Abstract
Aim
To investigate changes in body weight trajectories after the addition of individual sulphonylureas (SUs) to metformin in patients with type 2 diabetes.
Materials and methods
We conducted a retrospective observational cohort study, in a primary care setting in the Netherlands. Patients aged ≥18 years with type 2 diabetes who were included in the ZODIAC cohort between 1998 and 2012 and who received metformin monotherapy at inclusion (n = 29 195), and had used metformin as monotherapy for at least 1 year before receiving dual therapy through the addition of an SU for at least 1 year were eligible for inclusion. The primary outcome was within‐drug yearly change in body weight after receiving add‐on therapy with individual SUs during 5 years of follow‐up. The secondary outcome was within‐drug yearly change in glycated haemoglobin (HbA1c). Annual changes in weight and HbA1c were estimated with linear mixed models, adjusted for age, gender and diabetes duration.
Results
A total of 2958 patients were included. No significant weight changes were observed within and between any of the individual SUs after treatment intensification (p = 0.24). In addition, no significant difference in weight between the add‐on therapy combinations was observed (p = 0.26). The average HbA1c the year before intensification was 7.2% (55 mmol/mol) and dropped below 7.0% (53 mmol/mol) the year after.
Conclusions
In patients with type 2 diabetes treated in primary care, strict glycaemic control can be maintained with SUs used as add‐on therapy to metformin, without the offset of relevant weight changes.
Keywords: blood glucose, body weight, diabetes mellitus type 2, humans, metformin, prospective studies, sulphonylurea compounds
1. INTRODUCTION
Avoiding relevant increases in body weight, particularly after starting new glucose‐lowering agents, is an important treatment target in type 2 diabetes.1 Weight increase in patients with type 2 diabetes is problematic because it contributes to increased insulin resistance and disease progression.1, 2, 3 In trials investigating new glucose‐lowering agents, body weight is used as a separate endpoint. Except for lifestyle factors, the various glucose‐lowering agents have been reported to have either decreasing, neutral or increasing effects on body weight. Among the available agents, metformin is reported to have no effect on weight,4, 5, 6 while several other glucose‐lowering agents have been reported to cause small increases in weight.7, 8, 9, 10, 11, 12 The UK Prospective Diabetes Study reported an increase in weight of ~4 kg in the first 3 years after initiation of glibenclamide compared with metformin, after which weight remained relatively stable.7 The A Diabetes Outcome Progression Trial (ADOPT) reported that glibenclamide monotherapy caused a 1.6‐kg increase in weight in the first year compared with baseline, but remained stable after that for the next 5 years.13
The magnitude of weight changes in daily practice after starting sulphonylureas (SUs) is not entirely clear. Only a few observational cohort studies have investigated the effects of initiation of glucose‐lowering agents on weight; a meta‐analysis of just two prospective studies reported an increase of 2.06 kg [95% confidence interval (CI) 1.15‐2.96] for SUs as a group compared with placebo.14 A retrospective cohort study showed that monotherapy with any SU was associated with a 1.05‐kg/m2 (95% CI 0.90‐1.20) higher body mass index 12 months after initiation compared with metformin use.15
It is unclear whether this weight increase is a class effect or should be attributed to specific SU drugs. As a consequence, there is a growing interest in within‐class SU differences. Of the SUs available, gliclazide has a remarkable safety profile: its use is associated with exceptionally few hypoglycaemic events.16 Furthermore, gliclazide can even be used in patients with renal impairment without dose adjustment17, 18 and is possibly beneficial with respect to cardiovascular outcomes compared with other SUs.19 Together, this led to the incorporation of gliclazide as the preferred SU in the Dutch 2013 diabetes guidelines when treatment intensification after metformin is required.20 Evidence for within‐class differences in weight change after the start of individual SUs could have consequences regarding the preferred agent when treatment intensification is needed. The aim of the present study was to investigate within‐ and between SU‐group weight trajectories, in a prospective primary care cohort of patients with type 2 diabetes, after starting add‐on therapy with individual SUs, in addition to metformin.
2. METHODS
The present study is reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) recommendations.21
2.1. Study design and data collection
The present study (part of Clinicaltrials.gov #NCT02133118) was an analysis of patients included in the prospective Zwolle Outpatient Diabetes project Integrating Available Care (ZODIAC) study.22 The ZODIAC study started in 1998 and included patients diagnosed with type 2 diabetes who were treated exclusively in primary care. Since then, the ZODIAC study has expanded to more than 600 general practices in the north‐eastern and western part of the Netherlands. In the Netherlands, all inhabitants have a general practitioner and >80% of patients with type 2 diabetes are treated exclusively in primary care.23
As part of the ZODIAC study, the following data are collected by general practitioners and sent to the diabetes centre annually: presence of macrovascular complications; diabetes duration; medication use [diabetes medication, insulin (including type), and all other medication]; body weight; height (only at baseline); blood pressure; glycated haemoglobin (HbA1c); serum creatinine; urinary albumin‐creatinine ratio; and lipid profiles. All values are measured once at the yearly check‐up with the general practitioner. Any potential changes within the year were not recorded. All laboratory measurements were determined using standard laboratory procedures.
2.2. Patient selection
Eligible for selection were patients with type 2 diabetes, included in the ZODIAC cohort between 1998 and 2012, who were aged ≥18 years and who received metformin monotherapy during participation in the ZODIAC cohort, and metformin as monotherapy for at least 1 year before receiving dual therapy in the form of addition of an SU in the next year (new‐user design).24 Patients were not excluded on specific patient characteristics other than age (<18 years). Data were censored when follow‐up ended or when patients switched medication (including other SUs) during follow‐up; for example monotherapy with an SU or metformin, or when they subsequently received triple oral therapy or insulin therapy, or at the end of follow‐up, or death, or after 5 years of follow‐up. For each patient the baseline year was defined as the year before treatment intensification with an SU when they were using metformin monotherapy. Patients who achieved their HbA1c targets (HbA1c < 7%/53 mmol/mol) on metformin monotherapy were not included in the analysis as a comparator group, because this comparator group could have been subject to selection bias.
2.3. Outcome measures
The primary outcome of the study was mean yearly change in body weight compared with weight at baseline for each add‐on group separately for 5 years. Secondary outcome was mean change in HbA1c compared with baseline.
2.4. Statistical analysis
Quantitative variables are presented as means with standard deviation for normally distributed values, and median and interquartile range (IQR) for skewed variables. Pairwise comparisons with one‐way analysis of variance or Kruskal–Wallis tests were Bonferroni‐adjusted. Changes in body weight and HbA1c during follow‐up were estimated using linear mixed models. The use of linear mixed models allowed us to account for missing data. Patients with missing data on weight or HbA1c were not excluded; for example, if data on weight was missing in year 2 but available in year 1 and year 3, the patient was not included in the analysis in year 2 but was included in years 1 and 3. The changes in weight and HbA1c were modelled with a random intercept and slope, adjusted for baseline age, gender and diabetes duration. Age, gender and diabetes duration were modelled as fixed effects, with age and diabetes duration as continuous variables, while patients were modelled as a random effect. The model was adjusted for age, gender and diabetes duration because we hypothesized that these factors could have influenced both body weight and the choice of a specific SU. All analyses were performed using SPSS 20.0 software (SPSS Inc, Chicago, Illinois, USA).
2.5. Ethics
In the ZODIAC study, patients consented to anonymous use of their data for study purposes. The medical ethics committee of Isala, Zwolle, the Netherlands approved the ZODIAC study (METC reference numbers 03.0316 and 07.0335).
2.6. Patient involvement
Patients were not involved in the design of the study, the development of outcome measures or in the recruitment of patients.
3. RESULTS
From the total number of metformin monotherapy users at the start of the study (n = 29 195), 2958 patients (10.1%) were included in the analysis. The number of patients at each selection stage is shown in Figure S1, Supporting Information. Baseline characteristics are shown in Table 1. The mean age in the four treatment groups ranged from 62.1 to 63.5 years. Median weight was 91.9 kg in the gliclazide group, 92.2 kg in the glibenclamide group, 91.3 kg in the glimepiride group and 89.4 kg in the tolbutamide group.
Table 1.
Demographic and clinical characteristics of patients at baseline
| Metformin + glicazide n = 521 | Metformin + glibenclamide n = 43 | Metformin + glimepiride n = 964 | Metformin + tolbutamide n = 1427 | |
|---|---|---|---|---|
| Age at baseline, years | 63.1 (11.7) | 63.5 (12.3) | 62.1 (11.3) | 63.5 (11.5) |
| Gender: women, n (%) | 221 (42.5) | 21 (50.0) | 449 (46.6) | 674 (47.2) |
| HbA1c % | 7.1 (6.6‐7.6) | 7.0 (6.6‐7.7) | 7.0 (6.6‐7.5) | 7.0 (6.6‐7.5) |
| mmol/mol | 54 (49‐60) | 53 (54‐61) | 53 (49‐58) | 53 (49‐58) |
| n = 522 | n = 44 | n = 965 | n = 1427 | |
| Weight, kg | 91.9 (90.3, 93.4) | 92.2 (86.2, 98.2) | 91.3 (90.2, 92.4) | 89.4 (88.5, 90.3) |
| n = 520 | n = 43 | n = 963 | n = 1415 | |
| Body mass index | 30.9 (5.7) | 31.4 (6.1) | 30.7 (5.6) | 30.2 (5.0) |
| Systolic blood pressure, mm Hg | 140 (17) | 140 (14) | 139 (17) | 138 (16) |
| Diastolic blood pressure, mm Hg | 81 (10) | 83 (9) | 82 (10) | 80 (9) |
| Cholesterol HDL ratio | 3.7 (3.1‐4.7) | 3.9 (2.8‐4.8) | 3.7 (3.1‐4.6) | 3.7 (3.0‐4.6) |
| Creatinine, µmol/L | 78 (66‐90) | 70 (57‐83) | 76 (65‐88) | 76 (64‐88) |
| Albumin creatinine ratio | 1.0 (0.5‐2.9) | 0.8 (0.5‐1.0) | 1.0 (0.4‐2.1) | 0.8 (0.5‐2.0) |
| Macrovascular complications at baseline, % | 17.8 | 9.1 | 14.4 | 16.7 |
| Diabetes duration, years | 3.9 (1.8‐5.9) | 4.7 (1.3‐6.8) | 4.0 (2.0‐6.3) | 4.0 (1.9‐6.8) |
Data are mean (standard deviation) or median (interquartile range), unless otherwise indicated.
Baseline weight and HbA1c were available for 520 (99.6%) and 521 (99.8%) patients in the gliclazide add‐on group, for 43 (97.7%) and 43 (97.7%) patients in the glibenclamide add‐on group, for 963 (99.8%) and 964 (99.9%) patients in the glimepiride add‐on group and for 1415 (99.2%) and 1427 (100.0%) patients in the tolbutamide add‐on group, respectively.
3.1. Weight changes during follow‐up
The overall results of the linear mixed model analysis showed a non‐significant (p = 0.24) linear change in weight during the follow‐up period and a non‐significant (p = 0.26) difference in weight between the add‐on therapy combinations. In addition, the change in weight did not significantly differ between the add‐on therapy combinations (p value for interaction = 0.67). The regression parameters of the fixed effects of the mixed model analysis are shown in Table S1, Supporting Information. Estimated weights for the different add‐on therapy combinations at yearly time points are shown in Table 2 and Figure 1A. After 5 years, a non‐significant increase in weight was observed in the gliclazide and glibenclamide groups; in the glimepiride group no change was observed.
Table 2.
Estimated mean weight values corrected for age, gender and diabetes duration
| Treatment regimen | Estimated mean weight values, kg (95% CI) | |||
|---|---|---|---|---|
| Metformin + glicazide | Metformin + glibenclamide | Metformin + glimepiride | Metformin + tolbutamide | |
| Year 0 (baseline) | 91.8 (90.3, 93.4) | 92.2 (86.2, 98.2) | 91.3 (90.2, 92.4) | 89.4 (88.5, 90.3) |
| n = 520 | n = 43 | n = 963 | n = 1415 | |
| Year 1 | 91.9 (90.3, 93.5) | 92.4 (85.7, 99.1) | 91.3 (90.2, 92.4) | 89.7 (88.8, 90.6) |
| n = 510 | n = 39 | n = 953 | n = 1451 | |
| Year 2 | 92.2 (90.4, 93.9) | 91.6 (84.6, 98.6) | 90.6 (89.4, 91.9) | 89.9 (88.9, 91.0) |
| n = 373 | n = 36 | n = 681 | n = 1009 | |
| Year 3 | 92.1 (90.0, 94.1) | 92.7 (84.2, 101.2) | 91.0 (89.4, 92.6) | 89.6 (88.3, 90.8) |
| n = 243 | n = 26 | n = 434 | n = 657 | |
| Year 4 | 93.0 (90.2, 95.8) | 92.4 (81.7, 103.2) | 90.8 (88.6, 93.0) | 89.9 (88.2, 91.7) |
| n = 147 | n = 16 | n = 244 | n = 364 | |
| Year 5 | 95.7 (92.1, 99.3) | 95.5 (84.5, 106.4) | 91.4 (88.4, 94.4) | 90.8 (88.3, 93.3) |
| n = 94 | n = 13 | n = 148 | n = 206 | |
Figure 1.
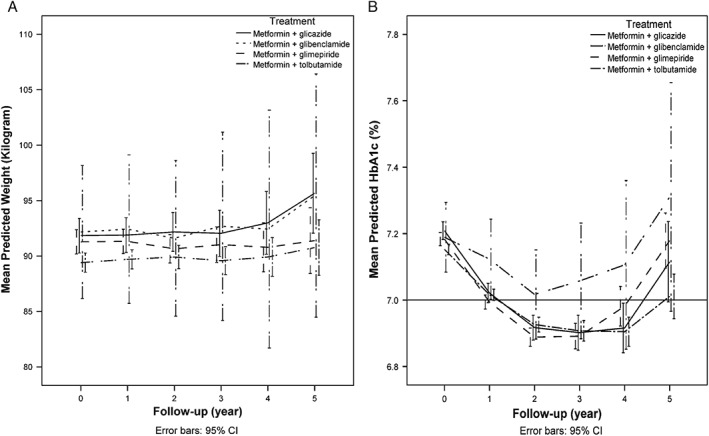
(A) Estimated mean body weight (kg) during follow‐up. Adjusted for age, gender and diabetes duration. (B) Estimated mean HbA1c (%) during follow‐up. Adjusted for age, gender, diabetes duration. Horizontal line indicates the treatment target at the time the study was performed.
3.2. Glucose control
The HbA1c results showed a non‐linear trend over time. A quadratic trend was introduced in the mixed model analysis for the changes in HbA1c during follow‐up. The results showed a significant overall quadratic trend (p < 0.005) in the HbA1c value during follow‐up. There was no significant (p = 0.37) difference between the different add‐on therapy combinations, and a non‐significant interaction effect (p = 0.14). In Table S2, Supporting Information the regression parameters of the fixed effects of the mixed model analysis are presented. Estimated HbA1c values for the different add‐on therapy combinations at different time periods are shown in Table 3 and Figure 1B. In the gliclazide, glimepiride and tolbutamide add‐on groups, the average HbA1c levels steadily decreased from 7.1% (54 mmol/mol) and remained below 7.0% (53 mmol/mol) during the first 4 years after intensification, and rose above 7.0% (53 mmol/mol) in the fifth year.
Table 3.
Estimated mean HbA1c values corrected for age, gender and diabetes duration
| Treatment regimen | Estimated mean HbA1c values, % (95% CI) | |||
|---|---|---|---|---|
| Metformin + glicazide | Metformin + glibenclamide | Metformin + glimepiride | Metformin + tolbutamide | |
| Year 0 (baseline) | 7.21 (7.18, 7.24) | 7.19 (7.08, 7.29) | 7.18 (7.16, 7.20) | 7.15 (7.14, 7.17) |
| n = 521 | n = 43 | n = 964 | n = 1427 | |
| Year 1 | 7.02 (6.99, 7.05) | 7.12 (7.00, 7.24) | 6.99 (6.97, 7.02) | 7.02 (6.99, 7.03) |
| n = 512 | n = 39 | n = 953 | n = 1459 | |
| Year 2 | 6.92 (6.88, 6.95) | 7.02 (6.88, 7.15) | 6.89 (6.86, 6.92) | 6.93 (6.90, 6.95) |
| n = 374 | n = 36 | n = 681 | n = 1014 | |
| Year 3 | 6.90 (6.85, 6.95) | 7.06 (6.88, 7.23) | 6.89 (6.85, 6.92) | 6.91 (6.88, 6.94) |
| n = 243 | n = 26 | n = 435 | n = 659 | |
| Year 4 | 6.92 (6.84, 6.99) | 7.11 (6.85, 7.36) | 6.98 (6.92, 7.04) | 6.90 (6.86, 6.95) |
| n = 147 | n = 16 | n = 245 | n = 364 | |
| Year 5 | 7.12 (6.99, 7.24) | 7.31 (6.97, 7.65) | 7.18 (7.10, 7.26) | 7.01 (6.94, 7.08) |
| n = 94 | n = 13 | n = 149 | n = 206 | |
4. DISCUSSION
In the present study, there was no evidence of a significant weight change within and between the four SU add‐on groups in the years after starting an SU. When used as add‐on therapy to metformin, increases in weight with similar glycaemic efficacy have been reported in randomized studies8, 10, 14, 25, 26, 27 investigating glibenclamide,7, 26, 28 gliclazide,29, 30 glimepiride,9 and glipizide.31 Most studies showed only modest increases in body weight (ranging from 0.5 to 3 kg) and most had a maximum follow‐up time of 12 months. One study with 10 years of follow‐up, showed that weight was gained only in the first 3 years after initiation of an SU.26
The present results contrast with results from randomized controlled trials (RCTs). The differences in weight change between this study and previous RCTs could partly have resulted from differences in design and baseline factors. Compared with RCTs, the patients included in the present study differed with respect to glycaemic control; the average baseline HbA1c level in most RCTs [ranging from 7.3% (56 mmol/mol) to 8.5% (69 mmol/mol)] was higher than the HbA1c 7.2% (55 mmol/mol) before treatment intensification in this cohort.9, 26, 27, 28, 29, 30, 31, 32 In addition, patients not only differed with respect to glycaemic control but probably also according to intensity of patient visits and patient counselling regarding lifestyle; factors possibly responsible for the lower average glycaemic control.
These results indicate that it is possible to maintain strict glycaemic control in the majority of patients in a real‐life setting without relevant weight increases when starting an SU. The effects of SUs on weight appear to be different from the effects found in observational studies. In such studies, significant or relevant weight increases have only been reported in people with unfavourable glycaemic control at baseline and only when SUs were analysed as one group.32, 33 Based on the CIs, however, we cannot exclude the possibility that there was a small weight increase in the present study. In contrast with previous observational studies, glycaemic control in patients included in the ZODIAC cohort was excellent.34 Moreover, when interpreting weight changes in observational cohort studies, there are several other possible explanations for weight increase besides the effects of medication. In the general population aged 55‐65 years, an increase of several decigrams per year is a phenomenon that is observed in several population studies performed in middle‐ and high‐income countries and therefore is not necessarily related to diabetes medication.35, 36
In the Netherlands, a form of protocol‐based care is deployed, which probably could be an explanation for the on average small and seemingly non‐relevant decrease in HbA1c. At the time the study was performed, patients treated in the Netherlands were checked at least once a year by their general practitioner and three times a year by practice nurses. During these visits there is a strong focus on lifestyle advice as well as glycaemic control.20 When patients’ HbA1c levels are above target, treatment is immediately intensified, even when they are only slightly above target. In the RCTs no structured lifestyle advice was described in the methods sections. Non‐adherence to lifestyle advice has been associated with worsening glycaemic control and weight gain,37, 38, 39 with subsequent increases in HbA1c leading to treatment intensification.40
The sample used in the present study has a high degree of generalizability and represents the majority of primary care‐treated patients in the Netherlands. Large regions of the Netherlands are participating in projects similar to that included the present study, and all patients with type 2 diabetes have the opportunity to opt out. In the Netherlands all patients have a primary care physician and >80% of all patients with type 2 diabetes are treated exclusively in primary care.23 In contrast to the observational design of the present study, RCTs often have strict selection criteria, limiting the number of patients eligible for inclusion and possibly leading to sample selection bias and therefore complicating the generalizability to daily practice.41
Strengths of the present study include the number of patients, the daily care setting and the new‐user design, thereby avoiding two potential types of bias: underascertainment of weight changes that occur early after the start of add‐on therapy and the inability to control for disease risk factors that may be altered by the study drugs themselves.24 Furthermore, >97% of all observations on weight and 99% of all observations on HbA1c from any study visit were complete at baseline and during follow‐up. The outcome measures weight and HbA1c were missing for a small percentage of patients but this posed no problem when using linear mixed models because all missing values are caused by random omissions in the registration and are consequently ‘missing completely at random.’ The decrease in the number of patients can mostly be explained by the study design. Patients entered the ZODIAC cohort at different time points and also entered into the present study at different time points; for example, a patient who entered the study in 1998 could have a maximum follow‐up of 6 years, while a patient who entered in 2009 could only have 3 years of follow‐up as the study cohort was censored at December 31, 2012. In addition, for a patient included in the ZODIAC cohort the maximum length of follow‐up was also shortened if this patient has, for example, 3 years of metformin monotherapy before receiving an add‐on, because in the present study follow‐up started in the year before receiving the add‐on. Table S5, Supporting Information shows the number of people censored because of changes in medication during follow‐up.
The study also has some important limitations. Although there were no significant within‐class differences in weight change after starting SUs in the first 5 years after treatment intensification, the number of patients in the gliclazide and glibenclamide group was small and a potentially relevant increase in weight could not be excluded. Furthermore, the decrease in number of patients in the tolbutamide and glibenclamide groups was relatively high compared with the other groups.
Secondly, baseline macrovascular complications differed between the SU groups. Although the Dutch diabetes guidelines do not base the choice of an oral glucose‐lowering agent on the presence of macrovascular complications, this theoretically could have influenced the choice of SU; however, the post hoc sensitivity analysis (Tables S3 and S4, Supporting Information) showed that the addition of macrovascular complications did not relevantly change the results. Third, the quality and reliability of our data were dependent on the accuracy of the data provided by practice nurses and general practitioners as part of a yearly benchmark. Information on doses and adherence to medication was not recorded within the ZODIAC cohort. Because data on medication adherence were not available, patients could have stopped medications during the following year which could possibly have resulted in misclassification. In addition, as patients may have been prescribed an SU at different time points during the year, the time between the first prescription of an SU and the measurement of body weight varied and was not always 1 year; however, there was no reason to assume that adherence and time of initiation within the year would substantially differ between groups. Furthermore, it is possible that patients who started to gain weight while using an SU wanted to try a different drug; however, we have no evidence that this is the case (Table S6, Supporting Information).The rapid decrease in number of patients after year 2 could complicate the interpretation of the results, but in the present study the CIs remain narrow. A common side effect of SUs as a group is severe hypoglycaemic events, which were not recorded in our database; however, the hazard of severe hypoglycaemia is very limited in gliclazide users,16 the most prescribed SU in the Netherlands. Furthermore, as no significant differences in weight were found, a dose–response analysis was not performed.
In conclusion, there was no evidence of relevant within‐class SU differences in weight during follow‐up. The results of the present study support the view that strict glycaemic control can be maintained in a substantial proportion of the primary care patients in the Netherlands with type 2 diabetes without clinically relevant weight change when adding SUs to metformin. In real life, when weight gain does occur, factors other than medication effects, for example, lifestyle‐associated factors, are likely to be more important.
Supporting information
Figure S1. Selection of patients. *The number of patients during follow‐up differ due to missing data.
Table S1. Parameter estimates of the fixed effects for the mixed model analysis of the change in weight.
Table S2. Parameter estimates of the fixed effects for the mixed model analysis of the change in HbA1c.
Table S3. Parameter estimates of the fixed effects for the sensitivity analysis of the change in weight.
Table S4. Parameter estimates of the fixed effects for the sensitivity analysis of the change in HbA1c.
Table S5. Reasons for censoring.
Table S6. Weight and HbA1c in year prior to censoring.
ACKNOWLEDGMENTS
D. S., R. W., G. W. D. L., K. H. G. and N. K. designed the study. D.S., R. W. and G. W. D. L. acquired the data. D.S., R.W., K.H.G. and G.W.D.L. analysed the data. D. S., R. W., N. K., K. J. J. H., H. J. G. B., G. H. B., K. H. G. and G. W. D. L. interpreted the data. D. S. and G. W. D. L. drafted the manuscript. D. S., R. W., N. K., S. T. H., K.J.J.H., G. H. B, H. J. G. B., K. H. G. and G. W. D. L. reviewed and edited the manuscript. All authors read and approved the final manuscripts. D. S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding information
None reported.
Schrijnders D, Wever R, Kleefstra N, Houweling ST, van Hateren KJJ., de Bock GH, Bilo HJG., Groenier KH, Landman GWD. Addition of sulphonylurea to metformin does not relevantly change body weight: a prospective observational cohort study (ZODIAC‐39), Diabetes Obes Metab 2016, 18:973–979. DOI:10.1111/dom.12700
REFERENCES
- 1. Eng C, Kramer CK, Zinman B, Retnakaran R. Glucagon‐like peptide‐1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta‐analysis. Lancet. 2014;384:2228–2234. [DOI] [PubMed] [Google Scholar]
- 2. Wieczorek A, Rys P, Skrzekowska‐Baran I, Malecki M. The role of surrogate endpoints in the evaluation of efficacy and safety of therapeutic interventions in diabetes mellitus. Rev Diabet Stud. 2008;5:128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Russell‐Jones D, Khan R. Insulin‐associated weight gain in diabetes–causes, effects and coping strategies. Diabetes Obes Metab. 2007;9:799–812. [DOI] [PubMed] [Google Scholar]
- 4. Mitri J, Hamdy O. Diabetes medications and body weight. Expert Opin Drug Saf. 2009;8:573–584. [DOI] [PubMed] [Google Scholar]
- 5. DeFronzo RA, Goodman AM. Efficacy of metformin in patients with non‐insulin‐dependent diabetes mellitus. The Multicenter Metformin Study Group. N Engl J Med. 1995;333:541–549. [DOI] [PubMed] [Google Scholar]
- 6. United Kingdom Prospective Diabetes Study Group . United Kingdom Prospective Diabetes Study 24: a 6‐year, randomized, controlled trial comparing sulfonylurea, insulin, and metformin therapy in patients with newly diagnosed type 2 diabetes that could not be controlled with diet therapy. Ann Intern Med. 1998;128:165–175. [DOI] [PubMed] [Google Scholar]
- 7. UK Prospective Diabetes Study (UKPDS) Group . Effect of intensive blood‐glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 8. Arechavaleta R, Seck T, Chen Y, et al. Efficacy and safety of treatment with sitagliptin or glimepiride in patients with type 2 diabetes inadequately controlled on metformin monotherapy: a randomized, double‐blind, non‐inferiority trial. Diabetes Obes Metab. 2011;13:160–168. [DOI] [PubMed] [Google Scholar]
- 9. Ferrannini E, Fonseca V, Zinman B, et al. Fifty‐two‐week efficacy and safety of vildagliptin vs. glimepiride in patients with type 2 diabetes mellitus inadequately controlled on metformin monotherapy. Diabetes Obes Metab. 2009;11:157–166. [DOI] [PubMed] [Google Scholar]
- 10. Hermann LS, Schersten B, Bitzen PO, Kjellstrom T, Lindgarde F, Melander A. Therapeutic comparison of metformin and sulfonylurea, alone and in various combinations. A double‐blind controlled study. Diabetes Care. 1994;17:1100–1109. [DOI] [PubMed] [Google Scholar]
- 11. Kaku K. Efficacy and safety of therapy with metformin plus pioglitazone in the treatment of patients with type 2 diabetes: a double‐blind, placebo‐controlled, clinical trial. Curr Med Res Opin. 2009;25:1111–1119. [DOI] [PubMed] [Google Scholar]
- 12. Kooy A, de Jager J, Lehert P, et al. Long‐term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med. 2009;169:616–625. [DOI] [PubMed] [Google Scholar]
- 13. Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–2743. [DOI] [PubMed] [Google Scholar]
- 14. Phung OJ, Scholle JM, Talwar M, Coleman CI. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA. 2010;303:1410–1418. [DOI] [PubMed] [Google Scholar]
- 15. Huizinga MM, Roumie CL, Greevy RA, et al. Glycemic and weight changes after persistent use of incident oral diabetes therapy: a Veterans Administration retrospective cohort study. Pharmacoepidemiol Drug Saf. 2010;19:1108–1112. [DOI] [PubMed] [Google Scholar]
- 16. Landman GW, de Bock GH, van Hateren KJ, et al. Safety and efficacy of gliclazide as treatment for type 2 diabetes: a systematic review and meta‐analysis of randomized trials. PLoS One. 2014;9:e82880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arnouts P, Bolignano D, Nistor I, et al. Glucose‐lowering drugs in patients with chronic kidney disease: a narrative review on pharmacokinetic properties. Nephrol Dial Transplant. 2014;29:1284–1300. [DOI] [PubMed] [Google Scholar]
- 18. National Kidney Foundation . KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis. 2012;60:850–886. [DOI] [PubMed] [Google Scholar]
- 19. Schramm TK, Gislason GH, Vaag A, et al. Mortality and cardiovascular risk associated with different insulin secretagogues compared with metformin in type 2 diabetes, with or without a previous myocardial infarction: a nationwide study. Eur Heart J. 2011;32:1900–1908. [DOI] [PubMed] [Google Scholar]
- 20. Rutten GEHM, De Grauw WJC, Nijpels G, et al. NHG‐standaard diabetes mellitus type 2 (derde herziening). Huisarts Wet. 2013;56:512–525. [Google Scholar]
- 21. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349. [DOI] [PubMed] [Google Scholar]
- 22. Ubink‐Veltmaat LJ, Bilo HJ, Groenier KH, Houweling ST, Rischen RO, Meyboom‐de Jong B. Prevalence, incidence and mortality of type 2 diabetes mellitus revisited: a prospective population‐based study in The Netherlands (ZODIAC‐1). Eur J Epidemiol. 2003;18:793–800. [DOI] [PubMed] [Google Scholar]
- 23. Edgar P, Sprangers N, van der Galiën O. Integrale bekostiging diabetes duur. Medisch Contact. 2012;16:991–992. [Google Scholar]
- 24. Ray WA. Evaluating medication effects outside of clinical trials: new‐user designs. Am J Epidemiol. 2003;158:915–920. [DOI] [PubMed] [Google Scholar]
- 25. Belsey J, Krishnarajah G. Glycaemic control and adverse events in patients with type 2 diabetes treated with metformin + sulphonylurea: a meta‐analysis. Diabetes Obes Metab. 2008;10(suppl 1):1–7. [DOI] [PubMed] [Google Scholar]
- 26. UK Prospective Diabetes Study (UKPDS) Group . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 27. Ristic S, Collober‐Maugeais C, Pecher E, Cressier F. Comparison of nateglinide and gliclazide in combination with metformin, for treatment of patients with type 2 diabetes mellitus inadequately controlled on maximum doses of metformin alone. Diabet Med. 2006;23:757–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marre M, Howlett H, Lehert P, Allavoine T. Improved glycaemic control with metformin‐glibenclamide combined tablet therapy (Glucovance) in type 2 diabetic patients inadequately controlled on metformin. Diabet Med. 2002;19:673–680. [DOI] [PubMed] [Google Scholar]
- 29. Charbonnel B, Schernthaner G, Brunetti P, et al. Long‐term efficacy and tolerability of add‐on pioglitazone therapy to failing monotherapy compared with addition of gliclazide or metformin in patients with type 2 diabetes. Diabetologia. 2005;48:1093–1104. [DOI] [PubMed] [Google Scholar]
- 30. Filozof C, Gautier JF. A comparison of efficacy and safety of vildagliptin and gliclazide in combination with metformin in patients with type 2 diabetes inadequately controlled with metformin alone: a 52‐week, randomized study. Diabet Med. 2010;27:318–326. [DOI] [PubMed] [Google Scholar]
- 31. Goke B, Gallwitz B, Eriksson J, Hellqvist A, Gause‐Nilsson I. Saxagliptin is non‐inferior to glipizide in patients with type 2 diabetes mellitus inadequately controlled on metformin alone: a 52‐week randomised controlled trial. Int J Clin Pract. 2010;64:1619–1631. [DOI] [PubMed] [Google Scholar]
- 32. Morgan CL, Jenkins‐Jones S, Evans M, Barnett AH, Poole CD, Currie CJ. Weight change in people with type 2 diabetes: secular trends and the impact of alternative antihyperglycaemic drugs. Diabetes Obes Metab. 2012;14:424–432. [DOI] [PubMed] [Google Scholar]
- 33. Kostev K, Rex J, Rockel T, Heilmaier C. Effects of selected antidiabetics on weight loss–a retrospective database analysis. Prim Care Diabetes. 2015;9:74–77. [DOI] [PubMed] [Google Scholar]
- 34. van Hateren KJ, Drion I, Kleefstra N, et al. A prospective observational study of quality of diabetes care in a shared care setting: trends and age differences (ZODIAC‐19). BMJ Open. 2012;2(4). pii: e001387. doi: 10.1136/bmjopen-2012-001387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dahl AK, Reynolds CA, Fall T, Magnusson PK, Pedersen NL. Multifactorial analysis of changes in body mass index across the adult life course: a study with 65 years of follow‐up. Int J Obes. 2014;38:1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wills AK, Hardy RJ, Black S, Kuh DJ. Trajectories of overweight and body mass index in adulthood and blood pressure at age 53: the 1946 British birth cohort study. J Hypertension. 2010;28:679–686. [DOI] [PubMed] [Google Scholar]
- 37. Chiu CJ, Wray LA. Factors predicting glycemic control in middle‐aged and older adults with type 2 diabetes. Prev Chronic Dis. 2010;7:A08. [PMC free article] [PubMed] [Google Scholar]
- 38. Khattab M, Khader YS, Al‐Khawaldeh A, Ajlouni K. Factors associated with poor glycemic control among patients with type 2 diabetes. J Diabetes Complications. 2010;24:84–89. [DOI] [PubMed] [Google Scholar]
- 39. Bjomtorp P. Biochemistry of obesity in relation to diabetes In: Ravussin E, section ed. International Textbook of Diabetes Mellitus. Chichester, England: Wiley; 1992:551–568. [Google Scholar]
- 40. Stanford J, Kaiser M, Ablah E, Dong F, Paull‐Forney B, Early J. The effect of weight loss on fasting blood sugars and hemoglobin A1c in overweight and obese diabetics and non diabetics. J Diabetes Mellitus. 2012;2(1):126–130. [Google Scholar]
- 41. Saunders C, Byrne CD, Guthrie B, et al. External validity of randomized controlled trials of glycaemic control and vascular disease: how representative are participants? Diabet Med. 2013;30:300–308. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Selection of patients. *The number of patients during follow‐up differ due to missing data.
Table S1. Parameter estimates of the fixed effects for the mixed model analysis of the change in weight.
Table S2. Parameter estimates of the fixed effects for the mixed model analysis of the change in HbA1c.
Table S3. Parameter estimates of the fixed effects for the sensitivity analysis of the change in weight.
Table S4. Parameter estimates of the fixed effects for the sensitivity analysis of the change in HbA1c.
Table S5. Reasons for censoring.
Table S6. Weight and HbA1c in year prior to censoring.


