Abstract
Multiple myeloma (MM) patient frailty has been delineated primarily by age and ECOG performance score (PS) and recently by the IMWG frailty score based on functional status [Activity of Daily Living (ADL) and Instrumental‐ADL scores], comorbidities [Charlson‐comorbidity‐index (CCI)] and age. It was hypothesized that N‐terminal natriuretic peptide type B (NT‐proBNP) might be both a more convenient measure of frailty and a predictor of overall survival (OS). Three‐hundred and fifty‐one consecutive symptomatic MM patients who were seen at Mayo Clinic within 30 days of diagnosis and who had blood stored were eligible. Data from the first visit was abstracted and used to calculate an ADL, CCI, and measure the NT‐proBNP level. The best cutoff of NT‐proBNP predicting OS was 300 ng/L. Variables predictive for OS were ECOG‐PS, age, CCI, ADL, ISS, revised‐ISS, and NT‐proBNP. On multivariate analysis age ≥70, PS ≥2, and NT‐proBNP ≥300 were independent predictors of survival. Patients were assigned a score of 1 for each of these variables, creating stages I–IV with scores of 0–3 points, respectively. The median OS from diagnosis was not reached, 58, 28, and 18 months (P < 0.0001), respectively. This frailty risk schema was independent of initial therapy and the revised‐ISS. NT‐proBNP is a useful predictor of survival independent of age and PS. It is a widely available biomarker that could be added to the panel of laboratory tests of newly diagnosed MM patients and serve as a simple and objective tool of determining frailty in clinical practice. Am. J. Hematol. 91:1129–1134, 2016. © 2016 Wiley Periodicals, Inc.
Introduction
Multiple myeloma (MM) is the second most common hematologic malignancy, with a higher incidence in elderly subjects 1, 2, 3. The introduction of immunomodulatory agents and proteasome inhibitors has improved the survival of patients with MM, including elderly subjects 4, 5, 6. However, there is a subgroup of frail subjects, most of whom are elderly, who are susceptible to side effects of chemotherapy and are often unable to tolerate full dose treatment 7, 8. The well‐known biologic and genetic prognostic factors, as well as age per se, are insufficient to explain this difference 5, 9, 10, 11. The International Myeloma Working Group (IMWG) showed that a frailty score that combined age, functional status, and comorbidities predicted survival and toxicity, and thus useful to determine the tolerability of treatment. This frailty profile was associated with increased risk of death, progression, non‐hematologic adverse events and treatment discontinuation 12. The determination of frailty adopted by Palumbo et al. consists of the Katz Activity of Daily Living (ADL) 13, the Lawton Instrumental Activity of Daily Living (IADL) 14 and the Charlson Comorbidity Index (CCI) 15, 16. These authors showed that patients' functional and health status have prognostic importance similar to that of myeloma‐related risk factors, such as the International Staging System (ISS) 17 and chromosomal abnormalities 18, 19, 20. In clinical practice, age, ECOG‐PS, and comorbidities are widely used by clinicians to assess vulnerability and, consequently, to empirically tailor therapy for patients with MM, but it is a challenge in a busy clinical practice to incorporate all of the frailty assessments proposed by Palumbo 12.
Brain natriuretic peptide (BNP) and the N‐amino terminal fragment of the prohormone BNP (NT‐proBNP) 21 are released predominantly from the ventricular myocardium in response to increased ventricular wall stress 22. They are measures of ventricular dysfunction and have a predictive utility for cardiovascular events and mortality 23, 24, 25, but because they are cleared by the kidney, and thus influenced by the glomerular filtration rate 26, 27, 28, thereby capturing the two most common organ systems that contribute to a patients' frailty. Moreover, the prognostic value of NT‐proBNP has been shown to be independent of traditional cardiovascular risk factors, prevalent cardiovascular disease, left ventricular dysfunction, and renal function 29.
Using this information, we tested the prognostic role of NT‐proBNP in the context of other host and tumor clinical features in an unselected population of MM patients prospectively evaluated at Mayo Clinic, Rochester, MN.
Methods
Patient population and study design
The study included 351 patients who were seen at the Mayo Clinic, Rochester, MN within 30 days of their multiple myeloma diagnosis from 1/1/2007 to 12/31/2011. Patients, who had biopsy proven organ involvement with light‐chain (AL) amyloidosis, at the time of NT‐proBNP sample collections were excluded from the current analysis. All patients that during the follow‐up had a subsequent biopsy proven diagnosis of AL amyloidosis were also excluded. Data were extracted from prospectively maintained databases and from review of medical records. Follow‐up information was collected prospectively and entered at the time of each visit. For patients followed up at other institutions, annual follow‐up letters were sent to patients to inquire about their disease status. All patients had consented to the use of their medical records. The study was conducted in accordance with the institutional guidelines with approval of the institutional review board (IRB) and in accordance with the principles of the Declaration of Helsinki.
Assessment
As part of the Mayo Clinic admission, all patients are required to complete a questionnaire about their past medical history, symptoms, and ADLs. Data from their first visit was abstracted and used to calculate the ADL score. The ADL score was adopted to assess self‐care activities and independence status. It was composed of questions regarding independence in bathing, dressing, toileting, transferring, and feeding. The presence of incontinence was not prospectively collect with the questionnaires. Therefore, we retrospectively abstracted from the clinical report this information. Each of these tasks had a score of 1 (best score was 6). The CCI was calculated after a complete medical history of all patients prior and at the time of the diagnosis. This calculation was based on the original CCI scale proposed by Charlson et al. in 1987 15, 16. Other prognostic systems considered were the Eastern Cooperative Oncology Group Performance status (ECOG‐PS) 30 ISS 17, chromosomal abnormalities t(4:14), t(11:14), t(14:16), del13, del17p, trisomies 31, 32, and the more recently defined R‐ISS which is defined as: (a) R‐ISS I, no high‐risk chromosome abnormalities [del(17p) and/or t(4;14) and/or t(14;16)], and normal LDH levels); (b) R‐ISS III: including ISS stage III and high‐risk chromosome abnormalities or high LDH level; and (c) R‐ISS II: including all the other possible combinations] 33.
NT‐proBNP was measured on sera frozen at −20°C under a bio‐bank IRB protocol. No indication of degradation of NT‐proBNP during long term storage was previously reported 34. The NT‐proBNP assay was run on the E170 Modular analyzer (Roche Diagnostics, Penzberg, Germany). The reference limits (97.5 percentiles of healthy subjects) in men and women are 87 and 150 ng/L, respectively, in subjects less than 50 years of age and 220 and 331.5 ng/L, respectively, in individuals more than 50 years of age (data from Roche from 712 normal subjects). Precision with this assay is excellent, but substantial biologic variability exists, especially at higher values 35. In particular, for subjects with more than 75 years of age, an NT‐proBNP values less than 300 ng/L have a 99% negative predictive value for excluding acute congestive heart failure. A cutoff of 1,200 ng/L for patients with an eGFR less than 60 mL/min yields a diagnostic sensitivity and specificity of 89% and 72% for acute congestive heart failure. Finally, a cutoff of 1,800 ng/L has been suggested in adults over 75 years of age in absence of renal failure.
Statistical analysis
Continuous data were described with median and range. Fisher's exact test was used to test differences in nominal variables. Differences in continuous variables between groups were compared using Wilcoxon signed‐rank test and correlations between them were compared using Spearman's rho. The best cutoff predicting survival of NT‐proBNP was defined according to the maximum likelihood approach. Overall survival (OS) was calculated from the time of beginning treatment until the date of death for any cause or the date patients was last known to be alive. Kaplan–Meier analysis was used for analyzing overall survival, and the differences between the groups were tested for statistical significance by means of the log‐rank test. Multivariate analysis of factors affecting survival was carried out using the Cox proportional hazards models. All analyses were performed using JMP 10.0 (SAS, Cary, NC).
Results
Baseline characteristics
The median age of the 351 patients was 65 years (interquartile range, IQR: 57–71). Table 1 details the clinical features and the baseline of NT‐proBNP. The cut‐point of NT‐proBNP levels of 300 ng/L was obtained with a maximum likelihood approach. This cutoff was able to distinguish two groups with a significant difference in survival (Fig. 1). Median NT‐proBNP in the overall cohort was 109 ng/L (IQR: 30–375 ng/L). The NT‐proBNP low and high populations were significantly different by almost every clinical feature with the exceptions of sex, serum calcium, monoclonal component concentration, and likelihood of being clonal kappa or lambda and for having high‐risk FISH (Table 1).
Table 1.
Baseline patient characteristics [median (interquartile range) – number (%)].
| Variable | All patients |
NT‐proBNP <300 ng/L
250 pts |
NT‐proBNP ≥300 ng/L
101 pts |
P |
|---|---|---|---|---|
| Age (years) | 65 (57–71) | 62 (56–70) | 70 (61–77) | <0.0001 |
| Age ≥70 | 114 (33) | 63 (25) | 51 (50) | 0.072 |
| Sex: male | 109 (56) | 146 (58) | 53 (51) | 0.158 |
| ECOG‐PS | ||||
| 0 | 155 (44) | 126 (50) | 29 (29) | 0.0003 |
| 1 | 129 (36) | 93 (37) | 36 (36) | 0.9026 |
| ≥2 | 66 (19) | 31 (12) | 35 (35) | <0.0001 |
| CCI ≥2 | 104 (29) | 57 (22) | 47 (46) | <0.0001 |
| ADL | ||||
|
≥1 ≥4 |
95 (27) 10 (3) |
196 (78) 4 (1) |
60 (59) 6 (6) |
0.0005 0.036 |
| Hemoglobin, g/dL | 10.9 (9.6–12.6) | 11.6 (10.1–13.4) | 9.7 (8.8–10.8) | <0.0001 |
| Calcium, mg/dL | 9.7 (9.2–10.2) | 9.7 (9.3–10.2) | 9.6 (9–10.2) | 0.4947 |
| Creatinine, mg/dL | 1 (0.8–1.3) | 0.9 (0.8–1.2) | 1.35 (0.9–2.62) | <0.0001 |
| Creatinine >2 mg/dL | 46 (13) | 13 (4) | 33 (9) | <0.0001 |
| eGFR mL/min | 72 (52–89) | 76 (60–91) | 52 (19–79) | <0.0001 |
| eGFR <30 mL/min | 43 (12) | 10 (3) | 33 (10) | <0.0001 |
| Albumin, g/dL | 3.5 (3.2–3.8) | 3.6 (3.3–3.8) | 3.4 (3.1–3.6) | 0.0002 |
| B2M, mg/dL | 3.95 (2.7–6.5) | 3.3 (2.5–4.8) | 7.56 (4.4–11.6) | <0.0001 |
| LDH ≥222 IU/L | 45 (15) | 25 (11) | 20 (24) | 0.01 |
| NT‐proBNP, ng/L | 109 (30–375) | 56 (0–123) | 864 (459–2238) | <0.0001 |
| BMPC, % | 40 (20–65) | 35 (20–55) | 52 (40–75) | <0.0001 |
| M‐comp, g/dL | 3 (1.8–4.1) | 2.9 (1.7–4.1) | 3.3 (1.9–4.4) | 0.3943 |
| Kappa: Lambda | 215 (62):121 (35) | 155 (63):85 (34) | 60 (61):36 (37) | 0.6239 |
| High risk MM FISH | 52 (19) | 32 (11) | 20 (7) | 0.1603 |
| ISS I | 84 (27) | 76 (33) | 8 (9) | <0.0001 |
| ISS 2 | 125 (40) | 105 (47) | 20 (23) | 0.0002 |
| ISS 3 | 101 (33) | 43 (19) | 58 (67) | <0.0001 |
| R‐ISS I | 46 (17) | 42 (22) | 4 (5) | <0.0001 |
| R‐ISS II | 177 (66) | 133 (69) | 44 (59) | <0.0001 |
| R‐ISS III | 43 (16) | 17 (8) | 26 (35) | <0.0001 |
|
Therapy, first‐line: Len‐based Prot‐inh‐based |
204 (63) 73 (22) |
159 (68) 41 (18) |
45 (49) 32 (35) |
0.0014 0.0011 |
| ASCT, yes | 138 (39) | 105 (42) | 33 (33) | 0.05 |
ECOG‐PS, Eastern Cooperative Oncology Group—Performance Status; CCI, Charlson comorbidity index; ADL, activity of daily leaving; eGFR, estimated glomerular filtration rate; B2M, beta‐2‐microglobulin; LDH, lactate dehydrogenase; NT‐proBNP, N‐amino terminal fragment of the B‐type brain natriuretic peptide; BMPC, estimated bone marrow plasma cells infiltrate; M‐comp, component where monoclonal protein migrates; FISH, fluorescent in situ hybridization; High risk MM by FISH, presence of del(17p) and/or translocation t(4,14) and/or translocation t(14,16); R‐ISS, revised‐international staging system, R‐ISS I, ISS stage I and standard‐risk MM by FISH and LDH <222 IU/L; R‐ISS II, not R‐ISS I or III; R‐ISS III, ISS stage III and either high‐risk MM by FISH or LDH ≥222 IU/L; Len‐based, lenalidomide containing regimen; Prot‐inh‐based, proteasome inhibitor containing regimen, ASCT, autologous stem cell transplant.
Figure 1.
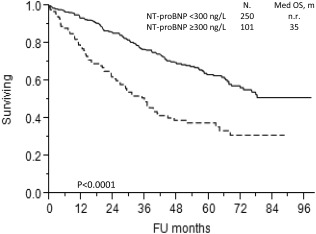
Kaplan–Meier curve for overall survival according to NT‐proBNP ≥300 ng/L (Continuous line: patients with NT‐proBNP <300 ng/L; dotted line: patients with NT‐proBNP ≥300 ng/L).
Ninety‐five (27%) patients had ADL ≥1 while CCI was ≥2 in 30%. The ADL tasks are listed in Supporting Information Table SI and the CCI score is listed in Supporting Information Table SII. The median (and IQR) NT‐proBNP based on co‐morbidities is shown in Supporting Information Table SII. As expected, patients with a history of myocardial infarction, congestive heart failure, atrial fibrillation, severe renal disease, or diabetes with organ damage had significantly elevated NT‐proBNP values, but patients with cerebrovascular disease, chronic pulmonary disease, peptic ulcer, mild liver disease, diabetes with no organ damage, or other cancers did not have higher levels. There was a trend toward higher levels among patients with pulmonary hypertension, peripheral artery disease, hypertension and connective tissue diseases (Supporting Information Tables SII and SIII).
Overall survival
The median overall survival (OS) of the entire cohort was 5.7 years at a median follow‐up of 5.4 years. On univariate analysis (Supporting Information Table SIV) the variables predicting survival were age [Relative risk (RR) 2.70 (1.97, 3.69)], ECOG‐PS [RR 3.45 (2.45, 4.81)], CCI [RR 1.96 (1.42, 2.68)] all with P < 0.0001, ADL [RR 1.64 (1.17, 2.27), P = 0.004], history of hypertension [RR 1.44 (1.05–1.96), P = 0.021], LDH ≥222 U/L [RR 2.29 (1.50, 3.40), P = 0.0002], NT‐proBNP ≥300 ng/L [RR 2.36 (1.71, 3.23)], and the ISS stages.
Despite relationships between NT‐proBNP and other clinical variables, NT‐proBNP retained independence as a prognostic marker for OS with each of these same variables (Supporting Information Table SV). In particular, all the proposed models were corrected by age and the prognostic ability of NT‐proBNP outperformed CCI, ADL, traditional ISS, and eGFR. When further multivariate modeling was performed, factors retaining significance were age, revised ISS, and performance status (Table 2a and b). Interestingly, there was an interaction between performance status and revised ISS driving NT‐proBNP off from the model (Table 2c). In contrast, when the traditional ISS and high‐risk FISH were used in the model as independent variables along with NT‐proBNP, ECOG‐PS, and age, ISS was not significant, but NT‐proBNP (Table 2d) as well as LDH (Table 2e) remained prognostic.
Table 2.
Proportional hazards predicting for overall survival
| a. Multivariate model | ||
| Variable | RR (CI 95%) | P |
| ECOG‐PS ≥2 | 2.60 (1.81, 3.70) | <0.0001 |
| Age ≥70 years | 2.17 (1.57, 2.99) | <0.0001 |
| NT‐proBNP ≥300 ng/L | 1.62 (1.15, 2.28) | 0.006 |
| b. Multivariate model | ||
| Variable | RR (CI 95%) | P |
| Revised ISS | 2.51 (1.15, 2.19) | <0.0001 |
| Age ≥70 years | 2.36 (1.68, 3.30) | <0.0001 |
| NT‐proBNP ≥300 ng/L | 1.67 (1.51, 2.39) | 0.007 |
| c. Multivariate model | ||
| Variable | RR (CI 95%) | P |
| ECOG‐PS ≥2 | 2.46 (1.66, 3.59) | <0.0001 |
| Age ≥70 years | 2.12 (1.50, 2.99) | <0.0001 |
| Revised ISS | 1.46 (1.06, 2.01) | 0.02 |
| NT‐proBNP ≥300 ng/L | 1.42 (0.97, 2.06) | 0.07 |
| d. Multivariate model a | ||
| Variable | RR (CI 95%) | P |
| ECOG‐PS ≥2 | 2.33 (1.54, 3.49) | <0.0001 |
| Age ≥70 years | 2.34 (1.60, 3.40) | <0.0001 |
| High‐risk FISH | 1.88 (1.24, 2.78) | 0.003 |
| NT‐proBNP ≥300 ng/L | 1.72 (1.16, 2.55) | 0.007 |
| e. Multivariate model a | ||
| Variable | RR (CI 95%) | P |
| ECOG‐PS ≥2 | 2.34 (1.50, 3.62) | 0.0003 |
| High‐risk FISH | 2.28 (1.46, 3.48) | 0.0005 |
| Age ≥70 years | 2.21 (1.48, 3.31) | 0.0001 |
| LDH ≥222 | 1.78 (1.08, 2.84) | 0.03 |
| NT‐proBNP ≥300 ng/L | 1.59 (1.05, 2.40) | 0.03 |
| f. Multivariate model | ||
| Variable | RR (CI 95%) | P |
| ECOG‐PS‐Age‐NT‐proBNP (3a)b | 2.10 (1.77, 2.50) | <0.0001 |
| High‐risk FISH | 1.87 (1.23, 2.76) | 0.004 |
| g. Multivariate model | ||
| Variable | RR (CI 95%) | P |
| ECOG‐PS‐Age‐NT‐proBNP (3a)b | 1.95 (1.62, 2.34) | <0.0001 |
| Revised ISS | 1.40 (1.02, 1.92) | 0.04 |
Traditional ISS as ordinal variable or as a dichotomous variable of ISS = 3 is not significant.
ECOG‐PS‐Age‐NT‐proBNP (3a) refers to PS ≥2, age ≥70, and NT‐proBNP ≥300 ng/L each scoring 1 point for a score and creating a staging from 1 to 4.
Using this information, we devised a frailty risk system that incorporated age ≥70, ECOG‐PS ≥2, and NT‐proBNP ≥300 ng/L. This divided patients into four groups with median OS from diagnosis of not reached with no risk factors, 58 months with one risk factor, 28 months with two and 18 months (P < 0.0001) for those with all three risk factors (Fig. 2A), P < 0.0001. This frailty score was independent of high‐risk cytogenetics (Table 2f) and of the revised ISS (Table 2g). As shown in Fig. 2B, there was excellent discrimination of the curves based on the new frailty score among the 220 standard risk FISH patients, P < 0.0001. For the 52 patients with high risk FISH, our new frailty score also performed well (Fig. 2C), P < 0.0001.
Figure 2.
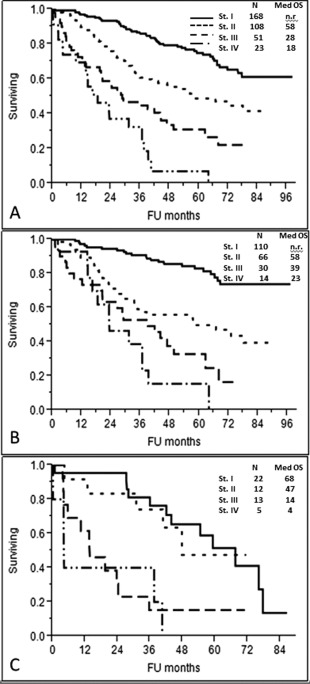
Kaplan–Meier curve for overall survival according to the frailty system based on Age ≥70, ECOG‐PS ≥2, and NT‐proBNP ≥300 ng/L (P < 0.0001) [different stages based on a score of 0 to 3 points, respectively]. A. Overall survival according to the frailty system in the entire cohort (P < 0.0001). B. Overall survival according to the frailty system in 220 patients with standard risk FISH (no high‐risk chromosome abnormalities), (P < 0.0001). C. Overall survival according to the frailty system in 52 patients with high risk FISH [presence of del17p and/or translocation t(4;14) and/or translocation t(14;16)], (P < 0.0001).
NT‐proBNP is prognostic independent of treatment
NT‐proBNP provided useful prognostic groupings in patients treated with lenalidomide‐based first line regimens (Supporting Information Fig. S1A) and also divided patients treated with a proteasome‐inhibitor as first line regimen (Supporting Information Fig. S1B), but this difference was not statistically significant, possibly because the limited number of patients. Amongst the 172 patients who did not undergo autologous stem cell transplant during the treatment course, NT‐proBNP ≥300 ng/L sharply discriminated two different groups (Supporting Information Fig. S1C).
This new frailty system based on age, ECOG‐PS and NT‐proBNP was able to discriminate different groups of patients amongst all the subjects treated with lenalidomide‐based and protasome‐inhibitor first line regimens (Supporting Information Figs. S1D and S1E, respectively) and also amongst patients who did not undergo autologous stem cell transplant (Supporting Information Fig. S1F). In all the different subgroups, Stage IV patients (Age ≥70, PS ≥2 and NT‐proBNP ≥300 ng/L) constituted only 6% of the patients.
Discussion
The present study demonstrates that NT‐proBNP is a good indicator of prognosis in unselected patients with multiple myeloma seen at a tertiary referral center. Interactions between variables, especially age, ECOG‐PS, NT‐proBNP, and the revised ISS were striking (Table 1). Any of these four variables when modeled with two other retained significance as predictors for OS, but when all four were included in a model, NT‐proBNP was forced out; however, when the revised ISS was split into its components, LDH and high‐risk FISH remained significant prognostic markers, NT‐proBNP retained significance, and the traditional ISS was forced out of the model. Hence the combination of age, ECOG‐PS, and NT‐proBNP resulted in a simple but robust frailty model that is independent of other prognostic factors such as FISH and LDH.
The purpose of this study was to determine whether NT‐proBNP could replace more complicated frailty scores despite an online tool (http://www.myelomafrailtyscorecalculator.net/). The identification of frail patients with an easily applicable, rapid and objective tool would be highly desirable to help identify specific tolerable but yet effective treatment approaches. Although direct comparisons cannot be made to the largest myeloma frailty study 12 our findings are compelling. As recommended by Palumbo et al, the combination of ADL, IADL and CCI scores resulted in a frailty score that identifies fit patients, “intermediate‐fit” patients and un‐fit ones. The ADL score includes one point each for not requiring assistance with any of the following: bathing, dressing, toileting, transferring, continence, and feeding (best score 6). The IADL score includes 1 point for each of the following abilities: use of telephone, shopping, food preparation, housekeeping, laundry, making transport, responsibility for own medications, and handle finances (best score 8). Finally, the CCI (worst score = 37) includes all the conditions reported in Supporting Information Table SII with different assigned weights. Using this very complex system of frailty, patients are deemed either fit, intermediately fit, or frail. Although these three categories were prognostic on univariate, the discriminatory ability between “fit” and “intermediate fitness'”when considered in the context of ISS, chromosome abnormalities, or therapy was not significant; only “frail” versus either intermediately fit or “fit” retained significance 12. Moreover, the authors did not include the revised ISS or performance status when evaluating their frailty/geriatric assessment model. We would, therefore, suggest that our NT‐proBNP risk system may perform as well as the geriatric assessment score and deserves further study. Due to the lack of the evaluation of the IADL score, the retrospective calculation of the CCI and of part of the ADL score, we were unable to do a direct comparison between the IMWG frailty index and our proposed frailty score.
The single blood test—NT‐proBNP—captures infirmity due to cardiac 23, 24, 25 and renal disease 26, 27, 28 but not due to either central or peripheral neurodegeneration; however, PS may compensate in part for these additional aspects of frailty. The NT‐proBNP threshold of 300 ng/L corresponds to the well‐established age‐independent cut‐off point for excluding acute heart failure 36 and provides information that is incremental to that obtained from established cardiovascular risk factors 37. In addition, natriuretic peptide levels may be elevated before the onset of clinically apparent cardiovascular disease in patients with light chain (AL) amyloidosis 38, 39. Subtle elevation of NT‐proBNP may predict existing cardiac amyloidosis which cannot be identified at echocardiography 38.
We demonstrated that the CCI ≥2 was forced out by NT‐proBNP in our modeling, but the simple ECOG‐PS was retained in every multivariate analysis constructed. An ECOG‐PS ≥2 had a risk for death that was higher than any single variable. In clinical practice, ECOG‐PS is widely used to assess fitness of cancer patients, and although more complex and time consuming scales are available 30, 40, this simple and reliable measure, in most cases, accurately provides the grade of frailty and disability of patients. It is intuitive that patients who are not ambulatory or up and about more than 50% of walking hours (i.e., an ECOG PS ≥2) would have deficits in ADL's and IADL's, which are used in more complex scoring system. According to the Palumbo's geriatric assessment scoring system, age greater than 80 alone assigns the “unfit” designation on a patient as would the presence of any one or two comorbidities, like diabetes with retinopathy, regardless of performance status.
In our dataset, age was also important in determining outcome, and it is due to the physiological changes of organ function 11. There is a definite relationship between age and natriuretic peptide levels which is likely consequent to age‐related changes in left ventricular compliance 41, as well as decreasing eGFR 42. In our cohort, the group of patients with elevated NT‐proBNP had a higher median age and a higher incidence of renal dysfunction. However, the prognostic role of NT‐proBNP was independent of age and outperformed eGFR. We chose the cutoff of age = 70 years because in clinical practice it is the cutoff for the consideration of autologous stem cell transplant as a treatment strategy in many centers. Currently, most European investigators choose a cutoff of 65 years in their clinical trial design, but this barrier can be increased to 70 years or even higher for fit patients 43. The ability of NT‐proBNP as prognostic marker was also confirmed in different models corrected by age with the different clinical variables. These data were in accordance with the assumption that biological age expressed by an individual performance status and, in our case by the combination of PS, NT‐proBNP and age, despite only chronological age, should be a major determinant for the treatment approach.
There are some limitations of this study. First, it did not include an IADL score. Second, it is a single center study than on average contained participants approximately 10 years younger than the IMWG study. Third, there was no validation cohort. Finally, patients were not uniformly treated, and one could argue that they received a particular therapy according to their fitness. However, we demonstrated that our system of NT‐proBNP, age, and performance status was an independent prognostic factor regardless of initial therapy. Also in balance, a potential advantage of the current study is that it included unselected newly diagnosed patients. All three studies included in the IMWG work had specific eligibility criteria that resulted in a lower percentage of patients with a CCI ≥2, only 17% as compared with our 30%.
In conclusion, we showed that patients with an NT‐proBNP ≥300 ng/L, an ECOG‐PS ≥2, and age ≥70 years should be considered as a “high risk” group. NT‐proBNP could be easily added to the traditional workup for newly diagnosed myeloma patients as a means of both assigning frailty risk and guide us to a more specific workup in the exclusion of a concomitant cardiac amyloidosis. The true challenge will be to understand how to manage frail patients at diagnosis. Exactly how therapy should be tailored among these frail patients is yet to be determined and beyond the scope of this work. In curative diseases like lymphoma, dose‐reductions are not recommended. In a disease that is not yet known to be curative, dose‐adjustment is likely the best approach 1. Regardless, further studies are needed to validate our findings regarding NT‐proBNP before it can be considered standard in multiple myeloma, but we would suggest that it will be important moving forward in these patient populations with a better stratification of the possible confounding factors.
Supporting information
Supporting Information
Acknowledgments
We express gratitude to Roche for supplying the reagent to run the assays.
Conflict of interest: Nothing to report
References
- 1. Palumbo A, Anderson K. Multiple myeloma. N Engl J Med 2011;364:1046–1060. [DOI] [PubMed] [Google Scholar]
- 2. Lenhoff S, Hjorth M, Westin J, et al. Impact of age on survival after intensive therapy for multiple myeloma: A population‐based study by the Nordic Myeloma Study Group. Br J Haematol 2006;133:389–396. [DOI] [PubMed] [Google Scholar]
- 3. Howlader NNA, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review, 1975‐2012. Bethesda, MD: National Cancer Institute; http://seer.cancer.gov/csr/1975_2012/, based on November 2014 SEER data submission, posted to the SEER web site, April 2015. [Google Scholar]
- 4. Pozzi S, Marcheselli L, Bari A, et al. Survival of multiple myeloma patients in the era of novel therapies confirms the improvement in patients younger than 75 years: A population‐based analysis. Br J Haematol 2013;163:40–46. [DOI] [PubMed] [Google Scholar]
- 5. Kumar SK, Dispenzieri A, Lacy MQ, et al. Continued improvement in survival in multiple myeloma: Changes in early mortality and outcomes in older patients. Leukemia 2013;28:1122–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood 2008;111:2516–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Palumbo A, Bringhen S, Ludwig H, et al. Personalized therapy in multiple myeloma according to patient age and vulnerability: A report of the European Myeloma Network (EMN). Blood 2011;118:4519–4529. [DOI] [PubMed] [Google Scholar]
- 8. Zweegman S, Palumbo A, Bringhen S, et al. Age and aging in blood disorders: Multiple myeloma. Haematologica 2014;99:1133–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chng WJ, Dispenzieri A, Chim CS, et al. IMWG consensus on risk stratification in multiple myeloma. Leukemia 2014;28:269–277. [DOI] [PubMed] [Google Scholar]
- 10. Siegel DS, Desikan KR, Mehta J, et al. Age is not a prognostic variable with autotransplants for multiple myeloma. Blood 1999;93:51–54. [PubMed] [Google Scholar]
- 11. Bringhen S, Mateos MV, Zweegman S, et al. Age and organ damage correlate with poor survival in myeloma patients: Meta‐analysis of 1435 individual patient data from 4 randomized trials. Haematologica 2013;98:980–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Palumbo A, Bringhen S, Mateos MV, et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: An International Myeloma Working Group report. Blood 2015;125:2068–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Katz S, Downs TD, Cash HR, et al. Progress in development of the index of ADL. Gerontologist 1970;10:20–30. [DOI] [PubMed] [Google Scholar]
- 14. Lawton MP, Brody EM. Assessment of older people: Self‐maintaining and instrumental activities of daily living. Gerontologist 1969;9:179–186. [PubMed] [Google Scholar]
- 15. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 16. Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol 1994;47:1245–1251. [DOI] [PubMed] [Google Scholar]
- 17. Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol 2005;23:3412–3420. [DOI] [PubMed] [Google Scholar]
- 18. Boyd KD, Ross FM, Chiecchio L, et al. A novel prognostic model in myeloma based on co‐segregating adverse FISH lesions and the ISS: Analysis of patients treated in the MRC Myeloma IX trial. Leukemia 2012;26:349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ross FM, Avet‐Loiseau H, Ameye G, et al. Report from the European Myeloma Network on interphase FISH in multiple myeloma and related disorders. Haematologica 2012;97:1272–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mikhael JR, Dingli D, Roy V, et al. Management of newly diagnosed symptomatic multiple myeloma: Updated Mayo Stratification of Myeloma and Risk‐Adapted Therapy (mSMART) consensus guidelines 2013. Mayo Clin Proc 2013;88:360–376. [DOI] [PubMed] [Google Scholar]
- 21. Hunt PJ, Yandle TG, Nicholls MG, et al. The amino‐terminal portion of pro‐brain natriuretic peptide (Pro‐BNP) circulates in human plasma. Biochem Biophys Res Commun 1995;214:1175–1183. [DOI] [PubMed] [Google Scholar]
- 22. Wiese S, Breyer T, Dragu A, et al. Gene expression of brain natriuretic peptide in isolated atrial and ventricular human myocardium: Influence of angiotensin II and diastolic fiber length. Circulation 2000;102:3074–3079. [DOI] [PubMed] [Google Scholar]
- 23. Costello‐Boerrigter LC, Boerrigter G, Redfield MM, et al. Amino‐terminal pro‐B‐type natriuretic peptide and B‐type natriuretic peptide in the general community: Determinants and detection of left ventricular dysfunction. J Am Coll Cardiol 2006;47:345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McKie PM, Rodeheffer RJ, Cataliotti A, et al. Amino‐terminal pro‐B‐type natriuretic peptide and B‐type natriuretic peptide: Biomarkers for mortality in a large community‐based cohort free of heart failure. Hypertension 2006;47:874–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McKie PM, Cataliotti A, Sangaralingham SJ, et al. Predictive utility of atrial, N‐terminal pro‐atrial, and N‐terminal pro‐B‐type natriuretic peptides for mortality and cardiovascular events in the general community: A 9‐year follow‐up study. Mayo Clin Proc 2011;86:1154–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khalifeh N, Haider D, Horl WH. Natriuretic peptides in chronic kidney disease and during renal replacement therapy: An update. J Investig Med 2009;57:33–39. [DOI] [PubMed] [Google Scholar]
- 27. Vickery S, Webb MC, Price CP, et al. Prognostic value of cardiac biomarkers for death in a non‐dialysis chronic kidney disease population. Nephrol Dial Transplant 2008;23:3546–3553. [DOI] [PubMed] [Google Scholar]
- 28. Palladini G, Foli A, Milani P, et al. Best use of cardiac biomarkers in patients with AL amyloidosis and renal failure. Am J Hematol 2012;87:465–471. [DOI] [PubMed] [Google Scholar]
- 29. Kistorp C, Raymond I, Pedersen F, et al. N‐terminal pro‐brain natriuretic peptide, C‐reactive protein, and urinary albumin levels as predictors of mortality and cardiovascular events in older adults. Jama 2005;293:1609–1616. [DOI] [PubMed] [Google Scholar]
- 30. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649–655. [PubMed] [Google Scholar]
- 31. Kumar S, Fonseca R, Ketterling RP, et al. Trisomies in multiple myeloma: Impact on survival in patients with high‐risk cytogenetics. Blood 2012;119:2100–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Avet‐Loiseau H, Durie BG, Cavo M, et al. Combining fluorescent in situ hybridization data with ISS staging improves risk assessment in myeloma: An International Myeloma Working Group collaborative project. Leukemia 2013;27:711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. A Palumbo, H Avet‐Loiseau, S Oliva, HM Lokhorst, H Goldschmidt, L Rosinol, et al. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. J Clin Oncol : official journal of the American Society of Clinical Oncology. 2015;33:2863–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Omland T, Sabatine MS, Jablonski KA, et al. Prognostic value of B‐Type natriuretic peptides in patients with stable coronary artery disease: The PEACE Trial. J Am Coll Cardiol 2007;50:205–214. [DOI] [PubMed] [Google Scholar]
- 35. Wu AH, Smith A, Wieczorek S, et al. Biological variation for N‐terminal pro‐ and B‐type natriuretic peptides and implications for therapeutic monitoring of patients with congestive heart failure. Am J Cardiol 2003;92:628–631. [DOI] [PubMed] [Google Scholar]
- 36. Januzzi JL, van Kimmenade R, Lainchbury J, et al. NT‐proBNP testing for diagnosis and short‐term prognosis in acute destabilized heart failure: An international pooled analysis of 1256 patients: The International Collaborative of NT‐proBNP Study. Eur Heart J 2006;27:330–337. [DOI] [PubMed] [Google Scholar]
- 37. Wang TJ, Larson MG, Levy D, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med 2004;350:655–663. [DOI] [PubMed] [Google Scholar]
- 38. Wechalekar AD, Gillmore JD, Wassef N, et al. Abnormal N‐terminal fragment of brain natriuretic peptide in patients with light chain amyloidosis without cardiac involvement at presentation is a risk factor for development of cardiac amyloidosis. Haematologica 2011;96:1079–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Palladini G, Campana C, Klersy C, et al. Serum N‐terminal pro‐brain natriuretic peptide is a sensitive marker of myocardial dysfunction in AL amyloidosis. Circulation 2003;107:2440–2445. [DOI] [PubMed] [Google Scholar]
- 40. Repetto L, Fratino L, Audisio RA, et al. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: An Italian Group for Geriatric Oncology Study. J Clin Oncol 2002;20:494–502. [DOI] [PubMed] [Google Scholar]
- 41. Arbab‐Zadeh A, Dijk E, Prasad A, et al. Effect of aging and physical activity on left ventricular compliance. Circulation 2004;110:1799–1805. [DOI] [PubMed] [Google Scholar]
- 42. McCullough PA, Duc P, Omland T, et al. B‐type natriuretic peptide and renal function in the diagnosis of heart failure: An analysis from the Breathing Not Properly Multinational Study. Am J Kidney Dis 2003;41:571–579. [DOI] [PubMed] [Google Scholar]
- 43. Chretien ML, Hebraud B, Cances‐Lauwers V, et al. Age is a prognostic factor even among patients with multiple myeloma younger than 66 years treated with high‐dose melphalan: The IFM experience on 2316 patients. Haematologica 2014;99:1236–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


