Summary
Aim
To compare atomoxetine (ATX) length of therapy (LoT) among adults with ADHD who reached the recommended dose of 80 mg/day (ATX ≥ 80) versus those who did not (ATX < 80) analyzed separately in patients prescribed ATX as monotherapy (mono) and in combination with other ADHD medications (combo).
Methods
This was a retrospective observational cohort study of the Truven Health Marketscan Commercial Claims Database from January 1, 2006–September 30, 2013, with a 6‐month preindex period free of ATX (1st ATX claim as index event) and a 1‐year follow‐up. LoT during follow‐up was calculated using prescription claim fill dates and included all days with medication on hand regardless of treatment gaps.
Results
Only 45.0% of the 36,076 mono and 77.9% of the 1548 combo patients reached an ATX dose of ≥80 mg/day in 1‐year follow‐up. When patients filled at least one 80 mg prescription, their total days of therapy over the course of a year were significantly greater than if they did not (mono: 159.3 vs. 65.6 days; combo: 237.4 vs. 172.0; P < 0.0001). Across all timepoints examined (Day 14, 30, 60, 90, 210) for mono and combo, ATX ≥ 80 versus ATX < 80 patients had greater mean doses (P < 0.0001). Combo patients had longer ATX LoT than mono patients regardless if they reached 80 mg or not (P < 0.0001), but mono patients LoT was 93.8 days longer for ATX ≥ 80 versus ATX < 80 patients compared to 65.5 days for combo patients. Of patients reaching 80 mg/day, 71.7% of mono and 62.8% of combo patients did so by Day 30. For mono ATX ≥ 80 and ATX < 80 patients, LoT was significantly (P < 0.0001) less in previously treated patients compared to naive patients.
Conclusion
Ensuring adult ADHD patients are treated with ATX at a target dose of 80 mg/day is an important clinical consideration for maximizing patient days on therapy, which can be important for treatment optimization.
Keywords: Adult, Atomoxetine, Attention‐deficit/hyperactivity disorder, Dosing, Length of therapy
Introduction
An estimated 4.4% of adults in the United States are affected by attention‐deficit hyperactivity disorder (ADHD) 1, which is characterized by a persistent pattern of inattention, hyperactivity, and/or impulsivity at all ages 2.
Atomoxetine (ATX) is a selective norepinephrine reuptake inhibitor indicated for the treatment of ADHD, and is the only nonstimulant approved for the treatment of ADHD in adults. For adults, ATX is recommended to be initiated at a total daily dose of 40 mg/day and increased after a minimum of 3 days to a target total daily dose of approximately 80 mg/day administered either as a single daily dose in the morning or as evenly divided doses in the morning and late afternoon/early evening. After 2–4 additional weeks, the dose may be increased to a maximum of 100 mg/day in patients who have not achieved an optimal response 3.
Results from clinical trials indicate that treatment using an adequate dose of atomoxetine for a sufficient duration of time is important for ADHD symptom improvement and, conversely, that suboptimal dosing may lead to lower efficacy 4. However, despite the recommended 80 mg/day target dose, real‐world data show that an approximately 60 mg/day average adult ATX dose is utilized in clinical settings, at least partially driven by lack of knowledge rather than clinical need 4. Additionally, although slower dose titration can benefit some patients, it may add the risk of premature drug discontinuation due to impatience waiting for efficacious results, particularly for patients who are not stimulant‐naive and who may be used to experiencing a quicker effect 4.
The finding that lower ATX doses could lead to lower persistency (time from initial atomoxetine dose to discontinuation) is supported by a post hoc analysis of data from a 12‐month prospective, observational study in pediatric and adolescent patients with ADHD 5. Medication persistence was assessed in 134 patients who were treated initially with low starting dose ATX (<0.5 mg/kg/day) or recommended starting dose ATX (0.5 mg/kg/day). Initial treatment with low‐dose ATX was associated with statistically significantly shorter medication persistence throughout the study. Patients who initially received low‐dose atomoxetine had higher discontinuation rates within the first 90 days (27.7% vs. 11.0%), after 180 days (45.8% vs. 15.3%), and after 365 days (51.8% vs. 21.1%; all P < 0.01) than patients who initially received standard‐dose atomoxetine. It is hypothesized that the same trend may be present in adults.
It is also hypothesized that a large percentage of adult ADHD patients treated with ATX therapy in real‐world clinical settings are not dosed to the recommended 80 mg/day target dose, and that not reaching the recommended dose leads to a shorter length of therapy (LoT) than when the target dose is achieved.
In a recent claims database study, Kabul and colleagues investigated dosing patterns over 1 year among 12,412 adults with claims for ADHD and newly prescribed ATX. Only 26.8% were treated consistently at the recommended dose range (80–100 mg/day), whereas 36.6% were suboptimally dosed (never reaching 80 mg/day) and about a third had fluctuating dosing 6. Kabul and colleagues used strict definitions to identify dosing cohorts and measures of persistency. They defined persistency as days until stopping the index ATX, not allowing for breaks in prescriptions longer than 30 days. Average persistency was not notably different between the recommended dosing and suboptimal dosing cohorts. For defining the dosing cohorts, the 12‐month follow‐up period started on Day 31 instead of Day 1 to allow for titration only during those 30 days. Patients prescribed ATX were excluded from analysis if during follow‐up they used ATX in combination with other ADHD medications or if their dosing fluctuated outside the ranges of the recommended dosing or suboptimal dosing definitions at any time during the follow‐up. This fluctuator population made up about a third of the overall patient population.
Reasons for poor adherence among patients with ADHD include adverse effects, dosing inconvenience, social stigma, patient attitude, lack of adequate symptom control, and also adequate symptom control 7, 8. Among adults, symptom relief combined with the general desire not to take medication chronically is a strong motivating factor 7. Patients have a tendency to stop treatment once they feel their symptoms are under control and often take drug holidays when they feel better or when they perceive there is less need to control their ADHD symptoms during that time – for example, during school breaks 7, 9. Despite drug holidays not being in the best interest of patients, the use of drug holidays that is common in the treatment of children with ADHD has generalized to the treatment of adults with ADHD 10.
Thus, in contrast to the Kabul study, the aim of the current study was to examine ATX dosing and its effects on LoT by expanding the patient sample to include more real‐world practice patterns by using less specific and rigid definitions of recommended and suboptimal dosing, include patients whose dose fluctuated over time, include patients who reached 80 mg/day at any time during the follow‐up period, and examine cumulative days of therapy during the follow‐up regardless of treatment breaks. Using this novel approach, among ADHD adults with prescription claims that reached 80 mg/day (ATX ≥ 80) versus did not reach 80 mg/day (ATX < 80), the objectives were to compare: LoT for ATX monotherapy patients (primary); LoT for ATX combination therapy patients; demographic, clinical characteristics, treatment patterns, and dosage patterns; LoT monotherapy versus combination therapy patients; LoT treatment naive versus not naive patients; and factors associated with reaching 80 mg/day.
Materials and Methods
Data Source
This retrospective analysis was conducted using administrative medical and pharmacy claims data from the Truven Health MarketScan Commercial Claims and Encounters Database for the period July 2005 through September 2014, including the preindex and follow‐up period. This database contains complete longitudinal records of inpatient and outpatient services, and prescription drug claims of more than 45 million employees and their dependents, covered under a variety of fee for service, fully capitated, and partially capitated health plans across all geographic regions of the United States. All study data are de‐identified and fully compliant with Health Insurance Portability and Accountability Act of 1996. Because this study used only de‐identified patient records and did not involve the collection, use, or transmittal of individually identifiable data, Institutional Review Board approval to conduct this study was not necessary.
Study Population
Adult patients (≥18 years) newly initiated on ATX (i.e., no ATX use in the prior 6 months) with at least one pharmacy claim for atomoxetine between January 1, 2006 and September 30, 2013 were identified. The date of the first ATX treatment episode was set as the index date. All patients were required to have at least one medical claim with an International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis code (ICD‐9‐CM) for ADHD (314.0x) and be continuously enrolled with medical and pharmacy benefits in the 6 months preindex and 12 months postindex. The study period spanned from July 1, 2005 through September 30, 2014 with a 6‐month preindex and 12‐month postindex (follow‐up) period.
Atomoxetine dosing was determined from pill strength based on the National Drug Code (NDC) and quantity dispensed and days’ supply information on pharmacy claims for ATX. Patients were either categorized as having reached (filled a prescription for) 80 mg/day dose at least one time or did not reach 80 mg/day dose.
Patients were categorized as monotherapy or combination therapy patients based on all ATX prescriptions during follow‐up including the index prescription. Each ATX prescription was evaluated for the number of days overlap with another non‐ATX ADHD‐indicated medication (amphetamine, methylphenidate, or alpha‐2 adrenergic). If any ATX prescription had >30 continuous days of overlap with another ADHD medication, the patient was considered a combination therapy patient.
Patients were categorized as having been treatment naive or not during the 6 months prior to their index event; that is, no prescriptions for ADHD‐indicated medications in the 6‐month preindex period.
Patients were characterized based on demographic information at the time of the index event including age and gender. Clinical characteristics based upon pre‐ and follow‐up period data included ADHD subtype and selected comorbid psychiatric disorders based on the presence of ≥1 medical claim with relevant ICD‐9‐CM diagnosis codes. Prescriber specialty and prior ADHD medication use, based on pharmacy claims with relevant NDC codes, were also captured. Prescriber specialty was determined based on the clinician specialty for the preindex office visit that fell closest to the date of the index ATX prescription.
LoT was defined as all prescription claim fill days over the 365‐day follow‐up period, including continuous and sporadic use such that LoT was calculated as cumulative days of therapy rather than persistency.
Statistical Analysis
All study variables were summarized descriptively and statistical tests of significance for differences between dosing cohorts (ATX ≥ 80 vs. ATX < 80) were performed with a P‐value ≤0.05 set as threshold for significance.
Based upon the data variable type, evidence of normality, and frequency size, the following tests were performed: independent t‐test, Wilcoxon rank‐sum test, Chi‐square test, or Fisher's exact test. For demographic and clinical characteristics the following tests were performed: Wilcoxon rank‐sum test (age); Chi‐square test (proxied prescriber specialty); and Fisher's exact test (gender, predominate ADHD subtype, preindex ADHD medication use, preindex comorbidities). Dosing patterns and LoT were assessed with the Wilcoxon rank‐sum test.
For demographic and clinical characteristic potential predictors (P ≤ 0.20 from univariate analysis) for reaching atomoxetine 80 mg/day dose, multivariate stepwise logistic regression was used to determine the P‐value, odds ratio, and odds ratio 95% confidence interval for each demographic and clinical characteristic showing a statistically significant (P ≤ 0.05 for monotherapy; P ≤ 0.10 for combination therapy) relationship with those who did or did not reach 80 mg/day, with a c‐statistic used to note multivariate model fit.
A sensitivity analysis was performed for dosing pattern variables and LoT analyses, repeating the current monotherapy analyses using the index dates of 01 January 2006–31 December 2011, which corresponds to the time period and Truven patient population used in the Kabul dosing and persistency study 6 discussed in this article.
Mean dosing was analyzed at Day 14, 30, 60, 90, and 210. Time 80 mg/day reached was analyzed for Days 1–7, 8–15, 16–30, 31–60, 61–90, 91–210, and ≥211.
Results
Demographic and Clinical Characteristics
Of the initial sample of 309,470 patients with at least one claim for ATX in the commercial database between January 1, 2006 and September 30, 2013, a total of 36,076 monotherapy (16,217 achieved 80 mg/day) and 1548 combination therapy (1206 achieved 80 mg/day) adult patients newly initiated on ATX met all of the study inclusion criteria (Figure 1).
Figure 1.
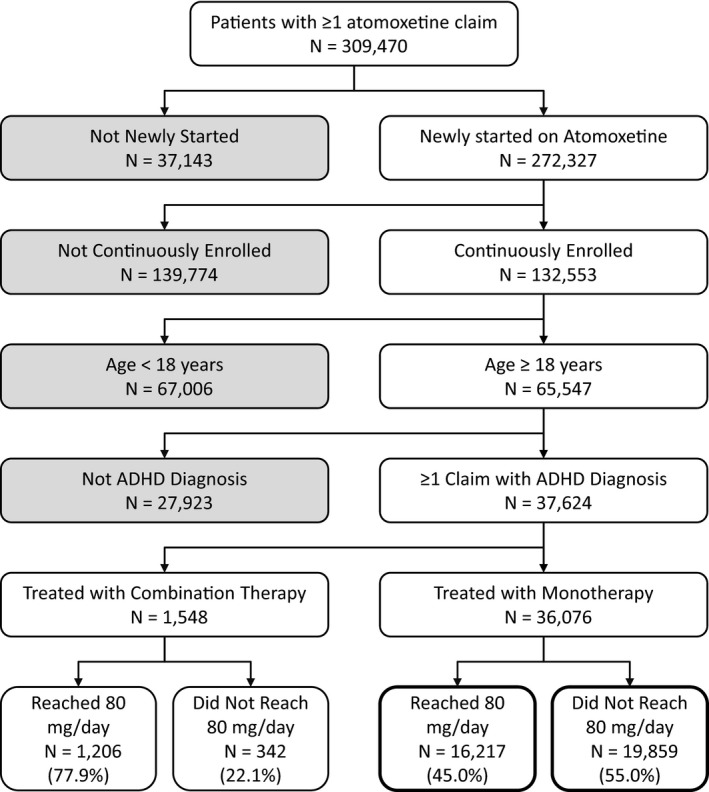
Patient disposition.
Of patients filling a claim for ATX, only 45.0% reached a dose of 80 mg/day or greater when used as monotherapy and 77.9% when used in combination therapy with other ADHD medications over the course of 1 year (Table 1).
Table 1.
Demographic and clinical characteristics
| Characteristics | Monotherapy | Combination therapy | ||||
|---|---|---|---|---|---|---|
| Reached 80 mg/day Dose (N = 16,217) | Did not reach 80 mg/day Dose (N = 19,859) | P‐value* | Reached 80 mg/day Dose (N = 1206) | Did not reach 80 mg/day Dose (N = 342) | P‐value* | |
| Demographic characteristics | ||||||
| Age at index, mean (SD) | 34.2 (12.8) | 32.1 (12.4) | <0.0001 | 36.2 (13.6) | 35.0 (13.6) | 0.1163 |
| Age group, N (%) | – | – | – | – | – | – |
| 18–24 | 5567 (34.3) | 8192 (41.3) | – | 371 (30.8) | 117 (34.2) | – |
| 25–44 | 6618 (40.8) | 7773 (39.1) | – | 447 (37.1) | 129 (37.7) | – |
| 45+ | 4032 (24.9) | 3894 (19.6) | – | 388 (32.2) | 96 (28.1) | – |
| Gender, N (%) | – | – | <0.0001 | – | – | 0.6684 |
| Male | 8682 (53.5) | 9877 (49.7) | – | 626 (51.9) | 173 (50.6) | – |
| Female | 7535 (46.5) | 9982 (50.3) | – | 580 (48.1) | 169 (49.4) | – |
| Clinical characteristics | ||||||
| Predominant ADHD subtype†, N (%) | – | – | 0.0002 | – | – | 0.9504 |
| Inattentive | 8373 (51.6) | 10,650 (53.6) | – | 501 (41.5) | 141 (41.2) | – |
| Hyperactive‐impulsive or combined | 7844 (48.4) | 9209 (46.4) | – | 705 (58.5) | 201 (58.8) | – |
| Proxied prescriber specialty‡, N (%) | – | – | <0.0001 | – | – | 0.8478 |
| Primary care | 9436 (60.6) | 12,656 (66.0) | – | 476 (41.4) | 134 (41.1) | – |
| Psychiatry | 4010 (25.8) | 4059 (21.2) | – | 486 (42.2) | 144 (44.2) | – |
| Neurology | 158 (1.0) | 287 (1.5) | – | 19 (1.7) | 4 (1.2) | – |
| Other | 1956 (12.6) | 2184 (11.4) | – | 170 (14.8) | 44 (13.5) | – |
| Preindex ADHD medication use§, N (%) | 4407 (27.2) | 5709 (28.8) | 0.0010 | 927 (76.9) | 259 (75.7) | 0.6645 |
| Long‐acting stimulants | 2174 (13.4) | 2806 (14.1) | 0.0478 | 469 (38.9) | 134 (39.2) | 0.9499 |
| Intermediate‐acting stimulants | 1573 (9.7) | 2109 (10.6) | 0.0041 | 339 (28.1) | 90 (26.3) | 0.5384 |
| Short‐acting stimulants | 610 (3.8) | 800 (4.0) | 0.1994 | 179 (14.8) | 44 (12.9) | 0.3839 |
| Prodrug stimulants | 964 (5.9) | 1114 (5.6) | 0.1802 | 219 (18.2) | 49 (14.3) | 0.1055 |
| Alpha‐2 adrenergic agonists | 269 (1.7) | 331 (1.7) | 0.9670 | 135 (11.2) | 34 (9.9) | 0.5565 |
| Preindex comorbidities N (%) | 11,039 (68.1) | 12,767 (64.3) | <0.0001 | 946 (78.4) | 240 (70.2) | 0.0018 |
| Anxiety | 4346 (26.8) | 5100 (25.7) | 0.0166 | 393 (32.6) | 93 (27.2) | 0.0645 |
| Bipolar/mania disorders | 1476 (9.1) | 1454 (7.3) | <0.0001 | 162 (13.4) | 41 (12.0) | 0.5259 |
| Conduct disturbance | 149 (0.9) | 196 (1.0) | 0.5146 | 21 (1.7) | 4 (1.2) | 0.6281 |
| Depression | 5351 (33.0) | 5916 (29.8) | <0.0001 | 498 (41.3) | 117 (34.2) | 0.0205 |
| Diabetes | 689 (4.3) | 687 (3.5) | 0.0001 | 62 (5.1) | 15 (4.4) | 0.6729 |
| Eating disorders | 155 (1.0) | 186 (0.9) | 0.8697 | 12 (1.0) | 4 (1.2) | 0.7638 |
| Gastrointestinal disorders | 3307 (20.4) | 4060 (20.4) | 0.9060 | 274 (22.7) | 70 (20.5) | 0.4177 |
| Hypertension | 2827 (17.4) | 2708 (13.6) | <0.0001 | 241 (20.0) | 53 (15.5) | 0.0722 |
| Oppositional Defiance Disorder | 61 (0.4) | 67 (0.3) | 0.5350 | 4 (0.3) | 2 (0.6) | 0.6190 |
| Personality disorders | 233 (1.4) | 236 (1.2) | 0.0398 | 30 (2.5) | 9 (2.6) | 0.8462 |
| Pervasive developmental disorders | 143 (0.9) | 100 (0.5) | <0.0001 | 33 (2.7) | 6 (1.8) | 0.4333 |
| Psychotic disorders | 356 (2.2) | 393 (2.0) | 0.1584 | 30 (2.5) | 3 (0.9) | 0.0875 |
| Sleep disorders | 2487 (15.3) | 2764 (13.9) | 0.0002 | 240 (19.9) | 51 (14.9) | 0.0413 |
| Substance abuse/dependence | 2216 (13.7) | 2630 (13.2) | 0.2445 | 174 (14.4) | 37 (10.8) | 0.0902 |
| Tics/Tourette's | 62 (0.4) | 88 (0.4) | 0.4110 | 17 (1.4) | 2 (0.6) | 0.2776 |
ADHD, attention‐deficit/hyperactivity disorder; SD, standard deviation. *Wilcoxon rank‐sum test (age); Chi‐square test (proxied prescriber specialty); Fishers Exact test (gender, predominate ADHD subtype, preindex ADHD medication use, preindex comorbidities). †Inattentive defined as ≥1 claims with ICD‐9 314.00 without any claims with ICD‐9 314.01; hyperactive‐impulsive or combined defined as ≥1 claim with ICD‐9 314.01. ‡Prescription claims do not list provider specialty; proxies from provider specialty on the office visit on index or in the 6 months preindex that fell closest to index. Prescriber specialty was a missing variable for 657 and 673 monotherapy and 55 and 16 combination therapy patients in the reached 80 mg/day and did not reach 80 mg/day cohorts, respectively. §Patients could have used more than one ADHD medication class in the 6 months preindex.
Some statistical differences in demographic and clinical characteristics were found between the ATX ≥ 80 and ATX < 80 groups (Table 1). This was more apparent in the monotherapy rather than combination therapy group, perhaps in part due to the large number of patients in the monotherapy group. However, patients reaching 80 mg/day versus those who did not were more likely to be older, male, and have more comorbid diagnostic claims overall, as well as for anxiety, bipolar/mania disorders, depression, diabetes, hypertension, personality disorders, pervasive development disorders, and sleep disorders. Patients in the ATX ≥ 80 group were more likely to have had their ATX prescribed by psychiatrists, whereas patients in the ATX < 80 group were more likely to have been prescribed their ATX by a primary care physician; although, the majority of prescriptions in both groups were provided by primary care physicians.
Overall, within the combination therapy patients, patients who did and did not reach 80 mg/day were similar in regard to demographics and clinical characteristics. Differences did exist in baseline demographics and clinical characteristics between monotherapy and combination therapy patients. A larger percentage of combination therapy patients were of the hyperactive‐impulsive or combined type, had a larger percentage of patients prescribed atomoxetine by psychiatrists, had a larger percentage of patients with preindex ADHD medication use, and had comorbid anxiety, depression, or sleep disorders. While there were fewer comorbidities for the combination therapy group that showed a statistically significant difference between the ATX ≥ 80 and ATX < 80 groups, this may be due to the smaller overall sample size, as trends were similar. Combination therapy patients achieving 80 mg/day had statistically significantly greater depression and sleep disorders.
Dosing Patterns
Across all timepoints examined, ATX ≥ 80 patients had statistically significantly greater mean doses compared to ATX < 80 patients (Table 2). In contrast to the ATX ≥ 80 group, patients in the ATX < 80 group had similar mean lowest and highest doses and did not have their dose increase over time.
Table 2.
Dosing patterns and length of therapy
| Patterns | Monotherapy | Combination therapy | ||||||
|---|---|---|---|---|---|---|---|---|
| n reached/n did not reach† | Reached 80 mg/day Dose | Did not reach 80 mg/day Dose | P‐value* | n reached/n did not reach† | Reached 80 mg/day Dose | Did not reach 80 mg/day Dose | P‐value* | |
| Dosing (mg/day) | – | – | – | – | – | – | – | – |
| Mean (SD) final dose | 16,217/19,859 | 71.6 (21.9) | 37.5 (13.2) | <0.0001 | 1206/342 | 68.7 (24.7) | 32.5 (14.4) | <0.0001 |
| Mean (SD) lowest dose | 16,217/19,859 | 59.1 (23.3) | 36.0 (13.4) | <0.0001 | 1206/342 | 53.5 (23.2) | 29.0 (13.7) | <0.0001 |
| Mean (SD) highest dose | 16,217/19,859 | 108.7 (29.0) | 40.2 (13.4) | <0.0001 | 1206/342 | 123.0 (28.8) | 43.8 (14.4) | <0.0001 |
| Mean (SD) dose at day 14 | 16,070/19,413 | 64.8 (25.5) | 37.0 (13.3) | <0.0001 | 1200/340 | 61.3 (27.8) | 31.3 (13.9) | <0.0001 |
| Mean (SD) dose at day 30 | 15,876/18,526 | 80.6 (31.7) | 38.3 (13.3) | <0.0001 | 1194/339 | 82.2 (38.1) | 36.3 (14.9) | <0.0001 |
| Mean (SD) dose at day 60 | 9605/5483 | 70.2 (26.0) | 38.2 (14.5) | <0.0001 | 962/262 | 71.5 (28.5) | 33.7 (14.6) | <0.0001 |
| Mean (SD) dose at day 90 | 8810/3759 | 71.5 (27.5) | 37.4 (14.9) | <0.0001 | 949/245 | 73.4 (29.5) | 34.7 (14.6) | <0.0001 |
| Mean (SD) dose at day 210 | 5558/1626 | 70.2 (25.7) | 35.6 (15.3) | <0.0001 | 706/123 | 72.0 (26.5) | 29.9 (13.2) | <0.0001 |
| Length of therapy (days) | – | – | – | – | – | – | – | – |
| Mean Length of Therapy (SD)‡ | 16,217/19,859 | 159.3 (111.8) | 65.6 (67.2) | <0.0001 | 1206/342 | 237.4 (95.9) | 172.0 (96.7) | <0.0001 |
ADHD, attention‐deficit/hyperactivity disorder; LoT, length of therapy; SD, standard deviation. *Wilcoxon rank‐sum test. †Only patients with a dose recorded at the examined time were included for that time. ‡Mean LoT days for all 36,076 monotherapy patients (107.7 ± 101.4) was significantly less than for the 1548 combination patients (222.9 ± 99.9; P < 0.0001); the mean LoT for monotherapy versus combination patients was significant less within both the patients reaching 80 mg/day as well as in the patients not reaching 80 mg/day (P < 0.0001).
Of those monotherapy patients who reached 80 mg/day, 71.7% did so by Day 30, while 20.4% did not get to 80 mg/day until after 60 days (Table 3). Only 45.0% of all monotherapy patients ever reached 80 mg/day. Of those patients who achieved 80 mg/day, about 72% of monotherapy patients reached 80 mg/day between Days 16–30 while about 72% of combination therapy patients reached 80 mg/day between Days 31–60. For all combination patients, 77.9% reached 80 mg/day.
Table 3.
Time 80 mg/day reached
| Day 80 mg/day dose achieved | Monotherapy | Combination therapy | ||||
|---|---|---|---|---|---|---|
| n (%) All patients (N = 36,076) | Cumulative % all patients (N = 36,076) | Cumulative % patients who reached 80 mg/day (N = 16,217) | n (%) All patients (N = 1548) | Cumulative % all patients (N = 1548) | Cumulative % of patients who reached 80 mg/day dose (N = 1206) | |
| Achieved 80 mg/day by day 1–7 | 8059 (22.3) | 22.3 | 49.7 | 460 (29.7) | 29.7 | 38.1 |
| Achieved 80 mg/day by day 8–15 | 404 (1.1) | 23.5 | 52.2 | 41 (2.7) | 32.4 | 41.5 |
| Achieved 80 mg/day by day 16–30 | 3172 (8.8) | 32.3 | 71.7 | 256 (16.5) | 48.9 | 62.8 |
| Achieved 80 mg/day by day 31–60 | 1277 (3.5) | 35.8 | 79.6 | 110 (7.1) | 56.0 | 71.9 |
| Achieved 80 mg/day by day 61–90 | 1209 (3.4) | 39.1 | 87.1 | 132 (8.5) | 64.5 | 82.8 |
| Achieved 80 mg/day by day 91–210 | 1529 (4.2) | 43.4 | 96.5 | 152 (9.8) | 74.4 | 95.4 |
| Achieved 80 mg/day by ≥day 211 | 567 (1.6) | 45.0 | 100.0 | 55 (3.6) | 77.9 | 100.0 |
| Did not achieve 80 mg/day | 19,859 (55.1) | 100.0 | NA | 342 (22.1) | 100.0 | NA |
LoT, length of therapy; NA, not applicable.
Length of Therapy
Patients in the ATX ≥ 80 group had prescription claims covering a statistically significantly greater number of cumulative days annually than patients in the ATX < 80 group (Table 2).
Combination therapy patients had statistically significantly longer LoT than monotherapy patients, regardless if they reached 80 mg/day or not (Table 2). Patients with combination therapy who reached 80 mg/day had the longest LoT. The difference in LoT between the ATX ≥ 80 and ATX < 80 groups was greater for the monotherapy patients (93.8 days) compared with the combination therapy patients (65.5 days).
A greater number of patients were treatment naïve than not naïve during the 6 months prior to their index ATX treatment in the monotherapy cohorts, whereas a greater number of patients were not treatment naïve in the combination therapy cohort (Table 4). When comparing LoT between the ATX ≥ 80 and ATX < 80 groups, the finding that ATX ≥ 80 patients had longer LoT than ATX < 80 patients held true within the naïve and nonnaïve subgroups. However, the LoT was statistically significantly longer for naive patients than it was for previously treated patients in the monotherapy cohort. This difference was not observed for combination therapy.
Table 4.
Length of therapy of treatment naive versus not naive patients
| Group | Treatment Naive† | Not Treatment Naive | |||
|---|---|---|---|---|---|
| n | Days Mean (SD) | n | Days Mean (SD) | P‐value* | |
| Monotherapy | |||||
| All | 25,960 | 112.0 (103.0) | 10,116 | 96.7 (96.3) | <0.0001 |
| Reached 80 mg/day | 11,810 | 164.0 (112.3) | 4407 | 146.9 (109.6) | <0.0001 |
| Did not reach 80 mg/day | 14,150 | 68.6 (69.3) | 5709 | 57.9 (60.8) | <0.0001 |
| Combination therapy | |||||
| All | 362 | 220.3 (96.2) | 1186 | 223.8 (101.0) | 0.4476 |
| Reached 80 mg/day | 279 | 233.2 (90.8) | 927 | 238.7 (97.4) | 0.2599 |
| Did not reach 80 mg/day | 83 | 177.0 (101.5) | 259 | 170.3 (95.3) | 0.8669 |
SD, standard deviation. *Wilcoxon rank‐sum test. †ADHD treatment naive is defined as not having ADHD treatment in the 6‐month preindex period; however, whether patients were treatment naive prior to this period is unknown.
The dosing pattern variable and LoT sensitivity analyses provided similar and consistent results to those in Table 2, with a mean LoT of 160.4 for ATX ≥ 80 and 67.1 for ATX < 80 monotherapy patients (P < 0.0001). The mean lowest and highest doses were 59.2 and 107.9 for ATX ≥ 80 and 36.5 and 40.5 for ATX < 80 monotherapy patients (P < 0.0001).
Predictors of Reaching Target Dose
Within the monotherapy patient group, several factors were suggestive of increasing the likelihood a patient would fill an 80 mg/day prescription during their follow‐up year (Table 5). Older patients (1.4% more likely per 1 year of age), males (22.6%), patients with a hyperactive‐impulsive or combined type diagnosis (6.7%), patients whose last provider type was a psychiatrist (25.4%) or other (15.2%) type versus the primary care/family practice reference group, and patients with a comorbidity diagnosis of bipolar (16.3%), depression (7.2%), hypertension (13.0%), or a pervasive development disorder (84.6% or almost twice as likely) were more likely to achieve 80 mg/day.
Table 5.
Demographic and clinical characteristic predictors of reaching atomoxetine 80 mg/day dose
| Variable | Monotherapy | Combination therapy | ||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P‐value* | Odds ratio | 95% CI | P‐value* | |
| Age | 1.014 | 1.012, 1.016 | <0.0001 | NA | NA | NA |
| Gender (male vs. female) | 1.226 | 1.174, 1.281 | <0.0001 | NA | NA | NA |
| ADHD type (hyperactive‐impulsive/combined vs. inattentive) | 1.067 | 1.022, 1.115 | 0.0035 | NA | NA | NA |
| Provider type (psychiatrist vs. primary care/family practice) | 1.254 | 1.189, 1.323 | <0.0001 | NA | NA | NA |
| Provider type (neurologist vs. primary care/family practice) | 0.701 | 0.576, 0.854 | – | NA | NA | NA |
| Provider type (other vs. primary care/family practice) | 1.152 | 1.077, 1.233 | – | NA | NA | NA |
| Preindex ADHD medication (intermediate‐acting stimulant, yes vs. no) | 0.873 | 0.813, 0.937 | 0.0002 | NA | NA | NA |
| Preindex ADHD medication (short‐acting stimulant, yes vs. no) | 0.894 | 0.800, 0.998 | 0.0466 | NA | NA | NA |
| Preindex ADHD medication (prodrug stimulant, yes vs. no) | NA | NA | NA | 1.339 | 0.956, 1.876 | 0.0894 |
| Comorbidity (bipolar/mania disorder, yes vs. no) | 1.163 | 1.073, 1.260 | 0.0002 | NA | NA | NA |
| Comorbidity (depression, yes vs. no) | 1.072 | 1.022, 1.125 | 0.0047 | 1.319 | 1.024, 1.699 | 0.0319 |
| Comorbidity (hypertension, yes vs. no) | 1.130 | 1.061, 1.204 | 0.0001 | NA | NA | NA |
| Comorbidity (pervasive development disorder, yes vs. no) | 1.846 | 1.408, 2.418 | <0.0001 | NA | NA | NA |
| Comorbidity (sleep disorder, yes vs. no) | NA | NA | NA | 1.368 | 0.982, 1.907 | 0.0642 |
NA, not applicable. *Multivariate stepwise logistic regression was used to determine the P‐value, odds ratio, and confidence interval for each demographic and clinical characteristic showing a statistically significant relationship with those who did or did not reach 80 mg/day, with a model fit c‐statistic of 0.574 for monotherapy and 0.560 for combination therapy, which suggests a moderate‐to‐weak fit.
Patients whose last provider type was a neurologist versus the primary care/family practice reference group (29.9%), and patients who received intermediate‐acting (12.7%) or short‐acting stimulants (10.6%) were less likely to achieve 80 mg/day.
Within the combination therapy patient group, patients who received prodrug stimulants (33.9%), and patients with a comorbidity diagnosis of depression (31.9%) or sleep disorder (36.8%) were more likely to achieve 80 mg/day. Comorbidity diagnosis of depression or sleep disorder as statistically significant predictors could be questionable as their P‐value was between 0.05 and 0.10.
Discussion
In this retrospective observational claims database study of 37,624 adult ADHD patients treated with ATX, 55.0% of monotherapy and 22.1% of combination therapy patients were dosed lower than the recommended 80 mg/day during a year follow‐up. When patients filled at least one 80 mg/day prescription during the follow‐up period, their cumulative days of therapy over the course of that year were significantly greater than if they did not. Achieving 80 mg/day was associated with an increase of about 94 treatment days for monotherapy patients. For a patient group known for being poor with their medication adherence 7, the increase in LoT could be clinically meaningful for their chronic ADHD symptom control 4.
Clinical trial data suggest that ATX response occurs incrementally over time 11. While ATX can have an onset of action in adults within 1–2 weeks of treatment 11, 12, clinically meaningful response can take 4–6 weeks 4. For responders, incrementally increasing response occurs in adults up to 24 weeks or longer 13, suggesting optimal response can take several months of treatment 14. Wietecha and colleagues demonstrated that ATX treatment in adults with ADHD was associated with small effect sizes after 4 weeks, moderate effect sizes by 6 months of treatment, and increased response rates during longer‐term treatment at the 80 mg/day adult target dose 15. In this Wietecha study, which assessed multiple dosing subgroups, increase in response rate over 1–26 weeks was most noticeable in the 80 mg/day group. Patients not staying on medication for an adequate duration may miss an opportunity for symptom improvement. This is of particular relevance to current findings that suggest patient underdosing is associated with much shorter treatment duration compared with those patients who are treated per recommended dosing levels. Thus, the current research expands upon the Wietecha findings regarding the importance for patients in reaching 80 mg/day dosing and staying on treatment long‐term to maximize the chance for treatment response – patients not being titrated to 80 mg/day during their ongoing treatment regimen are significantly less likely to stay on treatment. The clinical impact of this finding is important, as the current study of 36,076 patients treated with atomoxetine monotherapy showed that only 45% of patients ever reached the recommended 80 mg/day dosing.
ADHD is a chronic neurobiological disorder 16 and thus needs ongoing, long‐term treatment to maintain symptom control 17. The current findings that most patients do not stay on medication through a year after the start of their prescription and that many patients have breaks in their treatment adherence over time is in line with evidence from other studies that have reported nonadherence and discontinuation as issues for patients with ADHD 7, 17, 18, 19, 20.
There is growing evidence of the inappropriateness of breaks in dosing regimens for patients with ADHD 10, and that long‐term medication compliance is critical for long‐term treatment outcome 19. Intermittent dosing can negatively impact the efficacy of ADHD treatment, lead to reemergence of significant life impairments, increase outcome risks such as car accidents, substance abuse, and relationship or work disturbances, and may actually increase the overall side‐effect burden as patients frequently need to redevelop medication tolerance 9, 21. A 12‐month prospective observational study examining propensity‐matched patients who either discontinued study drug early or maintained treatment showed that pharmacotherapy effectiveness for their ADHD was significantly better in patients that did not discontinue 17.
There is evidence that underdosing of ATX can also lead to suboptimal efficacy 4, 17. The current data suggest underdosing could contribute to suboptimal treatment because it leads to significantly reduced LoT for a disorder known to be chronic. The finding that underdosing leads to shorter LoT is in contrast with previous data from the Kabul study showing no difference in LoT during a 12‐month follow‐up comparing patients with recommended dosing versus those suboptimally dosed 6. The mean dose for patients in the recommended dosing cohort was 83.1 mg/day, while the mean dose for patients in the suboptimal dosing cohort was 42.9 mg/day. The mean LoT for patients in the recommended dosing cohort was 131 days compared with 129 days for those in the suboptimal dosing cohort. Other studies have reported poor persistence to ADHD medications in general, with LoT varying by medication class and definitions used to capture these variables 7.
The contrast in the Kabul study results versus the current study results appears to be due to how patient cohorts and length of therapy were defined, which has been previously shown to result in the reporting of different adherence rates across studies 9. In the Kabul study 6, which utilized the same database and overlapped in study timing, patients (12,412) were grouped into 4 cohorts: (1) recommended dosing, 80–100 mg/day throughout follow‐up (26.8% of patients), (2) suboptimal dosing, <80 mg/day throughout the follow‐up (36.6% of patients), (3) above recommended dosing, >100 mg/day through the follow‐up (1.7% of patients), and (4) fluctuators, filled prescriptions that fluctuated across dosing groups throughout the follow‐up (34.9% of patients). LoT was defined as days until stopping index treatment (any gap of >30 days). Thus, patients continually dosed at 80 mg/day were compared to those never reaching 80 mg/day in regard to persistency. Above recommended and fluctuator patients were not included in analyses (over a third of patients). Additionally, patient's data after a break in their dosing were not included and thus their full dosing patterns over the course of a year could not be fully evaluated. In contrast, the current study looked at patients who reached 80 mg/day at least once versus those who never reached 80 mg/day in regard to cumulative treatment duration, allowing for fluctuation in dosing and gaps in treatment. No patients were excluded, and drug holidays were allowed, thereby addressing the clinically relevant limitations in the Kabul study 6, such as not allowing for treatment gaps that are common in this patient population 7, 9, 10. The current, less rigid dosing group and LoT definitions may better equate to real‐world clinical practice and patient adherence regimens. Cumulative days of therapy over time rather than consecutive days of therapy may be more clinically relevant for patients with ADHD who tend to start/stop medication over time.
The specific reasons for initial medication discontinuation that could affect LoT could not be measured in the claims data from either the Kabul or the current study. However, speculation behind the similar persistency between dosing cohorts in the Kabul study is possible. It could be that patients in the suboptimal group continued therapy longer than expected due to placebo effect and few side effects or due to low and slow titration prescribed by their physician to meet individual patients’ needs. Patients on recommended dosing could have continued therapy for a shorter than expected duration because of symptom control or adverse effects. This could have led to similar initial persistency, while not taking into account long‐term compliance over time and thereby masking the dosing group differences in overall adherence seen in the present study. Sensitivity analyses provide support for this theory. When LoT was reexamined in the Kabul study patient population using the current dosing group and LoT definitions, the LoT results were aligned with the overall study results – those reaching 80 mg/day had a significantly longer LoT.
In this study, monotherapy patients reaching 80 mg/day had a mean dose of 64.8 mg/day at Day 14 but had a mean dose of 80.6 mg/day at Day 30. This is logical as ATX is a titrated drug, although it does show that physicians are titrating patients at a much slower rate than the label‐recommended 40 mg/day for a minimum of 3 days followed by 80 mg/day thereafter. For patients that reach 80 mg/day, the mean dose thereafter fell to a consistent mean dose of about 70 mg/day, perhaps suggesting a drop in dose to aid in tolerability for some patients.
The observations that monotherapy patients never reaching 80 mg/day had similar mean lowest (36.0 mg/day) and highest (40.2 mg/day) doses, had a mean dose of 37.0 mg/day on Day 14, and had a mean dose of 38.3 mg/day on Day 30 suggests that physicians were not titrating their patients upward in dose after initial dosing.
Healthcare providers appear to be more open to ATX monotherapy for adult patients naive to other ADHD‐indicated treatments in the prior 6 months. The LoT was statistically significantly less in previously treated patients compared to naive patients in the overall monotherapy cohort, suggesting naive patients have a longer LoT. This finding could be because patients used to the feeling of stimulant therapy are more likely to discontinue ATX due to a perceived lack of efficacy, regardless of efficacy outcomes 22. Aligning with the ATX prescribing information, physicians prescribe ATX as a monotherapy a majority of the time.
Factors that increased the likelihood of a patient receiving and filling an 80 mg/day monotherapy prescription during their follow‐up year, included being older, male, hyperactive‐impulsive or combined type, psychiatrist prescriber, and having a bipolar, depression, hypertension, or pervasive development disorder as a comorbidity. However, the increased likelihood was from about 7 to 25% except for pervasive development disorder comorbidity that was about 85%, all of which are less than a 1‐fold change. Also, the multivariate model used to test statistical significance was found to only be of moderate‐to‐weak fit.
Study limitations to consider are that early dosing of 80 mg/day may be overestimated due to the dose algorithm used, such that some doses within the first 30 days of treatment could be added together but may actually be serial titration doses. This is based upon how NDC codes for prescription claims are entered into the Truven database. Also, only patients with doses recorded at the examined times were included. Data describing reasons for discontinuation and clinical outcome data are not available in an administrative claims database, so it was not possible to assess the true association between dosing cohorts and symptom control. Results were based upon medication prescribed and filled rather than actual patient‐level adherence, so it is not known whether or not the patients took their medication as intended. The analysis was limited to only those individuals with commercial health insurance and thus may not be generalizable to ADHD patients with other insurance types.
Collectively, the data highlight clinically important ATX treatment issues for adults with ADHD. First, patients are frequently underdosed, limiting their positive outcome potential. Second, patients do not adhere to taking their medication long‐term, also limiting the optimization of their treatment. Third, underdosing can exacerbate patient's lack of treatment compliance over time, synergistically setting the patient up for treatment failure. To maximize potential ATX efficacy, it is important for physicians to set appropriate expectations of ATX treatment with their patients around label‐based recommended target dosing, length of time to maximized efficacy and how this is different than for stimulants, and the importance of long‐term medication compliance. To our knowledge, this is the first study to show a data‐driven rather than anecdotal linkage between atomoxetine underdosing, medication adherence, and the importance of assessing cumulative days of therapy over time in the adult ADHD population.
Conclusion
A majority of adult ADHD patients treated with ATX were dosed lower than the recommended 80 mg/day. When patients filled at least one 80 mg/day prescription, their cumulative days of therapy over the course of a year were significantly greater than if they did not. Ensuring adult ADHD patients are treated with ATX at a target dose of 80 mg/day is an important clinical consideration for maximizing patient days on therapy, which may be important for optimizing a patient's chance for treatment response and maximal therapeutic benefit.
Conflict of Interest
Clemow, Nyhuis, and Robinson are employees and minor shareholders of Eli Lilly and Company and/or one of its subsidiaries.
Acknowledgements
Funding for this study was provided by Lilly USA, LLC.
References
- 1. Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: Results from the National Comorbidity Survey Replication. Am J Psychiatry 2006;163:716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Biederman J, Faraone SV. Attention‐deficit hyperactivity disorder. Lancet 2005;366:237–248. [DOI] [PubMed] [Google Scholar]
- 3. Strattera [package insert]. Indianapolis, IN: Eli Lilly and Company, 2014. [Google Scholar]
- 4. Clemow DB. Suboptimal dosing of Strattera (atomoxetine) for ADHD patients. Postgrad Med 2014;126:196–198. [DOI] [PubMed] [Google Scholar]
- 5. Treuer T, Feng Q, Desaiah D, et al. Predictors of pharmacological treatment outcomes with atomoxetine or methylphenidate in patients with attention‐deficit/hyperactivity disorder from China, Egypt, Lebanon, Russian Federation, Taiwan, and United Arab Emirates. Int J Clin Pract 2014;68:1152–1160. [DOI] [PubMed] [Google Scholar]
- 6. Kabul S, Alatorre C, Montejano LB, Farr AM, Clemow DB. Real‐world dosing patterns of atomoxetine in adults with attention‐deficit/hyperactivity disorder. CNS Neurosci Ther 2015;21:936–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gajria K, Lu M, Sikirica V, et al. Adherence, persistence, and medication discontinuation in patients with attention‐deficit/hyperactivity disorder – a systematic literature review. Neuropsychiratr Dis Treat 2014;10:1543–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frank E, Ozon C, Nair V, Othee K. Examining why patients with attention‐deficit/hyperactivity disorder lack adherence to medication over the long term: A review and analysis. J Clin Psychiatry 2015;76:e1459–e1468. [DOI] [PubMed] [Google Scholar]
- 9. Khoza S, Oladapo AO, Barner JC. Adherence to medication for attention deficit/hyperactivity disorder: Does time frame matter? J Pharm Health Serv Res 2011;2:157–163. [Google Scholar]
- 10. Faraone SV, Spencer TJ, Montano CB, Biederman J. Attention‐deficit/hyperactivity disorder in adults: A survey of current practice in psychiatry and primary care. Arch Intern Med 2004;164:1221–1226. [DOI] [PubMed] [Google Scholar]
- 11. Bushe CJ, Savill N. Systematic review of atomoxetine data in childhood and adolescent attention‐deficit hyperactivity disorder 2009–2011: Focus on clinical efficacy and safety. J Psychopharmacol 2014;28:204–211. [DOI] [PubMed] [Google Scholar]
- 12. Durell TM, Adler LA, Williams DW, et al. Atomoxetine treatment of attention‐deficit/hyperactivity disorder in young adults with assessment of functional outcomes, a randomized, double‐blind, placebo‐controlled trial. J Clin Psychopharmacol 2013;33:45–54. [DOI] [PubMed] [Google Scholar]
- 13. Young JL, Sarkis E, Qiao M, et al. Once‐daily treatment with atomoxetine in adults with attention‐deficit/hyperactivity disorder: A 24‐week, randomized, double‐blind, placebo controlled trial. Clin Neuropharmacol 2011;34:51–60. [DOI] [PubMed] [Google Scholar]
- 14. Clemow DB, Bushe CJ. Atomoxetine in patients with ADHD – a clinical and pharmacological review of the onset, trajectory, and duration of response: Implications for patients. J Psychopharmacol 2015;29:1221–1230. [DOI] [PubMed] [Google Scholar]
- 15. Wietecha LA, Clemow DB, Buchanan AS, Young JL, Sarkis EH, Findling RL. Atomoxetine increased effect over time in adults with Attention‐Deficit/Hyperactivity Disorder treated for up to 6 months: Pooled analysis of two double‐blind, placebo‐controlled, randomized trials. CNS Neurosci Ther 2016;22:546–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. American Psychiatric Association . Diagnostic Statistical Manual of Mental Disorders, 5th edn Washington, DC: American Psychiatric Association, 2013;59–66. [Google Scholar]
- 17. Wu SH, Wang K, Chen Y, et al. Exploratory analysis of early treatment discontinuation and clinical outcomes of patients with attention‐deficit/hyperactivity disorder. Asia Pac Psychiatry 2015. [Epub ahead of print] doi: 10.1111/appy.12231. [DOI] [PubMed] [Google Scholar]
- 18. Barner JC, Khoza S, Oladapo A. ADHD medication use, adherence, persistence and cost among Texas medicaid children. Curr Med Res Opin 2011;27:13–22. [DOI] [PubMed] [Google Scholar]
- 19. Wehmeier PM, Dittmann RW. Treatment compliance or medication adherence in children and adolescents on ADHD medication in clinical practice: Results from the COMPLY observational study. Atten Defic Hyperact Diord 2015;7:165–174. [DOI] [PubMed] [Google Scholar]
- 20. Adler LD, Nierenberg AA. Review of medication adherence in children and adolescents with ADHD. Postgrad Med 2010;122:184–191. [DOI] [PubMed] [Google Scholar]
- 21. Cascade E, Kalali AH, Weisler RH, et al. Seasonality and the changing adult/child prescription ratios in ADHD therapy. Psychiatry (Edgmont) 2008;5:23–25. [PMC free article] [PubMed] [Google Scholar]
- 22. Walker DJ, Mason O, Clemow DB, Day KA. Atomoxetine treatment in adults with attention‐deficit/hyperactivity disorder. Postgrad Med 2015;127:686–701. [DOI] [PubMed] [Google Scholar]


