Abstract
Women have a lifetime risk of major depression double that of men but only during their reproductive years. This sex difference has been attributed partially to activational effects of female sex steroids and also to the burdens of pregnancy, childbirth, and parenting. Men, in contrast, have a reproductive period difficult to delineate, and research on the mental health of men has rarely considered the effects of fatherhood. However, the couple goes through a number of potentially stressing events during the reproductive period, and both mothers and fathers are at risk of developing peripartum depression. This Review discusses the literature on maternal and paternal depression and the endocrine changes that may predispose a person to depression at this stage of life, with specific focus on the hypothalamus–pituitary axis, oxytocin, and testosterone levels in men. Important findings on sex differences in the neural correlates of maternal and paternal behavior have emerged, highlighting the relevance of the emotional brain in mothers and the sociocognitive brain in fathers and pointing toward the presence of a common parents' brain. Additionally, sex differences in neurogenesis and brain plasticity are described in relation to peripartum depression. © 2016 The Authors. Journal of Neuroscience Research Published by Wiley Periodicals, Inc.
Keywords: peripartum depression, sex, testosterone, cortisol, oxytocin, neuroimaging
Epidemiologic studies show a lifetime risk of major depression among women double that of men, and this sex difference is stable across diverse sociocultural backgrounds (Kessler et al., 1993; Bromet et al., 2011). This sex disparity is more profound during women's reproductive years (Soares and Zitek, 2008) and has been partially attributed to activational effects of female sex steroids. However, in recent years, differential programming during intrauterine life has also been suggested to account for this disparity (Goldstein et al., 2014).
Theories regarding the activational effects of female sex steroids are typically separated into hormonal excess or hormone deprivation. Periods of sex hormone excess that are associated with mood disturbances in women include the luteal phase of the menstrual cycle (i.e., premenstrual syndrome and premenstrual dysphoric disorder) and hormonal contraceptive use (Poromaa and Segebladh, 2012; Sundström Poromaa and Gingnell, 2014). Pregnancy may also be regarded as a period of sex hormone excess but is more complex than the other reproductive stages from psychiatric and endocrine perspectives. First, not only do estrogen and progesterone increase during pregnancy, but other hormones such as cortisol, corticotrophin‐releasing hormone (CRH), and testosterone increase, and the immune response is altered (Dorr et al., 1989; Harris et al., 1994; Skalkidou et al., 2012). Second, although pregnancy is a period with increased risk for relapse of depression (Cohen et al., 2006), it seems to protect against the most severe forms of depression, such as suicide and hospitalization (Munk‐Olsen et al., 2009; Esscher et al., 2015). Periods of hormonal deficiency include the postpartum period and perimenopause, both of which confer an increased risk for depression in women (Vesga‐Lopez et al., 2008; Soares, 2014). Perimenopause is characterized predominantly by estrogen deficiency, whereas, again, the postpartum period is more complex, characterized by deficiencies in sex hormones as well as hypothalamus–pituitary–adrenal (HPA) axis hormones (Skalkidou et al., 2012).
By comparison, the male reproductive period in relation to depression is far less often studied, mainly because other outcomes, such as aggression, drug abuse, and antisocial behavior, have gained greater scientific interest (Duke et al., 2014). Furthermore, the reproductive events are more difficult to delineate in men. Whereas the female reproductive period is marked by two dates, the first menstrual period (menarche) and the last menstrual period (menopause), the descriptors of male puberty and andropause are far less well characterized. Nevertheless, the prevalence of depression also increases in men at the time of puberty (Kessler et al., 1993), but, despite the 20–30‐fold increase in testosterone levels at that period, no conclusive relationship between male adolescent depression and testosterone levels has been established (Duke et al., 2014). After puberty, testosterone levels remain fairly stable, but, from the age of 40 years onward, a progressive decline is noted (Feldman et al., 2002). Androgen deficiency in men seems to be associated with an increased risk of depression, and, in a recent meta‐analysis, Amanatkar et al. (2014) concluded that testosterone treatment in hypogonadal men has beneficial effects on depressed mood.
However, hormonal influences are only partially responsible for depression onset; genetic vulnerability, life experiences, personality, psychosocial factors, and stress will also shape the individual risk for depression. Nevertheless, for the purpose of this Review, hormonal differences serve as a canvas for the events that take place during pregnancy and after childbirth. The topic for this Review is probably one of the few areas in neuroscience in which the male perspective is understudied. Most of the data on sex differences in mental health and parenting have been collected during the past 15 years.
MATERNAL AND PATERNAL DEPRESSION DURING PREGNANCY AND THE POSTPARTUM PERIOD
During the reproductive period, women and their partners go through a number of potentially stressing reproductive events, and childbirth and parenting are perhaps the most prominent ones, but the family planning process is not always smooth; the couple may suffer from miscarriages, perinatal loss, infertility, or demanding infertility treatments. In general, men and women show different patterns of grief following miscarriages, perinatal losses, and unresolved infertility (Kersting and Wagner, 2012; Martins et al., 2016). Also, the sex difference in prevalence of depression persists throughout various reproductive events such as infertility (Volgsten et al., 2008) and childbirth, with a female:male ratio of approximately 2:1 (Paulson and Bazemore, 2010).
Both maternal and paternal peripartum depression rates vary among studies, depending on the socioeconomic status of the population under investigation (Paulson and Bazemore, 2010). For instance, rates of 10–20% are reported for maternal depression in high‐income countries, whereas rates higher than 20% have been reported for low‐ and middle‐income countries (Biaggi et al., 2016). In addition to the between‐countries variability, the time and method of assessment play a role, and higher rates have been reported in studies that used self‐report questionnaires (Gavin et al., 2005; Vigod et al., 2013; Norhayati et al., 2015). With these precautions in mind, a meta‐analysis of 43 studies and 28,000 subjects estimated a rate of 23.8% for maternal depression in the period between the first pregnancy trimester and the end of the first year postpartum, whereas the corresponding estimate for paternal depression was 10.4%. The highest prevalence for maternal depression was typically during the first 3 postpartum months (Gavin et al., 2005), whereas paternal depression seemed to peak somewhat later, between 3 and 6 months postpartum (Paulson and Bazemore, 2010).
The postpartum period confers an increased risk of depression in comparison with other periods in women's lives (Vesga‐Lopez et al., 2008), but corresponding data in fathers have not been reported. Severe psychiatric morbidity in relation to childbirth, such as postpartum psychosis, postpartum psychiatric admissions, and postpartum suicide, have been described exclusively for mothers (Lindahl et al., 2005; Munk‐Olsen et al., 2009; Esscher et al., 2015; Langan Martin et al., 2016). It is quite possible that these are unique maternal experiences, but it remains to be established whether the postpartum period is a vulnerability period for severe psychiatric morbidity in fathers also.
A strong correlation between maternal and paternal depression is evident (Paulson and Bazemore, 2010), in which paternal depression onset (Paulson and Bazemore, 2010) typically follows the onset of maternal depression (Escriba‐Aguir and Artazcoz, 2011; Figueiredo and Conde, 2011; Wee et al., 2011). Maternal depression is therefore considered a strong risk factor for the development of depressive symptoms in fathers. Indeed, with a female partner suffering from depression, the prevalence of paternal peripartum depression may be as high as 24–50% (Goodman, 2004). Paternal depression is also a risk factor for maternal peripartum depression (Escriba‐Aguir and Artazcoz, 2011; Paulson et al., 2016).
Common risk factors for maternal and paternal depression include a history of mental disorders, neurotic personality traits, poor marital relationship, and poor social support (O'Hara and McCabe, 2013; Edward et al., 2015). However, some sex differences in risk factor profiles have been identified. When couples were examined together, negative life experiences were associated only with maternal depression (Escriba‐Aguir and Artazcoz, 2011), whereas an overprotective family of origin increased the risk only of paternal peripartum depression (Matthey et al., 2000). Furthermore, different stressors may apply to mothers and fathers during the peripartum period, diversely affecting their risk for depression. A cognitive–behavioral model for peripartum depression suggests that, for women with psychological vulnerability including a history of depression, childbirth could be a major stressful life event that stimulates the emergence of depressive symptoms (O'Hara et al., 1982, 1991). Several perinatal stressors have been implicated as risk factors for maternal depression, including pregnancy complications such as pre‐eclampsia and prenatal hospitalization, poor marital relationship and partner support, poor social support in the pre‐ and postnatal period, and stress related to infant health and childcare (Beck, 2001; Robertson et al., 2004; Blom et al., 2010). Nevertheless, delivery plays a central role in the stress experienced by women; fear of delivery or a negative delivery experience or deviation from the preferred mode of delivery increases the risk of postpartum depression (Tuohy and McVey, 2008; Raisanen et al., 2013; Haines et al., 2015; Houston et al., 2015). On the other hand, paternal stressors concern primarily fathers' ability to withstand their new role as a father (Morse et al., 2000). Finally, genetic influences on maternal postpartum depression have been described (Skalkidou et al., 2012) in interaction with environmental factors (Comasco et al., 2011; Mehta et al., 2012; Mitchell et al., 2012). Although fathers carrying a risk oxytocinergic genotype associated with lower oxytocin levels and impaired attachment were more frequently present in families with maternal postpartum depression (Apter‐Levy et al., 2013), genetic susceptibility to paternal peripartum depression has not been investigated.
CONSEQUENCES FOR THE OFFSPRING
When considering peripartum depression, it is of utmost importance to consider its association with subsequent offspring outcomes. Both antenatal and postnatal maternal depression have been associated with emotional disturbances in the child; increased risk for clinical depression in late adolescence (Pawlby et al., 2009); externalizing difficulties, including attention deficit hyperactivity disorder; and insecure, especially disorganized, mother–infant attachment. Furthermore, association with cognitive development, such as lower IQ score in early childhood, has been documented (for review see Stein et al., 2014). Although maternal peripartum depression is more strongly associated with unfavorable child outcomes, the paternal contribution is also considerable (Wilson and Durbin, 2010; Stein et al., 2014; Gutierrez‐Galve et al., 2015). Depressed fathers exhibit fewer positive and more negative behaviors, and their children are at increased risk of developing emotional and behavioral disorders (Wilson and Durbin, 2010; Stein et al., 2014). Furthermore, the prevalence of impaired bonding with the infant was highest among couples among whom both spouses had depression (Kerstis et al., 2016). It is of interest to note that, although the association in mothers is explained by factors related to direct mother–infant interaction, the major part of the respective association in fathers is explained by the mediating role of family environment, i.e., maternal depression and conflicts within the couple (Gutierrez‐Galve et al., 2015). Thus, father–infant interaction may be crucial in programming children's response to stress and thus contribute to their biopsychological development.
Parenting can influence mental and behavioral programming of the offspring as well as transgenerational transmission of parenting style and thus is of considerable strategic importance. To date, only one longitudinal investigation of socioemotional outcomes in the offspring has simultaneously considered parental mental health and parental neural correlates. Lower substantia nigra/midbrain reactivity to own‐infant cries mediated the association between anxious intrusive thoughts and harm‐avoidant behavior in mothers and socioemotional competence in the infant; on the other hand, higher thalamus/hypothalamus activations in response to own‐infant cries were associated with positive parenting in fathers and infant outcome (Kim et al., 2015). Although the relationship between maternal postpartum depression and neural signatures of reactivity to infant‐related cues has been studied only recently (for review see Moses‐Kolko et al., 2014), more studies are required to identify early neural parental markers mediating disrupted parental caregiving and subsequent deviant psychobiological behavior in the offspring (Fig. 1). From the offspring perspective, the identification of early objective biomarkers will allow prompt intervention, limiting the negative impact of disrupted parenting on the child's development.
Figure 1.
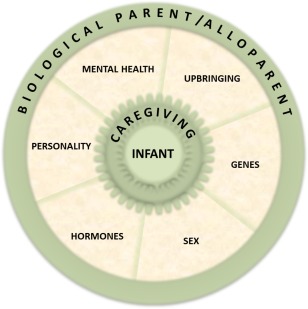
Complex interplay among factors that influence offspring mental health.
ENDOCRINE CHANGES IN PERIPARTUM DEPRESSION
The complex endocrine changes in women during pregnancy and the puerperium are well described and function to sustain the pregnancy, triggering delivery and preparing the mother for caring for the newborn. Most of the endocrine changes during pregnancy follow a common pattern, with a continuous increase throughout the 40 weeks of gestation followed by a drastic drop at parturition. Estrogens, progesterone, testosterone, prolactin, CRH, and cortisol all essentially adhere to this temporal plasma profile (Dorr et al., 1989; Stalla et al., 1989; Harris et al., 1994; Jung et al., 2011), with the most pronounced changes occurring in progesterone and CRH (approximately 100‐fold increase), followed by estrogen (approximately 50‐fold increase), cortisol (two‐ to threefold increase), and testosterone (50% increase) levels (Kuijper et al., 2013).
These complex endocrine changes that occur in mothers across pregnancy and the postpartum period have been well established in nonhuman mammals and humans to be at least partially responsible for a potent increase in females' positive appraisal of the offspring and promote maternal attachment and behavior (Bridges et al., 1978, 1985; Siegel and Rosenblatt, 1978; Fleming et al., 1989, 1997a,1997b; Pedersen, 1997; Feldman et al., 2007; MacKinnon et al., 2014; Lonstein et al., 2015). In contrast, the hormonal correlates of paternal care are not so well studied, possibly because of the less abrupt hormonal changes observed in fathers. Reports in the existing literature for nonhuman primates, focusing mostly on prolactin, oxytocin, and vasopressin increase as well as decrease in testosterone, are inconsistent (Nunes et al., 2001; Parker and Lee, 2001; Bales et al., 2004; Wynne‐Edwards and Timonin, 2007; Saltzman and Maestripieri, 2011).
Several of the hormonal alterations in pregnant and postpartum women have been, perhaps rightfully, held to be responsible for impairing women's mental health during the peripartum period. The foremost cited endocrine mediators in the development of maternal peripartum depression include the pregnancy hypercortisolism, the postpartum HPA axis hyporesponsiveness (Hellgren et al., 2013; Hannerfors et al., 2015; Iliadis et al., 2015, 2016), and the postpartum hypoestrogenism (Bloch et al., 2000).
In women, cortisol concentrations continuously increase throughout pregnancy only to drop abruptly after delivery of the placenta (Jung et al., 2011). Nevertheless, unlike the negative feedback that cortisol exerts on hypothalamic CRH, hypercortisolemia actually stimulates additional CRH production by the placenta, leading to an increase of its plasma levels and thus elevated maternal cortisol levels (Cheng et al., 2000). The abrupt expulsion of the placenta consequently results in a transient HPA axis suppression in mothers, with a duration of up to 4–6 weeks postpartum (Magiakou et al., 1996). However, although more than 40 articles on the HPA axis markers have been published on maternal peripartum depression, the findings can at best be described as inconsistent (Iliadis et al., 2016). In addition to having used different instruments for establishing depression (or depressed mood), most of the studies were, unfortunately, characterized by small numbers of women who fulfilled research criteria for peripartum depression. However, a rather consistent positive association of midpregnancy placental CRH levels with depressive symptoms during the first postpartum weeks has been documented (Yim et al., 2009; Hahn‐Holbrook et al., 2013; Glynn and Sandman, 2014; Iliadis et al., 2016). Higher pregnancy placental CRH levels indicate a more abrupt decrease after placental removal and might consequently lead to a more prolonged suppression of the HPA axis, supporting a crucial role of the transient HPA axis dysregulation after delivery in postpartum depression pathogenesis (Magiakou et al., 1996; Chrousos et al., 1998).
Oxytocin, released from the posterior pituitary, is of paramount importance during delivery, at which time it stimulates uterine contractions, but it is probably best known in relation to breastfeeding. Breastfeeding, also regulated by prolactin, is an important aspect of maternal behavior in mammalian species. Maternal prolactin levels rise throughout pregnancy and the postpartum period, but high levels of progesterone in pregnancy inhibit lactation. After the placental expulsion, prolactin stimulates lactogenesis, and oxytocin is involved mostly in milk expulsion. Studies on mothers often report a strong association of maternal perinatal depressive symptoms and breastfeeding problems or discontinuation (Dias and Figueiredo, 2015). This is, however, definitely influenced by individual and societal expectations on breastfeeding (Pope and Mazmanian, 2016). Both the direction of the aforementioned association and the underlying mechanisms are still under study. Some hypotheses involve the role of oxytocin as a buffer against stress reactivity (Cox et al., 2015) or the low levels of estrogen during breastfeeding (Perheentupa et al., 2004). Studies on fathers and breastfeeding focus mostly on attitudes toward breastfeeding and on how paternal education and support enhance breastfeeding duration (Hunter and Cattelona, 2014; Sherriff et al., 2014). Studies on how fathers are affected themselves and how their attachment to the infant is affected are sparse. One study actually showed that the degree of paternal involvement with the infant was inversely associated with breastfeeding (Ito et al., 2013). One could speculate that absence of breastfeeding would result in a more active paternal participation in neonatal feeding and a stronger father–infant bond. Moreover, oxytocin has been implicated in social bond formation and trust building among individuals of both genders (De Dreu, 2012; Lieberwirth and Wang, 2014). Studies on sex differences in oxytocin are scarce, but thus far no differences in oxytocin levels between mothers and fathers have been demonstrated (Feldman et al., 2010; Gordon et al., 2010a, 2010b; Atzil et al., 2012; Abraham et al., 2014). However, oxytocin was shown to be positively correlated with amygdalar and paralimbic reactivity to own‐infant‐related videos in mothers and negatively with emotional regulation substrates in fathers (Atzil et al., 2012). Furthermore, intranasal oxytocin has been shown to modulate reward and attachment of neural substrate reactivity to infant stimuli in fathers (Wittfoth‐Schardt et al., 2012), suggesting it as a potential treatment target for impaired parenting.
No articles on the neuroendocrine underpinnings of paternal depression have yet been published, but HPA axis dysfunction and hypogonadism could apply also to men because they undergo hormonal fluctuations somewhat similar to those of women, albeit on a smaller scale. In expectant fathers, free cortisol increases weeks before delivery (Berg and Wynne‐Edwards, 2001) but decreases postpartum and becomes lower than in nonfathers (Berg and Wynne‐Edwards, 2001). Expectant fathers also exhibit greater situational cortisol reactivity, possibly to be able to care for the infant without compromising the ability to respond to an external stressor (Storey et al., 2000; Wynne‐Edwards, 2001). After delivery, interaction with toddlers decreased paternal cortisol levels in a time‐dependent manner (Gettler et al., 2011a; Storey et al., 2011). Moreover, longitudinal studies on fathers have suggested a decrease in testosterone levels after birth (Berg and Wynne‐Edwards, 2001; Gettler et al., 2011b; Edelstein et al., 2015). Fathers have approximately 30% lower testosterone levels than age‐matched nonfathers (Storey et al., 2000; Berg and Wynne‐Edwards, 2001; Kuzawa et al., 2009; Muller et al., 2009; Gettler et al., 2011b), and those who spend more hours with their babies have even lower levels (Muller et al., 2009). It also seems that infant cues influence the production of free testosterone, although the direction depends on the paternal nurturing response (van Anders et al., 2012). A recent study showed that diurnal testosterone variability may differentially affect parenting quality in fathers and mothers; particularly, greater variability was associated with more optimal parenting in fathers, whereas the opposite was observed in mothers (Endendijk et al., 2016). Testosterone levels in fathers also positively correlated with caudate reactivity to infant visual stimuli (Kuo et al., 2012), whereas no relationship was found with neural response to infant‐related auditory stimuli (Mascaro et al., 2014). Overall, most human evidence supports the “challenge hypothesis,” which states that testosterone levels increase in a reproductive context (i.e., mating) and decrease in long‐term bonds and paternal care settings (Archer, 2006). Needless to say, given the long‐term consequences of maternal and paternal peripartum depression, additional well‐powered studies should be performed to elucidate the endocrine factors that are the major players for symptom development.
TRANSITION TO PARENTHOOD: SEX DIFFERENCES IN PARENTS' BRAINS DURING THE PERIPARTUM PERIOD
For humans, the transition to parenthood usually involves both partners and is associated with important changes in individual functioning (Nunes‐Costa et al., 2014). In addition, new parenting constellations, such as caregiving by single parents and homosexual parents, are emerging. Lesion studies of rodents indicate that the medial preoptic area of the hypothalamus, the ventral part of the bed nucleus of the stria terminalis, and the lateral septum, all of which are rich in steroid hormone and oxytocin receptors, form the main parenting brain system (Leckman et al., 2004; Leuner et al., 2010); however, this does not seem to apply to humans (Feldman, 2015). In support of the dopaminergic component of parenting, lesion studies of rodents also indicate an essential role of the reward brain in maternal behavior, including the ventral tegmental area, the nucleus accumbens, and the cingulate cortex (Swain et al., 2007). A relatively extensive literature has developed on a set of hypothalamic‐midbrain‐limbic‐paralimbic‐cortical neural pathways that interact in supporting human parental behaviors and feelings in response to infant cues (Swain et al., 2012; Nunes‐Costa et al., 2014).
Novel and evolving inputs that occur during parenting entail fine tuning of the parents' brains to anticipate the infant's needs, recognize and gate signals, integrate cognitive–affective processing, and develop a sense of the infant. Imaging of the neural substrates of parenting has been investigated primarily in mothers, whereas only a handful of studies have focused on fathers (Swain et al., 2014b). From the Lorberbaum and colleagues (1999) study of four women to a recent study by Swain and colleagues (Kim et al., 2015), brain reactivity of primary caregiver women has been measured with functional magnetic resonance imaging (MRI) while those women have been exposed to own‐infant valenced auditory or visual stimuli (Swain et al., 2014b). Despite the fact that methodological discrepancies and weaknesses warn the reader not to draw precipitate conclusions, interesting preliminary insights into sex differences in the parents' brain during the peripartum period can be gained.
Parenting's complex nature is, presumably, reflected in the various neurocircuitries assigned to its facets. As postulated by Swain et al. (2014a), arousal, salience, motivation, and reward dimensions as well as executive and empathy components mediate parent–infant bonding. Most of the studies have investigated mothers (Moses‐Kolko et al., 2014), and a few have investigated fathers (Kuo et al., 2012; Mascaro et al., 2014). At present, three studies that investigated sex differences in brain responsiveness to infant stimuli have pinpointed the relevance of the emotional brain in mothers and of the sociocognitive brain in fathers as well as the presence of a common parents' brain (Atzil et al., 2012; Abraham et al., 2014). Greater right amygdala activation distinguished mothers from fathers, highlighting a key role of the emotional and motivational limbic system, and fathers, compared with mothers, displayed positive activation of the left medial prefrontal cortex. Both fathers and mothers displayed synchronous activation of the embodied simulation, mentalizing, and empathy cortical networks that make up the anterior cingulate cortex, inferior frontal gyrus, cuneus, medial prefrontal cortex, temporal cortex, inferior parietal lobule, and insula (Atzil et al., 2012). These areas participate in the social and cognitive processing through which social understanding of others is derived. Perceiving and understanding infant stimuli and affective state as well as responding to them, which are essential to secure parent–infant attachment, involve the social brain network and mirror neurons (Moses‐Kolko et al., 2014). Another study showed a decreased activation of the right anterior cingulate and medial prefrontal cortices, also involved in the mentalizing network, in response to infant stimuli in women compared with men (Seifritz et al., 2003). Partial overlap with the main hubs of the default mode network, a task‐deactivated and at‐rest‐activated resting state network linked to empathy and the theory of mind (Deco et al., 2011; Li et al., 2014), further supports the role of these brain areas in caregiving. More recently, a common parenting brain response to infant stimuli was identified when comparing primary caregiving mothers, heterosexual secondary caregiving fathers, and homosexual primary caregiving fathers (Abraham et al., 2014). Overall, activation of subcortical and paralimbic structures involved in emotion processing and frontopolar‐medial prefrontal cortex and temporoparietal circuits of the mentalizing network have been observed. It is of relevance to sex differences and caregiving typology that the association with arousal and vigilance indexed by amygdalar reactivity was greater in mothers, whereas the mentalizing circuit with the superior temporal sulcus was predominant in fathers (Abraham et al., 2014). Concordantly, previous studies of fathers do support the involvement of social‐ rather than emotional network‐related areas (Kuo et al., 2012; Mascaro et al., 2014). Primary caregiving homosexual fathers displayed both maternal‐ and paternal‐like signatures, with activation of and connectivity between the amygdala and superior temporal sulcus, the latter being correlated with time spent in child caregiving in all fathers (Abraham et al., 2014). With emotion‐driven amygdalar activation as part of the salience, fear and reward networks are expected in the presence of infant stimuli in both biological and alloparents; one study showed, independently of sex, higher amygdala reactivity to infant crying stimuli in parents compared with nonparents (Seifritz et al., 2003). The involvement of the corticolimbic system in peripartum depression can be hypothesized in view of ample evidence of dysregulated emotion processing in depression and anxiety disorders (Moses‐Kolko et al., 2014). Differential time course of parenting is certainly a key factor to be considered in future studies as well as causal relationships.
In its universality, parenting behavior indeed occurs in nonbiological parents, possibly as part of a broad evolutionary feature of human sociality. Seifritz and colleagues (2003) found that parents showed stronger activation of amygdala, cingulate cortex, insula, left ventral prefrontal cortex, and left temporoparietal circuits for crying compared with nonparents, who showed a stronger activation from laughing (Seifritz et al., 2003). In addition, women but not men displayed deactivation of the right anterior cingulate cortex and medial prefrontal cortex in response to crying and laughing vocalization independently of their parental status, supporting the existence of a parents' brain in humankind (Seifritz et al., 2003).
Although these changes occur in healthy postpartum mothers, it is conceivable that increased emotional reactivity in concert with life stressors, maladaptive hormonal responses, and individual vulnerability may put susceptible women at increased risk of depression. Maternal postpartum depression, after it has been established, is associated with impaired salience and fear network activity as well as reduced corticolimbic responsiveness to infant‐related cues (Moses‐Kolko et al., 2014). No imaging studies of paternal peripartum depression have been performed to date.
BRAIN PLASTICITY DURING THE PERIPARTUM PERIOD: LINKS WITH PARENTAL DEPRESSION?
From a longitudinal perspective, in addition to the aforementioned hormonal effects, changes in neural circuitry take place during the perinatal period, altering the maternal and paternal behavior to prepare the new parents for childcare. Adult neurogenesis has surfaced as an essential mechanism that contributes to maternal neuroplasticity. Areas attracting the most attention for research in this area are the hippocampus (specifically the dentate gyrus) and the olfactory bulb, the two regions in which neurogenesis primarily takes place. To a lesser extent, neurogenesis has also been documented in other brain regions of mammals, including the amygdala (Bernier et al., 2002; Fowler et al., 2002; Akbari et al., 2007; Okuda et al., 2009; Lieberwirth et al., 2013), striatum (Bedard et al., 2002, 2006; Dayer et al., 2005; Luzzati et al., 2006; Inta et al., 2008; Ernst et al., 2014), neocortex (Gould et al., 1999; Dayer et al., 2005), and hypothalamus (Huang et al., 1998; Fowler et al., 2002; Kokoeva et al., 2005; Akbari et al., 2007; Leuner and Sabihi, 2016), but only scarce data exist with regard to its importance during the perinatal period, and its relation to parental behavior remains controversial (Raymond et al., 2006; Akbari et al., 2007; Ruscio et al., 2008; Lieberwirth et al., 2013). Neurogenesis depends on hormonal changes, and estrogen has been shown to promote neurogenesis in female but not male rats (Tanapat et al., 2005), whereas glucocorticoids attenuate it in both sexes (Saaltink and Vreugdenhil, 2014). With regard to the perinatal period, most of the literature on neurogenesis has been derived from animal research, and profound species differences in neurogenesis across pregnancy and postpartum have been noted. Thus, findings should be interpreted with caution because humans differ substantially in terms of reproductive endocrinology and strategy, maternal care, and degree of parental investment.
Some of the most consistent findings across species include findings of reduced cell proliferation during the postpartum period (Darnaudery et al., 2007; Leuner et al., 2007; Pawluski and Galea, 2007; Hillerer et al., 2014). For instance, in rat dams, an increased cortisol excretion and suppressed hippocampal neurogenesis were observed even on the first day postpartum (Leuner et al., 2007). This effect has been suggested to be adaptive in response to the high energy demands required for lactation and consequently subsides around the time of weaning (Leuner and Sabihi, 2016). In addition to hormonal effects, experiential stimuli in the peripartum period might reduce neurogenesis; specifically stress has been shown to be a suppressor (Saaltink and Vreugdenhil, 2014), whereas enriching learning experiences such as those derived from parent–infant interaction reinforce neurogenesis (Leuner et al., 2006; Pawluski and Galea, 2007; Ruscio et al., 2008). In young females, interaction with offspring seems to protect against adverse effects of stress on processes involved in memory and learning (Leuner and Shors, 2006). Additionally, there is evidence that expression of maternal behaviors is associated with survival of newly generated neurons in the hippocampus, emphasizing the important role of neurogenesis for adequate care of the offspring (Shors et al., 2016). Paternal neurogenesis has also been investigated in two biparental species, the California mouse and the prairie vole. In both of these studies, no sex differences appeared; i.e., fatherhood was also shown to reduce neurogenesis in the dentate gyrus of the hippocampus (Glasper et al., 2011; Lieberwirth et al., 2013). Studies investigating the potential hormonal mediators for reduced neurogenesis in fathers and the extent to which infant interaction plays a role in these events are lacking.
There is evidence that hippocampal neurogenesis is amplified after antidepressant treatment (Santarelli et al., 2003; Boldrini et al., 2012), whereas a reversible hippocampal volume loss, as measured by MRI, has been consistently observed in depressed humans compared with healthy controls (Videbech and Ravnkilde, 2004; Kronmuller et al., 2008; Kempton et al., 2011). These observations raise the possibility that neurogenesis is etiopathogenetically linked to peripartum depression (Lee et al., 2013). Plasticity of the human parents' brains, which just recently has begun to be explored in mothers and fathers, involves structural and functional changes. Sex‐ and region‐dependent variation in gray matter volume has been found during the first postpartum months (Kim et al., 2010, 2014). Increases in gray matter volume in the prefrontal cortex, central gyrus, parietal lobe, and midbrain have been noted in mothers (Kim et al., 2010). Fathers showed increases in striatum, lateral prefrontal cortex, and superior temporal gyrus but showed decreases in orbitofrontal, posterior, and cingulate cortices; insula; and fusiform gyrus (Kim et al., 2014). To date, one study has assessed the relationship between depressive symptoms and brain morphology plasticity in fathers, indicating a negative relationship with gray matter volume increase in the striatum, amygdala, and subgenual cortex but a positive relationship with posterior cingulate cortex and fusiform gyrus gray matter volume decrease (Kim et al., 2014). Finally, longitudinal studies of the maternal brain from delivery to the second postpartum month showed increased reactivity in emotional circuits (Gingnell et al., 2015) and decreased prefrontal cortex reactivity during tasks probing cognitive control (Bannbers et al., 2013). Given the importance of neurogenesis for maternal and paternal behavior, the suppressing effects of stress, the reinforcing effects of interaction with the child, and the links with depression, additional studies on brain structural and functional changes in the peripartum period are absolutely essential to delineate the events that ultimately lead to maternal and paternal depression. Abundant evidence of gonadal hormone fluctuations in brain anatomy and function has been provided both for healthy women and for psychiatric patients (Comasco et al., 2014; Toffoletto et al., 2014; Comasco and Sundström‐Poromaa, 2015). Therefore, it is plausible to hypothesize a sex‐dependent modulatory effect of prenatal and postnatal hormone changes that, in concert with constitutional factors and environmental stimuli, act on the parent's brain.
CONCLUSIONS AND FUTURE DIRECTIONS
In summary, both women and men go through a number of potentially stressing events during their reproductive periods, but the sex difference in depression prevalence rates persists. The most severe forms of psychiatric morbidity in relation to childbirth have been described exclusively for mothers, but whether the postpartum period is a vulnerability period also in fathers has not even been addressed. Both mothers and fathers go through endocrine changes throughout pregnancy and childbirth, albeit on a smaller scale for the fathers. Nevertheless, the pregnancy‐induced endocrine changes may put susceptible women at risk for depression, and the same may be true for fathers, who at the same time experience alterations in cortisol and testosterone levels.
Important information on sex differences in brain responsiveness to infant stimuli has recently emerged. Studies have pinpointed not only the relevance of the emotional brain in mothers and of the sociocognitive brain in fathers but also the presence of a common parents' brain. It is expected that future studies on maternal and paternal behavior, both important models for core human behaviors, will contribute to an increased understanding of the sex differences in the brain. Finally, parenting likely induces changes in brain plasticity in both mothers and fathers. Given the importance of neurogenesis for maternal and paternal behavior, the suppressing effects of stress, the reinforcing effects of interaction with the child, and the links with depression, additional studies on brain structural and functional changes in the peripartum period are required to delineate the events that ultimately lead to maternal and paternal depression. Overall, this Review highlights many areas that remain to be studied before sex differences in mental health and psychoneuroendocrinology in relation to childbirth have been fully explored.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to report.
SIGNIFICANCE Both women and men go through a number of potentially stressing events during their reproductive period, but the sex difference in depression prevalence rates persists. The most severe forms of psychiatric morbidity in relation to childbirth have thus far been described exclusively for mothers. Hormonal changes may put both mothers and fathers at increased risk of peripartum depression. A common parenting brain has begun to be described, and sex differences are being noted. Parenting induces changes in brain plasticity in both mothers and fathers.
REFERENCES
- Abraham E, Hendler T, Shapira‐Lichter I, Kanat‐Maymon Y, Zagoory‐Sharon O, Feldman R. 2014. Father's brain is sensitive to childcare experiences. Proc Natl Acad Sci U S A 111:9792–9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari EM, Chatterjee D, Levy F, Fleming AS. 2007. Experience‐dependent cell survival in the maternal rat brain. Behav Neurosci 121:1001–1011. [DOI] [PubMed] [Google Scholar]
- Amanatkar HR, Chibnall JT, Seo BW, Manepalli JN, Grossberg GT. 2014. Impact of exogenous testosterone on mood: a systematic review and meta‐analysis of randomized placebo‐controlled trials. Ann Clin Psychiatry 26:19–32. [PubMed] [Google Scholar]
- Apter‐Levy Y, Feldman M, Vakart A, Ebstein RP, Feldman R. 2013. Impact of maternal depression across the first 6 years of life on the child's mental health, social engagement, and empathy: the moderating role of oxytocin. Am J Psychiatry 170:1161–1168. [DOI] [PubMed] [Google Scholar]
- Archer J. 2006. Testosterone and human aggression: an evaluation of the challenge hypothesis. Neurosci Biobehav Rev 30:319–345. [DOI] [PubMed] [Google Scholar]
- Atzil S, Hendler T, Zagoory‐Sharon O, Winetraub Y, Feldman R. 2012. Synchrony and specificity in the maternal and the paternal brain: relations to oxytocin and vasopressin. J Am Acad Child Adolesc Psychiatry 51:798–811. [DOI] [PubMed] [Google Scholar]
- Bales KL, Kim AJ, Lewis‐Reese AD, Sue Carter C. 2004. Both oxytocin and vasopressin may influence alloparental behavior in male prairie voles. Horm Behav 45:354–361. [DOI] [PubMed] [Google Scholar]
- Bannbers E, Gingnell M, Engman J, Morell A, Sylven S, Skalkidou A, Kask K, Backstrom T, Wikstrom J, Poromaa IS. 2013. Prefrontal activity during response inhibition decreases over time in the postpartum period. Behav Brain Res 241:132–138. [DOI] [PubMed] [Google Scholar]
- Beck CT. 2001. Predictors of postpartum depression: an update. Nurs Res 50:275–285. [DOI] [PubMed] [Google Scholar]
- Bedard A, Cossette M, Levesque M, Parent A. 2002. Proliferating cells can differentiate into neurons in the striatum of normal adult monkey. Neurosci Lett 328:213–216. [DOI] [PubMed] [Google Scholar]
- Bedard A, Gravel C, Parent A. 2006. Chemical characterization of newly generated neurons in the striatum of adult primates. Exp Brain Res 170:501–512. [DOI] [PubMed] [Google Scholar]
- Berg SJ, Wynne‐Edwards KE. 2001. Changes in testosterone, cortisol, and estradiol levels in men becoming fathers. Mayo Clin Proc 76:582–592. [DOI] [PubMed] [Google Scholar]
- Bernier PJ, Bedard A, Vinet J, Levesque M, Parent A. 2002. Newly generated neurons in the amygdala and adjoining cortex of adult primates. Proc Natl Acad Sci U S A 99:11464–11469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biaggi A, Conroy S, Pawlby S, Pariante CM. 2016. Identifying the women at risk of antenatal anxiety and depression: a systematic review. J Affect Disord 191:62–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR. 2000. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry 157:924–930. [DOI] [PubMed] [Google Scholar]
- Blom EA, Jansen PW, Verhulst FC, Hofman A, Raat H, Jaddoe VW, Coolman M, Steegers EA, Tiemeier H. 2010. Perinatal complications increase the risk of postpartum depression. The Generation R Study. BJOG 117:1390–1398. [DOI] [PubMed] [Google Scholar]
- Boldrini M, Hen R, Underwood MD, Rosoklija GB, Dwork AJ, Mann JJ, Arango V. 2012. Hippocampal angiogenesis and progenitor cell proliferation are increased with antidepressant use in major depression. Biol Psychiatry 72:562–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges RS, Rosenblatt JS, Feder HH. 1978. Serum progesterone concentrations and maternal behavior in rats after pregnancy termination: behavioral stimulation after progesterone withdrawal and inhibition by progesterone maintenance. Endocrinology 102:258–267. [DOI] [PubMed] [Google Scholar]
- Bridges RS, DiBiase R, Loundes DD, Doherty PC. 1985. Prolactin stimulation of maternal behavior in female rats. Science 227:782–784. [DOI] [PubMed] [Google Scholar]
- Bromet E, Andrade LH, Hwang I, Sampson NA, Alonso J, de Girolamo G, de Graaf R, Demyttenaere K, Hu C, Iwata N, Karam AN, Kaur J, Kostyuchenko S, Lepine JP, Levinson D, Matschinger H, Mora ME, Browne MO, Posada‐Villa J, Viana MC, Williams DR, Kessler RC. 2011. Cross‐national epidemiology of DSM‐IV major depressive episode. BMC Med 9:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng YH, Nicholson RC, King B, Chan EC, Fitter JT, Smith R. 2000. Glucocorticoid stimulation of corticotropin‐releasing hormone gene expression requires a cyclic adenosine 3′,5′‐monophosphate regulatory element in human primary placental cytotrophoblast cells. J Clin Endocrinol Metab 85:1937–1945. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Torpy DJ, Gold PW. 1998. Interactions between the hypothalamic–pituitary–adrenal axis and the female reproductive system: clinical implications. Ann Intern Med 129:229–240. [DOI] [PubMed] [Google Scholar]
- Cohen LS, Altshuler LL, Harlow BL, Nonacs R, Newport DJ, Viguera AC, Suri R, Burt VK, Hendrick V, Reminick AM, Loughead A, Vitonis AF, Stowe ZN. 2006. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA 295:499–507. [DOI] [PubMed] [Google Scholar]
- Comasco E, Sundström‐Poromaa I. 2015. Neuroimaging the menstrual cycle and premenstrual dysphoric disorder. Curr Psychiatry Rep 17:77. [DOI] [PubMed] [Google Scholar]
- Comasco E, Sylven SM, Papadopoulos FC, Oreland L, Sundström‐Poromaa I, Skalkidou A. 2011. Postpartum depressive symptoms and the BDNF Val66Met functional polymorphism: effect of season of delivery. Arch Womens Ment Health 14:453–463. [DOI] [PubMed] [Google Scholar]
- Comasco E, Frokjaer VG, Sundström‐Poromaa I. 2014. Functional and molecular neuroimaging of menopause and hormone replacement therapy. Front Neurosci 8:388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox EQ, Stuebe A, Pearson B, Grewen K, Rubinow D, Meltzer‐Brody S. 2015. Oxytocin and HPA stress axis reactivity in postpartum women. Psychoneuroendocrinology 55:164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnaudery M, Perez‐Martin M, Del Favero F, Gomez‐Roldan C, Garcia‐Segura LM, Maccari S. 2007. Early motherhood in rats is associated with a modification of hippocampal function. Psychoneuroendocrinology 32:803–812. [DOI] [PubMed] [Google Scholar]
- Dayer AG, Cleaver KM, Abouantoun T, Cameron HA. 2005. New GABAergic interneurons in the adult neocortex and striatum are generated from different precursors. J Cell Biol 168:415–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Dreu CK. 2012. Oxytocin modulates cooperation within and competition between groups: an integrative review and research agenda. Horm Behav 61:419–428. [DOI] [PubMed] [Google Scholar]
- Deco G, Jirsa VK, McIntosh AR. 2011. Emerging concepts for the dynamical organization of resting‐state activity in the brain. Nat Rev Neurosci 12:43–56. [DOI] [PubMed] [Google Scholar]
- Dias CC, Figueiredo B. 2015. Breastfeeding and depression: a systematic review of the literature. J Affect Disord 171:142–154. [DOI] [PubMed] [Google Scholar]
- Dorr HG, Heller A, Versmold HT, Sippell WG, Herrmann M, Bidlingmaier F, Knorr D. 1989. Longitudinal study of progestins, mineralocorticoids, and glucocorticoids throughout human pregnancy. J Clin Endocrinol Metab 68:863–868. [DOI] [PubMed] [Google Scholar]
- Duke SA, Balzer BW, Steinbeck KS. 2014. Testosterone and its effects on human male adolescent mood and behavior: a systematic review. J Adolesc Health 55:315–322. [DOI] [PubMed] [Google Scholar]
- Edelstein RS, Wardecker BM, Chopik WJ, Moors AC, Shipman EL, Lin NJ. 2015. Prenatal hormones in first‐time expectant parents: longitudinal changes and within‐couple correlations. Am J Hum Biol 27:317–325. [DOI] [PubMed] [Google Scholar]
- Edward KL, Castle D, Mills C, Davis L, Casey J. 2015. An integrative review of paternal depression. Am J Mens Health 9:26–34. [DOI] [PubMed] [Google Scholar]
- Endendijk JJ, Hallers‐Haalboom ET, Groeneveld MG, van Berkel SR, van der Pol LD, Bakermans‐Kranenburg MJ, Mesman J. 2016. Diurnal testosterone variability is differentially associated with parenting quality in mothers and fathers. Horm Behav 80:68–75. [DOI] [PubMed] [Google Scholar]
- Ernst A, Alkass K, Bernard S, Salehpour M, Perl S, Tisdale J, Possnert G, Druid H, Frisen J. 2014. Neurogenesis in the striatum of the adult human brain. Cell 156:1072–1083. [DOI] [PubMed] [Google Scholar]
- Escriba‐Aguir V, Artazcoz L. 2011. Gender differences in postpartum depression: a longitudinal cohort study. J Epidemiol Community Health 65:320–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esscher A, Essen B, Innala E, Papadopoulos FC, Skalkidou A, Sundström‐Poromaa I, Hogberg U. 2015. Suicides during pregnancy and 1 year postpartum in Sweden, 1980–2007. Br J Psychiatry 208:462–469. [DOI] [PubMed] [Google Scholar]
- Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, Bremner WJ, McKinlay JB. 2002. Age trends in the level of serum testosterone and other hormones in middle‐aged men: longitudinal results from the Massachusetts Male Aging Study. J Clin Endocrinol Metab 87:589–598. [DOI] [PubMed] [Google Scholar]
- Feldman R. 2015. The adaptive human parental brain: implications for children's social development. Trends Neurosci 38:387–399. [DOI] [PubMed] [Google Scholar]
- Feldman R, Weller A, Zagoory‐Sharon O, Levine A. 2007. Evidence for a neuroendocrinological foundation of human affiliation: plasma oxytocin levels across pregnancy and the postpartum period predict mother‐infant bonding. Psychol Sci 18:965–970. [DOI] [PubMed] [Google Scholar]
- Feldman R, Gordon I, Schneiderman I, Weisman O, Zagoory‐Sharon O. 2010. Natural variations in maternal and paternal care are associated with systematic changes in oxytocin following parent–infant contact. Psychoneuroendocrinology 35:1133–1141. [DOI] [PubMed] [Google Scholar]
- Figueiredo B, Conde A. 2011. Anxiety and depression in women and men from early pregnancy to 3 months postpartum. Arch Womens Ment Health 14:247–255. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Cheung U, Myhal N, Kessler Z. 1989. Effects of maternal hormones on “timidity” and attraction to pup‐related odors in female rats. Physiol Behav 46:449–453. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Ruble D, Krieger H, Wong PY. 1997a. Hormonal and experiential correlates of maternal responsiveness during pregnancy and the puerperium in human mothers. Horm Behav 31:145–158. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Steiner M, Corter C. 1997b. Cortisol, hedonics, and maternal responsiveness in human mothers. Horm Behav 32:85–98. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Liu Y, Ouimet C, Wang Z. 2002. The effects of social environment on adult neurogenesis in the female prairie vole. J Neurobiol 51:115–128. [DOI] [PubMed] [Google Scholar]
- Gavin NI, Gaynes BN, Lohr KN, Meltzer‐Brody S, Gartlehner G, Swinson T. 2005. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol 106:1071–1083. [DOI] [PubMed] [Google Scholar]
- Gettler LT, McDade TW, Agustin SS, Kuzawa CW. 2011a. Short‐term changes in fathers' hormones during father‐child play: impacts of paternal attitudes and experience. Horm Behav 60:599–606. [DOI] [PubMed] [Google Scholar]
- Gettler LT, McDade TW, Feranil AB, Kuzawa CW. 2011b. Longitudinal evidence that fatherhood decreases testosterone in human males. Proc Natl Acad Sci U S A 108:16194–16199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingnell M, Bannbers E, Moes H, Engman J, Sylven S, Skalkidou A, Kask K, Wikstrom J, Sundström‐Poromaa I. 2015. Emotion reactivity is increased 4–6 weeks postpartum in healthy women: a longitudinal fMRI study. PLoS One 10:e0128964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasper ER, Kozorovitskiy Y, Pavlic A, Gould E. 2011. Paternal experience suppresses adult neurogenesis without altering hippocampal function in Peromyscus californicus . J Comp Neurol 519:2271–2281. [DOI] [PubMed] [Google Scholar]
- Glynn LM, Sandman CA. 2014. Evaluation of the association between placental corticotrophin‐releasing hormone and postpartum depressive symptoms. Psychosom Med 76:355–362. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Holsen L, Handa R, Tobet S. 2014. Fetal hormonal programming of sex differences in depression: linking women's mental health with sex differences in the brain across the lifespan. Front Neurosci 8:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman JH. 2004. Paternal postpartum depression, its relationship to maternal postpartum depression, and implications for family health. J Adv Nurs 45:26–35. [DOI] [PubMed] [Google Scholar]
- Gordon I, Zagoory‐Sharon O, Leckman JF, Feldman R. 2010a. Oxytocin, cortisol, and triadic family interactions. Physiol Behav 101:679–684. [DOI] [PubMed] [Google Scholar]
- Gordon I, Zagoory‐Sharon O, Leckman JF, Feldman R. 2010b. Prolactin, oxytocin, and the development of paternal behavior across the first six months of fatherhood. Horm Behav 58:513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Reeves AJ, Graziano MS, Gross CG. 1999. Neurogenesis in the neocortex of adult primates. Science 286:548–552. [DOI] [PubMed] [Google Scholar]
- Gutierrez‐Galve L, Stein A, Hanington L, Heron J, Ramchandani P. 2015. Paternal depression in the postnatal period and child development: mediators and moderators. Pediatrics 135:e339–e347. [DOI] [PubMed] [Google Scholar]
- Hahn‐Holbrook J, Schetter CD, Arora C, Hobel CJ. 2013. Placental corticotropin‐releasing hormone mediates the association between prenatal social support and postpartum depression. Clin Psychol Sci 1:253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines HM, Pallant JF, Fenwick J, Gamble J, Creedy DK, Toohill J, Hildingsson I. 2015. Identifying women who are afraid of giving birth: a comparison of the fear of birth scale with the WDEQ‐A in a large Australian cohort. Sex Reprod Healthcare 6:204–210. [DOI] [PubMed] [Google Scholar]
- Hannerfors AK, Hellgren C, Schijven D, Iliadis SI, Comasco E, Skalkidou A, Olivier JD, Sundström‐Poromaa I. 2015. Treatment with serotonin reuptake inhibitors during pregnancy is associated with elevated corticotropin‐releasing hormone levels. Psychoneuroendocrinology 58:104–113. [DOI] [PubMed] [Google Scholar]
- Harris B, Lovett L, Newcombe RG, Read GF, Walker R, Riad‐Fahmy D. 1994. Maternity blues and major endocrine changes: Cardiff puerperal mood and hormone study II. Br Med J 308:949–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellgren C, Akerud H, Skalkidou A, Sundström‐Poromaa I. 2013. Cortisol awakening response in late pregnancy in women with previous or ongoing depression. Psychoneuroendocrinology 38:3150–3154. [DOI] [PubMed] [Google Scholar]
- Hillerer KM, Neumann ID, Couillard‐Despres S, Aigner L, Slattery DA. 2014. Lactation‐induced reduction in hippocampal neurogenesis is reversed by repeated stress exposure. Hippocampus 24:673–683. [DOI] [PubMed] [Google Scholar]
- Houston KA, Kaimal AJ, Nakagawa S, Gregorich SE, Yee LM, Kuppermann M. 2015. Mode of delivery and postpartum depression: the role of patient preferences. Am J Obstet Gynecol 212:229 e221–e227. [DOI] [PubMed] [Google Scholar]
- Huang L, DeVries GJ, Bittman EL. 1998. Photoperiod regulates neuronal bromodeoxyuridine labeling in the brain of a seasonally breeding mammal. J Neurobiol 36:410–420. [DOI] [PubMed] [Google Scholar]
- Hunter T, Cattelona G. 2014. Breastfeeding initiation and duration in first‐time mothers: exploring the impact of father involvement in the early postpartum period. Health Promot Perspect 4:132–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliadis S, Sylven S, Jocelien O, Hellgren C, Hannefors AK, Elfstrom D, Sundström‐Poromaa I, Comasco E, Skalkidou A. 2015. Corticotropin‐releasing hormone and postpartum depression: a longitudinal study. Psychoneuroendocrinology 61:61. [Google Scholar]
- Iliadis SI, Sylven S, Hellgren C, Olivier JD, Schijven D, Comasco E, Chrousos GP, Sundström Poromaa I, Skalkidou A. 2016. Midpregnancy corticotropin‐releasing hormone levels in association with postpartum depressive symptoms. Depress Anxiety. doi: 10.1002/da.22529 [E‐pub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Inta D, Alfonso J, von Engelhardt J, Kreuzberg MM, Meyer AH, van Hooft JA, Monyer H. 2008. Neurogenesis and widespread forebrain migration of distinct GABAergic neurons from the postnatal subventricular zone. Proc Natl Acad Sci U S A 105:20994–20999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J, Fujiwara T, Barr RG. 2013. Is paternal infant care associated with breastfeeding? A population‐based study in Japan. J Hum Lact 29:491–499. [DOI] [PubMed] [Google Scholar]
- Jung C, Ho JT, Torpy DJ, Rogers A, Doogue M, Lewis JG, Czajko RJ, Inder WJ. 2011. A longitudinal study of plasma and urinary cortisol in pregnancy and postpartum. J Clin Endocrinol Metab 96:1533–1540. [DOI] [PubMed] [Google Scholar]
- Kempton MJ, Salvador Z, Munafo MR, Geddes JR, Simmons A, Frangou S, Williams SC. 2011. Structural neuroimaging studies in major depressive disorder. Meta‐analysis and comparison with bipolar disorder. Arch Gen Psychiatry 68:675–690. [DOI] [PubMed] [Google Scholar]
- Kersting A, Wagner B. 2012. Complicated grief after perinatal loss. Dialog Clin Neurosci 14:187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstis B, Aarts C, Tillman C, Persson H, Engstrom G, Edlund B, Ohrvik J, Sylven S, Skalkidou A. 2016. Association between parental depressive symptoms and impaired bonding with the infant. Arch Womens Ment Health 19:87–94. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. 1993. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity, and recurrence. J Affect Disord 29:85–96. [DOI] [PubMed] [Google Scholar]
- Kim P, Leckman JF, Mayes LC, Feldman R, Wang X, Swain JE. 2010. The plasticity of human maternal brain: longitudinal changes in brain anatomy during the early postpartum period. Behav Neurosci 124:695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Rigo P, Mayes LC, Feldman R, Leckman JF, Swain JE. 2014. Neural plasticity in fathers of human infants. Soc Neurosci 9:522–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Rigo P, Leckman JF, Mayes LC, Cole PM, Feldman R, Swain JE. 2015. A prospective longitudinal study of perceived infant outcomes at 18–24 months: neural and psychological correlates of parental thoughts and actions assessed during the first month postpartum. Front Psychol 6:1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokoeva MV, Yin H, Flier JS. 2005. Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science 310:679–683. [DOI] [PubMed] [Google Scholar]
- Kronmuller KT, Pantel J, Kohler S, Victor D, Giesel F, Magnotta VA, Mundt C, Essig M, Schroder J. 2008. Hippocampal volume and 2‐year outcome in depression. Br J Psychiatry 192:472–473. [DOI] [PubMed] [Google Scholar]
- Kuijper EA, Ket JC, Caanen MR, Lambalk CB. 2013. Reproductive hormone concentrations in pregnancy and neonates: a systematic review. Reprod Biomed Online 27:33–63. [DOI] [PubMed] [Google Scholar]
- Kuo PX, Carp J, Light KC, Grewen KM. 2012. Neural responses to infants linked with behavioral interactions and testosterone in fathers. Biol Psychol 91:302–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzawa CW, Gettler LT, Muller MN, McDade TW, Feranil AB. 2009. Fatherhood, pairbonding, and testosterone in The Philippines. Horm Behav 56:429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langan Martin J, McLean G, Cantwell R, Smith DJ. 2016. Admission to psychiatric hospital in the early and late postpartum periods: Scottish national linkage study. Br Med J Open 6:e008758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckman JF, Feldman R, Swain JE, Eicher V, Thompson N, Mayes LC. 2004. Primary parental preoccupation: circuits, genes, and the crucial role of the environment. J Neural Transm 111:753–771. [DOI] [PubMed] [Google Scholar]
- Lee MM, Reif A, Schmitt AG. 2013. Major depression: a role for hippocampal neurogenesis? Curr Top Behav Neurosci 14:153–179. [DOI] [PubMed] [Google Scholar]
- Leuner B, Sabihi S. 2016. The birth of new neurons in the maternal brain: hormonal regulation and functional implications. Front Neuroendocrinol 41:99–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Shors TJ. 2006. Learning during motherhood: a resistance to stress. Horm Behav 50:38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Gould E, Shors TJ. 2006. Is there a link between adult neurogenesis and learning? Hippocampus 16:216–224. [DOI] [PubMed] [Google Scholar]
- Leuner B, Mirescu C, Noiman L, Gould E. 2007. Maternal experience inhibits the production of immature neurons in the hippocampus during the postpartum period through elevations in adrenal steroids. Hippocampus 17:434–442. [DOI] [PubMed] [Google Scholar]
- Leuner B, Glasper ER, Gould E. 2010. Parenting and plasticity. Trends Neurosci 33:465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Mai X, Liu C. 2014. The default mode network and social understanding of others: what do brain connectivity studies tell us? Front Hum Neurosci 8:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberwirth C, Wang Z. 2014. Social bonding: regulation by neuropeptides. Front Neurosci 8:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberwirth C, Wang Y, Jia X, Liu Y, Wang Z. 2013. Fatherhood reduces the survival of adult‐generated cells and affects various types of behavior in the prairie vole (Microtus ochrogaster). Eur J Neurosci 38:3345–3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl V, Pearson JL, Colpe L. 2005. Prevalence of suicidality during pregnancy and the postpartum. Arch Womens Ment Health 8:77–87. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Levy F, Fleming AS. 2015. Common and divergent psychobiological mechanisms underlying maternal behaviors in nonhuman and human mammals. Horm Behav 73:156–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorberbaum JP, Newman JD, Dubno JR, Horwitz AR, Nahas Z, Teneback CC, Bloomer CW, Bohning DE, Vincent D, Johnson MR, Emmanuel N, Brawman‐Mintzer O, Book SW, Lydiard RB, Ballenger JC, George MS. 1999. Feasibility of using fMRI to study mothers responding to infant cries. Depress Anxiety 10:99–104. [DOI] [PubMed] [Google Scholar]
- Luzzati F, De Marchis S, Fasolo A, Peretto P. 2006. Neurogenesis in the caudate nucleus of the adult rabbit. J Neurosci 26:609–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon AL, Gold I, Feeley N, Hayton B, Carter CS, Zelkowitz P. 2014. The role of oxytocin in mothers' theory of mind and interactive behavior during the perinatal period. Psychoneuroendocrinology 48:52–63. [DOI] [PubMed] [Google Scholar]
- Magiakou MA, Mastorakos G, Rabin D, Dubbert B, Gold PW, Chrousos GP. 1996. Hypothalamic corticotropin‐releasing hormone suppression during the postpartum period: implications for the increase in psychiatric manifestations at this time. J Clin Endocrinol Metab 81:1912–1917. [DOI] [PubMed] [Google Scholar]
- Martins MV, Basto‐Pereira M, Pedro J, Peterson B, Almeida V, Schmidt L, Costa ME. 2016. Male psychological adaptation to unsuccessful medically assisted reproduction treatments: a systematic review. Hum Reprod Update 22:466–478. [DOI] [PubMed] [Google Scholar]
- Mascaro JS, Hackett PD, Gouzoules H, Lori A, Rilling JK. 2014. Behavioral and genetic correlates of the neural response to infant crying among human fathers. Soc Cogn Affect Neurosci 9:1704–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthey S, Barnett B, Ungerer J, Waters B. 2000. Paternal and maternal depressed mood during the transition to parenthood. J Affect Disord 60:75–85. [DOI] [PubMed] [Google Scholar]
- Mehta D, Quast C, Fasching PA, Seifert A, Voigt F, Beckmann MW, Faschingbauer F, Burger P, Ekici AB, Kornhuber J, Binder EB, Goecke TW. 2012. The 5‐HTTLPR polymorphism modulates the influence on environmental stressors on peripartum depression symptoms. J Affect Disord 136:1192–1197. [DOI] [PubMed] [Google Scholar]
- Mitchell C, Notterman D, Brooks‐Gunn J, Hobcraft J, Garfinkel I, Jaeger K, Kotenko I, McLanahan S. 2012. Role of mothers' genes and environment in postpartum depression. Proc Natl Acad Sci U S A 108:8189–8193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse CA, Buist A, Durkin S. 2000. First‐time parenthood: influences on pre‐ and postnatal adjustment in fathers and mothers. J Psychosom Obstet Gynaecol 21:109–120. [DOI] [PubMed] [Google Scholar]
- Moses‐Kolko EL, Horner MS, Phillips ML, Hipwell AE, Swain JE. 2014. In search of neural endophenotypes of postpartum psychopathology and disrupted maternal caregiving. J Neuroendocrinol 26:665–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MN, Marlowe FW, Bugumba R, Ellison PT. 2009. Testosterone and paternal care in East African foragers and pastoralists. Proc Biol Sci 276:347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munk‐Olsen T, Laursen TM, Mendelson T, Pedersen CB, Mors O, Mortensen PB. 2009. Risks and predictors of readmission for a mental disorder during the postpartum period. Arch Gen Psychiatry 66:189–195. [DOI] [PubMed] [Google Scholar]
- Norhayati MN, Hazlina NH, Asrenee AR, Emilin WM. 2015. Magnitude and risk factors for postpartum symptoms: a literature review. J Affect Disord 175:34–52. [DOI] [PubMed] [Google Scholar]
- Nunes S, Fite JE, Patera KJ, French JA. 2001. Interactions among paternal behavior, steroid hormones, and parental experience in male marmosets (Callithrix kuhlii). Horm Behav 39:70–82. [DOI] [PubMed] [Google Scholar]
- Nunes‐Costa RA, Figueiredo B, Moya‐Albiol L. 2014. The state of art of biological processes in paternal care. Psicol Reflex Crit 27:794–805. [Google Scholar]
- O'Hara MW, McCabe JE. 2013. Postpartum depression: current status and future directions. Annu Rev Clin Psychol 9:379–407. [DOI] [PubMed] [Google Scholar]
- O'Hara MW, Rehm LP, Campbell SB. 1982. Predicting depressive symptomatology: cognitive‐behavioral models and postpartum depression. J Abnorm Psychol 91:457–461. [DOI] [PubMed] [Google Scholar]
- O'Hara MW, Schlechte JA, Lewis DA, Wright EJ. 1991. Prospective study of postpartum blues. Biologic and psychosocial factors. Arch Gen Psychiatry 48:801–806. [DOI] [PubMed] [Google Scholar]
- Okuda H, Tatsumi K, Makinodan M, Yamauchi T, Kishimoto T, Wanaka A. 2009. Environmental enrichment stimulates progenitor cell proliferation in the amygdala. J Neurosci Res 87:3546–3553. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Lee TM. 2001. Central vasopressin administration regulates the onset of facultative paternal behavior in Microtus pennsylvanicus (meadow voles). Horm Behav 39:285–294. [DOI] [PubMed] [Google Scholar]
- Paulson JF, Bazemore SD. 2010. Prenatal and postpartum depression in fathers and its association with maternal depression: a meta‐analysis. JAMA 303:1961–1969. [DOI] [PubMed] [Google Scholar]
- Paulson JF, Bazemore SD, Goodman JH, Leiferman JA. 2016. The course and interrelationship of maternal and paternal perinatal depression. Arch Womens Ment Health 19:655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlby S, Hay DF, Sharp D, Waters CS, O'Keane V. 2009. Antenatal depression predicts depression in adolescent offspring: prospective longitudinal community‐based study. J Affect Disord 113:236–243. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Galea LA. 2007. Reproductive experience alters hippocampal neurogenesis during the postpartum period in the dam. Neuroscience 149:53–67. [DOI] [PubMed] [Google Scholar]
- Pedersen CA. 1997. Oxytocin control of maternal behavior. Regulation by sex steroids and offspring stimuli. Ann N Y Acad Sci 807:126–145. [DOI] [PubMed] [Google Scholar]
- Perheentupa A, Ruokonen A, Tapanainen JS. 2004. Transdermal estradiol treatment suppresses serum gonadotropins during lactation without transfer into breast milk. Fertil Steril 82:903–907. [DOI] [PubMed] [Google Scholar]
- Pope CJ, Mazmanian D. 2016. Breastfeeding and postpartum depression: an overview and methodological recommendations for future research. Depress Res Treat 2016:4765310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poromaa IS, Segebladh B. 2012. Adverse mood symptoms with oral contraceptives. Acta Obstet Gynecol Scand 91:420–427. [DOI] [PubMed] [Google Scholar]
- Raisanen S, Lehto SM, Nielsen HS, Gissler M, Kramer MR, Heinonen S. 2013. Fear of childbirth predicts postpartum depression: a population‐based analysis of 511,422 singleton births in Finland. Br Med J Open 3:e004047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond AD, Kucherepa NN, Fisher KR, Halina WG, Partlow GD. 2006. Neurogenesis of oxytocin‐containing neurons in the paraventricular nucleus (PVN) of the female pig in 3 reproductive states: puberty gilts, adult gilts, and lactating sows. Brain Res 1102:44–51. [DOI] [PubMed] [Google Scholar]
- Robertson E, Grace S, Wallington T, Stewart DE. 2004. Antenatal risk factors for postpartum depression: a synthesis of recent literature. Gen Hosp Psychiatry 26:289–295. [DOI] [PubMed] [Google Scholar]
- Ruscio MG, Sweeny TD, Hazelton JL, Suppatkul P, Boothe E, Carter CS. 2008. Pup exposure elicits hippocampal cell proliferation in the prairie vole. Behav Brain Res 187:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saaltink DJ, Vreugdenhil E. 2014. Stress, glucocorticoid receptors, and adult neurogenesis: a balance between excitation and inhibition? Cell Mol Life Sci 71:2499–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltzman W, Maestripieri D. 2011. The neuroendocrinology of primate maternal behavior. Prog Neuropsychopharmacol Biol Psychiatry 35:1192–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. 2003. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301:805–809. [DOI] [PubMed] [Google Scholar]
- Seifritz E, Esposito F, Neuhoff JG, Luthi A, Mustovic H, Dammann G, von Bardeleben U, Radue EW, Cirillo S, Tedeschi G, Di Salle F. 2003. Differential sex‐independent amygdala response to infant crying and laughing in parents versus nonparents. Biol Psychiatry 54:1367–1375. [DOI] [PubMed] [Google Scholar]
- Sherriff N, Panton C, Hall V. 2014. A new model of father support to promote breastfeeding. Community Pract 87:20–24. [PubMed] [Google Scholar]
- Shors TJ, Tobomicronn K, DiFeo G, Durham DM, Chang HY. 2016. Sexual conspecific aggressive response (SCAR): a model of sexual trauma that disrupts maternal learning and plasticity in the female brain. Sci Rep 6:18960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel HI, Rosenblatt JS. 1978. Duration of estrogen stimulation and progesterone inhibition of maternal behavior in pregnancy‐terminated rats. Horm Behav 11:12–19. [DOI] [PubMed] [Google Scholar]
- Skalkidou A, Hellgren C, Comasco E, Sylven S, Sundström Poromaa I. 2012. Biological aspects of postpartum depression. Womens Health 8:659–672. [DOI] [PubMed] [Google Scholar]
- Soares CN. 2014. Mood disorders in midlife women: understanding the critical window and its clinical implications. Menopause 21:198–206. [DOI] [PubMed] [Google Scholar]
- Soares CN, Zitek B. 2008. Reproductive hormone sensitivity and risk for depression across the female life cycle: a continuum of vulnerability? J Psychiatry Neurosci 33:331–343. [PMC free article] [PubMed] [Google Scholar]
- Stalla GK, Bost H, Stalla J, Kaliebe T, Dorr HG, Pfeiffer D, von Werder K, Muller OA. 1989. Human corticotropin‐releasing hormone during pregnancy. Gynecol Endocrinol 3:1–10. [DOI] [PubMed] [Google Scholar]
- Stein A, Pearson RM, Goodman SH, Rapa E, Rahman A, McCallum M, Howard LM, Pariante CM. 2014. Effects of perinatal mental disorders on the fetus and child. Lancet 384:1800–1819. [DOI] [PubMed] [Google Scholar]
- Storey AE, Walsh CJ, Quinton RL, Wynne‐Edwards KE. 2000. Hormonal correlates of paternal responsiveness in new and expectant fathers. Evol Hum Behav 21:79–95. [DOI] [PubMed] [Google Scholar]
- Storey AE, Noseworthy DE, Delahunty KM, Halfyard SJ, McKay DW. 2011. The effects of social context on the hormonal and behavioral responsiveness of human fathers. Horm Behav 60:353–361. [DOI] [PubMed] [Google Scholar]
- Sundström Poromaa I, Gingnell M. 2014. Menstrual cycle influence on cognitive function and emotion processing‐from a reproductive perspective. Front Neurosci 8:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Lorberbaum JP, Kose S, Strathearn L. 2007. Brain basis of early parent–infant interactions: psychology, physiology, and in vivo functional neuroimaging studies. J Child Psychol Psychiatry 48:262–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Perkins SC, Dayton CJ, Finegood ED, Ho SS. 2012. Parental brain and socioeconomic epigenetic effects in human development. Behav Brain Sci 35:378–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Dayton CJ, Kim P, Tolman RM, Volling BL. 2014a. Progress on the paternal brain: theory, animal models, human brain research, and mental health implications. Infant Ment Health J 35:394–408. [DOI] [PubMed] [Google Scholar]
- Swain JE, Kim P, Spicer J, Ho SS, Dayton CJ, Elmadih A, Abel KM. 2014b. Approaching the biology of human parental attachment: brain imaging, oxytocin, and coordinated assessments of mothers and fathers. Brain Res 1580:78–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Gould E. 2005. Ovarian steroids influence cell proliferation in the dentate gyrus of the adult female rat in a dose‐ and time‐dependent manner. J Comp Neurol 481:252–265. [DOI] [PubMed] [Google Scholar]
- Toffoletto S, Lanzenberger R, Gingnell M, Sundström‐Poromaa I, Comasco E. 2014. Emotional and cognitive functional imaging of estrogen and progesterone effects in the female human brain: a systematic review. Psychoneuroendocrinology 50:28–52. [DOI] [PubMed] [Google Scholar]
- Tuohy A, McVey C. 2008. Experience of pregnancy and delivery as predictors of postpartum depression. Psychol Health Med 13:43–47. [DOI] [PubMed] [Google Scholar]
- van Anders SM, Tolman RM, Volling BL. 2012. Baby cries and nurturance affect testosterone in men. Horm Behav 61:31–36. [DOI] [PubMed] [Google Scholar]
- Vesga‐Lopez O, Blanco C, Keyes K, Olfson M, Grant BF, Hasin DS. 2008. Psychiatric disorders in pregnant and postpartum women in the United States. Arch Gen Psychiatry 65:805–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B. 2004. Hippocampal volume and depression: a meta‐analysis of MRI studies. Am J Psychiatry 161:1957–1966. [DOI] [PubMed] [Google Scholar]
- Vigod SN, Tarasoff LA, Bryja B, Dennis CL, Yudin MH, Ross LE. 2013. Relation between place of residence and postpartum depression. Can Med Assoc J 185:1129–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgsten H, Skoog Svanberg A, Ekselius L, Lundkvist O, Sundström Poromaa I. 2008. Prevalence of psychiatric disorders in infertile women and men undergoing in vitro fertilization treatment. Hum Reprod 23:2056–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee KY, Skouteris H, Pier C, Richardson B, Milgrom J. 2011. Correlates of ante‐ and postnatal depression in fathers: a systematic review. J Affect Disord 130:358–377. [DOI] [PubMed] [Google Scholar]
- Wilson S, Durbin CE. 2010. Effects of paternal depression on fathers' parenting behaviors: a meta‐analytic review. Clin Psychol Rev 30:167–180. [DOI] [PubMed] [Google Scholar]
- Wittfoth‐Schardt D, Grunding J, Wittfoth M, Lanfermann H, Heinrichs M, Domes G, Buchheim A, Gundel H, Waller C. 2012. Oxytocin modulates neural reactivity to children's faces as a function of social salience. Neuropsychopharmacology 37:1799–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne‐Edwards KE. 2001. Hormonal changes in mammalian fathers. Horm Behav 40:139–145. [DOI] [PubMed] [Google Scholar]
- Wynne‐Edwards KE, Timonin ME. 2007. Paternal care in rodents: weakening support for hormonal regulation of the transition to behavioral fatherhood in rodent animal models of biparental care. Horm Behav 52:114–121. [DOI] [PubMed] [Google Scholar]
- Yim IS, Glynn LM, Dunkel‐Schetter C, Hobel CJ, Chicz‐DeMet A, Sandman CA. 2009. Risk of postpartum depressive symptoms with elevated corticotropin‐releasing hormone in human pregnancy. Arch Gen Psychiatry 66:162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]


