Abstract
Purpose
Whether inhaled medications improve long‐term survival in Chronic Obstructive Pulmonary Disease (COPD) is an open question. The purpose of this study is to assess the impact of adherence to inhaled drug use on 5‐year survival in COPD.
Methods
A population‐based cohort study in three Italian regions was conducted using healthcare linked datasets (hospitalization, mortality, drugs). Individuals (45+ years) discharged after COPD exacerbation in 2006–2009 were enrolled. Inhaled drug daily use during 5‐year follow‐up was determined through Proportion of Days Covered on the basis of Defined Daily Doses. Five levels of time‐dependent exposure were identified: (i) long‐acting β2 agonists and inhaled corticosteroids (LB/ICS) regular use; (ii) LB/ICS occasional use; (iii) LB regular use; (iv) LB occasional use; and (v) respiratory drugs other than LB. Cox regression models adjusted for baseline (socio‐demographic, comorbidities, drug use) and time‐dependent characteristics (COPD exacerbations, cardiovascular hospitalizations, cardiovascular therapy) were performed.
Results
A total of 12 124 individuals were studied, 46% women, mean age 73,8 years. Average follow‐up time 2,4 year. A total of 3415 subjects died (mortality rate = 11.9 per 100 person years). In comparison to LB/ICS regular use, higher risks of death for all remaining treatments were found, the highest risk for respiratory drugs other than LB category (HR = 1.63, 95%CI 1.43–1.87). Patients with regular LB use had higher survival than those with LB/ICS occasional use (HR = 0.89, 95%CI 0.79–0.99).
Conclusions
These findings support clinical guidelines and recommendations for the regular use of inhaled drugs to improve health status and prognosis among moderate–severe COPD patients. © 2016 The Authors. Pharmacoepidemiology and Drug Safety Published by John Wiley & Sons Ltd.
Keywords: adherence, bronchodilators, drug comparative effectiveness, inhaled corticosteroids, survival, pharmacoepidemiology
Introduction
Inhaled therapy is a key component of the management of stable Chronic Obstructive Pulmonary Disease (COPD) patients.1, 2, 3 Benefits in term of relieving symptoms, improving quality of life and preventing or treating exacerbations are supported by large evidence.4, 5, 6 However, several questions on inhaled agents are still open. Whether a particular inhalant is more beneficial than the others or whether a certain category of COPD patients is more susceptible to benefits from inhaled drugs have not been conclusively addressed.1, 6 Both the effect of inhaled corticosteroids (ICS) in reducing systemic and pulmonary inflammation and the role of combination therapy, e.g. long‐acting β2 agonists (LB) plus ICS, instead of monotherapy, are still controversial.7, 8, 9 The benefits over 3‐year period of follow‐up of combination therapy compared to monotherapy in two recent large clinical trials were moderate for COPD exacerbations and of borderline statistical significance for mortality.10, 11
A critical point for the effectiveness of pharmacological treatment is adherence.12, 13 In the case of COPD, as in other common chronic diseases, patients tend to receive fewer therapy for their disease than available or prescribed.14, 15, 16 Relatively few studies have evaluated the impact on health outcomes of non‐adherence; different study designs and methodology make it difficult to compare results.17, 18, 19
In the last decades the use of linked healthcare electronic databases has progressively increased and epidemiological studies based on them contribute to inform on adherence to drugs and their effectiveness in the real life practice.20, 21, 22 To our knowledge no studies have so far examined how the adherence to inhaled medications affects long‐term survival in COPD. The objective of this study is to measure the impact of regular use of inhaled drugs in people discharged after COPD exacerbation on 5‐year survival using a time‐dependent approach.
Methods
Study design, setting and data sources
A population‐based cohort study using linked health information systems from three Italian regions (Emilia Romagna, Lazio and Lombardia, about 19 million inhabitants) was carried out. Regional health information systems were used: Hospital Information Systems (HIS) (ICD‐9‐CM codes of the International Classification of Diseases), Drug Registers (PHARM) and Mortality Registers. Details on these datasets have been described elsewhere.23 Drugs dispensed to patients, which are reimbursable by the National Health Service, the Italian universal coverage healthcare system, are identified by the national drug register code, accordant with the international Anatomical Therapeutic Chemical (ATC) classification.24 The following information was available for each prescription: patient identification number, ATC code, number of packs, number of units per pack, dosage, unit cost per pack and prescription date. In compliance with the national privacy legislation, individual data were anonymized and a unique patient identifier allowed for record linkage.
Population
From the HIS, all 45+ years old aged patients discharged after a COPD exacerbation between 1 January 2006 and 31 December 2009 were enrolled using a standardized methodology.25, 26 We selected hospital discharges with COPD as the main diagnosis (ICD 9 CM codes 490, 491, 492, 494, 496) or secondary diagnosis in presence of a main diagnosis of acute respiratory failure (ICD 9 CM codes 518.81–518.84), dyspnea (786.0), cough (786.2) or abnormal sputum (786.4). The first hospitalization which met selection criteria during the enrollment period was selected. On the basis of the Regional Drug Register, the cohort was restricted to new users, subjects with no prescription for LB and ICS in the previous 6 months before index admission, with at least a prescription of respiratory drugs during the 90 days post discharge. Details on respiratory drugs ATC codes are reported in the Appendix.
Follow‐up and outcome
Each patient, with at least 90 days of follow‐up and alive at the 90° day after discharge, was followed from date of discharge to the end of the study 31 December 2010 or date of death, whichever occurred first. The outcome of interest was the non accidental causes mortality (all causes mortality excluding injury and poisoning, International Classification of Disease IX revision ICD9 codes: 001‐799.9).
Drug exposure
All prescriptions of LB and ICS dispensed during follow‐up were identified. According to standardized methodology, the number of Defined Daily Doses (DDD) available was translated into the number of days in which the patient was treated, counting one DDD per day and distributing all available DDD to the days of follow‐up.24, 27 For each day of follow‐up the time‐dependent use of inhaled drugs was calculated as the ratio between the cumulative number of days in which the drug was available, starting to discharge date and the day of follow‐up considered (proportion of days covered until that day, PDC). If a patient refilled a medication before exhausting the calculated days' supply of previous fill, the supply in excess was redistributed the first time that the patient had days without medication. Each patient contributed to different time‐dependent drug exposures, according to the type of drug and the level of coverage.
Five categories of time‐dependent drug exposure were defined. Overall time (in days) spent in each category along the follow‐up was classified according to the following values of the PDC for LB and ICS:
LB/ICS regular use: if PDCLB ≥ 75% and PDCICS ≥ 75%
LB/ICS occasional use: if 0 < PDCLB < 75% or 0 < PDCICS < 75%
LB regular use: if PDCLB ≥ 75% and PDCICS = 0
LB occasional use: if 0 < PDCLB < 75% and PDCICS = 0
Respiratory drugs other than LB: if PDCLB = 0
In the first 90 days from the discharge, the proportion of patients in the five categories was also measured. In the literature there is no consensus on acceptable therapy adherence, with definitions varying from >70% to >80% in clinical research settings. We decided to set the limit for optimal adherence at ≥75%, which is between these two definitions according to previous studies.28, 29 See the Appendix for details on respiratory drugs ATC codes.
Baseline and time‐dependent covariates
Patients were characterized at baseline according to socio‐demographic factors, diagnosis of respiratory failure (ICD9CM codes 518.81–518.84) or asthma (ICD9CM code 493), previous 2‐year COPD exacerbations (both severe defined as hospitalization for COPD and moderate defined as concomitant use of oral corticosteroids and antibacterials (ATC codes H02AB and J01), previous 6‐month use of respiratory medicaments and non‐respiratory drugs, and comorbidities.30 During the follow‐up, the days spent for severe or moderate COPD exacerbations or cardiovascular hospitalizations (ICD9CM codes 390–459 main diagnosis) were measured. Moreover, the regular use of cardiac therapies, antihypertensives or statins during follow‐up was measured (PDC ≥ 75%).28, 29 The year of enrollment was also introduced as potential confounder.
Details on codes for comorbidities, respiratory and non respiratory drugs are described in the Appendix.
Statistical analysis
The time‐dependent drug exposure starting from the 90° day post discharge was considered in the main analysis. In our model both the drug exposure and time‐dependent covariates could change value over the course of the observation period. We obtained multiple records for each individual, with each record corresponding to an interval of time during which both the drug exposure and the time‐dependent variables remain constant (counting process method). We applied the extended Cox model to estimate the hazard ratio (HR) and corresponding 95%CI of mortality associated to the different time‐dependent drug exposure, considering the period spent with LB/ICS regular use as reference. Baseline and time‐dependent covariates were taken into account through a stepwise bootstrap procedure. We perform proportional hazard model on our data using a SAS procedure called PROC PHREG, where we specified a starting and stopping time for each record.
The Cox models were replied for the subgroup of patients with previous exacerbations. A model was also run, for the whole population, to assess the effect on survival of LB regular use in comparison to respiratory drugs other than LB as reference. Cox regression survival curves were also analysed. For each time‐related drug exposure the attributable risk percentage, i.e. the percentage of deaths in the exposed group that can be attributed to the exposure (AR% = (HR − 1) / HR) was also calculated.31, 32
Sensitivity analysis
We performed several sensitivity analyses. First, to evaluate the role of recent exposure we replied the analyses measuring the exposure in a mobile window of 90‐day preceding each day of follow. Second, we replied the main analyses considering all nine possible combinations of time‐dependent exposure (instead of 5) including also the PDC 0.0–75% separately for LB and ICS. Third, analyses were replied choosing a PDC cutoff point ≥ 50% instead of 75%, and, fourth, considering shorter periods of follow‐up (1 and 2 years).
Statistical analyses were performed using SAS 9.2.
Results
A total of 12 124 subjects who met the recruiting criteria were included in the statistical analyses. The flow diagram for the selection of eligible study participants is shown in Figure 1.
Figure 1.
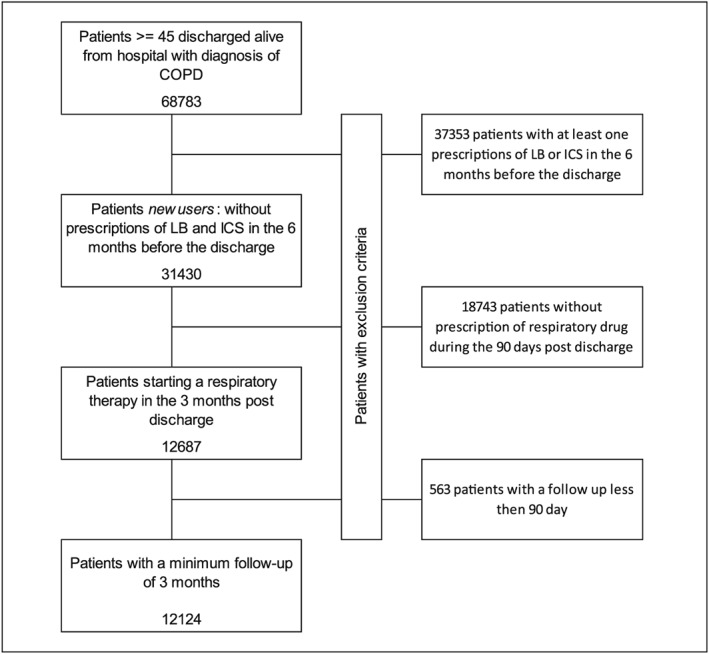
Flow chart of inclusion and exclusion criteria
Population characteristics
The characteristics of the cohort at baseline, according to the drug exposure category measured during the first 90 days post discharge, are displayed in Table 1. Most patients were resident in Lombardy (47.7%), male (54.0%) and were 75–84 years old aged (38.3%). More than 40% had a respiratory failure. Ischemic diseases (19.1%), heart failure (19.6%) and cerebrovascular diseases (16.9%) were the most common comorbidities. About 70% of patients were treated with antihypertensives, 36.3% with antiplatelets, 16.6% with statins and 16.8 with anti‐diabetic drugs. In the first 90 days, the proportion of LB/ICS regular users was 22.2%, while patients treated with respiratory drugs other than LB were 26.2. In this group, the proportions of women and of very old people (85+) were higher than in the other exposure groups (53.6% and 27.7% respectively).
Table 1.
Patient characteristics at baseline respect the treatment measured during the first 90 days post discharge
| LB/ICS regular use | LB/ICS occasional use | LB regular use | LB occasional use | Respiratory drugs other than LB | Total patients | |
|---|---|---|---|---|---|---|
| 22.2% | 22.3% | 15.0% | 14.3% | 26.2% | 12 124 | |
| Area of residence | ||||||
| Lazio | 28.9 | 24.1 | 28.0 | 25.8 | 33.4 | 28.4 |
| Emilia Romagna | 22.6 | 25.4 | 16.4 | 22.3 | 28.8 | 23.9 |
| Lombardia | 48.5 | 50.4 | 55.6 | 51.9 | 37.8 | 47.7 |
| Gender | ||||||
| Male | 58.7 | 53.7 | 60.2 | 54.7 | 46.4 | 54.0 |
| Female | 41.3 | 46.3 | 39.8 | 45.3 | 53.6 | 46.0 |
| Age | ||||||
| 45–54 | 5.6 | 4.7 | 4.5 | 3.6 | 2.8 | 4.2 |
| 55–64 | 16.6 | 12.5 | 14.9 | 11.4 | 7.2 | 12.2 |
| 65–74 | 30.6 | 25.4 | 28.1 | 27.5 | 21.4 | 26.2 |
| 75–84 | 33.4 | 39.1 | 37.9 | 40.3 | 41.0 | 38.3 |
| ≥85 | 13.8 | 18.2 | 14.7 | 17.2 | 27.7 | 19.0 |
| Year of enrollment | ||||||
| 2006 | 26.7 | 27.0 | 25.9 | 29.1 | 31.6 | 28.3 |
| 2007 | 22.3 | 24.8 | 26.0 | 24.8 | 25.8 | 24.7 |
| 2008 | 25.7 | 24.5 | 24.7 | 24.8 | 22.2 | 24.2 |
| 2009 | 25.3 | 23.8 | 23.4 | 21.3 | 20.4 | 22.8 |
| Previous exacerbations | ||||||
| Severe | 4.5 | 4.9 | 3.5 | 5.2 | 5.1 | 4.7 |
| Moderate | 7.8 | 6.1 | 7.3 | 7.6 | 7.4 | 7.2 |
| Concomitant respiratory diseases | ||||||
| Asthma | 1.2 | 1.5 | 1.2 | 1.1 | 1.0 | 1.2 |
| Respiratory failure | 48.5 | 41.0 | 42.8 | 42.2 | 40.4 | 42.9 |
| Comorbidities | ||||||
| Diabetes | 1.7 | 5.0 | 1.5 | 4.3 | 6.3 | 4.0 |
| Ischemic disease | 14.6 | 19.1 | 18.2 | 20.5 | 22.5 | 19.1 |
| Heart failure | 18.1 | 19.7 | 18.7 | 19.7 | 21.4 | 19.6 |
| Liver disease | 6.9 | 8.4 | 8.3 | 9.9 | 12.3 | 9.3 |
| Cerebrovascular diseases | 14.3 | 15.9 | 13.8 | 17.1 | 21.6 | 16.9 |
| Depression/psychiatric diseases | ||||||
| Use of drugs | ||||||
| Anti‐diabetic drugs | 15.8 | 15.6 | 17.4 | 18.2 | 17.3 | 16.8 |
| Antiplatelets | 32.4 | 34.2 | 36.6 | 38.3 | 40.1 | 36.3 |
| Antihypertensives | 68.1 | 67.0 | 68.7 | 71.9 | 73.2 | 69.8 |
| Statins | 16.8 | 15.5 | 17.2 | 18.0 | 16.3 | 16.6 |
| Cardiac therapies | 11.9 | 14.2 | 16.1 | 15.6 | 18.8 | 15.4 |
Drug exposure and covariates during follow‐up
Table 2 shows the distribution of time‐dependent drug exposure and covariates along the follow‐up. The percentage of days spent in the different drug exposure categories varied from to 11.1% in the respiratory drugs other than LB category to 45.7% for LB/ICS occasional use. The proportion of days spent in LB/ICS regular use was 13.0%. High levels of statins and cardiac therapy regular use during the follow‐up were observed (76.3% and 78.3% of overall follow‐up days, respectively). In the LB/ICS regular use category we observed a high proportion of days with regular statins therapy (83.8% versus 78.3% in the overall) and a low proportion of days with cardiovascular hospitalizations (8.6% versus 13.9%).
Table 2.
Time‐dependent characteristics according to the treatment measured during the follow‐up, starting from the 90° day after discharge
| LB/ICS regular use | LB/ICS occasional use | LB regular use | LB occasional use | Respiratory drugs other than LB | Total days | |
|---|---|---|---|---|---|---|
| 13.0% | 45.7% | 12.5% | 11.1% | 17.5% | 10 496 932 | |
| Regular use of | ||||||
| Statins | 16.3 | 16.6 | 12.8 | 14.6 | 14.6 | 15.5 |
| Antihypertensives | 76.4 | 76.0 | 74.6 | 76.3 | 78.2 | 76.3 |
| Cardiac therapies | 83.8 | 78.1 | 77.6 | 77.0 | 76.1 | 78.3 |
| Proxy of gravity | ||||||
| Moderate exacerbation | 31.4 | 32.1 | 27.1 | 22.2 | 23.2 | 28.7 |
| Severe exacerbation | 19.7 | 19.6 | 20.4 | 15.6 | 10.6 | 17.7 |
| Cardiovascular hosp | 8.6 | 15.8 | 12.7 | 13.8 | 13.6 | 13.9 |
Note: Figures represent the proportion of days spent in the follow‐up period.
Association of drug exposure and mortality
The associations between the drug exposure and the risk of death for the entire cohort and in the subgroup of patients with previous exacerbations are shown in Table 3. In comparison to the LB/ICS regular use category we found a higher risk of death for all the remaining categories. The highest risk was observed for the respiratory drugs other than LB category (HR = 1.63; 95%CI 1.43–1.87). Similar pattern of association resulted in the subgroup population (11.5% of the total) with stronger effects (HR = 2.11, 95%CI 1.47–3.03 for respiratory drugs other than LB category). The highest AR% value was found for respiratory drugs other than LB category (38.7% overall population, 52.6 % subgroup), while the absolute numbers of evitable deaths were higher in LB/ICS occasional use category (280 overall population, 82 subgroup). In the whole population, in comparison to LB/ICS occasional use, the exposure to LB regular use was associated with better survival (HR = 0.89; 95% 95% 0.79–0.99) (not shown in the table).
Table 3.
Association between inhaled drug use and survival (HR, 95%CI) both in the whole population under study and in the subgroup with previous exacerbations
| Cohort: N = 12 124, mean of follow‐up = 2.4 years, mortality rate = 11.9 per100 p. y. | |||||||
|---|---|---|---|---|---|---|---|
| Treatment | % time of follow‐up | Mortality rate | HR | CI95% | AR% | Number of preventable deaths | |
| LB/ICS regular use | 13.0 | 8.2 | 1 | — | — | — | — |
| LB/ICS occasional use | 45.7 | 11.3 | 1.26 | 1.11 | 1.43 | 20.6 | 280 |
| LB regular use | 12.5 | 9.7 | 1.13 | 0.97 | 1.32 | 11.6 | 39 |
| LB occasional use | 11.1 | 12.7 | 1.34 | 1.16 | 1.56 | 25.5 | 90 |
| Respiratory drugs other than LB | 17.6 | 17.0 | 1.63 | 1.43 | 1.87 | 38.7 | 261 |
| People with exacerbation: N = 1389, mean of follow‐up = 2.3 years, mortality rate = 14.9 per100 p. y. | |||||||
|---|---|---|---|---|---|---|---|
| Treatment | % time of follow‐up | Mortality rate | HR | CI95% | AR% | Number of preventable deaths | |
| LB/ICS regular use | 14.7 | 8.7 | 1 | — | — | — | — |
| LB/ICS occasional use | 45.2 | 14.4 | 1.65 | 1.17 | 2.32 | 39.4 | 82 |
| LB regular use | 13.7 | 11.9 | 1.45 | 0.96 | 2.19 | 30.8 | 17 |
| LB occasional use | 10.0 | 15.3 | 1.73 | 1.14 | 2.63 | 42.2 | 20 |
| Respiratory drugs other than LB | 16.5 | 23.6 | 2.11 | 1.47 | 3.03 | 52.6 | 51 |
p.y. = person years
Figure 2 shows adjusted survival probability curves considering the different exposure categories. LB/ICS regular use had the highest probability of survive. The lowest curve was found for the respiratory drugs other than LB category.
Figure 2.
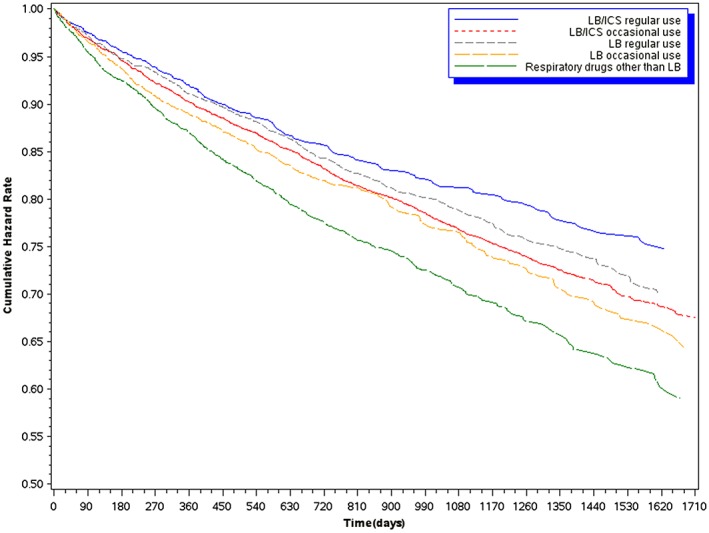
Survival Cox curves according to the different categories of time‐dependent exposure to inhaled drugs
Similar pattern of probability curves were found when we considered the recent exposure (Figure 3). Results were substantially confirmed both using different exposure classifications or cut off points and 1 or 2 year follow‐up time (data not shown).
Figure 3.
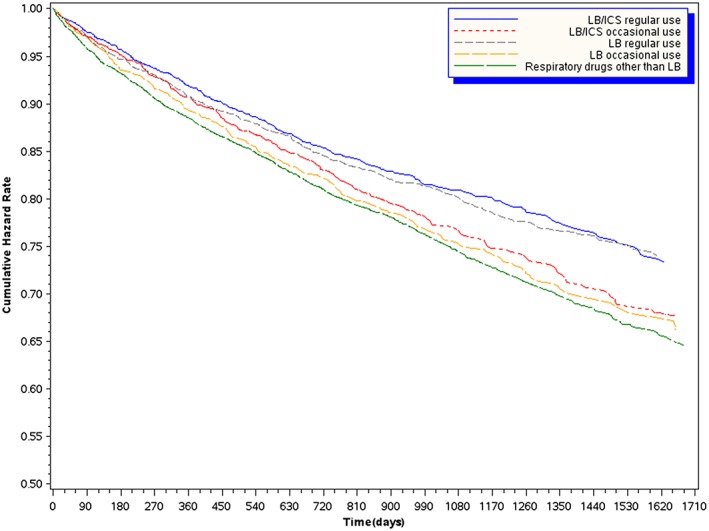
Survival Cox curves according to the different categories of time‐dependent exposure to inhaled drugs, measuring the exposure in a mobile window of 90‐day preceeding each day of follow‐up
Discussion
The present large multi‐centric Italian cohort study demonstrates the fundamental role of adherence to inhaled therapy in the 5‐year survival in patients with moderate–severe COPD. The beneficial effect is higher for the combined use of LB/ICS and it is more evident in the vulnerable group with previous exacerbations.
Non‐adherence is a common problem in chronic diseases.33, 34 Studies over the past decades have shown that adherence is significantly poor in COPD even if therapies can be life sustaining.13, 16 The average adherence rates in clinical trials have been estimated to be around 70–90% among COPD patients; however, in clinical practice, these rates are only in the range of 20–60%.14 In our previous work on 11 452 COPD patients in Lazio region with 2‐year follow‐up on drug treatment after discharge, only 34.8% received long‐acting bronchodilators continuously.28 Then, under real‐life conditions non‐adherence to medication regimens may represent a significant barrier to the optimal management of COPD patients. It has been shown that discontinuation of medication may increase the frequency of exacerbations, the number of hospitalization, the number of emergency department visits and health care costs.14, 15, 17, 18, 19
Based on the recent review by van Boven et al., only two studies analysed the impact of non adherence on mortality over a 3‐year follow‐up period: they are both clinical trials and results have limited generalizability.17, 35, 36 Reasons for non‐adherence are multiple. These include factors related to the characteristics of the patient, the disease, the therapies and also the doctor–patient relationship.12, 14, 16, 37 Age, current smoking, number of respiratory drugs, polypharmacy and depression have been associated with poor adherence.13, 14, 18, 38 The real‐life contest of this study contributes to understanding the extent to which suboptimal treatment adherence may reduce the clinical benefit of the therapy and may account for many of the observed differences between the efficacy (works under experimental conditions) and the real‐world effectiveness of the COPD drug treatment.
Risk and benefits of specific inhaled medicaments in COPD have been extensively evaluated in the last decades.7, 8, 39 However, whether one particular treatment is more effective than another is still an open question. A recent Cochrane meta‐analysis on long acting inhalers concludes that combination inhalers (LB and ICS) positively affect quality of life and lung function while Long‐acting muscarinc antagonists and LB inhalers had similar effects, particularly at 12 months: the benefit of adding ICS to LB was higher for these outcomes in COPD patients with reduced lung function (FEV1 that was less than 50% predicted).40 On the other hand, the superiority of ICS/LB over LB alone in preventing exacerbations has not been conclusively demonstrated.5, 8 This evidence supports current guidelines indicating long‐acting beta‐agonists as key component of therapy for COPD, with regular inhaled corticosteroid therapy as an adjunct in patients experiencing frequent exacerbations.1, 2, 3 However, the rationale of the ICS therapy in COPD is still a matter of scientific debate.41, 42 On one hand, COPD involves both chronic inflammation of the lung, particularly in peripheral airways and parenchyma, and systemic inflammation, which may contribute to skeletal muscle weakness and cachexia and increasing propensity to cardiovascular, metabolic and bone diseases. Many inflammatory mediators have been implicated in COPD, including lipids, free radicals, cytokines, chemokines and growth factors.41, 42 Considering that systemic inflammation derives from peripheral lung inflammation, inhaled antiinflammatory treatments are expected to reduce systemic inflammation and therefore reduce or treat comorbidities. On the other hand, the inflammatory process in COPD seems largely resistant to the antiinflammatory effects of corticosteroids and their use is associated with significant morbidity from side effects.5, 8, 41, 42 In this respect, COPD guidelines recommend long‐term treatment with ICS for patients with severe and very severe COPD and frequent exacerbations that are not adequately controlled by long‐acting bronchodilators.1, 2 The Agency for Healthcare Research and Quality—National Guidelines Clearinghouse summarizes that when to use combination therapy instead of monotherapy has not been clearly established and clinicians should weigh its potential benefits and harms on a case‐by‐case basis.43 Our study adds to the debate on the effectiveness of inhaled drugs. We found a higher 5‐year survival associated with LB/ICS in comparison to the LB monotherapy. This finding supports the hypothesis that inhaled anti‐inflammatory drugs may promote better prognosis by reducing the risk of exacerbations and by contrasting the impact of systemic inflammation and comorbidities. The finding of an even higher effect of adding ICS to LB among those with a history of previous COPD exacerbations support the hypothesis of the beneficial role of anti‐inflammatory drugs in the complex pathophysiology of the “frequent exacerbator phenotype”.44 In our study we assume that COPD patients discharged from hospital after an exacerbation have a moderate–severe disease and may be candidate to maintenance therapy with combined ICS/LABA according to guidelines.28 The fact that the regular LB use leads to a better impact on survival in comparison to the occasional LB/ICS use reinforces the importance of adherence to treatment for the health outcomes. Finally, the evidence of worst affect associated with respiratory drugs other than LB, which includes ICS monotherapy, are in keeping with current guidelines.1, 2, 3
In the last decades assessing the comparative effectiveness of health interventions through observational studies has become progressively a relevant challenge.45, 46 Besides the complex methodology in comparison to highly controlled research environments, findings from this kind of studies have the potential to better inform health care decisions to improve patient outcomes. Major methodological criticisms in the comparative effectiveness research area and possible solutions have been widely discussed.47, 48 In this field of research, electronic health care databases represent a rich source of data and they have been incorporated into several governative outcomes evaluation programme in many countries (USA, UK, Italy). The use of optimal study design and statistical approach are critical elements in population‐based studies, like the present work. In order to control for time‐varying confounders when studying time‐varying exposures, marginal structural model (MSM) is considered the preferred method.49, 50 Given the complex nature of drug exposure measurements in our study, we applied a time‐dependent Cox model. In two recent literature reviews which compared conventional models and MSM when studying causal effects of time‐dependent drug use, both discrepancies in effect estimates and suboptimal reporting of the applied methodology were found.51, 52 The lack of accurate information on time‐dependent confounders and on prescription determinants remains a challenge in comparative effectiveness research and limits the interpretation of the causal relationship between drug use and outcomes.51, 52, 53
The large number of people enrolled, the real‐life picture of inhaled drug use and their effectiveness, and the time‐dependent exposure measurements along the long follow‐up represent the main strengths of this study. There are some limits to be discussed. First, a major critical point is the definition of COPD patients. The discharge abstract does not contain information on clinical and functional characteristics limiting accurateness of our inclusion criteria. In a subsample of our study population we conducted a re‐abstract study on clinical records to evaluate diagnoses and severity. About 94% of reviewed cases were confirmed as being cases of COPD and with a moderate/severe level of disease, confirming previous result in our region.54, 55 Second, selection bias could be considered; however, all persons with a hospitalized COPD were enrolled with the same standardized criteria in the three regions, then we think this bias could have a limited impact in our study. Third, our pharmaceutical database does not contain information on the prescribed daily doses; therefore, the number of days in which the patient was treated was estimated on the basis of the DDDs.24, 27 Although this is a useful instrument for comparing the results from different studies, misclassification of drug utilization may have occurred.24, 56 Although this is well‐known methodology, we cannot exclude misclassification of drug utilization. It is to note of our source of data refers to prescription and we do not know the exact level of intake by the patients. However this is a known limitation of studies like that and it produces limited distortion in the estimates of association.57 Fourth, the choice of the measures of adherence and the cut‐off points was based on literature and our previous work.28, 29 Results from sensitivity analyses using different exposure measurements and classifications and shorter follow‐up confirm the main findings and support the validity of the study. Fifth, in our study, the HRs, the AR % and the number of preventable deaths were derived from Cox proportional hazard models in which a large number of potential confounders, both fixed and time‐dependent, were included. However, when quantifying the effect of an exposure by estimating the number of cases that were caused by it, it is necessary to take into account the mechanisms that produce effects. The observation of an exposed case does not reveal the mechanism that caused the case; people who have the exposure can develop the disease from a mechanism that does not include the exposure.32 In our study, besides the number of confounders included in the analysis, we cannot exclude the role of unknown factors we were not able to measure. To address this issue, one should consider that better compliance alone might be associated with improved outcomes (e.g. also in placebo groups of trials). However, only randomization can completely rule out residual confounding.
Our study shows the effectiveness of the regular use of combined LB/ICS on long‐term survival. Being regular users of LB is associated to better survival in comparison to suboptimal combined LB/ICS treatment. Considering that this study is based on patients discharged from hospitals, relevant impact on health outcomes of adherence to optimal treatment with inhalers could be expected in the overall COPD population.
Conflict of Interest
None.
Key Points.
Conclusive evidence on the impact of inhaled drugs on long‐term survival in chronic obstructive pulmonary disease patients is lacking.
From this large Italian population‐based cohort study, the continuous use of combined long‐acting β2 agonists (LB)/inhaled corticosteroids is associated with a higher 5‐year survival in comparison with regular use of LB in moderate–severe chronic obstructive pulmonary disease patients.
Patients with regular use of LB have a higher 5‐year survival than those occasionally treated with LB/inhaled corticosteroids.
Ethics Statement
Ethical approval was achieved from the regional authorities.
Funding
The project was partially funded by Agenzia Italiana del Farmaco (AIFA); Prot. FARM8ZBT93.
Author Contributions
NA, RP and VB conceived the idea of the study and together with MDM and UK were responsible for hypothesis delineation and design of the study. SC and MDM were responsible for the acquisition and preparation of the dataset. VB was responsible for undertaking the data analysis and produced the tables and graphs. DF, MD, MDM, RP and GF provided input into the data analysis. All authors contributed to the interpretation of the results. The initial draft of the manuscript was prepared by VB and NA and then circulated repeatedly among all authors for critical revision.
Supporting information
Supporting info item
Acknowledgements
We are grateful to Roberta Macci e Sandra Magliolo for their support in retrieving the cited articles.
Belleudi, V. , Di Martino, M. , Cascini, S. , Kirchmayer, U. , Pistelli, R. , Formoso, G. , Fusco, D. , Davoli, M. , Agabiti, N. , and on behalf of the OUTPUL Study Group (2016) The impact of adherence to inhaled drugs on 5‐year survival in COPD patients: a time dependent approach. Pharmacoepidemiol Drug Saf, 25: 1295–1304. doi: 10.1002/pds.4059.
The OUTPUL Study Group. Nera Agabiti (1) (principal investigator), Mirko Di Martino (1), Ursula Kirchmayer (1), Lisa Bauleo (1), Silvia Cascini (1), Valeria Belleudi (1), Luigi Pinnarelli (1), Danilo Fusco (1), Marina Davoli (1), Carlo A Perucci (2), Nicola Magrini (3), Giulio Formoso (3), Claudio Voci (3), Anna Maria Marata (3), Riccardo Pistelli (4), Vittoria Colamesta (4), Carlo Zocchetti (5).
- Department of Epidemiology, Lazio Regional Health Service, Roma, Italy.
- National Agency for Regional Health Services, Roma, Italy.
- Emilia‐Romagna Regional Health and Social Care Agency, Bologna, Italy.
- Department of Respiratory Physiology, Catholic University, Roma, Italy.
- Regional Health Authority, Lombardia Region, Milano, Italy.
References
- 1. Global Initiative for Chronic Obstructive Lung Disease . Global Strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. GOLD, Updated 2015 report. Available at: http://www.goldcopd.org/guidelines‐global‐strategy‐for‐diagnosis‐management.html (accessed March 2015)
- 2. Vestbo J, Hurd SS, Agustí AG, et al. R. GOLD executive summary. Am J Respir Crit Care Med 2013; 187: 347–365. [DOI] [PubMed] [Google Scholar]
- 3. National Clinical Guideline Centre . 2010. Chronic Obstructive Pulmonary Disease: Management of Chronic Obstructive Pulmonary Disease in Adults in Primary and Secondary Care. National Clinical Guideline Centre: London: Available at: http://guidance.nice.org.uk/CG101/Guidance/pdf/English (accessed March 2015) [Google Scholar]
- 4. Kew KM, Mavergames C, Walters JA. Long‐acting beta2‐agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2013; 10: CD010177. [DOI] [PubMed] [Google Scholar]
- 5. Nannini LJ, Poole P, Milan SJ, et al. Combined corticosteroid and long‐acting beta2‐agonist in one inhaler versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2013; 11: CD003794. doi:10.1002/14651858.CD003794.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oba Y, Lone NA. Comparative efficacy of inhaled corticosteroid and long‐acting beta agonist combinations in preventing COPD exacerbations: a Bayesian network meta‐analysis. Int J Chron Obstruct Pulmon Dis 2014; 12: 469–479. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Welsh EJ, Cates CJ, Poole P. Combination inhaled steroid and long‐acting beta2‐agonist versus tiotropium for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2013; 5: CD007891. doi:10.1002/14651858.CD007891.pub2. [DOI] [PubMed] [Google Scholar]
- 8. Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long‐acting beta2‐agonist in one inhaler versus long‐acting beta2‐agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012; 9: CD006829. doi:10.1002/14651858.CD006829.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barnes PJ. Inhaled corticosteroids in COPD: a controversy. Respiration 2010; 80: 89–95. [DOI] [PubMed] [Google Scholar]
- 10. Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007; 356: 775–789. [DOI] [PubMed] [Google Scholar]
- 11. Decramer M, Celli B, Kesten S, et al. Effect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): a prespecified subgroup analysis of a randomised controlled trial. Lancet 2009; 374: 1171–1178. [DOI] [PubMed] [Google Scholar]
- 12. Bryant J, McDonald VM, Boyes A, et al. Improving medication adherence in chronic obstructive pulmonary disease: a systematic review. Respir Res 2013; 14: 109. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bourbeau J, Bartlett SJ. Patient adherence in COPD. Thorax 2008; 63: 831–838. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ágh T, Inotai A, Mészáros Á. Factors associated with medication adherence in patients with chronic obstructive pulmonary disease. Respiration 2011; 82: 328–334. Epub 2011 Apr 1. [DOI] [PubMed] [Google Scholar]
- 15. Asche CV, Leader S, Plauschinat C, et al. Adherence to current guidelines for chronic obstructive pulmonary disease (COPD) among patients treated with combination of long‐acting bronchodilators or inhaled corticosteroids. Int J Chron Obstruct Pulmon Dis 2012; 7: 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bender BG. Nonadherence in chronic obstructive pulmonary disease patients: what do we know and what should we do next? Curr Opin Pulm Med 2014; 20: 132–137. Review. [DOI] [PubMed] [Google Scholar]
- 17. van Boven JF, Chavannes NH, van der Molen T, et al. Clinical and economic impact of non‐adherence in COPD: a systematic review. Respir Med 2014; 108: 103–113. Review. [DOI] [PubMed] [Google Scholar]
- 18. Yohannes AM, Alexopoulos GS. Pharmacological treatment of depression in older patients with chronic obstructive pulmonary disease: impact on the course of the disease and health outcomes. Drugs Aging 2014; 31: 483–492 Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Simoni‐Wastila L, Wei YJ, Qian J, et al. Association of chronic obstructive pulmonary disease maintenance medication adherence with all‐cause hospitalization and spending in a Medicare population. Am J Geriatr Pharmacother 2012; 10: 201–210. [DOI] [PubMed] [Google Scholar]
- 20. Mancia G, Zambon A, Soranna D, et al. Factors involved in the discontinuation of antihypertensive drug therapy: an analysis from real life data. J Hypertens 2014; 32: 1708–1715; discussion 1716. [DOI] [PubMed] [Google Scholar]
- 21. Jackson RS, Chang DC, Freischlag JA. Comparison of long‐term survival after open vs endovascular repair of intact abdominal aortic aneurysm among Medicare beneficiaries. JAMA 2012; 307: 1621–1628. [DOI] [PubMed] [Google Scholar]
- 22. Kirchmayer U, Di Martino M, Agabiti N, et al. Effect of evidence‐based drug therapy on long‐term outcomes in patients discharged after myocardial infarction: a nested case–control study in Italy. Pharmacoepidemiol Drug Saf 2013; 22: 649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kirchmayer U, Agabiti N, Belleudi V, et al. Socio‐demographic differences in adherence to evidence‐based drug therapy after hospital discharge from acute myocardial infarction: a population‐based cohort study in Rome, Italy. J Clin Pharm Ther 2012; 37: 37–44. [DOI] [PubMed] [Google Scholar]
- 24. WHO Collaborating Centre for Drug Statistics Methodology Norwegian Institute of Public Health . Available at http://www.whocc.no/filearchive/publications/1_2013guidelines.pdf (accessed May 2016).
- 25. Agabiti N, Belleudi V, Davoli M, et al. Profiling hospital performance to monitor the quality of care: the case of COPD. Eur Respir J 2010; 35: 1031–1038. [DOI] [PubMed] [Google Scholar]
- 26. Di Martino M, Agabiti N, Cascini S, et al. The effect on total mortality of adding inhaled corticosteroids to long‐acting bronchodilators for COPD: a real practice analysis in Italy. COPD 2015; 29: 1–12. [DOI] [PubMed] [Google Scholar]
- 27. Kirchmayer U, Cascini S, Agabiti N, et al. One‐year mortality associated with COPD treatment: a comparison of tiotropium and long‐acting beta2‐agonists in three Italian regions: results from the OUTPUL study. Pharmacoepidemiol Drug Saf 2016; 25: 578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Di Martino M, Agabiti N, Bauleo L, et al. Use patterns of long‐acting bronchodilators in routine COPD care: the OUTPUL study. COPD 2014; 11: 414–423. [DOI] [PubMed] [Google Scholar]
- 29. Koehorst‐Ter Huurne K, Movig K, van der Valk P, et al. The influence of type of inhalation device on adherence of COPD patients to inhaled medication. Expert Opin Drug Deliv 2016; 13: 469–475. [DOI] [PubMed] [Google Scholar]
- 30. Ferroni E, Belleudi V, Cascini S, et al. Role of tiotropium in reducing exacerbations of chronic obstructive pulmonary disease when combined with long‐acting B2‐agonists and inhaled corticosteroids: the OUTPUL study. J Clin Pharmacol 2016. doi:10.1002/jcph.750. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Natarajan S, Lipsitz SR, Rimm E. A simple method of determining confidence intervals for population attributable risk from complex surveys. Stat Med 2007; 26: 3229–3239. [DOI] [PubMed] [Google Scholar]
- 32. Rothman KJ, Greenland S. Modern Epidemiology (2nd edn). Lippincott – Raven Publications: California (USA), 1998. [Google Scholar]
- 33. Marcum ZA, Sevick MA, Handler SM. Medication nonadherence: a diagnosable and treatable medical condition. JAMA 2013; 309: 2105–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Naderi SH, Bestwick JP, Wald DS. Adherence to drugs that prevent cardiovascular disease: meta‐analysis on 376,162 patients. Am J Med 2012; 125(9): 882‐7.e1. [DOI] [PubMed] [Google Scholar]
- 35. Vestbo J, Anderson JA, Calverley PM, et al. Adherence to inhaled therapy, mortality and hospital admission in COPD. Thorax 2009; 64: 939e43. [DOI] [PubMed] [Google Scholar]
- 36. Turner J, Wright E, Mendella L, et al. Predictors of patient adherence to long‐term home nebulizer therapy for COPD. The IPPB Study Group Intermittent positive pressure breathing. Chest 1995; 108: 394e400. [DOI] [PubMed] [Google Scholar]
- 37. Takemura M, Mitsui K, Ido M, et al. Impact of a network system for providing proper inhalation technique by community pharmacists. J Asthma 2012; 49: 535–541. [DOI] [PubMed] [Google Scholar]
- 38. Qian J, Simoni‐Wastila L, Rattinger GB, et al. Association between depression and maintenance medication adherence among Medicare beneficiaries with chronic obstructive pulmonary disease. Int J Geriatr Psychiatry 2014; 29: 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Geake JB, Dabscheck EJ, Wood‐Baker R, et al. Indacaterol, a once‐daily beta2‐agonist, versus twice‐daily beta2‐agonists or placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015; 1: CD010139. doi:10.1002/14651858.CD010139.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kew KM, Dias S, Cates CJ. Long‐acting inhaled therapy (beta‐agonists, anticholinergics and steroids) for COPD: a network meta‐analysis. Cochrane Database Syst Rev 2014; 3: CD010844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.41. Barnes PJ. Cellular and molecular mechanisms of chronic obstructive pulmonary disease. Clin Chest Med 2014; 35: 71–86. Review. [DOI] [PubMed] [Google Scholar]
- 42. Anton E. How and when to use inhaled corticosteroids in chronic obstructive pulmonary disease? Expert Rev Respir Med 2013; 7(2 Suppl): 25–32. [DOI] [PubMed] [Google Scholar]
- 43. Agency for Healthcare Research and Quality—National Guidelines Clearinghouse—Diagnosis and Management of Stable Chronic Obstructive Pulmonary Disease (COPD). Available at: http://www.guideline.gov/syntheses/synthesis.aspx?id=48263 (accessed April 2016).
- 44. Wedzicha JA, Brill SE, Allinson JP, et al. Mechanisms and impact of the frequent exacerbator phenotype in chronic obstructive pulmonary disease. BMC Med 2013; 11: 181. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lapi F, Azoulay L, Yin H, et al. Concurrent use of diuretics, angiotensin converting enzyme inhibitors, and angiotensin receptor blockers with non‐steroidal anti‐inflammatory drugs and risk of acute kidney injury: nested case–control study. BMJ 2013; 346: e8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Franklin JM, Rassen JA, Bartels DB, et al. Prospective cohort studies of newly marketed medications: using covariate data to inform the design of large‐scale studies. Epidemiology 2014; 25: 126–133. [DOI] [PubMed] [Google Scholar]
- 47. Neugebauer R, Schmittdiel JA, Zhu Z, et al. High‐dimensional propensity score algorithm in comparative effectiveness research with time‐varying interventions. Stat Med 2014; 34: 753–781. [DOI] [PubMed] [Google Scholar]
- 48. Suissa S. Immortal time bias in pharmaco‐epidemiology. Am J Epidemiol 2008; 167(4): 492–499. Review. [DOI] [PubMed] [Google Scholar]
- 49. Gruber S, Logan RW, Jarrín I, et al. Ensemble learning of inverse probability weights for marginal structural modeling in large observational datasets. Stat Med 2015; 34: 106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Howe CJ, Cole SR, Mehta SH, et al. Estimating the effects of multiple time‐varying exposures using joint marginal structural models: alcohol consumption, injection drug use, and HIV acquisition. Epidemiology 2012; 23: 574–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yang S, Eaton CB, Lu J, et al. Application of marginal structural models in pharmacoepidemiologic studies: a systematic review. Pharmacoepidemiol Drug Saf 2014; 23: 560–571. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Suarez D, Borràs R, Basagaña X. Differences between marginal structural models and conventional models in their exposure effect estimates: a systematic review. Epidemiology 2011; 22: 586–588. [DOI] [PubMed] [Google Scholar]
- 53.53. de Keyser CE, Leening MJ, Romio SA, et al. Comparing a marginal structural model with a Cox proportional hazard model to estimate the effect of time‐dependent drug use in observational studies: statin use for primary prevention of cardiovascular disease as an example from the Rotterdam Study. Eur J Epidemiol 2014; 29: 841–850. [DOI] [PubMed] [Google Scholar]
- 54. Bauleo L, Agabiti N, Kirchmayer U et al. Accurateness of COPD diagnosis registered in the hospital discharge records: a reabstract study in Rome (Italy). XXXVIII Italian Epidemiology Association‐Annual Conference Naples November 5–7, 2014.
- 55. Fano V, D'Ovidio M, del Zio K, et al. The role of the quality of hospital discharge records on the comparative evaluation of outcomes: the example of chronic obstructive pulmonary disease (COPD). Epidemiol Prev 2012; 36: 172–179. [PubMed] [Google Scholar]
- 56. Di Martino M, Kirchmayer U, Agabiti N et al. The impact of time‐window bias on the assessment of the long‐term effect of medication adherence: the case of secondary prevention after myocardial infarction. BMJ Open 2015; 5(6: e007866). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Belleudi V, Fusco D, Kirchmayer U, et al. Definition of patients treated with evidence based drugs in absence of prescribed daily doses: the example of acute myocardial infarction. Pharmacoepidemiol Drug Saf 2011; 20: 169–176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting info item


