Abstract
Objective
To assess whether secukinumab treatment in patients with active psoriatic arthritis (PsA) is associated with sustained inhibition of radiographic progression.
Methods
In this phase III, double‐blind, placebo‐controlled study, 606 patients with PsA were randomized to receive intravenous (IV) secukinumab at a dose of 10 mg/kg (weeks 0, 2, 4) followed by subcutaneous secukinumab at a dose of 150 mg or 75 mg (the IV→150 mg and IV→75 mg groups, respectively) or placebo. Patients were stratified according to prior anti–tumor necrosis factor (anti‐TNF) exposure (71% were anti‐TNF naive). At week 16, placebo‐treated patients who had at least a 20% reduction in the tender and swollen joint counts (responders) continued to receive placebo until week 24; nonresponders were re‐randomized to receive secukinumab at a dose of 150 mg or 75 mg. The modified total Sharp/van der Heijde score (SHS) was determined at baseline, week 16 or 24, and week 52.
Results
In the overall population, radiographic progression was inhibited through 52 weeks; efficacy was demonstrated for both erosion and joint space narrowing scores and in patients who switched from placebo to secukinumab at week 24. Subgroup analyses showed that secukinumab reduced radiographic progression at week 24, regardless of previous anti‐TNF treatment. Among anti‐TNF–naive patients, the mean changes from baseline to week 24 in the modified total SHS were 0.05 in the pooled secukinumab group and 0.57 in the placebo group; among patients with an inadequate response or intolerance to anti‐TNF treatment, the mean changes were 0.16 and 0.58, respectively. Anti‐TNF–naive patients showed negligible progression through week 52. Inhibition of structural damage was observed through week 52 irrespective of concomitant methotrexate use. A high proportion of patients receiving secukinumab showed no progression (change in SHS of ≤ 0.5) from baseline to week 24 (82.3% of the IV→150 mg group and 92.3% of the IV→75 mg group) and from week 24 to week 52 (85.7% of the IV→150 mg group and 85.8% of the IV→75 mg group).
Conclusion
Secukinumab inhibited radiographic progression over 52 weeks of treatment in patients with active PsA.
Psoriatic arthritis (PsA), a chronic inflammatory arthritis characterized by structural damage to the joints, has been associated with reduced health‐related quality of life, disability, and reduced life expectancy 1, 2, 3. The joint changes in PsA are characterized radiographically by a combination of erosive and proliferative bone changes, including erosive joint destruction, fluffy periostitis, and pencil‐in‐cup deformities 4. Radiographic assessment of joints is recommended to aid in the diagnosis of PsA, provide information on disease severity, and assess the effect of treatment on disease progression 5.
Interleukin‐17A (IL‐17A) has been implicated in the pathogenesis of PsA, including progression of joint damage 6. Cells producing IL‐17A are observed in the joints of patients with PsA and correlate with disease activity, structural damage, and disease progression 6. To this end, drugs targeting IL‐17A are of interest in the search for new PsA treatments, and a number of these agents are currently in late‐stage clinical development.
Secukinumab is a fully human IgG1 monoclonal antibody that selectively binds to and neutralizes IL‐17A. In the placebo‐controlled, double‐blind, phase III FUTURE 1 study, secukinumab provided rapid, significant, and sustained improvements in key clinical domains of PsA, including signs and symptoms, physical functioning, and quality of life 7. Moreover, secukinumab significantly reduced radiographic progression, as measured by change from baseline to week 24 in the modified total Sharp/van der Heijde score (SHS) for PsA 8, compared with placebo 7. The current report describes 52‐week radiography results from FUTURE 1, and presents the results of analyses related to previous anti–tumor necrosis factor (anti‐TNF) therapy with concomitant methotrexate (MTX).
PATIENTS AND METHODS
Details of the FUTURE 1 study (ClinicalTrials.gov identifier: NCT01392326) design, patient eligibility criteria, and end points have been previously reported 7. Briefly, patients with PsA were randomized 1:1:1 to receive secukinumab (10 mg/kg intravenously [IV] at baseline and at weeks 2 and 4 followed by 150 mg [IV→150 mg group] or 75 mg [IV→75 mg group] administered subcutaneously [SC] every 4 weeks thereafter) or placebo (according to the same schedules). Randomization of the patients was stratified according to previous exposure to anti‐TNF therapy. A washout period of 4–10 weeks after anti‐TNF treatment was required prior to randomization. Concomitant MTX therapy was permitted. The study was approved by the institutional review board or ethics committees at participating sites and was conducted in accordance with the principles of the Declaration of Helsinki. All participants provided written informed consent.
At week 16, all patients were assessed for joint‐related responses (tender and swollen joint counts) and were classified as responders (≥20% improvement from baseline in tender and swollen joint counts) or nonresponders. Placebo‐treated patients were re‐randomized (1:1) to receive secukinumab 75 mg or 150 mg SC every 4 weeks without a loading dose beginning at either week 16 (nonresponders) or week 24 (responders). Nonresponders in the secukinumab treatment groups continued to receive their assigned treatment. Radiographic progression was measured using the modified total SHS for PsA, which is the sum of erosion and joint space narrowing (JSN) scores 8. The total possible erosion and JSN scores are 320 and 208, respectively, resulting in a maximum modified total SHS of 528. Radiographs of the hands/wrists and feet were obtained at baseline, week 16 (nonresponders) or week 24 (responders), and week 52. Radiographs were read independently by 2 readers (Dr. Charles Peterfy and Dr. Yan Chen, Spire Sciences, Boca Raton, FL), who were blinded with regard to radiograph chronology, type of therapy, and patient clinical status. The mean score was used for all analyses.
A nonparametric analysis of covariance model with treatment regimen and TNF inhibitor status as factors, and weight and baseline modified total SHS as covariates was used to evaluate the effect of secukinumab versus placebo at week 24. Prespecified analysis of stratified subgroups based on previous anti‐TNF therapy was also performed. A subgroup analysis according to baseline MTX treatment (none versus concomitant) was performed post hoc. For placebo‐treated patients who met the criteria for early escape at week 16 and patients who discontinued the study prior to week 24, linear extrapolation was used to impute values at week 24. Data for week 52 are presented for radiography completers (defined as patients who had radiographic assessments at baseline, week 16 or 24, and week 52), with no extrapolation for missing data. A preplanned sensitivity analysis was undertaken to evaluate whether radiography completer data and linearly extrapolated data were comparable.
Cumulative probability plots were generated to show the modified total SHS, erosion score, and JSN score for all patients according to dose group from baseline to week 24 and from week 24 to week 52. As recommended in published guidelines, a change of ≤0.5 in the modified total SHS was used to define no structural progression, and this was assessed from baseline to week 24 and from week 24 to week 52 in radiography completers 9. An exploratory exposure‐response analysis of change in the modified total SHS from week 24 to week 52 versus the trough secukinumab concentration (Cmin) at week 52 was also performed.
RESULTS
Patient disposition and baseline characteristics
A total of 606 patients were randomized (Figure 1). Demographics, disease characteristics, prior anti‐TNF treatment, and concomitant MTX treatment were comparable across the groups. Overall, 71% of patients were anti‐TNF naive, and 61% were receiving concomitant MTX (see Supplementary Table 1, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39685/abstract). The numbers of patients who had linear extrapolation applied at week 24 were as follows: 55 in the IV→150 mg group, 40 in the IV→75 mg group, and 109 in the placebo group.
Figure 1.
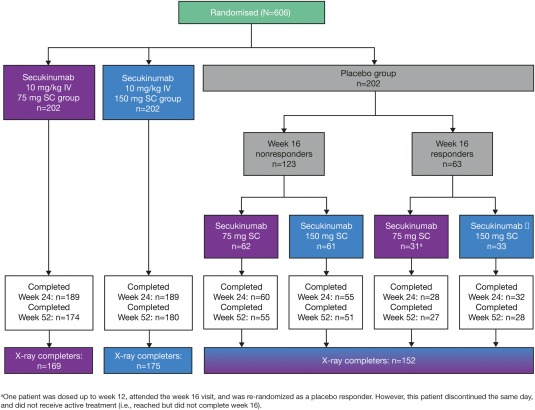
Patient disposition and flow through the trial from screening to week 52. At week 16, patients in the placebo group were classified as nonresponders or responders. Nonresponders were randomized to receive secukinumab 75 mg or 150 mg subcutaneously (SC) at week 16 and then every 4 weeks thereafter. Responders were randomized to receive secukinumab 75 mg or 150 mg SC at week 24 and then every 4 weeks thereafter. Patients who had radiographic assessments at baseline, week 16 or 24, and week 52 were defined as radiography (x‐ray) completers. IV = intravenous.
Radiographic progression at week 24.
Secukinumab‐treated patients showed significantly less radiographic progression from baseline to week 24 compared with placebo‐treated patients (Table 1 and Figure 2). The mean changes in the modified total SHS from baseline to week 24 were 0.08 in the pooled secukinumab group, 0.13 in the IV→150 mg group, and 0.02 in the IV→75 mg group, compared with 0.57 in the placebo group (all P < 0.05 versus placebo). In the pooled secukinumab group and the placebo group, the mean changes in the erosion score were 0.06 and 0.35, respectively, and the mean changes in the JSN score were 0.02 and 0.23, respectively. Radiographic progression was visualized using cumulative probability plots showing change from baseline to week 24 in the modified total SHS, the erosion score, and the JSN score. More placebo‐treated patients showed progression (and to a greater extent) than secukinumab‐treated patients (Figure 2 and Supplementary Figure 1, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39685/abstract).
Table 1.
Changes in the modified total SHS, erosion score, and JSN score from baseline to week 24 (full analysis set) and from week 24 to week 52 (radiography completers)a
| Baseline to week 24 | Week 24 to week 52 | |||||||
|---|---|---|---|---|---|---|---|---|
| Population | Secukinumab IV→150 mg | Secukinumab IV→75 mg | Secukinumab pooled | Placebo | Secukinumab 150 mg | Secukinumab 75 mg | Secukinumab pooled | Placebo→secukinumab |
| Overallb | ||||||||
| No. of patients | 185 | 181c | 366d | 179 | 175 | 169 | 344 | 152 |
| Modified SHS | 0.13 ± 1.18e | 0.02 ± 1.60e | 0.08 ± 1.40e | 0.57 ± 2.48 | 0.23 ± 2.41 | 0.20 ± 1.00 | 0.21 ± 1.86 | −0.03 ± 1.62 |
| Erosion score | 0.04 ± 0.60f | 0.08 ± 0.93f | 0.06 ± 0.78f | 0.35 ± 1.62 | 0.18 ± 1.41 | 0.12 ± 0.71 | 0.15 ± 1.12 | 0.01 ± 0.99 |
| JSN score | 0.10 ± 0.96 | −0.06 ± 0.93f | 0.02 ± 0.95 | 0.23 ± 1.24 | 0.05 ± 1.19 | 0.08 ± 0.59 | 0.06 ± 0.94 | −0.03 ± 0.91 |
| Anti‐TNF naiveg | ||||||||
| No. of patients | 135 | 128 | 263 | 129 | 130 | 121 | 251 | 116 |
| Modified SHS | 0.15 ± 1.09 | −0.06 ± 1.61f | 0.05 ± 1.37f | 0.57 ± 2.78 | −0.01 ± 0.81 | 0.14 ± 0.75 | 0.06 ± 0.78 | −0.12 ± 1.70 |
| Erosion score | 0.02 ± 0.53 | 0.00 ± 0.78 | 0.01 ± 0.66 | 0.29 ± 1.80 | 0.06 ± 0.66 | 0.09 ± 0.43 | 0.08 ± 0.56 | −0.02 ± 1.06 |
| JSN score | 0.13 ± 1.01 | −0.06 ± 0.96f | 0.04 ± 0.99 | 0.28 ± 1.37 | −0.07 ± 0.57 | 0.05 ± 0.43 | −0.01 ± 0.51 | −0.10 ± 0.95 |
| Anti‐TNF IRg | ||||||||
| No. of patients | 50 | 53 | 103 | 50 | 45 | 48 | 93 | 36 |
| Modified SHS | 0.10 ± 1.39f | 0.21 ± 1.57 | 0.16 ± 1.48f | 0.58 ± 1.45 | 0.91 ± 4.53 | 0.34 ± 1.46 | 0.61 ± 3.31 | 0.27 ± 1.29 |
| Erosion score | 0.08 ± 0.77f | 0.25 ± 1.19 | 0.17 ± 1.01 | 0.50 ± 1.02 | 0.51 ± 2.53 | 0.20 ± 1.15 | 0.35 ± 1.94 | 0.09 ± 0.70 |
| JSN score | 0.02 ± 0.82 | −0.05 ± 0.87 | −0.01 ± 0.84 | 0.09 ± 0.79 | 0.39 ± 2.12 | 0.13 ± 0.87 | 0.26 ± 1.59 | 0.19 ± 0.76 |
| Without concomitant MTXh | ||||||||
| No. of patients | 74 | 76 | 150 | 65 | 69 | 69 | 138 | 54 |
| Modified SHS | 0.12 ± 0.78 | 0.14 ± 1.21 | 0.13 ± 1.02 | 0.58 ± 3.24 | 0.04 ± 0.95 | 0.16 ± 1.13 | 0.10 ± 1.04 | 0.25 ± 1.43 |
| Erosion score | 0.02 ± 0.38 | 0.17 ± 0.90 | 0.10 ± 0.70 | 0.37 ± 2.30 | 0.08 ± 0.55 | 0.13 ± 0.97 | 0.10 ± 0.78 | 0.19 ± 0.96 |
| JSN score | 0.10 ± 0.67 | −0.03 ± 0.70 | 0.04 ± 0.68 | 0.21 ± 1.05 | −0.04 ± 0.65 | 0.03 ± 0.50 | −0.01 ± 0.58 | 0.06 ± 0.73 |
| Concomitant MTXh | ||||||||
| No. of patients | 111 | 105 | 216 | 114 | 106 | 100 | 206 | 98 |
| Modified SHS | 0.14 ± 1.38 | −0.07 ± 1.83f | 0.04 ± 1.61f | 0.57 ± 1.93 | 0.35 ± 3.00 | 0.22 ± 0.91 | 0.29 ± 2.24 | −0.18 ± 1.70 |
| Erosion score | 0.04 ± 0.71f | 0.01 ± 0.94f | 0.03 ± 0.83f | 0.34 ± 1.07 | 0.25 ± 1.75 | 0.11 ± 0.46 | 0.18 ± 1.30 | −0.09 ± 0.99 |
| JSN score | 0.10 ± 1.11 | −0.08 ± 1.07 | 0.01 ± 1.10 | 0.24 ± 1.33 | 0.10 ± 1.44 | 0.11 ± 0.64 | 0.11 ± 1.12 | −0.09 ± 1.00 |
Linear extrapolation was performed at week 24; no linear extrapolation was applied at week 52. Values are the mean ± SD. SHS = Sharp/van der Heidje score; JSN = joint space narrowing; IV = intravenous; anti‐TNF IR = inadequate response or intolerance to anti–tumor necrosis factor; MTX = methotrexate.
Included in the predefined hierarchical hypothesis testing strategy to adjust for multiplicity at week 24.
The erosion score was determined in 182 patients.
The erosion score was determined in 367 patients.
P < 0.05 versus placebo, adjusted for multiplicity of testing.
P < 0.05 versus placebo, unadjusted.
Prespecified subgroup analysis.
Post hoc subgroup analysis.
Figure 2.
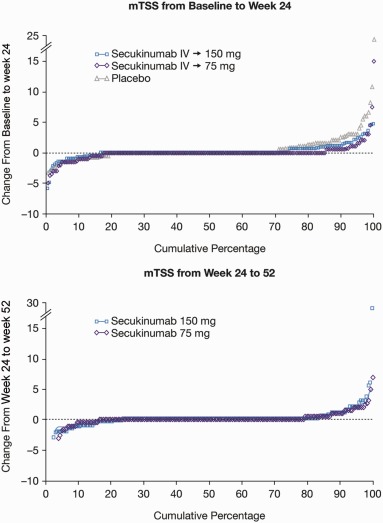
Cumulative probability plots of mean changes in the modified total Sharp/van der Heijde score (SHS; mTSS). Top, Mean changes from baseline to week 24 in patients receiving intravenous (IV) secukinumab followed by subcutaneous secukinumab at a dose of 150 mg (IV→150 mg) or 75 mg (IV→75 mg) and placebo‐treated patients. Data are for the full analysis set, with linear extrapolation for all patients who underwent radiographic assessment at week 16. Bottom, Mean changes from week 24 to week 52 in patients receiving secukinumab. Data are for radiography completers (patients who had radiographic assessments at baseline, week 16 or 24, and week 52), with no extrapolation applied.
The impact of applying linear extrapolation for missing values was evaluated in a sensitivity analysis, which considered an alternative, last observation carried forward (LOCF) approach for the patients who were nonresponders at week 16. The mean changes from baseline to week 24 using LOCF were 0.10 (secukinumab IV→150 mg; P = 0.0253), 0.03 (secukinumab IV→ 75 mg; P = 0.0314), and 0.46 (placebo). For the active secukinumab arms, using the LOCF approach produced results comparable with those obtained using linear extrapolation (mean changes of 0.13 in the IV→ 150 mg group and 0.02 in the IV→75 mg group); however, the value for the placebo arm was greater when the latter approach was used (mean change 0.57). Nevertheless, both approaches conclusively demonstrated that secukinumab treatment inhibited structural progression.
A separate sensitivity analysis, in which patients with a mean change in the modified total SHS of >10 were excluded (2 from the placebo group and 1 from the secukinumab IV→75 mg group), supported the robustness of these data. Removal of these 3 outliers decreased the mean change in the placebo and secukinumab IV→75 mg groups but had little effect on the level of statistical significance versus placebo (see Supplementary Table 2, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39685/abstract).
A prespecified analysis of patients stratified according to anti‐TNF therapy showed less progression in secukinumab‐treated patients compared with placebo‐treated patients among both anti‐TNF–naive patients and patients who had an inadequate response or intolerance to anti‐TNF therapy. The mean changes from baseline to week 24 in the modified total SHS among anti‐TNF–naive patients in the pooled secukinumab, IV→150 mg, IV→75 mg, and placebo groups were 0.05, 0.15, −0.06, and 0.57, respectively (Table 1). The mean changes from baseline to week 24 in the modified total SHS among patients with an inadequate response to anti‐TNF in the pooled secukinumab, IV→150 mg, IV→75 mg, and placebo groups were 0.16, 0.10, 0.21, and 0.58, respectively. In an exploratory post hoc analysis, inhibition of structural damage was also observed irrespective of concomitant MTX therapy (Table 1).
Radiographic assessment at week 52
The mean changes in the modified total SHS from week 24 to week 52 among patients initially randomized to receive secukinumab 150 mg and 75 mg were 0.23 and 0.20, respectively, compared with mean changes of 0.13 and 0.02, respectively, from baseline to week 24 (Table 1). From week 24 to week 52, the mean changes in the erosion score in the secukinumab 150 mg and 75 mg groups were 0.18 and 0.12, respectively, and the mean changes in the JSN score in the secukinumab 150 mg and 75 mg groups were 0.05 and 0.08, respectively. Inhibition of structural progression was also demonstrated in placebo‐treated patients who switched to secukinumab (Table 1). The mean change in the modified total SHS from week 24 to week 52 in placebo‐treated patients who switched to secukinumab was −0.03, compared with 0.57 in patients who received placebo from baseline to week 24. Cumulative probability plots indicated that both secukinumab doses were effective for inhibiting structural progression through 52 weeks of therapy (Figure 2 and Supplementary Figure 1, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39685/abstract).
To investigate the effect of using radiography completer data, a preplanned sensitivity analysis was conducted using linear extrapolation for patients who were missing week 52 data but had a postbaseline assessment at week 16 or week 24. The mean changes in the modified total SHS from baseline to week 52 using linear extrapolation and radiography completer data were 0.37 and 0.35, respectively (secukinumab IV→150 mg), 0.22 and 0.20, respectively (secukinumab IV→75 mg), and 0.70 and 0.46, respectively (placebo→secukinumab). Thus, although placebo → secukinumab data showed a higher progression rate using linear extrapolation of missing values, both data sets were comparable for both secukinumab groups, indicating that the radiography completer analysis offered a conservative estimate.
Structural progression from week 24 to week 52 in anti‐TNF–naive patients receiving secukinumab remained low (Table 1). The mean changes in the modified total SHS in the secukinumab 150 mg and 75 mg groups were −0.01 and 0.14, respectively. Among patients with an inadequate response or intolerance to anti‐TNF, the mean changes in the modified total SHS were 0.91 in the secukinumab 150 mg group and 0.34 in the secukinumab 75 mg group. Among patients who switched from placebo to secukinumab at week 24, low progression was observed in both the anti‐TNF–naive subgroup and the subgroup of patients with an inadequate response or intolerance to anti‐TNF treatment. The mean changes in the modified total SHS from week 24 to week 52 in anti‐TNF–naive patients and those with an inadequate response to anti‐TNF treatment in the placebo→secukinumab arm were −0.12 and 0.27, respectively. Furthermore, low radiographic progression was observed in the exploratory analysis in patients with and those without concomitant MTX therapy (Table 1).
An exposure‐response analysis showed a similar relationship between the secukinumab IV→SC and the placebo→secukinumab treatment groups at week 52 (see Supplementary Figure 2a, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39685/abstract). This suggested that the IV→SC and SC regimens have a comparable effect on radiographic progression, and that IV loading is not critical to prevent structural progression. Trends in subgroups stratified according to TNF inhibitor status were also apparent in this analysis. Patients who had an inadequate response to anti‐TNF treatment and low secukinumab concentrations were at particular risk of a higher rate of radiographic progression (see Supplementary Figure 2b). All 5 patients with the greatest increase in the modified total SHS were in the subgroup with an inadequate response to anti‐TNF treatment and had low (Cmin ≤10 μg/ml) drug concentrations.
Inhibition of structural progression
Data on radiography completers (no extrapolation applied) from the overall population demonstrated a sustained therapeutic effect (modified total SHS change from baseline of ≤0.5) of secukinumab on radiographic assessment from baseline to week 24 and from week 24 to week 52. In the secukinumab IV→150 mg group, 82.3% and 85.7% of patients had inhibition of structural progression from baseline to week 24 and from week 24 to week 52, respectively. In the secukinumab IV→75 mg group, 92.3% and 85.8% had inhibition of progression from baseline to week 24 and from week 24 to week 52, respectively. The proportion of placebo‐treated patients with inhibition of structural progression significantly increased from 75.7% (baseline to week 24) to 86.8% (week 24 to week 52) following secukinumab treatment (P = 0.0131). The switch from placebo to secukinumab at week 24 reduced the structural progression rate from 24.3% to 13.2%. Eleven of the 12 patients who had a high rate of progression (all with a modified total SHS of >3) had low (Cmin ≤15 μg/ml) secukinumab concentrations at week 52.
DISCUSSION
The results of the current study demonstrate that secukinumab inhibited radiographic disease progression in the overall PsA patient population, and that this inhibition was sustained for up to 52 weeks. Inhibition was observed for both erosion and JSN scores. Furthermore, progression in placebo‐treated patients was inhibited by switching to secukinumab treatment. In patients who showed the highest levels of radiographic progression, suboptimal secukinumab concentrations may have been linked to an increased likelihood of progression.
Subgroup analyses showed radiographic benefits of secukinumab up to week 24, regardless of prior anti‐TNF exposure. From week 24 to week 52, a low rate of radiographic progression was observed in secukinumab‐treated patients in the anti‐TNF–naive subgroup but not in secukinumab‐treated patients in the subgroup with an inadequate response to anti‐TNF treatment. Although these data should be interpreted with caution due to small patient numbers, this difference may be attributable to the difficulties associated with treatment in this latter population. The exploratory exposure‐response analysis indicated that insufficient secukinumab exposure after the IV loading period may have prevented adequate inhibition of radiographic progression in the subgroup with an inadequate response to anti‐TNF treatment, although there was no tendency toward a lower rate of progression in the higher‐dose secukinumab group during the loading period. Thus, patients with an inadequate response or intolerance to anti‐TNF therapy may require a higher dose of secukinumab to maintain acceptable secukinumab exposure levels for inhibition of structural damage.
Inhibition of structural damage was demonstrated through week 52, regardless of whether patients were receiving concomitant MTX, although these data should be interpreted with caution due to the fact that patients had active disease despite receiving MTX. The effect of concomitant MTX can be assessed only in an active comparator trial with both MTX‐naive patients and those who have been treated with MTX.
Compared with placebo, secukinumab significantly reduced radiographic progression, demonstrating a disease‐modifying effect. The average progression rate in all groups was low, although a small proportion of patients, especially those in the placebo groups, showed significant progression. Clinical trials are not appropriate for assessing the clinical relevance of this reduction; instead, this needs to be determined using long‐term observational cohorts. Also, it should be noted that the mean modified total SHS may not adequately reflect radiographic progression and should be interpreted with care, because it may be disproportionately influenced by outliers.
In FUTURE 1, the efficacy of secukinumab for inhibiting radiographic disease progression and improving clinical measures was comparable between the IV→ 150 mg and IV→75 mg dose arms after 24 weeks, due to the IV loading dose resulting in similar levels of exposure across both arms 7. In the recently published FUTURE 2 trial, which used subcutaneous loading and maintenance dosing but did not include a radiographic outcome measure, secukinumab 300 mg and 150 mg significantly improved a range of clinical outcomes in patients with PsA compared with placebo at week 24, while secukinumab 75 mg was ineffective 10. Consequently, the 150 mg and 300 mg secukinumab doses were recently approved for PsA in Europe.
A recent systematic review highlighted the inhibitory effect of anti‐TNF agents on structural damage in PsA 11. However, failure of anti‐TNF treatment, loss of efficacy, and intolerance in some patients highlight the unmet need for new therapies with an alternative mechanism of action 12. The radiographic findings from this study are consistent with those from other trials of TNF and IL‐12/23 inhibitors involving anti‐TNF–naive populations 13, 14, 15, 16, 17 and suggest that IL‐17A may offer an additional therapeutic option for patients with PsA.
The current study has several limitations. First, patients who were nonresponders at week 16 were not assessed at week 24, and radiographic data on these patients were estimated by linear extrapolation. Additionally, only erosions and JSN were assessed, and no information on bone formation was provided. However, this is a theoretical consideration, because a previous trial suggested that new bone development may not occur during the typical treatment period of 1 year 17. Of note, tender and swollen joint counts were chosen for this trial because these are commonly used in phase III studies of PsA. Newer measures such as the Simplified Disease Activity Index 18 and the Clinical Disease Activity Index 19 have not been used in this setting to date and therefore were not considered appropriate.
In conclusion, in the present study secukinumab treatment was associated with sustained inhibition of radiographic progression in patients with active PsA.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. van der Heijde had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. van der Heijde, Landewé, Mease, McInnes, Conaghan, Ligozio, Richards, Mpofu.
Acquisition of data. Mease, Conaghan, Mpofu.
Analysis and interpretation of data. van der Heijde, Landewé, Mease, McInnes, Conaghan, Pricop, Ligozio, Richards, Mpofu.
ROLE OF THE STUDY SPONSOR
Novartis Pharma provided writing and editorial assistance (performed by Jessica Breen, Joanna Chapman, and Ben Drever, Seren Communications, an Ashfield Company, part of UGD Healthcare, and by John Gallagher, Novartis Pharma. Publication of this article was not contingent upon approval by Novartis Pharma.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
We thank the patients who participated in the study and the study investigators. We also thank Didier Renard (Novartis Pharma) for his valued input.
Supported by Novartis Pharma AG.
Dr. van der Heijde has received research grants and/or consulting fees from AbbVie, Amgen, Astellas, AstraZeneca, Augurex, Bristol‐Myers Squibb, Boehringer‐Ingelheim, Celgene, Centocor, Chugai, Covagen, Daiichi, Eli‐Lilly, Galapagos, GlaxoSmithKline, Janssen Biologics, Merck, Novartis, Novo Nordisk, Otsuka, Pfizer, Roche, Sanofi Aventis, UCB, and Vertex (less than $10,000 each) and is the director of Imaging Rheumatology. Dr. Landewé has received consulting fees, speaking fees, and/or honoraria from Abbott/AbbVie, Ablynx, Amgen, AstraZeneca, Bristol‐Myers Squibb, Centocor, GlaxoSmithKline, Novartis, Merck, Pfizer, Roche, Schering‐Plough, UCB, and Wyeth (less than $10,000 each), has received research grants from Abbott, Amgen, Centocor, Novartis, Pfizer, Roche, Schering‐Plough, UCB, and Wyeth, and is director of Rheumatology Consultancy. Dr. Mease has received consulting fees and/or speaking fees from AbbVie, Amgen, Biogen Idec, Bristol‐Myers Squibb, Celgene, Covagen, Crescendo, Janssen, Lilly, Merck, Novartis, Pfizer, and UCB (less than $10,000 each) and research grants from AbbVie, Amgen, Biogen Idec, Bristol‐Myers Squibb, Celgene, Crescendo, Janssen, Lilly, Merck, Novartis, Pfizer, and UCB. Dr. McInnes has received consulting fees, speaking fees, and/or honoraria from Novartis, Bristol‐Myers Squibb, Janssen, AbbVie, Pfizer, and UCB (less than $10,000 each). Dr. Conaghan has received consulting fees and/or speaking fees from AbbVie, Janssen, Novartis, Pfizer, and Roche (less than $10,000 each) and a research grant from Bristol‐Myers Squibb. Drs. Pricop and Mpofu own stock or stock options in Novartis.
REFERENCES
- 1. Gladman DD, Antoni C, Mease P, Clegg DO, Nash P. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis 2005;64:ii14–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rosen CF, Mussani F, Chandran V, Eder L, Thavaneswaran A, Gladman DD. Patients with psoriatic arthritis have worse quality of life than those with psoriasis alone. Rheumatology (Oxford) 2012;51:571–6. [DOI] [PubMed] [Google Scholar]
- 3. Ogdie A, Haynes K, Troxel AB, Love TJ, Hennessy S, Choi H, et al. Risk of mortality in patients with psoriatic arthritis, rheumatoid arthritis and psoriasis: a longitudinal cohort study. Ann Rheum Dis 2014;73:149–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Day MS, Nam D, Goodman S, Su EP, Figgie M. Psoriatic arthritis. J Am Acad Orthop Surg 2012;20:28–37. [DOI] [PubMed] [Google Scholar]
- 5. Gottlieb A, Korman NJ, Gordon KB, Feldman SR, Lebwohl M, Koo JY, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 2. Psoriatic arthritis: overview and guidelines of care for treatment with an emphasis on the biologics. J Am Acad Dermatol 2008;58:851–64. [DOI] [PubMed] [Google Scholar]
- 6. Menon B, Gullick NJ, Walter GJ, Rajasekhar M, Garrood T, Evans HG, et al. Interleukin‐17+CD8+ T cells are enriched in the joints of patients with psoriatic arthritis and correlate with disease activity and joint damage progression. Arthritis Rheumatol 2014;66:1272–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mease PJ, McInnes IB, Kirkham B, Kavanaugh A, Rahman P, van der Heijde D, et al. Secukinumab inhibition of interleukin‐17A in patients with psoriatic arthritis. N Engl J Med 2015;373:1329–39. [DOI] [PubMed] [Google Scholar]
- 8. Van der Heijde D, Sharp J, Wassenberg S, Gladman DD. Psoriatic arthritis imaging: a review of scoring methods. Ann Rheum Dis 2005;64:ii61–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Van der Heijde D, Simon L, Smolen J, Strand V, Sharp J, Boers M, et al. How to report radiographic data in randomized clinical trials in rheumatoid arthritis: guidelines from a roundtable discussion. Arthritis Rheum 2002;47:215–8. [DOI] [PubMed] [Google Scholar]
- 10. McInnes IB, Mease PJ, Kirkham B, Kavanaugh A, Ritchlin CT, Rahman P, et al. Secukinumab, a human anti‐interleukin‐17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet 2015; 386:1137–46. [DOI] [PubMed] [Google Scholar]
- 11. Goulabchand R, Mouterde G, Barnetche T, Lukas C, Morel J, Combe B. Effect of tumour necrosis factor blockers on radiographic progression of psoriatic arthritis: a systematic review and meta‐analysis of randomised controlled trials. Ann Rheum Dis 2014;73:414–9. [DOI] [PubMed] [Google Scholar]
- 12. Mease PJ, Armstrong AW. Managing patients with psoriatic disease: the diagnosis and pharmacologic treatment of psoriatic arthritis in patients with psoriasis. Drugs 2014;74:423–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kavanaugh A, Ritchlin C, Rahman P, Puig L, Gottlieb AB, Li S, et al. Ustekinumab, an anti‐IL‐12/23 p40 monoclonal antibody, inhibits radiographic progression in patients with active psoriatic arthritis: results of an integrated analysis of radiographic data from the phase 3, multicentre, randomised, double‐blind, placebo‐controlled PSUMMIT‐1 and PSUMMIT‐2 trials. Ann Rheum Dis 2014;73:1000–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mease PJ, Gladman DD, Ritchlin CT, Ruderman EM, Steinfeld SD, Choy EH. Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double‐blind, randomized, placebo‐controlled trial. Arthritis Rheum 2005;52:3279–89. [DOI] [PubMed] [Google Scholar]
- 15. Van der Heijde D, Fleischmann R, Wollenhaupt J, Deodhar A, Kielar D, Woltering F. Effect of different imputation approaches on the evaluation of radiographic progression in patients with psoriatic arthritis: results of the RAPID‐PsA 24‐week phase III double‐blind randomised placebo‐controlled study of certolizumab pegol. Ann Rheum Dis 2014;73:233–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kavanaugh A, van der Heijde D, McInnes IB, Mease P, Krueger GG, Gladman DD. Golimumab in psoriatic arthritis: one‐year clinical efficacy, radiographic, and safety results from a phase III, randomized, placebo‐controlled trial. Arthritis Rheum 2012;64:2504–17. [DOI] [PubMed] [Google Scholar]
- 17. Mease PJ, Kivitz AJ, Burch FX, Siegel EL, Cohen SB, Ory P, et al. Etanercept treatment of psoriatic arthritis: safety, efficacy, and effect on disease progression. Arthritis Rheum 2004;50:2264–72. [DOI] [PubMed] [Google Scholar]
- 18. Smolen JS, Breedveld FC, Schiff MH, Kalden JR, Emery P, Eberl G, et al. A Simplified Disease Activity Index for rheumatoid arthritis for use in clinical practice. Rheumatology (Oxford) 2003;42:244–57. [DOI] [PubMed] [Google Scholar]
- 19. Aletaha D, Nell VP, Stamm T, Uffmann M, Pflugbeil S, Machold K, et al. Acute phase reactants add little to composite disease activity indices for rheumatoid arthritis: validation of a clinical activity score. Arthritis Res Ther 2005;7:R796–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


