Abstract
Use of the iron chelator deferiprone for treatment of iron overload in thalassemia patients is associated with concerns over agranulocytosis, which requires weekly absolute neutrophil counts (ANC). Here, we analyze all episodes of agranulocytosis (n = 161) and neutropenia (n = 250) during deferiprone use in clinical trials (CT) and postmarketing surveillance programs (PMSP). Rates of agranulocytosis and neutropenia in CT were 1.5% and 5.5%, respectively. Of the agranulocytosis cases, 61% occurred during the first 6 months of therapy and 78% during the first year. These events appeared to be independent of dose, and occurred three times more often in females than males. Their duration was not significantly shortened by use of G‐CSF. No patient with baseline neutropenia (n = 12) developed agranulocytosis during treatment, which raises questions about the validity of prior neutropenia as a contraindication to use. Only 1/7 novel neutropenia cases in CT progressed to agranulocytosis with continued treatment, indicating that neutropenia does not necessarily lead to agranulocytosis. The agranulocytosis fatality rate was 0% in CT and 15/143 (11%) in PMSP. Rechallenge with deferiprone produced agranulocytosis in 75% of patients in whom the event had already occurred, and in 10% with previous neutropenia. Weekly ANC monitoring allows early detection and interruption of therapy, but does not prevent agranulocytosis from occurring. Its relevance appears to decrease after the first year of therapy, when agranulocytosis occurs less often. Based upon analysis of data collected over the past 20 years, it appears that patient education may be the key to minimizing agranulocytosis‐associated risks during deferiprone therapy. Am. J. Hematol. 91:1026–1031, 2016. © 2016 The Authors. American Journal of Hematology Published by Wiley Periodicals, Inc.
Introduction
Deferiprone (Ferriprox®, ApoPharma Inc., Canada) is an oral iron chelator indicated for the treatment of iron overload in patients with thalassemia 1 Its use is associated with severe neutropenia, also called agranulocytosis, in 1–2% of patients 1, 2 and with less severe episodes of neutropenia in approximately 5% of patients 2, 3. The pathogenesis of and risk factors for deferiprone‐induced neutropenia are presently unidentified 1, 4, 5. To minimize the risk of developing agranulocytosis and its potential complications, all patients taking deferiprone are expected to have their blood absolute neutrophil count (ANC) monitored weekly; to discontinue therapy at the first sign of infection or of neutropenia (ANC < 1.5 × 109/L); and to avoid rechallenge 3, 6. However, the rationale for imposing weekly testing on both patients and health resources has been questioned 4, 5.
Thalassemia patients frequently experience transient episodes of mild or moderate neutropenia, independent of deferiprone 5, 7, 8, 9, and neutropenia during deferiprone therapy often does not progress to agranulocytosis, even with continued treatment 5, 10, 11. Indiscriminately stopping or interrupting deferiprone at onset of neutropenia may appear to be a prudent safety measure, but may fail to balance benefits and risks for individuals.
There is a need to identify risk factors for agranulocytosis during deferiprone therapy, the effectiveness of weekly ANC monitoring in avoiding its consequences, and the rate of its recurrence upon rechallenge. This article summarizes the data reviewed and the conclusions and recommendations offered by a panel of experts in thalassemia and drug‐induced agranulocytosis convened to evaluate fulfillment of those needs.
Methods
Data collected by ApoPharma on all episodes of neutropenia, including agranulocytosis, during clinical trials (CT) of deferiprone or reported from its postmarketing surveillance programs (PMSP), including literature‐reported cases, were reviewed as described below.
Clinical trials
Data from all participants who received at least one dose of deferiprone in ApoPharma‐sponsored trials from 1993 to August 31, 2014 were analyzed. Participants in more than one CT were counted only once, but events in all trials were included. Study participants enrolled after 1994 had their ANC monitored weekly (7 ± 3 days) while treated with deferiprone, some for more than 18 years. An ANC lower than 1.5 × 109/L required a confirmatory count within 24 hr. Agranulocytosis was defined as a confirmed ANC less than 0.5 × 109/L. Less severe episodes of neutropenia were classified as moderate (0.5 − < 1.0 × 109/L) or mild (1.0 − < 1.5 × 109/L). In this analysis, episodes of mild or moderate neutropenia are termed “neutropenia” and more severe cases “agranulocytosis.” Drug exposure, control groups, and so on are detailed in the Supporting Information section.
Postmarketing surveillance and literature review
All reported cases of agranulocytosis from the 63 countries where Ferriprox® has been available between its first market approval in 1999 and August 31, 2014 were analyzed. Reports were obtained from healthcare professionals (HCPs), consumers, regulatory agencies, and monitoring of the literature. PMSP data limitations preclude calculation of some values; for example, gender distribution. Details of data sourcing and assumptions are provided in the Supporting Information section.
Data analysis
Data analysis is described in the Supporting Information section. Rates per 100 patient‐years were normalized for exposure to deferiprone. Nonparametric tests were employed to analyze the difference in incidence and the rate of occurrence per patient‐year between groups where appropriate.
Results
The analysis included all 1,127 participants in the ApoPharma CTs and all episodes of neutropenia or agranulocytosis identified from the PMSP. Multiple reports of the same event were counted once.
Agranulocytosis (ANC < 0.5 × 109/L)
Frequency
Overall, 161 episodes of agranulocytosis have been reported during deferiprone therapy: 18 episodes in 17 (1.5%) of 1,127 CT subjects, for a rate of 1.1 per 100 patient‐years of drug exposure (total 1653.15 years), and 143 episodes in the PMSP, for a rate of 0.24 per 100 patient‐years (during an estimated 58,790 patient‐years of postmarketing exposure). No episodes of agranulocytosis occurred among 120 subjects given deferoxamine as comparator in the ApoPharma trials.
Demographics
Table 1 summarizes the characteristics of all 161 episodes. Among ApoPharma CT subjects, the incidence of agranulocytosis was higher in females (2.4%; 12/510) than in males (0.8%; 5/617) (P = 0.048). Of the 139 PMSP cases with known gender, 79 were females, and 60 males. Incidence did not differ significantly by race (Blacks 3.7%, Asians 2.2%, Caucasians 1.3%, and undefined 1.7%; P = 0.37).
Table 1.
Summary of the Characteristics of all 161 Episodes of Agranulocytosis and All 250 Episodes of Neutropenia Reported During Ferriprox Use in CT (1993–2014) and in Post‐Marketing Experience (1999–2014)
| Definition | Agranulocytosis | Neutropenia |
|---|---|---|
| No. of events | 161 | 250 |
| Age median | 26 years [n = 148] | 26 years [n = 219] |
| Age range | 4–86 years | 2–81 years |
| Sex—Male/Female/Unknown | 65/91/2 | 102/129/3 |
| Duration of eventa median | 11 days [n = 115] | 14 days [n = 129] |
| Duration of event range | 2–244 days | 2–380 days |
| Time to eventb median | 147 days (n = 137) | 242 days (n = 164) |
| Time to event range | 9 days–17 years | 0 day–17 years |
| Dose DFP (min–max) closest to onset of event | 15–226 mg/kg/d | 8–170 mg/kg/dc |
| G‐CSF treatment—Yes/No/Unknown | 90/33/38 | 14/115/121 |
| Hepatitis C status—Yes/No/Unknown | 17/44/100 | 35/66/149 |
| Splenectomy status—Yes/No/Unknown | 19/60/82 | 43/147/60 |
The drug exposure to deferiprone in CT for each patient was calculated as the number of days between the first and last date of medication minus the total interruption days. The total drug exposure for all patients in all CT was the sum of the drug exposure for all study participants.
Time to onset of the first episode of agranulocytosis or neutropenia was based on the time interval between the first dose of deferiprone and the diagnosis of agranulocytosis or neutropenia.
If doses were provided in units other than mg/kg/day, the patient's weight was used for calculation; if this was not available, a weight of 60 kg was assumed. If weight was not provided for children, the dose in mg/kg/day was calculated based on the age and sex of the patient according to the chart developed by the National Center of Health Statistics in collaboration with the National Center for Chronic Disease Prevention and Health Promotion.
Other hematological findings
Assessment of platelet, eosinophil, and lymphocyte counts showed thrombocytopenia in 7/143 PMSP agranulocytosis episodes, but none in CT episodes. Platelet counts were available in 4/7 PMSP cases, and ranged from 11 × 109/L to 72 × 109/L. Concurrent lymphopenia, considered an ominous sign in drug‐induced neutropenias 12, occurred in two cases, both from PMSP. One, in a patient with Diamond‐Blackfan Anemia (DBA), was fatal 13; the other, in a patient with superficial siderosis, resolved. There were no reports of rash or eosinophilia concomitant with agranulocytosis. Bone marrow examinations during agranulocytosis were available in 20 cases (6 CT and 14 PMSP), and often showed hypoplastic myelopoiesis (Supporting Information Table I); systematic analysis was hampered by inconsistent sample timing.
Dosage effect
No dose threshold for agranulocytosis was identified; the effect occurred over a range of 10–225 mg/kg/day. Time to onset did not correlate with the dose (r = −0.094; P = 0.2837). See Fig. 1.
Figure 1.
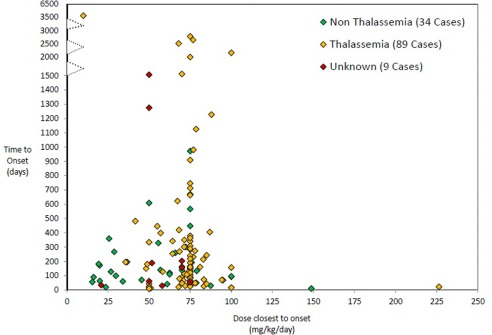
Episodes of agranulocytosis based on the time to onset, deferiprone dose closest to onset and patient's primary diagnosis. Data from episodes observed in CT and from episodes from the postmarketing surveillance programs for which information on time to onset and dose was available. There is no correlation between dose and time of onset of agranulocytosis (r = −0.094; P = 0.2837).
CT data do not indicate a higher incidence of agranulocytosis at the highest recommended dose of deferiprone, 100 mg/kg/day (range 100–226 mg/kg/day, 4/174 = 2.3%) than at lower doses (10–94 mg/kg/day, 13/953 = 1.4%; P = 0.32; Fisher's exact test). Four PMSP patients taking up to 250 mg/kg/day deferiprone had neither neutropenia nor agranulocytosis.
Baseline ANC
Twelve patients with mild‐to‐moderate neutropenia at baseline were subsequently treated with deferiprone for up to 2.5 years. In none of these patients was deferiprone use discontinued because of agranulocytosis. They included a patient with myelodysplasia (baseline ANC 0.5 × 109/L), whose ANC fluctuated between 0.2 and 1.2 × 109/L during 13 months of deferiprone therapy (see Supporting Information Fig. 1).
Primary disease
The incidence of agranulocytosis by primary disease was assessed in CT. Agranulocytosis was more frequent in patients with systemic iron overload due to conditions other than thalassemia syndromes than in patients with thalassemia or non‐systemic iron overload (P = 0.04) (Supporting Information).
Splenectomy
Agranulocytosis was not more frequent in non‐splenectomized (11/385; 2.9%) than in splenectomized thalassemia patients (4/243; 1.7%; P = 0.4267). Comparison in the PMSP population was precluded by lack of information on splenectomy.
Age and concomitant chelator therapy
Evaluation of age as a risk factor showed that 7 (2.7%) of 263 pediatric patients (<16 years old) experienced agranulocytosis versus 10 (1.2%) of 864 adults treated with deferiprone in CT (P = 0.08). One (1.1%) episode of agranulocytosis was seen in 92 patients receiving deferiprone concomitantly with deferoxamine or deferasirox in ApoPharma CT, which was not different from the incidence for deferiprone monotherapy (1.5%; P = 1.0).
Time to agranulocytosis
Median time to occurrence of agranulocytosis was 5 months (9 days–17 years) from the start of therapy. The incidence of agranulocytosis declined with increasing duration of therapy. Of the 18 agranulocytosis episodes in CT, 11 (61%) occurred within the first 6 months of therapy initiation, and 14 (78%) within 12 months (Fig. 2). Those episodes represent a rate of 3.2 events/100 patient‐years of drug exposure for the first 6 months of therapy in 1,127 patients; 1.1 events/100 patient‐years of exposure for the second six months of exposure in 592 patients; and 0.4 events/100 patient‐years of exposure after 1 year of therapy in 448 patients. Figure 3 shows the distribution of all episodes of agranulocytosis or neutropenia (CT and PMSP) by time to onset, where available. In the PMSP, the time to agranulocytosis was available in 118/143 cases, of which 76 (64%) occurred during the first six months of therapy and 94 (80%) within the first year.
Figure 2.
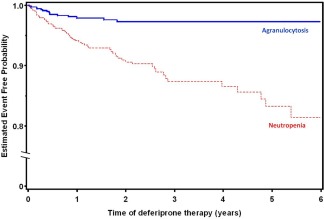
Kaplan‐Meier curves show the time to first occurrence of neutropenia or agranulocytosis in CT. The incidence of agranulocytosis declined with increasing duration of therapy. Of the 18 agranulocytosis episodes in CT, 11 (61%) occurred within the first 6 months of therapy initiation, and 14 (78%) within 12 months. Neutropenia occurred throughout the period of CTs. Two episodes of agranulocytosis and one episode of neutropenia that occurred after 6 years of deferiprone therapy were not included in the figure due to the small number of study subjects beyond that period. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 3.
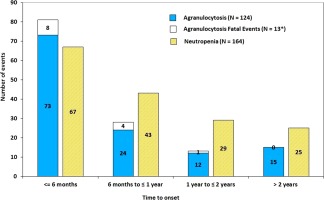
Numbers of agranulocytosis and neutropenia cases by time to onset in CT and postmarketing surveillance programs. Out of total reported 161 agranulocytosis and 250 neutropenia cases, only 134 agranulocytosis and 150 neutropenia cases have time to onset available. *There are 15 fatal cases in total, for 2 of which the time to event is not available. If patient reported multiple events, only first event is included. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Where available, weekly ANCs before onset of agranulocytosis revealed that in some cases the ANC dropped precipitously, and monitoring had not revealed signs of neutropenia prior to detection of agranulocytosis (Supporting Information).
Resolution and outcomes
Of 115 agranulocytosis episodes for which information was available, the median time to resolution was 11 days overall; 11 days in 76 episodes where G‐CSF was known to have been used; and 13 days in 22 episodes where G‐CSF was known not to have been used (P = 0.38; Wilcoxon Two‐Sample Test).
All 18 episodes of agranulocytosis that occurred in CT resolved. However, of the 143 cases that occurred in PMSP, 15 (11%) had fatal outcomes, an estimated rate of 0.03 fatalities/100 patient‐years post‐market exposure. Median time on therapy for the 15 PMSP events with a fatal outcome was 112 days (range 21 days to 15 months) for the 12 cases where this information was available. G‐CSF was used in 10 cases; was not used in two cases; and was unknown for the other three. In eight patients, fever was present up to 6 days prior to diagnosis of agranulocytosis. Nine of the 15 patients died of infections; one died of cerebral hemorrhage after resolution of the agranulocytosis; and one patient with DBA died after 2 months of pancytopenia, despite prolonged G‐CSF use 12, reportedly of circulatory collapse probably due to pulmonary embolism. Cause of death for the remaining four patients was not available (Supporting Information Tables II and III).
Eleven of the fatalities occurred between 1999 and 2006. Case analysis indicated that in most instances, deferiprone had not been discontinued at first evidence of infection or agranulocytosis, or the attending physician, unaware that a patient with signs of infection was at risk of drug‐dependent agranulocytosis, had managed the case inappropriately. Accordingly, an educational program for HCPs was instituted in 2006, emphasizing diligent ANC monitoring, particularly during infection, and interruption of deferiprone at the first evidence of neutropenia. Wallet‐sized cards were created for distribution to patients, reminding them of the importance of regular ANC monitoring and to stop taking deferiprone if they developed signs of infection. Patients were advised to show the card promptly to any HCP consulted about a possible infection. In subsequent ApoPharma surveys of HCPs, most respondents were aware of the protective measures highlighted. The reported agranulocytosis rate was essentially the same before and after implementation of the program (0.29 and 0.26 per 100 patient‐years, respectively; P = 0.56), but fatalities declined from 0.07 to 0.01 per 100 patient‐years (P = 0.013).
Neutropenia (ANC 0.5–1.5 × 109/L)
Overall, 250 episodes of neutropenia during deferiprone therapy were identified. In ApoPharma CT, 69 episodes (4.2 per 100 patient‐years' exposure) were reported in 62/1127 (5.5%) of deferiprone‐treated patients; in 35/506 females (6.9%) and in 27/621 (4.4%) males (P = 0.07). For the 129 cases in which information on duration was available, the median duration was 14 days (Table 1). However, of 120 patients treated with the CT comparator deferoxamine, 5 (4.2%) experienced six episodes of neutropenia (4.6 per 100 patient‐years). The incidence of neutropenia during deferoxamine use and its rate of occurrence were not significantly different from that in the deferiprone group (P = 0.67 and 0.81, respectively). The incidence of neutropenia in CT was also not dose‐related, being 12/174 (6.9%) at 100 mg/kg/day deferiprone and 50/953 (5.2%) at lower doses (P = 0.37; Fisher's exact test). There was no difference in the incidence of neutropenia between the first and second year of deferiprone therapy in CT (5.5% vs. 5.8%, respectively). Signs of infection within 14 days post diagnosis of neutropenia were observed in 3 (4.4%) of the 69 neutropenia cases in CT. Only one of the clinical trial subjects who experienced neutropenia required antibiotic therapy within 14 days after the onset of neutropenia.
Neutropenia incidence in CT suggested age dependency: 24 (9.1%) of 263 pediatric patients compared to 38 (4.4%) of 864 adults (P = 0.003).
The incidence of neutropenia in splenectomized patients was 12/243 (4.9%; 2.38/100 patient‐years' deferiprone exposure), compared with 42/385 (10.9%) (P = 0.0086) at a rate of 5.93/100 patient‐years (P = 0.0017) in non‐splenectomized patients. This difference may have been due to hypersplenism, a common condition in patients with thalassemia. However, as spleen sizes have not been reported, it is difficult to determine whether spleen size has a role in neutropenia.
To determine if neutropenia would proceed to agranulocytosis with continued deferiprone exposure, treatment was maintained in seven consecutive patients who experienced mild neutropenia; these findings have been reported separately 10. Briefly, these patients' ANC was monitored daily until resolution or a drop to <1.0 × 109/L. In five of the seven patients, the neutropenia resolved without recurrence. The sixth patient experienced two episodes of mild neutropenia that resolved despite continued therapy. The seventh patient experienced two episodes of mild neutropenia and a third episode of moderate neutropenia which progressed to agranulocytosis despite interruption of deferiprone.
Rechallenge
Three CT patients who experienced agranulocytosis were rechallenged because it was unclear if the events were related to deferiprone use. One of these patients experienced a second episode of agranulocytosis 15 days after rechallenge. Deferiprone was discontinued, and the event resolved. Another patient experienced an episode of neutropenia (ANC = 0.81 × 109/L) 35 days after rechallenge. Deferiprone was discontinued, and the neutropenia resolved. The third patient, with a diagnosis of myelodysplastic syndrome, experienced one episode of neutropenia (ANC = 1.4 × 109/L) fifteen days after rechallenge, which resolved despite continued deferiprone use. Approximately 1 month later, the patient experienced another episode of neutropenia; deferiprone was interrupted but subsequently the patient developed agranulocytosis. On resolution, the patient was rechallenged and experienced a third episode of agranulocytosis four months later. Deferiprone was again discontinued and re‐started on resolution of the event. The patient continued deferiprone use for another 7 years without recurrence of agranulocytosis.
Fifteen reports of rechallenge after episodes of agranulocytosis have been reported in the PMSP, with follow‐up data in 12 of them. Recurrence was observed in 9 (75%). Time to recurrence ranged from 1–5 months in the three episodes where this information was available.
Rechallenge in 10 CT patients in whom deferiprone had been interrupted at diagnosis of neutropenia (ANC 0.5–0.87 × 109/L) resulted in one patient experiencing agranulocytosis 64 days after rechallenge but no recurrence in the others, who were maintained on deferiprone for an additional 3 days to over 3 years. No PMSP data are available on rechallenge post episodes of neutropenia.
Discussion
Drug‐induced idiosyncratic agranulocytosis is a serious and sometimes fatal complication. Hence, attempts to identify factors that might reduce incidence of this event and minimize its complications are warranted. This goal requires large patient cohorts, but deferiprone is approved as an orphan drug, and the low incidence of agranulocytosis in the relative small cohort of patients treated with this medicine limits the potential database.
The first case of deferiprone‐induced agranulocytosis was reported in 1989 in a patient with DBA 14. Other cases in patients with thalassemia, myelodysplastic syndromes, and other anemias were subsequently reported 9, 15, 16. The incidence of agranulocytosis during deferiprone therapy was at that time reported to range from 0.5 to 3.6%, and from 0.2 to 0.43 per 100 patient‐years of exposure 9, 15, 17, 18. This report comprises the largest compilation of agranulocytosis or neutropenia episodes during deferiprone therapy. The overall incidence of agranulocytosis (1.5%; 1.1/100 patient‐years of drug exposure) in CT remains consistent with that reported in worldwide product labeling. The lower rate in the postmarketing surveillance program (0.24/100 patient‐years) may be due to the inherent underreporting of adverse events in the post marketing surveillance given that it is a voluntary process and/or it may reflect long‐term exposure to deferiprone, given the low incidence of agranulocytosis after the first year of therapy.
Assessment of primary disease as a risk factor indicates that the incidence of agranulocytosis in CT was higher among patients with non‐thalassemia systemic iron overload (6.7%) and asymptomatic HIV infection (7.1%) than in patients with thalassemia syndromes (1.7%) or neurodegenerative conditions involving brain iron overload (1.3%). However, the low frequency of deferiprone‐induced agranulocytosis, and the relatively low representation of conditions other than thalassemia syndromes, hampers comparisons of patient subpopulations. Larger cohorts (>300 patients) are required to establish if the primary disease is a significant risk determinant. Nonetheless, the DBA cases require attention. While the incidence of deferiprone‐induced agranulocytosis cannot be calculated in the absence of a known denominator, seven reported DBA cases suggest that agranulocytosis is more frequent in DBA than in thalassemia, as well as more severe 13, 14 (three cases were fatal, and several were associated with significantly diminished other leukocyte and platelet counts). Based on these data, we suggest that deferiprone therapy should not be initiated in patients with DBA, unless there is no alternative.
Neutropenia was observed more often in pediatric than in adult patients in CT with deferiprone, which is consistent with the higher occurrence of neutropenia in healthy children than in adults 19. However, for agranulocytosis, the difference in incidence between pediatric and adult populations was not significant. There was also no significant difference in the incidence of agranulocytosis in patients treated with deferiprone as monotherapy or in combination with another chelator. This finding appears consistent with the incidence of agranulocytosis reported in various publications of concurrent use of deferiprone and deferoxamine 20, 21, 22, 23, 24, 25, 26.
An important issue is whether episodes of neutropenia during deferiprone therapy is a harbinger of agranulocytosis or if they represent discrete events. In the initial ApoPharma studies, weekly monitoring of ANC was instituted to determine the incidence of neutropenia and agranulocytosis. Because it was unclear if neutropenia was the first sign of agranulocytosis, it was recommended that deferiprone therapy be discontinued at the first sign of neutropenia. Subsequent CT data, however, showed that thalassemia patients treated with comparator(s) experience transient episodes of mild or moderate neutropenia, which often would have gone undetected with less frequent monitoring 5, 7, 8. The CT data also show that in many of the 18 cases of agranulocytosis, the ANC dropped precipitously, and weekly monitoring of the ANC had not revealed signs of neutropenia.
These findings pose the question of whether mild or moderate neutropenia would progress to agranulocytosis with continued use of deferiprone. Accordingly, 294 patients with transfusion‐dependent anemias were enrolled into a noninterventional surveillance program designed to assess compliance in clinical practice with the recommended weekly ANC monitoring and interruption of deferiprone use at onset of neutropenia 10. If therapy was continued, the program assessed its impact on the occurrence of agranulocytosis. ANC monitoring was conducted at an average interval of 5 ± 4 weeks. Deferiprone was not always interrupted upon detection of neutropenia, but there was no greater incidence of agranulocytosis. Overall, one patient (0.3%) experienced agranulocytosis and 9 others (3%) experienced a total of 11 episodes of neutropenia. All neutropenia episodes resolved, and time to resolution was similar whether or not treatment had been interrupted. No case of neutropenia progressed to agranulocytosis. Data from the CT also suggest that deferiprone use was not associated with progression of pre‐existing mild‐to‐moderate neutropenia 10, 11. This is of particular relevance in geographic areas where ethnic neutropenia co‐exists with a high prevalence of patients requiring iron chelation therapy because of hemoglobinopathies 27, 28. A low neutrophil count should not be considered a contraindication to the use of deferiprone.
The characteristics of deferiprone‐induced agranulocytosis are typical of idiosyncratic drug reactions 1, 4, 15, 29 in that:
It is unpredictable and a rare event
Onset is delayed, occurrence peaks after 1–3 months, and time of first onset extends beyond one year
Incidence is not dose‐related within the therapeutic range. This is consistent with the lack of dependence of clozapine‐associated agranulocytosis and neutropenia on dose 30, but in contrast with the dose dependency of beta‐lactam antibiotic‐mediated agranulocytosis 31.
Idiosyncratic drug reactions are generally inconsistent with direct cytotoxicity, and there is growing evidence that most are mediated by the adaptive immune system 32. The manifestations of an “allergic” reaction, such as rash, that occur in some cases have not been recorded in deferiprone‐induced agranulocytosis 32.
No available tests are recognized as being of value in identifying those patients at risk of developing deferiprone‐induced agranulocytosis. Four episodes of agranulocytosis in CT were preceded by signs of infection or immune reactions. It is unclear whether those events may have played a role on the development of agranulocytosis. However, there would be merit in considering the potential contribution of a preceding infection along with other factors such as tolerance of alternative chelators in evaluating risk:benefit for rechallenge. Given the general clinical experience of precipitous drop in ANC in various cases, weekly monitoring of the neutrophil count has not been shown to be a reliable predictor of agranulocytosis. However, the concomitant use of medications known to be associated with neutropenia or agranulocytosis may increase the risk of developing agranulocytosis, as has been reported for clozapine‐induced agranulocytosis 33. A retrospective analysis suggested that exposure to interferon for treatment of hepatitis C infection increases the risk of neutropenia and agranulocytosis during deferiprone therapy 34.
Patients who experience deferiprone‐induced agranulocytosis are at increased risk of its recurrence on rechallenge, and the second episode is generally more severe 15, 29, 35. Nonetheless, rechallenge might be considered for those patients at risk of death due to iron toxicity and for whom therapy with other chelators has been shown to be ineffective, inadequate, or too toxic.
Fifteen episodes of agranulocytosis with fatal outcome have been reported since 1999, the majority of which show evidence of deficiencies in patient management. The implementation in 2006 of an educational program for health care professionals and patients in Europe was followed by a decline in fatal cases of deferiprone‐related agranulocytosis, suggesting that education may be an important contributor to minimizing risk.
Weekly ANC monitoring in patients treated with deferiprone is a demanding expectation, and may deter some from initiating therapy. While weekly monitoring may seem prudent, this analysis and another study 10 do not support its value in intervening in the occurrence of agranulocytosis or the severity of its consequences. The data evaluated here suggest that most cases of neutropenia do not progress to agranulocytosis, and that the development of agranulocytosis is frequently precipitous and not the outcome of a slow decline in neutrophil count. A normal routine ANC count thus may provide false security, as it does not preclude agranulocytosis occurring shortly afterwards 4. These observations underline the desirability of identifying a better approach to the minimization of the risks associated with agranulocytosis.
In that regard, the panel recommends that patient education should focus on stopping deferiprone therapy at first symptoms of a fever, and that measurement of ANC be conducted within 24 hr. In addition, we support that until novel predictive tools are available, weekly ANC monitoring, despite its shortcomings in terms of detecting an emerging episode of agranulocytosis, be maintained during the first year, but that ANC monitoring thereafter occurs at the time of blood transfusions (typically, every 2–4 weeks).
Our evaluation confirms that patients who experience agranulocytosis must cease taking deferiprone. The need to discontinue therapy at manifestation of mild to moderate neutropenia is less clear. Continuation of therapy may be reasonable in patients at high risk of iron toxicity if there are no signs of infection and the ANC can be monitored as frequently as needed. In other patients, deferiprone therapy might be temporarily suspended, and recommenced if considered appropriate on resolution of the neutropenia.
Concentrated efforts should be made to understand more about the factors underlying deferiprone‐related agranulocytosis and its mechanisms, and to identify genetic markers of increased susceptibility to the event.
Supporting information
Supporting Information
References
- 1. Galanello R, Campus S. Deferiprone chelation therapy for thalassemia major. Acta Haematol 2009;122:155–164. [DOI] [PubMed] [Google Scholar]
- 2.ApoPharma Inc. Ferriprox® US Prescribing Information (PI). http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021825lbl.pdf
- 3.ApoPharma Inc. Ferriprox® (Deferiprone) Summary of Product Characteristics (SmPC). http://ec.europa.eu/health/documents/communityregister/html/h108.htm.
- 4. Masera N, Tavecchia L, Longoni DV, et al. Agranulocytosis due to deferiprone: A case report with cytomorphological and functional bone marrow examination. Blood Transfus 2011;4:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. El‐Beshlawy AM, El‐Alfy MS, Sari TT, et al. Continuation of deferiprone therapy in patients with mild neutropenia may not lead to a more severe drop in neutrophil count. Eur J Haematol 2014;92:337–340. [DOI] [PubMed] [Google Scholar]
- 6. Cohen AR, Galanello R, Piga A, et al. Safety profile of the oral iron chelator deferiprone: A multicenter study. Br J Haematol 2000;108:305–312. [DOI] [PubMed] [Google Scholar]
- 7. Bertola U, Collell M, Piga A, et al. Neutropenia in homozygous b‐thalassemic patients on desferrioxamine (DFO) treatment. In: Proceedings of the 8th International Conference on Oral Chelation in the Treatment of Thalassemia and Other Diseases, Corfu, Greece; 1997. p 90.
- 8. Pennell DJ, Berdoukas V, Karagiorga M, et al. Randomized controlled trial of deferiprone or deferoxamine in beta thalassemia major patients with asymptomatic myocardial siderosis. Blood 2006;107:3738–3744. [DOI] [PubMed] [Google Scholar]
- 9. Cohen AR, Galanello R, Piga A, et al. Safety and effectiveness of long‐term therapy with the oral iron chelator deferiprone. Blood 2003;102:1583–1587. [DOI] [PubMed] [Google Scholar]
- 10. Elalfy M, Wali YA, Qari M, et al. Deviating from safety guidelines during deferiprone therapy in clinical practice may not be associated with higher risk of agranulocytosis. Pediatr Blood Cancer 2014;61:879–884. [DOI] [PubMed] [Google Scholar]
- 11. Elalfy MS, Sari TT, Lee CL, et al. The safety, tolerability, and efficacy of a liquid formulation of deferiprone in young children with transfusional iron overload. J Pediatr Hematol Oncol 2010;32:601–605. [DOI] [PubMed] [Google Scholar]
- 12. Julia A, Olona M, Bueno J, et al. Drug‐induced agranulocytosis: Prognostic factors in a series of 168 episodes. Br J Haematol 1991;79:366–371. [DOI] [PubMed] [Google Scholar]
- 13. Henter JI, Karlen J. Fatal agranulocytosis after deferiprone therapy in a child with Diamond‐Blackfan anemia. Blood 2007;109:5157–9. [DOI] [PubMed] [Google Scholar]
- 14. Hoffbrand AV, Bartlett AN, Veys PA, et al. Agranulocytosis and thrombocytopenia in patient with Blackfan‐Diamond Anaemia during oral chelator trial [letter]. Lancet 1989;2:457–458. [DOI] [PubMed] [Google Scholar]
- 15. Ceci A, Baiardi P, Felisi M, et al. The safety and effectiveness of deferiprone in a large‐scale, 3‐year study in Italian patients. Br J Haematol 2002;118:330–336. [DOI] [PubMed] [Google Scholar]
- 16. Hoffbrand AV. Recent advances in the understanding of iron metabolism and iron‐related diseases. Acta Haematol 2009;122:75–7. [DOI] [PubMed] [Google Scholar]
- 17. Al‐Refaie FN, Veys PA, Wilkes S, et al. Agranulocytosis in a patient with thalassaemia major during treatment with the oral iron chelator, 1,2‐dimethyl‐3‐hydroxypyrid‐4‐one. Acta Haematol 1993;89:86–90. [DOI] [PubMed] [Google Scholar]
- 18. Al‐Refaie FN, Hershko C, Hoffbrand AV, et al. Results of long‐term deferiprone (L1) therapy: A report by the International Study Group on Oral Iron Chelators. Br J Haematol 1995;91:224–9. [DOI] [PubMed] [Google Scholar]
- 19. Hsieh MM, Everhart JE, Byrd‐Holt DD, et al. Prevalence of neutropenia in the U.S. population: Age, sex, smoking status, and ethnic differences. Ann Intern Med 2007;146:486–492. [DOI] [PubMed] [Google Scholar]
- 20. Farmaki K, Anagnostopoulos G, Platis O, et al. Combined chelation therapy in patients with thalassemia major: A fast and effective method of reducing ferritin levels and cardiological complications. Hematolo J 2002;3:79. [Google Scholar]
- 21. Alymara V, Bourantas DK, Chaidos A, et al. Combined iron chelation therapy with desferrioxamine in thalassemic patients. Hematol J 2002;3:81. [Google Scholar]
- 22. Kattamis A, Kassou C, Ladis V, et al. Safety and efficacy of combining deferiprone and deferoxamine in iron chelation therapy in patients with thalassemia. Blood 2002;100:Pa120a. [Google Scholar]
- 23. Bansal RK. Combined chelation with desferrioxamine and deferiprone in thalassemia major. In: Proceedings of 12th International Conference on Oral Chelation in the Treatment of Thalassemia and Other Diseases, Santorini, Hellas,Vol. 120; 2002. pp 4–7.
- 24. Wonke N, Wright C, Hoffbrand AV. Combined therapy with deferiprone and desferrioxamine. Br J Haematol 1998;103:361–364. [DOI] [PubMed] [Google Scholar]
- 25. Balveer K, Pyar K, Wonke B. Combined oral and parenteral iron chelation in beta thalssaemia major. Med J Malaysia 2000;55:493–497. [PubMed] [Google Scholar]
- 26. Grady RW, Berdoukas VA, Rachmilewitz EA, Giarding PJ. Optimizing chelation therapy: Combining deferiprone and desferrioxamine. Blood 2000;96:604. [Google Scholar]
- 27. Denic S, Showqi C, Klein M, et al. Prevalence, phenotype and inheritance of benign neutropenia in Arabs. BMC Blood Disord 2009;9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thobakgale CF, Ndung'u T. Neutrophil counts in persons of African origin. Curr Opin Hematol 2014;21:50–57. [DOI] [PubMed] [Google Scholar]
- 29. Hoffbrand AV. Oral iron chelation. Semin Hematol 1996;33:1–8. [PubMed] [Google Scholar]
- 30. Atkin K, Kendall F, Gould D, et al. Neutropenia and agranulocytosis in patients receiving clozapine in the UK and Ireland. Br J Psychiatry 1996;169:483–488. [DOI] [PubMed] [Google Scholar]
- 31. Peralta FG, Sánchez MB, Roíz MP, et al. Incidence of neutropenia during treatment of bone‐related infections with piperacillin‐tazobactam. Clin Infect Dis 2003;37:1568–1572. 1; [DOI] [PubMed] [Google Scholar]
- 32. Johnston A, Uetrecht J. Current understanding of the mechanisms of idiosyncratic drug‐induced agranulocytosis. Expert Opin Drug Metab Toxicol 2015;11:243–257. [DOI] [PubMed] [Google Scholar]
- 33. Lahdelma L, Appelberg B. Clozapine‐induced agranulocytosis in Finland, 1982‐2007: Long‐term monitoring of patients is still warranted. J Clin Psychiatry 2012;73:837–842. [DOI] [PubMed] [Google Scholar]
- 34. Ricchi P, Ammirabile M, Costantini S, et al. The impact of previous or concomitant IFN therapy on deferiprone‐induced agranulocytosis and neutropenia: A retrospective study. Expert Opin Drug Saf 2010;9:875–881. [DOI] [PubMed] [Google Scholar]
- 35. Al‐Refaie FN, Wonke B, Hoffbrand AV. Deferiprone associated myelotoxicity. Eur J Haematol 1994;53:298–301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


