Abstract
Objective
This study was designed to investigate pharmacological interaction between magnesium laxative and antacid in patients receiving opioid analgesic.
Methods
Data obtained from a total of 441 eligible patients receiving opioid analgesic for the first time were retrospectively analysed. The incidence of constipation, defined as stool‐free interval of 3 days and more within the first week of opioid intake, was compared between patients who took laxative alone and those who received laxative in combination with antacid.
Key findings
Laxatives were prescribed in 74% of patients, among them 61% received antacids such as proton pump inhibitor and H2 receptor blocker. Magnesia was the most commonly used laxative (89%). Constipation occurred in 21% and 55% of patients with and without laxatives, respectively. Antacids reversed the laxative action of lower doses (<2000 mg/day) but not higher doses (>2000 mg/day) of magnesia without affecting the effects of other laxatives. Therefore, it is suggested that both acid‐dependent and acid‐independent mechanisms may operate in the laxative action of magnesia, in which the former may be involved in the action of lower doses of magnesia.
Conclusion
Care should be taken to avoid the unfavourable pharmacological interaction between low doses of magnesia and antacid.
Keywords: antacid, constipation, laxative, magnesia, opioid analgesic, pharmacological interaction
Introduction
Early palliative care, including pain relief, is essential in patients with cancer.1 Therefore, opioid analgesics are prescribed at an early stage of cancer, when patients complaint cancer‐related pain.2 However, opioid analgesics possess a number of adverse effects such as delirium, sedation and gastrointestinal dysfunction.3, 4 Among them, constipation is the most prevalent adverse reaction. It has been reported that the prevalence of constipation is up to 81% in patients receiving opioid analgesics.5 Therefore, laxatives are recommended to prescribe in patients with cancer receiving opioid analgesics for the prophylaxis of constipation. In our previous single‐institutional study,6 prophylactic medication with laxatives markedly reduces the incidence of constipation in patients with cancer who took opioid analgesics, in which the occurrence rates of constipation were 21% and 56% in patients with and without laxative, respectively. The most commonly prescribed laxative was magnesia (72%), followed by sennoside. Likewise, our multi‐institutional study consisting of 619 patients from 35 institutions in Japan (J‐RIGID) has demonstrated that prophylactic laxative significantly reduces the incidence of constipation (odds ratio, 0.432; 95% confidence interval (CI) 0.300 to 0.622; P < 0.001) in patients with cancer receiving opioid analgesics.7 Also, magnesia (88%) was the most common laxative as observed in our single‐institutional study.
It has been demonstrated that magnesia, an osmotic laxative, requires gastric acid to fulfil its action, in which the compound is soluble in gastric juice containing hydrochloride to form magnesium chloride,8 which is reacted with bicarbonate ion secreted from duodenum to produce the osmotic laxative magnesium bicarbonate.9 Therefore, it is conceivable that the laxative effect of magnesia is blocked by antacid.9
A number of patients receive opioid analgesic in combination with non‐steroidal anti‐inflammatory drugs (NSAIDs), according to the World Health Organization (WHO) analgesic ladder for the relief of cancer pain.10 Moreover, significant amount of patients take antacids such as proton pump inhibitors (PPI) and histamine H2 receptor blockers (H2RB) for prevention of mucosal toxicity induced by NSAIDs.11, 12 Therefore, it is likely that prophylactic effect of magnesia against constipation is diminished by the concomitant use of antacids in patients receiving opioid analgesics. In this study, we investigated whether or not the pharmacological interaction occurs between magnesia and antacids in patients with cancer receiving opioid analgesics.
Methods
Patients
Five hundred and fifty‐four adults (>18 years) were administrated with oral opioid analgesics for the first time during a period between January 2007 and October 2014. Among them, 113 patients were excluded due to the following exclusion criteria: patients receiving cancer chemotherapy or radiation therapy within 10 days before or 7 days after administration of opioid analgesics or being subjected to surgical operation within 7 days of opioid intake (N = 72), those whose Eastern Cooperative Oncology Group (ECOG) performance status was 3 or 4 (N = 9) and those who discharged within 7 days after start of opioid intake (N = 30). The reason for such exclusion of data was as follows: the use of 5‐HT3 receptor antagonist, high performance status and conduction of surgical operation are all related to the induction or deterioration of constipation. The 5‐HT3 receptor is known to be distributed through the gastrointestinal tract, where it plays an important role in the gastrointestinal motility. Therefore, patients receiving 5‐HT3 receptor antagonist for prevention of nausea and vomiting often reveal constipation as the side effect.13, 14 It has been reported that higher performance status is associated with more severe constipation in patients receiving opioid analgesic agents.15 Moreover, surgical operation often causes constipation, which becomes a risk for postoperative ileus.16
This study was conducted according to the guidelines for human studies determined by the Ethical Committee of Gifu University Graduate School of Medicine and the Government of Japan, and was approved by the Medical Review Board of Gifu University Graduate School of Medicine (approval no. 28–87).
Survey of the prevalence of prophylactic laxatives and the occurrence of constipation
The presence of the regular prescribing of laxatives for at least 7 days after start of opioid administration was regarded as the positive prophylactic medication. Constipation was defined as the absence of stool for at least consecutive 3 days during the first week of the initiation opioid use, as described previously.6, 7 Data were obtained from medical record and were coded anonymously.
Effect of antacids on the inhibitory action of laxative against opioid‐induced constipation
The occurrence of constipation was assessed in patients receiving opioid analgesic in the presence or absence of laxative. Then, the effects of various types of laxatives on the incidence of constipation were compared between patients who received antacids such as PPI and H2RB and those who did not.
Subsequently, the dose–response in the inhibitory effect of magnesia alone against opioid‐induced constipation was compared between patients treated with or without antacids.
Univariate and multivariate logistic regression analyses for the risk of opioid‐induced constipation in 248 patients receiving magnesia alone
Demographics of patients receiving magnesia alone as a laxative were compared between patients who showed constipation and those who did not. Subsequently, univariate and multivariate logistic regression analyses were carried out to determine the risk factors that affect the incidence of opioid‐induced constipation.
Statistical analyses
Patient data were coded anonymously and analysed using IBM SPSS version 22 (IBM Japan Ltd., Tokyo, Japan) and GraphPad Prism version 6.0 (GraphPad Software, San Diego, CA, USA). Nonparametric data were analysed by Mann–Whitney U‐test, chi‐square test or Kruskal–Wallis test, while parametric data were compared by t‐test. The occurrence of constipation for multiple comparisons was statistically compared by the Kruskal–Wallis test, followed by Scheffe's test. The odds ratio for the incidence of constipation associated with opioid analgesics, 95% CIs and forest plots were assessed by using GraphPad Prism version 6.0.
Results
Patient demographics are shown in Table 1. Among 441 eligible patients, 327 patients (74%) received prophylactic laxatives, in which 248 patients (76%) were administered with magnesia alone and 79 patients (24%) were given with other laxative alone or in combination with magnesia. Antacids were prescribed in 257 patients (58%) among all eligible patients. The most prevalent cancer was lung cancer (20.9%), followed by gastrointestinal cancer (20.0%), pancreas/gall bladder cancer (13.8%) and head and neck cancer (9.8%). Oxycodone sustained‐release tablet was the most commonly (88.7%) prescribed analgesic. Laxatives were prescribed in 74.1% of patients. The most common laxative was magnesia alone (75.8%). Antacids were administered in 58.3% of patients, which included PPI, including lansoprazole (47.1%), omeprazole (12.1%), rabeprazole (11.7%) and esomeprazole (11.3%) and H2RB such as famotidine (13.2%), cimetidine (3.9), ranitidine (0.4%) and roxatidine (0.4%). As shown in Figure 1, antacids were treated in 60.6% and 51.8% of patients with and without use of prophylactic laxative, respectively.
Table 1.
Patient demographics
| Number of patients (male/female) | 441 (251/190) |
| Age (min/max) | 64.4 (24–92) |
| Type of cancer | N |
| Lung | 92 (20.9%) |
| Gastrointestinal | 88 (20.0%) |
| Pancreas/gall bladder | 61 (13.8%) |
| Head and neck | 43 (9.8%) |
| Gynaecologic | 29 (6.6%) |
| Urological | 27 (6.1%) |
| Hematopoietic | 25 (5.7%) |
| Hepatic cell | 21 (4.8%) |
| Bone/soft tissue sarcoma | 15 (3.4%) |
| Breast | 16 (3.6%) |
| Skin | 5 (1.1%) |
| Thyroid | 2 (0.5%) |
| Others | 17 (3.9%) |
| Opioid analgesics | |
| Oxycodone sustained release | 391 (88.7%) |
| Morphine hydrochloride | 32 (7.3%) |
| Morphine sulfate | 1 (0.2%) |
| Codeine phosphate | 17 (3.9%) |
| Daily dose (morphine base, mg), 95% CI | 18.8 (10.0 to 30.0) |
| Prescription of laxatives | N |
| Presence | 327 (74.1%) |
| Absence | 114 (25.9%) |
| Laxatives | |
| Magnesia alone | 248 (75.8%) |
| Magnesia in combination with other laxatives | 44 (13.5%) |
| Sennoside alone | 30 (9.2%) |
| Picosulfate alone | 4 (1.2%) |
| Others | 1 (0.3%) |
| Prescription of antacids | |
| Presence | 257 (58.3%) |
| Absence | 184 (41.7%) |
| Antacids | |
| Proton pump inhibitors | |
| Lansoprazole | 121 (47.1%) |
| Omeprazole | 31 (12.1%) |
| Rabeprazole | 30 (11.7%) |
| Esomeprazole | 29 (11.3%) |
| H2 receptor blockers | |
| Famotidine | 34 (13.2%) |
| Cimetidine | 10 (3.9%) |
| Ranitidine | 1 (0.4%) |
| Roxatidine | 1 (0.4%) |
Figure 1.
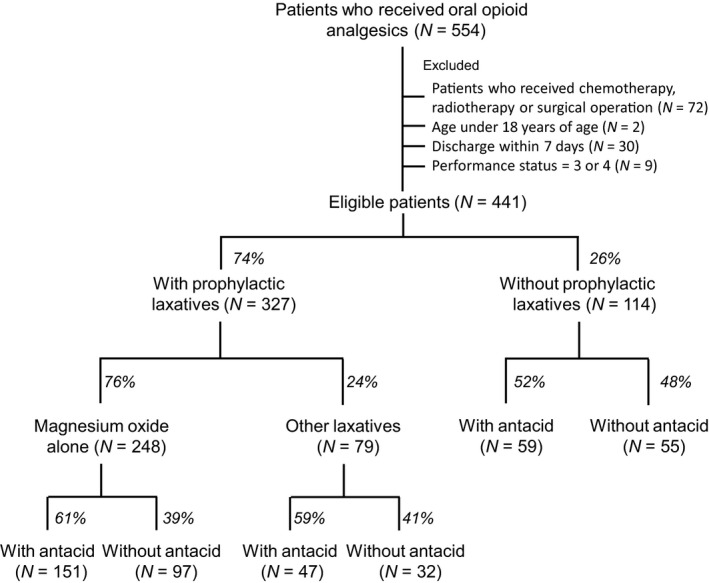
Study population.
Reversal by antacid of the action of magnesia but not of other laxative
The occurrence of opioid‐induced constipation was 58% and 53% in patients receiving no prophylactic laxative in the absence and presence of antacid, respectively (Figure 2a). In the absence of antacid, magnesia alone or in combination with other laxative significantly reduced the incidence of constipation (11% for magnesia alone P < 0.01; 18% for magnesia in combination with other laxative, P < 0.05), although sennoside did not significantly lower the incidence of constipation (36%). However, the laxative effect of magnesia alone was markedly reversed by the concomitant administration of antacid (11 vs 25%, P = 0.017), although antacid had no influence on the effect of sennoside (36 vs 37%) or magnesia used in combination with other laxative (18 vs 19%). As shown in Figure 2b, in the absence of antacid, magnesia alone inhibited the opioid‐induced constipation in a dose‐dependent fashion, in which significant effect was observed at all doses. Antacid reversed the effect of magnesia at daily doses lower than 2000 mg without affecting the effect of magnesia at daily doses higher than 2000 mg. Particularly, magnesia at daily doses lower than 1000 mg had no longer significant effect in the presence of antacid.
Figure 2.
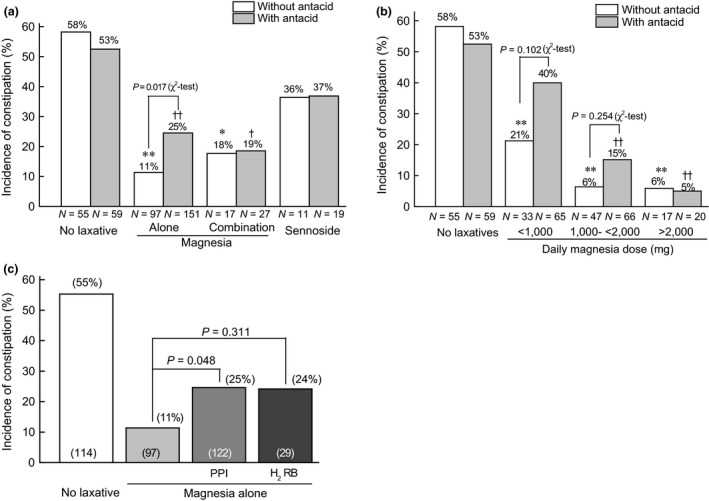
Comparison of the effects of different laxatives (a) and dose‐dependent effect of magnesia (b) between patients receiving opioid analgesics in combination with and without antacid, and comparison of inhibitory effects of PPI and H2 receptor blocker against laxative action of magnesia (c). *P < 0.05, **P < 0.01 versus no laxative without antacid, † P < 0.05, †† P < 0.01 vs no antacid, by Kruskal–Wallis test, followed by Scheffe's test. Data were also compared between the respective group with and without antacid by chi‐square test. In (c), data were statistically compared by Kruskal–Wallis test, followed by Scheffe's test.
As shown in Figure 2c, among antacids, PPI and H2RB showed a similar reversal action against magnesia (11 vs 25% for PPI, P = 0.048, or 24% for H2RB, P = 0.311).
Risk factors for opioid‐induced constipation in patient receiving magnesia
Demographics of patients treated with magnesia alone were compared in the presence or absence of opioid‐induced constipation. As shown in Table 2, antacid was prescribed significantly (P = 0.016) more prevalently in patients with constipation than in those without constipation. Moreover, daily dose of magnesia was significantly lower in patients with constipation than in those without constipation (1129 mg, 95% CI, 782 to 1812 vs 1456 mg, 990 to 3000, P < 0.001). A multivariate logistic regression analysis indicated that co‐administration of antacids and daily dose of magnesia <1000 mg were found to be significant risks for opioid‐induced constipation in patients who received magnesia (Figure 3).
Table 2.
Comparison of demographics between patients with constipation and those without constipation
| Presence of constipation | Absence of constipation | P values | |
|---|---|---|---|
| Number of patients (male/female) | 48 (27/21) | 200 (119/81) | P = 0.804a |
| Age (min/max) | 65.4 (24–83) | 63.8 (26–92) | P = 0.240b |
| Oxycodone sustained release | 47 (97.9%) | 176 (88%) | P = 0.0747a |
| Morphine hydrochloride | 1 (2.1%) | 13 (6.5%) | |
| Codeine phosphate | 0 (0%) | 11 (5.5%) | |
| Daily dose (morphine base, mg), 95% CI | 20.8 (15.0 to 43.5) | 18.1 (10.0 to 30.0) | P = 0.160b |
| Prescription of antacids | |||
| Presence | 11 (22.9%) | 86 (43%) | P = 0.016a |
| Absence | 37 (77.1%) | 114 (57%) | |
| Antacids | |||
| Proton pump inhibitors | |||
| Lansoprazole | 15 (40.5%) | 58 (50.9%) | P = 0.322c |
| Omeprazole | 5 (13.5%) | 15 (13.2%) | |
| Rabeprazole | 2 (5.4%) | 11 (9.6%) | |
| Esomeprazole | 8 (21.6%) | 8 (7.0%) | |
| H2 receptor blockers | |||
| Famotidine | 7 (18.9%) | 16 (14.0%) | |
| Cimetidine | 0 (0%) | 6 (5.3%) | |
| Daily dose of Magnesia (mg), 95% CI | 1129 (782 to 1812) | 1456 (990 to 3000) | P < 0.001b |
aChi‐square test, bMann–Whitney U‐test, cKruskal–Wallis test.
Figure 3.
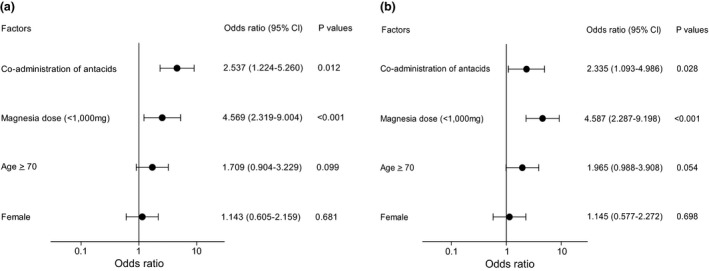
Univariate (a) and multivariate logistic regression analyses (b) for the risk of constipation in 248 patients who received magnesia alone for prevention of opioid‐induced constipation.
Discussion
A National Comprehensive Cancer Network (NCCN) guideline recommends using prophylactic treatment with laxatives such as stimulant laxative with or without stool softener or polyethylene glycol for prevention of opioid‐induced constipation.17 In our previous study consisting of 619 patients from 35 Japanese medical institutions,7 the prevalence of prophylactic treatment with laxative against opioid‐induced constipation was 73.7%, in which the incidence of constipation was 33.7% and 54.6% in patients with and without prophylactic laxative treatment, respectively (odds ratio, 0.432; 95% CI, 0.300 to 0.622). In addition, the most prevalent laxative used in the study was magnesia alone (62%) or in combination with other laxative (24%). Therefore, it is suggested that magnesia is the most commonly used laxative in Japan. Consistent with these data, in the present study, most patients (74.1%) received laxative for the prophylaxis of constipation and the incidence of constipation was 20.5% and 55.3% in patients with and without laxative, respectively.
On the other hand, magnesia neutralizes gastric acid, according to the following formulation: MgO + 2HCl → MgCl2 + H2O. The water‐soluble MgCl2 is converted in the duodenum to magnesium bicarbonate by the following formulation: MgCl2 + 2NaHCO3 → Mg(HCO3)2 + 2NaCl. Yamasaki et al.9 reported that magnesium bicarbonate produced in the duodenum increases the osmotic pressure within the intestines, stimulates water exudation and softens the stool, which leads to laxative effect of magnesia. Magnesia is approved in Japan as an ethical drug, and the dosage is 500–1000 mg/day for antacid use and 2000 mg/day for laxative use (manufacturer's instruction). However, in our previous study,7 a majority of patients (58.9%) received magnesia at doses lower than 1000 mg/day for the prophylaxis of opioid‐induced constipation. Also, in the present study, magnesia alone was most prevalently prescribed (76%), although most of them (85.1%) received at doses lower than the standard dose of 2000 mg/day.
Consistent with the data reported by Yamasaki et al.9, antacids such as PPI and H2RB reversed the laxative effect of magnesia at doses of <2000 mg/day. Moreover, the reversal by antacid of the laxative effect of magnesia was selective and was not observed for other types of laxatives, including sennoside. However, the reversal by antacids was no longer observed at magnesia doses of >2000 mg/day. In the present study, antacids had no influence on the laxative action of magnesia when administered in combination with other laxatives such as sennoside. At present, we could not explain the reason for the lack of influence of antacids on the laxative action of the combination of magnesia and sennoside. However, the dose of magnesia was slightly but not significantly higher in patients with antacids than in those without antacid (1551 ± 612 vs 1386 ± 709 mg, P = 0.175 by Mann–Whitney U‐test). In addition, the percentage of patients receiving magnesia at doses >1500 mg was higher but not significantly (P = 0.333 by Fisher's exact probability test) in antacid‐treated group (41%) than in the non‐treated group (24%). Therefore, it seems likely that the slightly higher dose of magnesia in antacid‐treated patients may explain the reason for the apparently no effect of antacid on the laxative action of the combined treatment with magnesia and other laxatives.
In general, opioid analgesics are prescribed in combination with NSAIDs for control of cancer pain, according to the WHO analgesic ladder.10 NSAIDs cause gastroduodenal ulceration by impairing mucosal defence and via direct toxic action, particularly in high‐risk patients, including elderly, patients using high dose of NSAIDs and those with a prior history of ulcer diseases.18 PPI and to a lesser extent H2RB are effective for protecting gastroduodenal cells against NSAID‐induced ulceration.19, 20 Therefore, it is likely that substantial amount of opioid users take antacids for protection of gastrointestinal tract from mucosal injury induced by NSAIDs. In the present study, approximately 60% of patients were prescribed with PPI and H2RB.
Taken together, care should be taken to avoid unfavourable pharmacological interaction between magnesia and antacids, particularly when magnesia is prescribed at daily doses less than 2000 mg. Replacement of low doses of magnesia by other laxative or high doses (>20 000 mg/day) of magnesia is recommended.
A multivariate logistic regression analysis also indicated that daily magnesia dose of <1000 mg was a significant risk for constipation in patients receiving magnesia in combination with opioid analgesic. Our present data were generally consistent with our previous findings indicating that prescription of magnesia at daily doses >1000 mg is a factor that significantly reduced the risk for constipation in patients receiving oral opioid analgesic.
On the other hand, the mechanisms underlying the laxative effect of magnesia seem to be complicated. It is conceivable that the requirement of gastric acid, as mentioned above, is limited to the action of lower doses (<2000 mg/day) of magnesia, but the effect of higher doses (>2000 mg/day) of magnesia is not dependent on the gastric pH. Izzo et al.21 reported that magnesium ion exerts an osmotic effect and causes water to be retained in the intestinal lumen. They also postulated that cholecystokinin or constitutive nitric oxide synthase (NOS) is involved in the pharmacological action of magnesium. Indeed, they showed in rats that the activation of NOS is implicated in the laxative action of magnesium sulfate: oral administration of magnesium sulfate stimulates the activity of NOS in gut tissue, which is blocked by the NOS inhibitor NG‐nitro‐l‐arginine methyl ester (L‐NAME).22 They also showed that the fluid retention induced in the intestinal lumen as well as the diarrhoea induced by magnesium sulfate are inhibited by L‐NAME.22 On the other hand, Vu et al.23 showed in healthy volunteers that magnesium sulfate given in combination with a fatty meal causes diarrhoea with a concomitant increase in postprandial plasma peptide YY and cholecystokinin.
On the other hand, aquaporin 3 is considered to play a role in the pathophysiology of magnesium‐induced diarrhoea. Ikarashi et al.24 reported that magnesium ion increases the expression of aquaporin 3 in the mucosal epithelial cells with the time course quite similar to the onset of diarrhoea in rats. They also showed that the enhancement of aquaporin 3 expression is mediated by the transcription factor cyclic AMP response element binding protein (CREB) through activation of cyclic AMP/protein kinase A pathway subsequent to the elevation of the intracellular magnesium ion in human colon cancer HT‐29 cells.25
Blood magnesium concentration is severely controlled by intestinal magnesium absorption, glomerular filtration and passive absorption through the paracellular pathway in the proximal tubule and the thick ascending loop of Henle via paracellin‐1 and claudin‐19, and active absorption by the distal convoluted tubule via the transient receptor potential cation channel subfamily M (TRPM6).26 However, in elderly patients with renal disturbance or those receiving high doses of magnesia, the occurrence of hypermagnesaemia should be taken into account.27, 28 Hypermagnesaemia is a rare but severe side effect of magnesia. In Japan, 15 cases of hypermagnesaemia associated with magnesia have been reported to the Pharmaceutical and Medical Devices Agency during the past 3 years from April 2005 to August 2008, in which the symptoms include nausea/vomiting, hypotension, bradycardia, muscle weakness at an early stage and respiratory depression, arrhythmia, disturbance in consciousness and cardiac arrest in severe case. Therefore, the possibility of the occurrence of hypermagnesaemia should be taken into account, when high doses of magnesia are prescribed. A case of hypermagnesaemia was reported in a woman with a history of coronary artery bypass surgery, hypertension and diabetes mellitus, even when low dose (1000 mg/day) of magnesia was given.29 Taken together, it is suggested that replacement by other class of laxatives rather than the dose elevation of magnesia is desirable in patients with renal dysfunction who received antacid. However, it does not seem to be appropriate to recommend prescribing sennoside for prevention of constipation as the effect of this compound was less marked than magnesia (Figure 2a). In the present study, oral picosulfate sodium, a laxative agent that stimulates colonic peristalsis and increases water retention within the gastrointestinal tract,30 was administered to four patients, all of whom showed no constipation. Moreover, in our previous multi‐institutional (J‐RIGID) study comparing the prophylactic effects of various laxatives in patients with cancer receiving opioid analgesics,7 oral picosulfate sodium was prescribed to eight patients among 466 patients who received prophylactic laxatives. Among eight patients receiving picosulfate sodium, constipation occurred only in one patient (12.5%). The incidence rate of constipation was 54.9% in patients receiving no prophylactic laxatives (N = 153), while 34.5% in those with laxatives (N = 466). Taken together, it is suggested that oral picosulfate sodium is useful for prevention of opioid‐induced constipation even in patients with renal insufficiency because the compound is hardly disposed by renal excretion.
There are several limitations in the present study: first, data were obtained by a retrospective chart review from a single medical institution. Second, a possibility of the involvement of other factors that affect the incidence of constipation could not excluded from the present study. Therefore, a prospective multi‐institutional study will be needed to clarify the influence of antacids on the laxative effect of magnesia.
In conclusion, a majority of patients with cancer receiving opioid analgesic for the first time were prescribed with magnesia for the prophylaxis of constipation. In addition, approximately 60% of the total patients received antacids, including PPI and H2RB. Such antacids reversed the laxative effect of lower doses (<2000 mg/day) but not higher doses (>2000 mg/day) of magnesia without affecting the effects of other laxatives. Therefore, care should be taken to avoid pharmacological interaction between magnesia and antacid.
Declarations
Conflict of interest
All Authors have no relevant conflict of interests to report.
Funding
No relevant funding related to this work was supported by all authors.
References
- 1. Glare PA. Early implementation of palliative care can improve patient outcomes. J Natl Compr Canc Netw 2013; 11 (Suppl. 1): S3–S9. [DOI] [PubMed] [Google Scholar]
- 2. Walsh TD. An overview of palliative care in cancer and AIDS. Oncology (Williston Park) 1991; 5 (9 Suppl.): 7–11. [PubMed] [Google Scholar]
- 3. Cherny N et al Strategies to manage the adverse effects of oral morphine: an evidence‐based report. J Clin Oncol 2001; 19: 2542–2554. [DOI] [PubMed] [Google Scholar]
- 4. McNicol E et al Management of opioid side effects in cancer‐related and chronic noncancer pain: a systematic review. J Pain 2003; 4: 231–256. [DOI] [PubMed] [Google Scholar]
- 5. Bell TJ et al The prevalence, severity, and impact of opioid‐induced bowel dysfunction: results of a US and European Patient Survey (PROBE 1). Pain Med 2009; 10: 35–42. [DOI] [PubMed] [Google Scholar]
- 6. Ishihara M et al Pharmaceutical interventions facilitate premedication and prevent opioid‐induced constipation and emesis in cancer patients. Support Care Cancer 2010; 18: 1531–1538. [DOI] [PubMed] [Google Scholar]
- 7. Ishihara M et al A multi‐institutional study analyzing effect of prophylactic medication for prevention of opioid‐induced gastrointestinal dysfunction. Clin J Pain 2012; 28: 373–381. [DOI] [PubMed] [Google Scholar]
- 8. Lindberg JS et al Magnesium bioavailability from magnesium citrate and magnesium oxide. J Am Coll Nutr 1990; 9: 48–55. [DOI] [PubMed] [Google Scholar]
- 9. Yamasaki M et al Interaction of magnesium oxide with gastric acid secretion inhibitors in clinical pharmacotherapy. Eur J Clin Pharmacol 2014; 70: 921–924. [DOI] [PubMed] [Google Scholar]
- 10. Ventafridda V et al WHO guidelines for the use of analgesics in cancer pain. Int J Tissue React 1985; 7: 93–96. [PubMed] [Google Scholar]
- 11. Lazzaroni M, Bianchi Porro G. Prophylaxis and treatment of non‐steroidal anti‐inflammatory drug‐induced upper gastrointestinal side‐effects. Dig Liver Dis 2001; 33 (Suppl. 2): S44–S58. [DOI] [PubMed] [Google Scholar]
- 12. Singh G, Triadafilopoulos G. Appropriate choice of proton pump inhibitor therapy in the prevention and management of NSAID‐related gastrointestinal damage. Int J Clin Pract 2005; 59: 1210–1217. [DOI] [PubMed] [Google Scholar]
- 13. Eisenberg P et al Improved prevention of moderately emetogenic chemotherapy‐induced nausea and vomiting with palonosetron, a pharmacologically novel 5‐HT3 receptor antagonist. Cancer 2003; 98: 2473–2482. [DOI] [PubMed] [Google Scholar]
- 14. Kovac AL. Benefits and risks of newer treatments for chemotherapy‐induced and postoperative nausea and vomiting. Drug Saf 2003; 26: 227–259. [DOI] [PubMed] [Google Scholar]
- 15. Fallon MT, Hanks GW. Morphine, constipation and performance status in advanced cancer patients. Palliat Med 1999; 13: 159–160. [DOI] [PubMed] [Google Scholar]
- 16. Lee TH et al Risk factors for postoperative ileus following orthopedic surgery: the role of chronic constipation. J Neurogastroenterol Motil 2015; 21: 121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology v.2.2015. Adult Cancer Pain. http://www.nccn.org/professionals/physician_gls/pdf/pain.pdf. [DOI] [PubMed]
- 18. Scheiman JM. NSAID‐induced peptic ulcer disease: a critical review of pathogenesis and management. Dig Dis 1994; 12: 210–222. [DOI] [PubMed] [Google Scholar]
- 19. Leandro G et al Prevention of acute NSAID‐related gastroduodenal damage: a meta‐analysis of controlled clinical trials. Dig Dis Sci 2001; 46: 1924–1936. [DOI] [PubMed] [Google Scholar]
- 20. Goldstein JL. Challenges in managing NSAID‐associated gastrointestinal tract injury. Digestion 2004; 69(Suppl. 1): 25–33. [DOI] [PubMed] [Google Scholar]
- 21. Izzo AA et al The osmotic and intrinsic mechanisms of the pharmacological laxative action of oral high doses of magnesium sulphate. Importance of the release of digestive polypeptides and nitric oxide. Magnes Res 1996; 9: 133–138. [PubMed] [Google Scholar]
- 22. Izzo AA et al Nitric oxide as a mediator of the laxative action of magnesium sulphate. Br J Pharmacol 1994; 113: 228–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vu MK et al The osmotic laxative magnesium sulphate activates the ileal brake. Aliment Pharmacol Ther 2000; 14: 587–595. [DOI] [PubMed] [Google Scholar]
- 24. Ikarashi N et al Effects of magnesium sulphate administration on aquaporin 3 in rat gastrointestinal tract. Biol Pharm Bull 2011; 34: 238–242. [DOI] [PubMed] [Google Scholar]
- 25. Ikarashi N et al A mechanism by which the osmotic laxative magnesium sulphate increases the intestinal aquaporin 3 expression in HT‐29 cells. Life Sci 2011; 88: 194–200. [DOI] [PubMed] [Google Scholar]
- 26. Muallem S, Moe OW. When EGF is offside, magnesium is wasted. J Clin Invest 2007; 117: 2086–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weisinger JR, Bellorin‐Font E. Magnesium and phosphorus. Lancet 1998; 352: 391–396. [DOI] [PubMed] [Google Scholar]
- 28. Onishi S, Yoshino S. Cathartic‐induced fatal hypermagnesemia in the elderly. Intern Med 2006; 45: 207–210. [DOI] [PubMed] [Google Scholar]
- 29. Weng YM et al Hypermagnesemia in a constipated female. J Emerg Med 2013; 44: e57–e60. [DOI] [PubMed] [Google Scholar]
- 30. Forth W et al The hydragogue and laxative effect of the sulfuric acid ester and the free diphenol of 4,4’‐dihydroxydiphenyl‐(pyridyl‐2)‐methane. Naunyn Schmiedebergs Arch Pharmacol 1972; 274: 46–53. [DOI] [PubMed] [Google Scholar]


