Abstract
Anti‐neutrophil cytoplasmic antibody (ANCA) is associated with small‐vessel vasculitis particularly in the kidneys and can induce the formation of neutrophil extracellular traps (NETs) from primed neutrophils. Recently we have reported that the induction of NETs correlates with ANCA affinity for myeloperoxidase (MPO) and disease activity in patients with MPO‐ANCA‐associated microscopic polyangiitis. To investigate whether MPO‐ANCA affinity is associated with the formation of NETs in vivo, we examined the occurrence of NETs in the renal tissues of patients with MPO‐ANCA‐associated microscopic polyangiitis and ANCA affinity by double immunofluorescence staining for NET components of citrullinated histone, MPO and PAD4 and by ELISA competition with MPO, respectively. We divided 30 MPO‐ANCA‐associated microscopic polyangiitis patients into 2 groups based on their ANCA affinity levels (IC50 for the high: 0.11 ± 0.04 µg/mL (Group1) and IC50 for the low: 0.66 ± 0.24 µg/mL (Group2)). Group1 showed a higher Birmingham vasculitis activity score (15.6 ± 5.7) and 73% of the patients presented clinically with rapidly progressive glomerulonephritis and histologically with focal/crescentic glomerulonephritis (GN). Group 2 showed a lower Birmingham vasculitis activity score (9.2 ± 4.9) and 73% of the patients presented clinically with chronic renal failure and histologically with mixed/sclerotic GN. Group 1 showed a much higher occurrence of NETs than Group 2. Our findings indicate that ANCA affinity was associated with the in vivo formation of NETs, which might be involved in the pathophysiology of patients with MPO‐ANCA‐associated microscopic polyangiitis.
Keywords: microscopic polyangiitis, myeloperoxidase anti‐neutrophil cytoplasmic antibody affinity, neutrophil extracellular traps, renal histopathology, vasculitis activity
Anti‐neutrophil cytoplasmic antibody (ANCA) is useful for diagnosing renal diseases associated with systemic small‐vessel vasculitis and for estimating disease activity. Patients with myeloperoxidase (MPO)‐ANCA‐associated microscopic polyangiitis (MPO‐AAV) often present with rapidly progressive glomerulonephritis (RPGN), and rarely with chronic renal failure (CRF).1, 2 MPO‐ANCA titres are high in untreated patients. The titre tends to change with disease status, but not consistently.3
We previously reported that MPO‐AAV patients could be classified into high‐affinity and low‐affinity groups based on ANCA affinity for MPO and this classification is interrelated with disease activity.4 ANCA IgG induces the formation of neutrophil extracellular traps (NETs) from activated neutrophils and is implicated in the presence of NETs in the kidney from ANCA‐positive patients.5, 6 By using the sera from patients with lupus nephritis, the ability to induce NETosis and to degrade NETs was found to be related to the disease manifestation.7We have recently reported that the ability of ANCA IgG to induce NETs correlates with microscopic polyangiitis disease activity and the ANCA affinity for MPO.6 The concomitant occurrences of NET components of extracellular MPO and citrullinated histones that are catalyzed by nuclear peptidylarginine deiminase 4 (PAD4) in the kidney from MPO‐AAV patients suffering from glomerulonephritis (GN) and fibrinoid necrosis were observed.8 In this study, we investigated the induction of NET formation in vivo and evaluated its correlation with ANCA affinity for MPO in MPO‐AAV patients.
Methods
Between January 2012 and December 2014, blood samples and renal biopsies were collected from 30 patients who had been newly diagnosed as having MPO‐AAV, and each patient gave written informed consent to participate in the clinical study.
The titre and affinity of serum MPO‐ANCA taken before the start of the therapy were determined using direct enzyme linked immunosorbent assay (ELISA) with the wells coated with 50 μL of 1 µg/mL leukocyte MPO (Lee Biosolutions, St. Louis, MO, USA).4 Affinity was defined as IC50 , which was the concentration of MPO producing 50% inhibition of MPO‐ANCA binding on the dose‐response curve in the liquid phase of ELISA as previously described.4
Formalin‐fixed renal biopsy sections were stained with periodic acid‐Schiff and then were evaluated according to the histopathologic classification of MPO‐AAV (i.e,, focal (F), crescentic (C), mixed (M), and sclerotic (S)).9 Double immunofluorescence staining for PAD4 and either MPO or citrullinated histone H3 was performed on 3‐µm‐thick sections of formalin fixed paraffin embedded tissues of renal biopsies. Briefly, sections were deparaffinized, and were performed the antigen retrieval treatment (microwaved in boiling 10 mM citrate buffer (pH 6.5) for 3 min three times at 1‐min intervals) on the sections for citrullinated histone H3 staining. Then the sections were blocked with a blocking buffer (Starting Block, PIERCE, Rockford, IL, USA), and incubated for 1 h at room temperature either with a mixture of rabbit anti‐human PAD4 antibody10 and mouse anti‐human MPO antibody (R&D Systems, Minneapolis, MN, USA), or with a mixture of rabbit anti‐human PAD4 antibody10 and mouse anti‐citrullinated histone H3 monoclonal antibody (Abcam, Cambridge, UK). The sections were incubated with a mixture of secondary antibodies Alexa Fluor 488‐conjugated donkey anti‐rabbit IgG and Alexa Fluor 594‐conjugated donkey anti‐mouse IgG (Rockland, Gilbertsville, PA, USA) for 30 min at room temperature. Counterstaining was performed with Hoechst 33342 (Dojindo, Kumamoto, Japan).6
The presence of NETs in the renal tissues of MPO‐AAV patients was assessed according to semiquantitative grading positivity of signals as follows: citrullinated histone H3 and MPO merging signal present with concomitant DNA and PAD4 and MPO merging signal present with concomitant DNA per glomerulus.
We evaluated systemic disease activity using the Birmingham vasculitis activity score (BVAS) and renal clinical symptoms (i.e,, rapidly progressive GN (RPGN) and chronic renal failure (CRF)). The correlations between MPO‐ANCA affinity and BVAS, renal clinical symptoms, histopathological findings, or renal NET formation levels were statistically evaluated. Data were expressed as mean ± SD or median with interquartile range (IQR). The Student t‐test, Fisher test, Mann‐Whitney U‐test, and Spearman's rank correlation coefficient were used for the statistical analysis. A P‐value of less than 0.05 was considered to indicate a statistically significant difference.
Results
The clinical and pathological findings of the patients who were divided into the two types of MPO‐ANCA affinity are described in Table 1. First, as shown in Figure 1, 30 patients could be divided into the two groups according to the levels of ANCA affinity for MPO: high‐affinity group G1 had 15 patients with IC50 < 0.30 µg/mL and low‐affinity group G2 15 patients with IC50 > 0.30 µg/mL. Table 1 shows the MPO‐ANCA titres and affinities, clinical findings, renal findings and NET occurrences in the two groups. The average IC50 value in G1 was 0.11 ± 0.04 µg/mL (n = 15), whereas that in G2 was 0.66 ± 0.24 µg/mL (n = 15). The difference between G1 and G2 was statistically significant (P < 0.001). The results on affinity from 30 patients newly enrolled in this study are consistent with those from 27 patients in a previous study.4 The median with IQR of MPO‐ANCA titre in G1 was 513 (287‐1680) EU and that in G2 was 622 (205‐979) EU, respectively. The difference between the two was not statistically significant (P = 0.330). Regarding the clinical findings, the mean age was higher in G1 (70 ± 4 years) than in G2 (66 ± 3 years). The BVAS was significantly higher in G1 (15.6 ± 5.7) than in G2 (9.2 ± 4.9) (P = 0.002). In terms of renal findings, 73% of the G1 patients presented with RPGN clinically and 73% with active fibrinoid crescentic GN (focal/crescentic) histologically, whereas 73% of the G2 patients showed CRF clinically and 73% mixed and sclerotic GN histologically. RPGN was more prevalent in G1 than in G2, whereas CRF was more prevalent in G2 than in G1. The renal biopsy from G1 showed fibrinoid necrosis (Fig. 2(A), (1)). Double immunofluorescence staining of a representative section revealed the codistribution of significant signals of MPO and citrullinated histone H3 in the fibrinoid necrosis lesion (Fig. 2(A), (2) (3) (4) (5)). NET formation was indicated by greater occurrences of MPO and citrullinated histone H3 merging signal present with concomitant DNA in a glomerulus (Fig. 2(B), (2) (3) (4) (5)) and MPO and PAD4 merging signal present with concomitant DNA in the glomerulus in crescentic type GN G1 than that in sclerotic GN G2 (Fig. 2(C), (2) (3) (4) (5)). As a whole, G1 had small but significant numbers of PAD4 and MPO merging signal per glomerulus and high citrullinated histone H3 and MPO merging signal per glomerulus, but G2 had almost no NETs (Table 1). The strong and significant correlation was found between MPO‐ANCA affinity and NET occurrences (citrullinated histone/MPO and PAD4/MPO) in the glomerulus (Fig. 3(A): r = −0.842, P < 0.001, and r = −0.818, P < 0.001, respectively), but the correlations were weak and not significant either between MPO‐ANCA titre and NET occurrences in the glomerulus (Fig. 3(B): r = 0.300, P = 0.054, and r = 0.194, P = 0.152, respectively) or between MPO‐ANCA titre and MPO‐ANCA affinity (r = −0.176, P = 0.176) as assessed by the Spearman's rank correlation coefficient.
Table 1.
Association between the two types of MPO‐ANCA affinity and occurrences of NETs in MPO‐AAV patients. Thirty patients studied were classified as high‐and low‐affinity groups with smaller than 0.30 of IC50 and larger than 0.30 of IC50, respectively
| Renal findings | NET occurrences§ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M/F (Gender) | Age (mean ± SD) | MPO‐ANCA affinity (IC50)* (µg/mL) (mean ± SD) | MPO‐ANCA titre (EU) ** (median (interquatile range) | BVAS*** (mean ± SD) | eGFR (mL/min per 1.73 m2) (mean ± SD) | ****RPGN(%)/CRF†(%) | Histopatholo.‡(%) | Cit histone H3/MPO | PAD4/MPO | ||
| High affinity | |||||||||||
| (G1) | 7 / 8 | 70 ± 4 | 0.11 ± 0.04 | 513 | 15.6 ± 5.7 | 21.25 ± 23.06 | 73 / 27 | F | 33 | 8.5 ± 2.5 | 2.5 ± 0.5 |
| (n = 15) | (287–1680) | C | 40 | ||||||||
| M | 27 | ||||||||||
| S | 0 | ||||||||||
| Low affinity | |||||||||||
| (G2) | 7 / 8 | 66 ± 3 | 0.66 ± 0.24 | 622 | 9.2 ± 4.9 | 31.11 ± 22.23 | 27 / 73 | F | 13 | ||
| (n = 15) | (205–979) | C | 14 | 0.0 ± 0.5 | 0.0 ± 0.5 | ||||||
| M | 33 | ||||||||||
| S | 40 | ||||||||||
P < 0.001,
P = 0.330,
P = 0.002,
P = 0.002.
CRF, chronic renal failure; RPGN, rapidly progressive glomerulonephritits.
C, crescentic; F, focal; M, mixed; S, sclerotic.
NET occurrences: mean numbers of signals double positive for citrullinated histone H3 and MPO or PAD4 and MPO concomitant with DNA per glomerular cross section; P‐values between high and low affinity groups.
Figure 1.
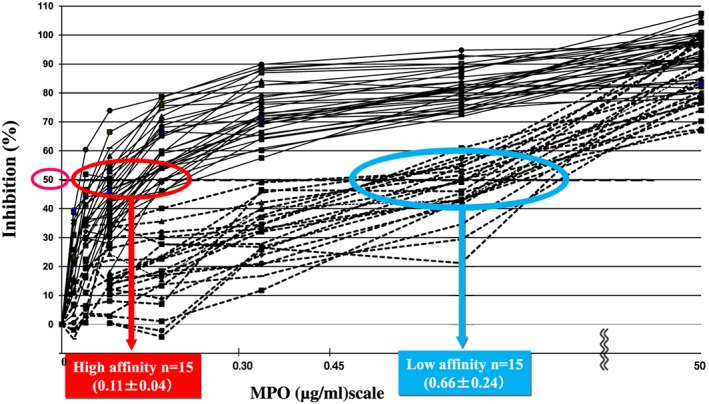
Two types of myeloperoxidase anti‐neutrophil cytoplasmic antibody (MPO‐ANCA) affinities: high and low affinities (n = 30) 30 MPA patients could be classified into a high‐affinity group (G1: solid line; 15 patients, and a low‐affinity group (G2: dotted line, 15 patients). Each point was assayed in triplicate.
Figure 2.
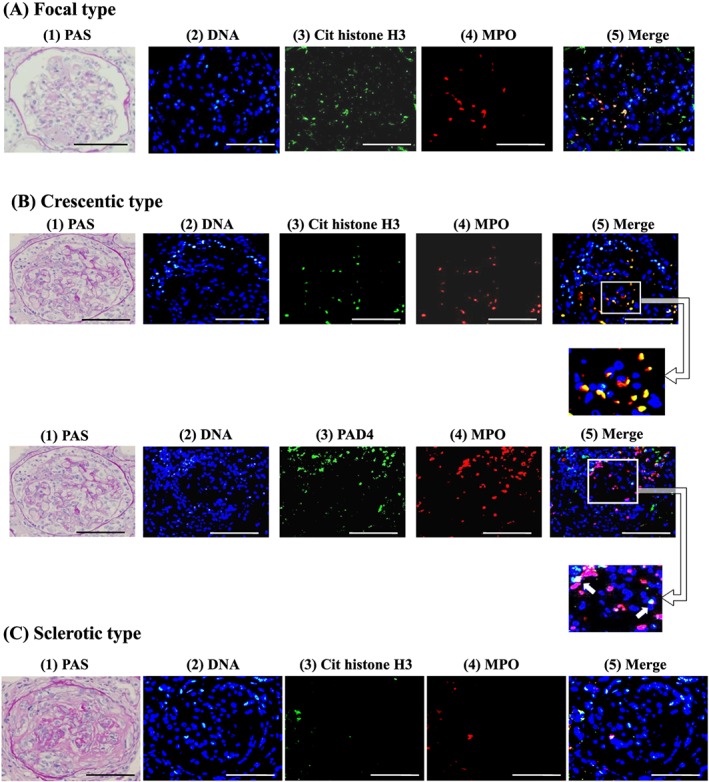
Representative photomicrographs of histology and neutrophil extracellular trap (NET) staining in the kidney Renal biopsy specimens from 30 patients with myeloperoxidase‐ANCA‐associated microscopic polyangiitis (MPO‐AAV) were examined by periodic acid‐Schiff staining and double immunofluorescences taining for NETs. (A) focal type of necrotizing GN, (B) crescentic type of GN, and (C) sclerotic type of GN. (1) Periodic acid‐Schiff‐staining in A, B, and C (×20), NET staining in A, B and C (2) DNA was stained as blue with Hoechst 33342. Scale bars indicate 100 µm. In A, (3) citrullinated histone H3 is depicted in green, (4) MPO in red, (5) merged images of (2–4). Solid arrows indicate merging signals. In B, on the upper panels (3) citrullinated histone H3 in green, (4) MPO in red, (5) merged images of 2–4. On the lower panels (3) PAD4 in green, (4) MPO in red, (5) merged images of 2–4. Square lines with arrow indicate higher magnification insets. In C, (3) citrullinated histone H3 in green, (4) MPO in red, (5) merged images of 2–4. Yellow indicates the colocalization of citrullinated histone H3 and MPO, PAD4 and citrullinated histone H3.
Figure 3.
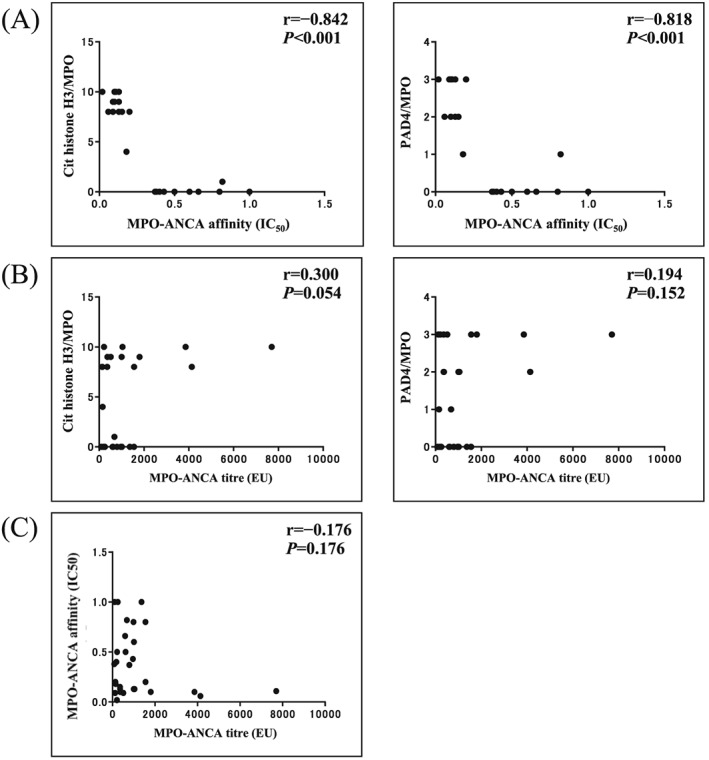
Relationships between myeloperoxidase anti‐neutrophil cytoplasmic antibody (MPO‐ANCA) affinity, MPO‐ANCA titre and neutrophil extracellular trap (NET) occurrences in glomerulus. (A) Correlation between MPO‐ANCA affinity and NET occurrences in the 30 subjects studied. The left panel indicated NET of citrullinated histone H3/MPO and the right panel PAD4/MPO. The Spearman's rank correlation coefficients for these were calculated to be r = −0.842, P < 0.001 and r = −0.818, P < 0.001, respectively, indicating, that ANCA affinity was significantly and strongly correlated with NET occurrence. (B) Correlation between MPO‐ANCA titre and NET occurrences. The left panel indicated NET of citrulinated histone H3/MPO and the right panel NET of PAD4/MPO. The Spearman's rank correlation coefficients for these were calculated to be r = 0.300, P = 0.054, r = 0.194, P = 0.152, respectively, indicating that correlation between MPO‐ANCA titre and NET occurrence was weak and not significant. (C) Correlation between MPO‐ANCA affinity (IC50) and MPO‐ANCA titre. The Spearman's rank correlation coefficient was calculated to be r = −0.176, P = 0.176, indicating that correlation between MPO‐ANCA affinity and titre was weak and not significant.
Discussion
We investigated the IC50 values of ANCA in the patient sera and evaluated their correlation to disease activity and histopathological findings. No significant correlation was found between MPO‐ANCA titres and MPO‐ANCA affinities in patients with MPO‐AAV (Fig. 3(C)). The progression of RPGN or CRF to end‐stage renal disease is an established clinical characteristic.2, 3, 4 It has been observed that the acute fibrinoid necrosis lesions of MPO‐AAV have leukocytoclasia (consisting of nuclear debris) and NETs in the same foci.8 The characteristic renal histopathological features in MPO‐AAV were classified into the following: (i) focal segmental and crescentic GN; (ii) mixed and scleroticGN.9 NETs comprise an extracellular fibrous material containing decondensed chromatin generated by PAD4‐mediated histone citrullination and inclusion of MPO and proteolytic enzymes in the nucleus and are released from activated neutrophils in MPO‐AAV.5, 6
Our findings indicated that the NETs mostly occurred in a focal glomerulus and a crescentic glomerulus in high affinity MPO‐ANCA‐positive patients. Furthermore, MPO‐ANCA affinity but not titre was definitely correlated with NET occurrences in the glomerulus (Fig. 3(A) and (B)). Consistent with our findings, O'Sullivan et al. showed that there is a wide range of extracellular MPO deposition in the kidneys of patients with MPO‐ANCA‐associated glomerulonephritis, and most of the extracellular MPOs are a constituent of NETs released from neutrophils present in the active glomerular lesions.11 NETosis is induced by stimulating tumour necrosis factor‐α (TNF‐α) primed neutrophils with purified ANCA IgGs.5, 6, 7 The NETs formed in vivo induce endothelial damage leading to extravasation of blood and then the activation of dendritic cells and regulatory T and B cells that might be involved in the genesis of autoimmune diseases such as MPO‐AAV and systemic lupus erythematosus.5, 6, 7, 12 In vitro NET is easily degraded by serum factors including DNase I, depending on the patient sera from MPO‐AAV.6 The heterogeneity of NET components observed in this study might be a reflection of the difference in clearance of NET component in vivo. The regulatory mechanism on the formation and degradation of NETs has become increasingly known, but the in vivo mechanism remains unclear.
Epitope mapping of MPO‐ANCA by epitope excision/mass spectrometry and ELISA with overlapping decapeptides revealed that a large number of MPO‐ANCA/epitope pairs where epitopes are classified as exclusive to active disease, persistent during remission and natural in healthy subjects. 13, 14 Clearly, among these epitopes conformational epitopes were much more than linear epitopes. Moreover, more MPO ANCAs with native MPO were undetectable with linear peptides and large segments of epitopes and thus most epitopes of MPO‐ANCA have been considered conformational.15 Studies on the relationship between high and low MPO‐ANCA affinities and pathogenic active and inactive epitopes are in progress. Further studies on the implications of epitope‐specific MPO‐ANCA in NET formation will aid in the understanding of the initiation and pathogenesis of autoimmune disease.
A limitation of this study was the relatively small number of enrolled patients. We plan to further investigate MPO‐ANCA affinity in relation to disease activity and renal histopathology in a larger number of MPO‐AAV patients with RPGN (n = 156) in Japan in reference to a recent study 16. This would provide new insights into the MPO‐ANCA biology and MPO‐AAV pathophysiology.
In conclusion, our study showed that MPO‐ANCA affinity may be useful for assessing vasculitis activity and is associated with the in vivo formation of NETs, which might be involved in the pathophysiology of MPO‐AAV patients.
Conflict of Interest Statement
None declared.
Acknowledgements
This study was supported in part by a Grant‐in‐Aid for Intractable Vasculitis Diseases Research and Progressive Renal Diseases Research, Research on Intractable Diseases, from the Ministry of Health, Labour and Welfare of Japan. The authors are grateful to Dr Edward Barroga, Associate Professor and Senior Medical Editor of Tokyo Medical University for editing the manuscript.
Yoshida, M. , Yamada, M. , Sudo, Y. , Kojima, T. , Tomiyasu, T. , Yoshikawa, N. , Oda, T. , and Yamada, M. (2016) Myeloperoxidase anti‐neutrophil cytoplasmic antibody affinity is associated with the formation of neutrophil extracellular traps in the kidney and vasculitis activity in myeloperoxidase anti‐neutrophil cytoplasmic antibody‐associated microscopic polyangiitis. Nephrology, 21: 624–629. doi: 10.1111/nep.12736.
References
- 1. Jennette JC, Falk RJ. Pathogenesis of antineutrophil cytoplasmic autoantibody‐mediated disease. Nat. Rev. Rheumatol. 2014; 10: 463–73. [DOI] [PubMed] [Google Scholar]
- 2. Yamagata K, Usui J, Saito C et al. ANCA‐associated systemic vasculitis in Japan: clinical features and prognostic changes. Clin. Exp. Nephrol. 2012; 16: 580–9. [DOI] [PubMed] [Google Scholar]
- 3. Land J, Rutgers A, Kallenberg CGM. Antineutrophil cytoplasmic autoantibody pathogenicity revisited: Pathogenic versus nonpathogenic anti‐neutrophil cytoplasmic autoantibody. Nephrol. Dial. Transplant. 2014; 29: 739–45. [DOI] [PubMed] [Google Scholar]
- 4. Yoshida M, Sasaki M, Nakabayashi I et al. Two types of myeloperoxidase antineutrophil cytoplasmic autoantibodies with a high affinity and a low affinity in small vessel vasculitis. Clin. Exp. Rheumatol. 2009; 27 (Suppl52): S28–32. [PubMed] [Google Scholar]
- 5. Kessenbrock K, Krumbholz M, Schönermarck UI et al. Netting neutrophils in autoimmune small‐vessel vasculitis. Nat. Med. 2009; 15: 623–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nakazawa D, Shida H, Tomaru U et al. Enhanced formation and disordered regulation of NETs in myeloperoxidase‐ANCA‐associated microscopic polyangiitis. J. Am. Soc. Nephrol. 2014; 25: 990–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hakkim A, Fürnrohr BG, Amann K, et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc. Natl. Acad. Sci. U. S. A. 2010; 107: 9813–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yoshida M, Sasaki M, Sugisaki K et al. Neutrophil extracellular trap components in fibrinoid necrosis of the kidney with myeloperoxidase‐ANCA‐associated vasculitis. Clin. Kidney J. 2013; 6: 308–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berden AE, Ferrario F, Hagen EC et al. Histopathologic classification of ANCA associated glomerulonephritis. J. Am. Soc. Nephrol. 2010; 21: 1628–36. [DOI] [PubMed] [Google Scholar]
- 10. Nakashima K, Hagiwara T, Yamada M. Nuclear localization of peptidylarginine deiminase V and histone deimination in granulocytes. J. Biol. Chem. 2002; 277: 49562–8. [DOI] [PubMed] [Google Scholar]
- 11. O'Sullivan KM, Lo CY, Summers SA et al. Renal participation of myeloperoxidase in antineutrophil cytoplasmic antibody (ANCA)‐associated glomerulonephritis. Kidney Int. 2015; 88: 1030–46 [DOI] [PubMed] [Google Scholar]
- 12. Villanueva E, Yalavarthi S, Berthier CC et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J. Immunol. 2011; 187: 538–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bruner BF, Vista ES, Wynn DM et al. Epitope specificity of myeloperoxidase antibodies: identification of candidate human immunodominant epitopes. Clin. Exp. Immunol. 2011; 164: 330–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roth AJ, Ooi JD, Hess JJ et al. Epitope specificity determines pathogenicity and detectability in ANCA‐associated vasculitis. J. Clin. Invest. 2013; 123: 1773–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gou SJ, Xu PC, Chen M, Zhao MH. Epitope analysis of anti‐myeloperoxidase antibodies in patients with ANCA‐associated vasculitis. PLoS One 2013; 8 (e60530): 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sada KE, Yamamura M, Harigai M et al. Classification and characteristics of Japanese patients with antineutrophil cytoplasmic antibody‐associated vasculitis in a nationwide, prospective, inception cohort study. Arthritis Res. Ther. 2014; 16 (R101): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]


