Abstract
Despite 10 years of post‐marketing safety monitoring of the phosphate binder lanthanum carbonate, concerns about aluminium‐like accumulation and toxicity persist. Here, we present a concise overview of the safety profile of lanthanum carbonate and interim results from a 5‐year observational database study (SPD405‐404; ClinicalTrials.gov identifier: NCT00567723). The pharmacokinetic paradigms of lanthanum and aluminium are different in that lanthanum is minimally absorbed and eliminated via the hepatobiliary pathway, whereas aluminium shows appreciable absorption and is eliminated by the kidneys. Randomised prospective studies of paired bone biopsies revealed no evidence of accumulation or toxicity in patients treated with lanthanum carbonate. Patients treated with lanthanum carbonate for up to 6 years showed no clinically relevant changes in liver enzyme or bilirubin levels. Lanthanum does not cross the intact blood–brain barrier. The most common adverse effects are mild/moderate nausea, diarrhoea and flatulence. An interim Kaplan–Meier analysis of SPD405‐404 data from the United States Renal Data System revealed that the median 5‐year survival was 51.6 months (95% CI: 49.1, 54.2) in patients who received lanthanum carbonate (test group), 48.9 months (95% CI: 47.3, 50.5) in patients treated with other phosphate binders (concomitant therapy control group) and 40.3 months (95% CI: 38.9, 41.5) in patients before the availability of lanthanum carbonate (historical control group). Bone fracture rates were 5.9%, 6.7% and 6.4%, respectively. After more than 850 000 person‐years of worldwide patient exposure, there is no evidence that lanthanum carbonate is associated with adverse safety outcomes in patients with end‐stage renal disease.
Keywords: end‐stage renal disease, hyperphosphatemia, lanthanum carbonate, long‐term safety, pharmacokinetics, phosphate binder
Summary at a Glance
Lanthanum carbonate is a non‐calcium‐based phosphate binder, and although it is a metal cation, its effects are not comparable with those of aluminium. This article discusses safety data of lanthanum with clinical studies showing no significant toxic effects after 10 years of follow‐up.
Introduction
An elevated serum phosphate level (hyperphosphatemia) is one of the major clinical manifestations of end‐stage renal disease (ESRD). Hyperphosphatemia is associated with cardiovascular events and increased all‐cause mortality in patients with ESRD.1, 2, 3, 4 Observational studies have shown that the treatment of hyperphosphatemia with phosphate‐binding agents reduces the risk of mortality compared with no treatment in patients with ESRD.5, 6, 7, 8 The main types of phosphate binder currently used in clinical practice are calcium based and non‐calcium based (including iron based).9 Lanthanum carbonate (LaC), a non‐calcium‐based binder, has been indicated for the management of hyperphosphatemia in patients with ESRD in the USA since 2005.10 As of 30 April 2015, the estimated total worldwide patient exposure to LaC was 851 634 person‐years (tablets, 626 636 person‐years; oral powder, 224 998 person‐years [data on file, Shire, 2015]). In total, 6287 patients have been exposed to LaC during completed phase 1–4 clinical trials. Studies evaluating the safety of LaC monotherapy over 2,11 312 and 6 years13 have consistently reported that LaC is well tolerated. Despite the positive safety profile of LaC, established by 17 phase 1–4 clinical studies11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 and 10 years of post‐marketing safety monitoring, questions over the long‐term safety of LaC persist. The historical toxicity of aluminium and the perception that lanthanum is chemically similar to aluminium engendered concerns that lanthanum could show aluminium‐like accumulation and toxicity in the bone and central nervous system.28, 29, 30, 31, 32 Owing to extensive safety concerns surrounding aluminium‐based phosphate binders, their use in clinical practice is now limited, and non‐aluminium‐based binders are instead favoured.33 A comparative review of the efficacy and safety of calcium, sevelamer and LaC concluded that all three non‐aluminium‐based binders were effective at reducing serum phosphate levels and that they were generally well tolerated; however, treatment with calcium carbonate was associated with higher rates of hypercalcaemia.34
To date, there has been no comprehensive safety review of LaC compared with aluminium. Here, we present a detailed review of the key pharmacokinetic, toxicological and clinical studies that established the positive long‐term safety profile of LaC and interim results from a 5‐year observational database study of LaC safety in patients with ESRD (SPD405‐404; ClinicalTrials.gov: NCT00567723).
Overview of key studies
Lanthanum pharmacokinetics
Given initial concerns that LaC treatment could lead to high systemic levels of lanthanum,28 low gastrointestinal absorption and low plasma levels of lanthanum were important target characteristics. A phase 1 study conducted in healthy individuals22 showed that the absolute bioavailability of lanthanum following a single 1000 mg oral dose of LaC was very low (mean ± SD: 0.00127 ± 0.0008%), indicating that lanthanum is minimally absorbed from the gut. Furthermore, any absorbed lanthanum was almost entirely (>99%) bound to plasma proteins.35 Hutchison et al. (2006)12 also demonstrated that mean plasma lanthanum concentrations plateaued at nanomolar levels (2.5–9.7 nmol/L) in dialysis patients receiving long‐term LaC treatment. Interestingly, plasma lanthanum levels were not statistically different at LaC doses of 750−3000 mg/day, indicating a nonlinear relationship between administered dose and plasma levels. This effect is probably caused by mechanisms that limit intestinal absorption of metals, including epithelial exfoliation.36
Because LaC is indicated for patients with impaired renal function, lanthanum excretion via a nonrenal route was an important characteristic. Damment et al. (2007)35 showed that 99.3% of an oral lanthanum dose was recovered in the faeces of rats and that 86.0% of an intravenous dose was recovered in the bile of bile duct‐cannulated rats. These results indicated that lanthanum is predominantly eliminated in the faeces via hepatobiliary excretion, although this has yet to be demonstrated in humans. Pennick et al. (2006)22 showed that only 0.00003% of an oral dose of lanthanum was eliminated in the urine of healthy human participants, reflecting both negligible renal clearance and the poor bioavailability of lanthanum. In light of the nonrenal clearance of lanthanum, the expectation was that patients with impaired renal function would not be more susceptible to systemic accumulation of lanthanum than healthy individuals. This was confirmed by data demonstrating similar lanthanum exposure profiles in patients with ESRD and individuals with normal renal function.37
In contrast to lanthanum, oral ingestion of aluminium results in appreciable absorption from the gastrointestinal tract in healthy participants (0.06–0.1%),38 ~50–80‐fold higher than that seen with lanthanum. Moreover, aluminium is mainly (~95%) eliminated via the kidney with negligible biliary excretion (~2%).39, 40, 41, 42 Thus, patients with ESRD are particularly vulnerable to the deleterious effects of aluminium ingestion.43
Based on these data, the pharmacokinetic profiles of LaC and aluminium appear to be quite different (Fig. 1).
Figure 1.
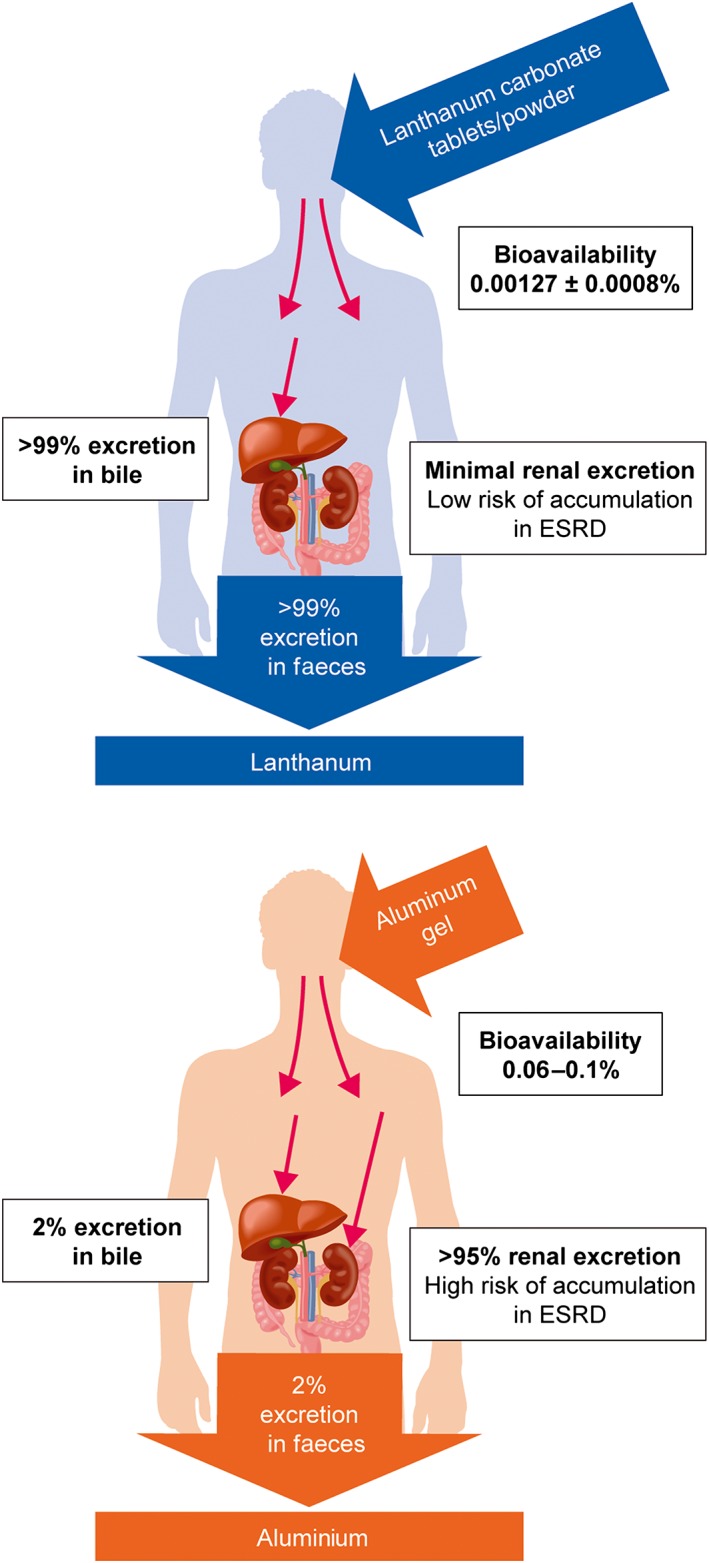
Comparison of the pharmacokinetics of lanthanum and aluminium. ESRD, end‐stage renal disease.
Effect of lanthanum on bone
Bone is known to be a major reservoir for divalent and trivalent cationic metals,44 and the pathophysiological effects of aluminium on bone health are well documented.28, 29, 30, 31, 32 Bone was therefore one of the key target organs in the safety evaluation of LaC. A phase 3, open‐label study compared the effects of LaC and calcium carbonate on the evolution of renal bone disease in patients with ESRD.16 Only 3.0% (1/33) of patients in the LaC group evolved towards adynamic bone compared with 20.0% (6/30) of patients receiving calcium carbonate. Additionally, a subset of patients in both groups had low bone turnover and hyperparathyroidism at baseline; however, these baseline characteristics evolved towards normalisation of bone turnover after 1 year of treatment in 71.0% (5/7) and 80.0% (4/5) of patients in the LaC group, respectively, and in 43.0% (3/7) and 50.0% (3/6) of patients in the calcium carbonate group, respectively. No statistical testing was performed because of the exploratory nature of this endpoint and the small sample size. A 2‐year, open‐label study45 also investigated the evolution of renal bone disease in patients with ESRD who were switched to LaC or maintained on their previous phosphate binder. The proportion of patients with improvement or no change was higher in the LaC group than in the phosphate binder group for bone activation frequency (66.7% vs 55.0%), bone formation rate (74.2% vs 57.1%) and bone volume (84.4% vs 62.5%) at 2 years.
Bronner et al. (2008)46 modelled the bone load of lanthanum in dialysis patients using bone lanthanum concentration data collected from three long‐term trials (up to 5 years) of LaC. The model predicted a sevenfold increase in total bone lanthanum from 1.0 µg/g to 6.6 µg/g after 10 years of LaC treatment. A 3‐year, prospective, open‐label study of dialysis patients, performed by Shigematsu et al. (2011),47 reported mean bone lanthanum levels at 3 years (4.3 µg/g) that were comparable with those predicted in the Bronner study.46 In addition, there was no evidence of bone toxicity: all patients with normal bone turnover at baseline remained normal, while two patients with osteitis fibrosa and two patients with adynamic bone disease evolved towards normal bone turnover. Another study48 reported mean bone lanthanum levels of 1.9 µg/g after 3 years of LaC treatment and observed no differences in osteoblast numbers or mineral apposition rates in bone biopsies of patients treated with LaC compared with those receiving calcium carbonate.
Persy et al. (2006)49 estimated the molar bone lanthanum (atomic mass, 138.9): calcium (atomic mass, 40.1) ratio to gain a better quantitative understanding of the potential for lanthanum to disrupt bone mineral structure. Assuming a maximum bone lanthanum concentration of 9.5 µg/g (0.0095 mg/g), a bone calcium concentration of 120 mg/g and homogeneous lanthanum distribution throughout the bone,50 the molar bone lanthanum : calcium ratio was estimated to be 2 × 10−5. This equates to 1 in 50 000 calcium atoms being replaced by lanthanum. Applying similar reasoning to aluminium, but assuming that it is localised in only 1% of the total bone volume (based on the observation that aluminium is localised at the osteoid‐calcification front), the molar bone aluminium : calcium ratio was estimated to be 6 × 10−2, equating to 1 in 16 calcium atoms being replaced by aluminium.
These studies indicate that, unlike aluminium, LaC is unlikely to accumulate to clinically relevant levels in bone and is not associated with bone toxicity.
Effect of lanthanum on the liver
Lanthanum is predominantly excreted via the hepatobiliary pathway.35 The subcellular localisation of lanthanum in liver tissue was determined using a combination of microscopy and spectroscopy techniques.51, 52 These studies demonstrated that lanthanum is present in the lysosomes of hepatocytes, and is concentrated both in the biliary pole of the hepatocyte and in the bile canaliculi, indicating that lanthanum undergoes transcellular transport in the liver. Hutchison et al. (2009)18 reported an analysis of hepatic biochemical tests and liver‐associated adverse events (AEs) in patients with ESRD who received LaC in four previous phase 3 trials and who were followed in a 2‐year extension study for a total treatment duration of up to 6 years (n = 93). Changes in transaminases, bilirubin and alkaline phosphatase observed during long‐term treatment were minimal and similar to those reported in previous trials. Overall, there was no evidence of an increase in the incidence or severity of liver‐related AEs with increasing treatment duration and no new types of liver‐related AEs in patients who received LaC for up to 6 years.
Effect of lanthanum on the central nervous system
The seminal study of Alfrey et al. (1976)53 was the first to present evidence of an association between aluminium accumulation in the brain and dialysis encephalopathy. Biochemical and epidemiological data subsequently confirmed that aluminium neurotoxicity is an aetiological factor in the development of this syndrome.54, 55, 56 In contrast to aluminium, lanthanum cannot cross the tight junctions of the intact blood–brain barrier (BBB). It is for this reason that lanthanum is used as a tracer to assess the integrity of the BBB.57, 58, 59, 60 Bervoets et al. (2009)51 reported no difference in brain levels of lanthanum in healthy rats and rats with chronic renal failure following treatment by oral gavage with LaC for 20 weeks. Lanthanum levels remained close to the limit of quantification (4 ng/g), and there was no evidence of increased levels with duration of treatment. A 2‐year, open‐label study61 found no evidence of a difference in the rate of cognitive decline in patients with ESRD randomised to LaC or ‘standard’ phosphate binder therapy.62
In summary, there is currently no evidence to indicate that lanthanum crosses the intact BBB.
Effect of lanthanum on the gastrointestinal tract
Lanthanum carbonate forms insoluble complexes with phosphate in the gastrointestinal tract;10, 63 but safety pharmacology studies have shown no clinically significant effects on gastric emptying, intestinal transit time or aspirin‐induced gastric lesions at LaC doses up to 13‐fold higher than the clinical dose (3000 mg/day).37 Hutchison et al. (2008)13 reported safety data in patients who received long‐term LaC monotherapy for up to 6 years. The majority of treatment‐related adverse effects were related to the gastrointestinal tract (mainly mild/moderate nausea, diarrhoea and flatulence) and were consistent with those observed with other phosphate binders.64 A meta‐analysis of 16 randomised controlled trials65 reported a higher rate of vomiting but a lower rate of abdominal pain with LaC compared with calcium‐based binders. No significant differences were found in the incidences of nausea, constipation or dyspepsia.
The risk of gastrointestinal obstruction and perforation is increased in patients with ESRD compared with the general population.66 There have also been reports of gastrointestinal obstruction, ileus, subileus and gastrointestinal perforation in patients treated with LaC.67, 68 However, analysis of pooled data from completed phase 2–4 clinical trials of LaC chewable tablet demonstrated a lower rate of gastrointestinal obstruction and ileus/subileus compared with active comparators (0.3% [18/5634] vs 0.8% [10/1184], respectively), and a similar incidence of gastrointestinal perforation (0.09% [5/5634] vs 0.08% [1/1184]) [data on file, Shire, 2015]. It should be noted that the current LaC label instructs that LaC chewable tablets must be chewed completely before swallowing, in order to prevent such adverse gastrointestinal events. The tablets may also be crushed completely to aid chewing, but intact tablets must not be swallowed whole.69 These data, along with the findings from a cumulative review of safety data from all sources, show that there is currently not sufficient evidence to indicate that these AEs are directly related to LaC therapy.
Published case studies have demonstrated the presence of lanthanum particles in the gastrointestinal tract using radiographic techniques.70, 71, 72, 73, 74 Additionally, two recent reports have documented lanthanum deposition in the human gastric mucosa75, 76; however, the clinical significance of these observations is unclear. Absorption from the gastrointestinal tract has also been documented in the juvenile rat, with lanthanum detected in all measured tissues after dosing. Lanthanum levels of up to 90 000 ng/g wet tissue weight were observed in juvenile rats administered 2000 mg/kg doses of LaC by oral gavage (>16 times the human dose of 3 g/day). Dosing was then followed by a 28−29‐day treatment‐free period, where tissue levels of LaC decreased, except for in the glandular mucosa of the stomach where they remained high [Shire, unpublished data]; however, these data were not considered clinically significant. Overall, there is no evidence of an increase in the incidence of gastrointestinal‐related AEs with increasing exposure to LaC.
Effect of lanthanum on the haematopoietic system
Hutchison et al. (2008)13 reported that 6 years of LaC treatment did not result in adverse effects on haematological laboratory parameters, including serum total iron, ferritin and haemoglobin, transferrin saturation, haematocrit and mean cell volume. A post hoc analysis of two phase 3 studies also showed no evidence of iron accumulation in patients treated with LaC.77 In contrast, aluminium toxicity is known to result in a microcytic, hypochromic anaemia that is refractory to iron and recombinant erythropoietin therapy.78, 79 The mechanism by which aluminium disrupts erythropoiesis is unclear but may involve effects on bone marrow erythroid progenitor cells80 or haemoglobin synthesis.79, 81
The conclusion from these studies is that LaC does not seem to have an effect on iron or haematological parameters.
Overview of Spd405‐404 study
Background
The primary objectives of the SPD405‐404 study are to compare all‐cause mortality and bone fractures requiring hospitalisation in patients who received LaC with patients who received other phosphate binders.
Patients
Patients in the USA (≥18 years) who received a minimum of 12 consecutive weeks of treatment with LaC were recruited into SPD405‐404 and formed the test group. The control arms (historical and concomitant), which included adult patients with ESRD receiving dialysis for at least 12 consecutive weeks, were identified from the United States Renal Data System (USRDS) database.82 The historical control group comprised individuals whose data were in the database prior to 1 January 2000 (5 years before LaC became available in the USA), and as such, the comparison has limitations due to changes in standards of care; the concomitant therapy control group instead comprised patients who were treated for hyperphosphatemia with any phosphate binder other than LaC (same era as the test group). For each patient in the test group, a maximum of four other patients were selected from the USRDS database for inclusion in each of the control groups. Patients in the control groups were matched to those in the test group by age (5‐year categories), sex and duration of dialysis (integer year categories, historical control group) or first year of commencement of dialysis (concomitant control group). To be eligible for matching, patients in the USRDS database (historical and concomitant therapy control groups) must have been alive and at least 18 years old at their respective screening date and have (1) at least one post‐screening record in the database (i.e. treatment or hospitalisation data); (2) Medicare as the primary insurer at screening; and (3) more than 12 consecutive weeks of dialysis at screening. It should be noted that control group membership was assigned based on the data available in the downloaded USRDS database. This study is being conducted in accordance with the ethical principles set out in the Declaration of Helsinki (1989). The protocol was approved at each centre by an Institutional Review Board. Patients in the test group provided written, informed consent before participating in the study.
Study design
This is a phase 4, observational database study of patients with ESRD in the USA. Patients will enter into the study at the screening assessment and will be contacted every 6 months during the observational period to record information on exposure to LaC or any other phosphate binder. Participant follow‐up for primary and secondary endpoints is planned for up to 5 years via the USRDS database. Control arms were matched from USRDS data. Primary endpoints are time to and incidence of all‐cause mortality, and time to first event and incidence of bone fractures requiring hospitalisation. Secondary endpoints are the time to first event and incidence of gastrointestinal disease, liver disease, malignancy, and major infectious episodes requiring hospitalisation. Endpoint data are obtained from the USRDS database. The USRDS records and data from SPD405‐404 case report forms will be used in the summary and analysis. The USRDS data will be used if there are discrepancies between the databases. The interim analysis presented here was based on USRDS data through 2014, which was downloaded in May 2015.
Survival curves were generated using the Kaplan−Meier method. Statistical analyses were performed using SAS® version 9.4 (SAS Institute, Cary, NC, USA). Analyses were performed on the full analysis set, which comprises all patients enrolled in the study who had received LaC treatment for a minimum of 12 consecutive weeks and had at least one post‐screening record in the USRDS database, plus their matched control patients. A multivariate regression analysis was conducted to compare time to all‐cause mortality between the test group and the historical and concomitant therapy control groups, respectively, using a Cox proportional hazards model. The model adjusted for patient baseline characteristics and included the following covariate terms: age category, sex, body mass index, region, year of starting dialysis (test vs concomitant), duration of dialysis at screening (test vs historical), study group, type of dialysis, presence of diabetes and other comorbidities. Covariates were included using stepwise regression covariate selection with α = 0.1 entry and removal significance levels. The stepwise regression began with the fixed terms in the model (age category, sex and study group). The fixed terms were not removed from the stepwise procedure.
Interim results
In total, 2136 patients were enrolled in the study, of whom 2027 were included in the full analysis set, along with 8112 historical and 8103 concomitant therapy matched control patients. Baseline demographic characteristics in the test and control groups were similar (Table 1). A Kaplan−Meier analysis revealed that the median survival with 5 years follow‐up was 51.6 months (95% CI: 49.1, 54.2) in patients who received LaC (test group) (Fig. 2), 48.9 months (95% CI: 47.3, 50.5) in patients treated with any other phosphate binder (concomitant therapy control group) (Fig. 2) and 40.3 months (95% CI: 38.9, 41.5) in patients before the availability of LaC (historical control group). Kaplan−Meier estimates of survival (95% CI) at month 60 were 0.44 (0.42, 0.46) versus 0.43 (0.42, 0.44) versus 0.35 (0.34, 0.36) for the test, concomitant therapy and historical control groups, respectively. A Cox proportional hazards model was also fitted; hazard ratios (95% CI) for the test group versus the concomitant therapy control group were 0.96 (0.90, 1.03), P = 0.262 and 0.76 (0.71, 0.82), P < 0.001 for the test group versus historical control group. The incidence of bone fractures was similar in the test, concomitant therapy and historical control groups: 5.9%, 6.7%, and 6.4%, respectively (Table 2). Given that the ‘other’ phosphate binders were mostly calcium based, these findings are consistent with the results of Jamal et al. 83 With regard to pathophysiological bone changes that are a common complication in patients with ESRD,84 bone fracture rates were similar across the three groups, corroborating that long‐term LaC therapy is not associated with bone toxicity. However, it must be noted that there were very few bone fracture events.
Table 1.
SPD405‐404 study: patient disposition and baseline demographic characteristics
| Test group | Concomitant therapy control group | Historical control group | |
|---|---|---|---|
| Enrolled patients, n | 2136 | N/A | N/A |
| Safety analysis set, n (%)† | 2029 (95.0) | N/A | N/A |
| Full analysis set, n (%)‡ | 2027 (94.9) | 8103 | 8112 |
| Transplant recipients, n (%) | 290 (14.3) | 873 (10.8) | 1225 (15.1) |
| Length of follow‐up, months, mean (SD) | 37.5 (21.39) | 37.4 (21.72) | 33.5 (21.58) |
| Length of follow‐up for living patients w/o transplant, n (%)§ | |||
| < 60 months | 3 (0.1) | 5 (0.1) | 3 (< 0.1) |
| 60 months | 718 (35.4) | 2934 (36.2) | 2202 (27.1) |
| Died during the follow‐up period¶, n (%) | 1050 (51.8) | 4372 (54.0) | 4846 (59.7) |
| Transplant recipient during the follow‐up period, n (%) | 290 (14.3) | 873 (10.8) | 1225 (15.1) |
| Age, years, mean (SD) | 57.1 (14.54) | 56.7 (14.58) | 57.1 (14.59) |
| Sex, n (%) | |||
| Male | 1178 (58.1) | 4707 (58.1) | 4714 (58.1) |
| Female | 849 (41.9) | 3396 (41.9) | 3398 (41.9) |
| Race, n (%) | |||
| White | 970 (47.9) | 3940 (48.6) | 4084 (50.3) |
| Non‐white | 1055 (52.0) | 4163 (51.4) | 4025 (49.6) |
| Black/African American | 959 (47.3) | 3529 (43.6) | 3359 (41.4) |
| Asian | 37 (1.8) | 238 (2.9) | 189 (2.3) |
| Other | 59 (2.9) | 396 (4.9) | 477 (5.9) |
| Missing | 2 (0.1) | 0 | 3 (< 0.1) |
| BMI, kg/m2, mean (SD) | 30.31 (8.33) | 29.00 (8.02) | 26.34 (7.14) |
| Serum phosphate, mg/dL, mean (SD) | 5.89 (1.77) | N/A | N/A |
| Presence of diabetes, n (%) | 895 (44.2) | 3920 (48.4) | 2795 (34.5) |
| Duration of ESRD, years, mean (SD) | 5.36 (5.01) | 5.29 (4.96) | 5.47 (4.96) |
Percentages for the safety analysis set and full analysis set are based on enrolled patients. Other percentages are based on the full analysis set.
The safety analysis set comprises all patients who were registered to the study and had previously received lanthanum carbonate treatment for a minimum of 12 consecutive weeks (test group only).
The full analysis set consists of patients in the safety analysis set who also had at least one post‐screening record in the United States Renal Data System database (test group) and their matched control patients (concomitant therapy and historical control groups).
The length of follow‐up for living patients without a transplant is from screening until the date of their last available claim record in the United States Renal Data System database; the length of follow‐up for patients who died or received a transplant is from screening until their death or transplant. The maximum length of follow‐up is 60 months.
Thirty‐four patients (1.7%) in the test group, 81 patients (1.0%) in the concomitant therapy control group and 164 patients (2.0%) in the historical control group had a transplant and died during the follow‐up period.
BMI, body mass index; ESRD, end‐stage renal disease; N/A, not available; SD, standard deviation; w/o, without.
Figure 2.

Kaplan–Meier analysis of time to all‐cause mortality based on United States Renal Data System data from 2014 (full analysis set). Horizontal reference line shows median survival time. CI, confidence interval.
Table 2.
SPD405‐404 study: rates of bone fracture requiring hospitalisation based on United States Renal Data System data from 2014 (Full analysis set†)
| Test group (N = 2027) | Concomitant therapy control group (N = 8103) | Historical control group (N = 8112) |
|---|---|---|
| n (%) | n (%) | n (%) |
| 120 (5.9) | 546 (6.7) | 519 (6.4) |
The full analysis set comprises patients in the safety analysis set who also had at least one post‐screening record in the United States Renal Data System database (test group) and their matched control patients (concomitant therapy and historical control groups).
Limitations of the USRDS include lack of continuous validation of its data capture, lack of complete comorbidity and laboratory data, survival bias, attrition bias, differences between test and historical control arms due to the different landscape in standard of care, limitations in matching control from registry database and lack of accuracy of cause‐of‐death reporting.82
Conclusions
LaC is a well tolerated phosphate binder that has been indicated for the management of hyperphosphatemia in patients with ESRD since 2005. Initial concerns that lanthanum could show aluminium‐like accumulation and toxicity have been refuted by the pharmacokinetic, toxicological and clinical evidence summarised here. It is clear that the pharmacokinetic paradigms of lanthanum and aluminium are different: lanthanum is minimally absorbed and mainly eliminated via the hepatobiliary pathway, whereas aluminium shows appreciable absorption and is mainly eliminated via the kidneys. Randomised prospective studies of paired bone biopsies revealed no evidence of aluminium‐like accumulation or toxicity, or mineralisation defects in patients treated with LaC. Patients treated with LaC for up to 6 years showed no clinically relevant changes in liver enzyme or bilirubin levels and no increase in the incidence of liver‐associated AEs. Lanthanum does not cross the intact BBB, and there is no evidence of an adverse effect of lanthanum on cognitive functioning. Similar to other oral phosphate binders, the majority of adverse reactions in patients taking LaC are related to the gastrointestinal tract (mainly nausea, diarrhoea and flatulence) and are primarily mild/moderate in intensity. Recent reports have documented lanthanum deposition in the gastric mucosa; however, the clinical significance of this is unclear.
Interim results from study SPD405‐404, a 5‐year observational database study, indicate that all‐cause mortality and bone fracture rates in patients treated with LaC are comparable with those in patients treated with other phosphate binders. Overall, after 10 years of continuous post‐marketing safety monitoring and more than 850 000 person‐years of worldwide patient exposure to LaC, there is no evidence that LaC is associated with adverse safety outcomes in patients with ESRD.
Acknowledgements
Alastair J Hutchison has acted as a consultant to Shire and been an invited speaker at Shire‐sponsored clinical meetings. Rosamund J Wilson is a consultant to Shire. Svetlana Garafola and J. Brian Copley are employees of Shire. This analysis was funded by Shire Development LLC. Writing and editorial support was provided by Fernando Gibson, PhD, and Luci Witcomb, PhD, employees of PharmaGenesis London, UK, with funding from Shire Development LLC. The SPD405‐404 study (ClinicalTrials.gov Identifier: NCT00567723) and analysis was funded by Shire Development LLC.
Hutchison, A. J. , Wilson, R. J. , Garafola, S. , and Copley, J. B. (2016) Lanthanum carbonate: safety data after 10 years. Nephrology, 21: 987–994. doi: 10.1111/nep.12864.
References
- 1. Block GA, Hulbert‐Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am. J. Kidney Dis. 1998; 31: 607–17. [DOI] [PubMed] [Google Scholar]
- 2. Melamed ML, Eustace JA, Plantinga L et al. Changes in serum calcium, phosphate, and PTH and the risk of death in incident dialysis patients: a longitudinal study. Kidney Int. 2006; 70: 351–7. [DOI] [PubMed] [Google Scholar]
- 3. Slinin Y, Foley RN, Collins AJ. Calcium, phosphorus, parathyroid hormone, and cardiovascular disease in hemodialysis patients: the USRDS waves 1, 3, and 4 study. J. Am. Soc. Nephrol. 2005; 16: 1788–93. [DOI] [PubMed] [Google Scholar]
- 4. Young EW, Albert JM, Satayathum S et al. Predictors and consequences of altered mineral metabolism: the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2005; 67: 1179–87. [DOI] [PubMed] [Google Scholar]
- 5. Komaba H, Kakuta T, Suzuki H, Hida M, Suga T, Fukagawa M. Survival advantage of lanthanum carbonate for hemodialysis patients with uncontrolled hyperphosphatemia. Nephrol. Dial. Transplant. 2015; 30: 107–14. [DOI] [PubMed] [Google Scholar]
- 6. Isakova T, Gutierrez OM, Chang Y et al. Phosphorus binders and survival on hemodialysis. J. Am. Soc. Nephrol. 2009; 20: 388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lopes AA, Tong L, Thumma J et al. Phosphate binder use and mortality among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS): evaluation of possible confounding by nutritional status. Am. J. Kidney Dis. 2012; 60: 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cannata‐Andia JB, Fernandez‐Martin JL, Locatelli F et al. Use of phosphate‐binding agents is associated with a lower risk of mortality. Kidney Int. 2013; 84: 998–1008. [DOI] [PubMed] [Google Scholar]
- 9. Hutchison AJ, Smith CP, Brenchley PE. Pharmacology, efficacy and safety of oral phosphate binders. Nat. Rev. Nephrol. 2011; 7: 578–89. [DOI] [PubMed] [Google Scholar]
- 10. Albaaj F, Hutchison AJ. Lanthanum carbonate (Fosrenol): a novel agent for the treatment of hyperphosphataemia in renal failure and dialysis patients. Int. J. Clin. Pract. 2005; 59: 1091–6. [DOI] [PubMed] [Google Scholar]
- 11. Finn WF. Lanthanum carbonate versus standard therapy for the treatment of hyperphosphatemia: safety and efficacy in chronic maintenance hemodialysis patients. Clin. Nephrol. 2006; 65: 191–202. [DOI] [PubMed] [Google Scholar]
- 12. Hutchison AJ, Maes B, Vanwalleghem J et al. Long‐term efficacy and tolerability of lanthanum carbonate: results from a 3‐year study. Nephron Clin. Pract. 2006; 102: c61–71. [DOI] [PubMed] [Google Scholar]
- 13. Hutchison AJ, Barnett ME, Krause R, Kwan JT, Siami GA. Long‐term efficacy and safety profile of lanthanum carbonate: results for up to 6 years of treatment. Nephron Clin. Pract. 2008; 110: c15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Al‐Baaj F, Speake M, Hutchison AJ. Control of serum phosphate by oral lanthanum carbonate in patients undergoing haemodialysis and continuous ambulatory peritoneal dialysis in a short‐term, placebo‐controlled study. Nephrol. Dial. Transplant. 2005; 20: 775–82. [DOI] [PubMed] [Google Scholar]
- 15. Chiang SS, Chen JB, Yang WC. Lanthanum carbonate (Fosrenol) efficacy and tolerability in the treatment of hyperphosphatemic patients with end‐stage renal disease. Clin. Nephrol. 2005; 63: 461–70. [DOI] [PubMed] [Google Scholar]
- 16. D'Haese PC, Spasovski GB, Sikole A et al. A multicenter study on the effects of lanthanum carbonate (Fosrenol) and calcium carbonate on renal bone disease in dialysis patients. Kidney Int. Suppl. 2003: S73–8. [DOI] [PubMed] [Google Scholar]
- 17. Finn WF, Joy MS, Hladik G. Efficacy and safety of lanthanum carbonate for reduction of serum phosphorus in patients with chronic renal failure receiving hemodialysis. Clin. Nephrol. 2004; 62: 193–201. [DOI] [PubMed] [Google Scholar]
- 18. Hutchison AJ, Barnett ME, Krause R, Siami GA. Lanthanum carbonate treatment, for up to 6 years, is not associated with adverse effects on the liver in patients with chronic kidney disease Stage 5 receiving hemodialysis. Clin. Nephrol. 2009; 71: 286–95. [PubMed] [Google Scholar]
- 19. Hutchison AJ, Gill M, Copley JB, Poole L, Wilson RJ. Lanthanum carbonate versus placebo for management of hyperphosphatemia in patients undergoing peritoneal dialysis: a subgroup analysis of a phase 2 randomized controlled study of dialysis patients. BMC Nephrol. 2013; 14: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Joy MS, Finn WF. Randomized, double‐blind, placebo‐controlled, dose‐titration, phase III study assessing the efficacy and tolerability of lanthanum carbonate: a new phosphate binder for the treatment of hyperphosphatemia. Am. J. Kidney Dis. 2003; 42: 96–107. [DOI] [PubMed] [Google Scholar]
- 21. Mehrotra R, Martin KJ, Fishbane S, Sprague SM, Zeig S, Anger M. Higher strength lanthanum carbonate provides serum phosphorus control with a low tablet burden and is preferred by patients and physicians: a multicenter study. Clin. J. Am. Soc. Nephrol. 2008; 3: 1437–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pennick M, Dennis K, Damment SJ. Absolute bioavailability and disposition of lanthanum in healthy human subjects administered lanthanum carbonate. J. Clin. Pharmacol. 2006; 46: 738–46. [DOI] [PubMed] [Google Scholar]
- 23. Pierce D, Hossack S, Robinson A, Zhang P, Martin P. Assessment of pharmacodynamic equivalence and tolerability of lanthanum carbonate oral powder and tablet formulations: a single‐center, randomized, open‐label, 2‐period crossover study in healthy subjects. Clin. Ther. 2012; 34: 1290–1300. [DOI] [PubMed] [Google Scholar]
- 24. Shigematsu T. Multicenter prospective randomized, double‐blind comparative study between lanthanum carbonate and calcium carbonate as phosphate binders in Japanese hemodialysis patients with hyperphosphatemia. Clin. Nephrol. 2008; 70: 404–10. [DOI] [PubMed] [Google Scholar]
- 25. Sprague SM, Abboud H, Qiu P, Dauphin M, Zhang P, Finn W. Lanthanum carbonate reduces phosphorus burden in patients with CKD stages 3 and 4: a randomized trial. Clin. J. Am. Soc. Nephrol. 2009; 4: 178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vemuri N, Michelis MF, Matalon A. Conversion to lanthanum carbonate monotherapy effectively controls serum phosphorus with a reduced tablet burden: a multicenter open‐label study. BMC Nephrol. 2011; 12: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu J, Zhang YX, Yu XQ et al. Lanthanum carbonate for the treatment of hyperphosphatemia in CKD 5D: multicenter, double blind, randomized, controlled trial in mainland China. BMC Nephrol. 2013; 14: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Canavese C, Mereu C, Nordio M, Sabbioni E, Aime S. Where have all the lanthanum salts gone, long time passing? Kidney Int. 2003; 64: 2327–8. [DOI] [PubMed] [Google Scholar]
- 29. Canavese C, Mereu C, Nordio M, Sabbioni E, Aime S. Blast from the past: the aluminum's ghost on the lanthanum salts. Curr. Med. Chem. 2005; 12: 1631–6. [DOI] [PubMed] [Google Scholar]
- 30. Drueke TB. Lanthanum carbonate as a first‐line phosphate binder: the “cons”. Semin. Dial. 2007; 20: 329–32. [DOI] [PubMed] [Google Scholar]
- 31. Frazao JM, Adragao T. Non‐calcium‐containing phosphate binders: comparing efficacy, safety, and other clinical effects. Nephron Clin. Pract. 2012; 120: c108–19. [DOI] [PubMed] [Google Scholar]
- 32. Molony DA, Murthy B. Accumulation of metals and minerals from phosphate binders. Blood Purif. 2005; 23 (Suppl 1): 2–11. [DOI] [PubMed] [Google Scholar]
- 33. Clinical practice guidelines for bone metabolism and disease in chronic kidney disease . The National Kidney Foundation Kidney Disease Outcomes Quality Initative (NFK KDOQI)™. Available from: http://www2.kidney.org/professionals/kdoqi/guidelines_bone/guide5.htm [Accessed 27 April 2016].
- 34. Sprague SM. A comparative review of the efficacy and safety of established phosphate binders: calcium, sevelamer, and lanthanum carbonate. Curr. Med. Res. Opin. 2007; 23: 3167–75. [DOI] [PubMed] [Google Scholar]
- 35. Damment SJ, Pennick M. Systemic lanthanum is excreted in the bile of rats. Toxicol. Lett. 2007; 171: 69–77. [DOI] [PubMed] [Google Scholar]
- 36. Floren C, Tekaya L, Escaig F, Labejof L, Mouthon G, Galle P. Analytical microscopy observations of rat enterocytes after oral administration of soluble salts of lanthanides, actinides and elements of group III‐A of the periodic chart. Cell. Mol. Biol. 2001; 47: 419–25. [PubMed] [Google Scholar]
- 37. Damment SJ, Pennick M. Clinical pharmacokinetics of the phosphate binder lanthanum carbonate. Clin. Pharmacokinet. 2008; 47: 553–63. [DOI] [PubMed] [Google Scholar]
- 38. Drueke TB. Intestinal absorption of aluminium in renal failure. Nephrol. Dial. Transplant. 2002; 17 (Suppl 2): 13–6. [DOI] [PubMed] [Google Scholar]
- 39. Krewski D, Yokel RA, Nieboer E et al. Human health risk assessment for aluminium, aluminium oxide, and aluminium hydroxide. J. Toxicol. Environ. Health B Crit. Rev. 2007; 10 (Suppl 1): 1–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lote CJ, Saunders H. Aluminium: gastrointestinal absorption and renal excretion. Clin. Sci. (Lond.) 1991; 81: 289–95. [DOI] [PubMed] [Google Scholar]
- 41. Savory J, Bertholf RL, Wills MR. Aluminium toxicity in chronic renal insufficiency. Clin. Endocrinol. Metab. 1985; 14: 681–702. [DOI] [PubMed] [Google Scholar]
- 42. Wilhelm M, Jager DE, Ohnesorge FK. Aluminium toxicokinetics. Pharmacol. Toxicol. 1990; 66: 4–9. [DOI] [PubMed] [Google Scholar]
- 43. Cannata‐Andia JB, Fernandez‐Martin JL. The clinical impact of aluminium overload in renal failure. Nephrol. Dial. Transplant. 2002; 17 (Suppl 2): 9–12. [DOI] [PubMed] [Google Scholar]
- 44. Principles of Bone Biology. Waltham, MA, USA: Academic Press, 2008. [Google Scholar]
- 45. Malluche HH, Siami GA, Swanepoel C et al. Improvements in renal osteodystrophy in patients treated with lanthanum carbonate for two years. Clin. Nephrol. 2008; 70: 284–95. [PubMed] [Google Scholar]
- 46. Bronner F, Slepchenko BM, Pennick M, Damment SJ. A model of the kinetics of lanthanum in human bone, using data collected during the clinical development of the phosphate binder lanthanum carbonate. Clin. Pharmacokinet. 2008; 47: 543–52. [DOI] [PubMed] [Google Scholar]
- 47. Shigematsu T, Tokumoto A, Nakaoka A, Arisaka H. Effect of lanthanum carbonate treatment on bone in Japanese dialysis patients with hyperphosphatemia. Ther. Apher. Dial. 2011; 15: 176–84. [DOI] [PubMed] [Google Scholar]
- 48. Spasovski GB, Sikole A, Gelev S et al. Evolution of bone and plasma concentration of lanthanum in dialysis patients before, during 1 year of treatment with lanthanum carbonate and after 2 years of follow‐up. Nephrol. Dial. Transplant. 2006; 21: 2217–24. [DOI] [PubMed] [Google Scholar]
- 49. Persy VP, Behets GJ, Bervoets AR, De Broe ME, D'Haese PC. Lanthanum: a safe phosphate binder. Semin. Dial. 2006; 19: 195–9. [DOI] [PubMed] [Google Scholar]
- 50. Behets GJ, Verberckmoes SC, Oste L et al. Localization of lanthanum in bone of chronic renal failure rats after oral dosing with lanthanum carbonate. Kidney Int. 2005; 67: 1830–6. [DOI] [PubMed] [Google Scholar]
- 51. Bervoets AR, Behets GJ, Schryvers D et al. Hepatocellular transport and gastrointestinal absorption of lanthanum in chronic renal failure. Kidney Int. 2009; 75: 389–98. [DOI] [PubMed] [Google Scholar]
- 52. Yang Z, Schryvers D, Roels F, D'Haese PC, De Broe ME. Demonstration of lanthanum in liver cells by energy‐dispersive X‐ray spectroscopy, electron energy loss spectroscopy and high‐resolution transmission electron microscopy. J. Microsc. 2006; 223: 133–9. [DOI] [PubMed] [Google Scholar]
- 53. Alfrey AC, LeGendre GR, Kaehny WD. The dialysis encephalopathy syndrome. Possible aluminum intoxication. N. Engl. J. Med. 1976; 294: 184–8. [DOI] [PubMed] [Google Scholar]
- 54. Rozas VV, Port FK, Rutt WM. Progressive dialysis encephalopathy from dialysate aluminum. Arch. Intern. Med. 1978; 138: 1375–7. [PubMed] [Google Scholar]
- 55. Alfrey AC. Dialysis encephalopathy. Kidney Int. Suppl. 1986; 18: S53–7. [PubMed] [Google Scholar]
- 56. Alfrey AC. Dialysis encephalopathy. Clin. Nephrol. 1985; 24 (Suppl 1): S15–9. [PubMed] [Google Scholar]
- 57. Evans CH. Biochemistry of the Lanthanides. New York: Plenum Press, 1990. [Google Scholar]
- 58. Kato M, Sugihara J, Nakamura T, Muto Y. Electron microscopic study of the blood‐brain barrier in rats with brain edema and encephalopathy due to acute hepatic failure. Gastroenterol. Jpn. 1989; 24: 135–42. [DOI] [PubMed] [Google Scholar]
- 59. Xu J, Ling EA. Studies of the ultrastructure and permeability of the blood‐brain barrier in the developing corpus callosum in postnatal rat brain using electron dense tracers. J. Anat. 1994; 184 (Pt 2): 227–37. [PMC free article] [PubMed] [Google Scholar]
- 60. LaRue B, Hogg E, Sagare A et al. Method for measurement of the blood‐brain barrier permeability in the perfused mouse brain: application to amyloid‐beta peptide in wild type and Alzheimer's Tg2576 mice. J. Neurosci. Methods 2004; 138: 233–42. [DOI] [PubMed] [Google Scholar]
- 61. Altmann P, Barnett ME, Finn WF. Cognitive function in Stage 5 chronic kidney disease patients on hemodialysis: no adverse effects of lanthanum carbonate compared with standard phosphate‐binder therapy. Kidney Int. 2007; 71: 252–9. [DOI] [PubMed] [Google Scholar]
- 62. Wesnes KA, Simpson PM, White L, Pinker S. The Cognitive Drug Research Computerized Assessment Systems for Elderly, AAMI & Demented Patients. J Psychopharmacol 1992; 6: 108. [DOI] [PubMed] [Google Scholar]
- 63. Swainston Harrison T, Scott LJ. Lanthanum carbonate. Drugs 2004; 64: 985–98. [DOI] [PubMed] [Google Scholar]
- 64. Navaneethan SD, Palmer SC, Craig JC, Elder GJ, Strippoli GF. Benefits and harms of phosphate binders in CKD: a systematic review of randomized controlled trials. Am. J. Kidney Dis. 2009; 54: 619–37. [DOI] [PubMed] [Google Scholar]
- 65. Zhang C, Wen J, Li Z, Fan J. Efficacy and safety of lanthanum carbonate on chronic kidney disease‐mineral and bone disorder in dialysis patients: a systematic review. BMC Nephrol. 2013; 14: 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cooney DR, Cutshall WD, Madura JA, Winegarner FG. Small bowel obstruction and ileal perforation: complications of uremia. J. Indiana State Med. Assoc. 1976; 69: 781–4. [PubMed] [Google Scholar]
- 67. Kurita N, Uchihara H. Fecalith formation and colonic perforation after lanthanum carbonate granules administration. Am. J. Kidney Dis. 2014; 63: 861–2. [DOI] [PubMed] [Google Scholar]
- 68. Korzets A. Lanthanum carbonate/oxycodone constipation, peritonitis and perforated colonic diverticulum in an elderly patient: case report. Reactions Weekly 2012; 1421: 34. [Google Scholar]
- 69. Highlights of prescribing information. FOSRENOL (lanthanum carbonate) chewable tablets, for oral use. US Food and Drug Administration. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021468s020,204734s001lbl.pdf (Accessed 8 June 2016).
- 70. Cerny S, Kunzendorf U. Images in clinical medicine. Radiographic appearance of lanthanum. N Engl J Med 2006; 355: 1160. [DOI] [PubMed] [Google Scholar]
- 71. Chuang CL, Chiou SY, Li SY, Jian DY, Chen JY. The case: a peritoneal dialysis patient with an unusual abdominal film. Treatment with lanthanum carbonate. Kidney Int. 2007; 72: 1291–2. [DOI] [PubMed] [Google Scholar]
- 72. Muller C, Chantrel F, Faller B. A confusional state associated with use of lanthanum carbonate in a dialysis patient: a case report. Nephrol. Dial. Transplant. 2009; 24: 3245–7. [DOI] [PubMed] [Google Scholar]
- 73. Kato A, Takita T, Furuhashi M. Accumulation of lanthanum carbonate in the digestive tracts. Clin. Exp. Nephrol. 2010; 14: 100–1. [DOI] [PubMed] [Google Scholar]
- 74. Crush L, O'Connor OJ, Plant W, Clarkson MR, Shanahan F, Maher MM. Perplexing plain abdominal x‐ray. Radiographic opacities were due to the lanthanum. Gut 2011; 60: 218–54. [DOI] [PubMed] [Google Scholar]
- 75. Makino M, Kawaguchi K, Shimojo H, Nakamura H, Nagasawa M, Kodama R. Extensive lanthanum deposition in the gastric mucosa: the first histopathological report. Pathol. Int. 2015; 65: 33–7. [DOI] [PubMed] [Google Scholar]
- 76. Haratake J, Yasunaga C, Ootani A, Shimajiri S, Matsuyama A, Hisaoka M. Peculiar histiocytic lesions with massive lanthanum deposition in dialysis patients treated with lanthanum carbonate. Am. J. Surg. Pathol. 2015; 39: 767–71. [DOI] [PubMed] [Google Scholar]
- 77. Wilson RJ, Johnson S, Marelli C. Lanthanum carbonate versus standard phosphate binder therapy: iron parameters in patients with end‐stage renal disease. Hemodial. Int. 2016; 20: 156. [Google Scholar]
- 78. Alfrey AC. Aluminum toxicity in patients with chronic renal failure. Ther. Drug Monit. 1993; 15: 593–7. [DOI] [PubMed] [Google Scholar]
- 79. Jeffery EH, Abreo K, Burgess E, Cannata J, Greger JL. Systemic aluminum toxicity: effects on bone, hematopoietic tissue, and kidney. J. Toxicol. Environ. Health 1996; 48: 649–65. [DOI] [PubMed] [Google Scholar]
- 80. Garbossa G, Galvez G, Castro ME, Nesse A. Oral aluminum administration to rats wih normal renal function. 1. Impairment of erythropoiesis. Hum. Exp. Toxicol. 1998; 17: 312–7. [DOI] [PubMed] [Google Scholar]
- 81. Cannata‐Andia JB, Fernandez‐Martin JL. The clinical impact of aluminium overload in renal failure. Nephrol. Dial. Transplant. 2002; 17 (Suppl 2): 9–12. [DOI] [PubMed] [Google Scholar]
- 82. Foley RN, Collins AJ. The USRDS: what you need to know about what it can and can't tell us about ESRD. Clin. J. Am. Soc. Nephrol. 2013; 8: 845–51. [DOI] [PubMed] [Google Scholar]
- 83. Jamal SA, Vandermeer B, Raggi P et al. Effect of calcium‐based versus non‐calcium‐based phosphate binders on mortality in patients with chronic kidney disease: an updated systematic review and meta‐analysis. Lancet 2013; 382: 1268–77. [DOI] [PubMed] [Google Scholar]
- 84. Moe S, Drueke T, Cunningham J et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2006; 69: 1945–53. [DOI] [PubMed] [Google Scholar]


