Summary
Introduction
Data on statin safety in Asian patients are limited compared with evidence from Western populations.
Aim
This study assessed atorvastatin safety among Asian patients enrolled in 58 randomized clinical trials.
Methods
Data from 52 short‐term trials (median exposure 4–72 weeks) and six long‐term cardiovascular outcomes trials (median exposure 3.1–4.9 years) conducted across the atorvastatin 10–80‐mg dose range were analyzed retrospectively to assess the incidence of safety endpoints.
Results
A total of 77 952 patients were identified (49 974 received atorvastatin), among whom 3191 were Asian (2519 received atorvastatin). In the short‐term trials, the incidence of all‐causality adverse events (AEs) and serious AEs (SAEs) in Asian patients treated with atorvastatin was similar to or lower than that observed with other statins or placebo, and discontinuations due to treatment‐related AEs/SAEs were infrequent (2.0% across all doses). These observations were confirmed in the long‐term trials. Treatment‐related SAEs were rare (n = 4) among Asian patients receiving atorvastatin. No cases of rhabdomyolysis were observed in atorvastatin‐treated Asian patients, and the incidence of myalgia was 1.8% in the short‐term studies and 6.7% in the long‐term trials. Elevations (>3× the upper limit of normal) in liver transaminases were observed in ~2% of Asian patients receiving atorvastatin; renal AEs occurred in <2%.
Conclusion
The incidence of AEs/SAEs with atorvastatin 10–40‐mg in patients of Asian origin was low and comparable to placebo. Further evaluation of atorvastatin 80‐mg is required owing to the limited number of Asian patients (n = 281; 11.2%) who received this dose.
Keywords: Adverse event, Asian, Atorvastatin, Cardiovascular disease, Data pooling, Hyperlipidemia
1. Introduction
Cardiovascular disease (CVD) is a leading cause of death worldwide,1 particularly in Asian countries such as China, where it represents a huge economic burden.2 Studies from the Asia‐Pacific region have shown that classical cardiovascular (CV) risk factors, such as blood pressure and lipid levels, are as relevant in Eastern as in Western populations.3, 4 Therefore, CV risk reduction strategies that have proved successful in Western populations will likely have a similar impact in preventing CV events in Asian patients.3 The reduction in low‐density lipoprotein cholesterol (LDL‐C) levels with statins has been shown to be highly effective for reducing CV risk in a wide range of patient populations,5, 6 including Asian patients.7, 8, 9, 10 Recent dyslipidemia treatment guidelines have emphasized the importance of LDL‐C lowering with statins for the prevention of CVD,11, 12 including high‐intensity statin therapy to achieve ≥50% reduction in LDL‐C.12 Despite this overwhelming evidence to support lipid lowering through the use of statins to reduce CV risk, dyslipidemia is often undertreated in Asian populations, with reports of up to 60% of high‐risk patients not receiving a statin,13, 14 and low rates of goal attainment in those patients receiving lipid‐lowering treatment (~50%), most notably in those at very high CV risk (~30%).15, 16
Long‐term CV outcomes trials with atorvastatin,17, 18, 19, 20, 21, 22, 23 as well as other statins,24, 25 have demonstrated the safety of this class in a range of populations. However, compared with the wealth of evidence from Western populations, the availability of statin safety data from Asian populations is limited, which may be a contributing factor in the underutilization of statins for the treatment of dyslipidemia in this ethnic group. Previous retrospective analyses of pooled clinical trial data support the overall safety of atorvastatin across the 10–80‐mg dose range.26, 27 This current retrospective analysis assessed the safety of atorvastatin among Asian patients enrolled in randomized clinical trials.
2. Methods
2.1. Data sources and grouping
There were 101 well‐controlled, well‐documented Pfizer‐sponsored atorvastatin clinical trials that began in or after 1992 and were completed by 30 March 2012. With one exception,22 these trials are part of a pooled repository database; however, available data from the remaining trial were included in this analysis. A programmatic search for patient race or ethnicity captured on case report forms (CRFs) revealed 58 trials that had enrolled ≥1 patient self‐identified as Asian, Oriental, South Asian, Indian, or Pacific Islander.
Data from these 58 trials were grouped by trial duration (Fig. 1) so that the several large trials did not confound results from the smaller trials. Six long‐term trials18, 20, 21, 22, 23, 28 (see footnote to Table 1 for trial details) investigating the effect of atorvastatin on CV outcomes were analyzed both individually and pooled according to treatment group; the median study duration was 3.1–4.9 years. The remaining 52 short‐term studies (≤2 years) were pooled according to treatment group; the median study duration was 4–72 weeks. All atorvastatin doses (10–80‐mg) were investigated (Fig. 1).
Figure 1.
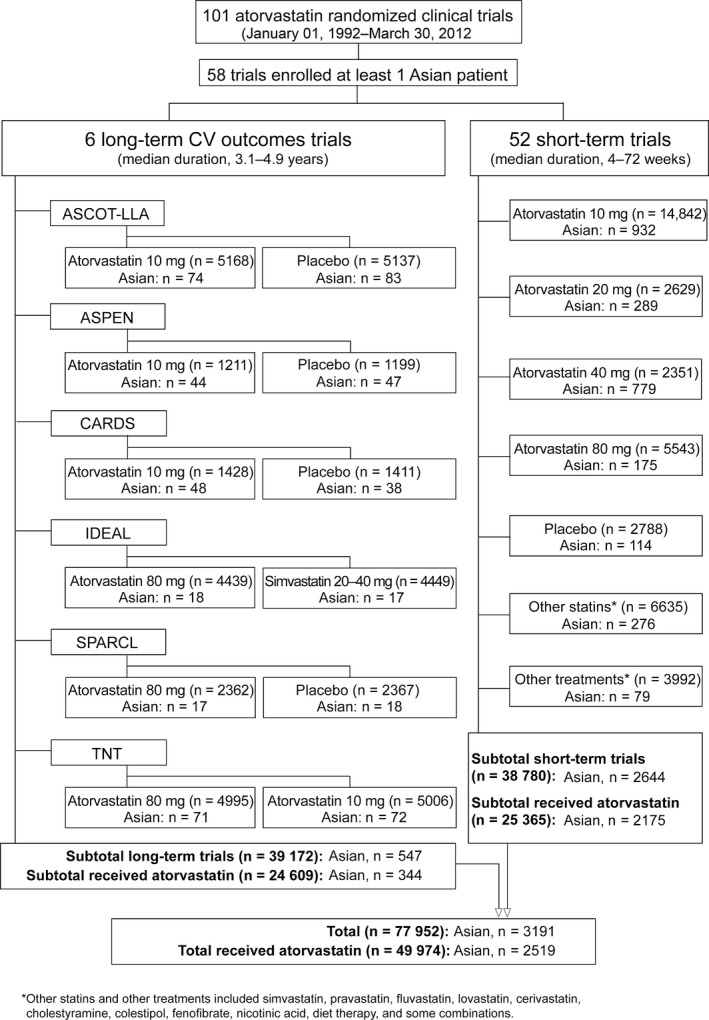
Identification of Asian patients in randomized clinical trials of atorvastatin. Details of the long‐term CV outcomes trials are provided in the footnote to Table 1. CV, cardiovascular
Table 1.
Baseline demographics and characteristics of Asian patients in long‐term CV outcomes trials of atorvastatin stratified by treatmenta
| ATV 10 mg | ATV 80 mg | Placebo | |
|---|---|---|---|
| No. of Asian patients | 238 | 106 | 186 |
| Age (years) | 58.3 (8.1) | 59.1 (9.7) | 58.2 (9.4) |
| Age ≥65 years, n (%) | 57 (23.9) | 35 (33.0) | 46 (24.7) |
| Age ≥70 years, n (%) | 13 (5.5)b | 15 (14.2)b | 18 (9.7) |
| Male gender, n (%) | 188 (79.0) | 87 (82.1) | 142 (76.3) |
| Smoking status, n (%) | |||
| Current smoker | 33 (13.9) | 7 (6.6)c | 28 (15.1) |
| Non/ex‐smoker | 205 (86.1) | 99 (93.4)c | 158 (84.9) |
| BMI (kg/m2) | 26.7 (3.5) | 25.9 (2.9)c | 27.2 (4.1) |
| BP (mm Hg) | |||
| SBP | 139.2 (19.5)b , c | 128.7 (17.9)b , c | 143.3 (21.6) |
| DBP | 82.9 (10.6)b , c | 79.7 (10.7)b , c | 86.0 (10.6) |
| Lipids (mg/dL) | |||
| LDL‐C | 109.0 (30.3)c | 103.6 (26.9)c | 121.0 (26.5) |
| HDL‐C | 48.6 (11.0) | 47.1 (10.5) | 49.5 (12.7) |
| Total cholesterol | 187.5 (35.5)b , c | 179.2 (29.8)b , c | 201.2 (29.5) |
| Triglycerides | 148.5 (77.5) | 144.8 (61.3) | 155.2 (72.3) |
| BUN (mg/dL) | 15.2 (4.5) | 16.0 (5.4) | 14.8 (4.1) |
| Creatinine (mg/dL) | 1.12 (0.19)b | 1.18 (0.21)b | 1.14 (0.22) |
| HbA1c (%)d | 7.7 (1.5)b | 6.5 (1.4)b , c | 7.8 (1.5) |
Values are mean (SD) or n (%). To convert mg/dL to mmol/L for cholesterol, divide by 38.67; for triglycerides, divide by 88.57; for BUN, multiply by 0.357. To convert mg/dL to μmol/L for creatinine, multiply by 88.4. Trials and endpoints included: ASCOT‐LLA, Anglo‐Scandinavian Cardiac Outcomes Trial–Lipid‐Lowering Arm (primary endpoint: nonfatal myocardial infarction [MI] and fatal coronary heart disease [CHD]); ASPEN, Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in Non‐Insulin‐Dependent Diabetes Mellitus (primary endpoint: time to the first occurrence of cardiovascular death, nonfatal or silent MI, nonfatal stroke, recanalization, coronary artery bypass grafting, resuscitated cardiac arrest, or worsening or unstable angina requiring hospitalization); CARDS, Collaborative Atorvastatin Diabetes Study (primary endpoint: time to first occurrence of acute CHD event, coronary revascularization, or stroke); IDEAL, Incremental Decrease in End Points Through Aggressive Lipid Lowering study (primary endpoint: composite of major coronary event, defined as coronary death, hospitalization for nonfatal acute MI, or resuscitated cardiac arrest); SPARCL, Stroke Prevention by Aggressive Reduction in Cholesterol Levels study (primary end point: a first nonfatal or fatal stroke); TNT, Treating to New Targets study (primary endpoint: the occurrence of a first major cardiovascular event, defined as death from CHD, nonfatal nonprocedure‐related MI, resuscitation after cardiac arrest, or fatal or nonfatal stroke). ATV, atorvastatin; BMI, body mass index; BP, blood pressure; BUN, blood urea nitrogen; DBP, diastolic blood pressure; HbA1c, glycosylated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; SD, standard deviation.
Atorvastatin and placebo treatment groups are shown; for simvastatin 20–40 mg in IDEAL, see Table S1.
P < 0.05 versus other atorvastatin dose.
P < 0.05 versus placebo.
Approximately 25% of all patients in TNT had values for HbA1c; for the Asian participants in TNT, this rate was about 40%.
2.2. Overall safety analyses
This analysis utilized MedDRA (Medical Dictionary for Regulatory Activities) for adverse event (AE) terms. As MedDRA has been updated during the 20‐year period covered by the clinical trial database used for this analysis, and some trials20, 21, 23 utilized coding dictionaries other than MedDRA, AEs from all trials were mapped to the latest MedDRA terms. Therefore, calculated incidences of AEs for individual trials may differ from rates originally reported.
AEs were collected at each clinic visit based on symptoms reported by the patient, physical examination findings, and abnormal laboratory tests. AEs were reported from initiation of treatment and up to 30 days after trial discontinuation. The intensity of AEs and their relation to study medication were determined by the investigator. Treatment‐related AEs were characterized as possibly, probably, or definitely related to study medication. Any AE for which treatment‐relatedness was not assessed, or was assessed as unknown on the CRF, was considered related to study drug. Serious AEs (SAEs) were defined according to Food and Drug Administration criteria previously used to evaluate atorvastatin safety.26 In studies with CV events as endpoints, CV SAEs were not summarized under the safety results as these were considered to be efficacy endpoints.
Incidences of AEs, treatment‐related AEs, SAEs, treatment‐related SAEs, and study discontinuation due to treatment‐related AEs/SAEs were extracted from the database for all 58 trials. For ASCOT‐LLA, only a summary of overall AEs, SAEs, and discontinuations due to treatment‐related AEs/SAEs was available.
2.3. Musculoskeletal, hepatic, and renal safety analyses
Musculoskeletal AEs (myalgia, myopathy, rhabdomyolysis), creatine kinase (CK) elevations >10× the upper limit of normal (ULN), hepatic transaminase elevations >3× ULN, and renal AEs were evaluated. The normal range for each laboratory parameter was determined by the designated laboratories for each study. Postbaseline incidences of alanine transaminase (ALT) and aspartate transaminase (AST) elevations were included for the hepatic safety analysis. The renal safety analysis included renal failure (both acute and chronic) or renal impairment, and other renal safety parameters including hematuria, albuminuria, microalbuminuria, or proteinuria. Investigator‐reported AEs were used to define renal AEs and were not linked to laboratory data. For ASCOT‐LLA, only ALT elevations were available.
2.4. Statistical analyses
The goal of this analysis was to summarize the safety of atorvastatin in Asian patients across the 58 trials; hence, descriptive statistics are shown for the majority of data. However, for the pooled treatment groups in the long‐term CV outcomes trials, baseline characteristics were compared using a chi‐square test for categorical variables and t‐test for continuous variables, and rates of AEs, SAEs, and discontinuations due to treatment‐related AEs/SAEs were compared using logistic regression adjusted for: age (<70 vs ≥70 years of age); current smoking status; body mass index (BMI); systolic blood pressure (SBP), diastolic blood pressure (DBP), LDL‐C, total cholesterol, and serum creatinine levels; and history of coronary artery bypass graft, coronary angioplasty, CVD, diabetes, myocardial infarction, or hypertension. Statistical analyses were performed using SAS 9.2 or later (SAS Institute, Cary, NC, USA). Two‐sided P‐values < 0.05 were considered statistically significant.
3. Results
3.1. Patient population
Overall, of 77 952 patients identified, 3191 (4.1%) were Asian, of whom 2519 received atorvastatin, 300 received placebo, 293 received other statins, and 79 received other lipid‐lowering treatments (Fig. 1). In the six long‐term CV outcomes trials, 547 of the 39 172 patients were Asian, of whom 344 received atorvastatin, 186 received placebo, and 17 received another statin (simvastatin in IDEAL). In the 52 short‐term trials, 2644 of the 38 780 patients were Asian, of whom 2175 received atorvastatin, 114 received placebo, 276 received other statins, and 79 received other lipid‐lowering treatments.
Baseline variables of Asian patients included in the long‐term CV outcomes trials are shown stratified by study in Table S1, and by treatment group in Table 1. As in the overall trial populations,18, 20, 21, 22, 23, 28 treatment and comparator groups for Asian patients in the individual long‐term trials were generally well‐matched (Table S1). There were notable exceptions in some baseline variables such as: age ≥65 years and current smokers in ASCOT‐LLA; smoking status and triglyceride levels in ASPEN; age ≥65 years and smoking status in CARDS; age, smoking status, and SBP in IDEAL; age, age ≥65 years, male gender, smoking status, and triglyceride levels in SPARCL; and age ≥65 years in TNT (Table S1). Pooled treatment groups for Asian patients in the long‐term trials were well‐matched (Table 1), with the exception of higher SBP, DBP, LDL‐C, and total cholesterol levels in placebo patients vs patients receiving atorvastatin 10‐ or 80‐mg (all P < 0.05). Additionally, placebo patients were more likely to be current smokers with a higher BMI and glycosylated hemoglobin level compared with patients receiving atorvastatin 80‐mg (all P < 0.05). Patients receiving atorvastatin 10‐mg had higher SBP, DBP, total cholesterol, and glycosylated hemoglobin levels, but had lower serum creatinine levels and were less likely to be ≥70 years of age, vs patients receiving atorvastatin 80‐mg (all P < 0.05). Baseline variables of Asian patients included in the short‐term trials are shown stratified by treatment group in Table 2. Pooled treatment groups for Asian patients in the short‐term trials were well‐matched (Table 2), with the exception that placebo patients were more likely to be older males who were current or ex‐smokers, with lower cholesterol levels than those in other treatment groups.
Table 2.
Baseline demographics and characteristics of Asian patients in short‐term trials of atorvastatin stratified by treatment
| All ATV doses | ATV 10 mg | ATV 20 mg | ATV 40 mg | ATV 80 mg | Placebo | Other statinsa | Other treatmentsa | |
|---|---|---|---|---|---|---|---|---|
| No. of Asian patients | 2175 | 932 | 289 | 779 | 175 | 114 | 276 | 79 |
| Age (years) | 58.0 (11.2) | 57.0 (11.3) | 57.8 (11.4) | 59.8 (10.2) | 55.6 (12.9) | 60.3 (11.8) | 57.3 (10.8) | 52.0 (13.4) |
| n | 2173 | 930 | 289 | 779 | 175 | 114 | 275 | 79 |
| Age ≥65 years, n (%) | 639 (29.4) | 241 (25.9) | 87 (30.1) | 262 (33.6) | 49 (28.0) | 39 (34.2) | 71 (25.7) | 13 (16.5) |
| Age ≥70 years, n (%) | 340 (15.6) | 133 (14.3) | 44 (15.2) | 142 (18.2) | 21 (12.0) | 26 (22.8) | 36 (13.0) | 10 (12.7) |
| Male gender, n (%) | 1174 (54.0) | 469 (50.3) | 148 (51.2) | 465 (59.7) | 92 (52.6) | 82 (71.9) | 120 (43.5) | 49 (62.0) |
| Smoking status, n (%) | ||||||||
| Current smoker | 188 (8.6) | 98 (10.5) | 33 (11.4) | 31 (4.0) | 26 (14.9) | 22 (19.3) | 12 (4.4) | 10 (12.7) |
| Non/ex‐smoker | 1058 (48.6) | 571 (61.3) | 181 (62.6) | 186 (23.9) | 120 (68.6) | 83 (72.8) | 107 (38.8) | 62 (78.5) |
| Unknown | 929 (42.7) | 263 (28.2) | 75 (26.0) | 562 (72.1) | 29 (16.6) | 9 (7.9) | 157 (56.9) | 7 (8.9) |
| BMI (kg/m2) | 25.6 (7.7) | 26.0 (11.1) | 25.8 (3.5) | 25.2 (3.4) | 25.8 (3.7) | 25.5 (3.9) | 25.1 (3.5) | 25.5 (4.2) |
| n | 2139 | 921 | 284 | 770 | 164 | 109 | 262 | 79 |
| BP (mm Hg) | ||||||||
| SBP | 129.3 (17.3) | 129.3 (16.4) | 131.6 (15.7) | 129.2 (18.4) | 125.6 (18.1) | 127.7 (19.3) | 128.7 (18.4) | 116.6 (15.7) |
| DBP | 81.1 (16.1) | 81.0 (14.1) | 85.1 (19.4) | 79.1 (14.9) | 84.7 (21.6) | 74.4 (12.2) | 78.7 (10.4) | 71.9 (10.1) |
| n | 2067 | 845 | 287 | 772 | 163 | 111 | 272 | 13 |
| Lipids (mg/dL)b | ||||||||
| LDL‐C | 163.4 (37.7) | 161.3 (38.0) | 153.6 (36.1) | 172.2 (34.1) | 181.7 (37.4) | 135.7 (40.9) | 186.3 (34.6) | 204.5 (90.0) |
| n | 1324 | 738 | 247 | 243 | 96 | 38 | 273 | 15 |
| HDL‐C | 49.1 (12.2) | 48.8 (12.4) | 48.7 (11.6) | 49.7 (12.1) | 50.0 (12.7) | 44.5 (9.9) | 50.1 (12.9) | 45.6 (14.4) |
| n | 1370 | 756 | 257 | 254 | 103 | 38 | 273 | 15 |
| Total cholesterol | 243.0 (42.9) | 241.0 (45.7) | 232.5 (38.8) | 251.1 (33.4) | 263.5 (41.8) | 207.2 (49.5) | 271.9 (36.2) | 288.1 (93.6) |
| n | 1349 | 749 | 250 | 247 | 103 | 38 | 272 | 15 |
| Triglycerides | 168.8 (86.2) | 174.0 (92.7) | 159.4 (77.8) | 161.1 (75.3) | 172.5 (78.2) | 164.4 (101.1) | 178.6 (79.6) | 190.7 (94.8) |
| n | 1349 | 749 | 250 | 247 | 103 | 38 | 273 | 15 |
| BUN (mg/dL)b | 15.3 (4.8) | 15.4 (4.7) | 15.6 (5.2) | 15.4 (5.0) | 14.3 (4.5) | 11.6 (3.2) | 15.2 (7.3) | 16.1 (2.6) |
| n | 870 | 473 | 162 | 144 | 91 | 18 | 248 | 10 |
| Creatinine (mg/dL)c | 0.98 (0.22) | 0.99 (0.23) | 0.95 (0.23) | 0.97 (0.23) | 0.97 (0.18) | 0.97 (0.24) | 1.01 (0.22) | 1.18 (0.10) |
| n | 1082 | 664 | 160 | 199 | 59 | 18 | 253 | 11 |
| HbA1c (%)b | 7.6 (1.6) | 7.3 (1.7) | 7.8 (1.4) | 7.9 (1.3) | 7.9 (1.8) | 7.3 (1.1) | 6.7 (1.8) | 7.7 (1.6) |
| n | 508 | 210 | 142 | 97 | 59 | 19 | 80 | 2 |
Values are mean (SD) or n (%). To convert mg/dL to mmol/L for cholesterol, divide by 38.67; for triglycerides, divide by 88.57; for BUN, multiply by 0.357. To convert mg/dL to μmol/L for creatinine, multiply by 88.4. ATV, atorvastatin; BMI, body mass index; BP, blood pressure; BUN, blood urea nitrogen; DBP, diastolic blood pressure; HbA1c, glycosylated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; SD, standard deviation; SIM, simvastatin.
Other statins and other treatments included simvastatin, pravastatin, fluvastatin, lovastatin, cerivastatin, cholestyramine, colestipol, fenofibrate, nicotinic acid, diet therapy, and some combinations.
Baseline measurements of lipids, BUN, creatinine, and HbA1c were not available for some patients.
The mean (SD) creatinine of Study A2581045, a multicenter, randomized, open‐label, parallel‐group, dose‐response, 6‐week study evaluating the efficacy and safety of atorvastatin 10, 20, 40, and 80 mg in 130 Philippine dyslipidemia patients, was 10.8 (28.6) mg/dL, which was considered as an outlier and therefore excluded from the current analysis.
3.2. Treatment exposure
Asian patients enrolled in the six long‐term CV outcomes trials, in which 106 patients received atorvastatin 80‐mg (Fig. 1), had a median exposure to atorvastatin of ~3–5 years (Table S2). For the 52 short‐term trials, where 175 patients received atorvastatin 80‐mg (Fig. 1), the median exposure to atorvastatin was ~1–3 months (Table S2).
3.3. Overall safety
In long‐term CV outcomes trials that compared atorvastatin 10‐mg with placebo, the proportion of Asian patients who experienced any AE in each of the atorvastatin groups was similar to that observed in the corresponding placebo group (Table 3). In trials that compared high‐dose atorvastatin therapy (80‐mg) with placebo or lower‐dose statin therapy, a higher proportion of Asian patients reported AEs in the atorvastatin 80‐mg group compared with the control group in IDEAL and SPARCL (Table 3); however, similar AE rates were observed for atorvastatin 80‐ and 10‐mg in TNT. Although the incidence of SAEs between treatment groups varied across trials (Table 3), high‐dose atorvastatin was generally associated with higher SAE rates in Asian patients compared with lower statin doses or placebo, apart from atorvastatin 80‐mg vs 10‐mg in TNT. With the exception of CARDS and ASCOT‐LLA, the proportion of Asian patients with treatment‐related AEs/SAEs that led to discontinuation was higher in the atorvastatin groups (10‐ and 80‐mg) vs comparators (placebo, simvastatin 20–40‐mg, atorvastatin 10‐mg; Table 3). However, within each trial, the number of Asian patients who discontinued atorvastatin therapy due to treatment‐related AEs/SAEs was ≤8 (Table 3). When the long‐term trials were pooled by treatment group, no significant differences in the rates of AEs, SAEs, or treatment‐related AE/SAE discontinuations were seen between treatment groups, with the exception of treatment‐related AE/SAE discontinuations in the atorvastatin 80‐mg vs 10‐mg group (Table 3; P = 0.011); the corresponding atorvastatin 80‐mg vs placebo treatment difference did not reach statistical significance (Table 3; P = 0.200).
Table 3.
Safety findings for Asian patients in long‐ and short‐term trials of atorvastatin
| AEs | SAEs | Treatment‐related AEs/SAEs leading to discontinuation | |
|---|---|---|---|
| Long‐term trialsa | |||
| ASCOT‐LLA | |||
| ATV 10 mg | 58 (78.4) | 5 (6.8) | 3 (4.1) |
| Placebo | 74 (89.2) | 16 (19.3) | 4 (4.8) |
| ASPEN | |||
| ATV 10 mg | 42 (95.5) | 12 (27.3) | 5 (11.4) |
| Placebo | 44 (93.6) | 13 (27.7) | 3 (6.4) |
| CARDS | |||
| ATV 10 mg | 44 (91.7) | 15 (31.3) | 2 (4.2) |
| Placebo | 36 (94.7) | 12 (31.6) | 2 (5.3) |
| IDEAL | |||
| ATV 80 mg | 17 (94.4) | 9 (50.0) | 3 (16.7) |
| SIM 20–40 mg | 15 (88.2) | 4 (23.5) | 2 (11.8) |
| SPARCL | |||
| ATV 80 mg | 16 (94.1) | 7 (41.2) | 3 (17.7) |
| Placebo | 16 (88.9) | 5 (27.8) | 1 (5.6) |
| TNT | |||
| ATV 80 mg | 68 (95.8) | 19 (26.8) | 7 (9.9) |
| ATV 10 mg | 70 (97.2) | 29 (40.3) | 1 (1.4) |
| Pooled long‐term trials | |||
| ATV 10 mg | 214 (89.9) | 61 (25.6) | 11 (4.6)b |
| ATV 80 mg | 101 (95.3) | 35 (33.0) | 13 (12.3)b |
| Placebo | 170 (91.4) | 46 (24.7) | 10 (5.4) |
| Pooled short‐term trials | |||
| ATV all doses | 755 (34.7) | 69 (3.2) | 43 (2.0) |
| ATV 10 mg | 263 (28.2) | 22 (2.4) | 17 (1.8) |
| ATV 20 mg | 88 (30.5) | 2 (0.7) | 5 (1.7) |
| ATV 40 mg | 324 (41.6) | 36 (4.6) | 13 (1.7) |
| ATV 80 mg | 80 (45.7) | 9 (5.1) | 8 (4.6) |
| Placebo | 65 (57.0) | 10 (8.8) | 3 (2.6) |
| Other statinsc | 97 (35.1) | 11 (4.0) | 5 (1.8) |
| Other treatmentsc | 12 (15.2) | 0 (0) | 1 (1.3) |
Values are n (%). AE, adverse event; ATV, atorvastatin; SAE, serious adverse event; SIM, simvastatin.
Details of the long‐term CV outcomes trials are provided in the footnote to Table 1.
P < 0.05 versus other atorvastatin dose.
Other statins and other treatments included simvastatin, pravastatin, fluvastatin, lovastatin, cerivastatin, cholestyramine, colestipol, fenofibrate, nicotinic acid, diet therapy, and some combinations.
In the 52 short‐term trials, Asian patients treated with atorvastatin generally had similar or lower rates of all‐causality AEs and SAEs, and discontinuations due to treatment‐related AEs/SAEs, compared to those treated with other statins or placebo, with the exception of treatment‐related AE/SAE discontinuations in Asian patients who received atorvastatin 80‐mg (Table 3). In general, higher doses of atorvastatin were associated with higher AE and SAE rates in Asian patients compared with lower atorvastatin doses (Table 3); however, a similar proportion of Asian patients who received atorvastatin 10–40‐mgdiscontinued study treatment due to treatment‐related AEs/SAEs (Table 3). In the pooled short‐term trials, Asian patients treated with atorvastatin also had similar or lower rates of AEs, SAEs, and treatment‐related discontinuations compared to non‐Asian patients (Table S3).
Treatment‐related SAEs were rare among Asian patients who received atorvastatin (n = 4): two in the long‐term trials (one each in CARDS and SPARCL) and two in the short‐term trials (one each with atorvastatin 40‐ and 80‐mg). The treatment‐related SAEs were arthritis and myopathy (in the same CARDS patient), hepatic enzyme elevations (in the SPARCL patient and the patient on short‐term atorvastatin 40‐mg), and hepatitis (in the patient on short‐term atorvastatin 80‐mg). The myopathy and hepatic enzyme elevation cases resolved on treatment discontinuation; no outcome information was available for the case of hepatitis.
Among the seven body systems investigated (excluding ASCOT‐LLA), the most frequently reported all‐causality AEs in Asian patients across most trials were gastrointestinal disorders, followed by nervous system disorders (Table S4). The incidence of these AEs between treatment groups varied from trial to trial with no evidence of a treatment‐ or dose‐related trend.
3.4. Musculoskeletal safety
Across 57 trials (excluding ASCOT‐LLA), there were no cases of rhabdomyolysis in Asian patients treated with atorvastatin (Table S5; Fig. 2A), with one case reported in a patient treated with another statin in a short‐term trial (Table S5). Among the 390 Asian participants in the five long‐term trials with available musculoskeletal safety data (excluding ASCOT‐LLA; 270 received atorvastatin), 20 incidences of myalgia (all‐causality) were reported: 11 patients received atorvastatin 80‐mg, seven received atorvastatin 10‐mg, and two received placebo (Table S5), corresponding to myalgia rates of 6.7% (18/270) for atorvastatin (Fig. 2A) and 1.9% (2/103) for placebo (Table S5). Of the 2644 Asian participants in the 52 short‐term trials (2175 received atorvastatin), 45 experienced myalgia: 39 received atorvastatin, three received another statin, two received placebo, and one received other therapy (Table S5), corresponding to myalgia rates of 1.8% (39/2175), 1.1% (3/276), 1.8% (2/114), and 1.3% (1/79), respectively (Table S5; Fig. 2A).
Figure 2.
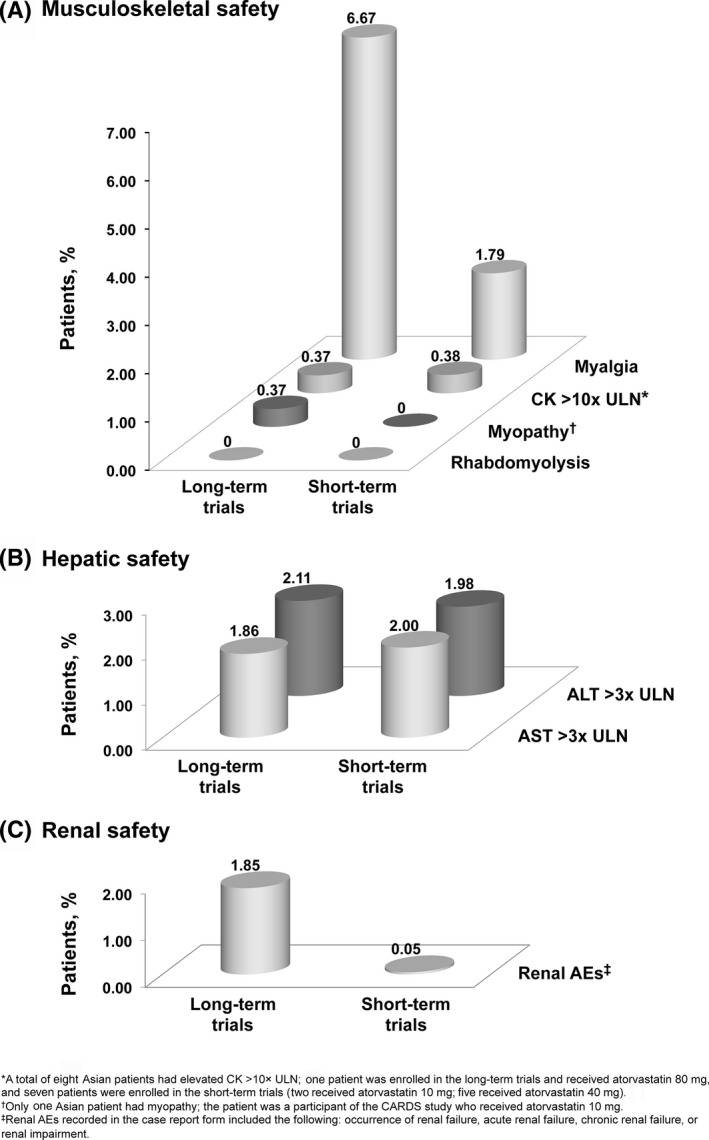
Proportion of atorvastatin‐treated Asian patients experiencing musculoskeletal (A), hepatic (B), and renal (C) AEs. No musculoskeletal AE data were available for ASCOT‐LLA; hence, the long‐term trials included ASPEN, CARDS, SPARCL, IDEAL, and TNT. AE, adverse event; ALT, alanine transaminase; AST, aspartate transaminase; CK, creatine kinase; ULN, upper limit of normal
Among the 3034 Asian patients included in the musculoskeletal safety analysis, there was one report of myopathy, in a CARDS patient who received atorvastatin 10‐mg (Table S5; Fig. 2A). An elevation of CK >10× ULN occurred in eight atorvastatin‐treated Asian patients: one patient in IDEAL who received atorvastatin 80‐mg and seven patients in the short‐term trials (two received atorvastatin 10‐mg; five received atorvastatin 40‐mg; Table S5; Fig. 2A).
3.5. Hepatic safety
Of the 2740 Asian patients with hepatic safety data, an elevation of ALT >3× ULN occurred in 45 patients who received atorvastatin, with a rate of 2.1% (7/331) in the long‐term trials (including ASCOT‐LLA; 2.3% [4/174] for placebo) and 2.0% (38/1921) in the short‐term trials (0.9% [1/108] for placebo; Table S5; Fig. 2B). An elevation of AST >3× ULN occurred in 43 Asian patients receiving atorvastatin, with a rate of 1.9% (5/269) in long‐term trials (excluding ASCOT‐LLA; no placebo cases) and 2.0% (38/1901) in short‐term trials (1.1% [1/89] for placebo; Table S5; Fig. 2B).
3.6. Renal safety
All‐causality renal AEs were reported in 1.9% (5/270) of Asian patients who received atorvastatin in the long‐term trials (excluding ASCOT‐LLA; no placebo cases; Table S5; Fig. 2C), and in one atorvastatin patient (0.05% [1/2175]) and three placebo patients (2.6% [3/114]) in the short‐term trials (Table S5; Fig. 2C). The occurrence of albuminuria, hematuria, microalbuminuria, or proteinuria was infrequent with no evidence of a dose‐related trend in Asian patients who received atorvastatin (Table S5).
4. Discussion
Despite overwhelming evidence that supports statin‐mediated lipid lowering to reduce CV risk,5, 6, 7, 8, 9, 10, 11, 12 dyslipidemia is often undertreated in Asian populations. A retrospective analysis of hospital medical records in China demonstrated that approximately 60% of patients with a history of atherosclerotic CVD were not prescribed a statin.13 Similar results were obtained in a retrospective analysis of Taiwan's National Health Insurance database, where approximately 40% of hyperlipidemic patients with diabetes and CHD received a statin.14 Furthermore, pan‐Asian national surveys have revealed that in patients receiving lipid‐lowering treatment, the recommended LDL‐C goal was achieved in only one‐half of these patients overall, and in only a third of those at very high CV risk.15, 16 The limited availability of statin safety data from Asian populations, combined with perceived safety concerns due, in part, to differences in statin pharmacokinetics observed between Asian and Western patients,29, 30 may have contributed to undertreatment in this ethnic group. This analysis of data from 52 pooled short‐term studies and six long‐term trials of atorvastatin across the 10–80‐mg dose range in 3191 Asian patients provides valuable information on the safety and tolerability of atorvastatin therapy in this ethnic group.
The pooled analysis of the short‐term trials demonstrated that the incidence of all‐causality AEs and SAEs in Asian patients treated across the atorvastatin 10–80‐mg dose range was similar to or lower than that observed with other statins or placebo and in non‐Asian patients. Discontinuations due to treatment‐related AEs/SAEs were infrequent, with no discernible differences between treatment groups apart from the atorvastatin 80‐mg dose. Although high‐dose atorvastatin (80‐mg) was associated with higher AE/SAE rates compared with lower atorvastatin doses (10–40‐mg), far fewer Asian patients in the short‐term trials received atorvastatin at the 80‐mg dose (175 of 2175 patients that received atorvastatin), and this may confound the interpretation of AE rates for this dose. A statistical comparison between pooled treatment groups for these short‐term trials was not appropriate due to the diverse nature of the 52 studies in terms of trial design, characteristics of enrolled study population, length of treatment duration, and treatment doses/comparators.
The safety findings from these pooled short‐term studies were supported by the analysis of long‐term trials that enrolled a wide range of patients, including those with diabetes,18, 28 stable CHD,20 hypertension,22 recent myocardial infarction,21 or stroke.23 After adjusting for differences in baseline characteristics, no significant differences in the rates of AEs or SAEs were observed between pooled treatment groups; however, there was a higher rate of treatment‐related AE/SAE discontinuations with high‐dose atorvastatin (80‐mg). It should be noted that—as for the short‐term studies—these long‐term studies also have varied trial designs, study populations, treatment durations, and treatment arms. Hence, these inferential statistics should be interpreted with caution as the differences in AE rates between treatment arms might be due to differences between study populations and trial durations represented in that treatment group and not due to the treatment per se.
The results of our analysis of atorvastatin safety in Asian patients are consistent with previous analyses of atorvastatin safety in the wider atorvastatin clinical trial population by Newman et al.26, 27 In an analysis of data from short‐term studies (2–78 weeks),26 all‐causality AEs were experienced by 65% of patients who received atorvastatin at any dose (n = 9416); 51%, 25%, 44%, and 51% of patients in the atorvastatin 10‐mg (n = 6343), 20‐mg (n = 242), 40‐mg (n = 186), and 80‐mg (n = 2345) dose groups, respectively, experienced an AE. A subsequent analysis,27 which included data from longer‐term trials (up to 52 months) and compared low‐ vs high‐dose atorvastatin, found an incidence of AEs of 53% and 48% in the 10‐ and 80‐mg dose groups, respectively. Our analysis of Asian safety data from pooled short‐term trials (4–72 weeks) found that all‐causality AEs were experienced by 35% of Asian patients treated with atorvastatin at any dose, with dose‐specific AE rates ranging from 28% (atorvastatin 10‐mg) to 46% (atorvastatin 80‐mg). SAEs were previously reported in ≤10% of atorvastatin patients, and discontinuations due to treatment‐related AEs in ≤3% of patients.26, 27 The corresponding proportions from our analysis of Asian patients enrolled in short‐term studies were ≤5% for both SAEs (5.1% for atorvastatin 80‐mg; 3.2% across all doses) and discontinuations due to treatment‐related AEs/SAEs (4.6% for atorvastatin 80‐mg; 2.0% across all doses). Notably higher proportions of Asian patients experienced all‐causality AEs and SAEs, and discontinuations due to treatment‐related AEs/SAEs, in the long‐term CV outcomes trials (3.1–4.9 years). This may be due to longer durations of exposure increasing the reporting period for AEs, combined with the increased rigor of monitoring and reporting of adverse outcomes associated with CV endpoint trials conducted in high‐risk patients, and smaller patient numbers within these treatment groups.
Perceived safety risks related to musculoskeletal, hepatic, and renal AEs may partly explain undertreatment with statins in various patient populations. In this analysis, no direct relationship was observed between atorvastatin dose and incidence of musculoskeletal AEs in Asian patients. The myalgia rate in atorvastatin‐treated Asian patients was low and comparable to that observed in the wider atorvastatin clinical trial population (1.8% in short‐term studies; 6.7% in long‐term trials; ≤4% in previous analyses26, 27). No cases of rhabdomyolysis were observed in atorvastatin‐treated Asian patients. Furthermore, incidences of any elevation in ALT or AST >3× ULN were comparable across the previous analyses26, 27 and this study (≤3%).
Although a growing body of evidence demonstrates the efficacy and safety of atorvastatin in Asian populations of both East Asian31, 32, 33, 34, 35, 36, 37 and South Asian34, 38, 39, 40, 41, 42, 43, 44 origin, many of these previous studies evaluated the safety profile of atorvastatin doses up to 20‐mg over relatively short study durations (6–12 weeks).31, 33, 35, 36, 41, 42, 43 This current analysis has extended these observations to atorvastatin doses up to 80‐mg and provides strong evidence to support the safety of conventional doses of atorvastatin (10–40‐mg daily) in Asian patients over the short‐ and longer‐term (up to 4.9 years) for intensive lipid lowering. The inclusion of a number of Asian patients who received atorvastatin 80‐mg (n = 281) is of clinical importance, given the potential for intensive statin therapy to attain lipid goals specified in Asian dyslipidemia treatment guidelines.45, 46, 47 However, given the paucity of clinical trial data with the atorvastatin 80‐mg dose in Asian populations and with ~10% of Asian patients in this current analysis receiving this highest dose, the safety of atorvastatin 80‐mg in patients of Asian origin requires further evaluation.
The rigorous study conduct and strict AE monitoring within the clinical trials included in this analysis are obvious strengths of this study. Limitations include those that are inherent to pooled subgroup analyses. As previously discussed, the included trials, which were conducted across an extended period of time (1992–2012), employed various study designs with different drug doses/comparators, treatment durations, and study populations. Furthermore, the loss of randomization through the re‐assignment of patients to specific subgroups may lead to differences in baseline factors between subgroups and confounding of the results. The smaller sample sizes may also limit the ability to detect differences between treatment groups, particularly for the long‐term trials where the number of Asian patients was limited relative to the short‐term studies.
Statin therapy has been recommended to reduce CVD outcomes in patients at CV risk.11, 12 This retrospective analysis of pooled AE data from Asian patients in randomized clinical trials provides important additional information on the safety profile of atorvastatin in a previously understudied ethnic group. The consistently low incidence of AEs/SAEs with atorvastatin 10–40‐mg, comparable to that of the placebo group, is reassuring, while more studies are needed for atorvastatin 80‐mg. The next challenge is to ensure that this evidence is translated into real‐world practice to benefit Asian patients at CV risk.
Funding
This study was funded by Pfizer. Editorial support was provided by Shuang Li and Shirley Smith of Engage Scientific Solutions and was funded by Pfizer.
Conflict of Interest
J.C.N.C. is the chief executive officer (pro bono) of the Asia Diabetes Foundation, which has received a research grant from Pfizer to conduct a quality improvement program for diabetes. J.C.N.C. and A.P.S.K. have received speaker's honoraria and travel support from Pfizer, which have been donated to the Chinese University of Hong Kong to support diabetes research, and their institution has received research grants and sponsorships for educational activities from Pfizer. W.B., R.F., and R.L. are employees of Pfizer with ownership of stock in Pfizer.
Supporting information
References
- 1. World Health Organization [Internet] . Cardiovascular diseases (CVDs); June 2016. http://www.who.int/mediacentre/factsheets/fs317/en/index.html. Accessed July 11, 2016.
- 2. World Health Organization [Internet] . The impact of chronic disease in China; October 2005. http://www.who.int/chp/chronic_disease_report/media/china.pdf. Accessed July 11, 2016.
- 3. Asia Pacific Cohort Studies Collaboration . A comparison of the associations between risk factors and cardiovascular disease in Asia and Australasia. Eur J Cardiovasc Prev Rehabil. 2005;12:484–491. [DOI] [PubMed] [Google Scholar]
- 4. Asia Pacific Cohort Studies Collaboration . Joint effects of systolic blood pressure and serum cholesterol on cardiovascular disease in the Asia Pacific region. Circulation. 2005;112:3384–3390. [DOI] [PubMed] [Google Scholar]
- 5. Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol‐lowering treatment: prospective meta‐analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. [DOI] [PubMed] [Google Scholar]
- 6. Fulcher J, O'Connell R, Voysey M, et al. Efficacy and safety of LDL‐lowering therapy among men and women: meta‐analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385:1397–1405. [DOI] [PubMed] [Google Scholar]
- 7. Matsuzaki M, Kita T, Mabuchi H, et al. Large scale cohort study of the relationship between serum cholesterol concentration and coronary events with low‐dose simvastatin therapy in Japanese patients with hypercholesterolemia. Circ J. 2002;66:1087–1095. [DOI] [PubMed] [Google Scholar]
- 8. Mabuchi H, Kita T, Matsuzaki M, et al. Large scale cohort study of the relationship between serum cholesterol concentration and coronary events with low‐dose simvastatin therapy in Japanese patients with hypercholesterolemia and coronary heart disease: secondary prevention cohort study of the Japan Lipid Intervention Trial (J‐LIT). Circ J. 2002;66:1096–1100. [DOI] [PubMed] [Google Scholar]
- 9. Nakamura H, Arakawa K, Itakura H, et al. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet. 2006;368:1155–1163. [DOI] [PubMed] [Google Scholar]
- 10. Ting RZ, Yang X, Yu LW, et al. Lipid control and use of lipid‐regulating drugs for prevention of cardiovascular events in Chinese type 2 diabetic patients: a prospective cohort study. Cardiovasc Diabetol. 2010;9:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Catapano AL, Reiner Z, De Backer G, et al. ESC/EAS guidelines for the management of dyslipidaemias. Atherosclerosis. 2011;217:3–46. [DOI] [PubMed] [Google Scholar]
- 12. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2889–2934. [DOI] [PubMed] [Google Scholar]
- 13. Zhang D, Ge L, Li J, et al. Current statin use for patients with atherosclerotic cardiovascular disease in 39 large Chinese hospitals. Zhonghua Xin Xue Guan Bing Za Zhi. 2011;39:397–401. [PubMed] [Google Scholar]
- 14. Lin YC, Yang CC, Chen YJ, Peng WC, Li CY, Hwu CM. Utilization of statins and aspirin among patients with diabetes and hyperlipidemia: Taiwan, 1998–2006. J Chin Med Assoc. 2012;75:567–572. [DOI] [PubMed] [Google Scholar]
- 15. Kim HS, Wu Y, Lin SJ, et al. Current status of cholesterol goal attainment after statin therapy among patients with hypercholesterolemia in Asian countries and region: the Return on Expenditure Achieved for Lipid Therapy in Asia (REALITY–Asia) study. Curr Med Res Opin. 2008;24:1951–1963. [DOI] [PubMed] [Google Scholar]
- 16. Park JE, Chiang CE, Munawar M, et al. Lipid‐lowering treatment in hypercholesterolaemic patients: the CEPHEUS Pan‐Asian survey. Eur J Prev Cardiol. 2012;19:781–794. [DOI] [PubMed] [Google Scholar]
- 17. Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. [DOI] [PubMed] [Google Scholar]
- 18. Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo‐controlled trial. Lancet. 2004;364:685–696. [DOI] [PubMed] [Google Scholar]
- 19. Koren MJ, Hunninghake DB, ALLIANCE Investigators. Clinical outcomes in managed‐care patients with coronary heart disease treated aggressively in lipid‐lowering disease management clinics: the ALLIANCE study. J Am Coll Cardiol. 2004;44:1772–1779. [DOI] [PubMed] [Google Scholar]
- 20. LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–1435. [DOI] [PubMed] [Google Scholar]
- 21. Pedersen TR, Faergeman O, Kastelein JJ, et al. High‐dose atorvastatin vs usual‐dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA. 2005;294:2437–2445. [DOI] [PubMed] [Google Scholar]
- 22. Sever PS, Dahlöf B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower‐than‐average cholesterol concentrations, in the Anglo‐Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm (ASCOT‐LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149–1158. [DOI] [PubMed] [Google Scholar]
- 23. Amarenco P, Bogousslavsky J, Callahan A III, et al. High‐dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–559. [DOI] [PubMed] [Google Scholar]
- 24. Scandinavian Simvastatin Survival Study Group . Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 25. Heart Protection Study Collaborative Group . MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high‐risk individuals: a randomised placebo‐controlled trial. Lancet. 2002;360:7–22.12114036 [Google Scholar]
- 26. Newman CB, Palmer G, Silbershatz H, Szarek M. Safety of atorvastatin derived from analysis of 44 completed trials in 9,416 patients. Am J Cardiol. 2003;92:670–676. [DOI] [PubMed] [Google Scholar]
- 27. Newman C, Tsai J, Szarek M, Luo D, Gibson E. Comparative safety of atorvastatin 80 mg versus 10 mg derived from analysis of 49 completed trials in 14,236 patients. Am J Cardiol. 2006;97:61–67. [DOI] [PubMed] [Google Scholar]
- 28. Knopp RH, d'Emden M, Smilde JG, Pocock SJ. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in Non‐Insulin‐Dependent Diabetes Mellitus (ASPEN). Diabetes Care. 2006;29:1478–1485. [DOI] [PubMed] [Google Scholar]
- 29. Birmingham BK, Bujac SR, Elsby R, et al. Impact of ABCG2 and SLCO1B1 polymorphisms on pharmacokinetics of rosuvastatin, atorvastatin and simvastatin acid in Caucasian and Asian subjects: a class effect? Eur J Clin Pharmacol. 2015;71:341–355. [DOI] [PubMed] [Google Scholar]
- 30. Wang P. Statin dose in Asians: is pharmacogenetics relevant? Pharmacogenomics. 2011;12:1605–1615. [DOI] [PubMed] [Google Scholar]
- 31. Japan Cholesterol Lowering Atorvastatin Study (J‐CLAS) Group . Efficacy of atorvastatin in primary hypercholesterolemia. Am J Cardiol. 1997;79:1248–1252. [PubMed] [Google Scholar]
- 32. Wang KY, Ting CT. A randomized, double‐blind, placebo‐controlled, 8‐week study to evaluate the efficacy and safety of once daily atorvastatin (10 mg) in patients with elevated LDL‐cholesterol. Jpn Heart J. 2001;42:725–738. [DOI] [PubMed] [Google Scholar]
- 33. Wu CC, Sy R, Tanphaichitr V, Hin AT, Suyono S, Lee YT. Comparing the efficacy and safety of atorvastatin and simvastatin in Asians with elevated low‐density lipoprotein‐cholesterol—a multinational, multicenter, double‐blind study. J Formos Med Assoc. 2002;101:478–487. [PubMed] [Google Scholar]
- 34. Zhu JR, Tomlinson B, Ro YM, Sim KH, Lee YT, Sriratanasathavorn C. A randomised study comparing the efficacy and safety of rosuvastatin with atorvastatin for achieving lipid goals in clinical practice in Asian patients at high risk of cardiovascular disease (DISCOVERY‐Asia study). Curr Med Res Opin. 2007;23:3055–3068. [DOI] [PubMed] [Google Scholar]
- 35. Kurabayashi M, Yamazaki T. Superior benefit of aggressive lipid‐lowering therapy for high‐risk patients using statins: the SUBARU study—more hypercholesterolemic patients achieve Japan Atherosclerosis Society LDL‐C goals with rosuvastatin therapy than with atorvastatin therapy. J Atheroscler Thromb. 2008;15:314–323. [DOI] [PubMed] [Google Scholar]
- 36. Park JS, Kim YJ, Choi JY, et al. Comparative study of low doses of rosuvastatin and atorvastatin on lipid and glycemic control in patients with metabolic syndrome and hypercholesterolemia. Korean J Intern Med. 2010;25:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ye YC, Zhao XL, Zhang SY. Use of atorvastatin in lipid disorders and cardiovascular disease in Chinese patients. Chin Med J (Engl). 2015;128:259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Patel JV, Gupta S, Lie F, Hughes EA. Efficacy and safety of atorvastatin in South Asian patients with dyslipidemia: an open label noncomparative pilot study. Vasc Health Risk Manag. 2005;1:351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Deedwania PC, Gupta M, Stein M, Ycas J, Gold A. Comparison of rosuvastatin versus atorvastatin in South‐Asian patients at risk of coronary heart disease (from the IRIS trial). Am J Cardiol. 2007;99:1538–1543. [DOI] [PubMed] [Google Scholar]
- 40. Deerochanawong C, Buranakitjaroen P, Nitiyanant W, et al. The Atorvastatin Goal Achievement Across Risk Levels (ATGOAL) study in Thailand. J Med Assoc Thai. 2007;90:72–81. [PubMed] [Google Scholar]
- 41. Adsule SM, Baig MS, Gade PR, Khandelwal PN. A comparative evaluation of safety and efficacy of rosuvastatin, simvastatin, and atorvastatin in patients of type 2 diabetes mellitus with dyslipidemia. Int J Diabetes Dev Ctries. 2009;29:74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pola P, Kumar R, Reddy AP, et al. Efficacy of low dose atorvastatin in diabetic dyslipidaemia. J Indian Med Assoc. 2009;107:807–809. [PubMed] [Google Scholar]
- 43. Sansanayudh N, Wongwiwatthananukit S, Putwai P, Dhumma‐Upakorn R. Comparative efficacy and safety of low‐dose pitavastatin versus atorvastatin in patients with hypercholesterolemia. Ann Pharmacother. 2010;44:415–423. [DOI] [PubMed] [Google Scholar]
- 44. Gupta M, Martineau P, Tran T, et al. Low‐density lipoprotein cholesterol and high‐sensitivity C‐reactive protein lowering with atorvastatin in patients of South Asian compared with European origin: insights from the Achieve Cholesterol Targets Fast with Atorvastatin Stratified Titration (ACTFAST) study. J Clin Pharmacol. 2012;52:850–858. [DOI] [PubMed] [Google Scholar]
- 45. Teramoto T, Sasaki J, Ishibashi S, et al. Executive summary of the Japan Atherosclerosis Society (JAS) guidelines for the diagnosis and prevention of atherosclerotic cardiovascular diseases in Japan—2012 version. J Atheroscler Thromb. 2013;20:517–523. [DOI] [PubMed] [Google Scholar]
- 46. Joint Committee for Developing Chinese Guidelines on Prevention and Treatment of Dyslipidemia in Adults . Chinese guidelines on prevention and treatment of dyslipidemia in adults. Zhonghua Xin Xue Guan Bing Za Zhi. 2007;35:390–419. [PubMed] [Google Scholar]
- 47. Son J‐I, Chin SO, Woo J‐T. Treatment guidelines for dyslipidemia: summary of the expanded second version. J Lipid Atheroscler. 2012;1:45–59. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


