induction of agsA in response to Calcofluor White induced cell wall stress is RlmA dependent (Damveld et al., 2005a) it was previously suggested that the transcriptomic responses in the ugmA mutant were mediated via the CWI pathway (Damveld et al., 2008). To establish the role of the CWI pathway in cell wall remodelling in the ΔugmA mutant more firmly, the phosphorylation state of the CWI‐related MAP kinase (CWI‐MpkA) was evaluated. Therefore, proteins extracts were prepared from wild‐type and ΔugmA strains and analysed by Western blot using anti‐phospho p44/42. The total amount of MpkA was detected by using the anti p44/42 MAPK antibody. As shown in Fig. 1, the MAP kinase in the ΔugmA mutant displays at various time points during growth a consistent higher phosphorylation status compared the wild‐type strain. Quantification of the signal indicates a threefold higher level of phosphorylation (Fig. 1). These results indicate that loss of galactofuranose biosynthesis signals via MpkA phosphorylation in the A. niger CWI pathway.
Figure 1.
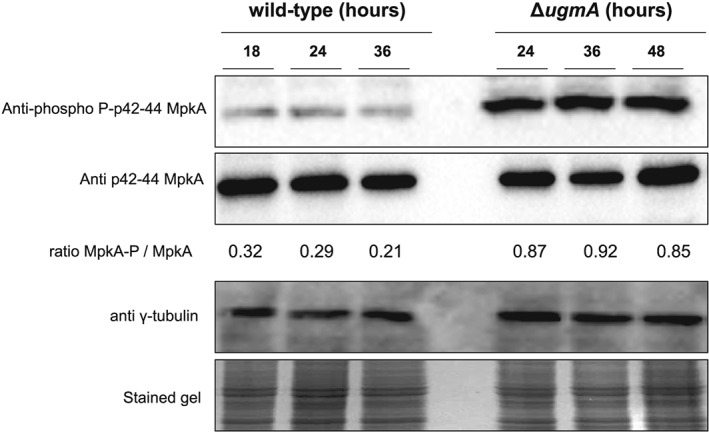
The ΔugmA mutant strain has increased and constitutive CWI pathway activation. Conidia from the wild‐type and mutant strain were grown in liquid CM medium. The samples were collected at the indicated time points for Western blot preparation. The phosphorylated fractions and the total MpkA amount were detected using anti‐phospho p44/42 MAPK and anti‐p44/42 MAPK antibodies respectively. The γ‐tubulin antibody and the Coomassie Brilliant Blue stained gel were used as loading sample controls. Densitometry analysis of western blots showing the ratio of phosphorylated MpkA/MpkA expressed as the relative abundance (arbitrary units).
Growth analysis of the ΔugmA mutant of A. niger
To perform genome‐wide expression analysis of the ΔugmA mutant, we initially cultivated it in a bioreactor under conditions normally used for wild‐type A. niger batch cultivations (Jørgensen et al., 2010; Nitsche et al., 2012a, 2012b). According to this standard growth protocol, the pH of the cultivations is set at pH 3.0 during spore germination and kept constant during the exponential and post‐exponential growth phases. Starting the cultivation at pH 3.0 prevents aggregation of the conidiospores and thereby prevents pellet formation. The pH is normally kept continuously at 3.0, which is close to the normal ambient pH created by A. niger through the secretion of acids. However, we noticed very poor growth of the ΔugmA at pH 3.0 (Table 1) compared to the wild‐type strain (N402) and therefore also examined growth of the ΔugmA mutant at other pH values. In the bioreactor cultures run at pH 4.0, pH 5.0 and pH 6.0 were started at pH 3.0 to prevent spore aggregation and the pH was set to the desired pH after germination of the spores which takes about 5 h. The maximum growth rate of the ΔugmA was strongly improved at pH 4.0 and 5.0, but still slightly lower than in the wild‐type (Table 1). At pH 6.0, the maximum growth rates of both the wild‐type and the ΔugmA mutant were significantly lower (Table 1). The morphology of the ΔugmA mutant was still aberrant at pH 5.0 compared to the wild‐type strain and characterized by short hyphal fragments and increased branching (Fig. 2A). At all pH conditions, the ΔugmA mutant did not produce any Galf‐containing glycostructures in the culture medium, whereas Galf was present in the culture medium of the wild‐type strain (Fig. 2B shows data for pH 5.0; data obtained at the other pHs not shown).
Table 1.
Growth characteristics of the wild‐type (N402) and the ΔugmA mutant in pH‐controlled bioreactors.
| N402 | ΔugmA | |||
|---|---|---|---|---|
| pH | μmax (h−1)* | Max. biomass (g/L) | μmax(h−1) | Max. biomass (g/L) |
| 3.0 | 0.22 ± 0.02 | 4.81 ± 0.53 | 0.04 | 2.43 |
| 4.0 | 0.23 | 3.79 | 0.20 | 4.24 |
| 5.0 | 0.25 ± 0.04 | 3.37 ± 0.02 | 0.20 ± 0 | 3.45 ± 0.01 |
| 6.0 | 0.14 ± 0.01 | 1.28 ± 0.18 | 0.11 ± 0.01 | 1.57 ± 0.05 |
The averages and standard deviations of the growth rate are based on independent bioreactor cultivations.
Figure 2.
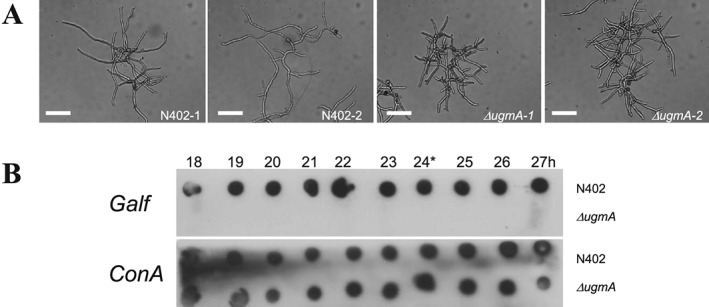
Cultivation of the wild‐type (N402) and the ΔugmA mutant in bioreactors.
A. Hyphal morphology of N402 and ΔugmA mutant. Note the compact growth phenotype of the ΔugmA mutant.
B. Dot blot assay to detect the presence of Galf residues on secreted glycoconjugates from A. niger during batch growth. A. niger wild‐type strain (N402) and ΔugmA mutant were grown for the times indicated from inoculation of the spores, and cell‐free medium was spotted on nitrocellulose filter paper. The blots were incubated with the anti‐Galf antibody (L10) (Heesemann et al., 2011) to detect the presence of Galf or incubated with ConA‐PO to detect mannoproteins.
Comparison of the transcriptome of the ΔugmA mutant with the wild‐type strain
To identify genes in A. niger that are differentially expressed in the ΔugmA mutant compared to the wild‐type strain, RNA was extracted from mycelium of the wild‐type (N402) and the ΔugmA mutant after growth at pH 5. The maximum growth rate of the ΔugmA strain (0.20 h−1) was slightly lower than the growth rate of the wild‐type strain (0.25 h−1). Biomass accumulation profiles of the wild‐type (N402) and ΔugmA mutant at pH 5 were reproducible (Table 1) and mycelium for RNA extraction was harvested when 60% of the maximum biomass was reached. The RNA samples were analysed by Northern blot to examine whether marker genes known or expected to be induced in the ΔugmA mutant were up‐regulated. As the ΔugmA mutant was identified in a screen for mutants with induced expression of an agsA‐driven reporter gene (Damveld et al., 2008), we expected the agsA gene to be higher expressed in the ΔugmA. As shown in Fig. 3, the agsA gene was indeed strongly induced in the ΔugmA mutant compared to the wild‐type strain. We also tested the expression of three other genes (gfaA: glutamine:fructose‐6‐phosphate amidotransferase, which is responsible for the first step in chitin synthesis) and two genes encoding putative cell wall proteins (PhiA and PirA), which were previously shown to be induced by cell wall stress (Ram et al., 2004; Meyer et al., 2007). These genes were also clearly induced in the ΔugmA mutant indicating that a typical cell wall stress response was activated in the ΔugmA mutant (Fig. 3).
Figure 3.
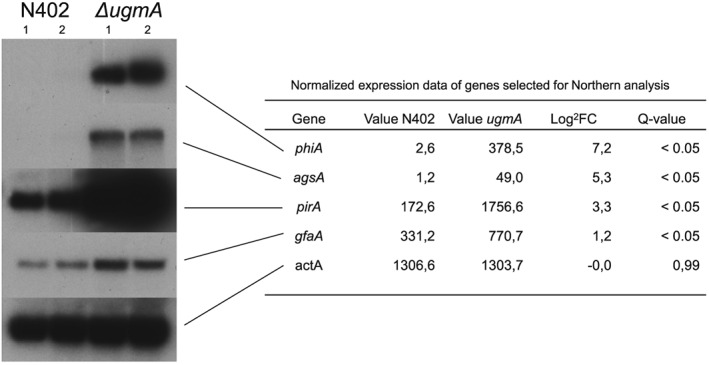
Northern blot analysis of selected genes during batch growth in bioreactors. RNA was isolated from mycelium grown at pH 5.0 for 18 h, when about 60% of the glucose was consumed, and analysed by Northern blot. Hybridization with an actin probe was used to confirm similar loading.
Genome‐wide expression analysis using RNA‐seq revealed in total 741 genes that were differentially expressed between the wild‐type and the ΔugmA mutant. Differential expression was defined as a statistically significant, differential expression with a false discovery rate (FDR) <0.05. In total, 432 genes were relatively higher expressed in the ΔugmA mutant, and we will refer to these genes as UgmAup and 309 genes were relatively lower expressed in the ΔugmA mutant (UgmAdown). The normalized expression values for the genes monitored by Northern blot are shown in Fig. 3 and agree well with the Northern blot results. A comprehensive list of all differentially expressed genes including statistical significance and transcript ratios is presented in Table S1 (UgmAup) and Table S2 (UgmAdown).
To get a better insight into the processes that are affected in the ΔugmA mutant, we performed GO‐enrichment analysis of the genes that were significantly higher expressed in the ΔugmA mutant using FetGOat (Nitsche et al., 2012a, 2012b) (Tables 2 and 3). Twenty‐one GO terms (Biological Processes) were overrepresented in the ΔugmA mutant (Table S3). Among those GO terms, six terminal nodes were present (Table 2). GO enrichment analysis of the genes down‐regulated in the ΔugmA mutant identified three GO terms that were related to zinc metabolism (Table S4). The three genes in the terminal node (GO:0071577; zinc ion transmembrane transport) (Table 3) all encode putative zinc transporters, suggesting a lower need for zinc in the ΔugmA mutant. Closer examination of the genes and GO terms upregulated and enriched in the ΔugmA mutant revealed two groups of overrepresented genes. The first group of genes encodes proteins related to chitin biosynthesis and included genes encoding proteins involved in UDP‐N‐acetyl‐glucosamine biosynthesis (gnaA, gfaA and gfaB) as well as chitin polymerization (chsA and chsB) (Table 4). The second group encodes glycosyl hydrolases (GH) and includes genes encoding proteins that act on the plant cell wall polysaccharides cellulose, xylan and galactan. In total, 18 plant cell wall degradation genes were highly expressed in the ΔugmA mutant. (See below)
Table 2.
Enriched GO‐terms in the up‐regulated genes in the ΔugmA strain.
| Terminal GO term | Description GO term | Systematic name | Gene name | Description |
|---|---|---|---|---|
| GO:0045490 | Pectin catabolic processes | An14g04200 | rhgB | Rhamnogalacturonan hydrolase |
| An01g11520 | pgaI | Polygalacturonase | ||
| An04g09690 | Putative pectin methylesterase | |||
| An16g02730 | Putative arabinan endo‐1,5‐alpha‐L‐arabinosidase | |||
| An02g10550 | abnC | Putative endo‐alpha‐1,5‐arabinanase | ||
| GO:0006031 | Chitin biosynthetic processes | An09g04010 | chsB | Putative chitin synthase, class III; induced by caspofungin |
| An03g05940 | gfaB | Putative glutamine:fructose‐6‐phosphate amidotransferase | ||
| An07g05570 | chsA | Chitin synthase class I | ||
| An12g07840 | gnaA | Putative glucosamine‐6‐phosphate N‐acetyltransferase | ||
| An18g06820 | gfaA | Putative glutamine:fructose‐6‐phosphate amidotransferase | ||
| GO:0005996 | Monosaccharide metabolic processes | An01g11520 | pgaI | Polygalacturonase |
| An02g10550 | abnC | Putative endo‐alpha‐1,5‐arabinanase | ||
| An14g04200 | rhgB | Rhamnogalacturonan hydrolase | ||
| An03g00960 | axhA | 1,4‐Beta‐D‐arabinoxylan arabinofuranohydrolase | ||
| An14g01800 | Putative alpha‐galactosidase | |||
| An04g02670 | Predicted oxidoreductase activity | |||
| An09g02240 | nag1 | N‐Acetyl‐beta‐glucosaminidase | ||
| An02g11150 | aglB | Putative alpha‐galactosidase variant B | ||
| An01g12550 | msdS | Mannosyl‐oligosaccharide 1,2‐alpha‐mannosidase | ||
| An04g03200 | Mannose‐6‐phosphate isomerase | |||
| An01g00780 | xynB | Endo‐1,4‐xylanase | ||
| An12g07840 | gnaA | Putative glucosamine‐6‐phosphate N‐acetyltransferase | ||
| GO:0010383 | Cell wall polysaccharide metabolic processes | An14g02760 | eglA | Putative secreted endoglucanase A; xylose‐induced |
| An02g11150 | aglB | Putative alpha‐galactosidase variant B; expression is induced on xylan | ||
| An18g06820 | gfaA | Putative glutamine:fructose‐6‐phosphate amidotransferase | ||
| An03g05940 | gfaB | Putative glutamine:fructose‐6‐phosphate amidotransferase | ||
| An01g00780 | xynB | Endo‐1,4‐xylanase | ||
| An14g01800 | Putative alpha‐galactosidase | |||
| GO:0009251 | Glucan metabolic processes | An02g00850 | Glucoamylase (exo‐1,4‐glucosidase/amyloglucosidase); beta‐glucanase | |
| An16g06800 | eglB | Putative endoglucanase | ||
| An15g04900 | Putative endo‐glucanase | |||
| An03g05290 | bgtB | Glucan 1,3‐beta‐glucosidase; putative glucanotransferase (caspofungin induced) | ||
| An07g08950 | eglC | Endoglucanase; specific for substrates with beta‐1,3 and beta (XlnR controlled) | ||
| GO:0070882 | Cellular cell wall organization or biogenesis | An05g00340 | Unknown function, Has domain(s) with predicted hydrolase | |
| An14g02760 | eglA | Putative secreted endoglucanase A; abundantly expressed on D‐xylose; irreversibly inhibited by palladium ion; XlnR regulated | ||
| An10g00430 | Strong similarity to agglutinin core protein Aga1 | |||
| An08g03510 | Serine‐rich protein | |||
| An06g01900 | Similarity to polyphosphoinositide binding protein Ssh2p | |||
| An07g05570 | chsA | Chitin synthase class I | ||
| An14g01800 | putative Alpha‐galactosidase | |||
| An02g07670 | Serine‐rich protein | |||
| An02g11150 | aglB | Putative alpha‐galactosidase variant B; expression is induced on xylan; XlnR regulated | ||
| An18g06820 | gfaA | Putative glutamine:fructose‐6‐phosphate amidotransferase (CA‐induced) | ||
| An03g05940 | gfaB | Putative glutamine:fructose‐6‐phosphate amidotransferase (CA‐induced) | ||
| An01g09050 | Ortholog(s) have FAD transmembrane transporter activity |
Table 3.
Enriched GO‐term in genes downregulated in the ΔugmA strain.
| Terminal GO term | Description GO term | Systematic name | Gene name | Description |
|---|---|---|---|---|
| GO:0071577 | Zinc ion transmembrane transport | An01g01620 | Putative high‐affinity zinc uptake transmembrane transporter | |
| An12g10320 | zrtA | Putative high‐affinity zinc transport protein | ||
| An01g06690 | Putative low‐affinity zinc ion transmembrane transporter |
Table 4.
Differentially expressed cell wall modifying genes (FC > 1.3; FDR < 0.05).
| Upregulated genes | Normalized expression value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| beta‐1,3‐glucan | Description | Gene name | N402 | ΔugmA | log2 FC | Q‐value | RlmA‐box | MsnA‐box | CrzA‐box | |
| An10g00400 | Putative 1,3‐beta‐glucanosyltransferase GH72; | gelA | 335.5 | 637.5 | 0.93 | 0.028 | up | n | y | y |
| An16g06120 | Putative 1,3‐beta‐glucanosyltransferase GH72 | gelF | 1.6 | 47.2 | 4.91 | 0.001 | up | n | n | n |
| An02g09050 | Putative 1,3‐beta‐glucanosyltransferase GH72 | gelG | 2.2 | 5.4 | 1.30 | 0.035 | up | n | y | y |
| An03g05290 | Putative beta‐1,3‐glucanosyltransferase GH17 | bgtB | 668.5 | 1601.5 | 1.26 | 0.013 | up | y | y | y |
| An13g02510 | Putative transglycosidase of GH16‐family ScCrh1 | crhE | 1.5 | 11.4 | 2.91 | 0.001 | up | n | y | n |
| An01g12450 | Putative exo‐beta‐1,3‐glucanase GH55‐family | bxgA | 73.2 | 193.5 | 1.40 | 0.001 | up | n | y | n |
| Alpha‐glucan | ||||||||||
| An04g09890 | Putative alpha1,3‐glucan synthase | agsA | 1.2 | 49.0 | 5.34 | 0.001 | up | y | y | n |
| An08g09610 | Putative alpha‐1,3‐glucanase GH71 | agnD | 51.8 | 91.8 | 0.82 | 0.035 | up | n | y | y |
| Chitin | ||||||||||
| An09g04010 | Putative chitin synthase class‐III | chsB | 144.3 | 282.8 | 0.97 | 0.016 | up | n | y | n |
| An12g10380 | Putative chitin synthase class‐III | chsE | 80.8 | 160.3 | 0.99 | 0.007 | up | y | y | n |
| An07g05570 | Putative chitin synthase class‐II | chsA | 20.5 | 42.5 | 1.05 | 0.001 | up | n | y | y |
| An04g04670 | Putative class‐V chitinase (GH18) | cfcC | 36.8 | 72.4 | 0.98 | 0.006 | up | y | y | y |
| Galactomannan | ||||||||||
| An02g08670 | UDP‐galactofuranose transporter | ugtA | 245.9 | 456.4 | 0.89 | 0.035 | up | y | n | n |
| An12g08720 | UDP‐galactofuranose transferase | gfsA | 98.0 | 203.7 | 1.06 | 0.002 | up | y | y | n |
| CWP‐linking | ||||||||||
| An14g03520 | Putative endo‐mannanase (GH76‐family) with a possible role in GPI‐CWP incorporation | dfgC | 42.0 | 107.8 | 1.36 | 0.001 | up | y | y | n |
| Downregulated genes | ||||||||||
| beta‐1,3‐glucan | ||||||||||
| An19g00090 | Putative exo‐beta‐1,3‐glucanase GH55‐family | bgxC | 2.9 | 1.0 | −1.55 | 0.011 | down | n | n | n |
| Chitin | ||||||||||
| An09g06400 | Putative class‐III chitinase (GH18) | ctcA | 255.7 | 2.2 | −6.86 | 0.001 | down | n | n | n |
| Galactomannan | ||||||||||
| An02g11320 | UDP‐glucose 4‐epimerase | ugeC | 72.6 | 33.0 | −1.14 | 0.002 | down | n | n | n |
| An02g08660 | UDP‐galactose mutase | ugmA | 167.8 | 5.5 | −4.93 | 0.001 | down | n | n | n |
| CWP‐linking | ||||||||||
| An11g01240 | Putative endo‐mannanase (GH76‐family) with a possible role in GPI‐CWP incorporation | dfgH | 10.2 | 4.1 | −1.31 | 0.022 | down | n | n | n |
Consensus bindings sites for RlmA (TAWWWWTAG), MsnA (AGGGG) and CrzA (GWGGCS) were used.
Cell wall remodelling in the ΔugmA mutant
Table 4 lists the differential expression of the genes directly involved in the biosynthesis of the cell wall polysaccharides β‐glucan, α‐glucan, chitin and galactomannan (Pel et al., 2007). In addition to agsA and genes involved in UDP‐N‐acetyl‐glucosamine biosynthesis (gnaA, gfaA and gfaB), differentially expressed genes include genes encoding proteins involved in chitin polymerization (chsA and chsB), a putative 1,3‐β‐glucanosyltransferase gelF and a gene encoding a putative glucan‐chitin cross‐linking enzyme (crhE). Although several chitin synthase‐encoding genes showed a statistically significant induction, their fold‐changes were limited, varying between 1.5 and 2. The strongest down‐regulated gene in the ΔugmA mutants compared to wild‐type is a chitinase (ctcA)‐encoding gene showing a >100‐fold decrease in expression (Table 4).
The genome of A. niger is predicted to contain 117 glycosylphosphatidylinositol (GPI)‐anchored proteins (Pel et al., 2007). GPI‐anchored proteins can either remain attached to the plasma membrane (mostly cell wall biosynthetic enzymes) or can become covalently linked to the β‐glucan part of the cell wall (Frieman and Cormack, 2003, 2004). Table 5 summarizes the differentially expressed, putative GPI‐anchored protein‐encoding genes. Expression of 17 GPI‐anchored protein‐encoding genes was up‐regulated in the ΔugmA and 16 was down‐regulated, suggesting important changes in the composition of membrane‐ and cell wall‐localized GPI‐anchored proteins. The set of down‐regulated genes also includes the gene encoding CwpA, which is currently the only cell wall protein from A. niger for which a covalent linkage to the cell wall has been experimentally confirmed (Damveld et al., 2005a, 2005b, 2005c).
Table 5.
Differentially expressed genes encoding putative (GPI)‐anchored cell wall proteins*.
| Normalized expression value | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Upregulated | Description | Gene name | N402 | ΔugmA | log2 FC | Q‐value | RlmA‐box | MsnA‐box | CrzA‐box |
| An01g12240 | Unknown function | 4.2 | 385.3 | 6.54 | 0.001 | y | n | y | |
| An02g00850 | Putative glucanase related to MLG1 ‐ Neurospora crassa | 4.7 | 57.0 | 3.61 | 0.001 | y | n | y | |
| An03g02510 | Unknown function | 0.92 | 2.9 | 1.66 | 0.046 | n | y | n | |
| An03g05530 | Putative endo‐beta‐1.4‐glucanase | 16.9 | 54.0 | 1.68 | 0.001 | y | y | n | |
| An03g05620 | Putative hydrolases or acyltransferases | 1.0 | 9.1 | 3.19 | 0.002 | n | n | n | |
| An04g07160 | Unknown function | 52.7 | 162.9 | 1.63 | 0.001 | n | y | n | |
| An09g03260 | Endo‐polygalacturonase D | pgaD | 1.5 | 4.3 | 1.57 | 0.032 | n | y | y |
| An10g00390 | Putative cellobiose dehydrogenase CDH | 2.9 | 11.8 | 2.05 | 0.001 | n | y | n | |
| An12g07430 | Putative dioxygenase C | 4.2 | 32.2 | 2.95 | 0.001 | n | y | n | |
| An13g02670 | Unknown function | 0.6 | 183.2 | 8.27 | 0.001 | n | y | n | |
| An14g01068 | Unknown function | 112.1 | 275.2 | 1.30 | 0.001 | y | y | y | |
| An14g01820 | Cell wall protein binB | 2.6 | 378.6 | 7.17 | 0.001 | y | y | y | |
| An14g01840 | Temperature‐shock induced protein Tir3 | 107.8 | 719.7 | 2.74 | 0.002 | n | n | y | |
| An14g02170 | Putative cutinase | 0.26 | 2.0 | 2.97 | 0.123 | n | n | n | |
| An15g07790 | Unknown function | n.d. | 1.3 | inf | 0.001 | y | y | y | |
| An18g03730 | Unknown function | 507.6 | 3073.2 | 2.60 | 0.001 | n | y | y | |
| Downregulated | |||||||||
| An02g09010 | Unknown function | 7.6 | 2.7 | −1.47 | 0.007 | n | n | n | |
| An02g13220 | Putative lysophospholipase phospholipase | 397.4 | 156.5 | −1.34 | 0.001 | n | n | n | |
| An07g04620 | N. crassa Ham7 required for cell fusion. | ham‐7 | 136.8 | 13.4 | −3.35 | 0.001 | n | n | n |
| An07g06210 | Unknown function | 45.9 | 19.1 | −1.27 | 0.003 | n | n | n | |
| An08g07090 | Similarity to protein Sim1 | 105.0 | 43.7 | −1.27 | 0.001 | n | n | n | |
| An08g09850 | Putative phosphatase precursor | 3.5 | 0.9 | −2.01 | 0.013 | n | n | n | |
| An09g02340 | Similarity to self‐pruning protein SP ‐ Lycopersicon esculentum | 76.4 | 26.1 | −1.55 | 0.001 | n | n | n | |
| An12g07750 | Unknown function | 78.9 | 23.1 | −1.77 | 0.001 | n | n | n | |
| An13g02130 | Putative aspartyl protease; | 12.0 | 2.6 | −2.22 | 0.001 | n | n | n | |
| An14g02100 | Cell wall protein A (CwpA) Aspergillus niger | cwpA | 65.9 | 1.2 | −5.77 | 0.001 | n | n | n |
| An16g01780 | Unknown function | 94.8 | 45.6 | −1.06 | 0.002 | n | n | n | |
| An16g07920 | Unknown function | 165.4 | 46.8 | −1.82 | 0.001 | n | n | n | |
| An16g07950 | Unknown function | 872.5 | 359.7 | −1.28 | 0.001 | n | n | n | |
| An18g00630 | Unknown function | 23.5 | 7.9 | −1.58 | 0.004 | n | n | n | |
| An18g01320 | Putative aspartyl protease | 80.5 | 45.0 | −0.84 | 0.037 | n | n | n | |
| An18g04070 | Unknown function | 1322.2 | 230.5 | −2.52 | 0.001 | n | n | n | |
| An18g06360 | Similarity to surface antigen Csa1 ‐ | 14.7 | 5.6 | −1.38 | 0.035 | n | n | n | |
GPI‐anchored proteins known to be directly involved in cell wall biosynthesis (Table 4) are not included in this table.
The major pathway for cell wall remodelling is the CWI pathway, which has been studied most extensively in S. cerevisiae. This pathway comprises among other proteins, the cell wall stress sensors (Wsc‐ and Mid‐proteins), the Rho1 GTPase module, Pkc1 and the Bck1‐Mkk1/Mkk2‐Mpk1 MAP kinase signalling cascade. Activation of the CWI pathway results in activation of the Rlm1 transcription factor, which can bind to a conserved motif in promoter regions of cell wall stress‐induced genes and activate their transcription. Homologs of these proteins have been identified in Aspergilli and have been shown to be also involved in the cell wall stress response (Damveld et al., 2005a, 2005b, 2005c; Dichtl et al., 2012). From the list of genes related to the CWI pathway of A. niger (Pel et al., 2007), three genes encoding a putative cell wall stress sensor protein WscC (An07g04070), a small GTPase RhoB (An16g04200) and MkkA (An18g03740) were transcriptionally induced (Table 6). No CWI‐related genes were down‐regulated in the ΔugmA strain.
Table 6.
Upregulated cell wall integrity pathway‐related genes.
| Normalized expression value | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gene identifier | Description | Gene name | N402 | ΔugmA | log2 FC | Q‐value | RlmA‐box | MsnA‐box | CrzA‐box |
| An07g04070 | Putative plasma membrane cell wall sensor | wscC | 105.5 | 310.7 | 1.56 | 0.001 | n | y | y |
| An16g04200 | Putative Rho‐related GTPase; ScRho2‐ and SpRho2‐like | rhoB | 118.8 | 255.8 | 1.11 | 0.003 | y | n | y |
| An18g03740 | MAPKK with putative function in CWI‐signalling | mkkA | 44.8 | 122.8 | 1.46 | 0.001 | n | n | n |
Identification of transcription factors important to sustain growth of the ΔugmA strain
The 432 significantly up‐regulated genes in the ΔugmA strain were analysed using the TFBSF tool (Meyer et al., 2009). The available 1 kb promoter regions of 419 of the 432 up‐regulated genes were analysed for the presence of binding sites established for 118 fungal transcription factors. The number of genes with these binding sites in the group of 419 induced genes was determined and it was evaluated whether the genes with these binding sites are statistically significant over‐ or under‐represented compared to groups of the same size comprising randomly selected genes of A. niger (500 000 bootstrap samples, FDR ≤0.0005) (Table S5).
Using this in silico approach, four transcription factor binding sites were found to be enriched (Table 7). These are SteA (ScSte12‐homolog), PacC (ScRim101‐homolog), MsnA (ScMsn2/4‐homolog) and RlmA (ScRlm1‐homolog). The identification of the RlmA transcription factor binding site is in agreement with the function of the RlmA transcription factor in the CWI pathway and its requirement for the induction of agsA (Damveld et al., 2005a, 2005b, 2005c). A recent study addressing the role of transcription factors in response to cell wall perturbing drugs identified RlmA, MsnA and CrzA transcription factors as being involved in cell wall remodelling in A. niger (Fiedler et al., 2014). Although CrzA binding sites were not identified as being overrepresented in the ΔugmA induced gene set, we decided to include CrzA in our subsequent studies.
Table 7.
Transcription factor binding site enrichment analysis of ΔugmA‐induced genes.
| S. cerevisiae transcription factor | A. niger ortholog | ReqGN | ReqPG | RanGN | RanPG | p‐Value | FDR |
|---|---|---|---|---|---|---|---|
| Rim101p | PacC | 316.00 | 75.42 | 270.08 | 64.46 | 2.00E − 06 | 1.18E − 04 |
| Ste12p | SteA | 229.00 | 54.65 | 161.53 | 38.55 | 2.00E − 06 | 1.18E − 04 |
| Msn2p/Msn4p | MsnA | 291.00 | 69.45 | 246.47 | 58.82 | 4.00E − 06 | 1.57E − 04 |
| Rlm1p | RlmA | 81.00 | 19.33 | 51.10 | 12.20 | 1.00E − 05 | 2.95E − 04 |
ReqGN: number of genes with at least one putative TF binding site in the gene set up‐regulated in the ΔugmA mutant.
ReqPG: percent of genes with at least one putative TF binding site in the gene set up‐regulated in the ΔugmA mutant.
RanGN: average number of genes with at least one putative TF binding site in random gene sets.
RanPG: average percent of genes with at least one putative TF binding site in random gene sets.
p‐Value: probability to get equal/more extreme number of genes with at least one putative TF binding site in random gene sets compared to the gene set up‐regulated in the ΔugmA mutant.
FDR: FDR corrected p‐value.
To evaluate the importance of the five transcription factors (SteA, PacC, MsnA, RlmA and CrzA) with respect to growth of the ΔugmA strain, ΔugmA/ΔTF double mutants were generated and compared to the growth phenotype of the single mutants. The rationale of this analysis is that if a transcription factor is important for maintaining CWI in the ΔugmA mutant, the ΔugmA/ΔTF double mutant would have a more severe phenotype or even lead to synthetic lethality. As shown in Fig. 4, deletion of ugmA results in a severe reduction in radial growth and reduced conidiation, as previously observed (Damveld et al., 2008). Single deletion of the transcription factors RlmA and SteA did not affect growth on MM. Deletion of the msnA gene, resulted in limited growth reduction but the ΔmsnA colony still produced spores. Single deletions of pacC and crzA severely reduced growth, indicative of an important function for these transcription factors during vegetative growth. Somewhat surprisingly the ΔcrzA strain grew and sporulated much better at 37 °C compared to growing this mutant at lower temperatures. The strong growth phenotype of the ΔpacC and the ΔcrzA by itself made it difficult to assess whether their function is specifically required for the ΔugmA mutant as it is likely that deletion of pacC and crzA in the ΔugmA mutant will further reduce growth anyway. Analysis of the ΔugmA/ΔTF double mutants showed a strong reduction in growth when the ugmA deletion was combined with the deletion of the msnA or rlmA transcription factor. In addition, the growth of the ΔugmA/ΔcrzA double mutant at 37 °C also was severely reduced. The synthetic growth defects of the ΔugmA/ΔrlmA, ΔugmA/ΔmsnA and ΔugmA/ΔcrzA double mutant (at 37 °C) suggest that RlmA, MsnA and CrzA target genes are important for survival of the ΔugmA mutant. Combining ΔugmA with deletion of ΔsteA did not show a synthetic growth phenotype indicating that SteA and SteA target genes are not important for survival of the ΔugmA mutant. Combining the ΔugmA with ΔpacC resulted also in very poorly growing colonies, but because single deletion of pacC also resulted in poor growth, it is not possible to conclude whether PacC target genes are specifically required for ΔugmA survival.
Figure 4.

Growth analysis of single and double mutants. Spores of single and double mutants were spotted in the centre of a MM plate and grown for four days at the indicated temperature. Synergistic growth reduction of the ΔugmA/msnA and ΔugmA/rlmA is indicative of an important role of the MsnA and RlmA transcription factors in survival of the ugmA mutant.
Discussion
Biosynthesis of Galf‐containing glycoconjugates is important for securing the integrity of the fungal cell wall. Galf‐containing glycoconjugates are found in several ascomycetous clades, including the Eurotiales (Aspergilli and Penicillium ssp), Sordariales (Neurospora and Magnaporthe ssp), Hypocreales (Trichoderma and Fusarium spp), but are absent in the Saccharomycetales (Saccharomyces and Candida ssp). In the opportunistic pathogen A. fumigatus, Galf is an important virulence factor and Galf‐mutants display reduced virulence (Schmalhorst et al., 2008). In addition, Galf is an important diagnostic tool to detect early‐stage A. fumigatus infections in humans (Alexander and Pfaller, 2006). In the past few years the genes encoding the enzymes required for the synthesis of Galf‐containing glycostructures such as galactomannan, N‐ and O‐glycans, and glycolipids have been identified. Analysis of mutants in genes involved in the biosynthesis of Galf in several Aspergilli has shown that Galf‐mutants have an altered morphology, a different cell wall structure and are in general more susceptible to cell wall synthesis inhibitors as well as cell wall assembly‐disturbing compounds (Damveld et al., 2008; Engel et al., 2009; Park et al., 2014; Afroz et al., 2011; 2012, 2014). The genes involved in Galf biosynthesis include ugeA (encoding UDP‐glucose‐epimerase), ugmA (encoding UDP‐galactose‐mutase), ugtA and ugtB (encoding a UDP‐Galf transporters) and gfsA (encoding a galactofuranosyltransferase) (Komachi et al., 2013). Genes encoding these enzymes have been characterized in the three best studied Aspergillus species (A. fumigatus, A. nidulans and A. niger). In A. niger, the biosynthesis of Galf has also been largely elucidated. By performing a genetic screen for cell wall mutants both the ugeA and ugmA genes have been identified (Damveld et al., 2008; Park et al., 2014). Because of genetic redundancy of the UDP‐Galf transporters and UDP‐Galf transferases in A. niger these genes were not identified in the genetic screen, but simultaneous disruption of the two redundant UDP‐Galf transporters (UgtA and UgtB) and simultaneous disruption of the three putative UDP‐Galf transferases lead to similar phenotypes as the ΔugeA and ΔugmA mutants (Park et al., 2015; Arentshorst et al., 2015). In addition to increased α‐glucan synthesis and, presumably, α‐glucan levels, ΔugmA mutants have increased chitin levels (Afroz et al., 2011) and show increased expression of gfaA and gfaB (Fig. 3 and Table 4), which both encode glutamine:fructose‐6‐phosphate amidotransferases and are required for the biosynthesis of UDP‐N‐acetylglucosamine, the precursor of chitin. These observations indicated that the absence of Galf‐biosynthesis induces a cell wall remodelling programme to counteract the loss of Galf‐containing glycostructures.
In addition to cell wall remodelling enzymes, a group of 18 differentially expressed genes in the ΔugmA strain was identified which encode glycosyl hydrolases (GH) that act on the plant cell wall polysaccharides cellulose, xylan and galactan (Table 8). Several of these genes (xynB, eglB, eglC, aglB) are known to be controlled by the XlnR transcription factor (van Peij et al., 1998b). XlnR encodes a Zn(II)2Cys6 transcription factor that positively regulates the expression of genes involved in xylose metabolism (van Peij et al., 1998a, 1998b). Although the mechanism underlying the induced expression of plant cell wall‐degrading enzymes in the ΔugmA mutant is unknown, it could be related to a possible accumulation of UDP‐galactose as the pathway to UDP‐Galf is blocked by the absence of UgmA. Accumulation of UDP‐galactose (in the pyranose form) could have an effect on the intracellular galactoglycome, which has been shown to be linked to the production of plant cell wall‐degrading enzymes (Karaffa et al., 2013).
Table 8.
Differentially expressed genes encoding plant cell wall‐degrading enzymes.
| Description | Gene name | N402 | ΔugmA | log2 FC | Q‐value | XlnR site* | |
|---|---|---|---|---|---|---|---|
| An02g00730 | Similarity cutinase A gene | 0 | 37.8 | inf | 0.001 | Y (2x) | |
| An03g00960 | Arabinofuranosidase active on arabinoxylan | axhA | 0.7 | 60.3 | 6.4 | 0.001 | Y (3x) |
| An15g07760 | Putative beta‐mannanase | 0.7 | 32.4 | 5.6 | 0.001 | Y (2x) | |
| An02g10550 | Endo‐alpha‐1,5‐arabinanase | abnA | 2.1 | 78.5 | 5.2 | 0.001 | Y (3x) |
| An15g04550 | Xylanase A | xynA | 18.2 | 323.5 | 4.2 | 0.001 | Y (4x) |
| An15g04570 | Putative endo‐glucanase IV | 8.5 | 112.7 | 3.7 | 0.001 | Y (2x) | |
| An14g04200 | Rhamnogalacturonase | rhgB | 6.3 | 69.6 | 3.5 | 0.001 | Y (2x) |
| An15g04900 | Putative endoglucanase IV | 3.9 | 40.6 | 3.4 | 0.001 | Y (6x) | |
| An08g05230 | Putative endoglucanase IV | 8.4 | 86.7 | 3.4 | 0.001 | Y (1x) | |
| An06g02070 | Rhamnogalacturonase rhgA ‐ | rhgA | 0.8 | 8.4 | 3.4 | 0.001 | Y (1x) |
| An14g02670 | Putative endoglucanase IV | 3.6 | 31.1 | 3.1 | 0.001 | Y (2x) | |
| An04g09690 | Pectin methylesterase | pme1 | 0.7 | 5.6 | 3.0 | 0.001 | Y (6x) |
| An01g00780 | Xylanase B | xynB | 35.3 | 241.7 | 2.8 | 0.001 | Y (6x) |
| An14g01800 | Putative alpha‐galactosidase | 0.6 | 3.2 | 2.5 | 0.014 | Y (1x) | |
| An13g03140 | Putative endo‐beta‐1,4‐glucanase | 2.8 | 13.4 | 2.2 | 0.001 | N | |
| An09g03260 | Endo‐polygalacturonase D* | pgaD | 1.5 | 4.3 | 1.6 | 0.03 | Y (4x) |
| An02g11150 | Alpha‐galactosidase | aglB | 60.3 | 141.8 | 1.2 | 0.04 | Y (2x) |
| An16g02730 | Endo‐1,5‐alpha‐arabinase | abnA | 42.3 | 91.6 | 1.1 | 0.003 | Y (2x) |
Consensus binding sites for XlnR (GGCTRR or GGNTAAA)
We previously studied the genome‐wide cell wall stress response in A. niger by challenging germlings with antifungals affecting cell wall synthesis or assembly. These treatments include the exposure to the β‐1,3‐glucan synthase inhibitor CA and the ergosterol biosynthesis inhibitor FP (Meyer et al., 2007) as well as exposure to sphingolipid inhibitor aureobasidin A (AbaA), and calcineurin inhibitor FK506 (Fiedler et al., 2014). Compound‐induced genes in these studies were defined based on a FDR < 0.05 and resulted in 134 differentially expressed genes for CA, 178 for AbaA, 22 for FP and 49 for FK506 (Fiedler et al., 2014; Table S6). We were interested whether the sets of genes up‐regulated in the ΔugmA mutant showed overlap with the gene sets up‐regulated by the various treatments. As shown in Fig. 5, the largest overlap of the 432 genes up‐regulated in the ΔugmA mutant was observed with the CA‐induced gene set (35 genes overlapping) and AbaA (33 genes overlapping) (Fig. 5). No overlapping genes were identified between the ΔugmA‐induced genes and FP‐ or FK506‐treated germlings (data not shown). The gene identities for the different groups of genes are given in Table S6. The numbers also show that there is only a limited overlap of genes that are commonly induced. In fact, when comparing CA‐induced, AbaA‐induced and ΔugmA‐induced gene expression nine genes were commonly induced (Table 9). The majority of these nine genes encode proteins with unknown functions. Two proteins An04g03870 and An06g01900 encode homologs of the S. cerevisiae Dpp1p and Csr1p proteins. Dpp1p is a diacylglycerol diphosphate phosphatase, which synthesizes the second messenger diacylglycerol, a potential activator of protein kinase C of the CWI signalling cascade. Csr1p is a phosphatidylinositol transfer protein belonging to the Sec14‐family, which has a potential role in regulating lipid and fatty acid metabolism and indicates turnover of phosphoinositides in response to different forms of cell wall stress, possibly, to relocate proteins involved in CWI signalling, such as Wsc1p, Rho1p, Rom2p and Pkc1p to the proper location at the membrane (Fernández‐Acero et al., 2015). The limited overlap of genes between the various treatments could have multiple reasons and might relate to the fact that treatments with antifungals provoke both short‐term and transient responses, while the ΔugmA mutant is fully adapted to the lack of Galf biosynthesis and therefore represents a long‐term adaptation. Alternatively, a noticeable difference between the ΔugmA transcriptome (this study) and transcriptome in response to chemical compounds (previous studies) is that the ugmA study was performed with mycelia while the chemical compound studies were performed with germlings. The limited overlap could also be well related to differences between the expression of cell wall biosynthetic genes during spore germination and vegetative growth.
Figure 5.
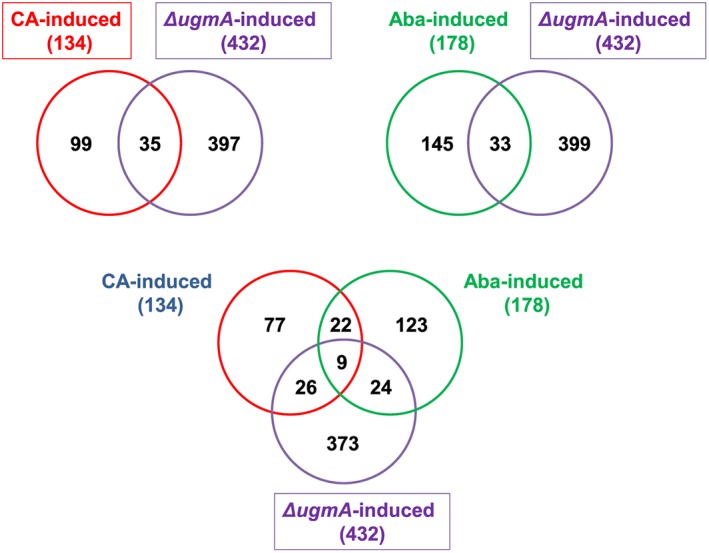
Overview of the number of genes overlapping between different cell wall stress conditions. Caspofungin (CA)‐induced genes (Meyer et al., 2007) and Aureobasidin A (AbaA)‐induced genes (Fiedler et al., 2014) were compared to ΔugmA‐induced genes (this study).
Table 9.
Description of commonly induced genes after CA or AbaA treatments and in the ΔugmA mutant.
| Gene# | Description* | S. cerevisiae homolog |
|---|---|---|
| An04g03870 | Diacylglycerol diphosphate phosphatase activity | Ddp1 |
| An05g00760 | Protein with unknown function | |
| An06g01900 | Sec14 family; member phosphatidylinositol transfer protein | Csr1 |
| An07g05820 | Protein with unknown function | |
| An12g06380 | Protein of unknown function, highly repetitive; ortholog in A. fumigatus is farnesol‐responsive | |
| An14g01070 | Protein with unknown function | |
| An14g01840 | GPI anchored protein; strongly induced in ugmA | |
| An15g07090 | Protein with unknown function | |
| An17g01000 | Putative anion transporter |
Taken from AspGD (http://www.ncbi.nlm.nih.gov/pubmed/?term=aspGD).
It is therefore striking that, in contrast to the limited overlap of individual antifungal‐responsive genes with ΔugmA‐responsive genes, the transcription factor binding site enrichment analysis showed remarkable similarities and identified RlmA and MsnA transcription factor binding sites as overrepresented under all conditions (Fig. 6). Possibly, these transcription factors play an important role in combination with other factors that co‐regulate and fine‐tune the expression of different target genes.
Figure 6.
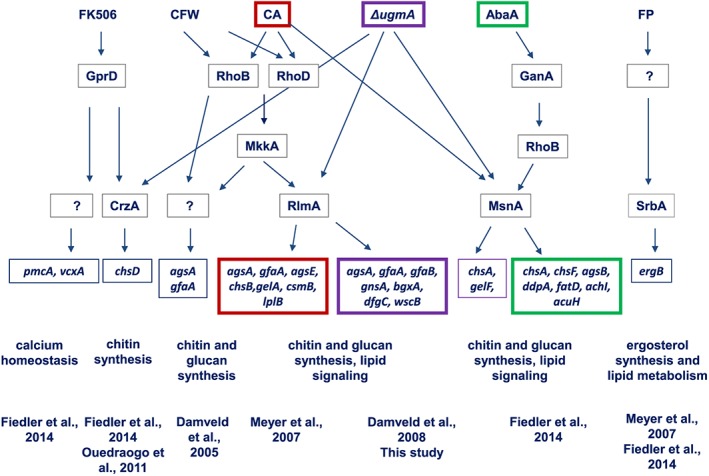
Overview of key transcription factors and some of their putative target genes involved in the cell wall salvage gene network of A. niger deduced from transcriptomic and phenotypic analysis. On the upper row, several cell wall stress conditions including FK506 (Calcineurin inhibitor), CFW (Calcofluor white), caspofungin (CA), ΔugmA (lack of galactofuranose biosynthesis), Aureobasidin A (AbaA) and fenpropimorph (FP). Arrows indicate (putative) signalling events between and the proteins involved in signalling based on transcriptomic or functional genomics studies. Boxed genes are the possible target genes of various transcription factors and the relation with the inducing condition. Major biological processes affected by the different treatments are depicted under the boxed genes. Despite limited overlap of the target genes induced by chemical compounds and in the ΔugmA mutant, the RlmA, MsnA and CrzA transcription factors are likely to play a key role in orchestrating the response.
Growth analysis of the ΔugmA/ΔrlmA and ΔugmA/ΔmsnA double mutants indicated an important role of the RlmA and MsnA transcription factors in securing CWI of the ΔugmA mutant. It will be of interest in future studies to determine the cell wall composition of the double mutants in comparison with single mutants and to identify RlmA or MsnA target genes via transcriptome analysis of CHIPseq analysis. In contrast to RlmA and MsnA, the role of SteA seems to be dispensable for the ΔugmA mutant, although its binding site was overrepresented in the ΔugmA up‐regulated gene set. In. S. cerevisiae, the ScSte12 transcription factor is activated by the ScFus3/ScKss1 MAPK signalling cascade and is responsible for the activation of genes involved in mating or pseudohyphal growth (Roberts and Fink, 1994). In agreement with this, ScSte12 is involved in the regulation of fungal development and pathogenicity in various filamentous fungi (see for review Wong Sak Hoi and Dumas, 2010). Single deletion of the ScSTE12 homolog in A. niger did not result in a detectable growth phenotype under the selected culture conditions (Fig. 4, and data not shown). The role of the PacC and CrzA transcription factors in rescuing the ΔugmA strain is more difficult to examine as single deletion of the corresponding genes in itself results in a severe growth phenotype (Fig. 4). However, both transcription factors have been shown to be involved in fungal cell wall remodelling. In S. cerevisiae, the ScRim101/PacC pathway is essential for cell wall assembly in the absence of CWI signalling created by disrupting the ScSLT2/ScMPK1 gene (Castrejon et al., 2006), indicating that the ScPkc1–Slt2 CWI pathway and the ScRim101/PacC pathway are partially redundant. In Cryptococcus neoformans, deletion of Rim101 results in down‐regulation of several cell wall‐related genes, indicating that Rim101 is important for their expression (O'Meara et al., 2014). Several studies have shown that the Crz1/CrzA transcription factor is also important for cell wall remodelling. An important role of the calcium/calcineurin‐responsive transcription factor for cell wall maintenance was first discovered in S. cerevisiae (Yoshimoto et al., 2002), but its role has been shown to be conserved in filamentous fungi, including Aspergillus species (Steinbach et al., 2007; Fortwendel et al., 2010; Fiedler et al., 2014). A role for CrzA‐mediated cell wall remodelling in the ΔugmA mutant was not inferred from the TF enrichment analysis as the CrzA transcription factor binding site was not enriched in the ΔugmA‐upregulated gene set. However, the phenotype of the ΔugmA/ΔcrzA double mutant at 37 °C does suggest an important role for CrzA in cell wall remodelling in the ΔugmA mutant at least at higher temperatures. As in Candida albicans the calcineurin/Crz1p pathway is activated in response to membrane stress (Zakrzewska et al., 2005), this might imply that deletion of ugmA does result in significant membrane stress.
Several studies have shown that cross‐talk between several stress response pathways or pathways acting in parallel is important to cope with cell wall stress (Burgwun Fuchs and Mylonakis, 2009; Ouedraogo et al., 2011; Verwer et al., 2012; García et al., 2015). Efficient inhibition of a fungal pathogen can be accomplished by the combination of two drugs, one targeting cell wall synthesis and the other by interfering with the adaptive mechanism. An elegant and promising example for such an approach is the recent study by Valiante et al., who showed that the efficacy of caspofungin treatment was strongly increased by simultaneous inhibition of the High Osmolarity Glycerol response pathway by the newly identified drug humidimycin (Valiante et al., 2015). Our study has shown that inhibition of Galf biosynthesis is by itself not fungicidal, but that Galf‐deficient cells become highly dependent on the RlmA and MsnA transcription factors. The strong synthetic growth defect of the double mutants shows the importance of activation of the CWI pathway to maintain CWI in the absence of Galf biosynthesis and underscores the potential of using combinations of antifungals that act synergistically to combat fungal infections.
Experimental procedures
Strains and culture conditions
Aspergillus niger strains used in this study are listed in Table 10. Strains were cultivated in minimal medium (MM) (Bennett and Lasure, 1991) containing 1% (w/v) glucose (or other carbon sources as indicated), 7 mM KCl, 11 mM KH2PO4, 70 mM NaNO3, 2 mM MgSO4, 76 nM ZnSO4, 178 nM H3BO3, 25 nM MnCl2, 18 nM FeSO4, 7.1 nM CoCl2, 6.4 nM CuSO4, 6.2 nM Na2MoO4, 174 nM EDTA; or in complete medium (CM) containing, in addition to MM, 0.1% (w/v) casamino acids and 0.5% (w/v) yeast extract. When required, 10 mM uridine, 2.5 µg/ml nicotinamide or 100 µg/ml hygromycin was added. Fermentation medium (FM) initially adjusted to pH 3 is composed of 0.75% glucose, 0.45% NH4Cl, 0.15% KH2PO4, 0.05%KCl, 0.05% MgSO4, 0.1% trace element solution and 0.003% yeast extract as described (Jørgensen et al., 2010).
Table 10.
Strains used in this study.
| Strain | Genotype | Description | Reference |
|---|---|---|---|
| N402 | cspA1 | derivative of N400 | Bos et al., 1988 |
| MA87.6 | cspA1, pyrG378, kusA::amdS, ugmA::AOpyrG | ΔugmA in MA70.15 | Damveld et al., 2008 |
| MA169.4 | cspA1, pyrG378, kusA::DR‐amdS‐DR | ku70 disruption in AB4.1 | Carvalho et al., 2010 |
| MA234.1 | cspA1, kusA::DR‐amdS‐DR | Restored pyrG in MA169.4 | This study |
| MA417.1 | cspA1, kusA::DR‐amdS‐DR, rlmA::hygB | ΔrlmA in MA234.1 | This study |
| MA306.1 | cspA1, kusA::DR‐amdS‐DR, crzA::hygB | ΔcrzA in MA234.1 | This study |
| MA513.1 | cspA1, kusA::DR‐amdS‐DR, msnA::hygB | ΔmsnA in MA234.1 | This study |
| MA527.2 | cspA1, kusA::DR‐amdS‐DR, steA::hygB | ΔsteA in MA234.1 | This study |
| EA13.1 | cspA1, kusA::DR‐amdS‐DR, pacC::hygB | ΔpacC in MA234.1 | Alazi et al., in prep |
| MA322.1 | cspA1, pyrG378, kusA::DR‐amdS‐DR, ΔnicB::AOpyrG | ΔnicB::AOpyrG in MA169.4 | Niu et al., 2016 |
| MA323.1 | cspA1, pyrG378, kusA::DR‐amdS‐DR, ΔnicB | ΔnicB::AOpyrG in MA169.4, followed by AOpyrG loopout | Niu et al., 2016 |
| MB2.1 | cspA1, pyrG378, kusA::DR‐amdS‐DR, ΔnicB, ugmA::AnidnicB | ΔugmA in MA323.1 | This study |
| MB3.1 | cspA1, pyrG378, kusA::DR‐amdS‐DR, ΔnicB, ugmA::AnidnicB, rlmA::AOpyrG | ΔugmAΔrlmA (ΔrlmA in MB2.1) | This study |
| MB4.1 | cspA1, pyrG378, kusA::DR‐amdS‐DR, ΔnicB, ugmA::AnidnicB, crzA::AOpyrG | ΔugmAΔcrzA (ΔcrzA in MB2.1) | This study |
| MB5.1 | cspA1, pyrG378, kusA::DR‐amdS‐DR, ΔnicB, ugmA::AnidnicB, msnA::AOpyrG | ΔugmAΔmsnA (ΔmsnA in MB2.1) | This study |
| MB6.1 | cspA1, pyrG378, kusA::DR‐amdS‐DR, ΔnicB, ugmA::AnidnicB, steA::AOpyrG | ΔugmAΔsteA (ΔsteA in MB2.1) | This study |
| MB7.1 | cspA1, pyrG378, kusA::DR‐amdS‐DR, ΔnicB, ugmA::AnidnicB, pacC::AOpyrG | ΔugmAΔpacC (ΔpacC in MB2.1) | This study |
Protein extraction and immunoblotting analysis
1 × 107 conidia of A. niger N402 and ΔugmA strain were grown at 30 °C (200 rpm) in liquid CM during 18, 24 and 36 h or 24, 36 and 48 h for the wild‐type and ΔugmA strains respectively. For protein extraction, 0.5 ml of lysis buffer added to the ground mycelia as described previously (Rocha et al., 2015). The supernatants were collected and the protein concentrations were determined according to the Lowry method modified by Hartree (Hartree, 1972). A 50 µg quantity of protein from each sample was resolved in a 12% (w/v) SDS‐PAGE and transferred to polyvinylidene difluoride (PVDF) membranes (GE Health Care). The phosphorylation of MpkA was examined using anti‐phospho p44/42 and the total amount of MpkA was detected by using the anti p44/42 MAPK antibody (9101 and 4370, respectively; Cell Signaling Technologies) according to the manufacturer's instructions. The primary antibody was detected with HRP‐conjugated secondary antibody raised in rabbits (A0545; Sigma). Anti γ‐tubulin (yN‐20; Santa Cruz Biotechnology) was used as the loading control in these experiments. Anti γ‐tubulin antibodies were detected with peroxidase HRP‐conjugated secondary antibody (sc‐2020; Santa Cruz Biotechnology). All the primary antibody detections here were performed at room temperature with the specified secondary antibody in TBST buffer (137 mM NaCl, 20 mM Tris and 0.1% Tween‐20). Chemoluminescent detection was performed by using an ECL Prime Western Blot detection kit (GE Health Care). Images were generated by exposing the membranes to the ChemiDoc XRS gel imaging system (BioRad). The images were subjected to densitometric analysis in ImageJ software to achieve the ratio of phosphorylated/total MpkA for each time point.
Construction of single and double mutant knock‐out mutants
For the construction of single deletion mutants, flanks of the gene to be deleted were PCR‐amplified and split marker fragments with selection markers AOpyrG or hygB were created using fusion PCR (Arentshorst et al., 2015). The split marker fragments were either transformed to strain MA169.4 (ku70−, pyrG−) for pyrG selection (Carvalho et al., 2010) or to strain MA234.1 (ku70−) for hygB selection. Strain MA234.1 (ku70−) was obtained by transformation of strain MA169.4 (ku70−, pyrG−) with a 3.8 kb XbaI fragment containing the A. niger pyrG gene, resulting in the full restoration of the pyrG locus in strain MA234.1. For the construction of ΔugmA/transcription factor double deletion mutants, parental strain MA323.1 (ΔnicB‐, pyrG‐) (Niu et al., 2016) was transformed with ugmA‐nicB split marker fragments (Arentshorst et al., 2015) to obtain ΔugmA mutant strain MB2.1 (ΔugmA::nicB, pyrG−). Aspergillus oryzae pyrG (AOpyrG) split marker fragments of all transcription factors were transformed to this strain, resulting in double deletion mutants. All strains used in this study are listed in Table 10. All primers used in this study are listed in Table S7 Proper deletion of the genes in the various mutants was verified by Southern blot analysis or diagnostic PCR (data not shown).
Bioreactor cultivation conditions
Details on the medium composition and batch cultivation conditions with 6.6 L BioFlo3000 bioreactors (New Brunswick Scientific, NJ, USA) were performed as described by Jørgensen et al. (2010), except that glucose was used as a carbon source in this study. The cultivation program involves two consecutive phases: in the first phase, carried at out 30 °C and at pH 3, head space aeration was used and the agitation speed was kept at 250 rpm for the first 5 h. In the second phase, after spore germination, the pH was slowly increased by the addition of NaOH to the desired pH value (set at 3.0, 4.0, 5.0 or 6.0) and increased agitation speed (750 rpm) and sparger aeration. At the same time, 0.01% (v/v) polypropyleneglycol P2000 was added as antifoam agent. Biomass was determined by weighing lyophilized mycelium from a known mass of culture broth. Culture broth was filtered through GF/C glass microfiber filters (Whatman). The filtrate was collected and frozen for use in solute analyses. The mycelium was washed with demineralised water, rapidly frozen in liquid nitrogen and stored at −80 °C for RNA isolation and microscopic analyses. Mycelium samples harvested from bioreactor runs JH04 and JH08 (N402 at pH 5.0) and JH05 and JH09 (ΔugmA at pH 5.0) were used for RNA extraction. Dot blot analysis using the anti‐galactofuranose antibody L10 (Heesemann et al., 2011) was performed as described (Park et al., 2014)
RNA isolation and quality control
Mycelium intended for gene‐expression analyses was separated from culture medium and frozen in liquid nitrogen within 15–20 s from sampling and stored at −80 °C. RNA was extracted from mycelium in liquid nitrogen using TRIzol reagent (InVitrogen). Frozen ground mycelium (≈200 mg) was directly suspended in 800 µl Trizol reagent and vortexed vigorously for 1 min. After centrifugation for 5 min at 10 000 ×g, 450 µl of the supernatant was transferred to a new tube. Chloroform (150 µl) was added and after a 3 min incubation at room temperature, samples were centrifuged and the upper aqueous phase was transferred to a new tube to which 400 µl of isopropanol was added, followed by a 10 min incubation at room temperature and centrifugation for 10 min at 10,000 ×g. The pellet was washed with 75% (v/v) ethanol and finally dissolved in 100 µl H2O. RNA samples for RNA sequencing were additionally purified on NucleoSpin RNA II columns (Machery‐Nagel) according to the manufacturer's instructions. RNA quantity and quality were determined using a Nanodrop ND1000 spectrophotometer.
RNA seq analysis
RNA sequencing was outsourced to ServiceXS (Leiden, The Netherlands). cDNA library constructions were performed using the Illumina mRNA‐Seq Sample preparation kit according to the instructions of the supplier. In brief, mRNA was isolated from total RNA using oligo‐dT magnetic beads. After fragmentation of the mRNA, cDNA synthesis was performed and the cDNA was ligated with the sequencing adapters before PCR amplification of the resulting product. The quality and yield after sample preparation were measured with a DNA 1000 Lab‐on‐a‐Chip. Clustering and mRNA sequencing using the Illumina cBot and HiSeq 2000 were performed according to the manufacturer's protocol. For each RNA sample at least 3.2 Gb of sequence data were obtained that passed the quality control (% ≥ Q30). Image analysis, base calling and quality checks were performed with the Illumina data analysis pipeline RTA v1.13.48 and/or OLBv1.9 and CASAVA v1.8.2. Quality‐filtered sequence tags are available upon request.
Transcriptomic data analysis
RNA‐Seq analysis was performed essentially as described previously (Wang et al., 2015) except for the transfer of genome annotations. Annotations were transferred from the CBS 513.88 genome to the N402 genome based on bidirectional alignments created with LAST (version 417) (Kiełbasa et al., 2011), with tandem repeat sections masked with TRF (Benson, 1999). Alignments were combined and chained using CLASP (Otto et al., 2011). Gaps in the global alignment were improved by performing realignment using either LAST (for large gaps, in sensitive mode) or Needleman–Wunsch (for smaller gaps). Chains with scores > 150 were accepted for alignment transfer. In total, 13 412 (of 14070) genes were transferred. The normalized, RNAseq‐based expression data are summarized in Table S8.
Gene ontology (GO) and transcription factor binding site enrichment analysis
Over‐represented GO terms in sets of differentially expressed genes (FDR at q < 0.05) were determined by Fisher's exact test (Fisher, 1922). An improved GO annotation for the A. niger CBS 513.88 genome was based on orthology mappings from A. nidulans FGSC A4 (Nitsche et al., 2012a, 2012b). Transcription factor binding site enrichment analysis of genes differentially higher expressed in the ΔugmA mutant was performed using a Perl script named the transcription factor binding site finder (TFBSF) as described previously (Meyer et al., 2009).
Supporting information
Table S1. Differentially expressed genes up‐regulated in ΔugmA mutant.
Table S2. Differentially expressed genes down‐regulated in ΔugmA mutant.
Table S3. GO‐terms (Biological Processes) over‐represented in the genes up‐regulated in the ΔugmA mutant.
Table S4. GO‐terms (Biological Processes) over‐represented in the genes down‐regulated in the ΔugmA mutant.
Table S5. Transcription factor binding sites enriched in the promoter of regions of genes up‐regulated in the ΔugmA mutant.
Table S6. Gene identities of genes up‐regulated after treatment with caspofungin, fenpropimorph, aureobasidin A, FK506 and in the ΔugmA mutant.
Table S7. Primers used in this study.
Table S8. RNAseq expression data N402 and ΔugmA mutant.
Supporting info item
Acknowledgements
We thank Sabine van der Kaay, Noortje Dannenberg and Norman van Reijn for their contributions in strain constructions and bioinformatic analyses. We thank Frank Ebel for the L10 antibody. We thank Frans Klis for reading the manuscript and helpful comments. This work was financially supported by the Dutch Technology Foundation (STW).
Park, J. , Hulsman, M. , Arentshorst, M. , Breeman, M. , Alazi, E. , Lagendijk, E. L. , Rocha, M. C. , Malavazi, I. , Nitsche, B. M. , van den Hondel, C. A. M. J. J. , Meyer, V. , and Ram, A. F. J. (2016) Transcriptomic and molecular genetic analysis of the cell wall salvage response of Aspergillus niger to the absence of galactofuranose synthesis. Cellular Microbiology, 18: 1268–1284. doi: 10.1111/cmi.12624.
References
- Afroz, S. , El‐Ganiny, A.M. , Sanders, D.A. , and Kaminskyj, S.G. (2011) Roles of the Aspergillus nidulans UDP‐galactofuranose transporter, UgtA in hyphal morphogenesis, cell wall architecture, conidiation, and drug sensitivity. Fungal Genet Biol 48: 896–903. [DOI] [PubMed] [Google Scholar]
- Alam, M.K. , El‐Ganiny, A.M. , Afroz, S. , Sanders, D.A.R. , Liu, J. , and Kaminskyj, S.G.W. (2012) Aspergillus nidulans galactofuranose biosynthesis affects antifungal drug sensitivity. Fungal Genet Biol 49: 1033–1043. [DOI] [PubMed] [Google Scholar]
- Alam, M.K. , van Straaten, K.E. , Sanders, D.R. , and Kaminskyj, S.G.W. (2014) Aspergillus nidulans cell wall composition and function change in response to hosting several Aspergillus fumigatus UDP‐galactopyranose mutase activity mutants. PLoS One 9 e85735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, B.D. , and Pfaller, M.A. (2006) Contemporary Tools for the Diagnosis and Management of Invasive Mycoses. Clin Infect Dis 43: S15–S27. [Google Scholar]
- Arentshorst, M. , Jing, N. , and Ram, A.F. (2015) Efficient generation of Aspergillus niger knock out strains by combining NHEJ mutants and a split marker approach In Genetic Transformation Systems in Fungi, Volume 1. van den Berg M.A., Maruthachalam K, (eds). Switzerland: Springer International Publishing, pp. 263–272. [Google Scholar]
- Bennett, J.W. , and Lasure, L.L. (1991) More Gene Manipulations in Fungi. San Diego: Academic Press. [Google Scholar]
- Benson, G. (1999) Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Res 27: 573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos, C.J. , Debets, A.J. , Swart, K. , Huybers, A. , Kobus, G. , and Slakhorst, S.M. (1988) Genetic analysis and the construction of master strains for assignment of genes to six linkage groups in Aspergillus niger . Curr Genet 14: 437–443. [DOI] [PubMed] [Google Scholar]
- Bose, S. , Dutko, J.A. , and Zitomer, R.S. (2005) Genetic factors that regulate the attenuation of the general stress response of yeast. Genetics 169: 1215–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho, N.D.S.P. , Arentshorst, M. , Jin Kwon, M. , Meyer, V. , and Ram, A.F.J. (2010) Expanding the ku70 toolbox for filamentous fungi: establishment of complementation vectors and recipient strains for advanced gene analyses. Appl Microbiol Biotechnol 87: 1463–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrejon, F. , Gomez, A. , Sanz, M. , Duran, A. , and Roncero, C. (2006) The RIM101 pathway contributes to yeast cell wall assembly and its function becomes essential in the absence of mitogen‐activated protein kinase Slt2p. Eukaryot Cell 5: 507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damveld, R.A. , Arentshorst, M. , Franken, A. , vanKuyk, P.A. , Klis, F.M. , van den Hondel, C.A.M.J.J. , and Ram, A.F.J. (2005a) The Aspergillus niger MADS‐box transcription factor RlmA is required for cell wall reinforcement in response to cell wall stress. Mol Microbiol 58: 305–319. [DOI] [PubMed] [Google Scholar]
- Damveld, R.A. , Arentshorst, M. , VanKuyk, P.A. , Klis, F.M. , van den Hondel, C.A.M.J.J. , and Ram, A.F.J. (2005b) Characterisation of CwpA, a putative glycosylphosphatidylinositol‐anchored cell wall mannoprotein in the filamentous fungus Aspergillus niger . Fungal Genet Biol 42: 873–885. [DOI] [PubMed] [Google Scholar]
- Damveld, R.A. , Franken, A. , Arentshorst, M. , Punt, P.J. , Klis, F.M. , van den Hondel, C.A.M.J.J. , and Ram, A.F.J. (2008) A novel screening method for cell wall mutants in Aspergillus niger identifies UDP‐galactopyranose mutase as an important protein in fungal cell wall biosynthesis. Genetics 178: 873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damveld, R.A. , vanKuyk, P.A. , Arentshorst, M. , Klis, F.M. , van den Hondel, C.A.M.J.J. , and Ram, A.F.J. (2005c) Expression of agsA, one of five 1,3‐alpha‐D‐glucan synthase‐encoding genes in Aspergillus niger, is induced in response to cell wall stress. Fungal Genet Biol 42: 165–177. [DOI] [PubMed] [Google Scholar]
- Dichtl, K. , Helmschrott, C. , Dirr, F. , and Wagener, J. (2012) Deciphering cell wall integrity signalling in Aspergillus fumigatus: identification and functional characterization of cell wall stress sensors and relevant Rho GTPases. Mol Microbiol 83: 506–519. [DOI] [PubMed] [Google Scholar]
- Dichtl, K. , Samantaray, S. , Aimanianda, V. , Zhu, Z. , Prévost, M.‐C. , Latgé, J.‐P. , et al. (2015) Aspergillus fumigatus devoid of cell wall β‐1,3‐glucan is viable, massively sheds galactomannan and is killed by septum formation inhibitors. Mol Microbiol 95: 458–471. [DOI] [PubMed] [Google Scholar]
- Engel, J. , Schmalhorst, P.S. , Dörk‐Bousset, T. , Ferrières, V. , and Routier, F.H. (2009) A single UDP‐galactofuranose transporter is required for galactofuranosylation in Aspergillus fumigatus . J Biol Chem 284: 33859–33868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández‐Acero, T. , Rodríguez‐Escudero, I. , Molina, M. , and Cid, V.J. (2015) The yeast cell wall integrity pathway signals from recycling endosomes upon elimination of phosphatidylinositol (4,5)‐bisphosphate by mammalian phosphatidylinositol 3‐kinase. Cell Signal 27: 2272–2284. [DOI] [PubMed] [Google Scholar]
- Fiedler, M.R. , Lorenz, A. , Nitsche, B.M. , van den Hondel, C.A. , Ram, A.F. , and Meyer, V. (2014) The capacity of Aspergillus niger to sense and respond to cell wall stress requires at least three transcription factors: RlmA, MsnA and CrzA. Fungal Biol Biotechnol 1: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, R.A. (1922) On the mathematical foundations of theoretical statistics. Philos Trans R Soc A Math Phys Eng Sci 222: 309–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine, T. , Simenel, C. , Dubreucq, G. , Adam, O. , Delepierre, M. , Lemoine, J. , et al. (2000) Molecular organization of the alkali‐insoluble fraction of Aspergillus fumigatus cell wall. J Biol Chem 275: 27594–27607. [DOI] [PubMed] [Google Scholar]
- Fortwendel, J.R. , Juvvadi, P.R. , Perfect, B.Z. , Rogg, L.E. , Perfect, J.R. , and Steinbach, W.J. (2010) Transcriptional regulation of chitin synthases by calcineurin controls paradoxical growth of Aspergillus fumigatus in response to caspofungin. Antimicrob Agents Chemother 54: 1555–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Free, S.J. (2013) Fungal cell wall organization and biosynthesis. Adv Genet 81: 33–82. [DOI] [PubMed] [Google Scholar]
- Frieman, M.B. , and Cormack, B.P. (2003) The omega‐site sequence of glycosylphosphatidylinositol‐anchored proteins in Saccharomyces cerevisiae can determine distribution between the membrane and the cell wall. Mol Microbiol 50: 883–896. [DOI] [PubMed] [Google Scholar]
- Frieman, M.B. , and Cormack, B.P. (2004) Multiple sequence signals determine the distribution of glycosylphosphatidylinositol proteins between the plasma membrane and cell wall in Saccharomyces cerevisiae. Microbiology 150: 3105–3114. [DOI] [PubMed] [Google Scholar]
- Fuchs, B.B. , and Mylonakis, E. (2009) Our paths might cross: the role of the fungal cell wall integrity pathway in stress response and cross talk with other stress response pathways. Eukaryot Cell 8: 1616–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García, R. , Botet, J. , Rodríguez‐Peña, J.M. , Bermejo, C. , Ribas, J.C. , Revuelta, J.L. , et al. (2015) Genomic profiling of fungal cell wall‐interfering compounds: identification of a common gene signature. BMC Genomics 16: 683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastebois, A. , Clavaud, C. , Aimanianda, V. , and Latgé, J.‐P. (2009) Aspergillus fumigatus: cell wall polysaccharides, their biosynthesis and organization. Future Microbiol 4: 583–595. [DOI] [PubMed] [Google Scholar]
- Guest, G.M. , and Momany, M. (2000) Analysis of Cell Wall Sugars in the Pathogen Aspergillus fumigatus and the Saprophyte Aspergillus nidulans . Mycologia 92: 1047–1050. [Google Scholar]
- Han, K.‐H. , and Prade, R.A. (2002) Osmotic stress‐coupled maintenance of polar growth in Aspergillus nidulans . Mol Microbiol 43: 1065–1078. [DOI] [PubMed] [Google Scholar]
- Hartree, E.F. (1972) Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem 48: 422–427. [DOI] [PubMed] [Google Scholar]
- Heesemann, L. , Kotz, A. , Echtenacher, B. , Broniszewska, M. , Routier, F. , Hoffmann, P. , and Ebel, F. (2011) Studies on galactofuranose‐containing glycostructures of the pathogenic mold Aspergillus fumigatus . Int J Med Microbiol 301: 523–530. [DOI] [PubMed] [Google Scholar]
- Hong, S.‐Y. , Roze, L.V. , and Linz, J.E. (2013) Oxidative stress‐related transcription factors in the regulation of secondary metabolism. Toxins (Basel) 5: 683–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen, T.R. , Nitsche, B.M. , Lamers, G.E. , Arentshorst, M. , a van den Hondel, C. , and Ram, A.F. (2010) Transcriptomic insights into the physiology of Aspergillus niger approaching a specific growth rate of zero. Appl Environ Microbiol 76: 5344–5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaffa, L1. , Coulier, L. , Fekete, E. , Overkamp, K.M. , Druzhinina, I.S. , Mikus, M. , et al. (2013) The intracellular galactoglycome in Trichoderma reesei during growth on lactose. Appl Microbiol Biotechnol 97(12): 5447–5456. [DOI] [PubMed] [Google Scholar]
- Kiełbasa, S.M. , Wan, R. , Sato, K. , Horton, P. , and Frith, M.C. (2011) Adaptive seeds tame genomic sequence comparison. Genome Res 21: 487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komachi, Y. , Hatakeyama, S. , Motomatsu, H. , Futagami, T. , Kizjakina, K. , Sobrado, P. , et al. (2013) GfsA encodes a novel galactofuranosyltransferase involved in biosynthesis of galactofuranose antigen of O‐glycan in Aspergillus nidulans and Aspergillus fumigatus . Mol Microbiol 90: 1054–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin, D.E. (2011) Regulation of cell wall biogenesis in Saccharomyces cerevisiae: the cell wall integrity signaling pathway. Genetics 189: 1145–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, V. , Arentshorst, M. , Flitter, S.J. , Nitsche, B.M. , Kwon, M.J. , Reynaga‐Peña, C.G. , et al. (2009) Reconstruction of signaling networks regulating fungal morphogenesis by transcriptomics. Eukaryot Cell 8: 1677–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, V. , Damveld, R.A. , Arentshorst, M. , Stahl, U. , van den Hondel, C.A.M.J.J. , and Ram, A.F.J. (2007) Survival in the presence of antifungals: genome‐wide expression profiling of Aspergillus niger in response to sublethal concentrations of caspofungin and fenpropimorph. J Biol Chem 282: 32935–32948. [DOI] [PubMed] [Google Scholar]
- Nitsche, B.M. , Jørgensen, T.R. , Akeroyd, M. , Meyer, V. , and Ram, A.F.J. (2012a) The carbon starvation response of Aspergillus niger during submerged cultivation: insights from the transcriptome and secretome. BMC Genomics 13: 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche, B.M. , Ram, A.F.J. , and Meyer, V. (2012b) The use of open source bioinformatics tools to dissect transcriptomic data. Methods Mol Biol 835: 311–331. [DOI] [PubMed] [Google Scholar]
- Niu, J. , Arentshorst, M. , Seelinger, F. , Ram, A.F. , and Ouedraogo, J.P. (2016) A set of isogenic auxotrophic strains for constructing multiple gene deletion mutants and parasexual crossings in Aspergillus niger . Arch Microbiol available online: doi:10.1007/s00203‐016‐1240‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Meara, T.R. , Xu, W. , Selvig, K.M. , O'Meara, M.J. , Mitchell, A.P. , and Alspaugh, J.A. (2014) The Cryptococcus neoformans Rim101 transcription factor directly regulates genes required for adaptation to the host. Mol Cell Biol 34: 673–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto, C. , Hoffmann, S. , Gorodkin, J. , and Stadler, P.F. (2011) Fast local fragment chaining using sum‐of‐pair gap costs. Algorithms Mol Biol 6: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouedraogo, J.P. , Hagen, S. , Spielvogel, A. , Engelhardt, S. , and Meyer, V. (2011) Survival strategies of yeast and filamentous fungi against the antifungal protein AFP. J Biol Chem 286: 13859–13868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J. , Tefsen, B. , Arentshorst, M. , Lagendijk, E. , Hondel, C.A.V.D. , Die, I.V. , and Ram, A.F.J. (2014) Identification of the UDP‐glucose‐4‐epimerase required for galactofuranose biosynthesis and galactose metabolism in A. niger . Fungal Biol Biotechnol 1: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J. , Tefsen, B. , Heemskerk, M. , Lagendijk, E. , Hondel, C.A.V.D. , Die, I.V. , and Ram, A.F.J. (2015) Identification and functional analysis of two Golgi‐localized UDP‐galactofuranose transporters with overlapping functions in Aspergillus niger . BMC Microbiol 15: 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Peij, N.N. , Gielkens, M.M. , de Vries, R.P. , Visser, J. , and de Graaff, L.H. (1998a) The transcriptional activator XlnR regulates both xylanolytic and endoglucanase gene expression in Aspergillus niger . Appl Environ Microbiol 64: 3615–3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Peij, N.N. , Visser, J. , and de Graaff, L.H. (1998b) Isolation and analysis of xlnR, encoding a transcriptional activator co‐ordinating xylanolytic expression in Aspergillus niger . Mol Microbiol 27: 131–142. [DOI] [PubMed] [Google Scholar]
- Pel, H.J. , de Winde, J.H. , Archer, D.B. , Dyer, P.S. , Hofmann, G. , Schaap, P.J. , et al. (2007) Genome sequencing and analysis of the versatile cell factory Aspergillus niger CBS 513.88. Nat Biotechnol 25: 221–231. [DOI] [PubMed] [Google Scholar]
- Ram, A.F.J. , Arentshorst, M. , Damveld, R.A. , VanKuyk, P.A. , Klis, F.M. , and van den Hondel, C.A.M.J.J. (2004) The cell wall stress response in Aspergillus niger involves increased expression of the glutamine : fructose‐6‐phosphate amidotransferase‐encoding gene (gfaA) and increased deposition of chitin in the cell wall. Microbiology 150: 3315–3326. [DOI] [PubMed] [Google Scholar]
- Rocha, M.C. , Godoy, K.F. , de Castro, P.A. , Hori, J.I. , Bom, V.L. , Brown, N.A. , et al. (2015) The Aspergillus fumigatus pkcAG579R mutant is defective in the activation of the Cell Wall Integrity pathway but is dispensable for virulence in a neutropenic mouse infection model. PLoS One 10: e0135195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, R.L. , and Fink, G.R. (1994) Elements of a single MAP kinase cascade in Saccharomyces cerevisiae mediate two developmental programs in the same cell type: mating and invasive growth. Genes Dev 8: 2974–2985. [DOI] [PubMed] [Google Scholar]
- Schmalhorst, P.S. , Krappmann, S. , Vervecken, W. , Rohde, M. , Müller, M. , Braus, G.H. , et al. (2008) Contribution of galactofuranose to the virulence of the opportunistic pathogen Aspergillus fumigatus . Eukaryot Cell 7: 1268–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach, W.J. , Cramer, R.A. Jr , Perfect, B.Z. , Henn, C. , Nielsen, K. , Heitman, J. , and Perfect, J.R. (2007) Calcineurin inhibition or mutation enhances cell wall inhibitors against Aspergillus fumigatus . Antimicrob Agents Chemother 51: 2979–2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tefsen, B. , Ram, A.F.J. , Die, I.V. , and Routier, F.H. (2012) Galactofuranose in eukaryotes: aspects of biosynthesis and functional impact. Glycobiology 22: 456–469. [DOI] [PubMed] [Google Scholar]
- Valiante, V. , Macheleidt, J. , Föge, M. , and Brakhage, A.A. (2015) The Aspergillus fumigatus cell wall integrity signaling pathway: drug target, compensatory pathways, and virulence. Front Microbiol 6: 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwer, P.E. , van Duijn, M.L. , Tavakol, M. , Bakker‐Woudenberg, I.A. , and van de Sande, W.W. (2012) Reshuffling of Aspergillus fumigatus cell wall components chitin and β‐glucan under the influence of caspofungin or nikkomycin Z alone or in combination. Antimicrob Agents Chemother 56: 1595–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, F. , Krijgsheld, P. , Hulsman, M. , de Bekker, C. , Müller, W.H. , Reinders, M. , et al. (2015) FluG affects secretion in colonies of Aspergillus niger . Antonie Van Leeuwenhoek 107: 225–240. [DOI] [PubMed] [Google Scholar]
- Wong Sak Hoi, J. , and Dumas, B. (2010) Ste12 and Ste12‐like proteins, fungal transcription factors regulating development and pathogenicity. Eukaryot Cell 9: 480–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto, H. , Saltsman, K. , Gasch, A.P. , Li, H.X. , Ogawa, N. , Botstein, D. , et al. (2002) Genome‐wide analysis of gene expression regulated by the calcineurin/Crz1p signaling pathway in Saccharomyces cerevisiae . J Biol Chem 277: 31079–31088. [DOI] [PubMed] [Google Scholar]
- Zakrzewska, A. , Boorsma, A. , Brul, S. , Hellingwerf, K.J. , and Klis, F.M. (2005) Transcriptional response of Saccharomyces cerevisiae to the plasma membrane‐perturbing compound chitosan. Eukaryot Cell 4: 703–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Differentially expressed genes up‐regulated in ΔugmA mutant.
Table S2. Differentially expressed genes down‐regulated in ΔugmA mutant.
Table S3. GO‐terms (Biological Processes) over‐represented in the genes up‐regulated in the ΔugmA mutant.
Table S4. GO‐terms (Biological Processes) over‐represented in the genes down‐regulated in the ΔugmA mutant.
Table S5. Transcription factor binding sites enriched in the promoter of regions of genes up‐regulated in the ΔugmA mutant.
Table S6. Gene identities of genes up‐regulated after treatment with caspofungin, fenpropimorph, aureobasidin A, FK506 and in the ΔugmA mutant.
Table S7. Primers used in this study.
Table S8. RNAseq expression data N402 and ΔugmA mutant.
Supporting info item


