Abstract
Explaining the overwhelming success of sex among eukaryotes is difficult given the obvious costs of sex relative to asexuality. Different studies have shown that sex can provide benefits in spatially heterogeneous environments under specific conditions, but whether spatial heterogeneity commonly contributes to the maintenance of sex in natural populations remains unknown. We experimentally manipulated habitat heterogeneity for sexual and asexual thrips lineages in natural populations and under seminatural mesocosm conditions by varying the number of hostplants available to these herbivorous insects. Asexual lineages rapidly replaced the sexual ones, independently of the level of habitat heterogeneity in mesocosms. In natural populations, the success of sexual thrips decreased with increasing habitat heterogeneity, with sexual thrips apparently only persisting in certain types of hostplant communities. Our results illustrate how genetic diversity‐based mechanisms can favor asexuality instead of sex when sexual lineages co‐occur with genetically variable asexual lineages.
Keywords: Asexuality, evolution of sex, parthenogenesis, Thysanoptera, Tangled Bank
Sex: What is it good for? Identifying the factors that drive the maintenance of sexual reproduction in natural populations remains a central goal in evolutionary biology. Sexual reproduction is associated with many direct costs, especially in metazoans, where males often provide little or no resources to their offspring (reviewed by Lehtonen et al. 2012). Since only females can directly produce offspring, sexual females who invest equally in sons and daughters experience a twofold cost relative to asexual females who solely produce daughters (Williams 1975; Maynard Smith 1978). This cost translates into dramatically slower growth rates of the sexual population relative to asexual lines. Even in cases where males do contribute resources to their offspring, sex is typically still costly because it requires the attraction of mates and eventually mating (Bell 1982; Lehtonen et al. 2012). Sex also generates indirect costs because it breaks up associations between alleles, including beneficial associations that were generated by selection in past generations (reviewed in Agrawal 2006; Otto 2009). These multifaceted costs have motivated a scientific quest for identifying mechanisms and situations that can generate benefits for sex and, therefore, could help explain the overwhelming success of this reproductive mode (e.g., Maynard Smith 1971; Charlesworth 1976; Barton 1995; Peters and Lively 1999; Lenormand and Otto 2000; Gandon and Otto 2007; Agrawal 2009).
Theoretical approaches have identified a number of possible mechanisms that could generate indirect benefits for sex, with support from experimental evolution studies under laboratory conditions for some of them (e.g., Colegrave 2002; Goddard et al. 2005; Cooper 2007; Morran et al. 2009; Becks and Agrawal 2010; Morran et al. 2011; McDonald et al. 2016). However, it remains unknown whether any of the suggested benefits of sex may be sufficient to outweigh the costs sex generates under natural conditions.
Here, we evaluate whether spatial habitat heterogeneity, a situation that can generate benefits for sex under some conditions, contributes to the maintenance of sex in natural populations. Two classes of theoretical arguments propose that sex can generate benefits under spatial heterogeneity. In the first class, spatial habitat heterogeneity causes spatial variation in selection. Spatial variation in selection can generate locally adapted allele associations with locally maladapted associations introduced by migration (Lenormand 2002). Sex can then provide benefits because it breaks such maladaptive allele associations, provided migration rates are high enough to regularly introduce locally maladapted associations and low enough to not constrain local adaptation via gene flow (Pylkov et al. 1998; Lenormand and Otto 2000; Agrawal 2009). These theoretical predictions were supported by results from an experimental evolution approach in cyclically asexual rotifers. By controlling migration rates between similar and different rearing environments, Becks and Agrawal (2010) showed that higher rates of sex are maintained under migration between different environments. However, theory also showed that spatial variation only favors sex if specific types of epistasis occur in combination with the appropriate migration rates and strengths of selection (Pylkov et al. 1998; Lenormand and Otto 2000), raising the question of how frequently natural populations might fit the theoretical conditions predicted to favor sex under habitat heterogeneity.
The second class of arguments, referred to as “Tangled Bank” models, posit that there is genetically based variation among individuals for exploiting different niches (e.g., Ghiselin 1974; Maynard Smith 1978; Young 1981; Bell 1982; Case and Taper 1986; Doncaster et al. 2000; Song et al. 2011). Genetically identical individuals should, therefore, experience more intense competition than genetically different individuals. Under the assumption that sexual lineages comprise more genetic variation for niche exploitation than asexual ones, sexual lineages can be favored via at least two mechanisms. First, sexual reproduction could reduce competition among siblings because sexually produced offspring display more genetic variation for resource use than asexually produced ones (Maynard Smith 1978; Young 1981). Second, intraspecific competition can negatively impact population growth rates. The negative impact of an asexual lineage on its own growth rate should be more severe than the impact on the growth rate of the sexual lineage (given sexual individuals are genetically variable and different from asexual individuals), which can maintain the coexistence of sexual and asexual lineages (Case and Taper 1986; Doncaster et al. 2000; see also Song et al. 2011). Empirical tests of Tangled Bank models have used experimental populations consisting either of a single clone (low genetic diversity) or multiple clones (high genetic diversity) of an asexual species and found that genetic diversity can indeed provide benefits (e.g., Antonovics and Ellstrand 1984; Ellstrand and Antonovics 1985; Weeks and Hoffmann 1998, 2008). However, there are currently no experimental tests of whether Tangled Bank mechanisms contribute to the maintenance of competing sexual and asexual lineages in natural populations.
We evaluated the possible contribution of habitat heterogeneity to the maintenance of sex in natural populations by using grassthrips of the genus Aptinothrips (Thysanoptera) as a model system. This genus comprises four species of small (∼1 mm) insects, which live and feed on a variety of grasses (Poaceae) (Palmer 1975). Breeding experiments with Aptinothrips females from Western Europe revealed reproductive polymorphism in this area. Two Aptinothrips species reproduce exclusively asexually (A. stylifer and A. karnyi), one species (A. elegans) reproduces sexually, and the forth species (A. rufus) is characterized by mixed reproduction, with some females reproducing sexually and others asexually (van der Kooi and Schwander 2014). As for most thrips (Hamilton 1967; Lewis 1973), sex ratios for sexual Aptinothrips lineages in field populations vary extensively over space and time and are typically highly female‐biased (van der Kooi and Schwander 2014). Female‐bias is most likely caused by a combination of low investment into males, sex‐specific behaviors, and males being very short‐lived (Lewis 1973; Mound 1991). Sexual and asexual species of Aptinothrips form mixed populations across a large part of their distribution (van der Kooi and Schwander 2014). Several independent transitions to asexuality have been described, and clonal diversity is extremely high in the widely distributed asexual A. rufus (van der Kooi and Schwander 2014; Fontcuberta et al. 2016).
We took advantage of the reproductive mode polymorphism among Aptinothrips species and lineages to test whether the success of sexual lineages is affected by spatial heterogeneity in selection. Because Aptinothrips are dependent on grasses for their entire life cycle, we assumed that different grass species would correspond to different habitat types for the thrips, as host plants often represent different ecological niches for herbivorous insects (Jaenike 1990). We first verified that different grasses do indeed represent habitats of variable quality for Aptinothrips, by measuring reproductive success of females on five different grass species. We then used two complementary experimental approaches to test whether grass diversity (i.e., the level of habitat heterogeneity) affected the relative success of competing sexual and asexual lineages. For the first approach, we generated mesocosms comprising either a single grass species (homogeneous habitats) or a mix of three grass species (heterogeneous habitats). Sexual and asexual grassthrips were then introduced into these habitats and their relative proportions followed over 12 generations. This setup thus mimics a situation where a largely empty habitat is simultaneously colonized by sexual and asexual individuals. In the second approach, we took advantage of experimental hay meadows where grass diversity was manipulated via fertilization and irrigation treatments (Andrey et al. 2014). The original purpose of these treatments was to investigate the effects of fertilization and irrigation on the biodiversity of extensively managed, montane, and subalpine hay meadows, to formulate management recommendations that would simultaneously optimize biodiversity and hay yield. Sexual and asexual thrips co‐occur in such extensively managed hay meadows. In contrast to the mesocosm experiments, the meadow treatments thus correspond to a modification of a habitat with established sexual and asexual populations and not to a situation where empty habitat is colonized. We collected grassthrips from these experimental meadows to test whether sexual Aptinothrips were favored in treatments generating increased grass diversity.
In summary, our aim was to experimentally manipulate habitat heterogeneity for thrips under the most natural conditions possible and to test whether the level of habitat heterogeneity affects the relative success of sexual and asexual thrips. Our approach, therefore, does not generate artificial conditions that would favor sex specifically via Tangled Bank mechanisms or because it would break up maladaptive gene combinations introduced by migration. Rather, our approach allows us to infer whether habitat heterogeneity contributes to the maintenance of sex under natural conditions independently of the mechanism through which sex would generate benefits. In contrast to hypotheses and experiments under artificial conditions, we show that habitat heterogeneity under natural conditions always favors asexual, rather than sexual, reproduction.
Methods
THRIPS FITNESS ON DIFFERENT GRASS SPECIES
To test whether grass species represent habitats of variable quality for thrips, we measured reproductive success on different grasses for females of the sexual species A. elegans, a sexual A. rufus lineage, and asexual A. rufus. We grew five grass species in 50 ml falcon tubes, with 24–66 replicates per Aptinothrips species and grass species: Molinia caerulea (moor‐grass), Bromus hordeaceus (brome), Festuca rubra (fescue), Triticum aestivum (wheat), and Secale cereale (rye). One field‐collected, adult female was added to each tube and allowed to reproduce for 24 days at 23°C and 10:14 night/day cycles. Reproductive success of each female was then determined by counting all offspring present in a tube and these offspring numbers were compared between treatments using a GLM with a poisson‐distributed error and an empirically estimated scale parameter. Whether thrips species, grass species, and their interaction explained a significant amount of deviance in the GLM was evaluated using a χ2‐test.
MESOCOSM EXPERIMENT
Based on the results from the previous experiment, we chose three easy‐to‐grow grass species that varied in their quality for thrips: wheat, fescue, and rye. With these grasses, we generated four different types of mesocosms: one with a heterogeneous habitat (the three species mixed in equal proportions) and three with homogeneous habitats consisting only of one grass species. Mesocosms were generated in transparent, cylindrical cages (15 cm diameter, 25 cm high), randomly placed on watering tables in a greenhouse under a light regime of L/D = 14:10 superimposed over a natural light regime. These mesocosms were used to test whether habitat heterogeneity affects the relative success of competing sexual and asexual lineages in two different assays, each running over approximately 12 thrips generations (thrips have overlapping generations). In the first assay, we used two Aptinothrips lineages that frequently co‐occur in natural populations but belong to different species: sexual A. elegans and asexual A. rufus. Females for this assay were sampled in a hay meadow in Switzerland (N46.657906, W6.533641), where the two species are sympatric with a ratio of 49:51 (n > 3000) and that is mainly composed of the grasses Arrhenatherum elatius, Dactylis glomerata, and Bromus hordeaceus. Aptinothrips elegans and A. rufus females were sorted by species under a dissecting scope at 40× magnification, after CO2 anesthetization. The two species are very similar but can be distinguished by the shape of antennal segments and position of hairs on the abdomen (Palmer 1975).
In the second competition assay, we used A. rufus sexual and asexual strains from laboratory stock populations to account for eventual species differences in the first assay. The stock populations have been maintained in the laboratory for over 3 years on wheat (>30 generations). Under the laboratory conditions used (23°C and 10:14 night/day cycles), the sexual A. rufus populations were characterized by an approximately constant proportion of 0.32 males.
For each competition assay, we prepared a total of 160 mesocosms (40 heterogeneous ones and 40 homogeneous ones for each of the three grass species). Six asexual and six mated sexual females were then introduced into each of the 160 mesocosms. This is below the carrying capacity of these mesocosms, which means that competition occurred after a colonisation episode. Furthermore, it is important to note that given the very high clonal diversities in asexual A. rufus populations, the six asexual females per mesocosm most likely comprised several different clones. To quantify the frequency of sexual and asexual lineages present, we removed randomly without replacement ten heterogeneous mesocosms and 30 homogeneous ones (10 per grass species) at three successive intervals of 40 ± 2 days (approximately two thrips generations). Thrips in the remaining ten heterogeneous mesocosms and 30 homogeneous ones were counted after a final interval of 120 days (240 ± 6 days from the start of the experiment). In the sexual A. elegans versus asexual A. rufus assay, we determined the frequency of sexuals and asexuals by counting the number of adult females of each species. In the sexual versus asexual A. rufus assay, we counted the proportion of males among adults because sexual and asexual A. rufus females cannot be distinguished morphologically.
Because the mesocosm conditions differed from the conditions in the field (notably because of different grasses), we may have selected for adaptation to new host plants. To test for evidence of adaptation, we collected individuals alive from the 40 mesocosms of the first interval of competing A. elegans versus asexual A. rufus. Individual A. elegans and A. rufus females were then transferred to 50 ml tubes with the same habitat as the mesocosm they were extracted from and allowed to reproduce for 24 days as described above. Reproductive output for each thrips species was then compared to the reproductive output under the same conditions prior to the experiment. Thrips from all the remaining mesocosms were counted after extraction into alcohol (adults and larvae) via heat gradients on Berlese funnels.
FIELD EXPERIMENT
To test the effect of habitat heterogeneity on the success of sexual and asexual grassthrips in natural populations, we took advantage of experimental hay meadows in the inner European Alps (Valais, SW Switzerland), where sexual A. elegans co‐occur with asexual A. rufus, A. stylifer, and A. karnyi. These meadows have been extensively managed for a long time before the start of the experiment. Within each meadow, grass diversity was manipulated via six different irrigation and fertilization treatments (Andrey et al. 2014). These meadows are strongly water and nutrient limited and are occupied by xerophilic species, such that low or intermediate management intensities were expected to increase plant diversity in general and grass diversity in particular (Peter et al. 2009). Precise details on site selection and experimental setups are presented elsewhere (Andrey et al. 2014). Briefly, six circular plots (20 m diameter) were delineated at each of 11 study sites (66 different plots; see Fig. S1) and randomly assigned to the treatments. In the first treatment (“standard”), hay meadows were managed as prior to the start of the experiment with grass cut once per year and no other manipulation. The second treatment consisted in watering the plots once per week, the third treatment in fertilizing them. For the forth to sixth treatments, plots were simultaneously watered and fertilized in an increasing intensity (see Table S1).
Treatments were initiated in spring 2010 and continued over five years, inducing a gradual transition from the original to new plant communities (Andrey et al. 2014) over ∼40–50 thrips generations. To estimate the final grass diversity in each plot, we used coverage data for grass species from exhaustive plant inventories in rectangles of 2 × 4 m within each plot, conducted in May–June 2014; thrips were collected in June 2014 (see Supporting Information).
STATISTICAL ANALYSES
All analyses were conducted in R 3.1.1 (R Core Team 2014). We computed Shannon–Wiener ɑ‐diversity indices from grass species coverage in experimental plots using the R package vegan (Oksanen et al. 2015) to use these diversities as a proxy for habitat heterogeneity. We tested the effects of the treatments on habitat heterogeneity (i.e., grass diversity) using a linear mixed model (LMM). The effect of the treatment and of habitat heterogeneity on the weighted counts of sexual Aptinothrips was tested using generalized LMMs (GLMMs) implemented in the R package lme4 (Bates et al. 2015). Because the residuals in some of our models were overdispersed, we corroborated each GLMM result with an ANOVA using the proportion of sexual Aptinothrips rather than their weighted counts (see Supporting Information).
Because our analyses revealed a negative effect of grass diversity on the success of sexual thrips (see results), we investigated whether specific types of grass communities could favor sexual Aptinothrips. We first generated a grass community distance dendrogram using Bray‐Curtis distances, as implemented in the R package vegan. We then used this dendrogram to test whether similar communities were characterized by similar sexual thrips abundances by calculating Pagel's ɑ with the R package phytools (Revell 2012). Pagel's ɑ is typically used to assess whether phylogenetically related species share similar values of a quantitative trait. In our analyses Pagel's ɑ thus indicates whether similar grass communities comprise more similar abundances of sexual thrips than expected by chance. For visual evaluation, we further performed a principal component analysis (PCA) as implemented in the R package vegan, using a Hellinger transformation for scaling grass cover data (Legendre and Gallagher 2001). Additional grass community analyses are described in the Supporting Information.
Results
Grass species vary significantly in quality for thrips as revealed by the number of progeny females produced on the five species wheat, brome, fescue, rye, and moor‐grass (thrips species effect: df = 2, P < 0.0001; grass species effect: df = 4, P < 0.0001; interaction: df = 8, P = 0.11; Fig. 1). Females of the three tested thrips species/lineages were unable to reproduce on moor‐grass and we found up to sevenfold differences in reproductive output among the four remaining grasses. Given this broad quality variation among grasses, grass diversity is a suitable proxy for the level of habitat heterogeneity in a thrips population.
Figure 1.
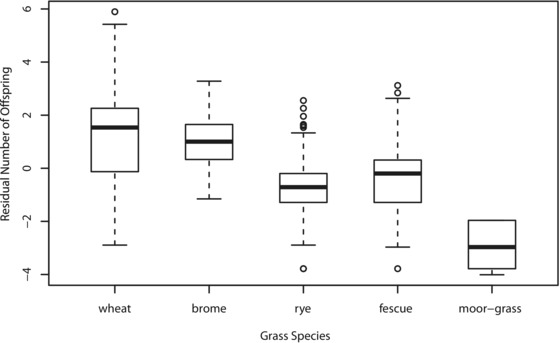
Grassthrips females produce different numbers of offspring depending on the grass species they breed on, indicating that different grasses correspond to habitats of different quality. N = 24–66 females per thrips‐ and grass species; residual offspring numbers after accounting for variation among thrips species are presented.
We then used three of the five grass species to generate heterogeneous (three species mixed) and homogeneous (one species) mesocosms in which we followed the relative proportion of sexual and asexual thrips over time. Within 80 days of the experiment (approximately four thrips generations), each mesocosm comprised close to 300 individuals, a number that remained approximately constant until the 12th generation (Fig. 2). Independently of the mesocosm type (heterogeneous or different homogeneous ones) and the sexual lineage we used (sexual A. rufus or A. elegans), sexual females were always completely replaced by asexual ones after 12 generations (Fig. 2). We therefore found no positive effect of habitat heterogeneity on the maintenance of sexual thrips.
Figure 2.
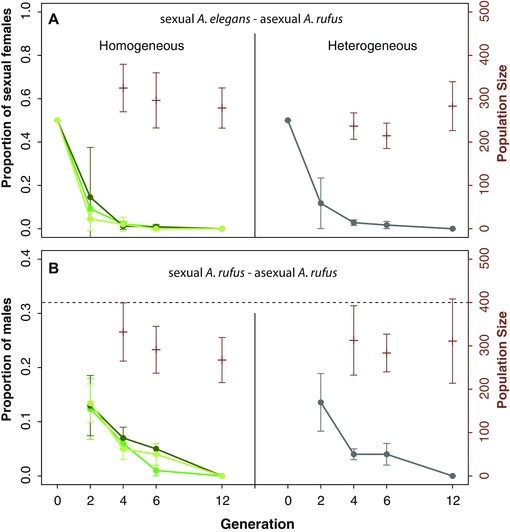
(A) Proportion of sexual A. elegans females and (B) proportion of A. rufus males in mesocosms across different generations; the dotted line in panel (B) indicates the proportion of males in laboratory populations of sexual A. rufus (i.e., the proportion of males corresponding to the fixation of sexual lineages). Generation 0 started with six asexual females and six mated sexual females in all mesocosms, n = 10 mesocosms per generation and mesocosm type. The proportions of males, respectively sexual and asexual lineages, were not determined for generations 8–10. Different colours in the homogeneous mesocosms indicate different grass species used in the homogeneous mesocosms (dark green: fescue, intermediate: wheat, light green: rye). Right axis: population size, as measured by the total number of Aptinothrips in the mesocosm. Error bars represent standard errors.
In natural populations, the sexual thrips species A. elegans frequently co‐occurs with asexual A. rufus, with individuals used in the mesocosm experiments stemming from a location with approximately equal frequencies of both species. Nevertheless, the rate of population increase of asexual A. rufus was much higher than of sexual A. elegans in all mesocosms, leading to complete disappearance of A. elegans after 4–12 generations. After approximately six generations, A. elegans was completely extinct in 10 homogeneous rye mesocosms, and in seven respectively eight of the homogeneous fescue and wheat ones (without a single A. elegans individual among over 7000 thrips). Aptinothrips elegans females were still present in the 15 remaining mesocosms, but at a very low frequency in 14 of the 15 (one to five individuals among several hundred asexual females). Only one homogeneous wheat mesocosm still comprised 12 A. elegans females (among 369 asexual females) after six generations. However, these residual individuals from sexual lineages were completely replaced by asexual females by generation 12, at which point we did not find a single A. elegans in any of the 40 mescosms (combined over 11,000 females). Thus, although the final extinction of sexuals was somewhat delayed in heterogeneous mesocosms (after six generations: complete extinction in 25 out of 30 homogeneous habitats vs. 0 out of 10 heterogeneous ones; Fisher's exact test P = 0.01), sexuals were unable to persist when in competition with asexuals, whatever the conditions.
Part of the success of asexual A. rufus, when in competition with A. elegans under the used rearing conditions stems from an approximately twofold fecundity advantage of A. rufus that is unrelated to reproductive mode but characterizes different species (see below; Fig. 3). However, we also found complete extinction of the sexual lineages after 12 generations for the mesocosms where asexual A. rufus were in competition with sexual A. rufus lineages. After generation 12, we found not a single male among over 12,000 females (Fig. 2). In contrast to the first competition experiment where sexual lineages were largely extinct by generation six, the proportion of males present at generation six (0–0.11 per mesocosm, Fig. 2) indicated that most mesocosms still comprised sexual lineages. Consequently, we did not find any difference between homogeneous and heterogeneous mesocosms in the residual persistence of sexuals (number of mesocosms without males after six generations: 4 out of 30 homogeneous ones vs. 1 out of 10 heterogeneous ones, P > 0.99).
Figure 3.
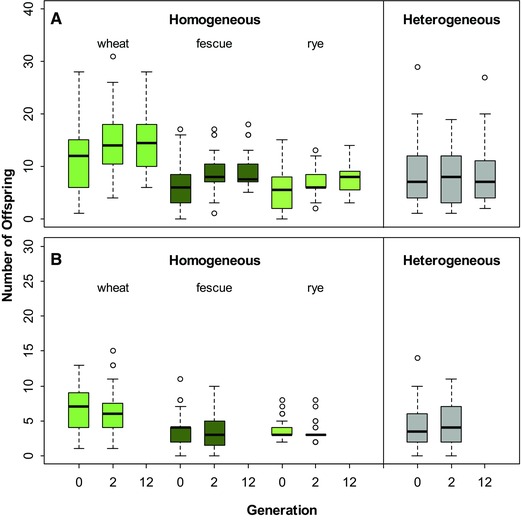
Fitness (number of offspring produced in 24 days) of asexual A. rufus (A) and of sexual A. elegans females (B) prior to the experiment (Generation 0) and after approximately two and 12 (for A. rufus) generations of adaptation to the new conditions.
Asexual A. rufus and sexual A. elegans used in the first mesocosm experiment were collected from a meadow comprising the grass species Arrhenatherum elatius, Dactylis glomerata, and Bromus hordeaceus and without any of the three species used in the mesocosm experiment. We therefore expected to observe some adaptation to the new grass species in the mesocosms. We tested for evidence of adaptation by measuring reproductive output of individual females before the start of the experiment and after two generations in the mesocosms. For asexual A. rufus in homogeneous mesocosms, we found, as expected, that the average number of offspring females produced within 24 days increased after ca. two generations of selection (grass species: df = 2, P < 0.0001; generation: df = 2, P < 0.0001; Fig. 3A). The reproductive output increase after two generations was accompanied by a significant decrease in variation among females in offspring number on wheat and fescue (one‐tailed Ansari–Bradley tests, AB = 1478.5, P = 0.02 and AB = 1451, P = 0.05, respectively) as expected if the frequency of genotypes poorly performing on a given plant decreased as a consequence of directional selection. A significant variation decrease was not observed on rye (AB = 1749, P = 0.35). In contrast to the improved reproductive output after two generations of selection in homogeneous habitats, we found no evidence for adaptation in the heterogeneous mesocosms (generation effect: df = 2, P = 0.98, Fig. 3A) as expected if adaptation to the new conditions was hampered by the presence of multiple plants (i.e., spatially fluctuating selection).
Sexual reproduction is expected to facilitate adaptation to new biotic and abiotic conditions. However, in contrast to asexual A. rufus, we found no evidence for adaptation to mesocosm conditions in sexual A. elegans. The reproductive output of sexual females did not improve significantly after two generations in the mesocosms, neither for homogeneous (plant effect: df = 2, P < 0.0001; generation effect: df = 1, P = 0.166) or heterogeneous habitats (generation effect: df = 1, P = 0.293; Fig. 3B). No measurement of fitness was conducted past the second generation in A. elegans because we were unable to collect enough A. elegans females from the mesocosms at that point.
In summary, our results from the mesocosm experiments show that sexual lineages (A. elegans and A. rufus) are unable to persist when in competition with asexual A. rufus lineages in homogeneous habitats consisting of a single grass species and in heterogeneous habitats consisting of a mix of three species. However, although a mix of three grasses corresponds to a more complex habitat for thrips than a single grass species, a mix of three grasses may not be representative of natural populations, where typically more than three different grass species co‐occur within a field. Therefore, the most relevant insights into the effect of habitat heterogeneity on the success of sexual thrips stem from diversity variation in natural populations.
In the natural setting represented by 66 plots in 11 hay meadows, 29 different grass species were inventoried, with an average of 8.6 (range 2–13) species per plot. The fertilization and irrigation treatments applied to the plots significantly changed grass diversity (LMM with meadow location as random effect: P = 0.04; Fig. 4A). The treatments also influenced the proportion of sexual thrips in each plot (GLMM, χ2 = 26.62, df = 5, P = 0.00007; LMM: P = 0.0002; Fig. 4B). However, contrary to the predictions, the frequency of sexual thrips was negatively affected by treatments increasing grass diversity (i.e., the level of habitat heterogeneity). This effect was most obvious when directly using grass diversity independently of fertilization and irrigation treatments, with a significant negative effect of the grass species diversity on the proportion of sexuals (GLMM χ2 = 4.96, df = 1, P = 0.026; LMM: P = 0.011, see also Supporting Information). This negative correlation was also observed within standard plots only (R 2 = 0.46, P = 0.02), indicating it was not solely driven by the shifts in grass communities following the irrigation and fertilization treatments.
Figure 4.
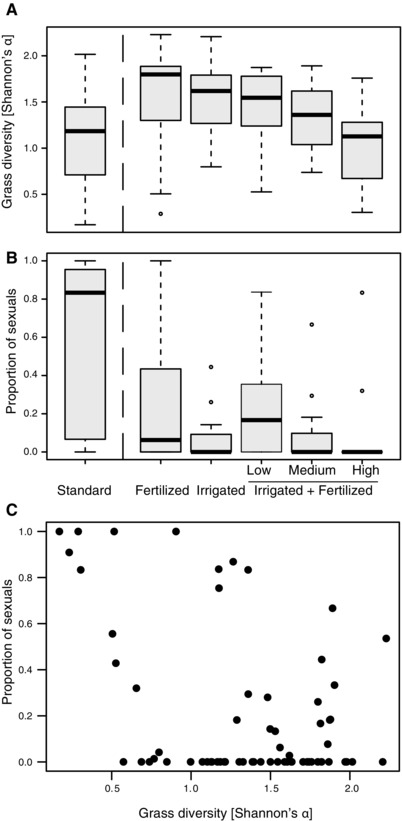
(A) Habitat heterogeneity (measured as grass diversity indices) among plots in experimental hay meadows exposed to fertilization and irrigation treatments. Combined irrigating and fertilizing treatments (irrigated + fertilized) were applied at three different intensity levels (low, intermediate, and high). (B) Proportion of individuals from sexual Aptinothrips species with respect to plot treatment. (C) Correlation between grass diversity and the proportion of sexual thrips independently of plot treatments.
Given the unexpected finding that the frequency of sexual thrips was negatively affected by increased grass diversities in the experimental plots, we examined the plots in which a high frequency of sexual thrips was maintained. Specifically, we examined whether plots with a high frequency of sexual thrips comprised specific grass communities, which would indicate that sexual thrips only occur in a specific type of niche. To this end, we grouped plots by their grass species composition in a dendrogram, using Bray–Curtis distances to quantify the differences between plots. Mapping the proportions of sexual thrips on this dendrogram revealed that plots with similar frequencies of sexual thrips were also characterized by similar grass communities (Pagel's ɑ = 0.55, P < 0.0001; Fig. 5A). Similarly, plots with high frequencies of sexual thrips tended to be clustered when the grass communities of the 66 plots were displayed along the first two axes obtained from a PCA (Fig. 5B).
Figure 5.

(A) Similarities of grass communities in the experimental plots displayed as a dendrogram. (B) Principal component analysis of the grass communities in the experimental plots. Plot labels are shaded proportionally to the frequency of sexual Aptinothrips species.
In combination, the grass community analyses support the idea that sexual thrips occur in a specific type of niche. As a first step to characterize this niche, we identified the grass species that were the most strongly correlated with sexual thrips abundances. Only the grass species Bromus erectus was consistently associated with sexual A. elegans (see Supplementary Methods and Results and Tables S2 and S3 in the Supporting Information), with a strong correlation between the coverage of B. erectus in the plots and the proportion of sexual thrips (R 2 = 0.58, P < 0.0001).
Discussion
Explaining the overwhelming success of sexual reproduction has been a challenge in evolutionary biology for decades. Recent theoretical approaches have identified plausible mechanisms that could provide benefits to sex and therefore help explain its maintenance. For some of these mechanisms, experimental evolution has provided a “proof of principle” that the predicted mechanisms can work in real organisms given the appropriate conditions (Colegrave 2002; Goddard et al. 2005; Cooper 2007; Morran et al. 2009; Becks and Agrawal 2010; Morran et al. 2011; Becks and Agrawal 2012). However, these conditions may not be realized in natural populations such that these approaches provide little insights into the maintenance of sex in nature. Indeed, it is impossible to know whether any benefit to sex detected under artificial conditions (that may include controlled migration rates, specific population densities or sizes) would outweigh its immediate costs expressed under natural conditions.
To fill this gap, we tested whether one of the classical conditions predicted to provide benefits to sex, habitat heterogeneity, can contribute to the maintenance of sex under natural conditions. By analyzing the frequency of sexual and asexual grassthrips in experimental hay meadows, we found that an increase of habitat heterogeneity (measured via grass diversity) results in a decrease of sexual grassthrips and favors asexual ones. The frequency of sexuals and grass diversity was also negatively correlated in unmanipulated grass communities. In seminatural mesocosm conditions, habitat heterogeneity did not affect the outcome of competition between sexual and asexual grassthrips; asexual grassthrips completely replaced their sexual relatives within 4–12 generations. In combination, these experiments show that the potential benefits that sex confers under habitat heterogeneity are negligible compared to its costs when sexual lineages compete with asexuals under natural or seminatural conditions.
Experimental evolution studies have also shown that sex can facilitate adaptation to new environments (Colegrave 2002; Goddard et al. 2005; Cooper 2007; Morran et al. 2009; Becks and Agrawal 2012). Our field and mesocosm experiments both involved a shift of environmental conditions. The treatments applied to the experimental hay meadows were initiated in spring 2010 and induced a gradual transition from the original to new plant communities (Andrey et al. 2014). Unexpectedly, asexual grassthrips were more successful than sexual ones under these changing conditions. The mesocosm habitats also represented new conditions for the thrips. Yet in both competition assays, the one involving sexual A. elegans and asexual A. rufus collected from the field, and the one involving sexual and asexual A. rufus from laboratory populations, asexuals completely replaced their sexual counterparts. Part of the success of asexuals in the mesocosm experiments could stem from a colonisation advantage at the beginning of the experiment when only 12 individuals were present. However most mesocosms still contained sexuals when the carrying capacity was reached (by generation four), indicating that a colonization advantage of asexuals is unlikely to fully explain the results. For asexual A. rufus collected in the field, we further observed an increase of average fitness in two of the three homogeneous mesocosms, and a decrease in fitness variation among females, as expected under adaptation following directional selection. Surprisingly however, we did not find similar patterns for sexual A. elegans. A possible explanation for this pattern is that the response to selection in sexuals is slow, perhaps because of epistasis effects among loci involved in adaptation to different grasses, while genotype sorting in asexuals may happen more rapidly. However, the most likely explanation for these unexpected findings is that natural populations of asexual Aptinothrips are characterized by more standing genetic variation for adaptation to different host plants and that the sexual species A. elegans is specialized on one or a few grass species.
The same explanation could account for the unexpected success of asexual thrips in experimental plots with high grass diversity. As asexual thrips would be able to efficiently exploit many different species of grasses, and sexual thrips only few of them, the niche space available to asexual thrips would increase as grass diversities increase. Additional studies are required to infer the fundamental niche of the different sexual and asexual grassthrips lineages and their level of specialism vs generalism with respect to host plant use.
The proposition that increased genetic variation improves a lineage's ability to exploit alternative niches is at the root of Tangled Bank related theories (reviewed in Bell 1982; Song et al. 2011). However, in contrast to our findings, Tangled Bank theories assume that sexual species, rather than asexual ones, would feature extensive genetic variation for exploiting alternative niches. Thus, these theories in fact propose that there is an advantage for genetically based diversity in niche usage; they do not directly show an advantage for sex. In systems where such diversity is larger in asexuals than sexuals, as appears to be the case in grassthrips, Tangled Bank mechanisms predict an advantage for asexual species. Sexuals would then only persist in specific niches. Such niche‐specific persistence might however be transient, pending colonization of that niche by a new set of asexual clones.
What mechanisms could generate genetic variation for niche usage in asexuals? Asexual A. rufus populations are distributed throughout the world (van der Kooi and Schwander 2014; Fontcuberta et al. 2016), and the consequently enormous population sizes may be sufficient for mutation, selection, and migration to generate clones adapted to different grass species. Furthermore, asexual A. rufus populations consist of a diverse mixture of clones, including clones that independently derived from sexual ancestors (Fontcuberta et al. 2016). Such high levels of clonal diversity may allow different clones to be selected on alternative plant species. More generally, given that populations of asexual “species” are often a mixture of different and independently derived clones (e.g., Neiman et al. 2005; Janko et al. 2008; Ros et al. 2008; Rabeling et al. 2011), the scope for “genetic diversity” based advantages of sex may be more limited in natural populations than typically assumed (e.g., Judson 1997; Ladle et al. 1993; Lively 2010; Neiman and Schwander 2011).
In summary, we maintained competing sexual and asexual thrips in spatially (and temporally) heterogeneous habitat conditions, conditions that have been shown to provide benefits to sex. In spite of this, sexual species decreased in frequency and, in the mesocosm experiments, went extinct. These findings indicate that habitat heterogeneity is unlikely to maintain sex in natural populations given the direct costs sex generates. The question, of course, remains; whether sex in natural grassthrips populations is on the verge of extinction or whether sexual lineages are maintained by parasite‐imposed selection or other factors unrelated to variation in grass composition over space or time. Answering these questions is a task for future studies.
Associate Editor: J. Engelstaedter
Handling Editor: M. Servedio
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Figure S1. Locations of experimental hay meadows.
Figure S2. Number of thrips of each species in the different treatments.
Figure S3. Distribution of F‐values in the bootstrap LMM analyses.
Figure S4. Correlation between grass diversity and the proportion of sexual thrips separately for each hay meadow.
Table S1. Details on the treatments applied to the hay meadows used in the field experiment.
Table S2. Contribution of the different inventoried grass species to the structuring of plant communities.
Table S3. Contribution of the different inventoried grass species to the clustering of plant communities
ACKNOWLEDGMENTS
We thank Bärbel Koch for her help with the botanical inventories and Aline Andrey and Stéphane Mettaz for managing the experimental hay meadows. We also thank Maurine Neiman, Denis Roze, Jan Engelstaedter, and two anonymous reviewers for discussions and comments on the manuscript, and Daniel L. Jeffries for proofreading. This study was supported by Swiss FNS grants PP00P3_139013 to T.S. and 31003A_125398 and 31003A_149656 to R.A.
DATA ARCHIVING
The doi for our data is 10.5061/dryad.h2m12.
LITERATURE CITED
- Agrawal, A. F. 2006. Evolution of sex: why do organisms shuffle their genotypes? Curr. Biol. 16:R696–R704. [DOI] [PubMed] [Google Scholar]
- Agrawal, A. F. . 2009. Spatial heterogeneity and the evolution of sex in diploids. Am. Nat. 174:S54–S70. [DOI] [PubMed] [Google Scholar]
- Andrey, A. , Humbert J.‐Y., Pernollet C., and Arlettaz R.. 2014. Experimental evidence for the immediate impact of fertilization and irrigation upon the plant and invertebrate communities of mountain grasslands. Ecol. Evol. 4:2610–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonovics, J. , and Ellstrand N. C.. 1984. Experimental studies of the evolutionary significance of sexual reproduction. I. A test of the frequency‐dependent selection hypothesis. Evolution 38:103–115. [DOI] [PubMed] [Google Scholar]
- Barton, N. H. 1995. A general model for the evolution of recombination. Genet. Res. 65:123–144. [DOI] [PubMed] [Google Scholar]
- Bates, D. , Maechler M., Bolker B., Walker S.. 2015. lme4: linear mixed‐effects models using Eigen and S4. R package version 11–9. Available at https://CRAN.R‐project.org/package=lme4. [Google Scholar]
- Becks, L. , and Agrawal A. F.. 2010. Higher rates of sex evolve in spatially heterogeneous environments. Nature 468:89–92. [DOI] [PubMed] [Google Scholar]
- Becks, L. . 2012. The evolution of sex is favoured during adaptation to new environments. PLoS Biol. 10:e1001317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell, G. 1982. The masterpiece of nature. The evolution and genetics of sexuality. California Univ. Press, Berkeley. [Google Scholar]
- Case, T. J. , and Taper M. L.. 1986. On the coexistence and coevolution of asexual and sexual competitors. Evolution 40:366–387. [DOI] [PubMed] [Google Scholar]
- Charlesworth, B. 1976. Recombination modification in a fluctuating environment. Genetics 83:181–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colegrave, N. 2002. Sex releases the speed limit on evolution. Nature 420:664–666. [DOI] [PubMed] [Google Scholar]
- Cooper, T. F. 2007. Recombination speeds adaptation by reducing competition between beneficial mutations in populations of Escherichia coli . Plos Biol. 5:1899–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doncaster, C. P. , Pound G. E., and Cox S. J.. 2000. The ecological cost of sex. Nature 404:281–285. [DOI] [PubMed] [Google Scholar]
- Ellstrand, N. C. , and Antonovics J.. 1985. Experimental studies of the evolutionary significance of sexual reproduction II. A test of the density‐dependent selection hypthesis. Evolution 39:657–666. [DOI] [PubMed] [Google Scholar]
- Fontcuberta, A. , Dumas Z., and Schwander T.. 2016. Extreme genetic diversity in asexual grassthrips populations. J. Evol. Biol. 29:887–899. [DOI] [PubMed] [Google Scholar]
- Gandon, S. , and Otto S. P.. 2007. The evolution of sex and recombination in response to abiotic or coevolutionary fluctuations in epistasis. Genetics 175:1835–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiselin, M. T. 1974. The economy of nature and the evolution of sex. California Univ. Press, Berkley. [Google Scholar]
- Goddard, M. R. , Charles H., Godfray J., and Burt A.. 2005. Sex increases the efficacy of natural selection in experimental yeast populations. Nature 434:636–640. [DOI] [PubMed] [Google Scholar]
- Hamilton, W. D. 1967. Extraordinary sex ratios. Science 156:477–488. [DOI] [PubMed] [Google Scholar]
- Jaenike, J. 1990. Host specialization in phytophagous insects. Ann. Rev. Ecol. Evol. Syst. 21:243–273. [Google Scholar]
- Janko, K. , Drozd P., Flegr J., and Pannell J. R.. 2008. Clonal turnover versus clonal decay: a null model for observed patterns of asexual longevity, diversity and distribution. Evolution 62:1264–1270. [DOI] [PubMed] [Google Scholar]
- Judson, O. 1997. A model of asexuality and clonal diversity: cloning the red queen. J. Theoret. Biol. 186:33–40. [Google Scholar]
- Ladle, R. J. , Johnstone R. A., and Judson O. P.. 1993. Coevolutionary dynamics of sex in a metapopulation: escaping the Red Queen. Proc. R Soc. Lond B Biol. Sci. 253:155–160. [Google Scholar]
- Legendre, P. , and Gallagher E. D.. 2001. Ecologically meaningful transformations for ordination of species data. Oecologia 129:271–280. [DOI] [PubMed] [Google Scholar]
- Lehtonen, J. , Jennions M. D., and Kokko H.. 2012. The many costs of sex. Trends Ecol. Evol. 27:172–178. [DOI] [PubMed] [Google Scholar]
- Lenormand, T. 2002. Gene flow and the limits to natural selection. Trends Ecol. Evol. 17:183–189. [Google Scholar]
- Lenormand, T. , and Otto S. P.. 2000. The evolution of recombination in a heterogeneous environment. Genetics 156:423–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, T. 1973. Thrips. Their biology, ecology and economic importance. Academic Press, London. [Google Scholar]
- Lively, C. M. 2010. A review of Red Queen models for the persistence of obligate sexual reproduction. J. Heredity 101(Suppl 1):S13–S20. [DOI] [PubMed] [Google Scholar]
- Maynard Smith, J. 1971. What use is sex? J. Theoret. Biol. 30:319–335. [DOI] [PubMed] [Google Scholar]
- Maynard Smith, J. . 1978. The evolution of sex. Cambridge Univ. Press, New York. [Google Scholar]
- McDonald, M. J. , Rice D. P., and Desai M. M.. 2016. Sex speeds adaptation by altering the dynamics of molecular evolution. Nature 531:233–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morran, L. T. , Parmenter M. D., and Phillips P. C.. 2009. Mutation load and rapid adaptation favour outcrossing over self‐fertilization. Nature 462:350–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morran, L. T. , Schmidt O. G., Gelarden I. A., Parrish R. C. II, and Lively C. M.. 2011. Running with the Red Queen: host‐parasite coevolution selects for biparental sex. Science 333:216–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mound, L. A. 1991. Patterns of sexuality in Thysanoptera Pp. 2–14 in Cameron E. A., Teulon D. A., McCormick L. H. and Holb T. E., eds. The 1991 conference on Thrips (Thysanoptera): Insect and disease considerations in sugar maple management. U.S.D.A. Forest Service, General Technical Report, Pennsylvania State University. [Google Scholar]
- Neiman, M. , Jokela J., and Lively C. M.. 2005. Variation in asexual lineage age in Potamopyrgus antipodarum, a New Zealand snail. Evolution 59:1945–1952. [PubMed] [Google Scholar]
- Neiman, M. , and Schwander T.. 2011. Using parthenogenetic lineages to identify advantages of sex. Evol. Biol. 38:115–123. [Google Scholar]
- Oksanen, J. , Blanchet F., Kindt R., Legendre P., O'Hara R., Simpson G., Solymos P., Stevens M., and Wagner H.. 2015. vegan: community ecology package. R package version 23‐0. Available at http://CRAN.R‐project.org/package=vegan. [Google Scholar]
- Otto, S. P. 2009. The evolutionary enigma of sex. Am. Nat. 174:S1–S14. [DOI] [PubMed] [Google Scholar]
- Palmer, J. M. 1975. The grass‐living genus Aptinothrips Haliday (Thysanoptera: Thripidae). J. Entomol. 44:175–188. [Google Scholar]
- Peter, M. , Gigon A., Edwards P. J., and Luescher A.. 2009. Changes over three decades in the floristic composition of nutrient‐poor grasslands in the Swiss Alps. Biodiv. Conserv. 18:547–567. [Google Scholar]
- Peters, A. D. , and Lively C. M.. 1999. The red queen and fluctuating epistasis: a population genetic analysis of antagonistic coevolution. Am. Nat. 154:393–405. [DOI] [PubMed] [Google Scholar]
- Pylkov, K. V. , Zhivotovsky L. A., and Feldman M. W.. 1998. Migration versus mutation in the evolution of recombination under multilocus selection. Genet. Res. 71:247–256. [DOI] [PubMed] [Google Scholar]
- Rabeling, C. , Gonzales O., Schultz T. R., Bacci M. Jr., Garcia M. V., Verhaagh M., Ishak H. D., and Mueller U. G.. 2011. Cryptic sexual populations account for genetic diversity and ecological success in a widely distributed, asexual fungus‐growing ant. Proc. Natl. Acad. Sci. USA 108:12366–12371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revell, L. J. 2012. Phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3:217–223. [Google Scholar]
- Ros, V. I. , Breeuwer J. A., and Menken S. B.. 2008. Origins of asexuality in Bryobia mites (Acari: Tetranychidae). BMC Evol. Biol. 8:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, Y. , Drossel B., and Scheu S.. 2011. Tangled Bank dismissed too early. Oikos 120:1601–1607. [Google Scholar]
- van der Kooi, C.J. , and Schwander T.. 2014. Evolution of asexuality via different mechanisms in grass thrips (Thysanoptera: Aptinothrips). Evolution 68:1883–1893. [DOI] [PubMed] [Google Scholar]
- Weeks, A. R. , and Hoffmann A. A.. 1998. Intense selection of mite clones in a heterogeneous environment. Evolution 52:1325–1333. [DOI] [PubMed] [Google Scholar]
- Weeks, A. R. . 2008. Frequency‐dependent selection maintains clonal diversity in an asexual organism. Proc. Natl. Acad. Sci. USA 105:17872–17877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, G. C. 1975. Sex and evolution. Princeton Univ. Press, Princeton, NJ. [Google Scholar]
- Young, J. P. W. 1981. Sib competition can favour sex in two ways. J. Theoret. Biol. 88:755–756. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Figure S1. Locations of experimental hay meadows.
Figure S2. Number of thrips of each species in the different treatments.
Figure S3. Distribution of F‐values in the bootstrap LMM analyses.
Figure S4. Correlation between grass diversity and the proportion of sexual thrips separately for each hay meadow.
Table S1. Details on the treatments applied to the hay meadows used in the field experiment.
Table S2. Contribution of the different inventoried grass species to the structuring of plant communities.
Table S3. Contribution of the different inventoried grass species to the clustering of plant communities


