Abstract
Objective
To assess joint disease activity by ultrasound (US) in patients with rheumatoid arthritis (RA) initiating treatment with adalimumab (ADA) plus methotrexate (MTX).
Methods
Data for this post hoc analysis originated from the MUSICA trial (ClinicalTrials.gov identifier: NCT01185288), which evaluated the efficacy of initiating ADA (40 mg every other week) plus 7.5 or 20 mg/week MTX in 309 patients with RA with an inadequate response to MTX. Synovial vascularization over 24 weeks was assessed bilaterally at metacarpophalangeal joint 2 (MCP2), MCP3, MCP5, metatarsophalangeal joint 5, and the wrists by power Doppler US (PDUS). A semiquantitative 4‐grade scale was used. Disease activity was assessed using the Disease Activity Score in 28 joints using the C‐reactive protein level (DAS28‐CRP) and Simplified Disease Activity Index (SDAI). The correlation between continuous variables was assessed using Pearson's correlation coefficient.
Results
After 24 weeks of treatment with ADA plus MTX, rapid improvements in the mean synovial vascularity score were observed; the greatest improvements were in MCP2 (−0.5), MCP3 (−0.4), and the wrist (−0.4). At week 24, patients with the lowest DAS28‐CRP (<2.6) had the lowest mean 5‐joint and 3‐joint composite synovial vascularity scores. The 5‐joint and 3‐joint scores were strongly correlated (ρ > 0.9). Synovial vascularity scores correlated poorly with DAS28, swollen joint count in 66 joints (SJC66), SJC28, tender joint count in 68 joints (TJC68), TJC28, Clinical Disease Activity Index (CDAI), SDAI, physician's global assessment, patient's global assessment of pain, and disease duration (ρ < 0.2). Thirty‐two (70%) of 46 patients with a DAS28‐CRP of <2.6, and 11 (58%) of 19 patients with an SDAI indicating remission had at least 1 joint with a synovial vascularity score of ≥1.
Conclusion
PDUS detects changes in synovial vascularity in RA patients treated with ADA plus MTX, and residual synovial vascularity in patients in whom clinical disease control has been achieved.
In patients with rheumatoid arthritis (RA), satisfactory disease control may be achieved using synthetic or biologic disease‐modifying antirheumatic drugs (DMARDs) as monotherapy or in combination. The timely initiation of effective therapy can prevent the progression of structural damage and functional disability. However, studies using sensitive ultrasonographic imaging have demonstrated that some patients, who attain remission of disease activity based on clinical and laboratory assessments, may have residual synovitis 1, 2. Power Doppler ultrasound (PDUS) is sensitive to a low vascular flow within the synovial membrane, indicating vascularization of the pannus, a sign of inflammatory activity in the joint. This vascularized pannus, if not detected and treated, may progress to bone damage 1, 3. Ultrasonography can be used to detect other joint changes, including synovitis and bone erosion, and to monitor response to treatment and predict disease progression and relapse 4, 5, 6.
US is more sensitive than clinical examination for detecting the presence of synovial fluid and synovitis 2. Thus, US can complement clinical assessment by providing a more accurate picture of disease state and treatment response. Moreover, the technology is noninvasive, does not use ionizing radiation or intravenous contrast, is relatively inexpensive, and can rapidly evaluate multiple sites, making it attractive for use in clinical practice. In the last decade, the Outcome Measures in Rheumatology (OMERACT) US task force provided standardized US definitions 7. However, consensus is still needed regarding the specific joints that should be evaluated. Summed scores from 78, 44, 28, 12, and 7 joints have been evaluated and compared for their performance in assessing disease activity and response to treatment 8, 9, 10, 11. Comprehensive scores may be expected to provide more information but may be less feasible for use in clinical practice. In this study, we assessed the utility of summed scores for 5 bilateral joints (metacarpophalangeal joint 2 [MCP2], MCP3, MCP5, metatarsophalangeal joint 5 [MTP5], and the wrist) and 3 bilateral joints (MCP2, MCP3, and the wrist) of the hand and foot that are commonly affected in RA. One of the concerns expressed about sonography is the lack of standardization of scanning protocols and variability introduced by different machines and readers. In this study, we report data from the MUSICA trial, which used standardized equipment and protocols for scan acquisition and blinded centralized scan reading 12.
Multicenter Double‐Blind Randomized Parallel Arm Study to Determine the Effect of MTX Dose on Clinical and Ultrasonographic Signs in Subjects with Moderately to Severely Active Rheumatoid Arthritis Treated with Adalimumab (MUSICA; ClinicalTrials.gov identifier: NCT01185288) was a large, randomized trial undertaken to assess clinical and US outcomes in patients with RA with a prior inadequate response to methotrexate (MTX), in response to initiation of adalimumab (ADA) therapy in combination with 1 of 2 doses of MTX. US was used to assess ongoing changes in synovial vascularity, synovial hypertrophy, and bony erosions during 24 weeks of treatment with ADA plus MTX. The objectives of this post hoc analysis were to assess the sensitivity of detection of synovial vascularity bilaterally at 5 joints, to assess the accuracy of imaging at 3 joints versus 5 joints, and to assess the association of clinical responses with PDUS scores.
PATIENTS AND METHODS
Patients and study design
MUSICA was a randomized, double‐blind, controlled, 24‐week clinical trial enrolling 309 patients to test the noninferiority of a lower dose compared to a higher dose of MTX upon the addition of ADA 12. The primary end point was noninferiority of low‐dosage MTX at week 24, assessed using the Disease Activity Score in 28 joints using the C‐reactive protein level (DAS28‐CRP). Patients were at least 18 years of age and had active RA for any duration of time. Patients had to have a DAS28‐CRP of ≥3.2 at baseline and ≥5 swollen joints of 66 joints assessed and ≥5 tender joints of 68 joints assessed at screening or baseline. In addition, patients must have received a stable MTX dosage of ≥15 mg/week for at least 12 weeks prior to screening, and must have had a demonstrated inadequate response to MTX. Prior treatment with 1 biologic agent was allowed in <10% of the patients enrolled. All enrolled patients were required to be naive for ADA. Patients were randomized (1:1) in a blinded manner to receive an MTX dosage of 7.5 mg/week or an MTX dosage of 20 mg/week, both in combination with open‐label ADA at a dosage of 40 mg every other week.
US and clinical assessments
Musculoskeletal US was performed at baseline and at weeks 4, 8, 12, 16, 20, and 24 to assess the severity of inflammation and RA disease activity at prespecified joints, using uniform machines (Esaote MyLab 5 with an 18‐MHz transducer) and settings (pulse repetition frequency and frequency range), by independent experienced sonographer rheumatologists (GSK, MJN, JRG, and DKM) who collaborated with AbbVie to design training.
Bilateral images of the following prespecified joints were obtained: dorsal and volar PD images of MCP2, MCP3, and MCP5, and only dorsal images of MTP5 and the wrists. The radiocarpal and intercarpal joints were included by using the dorsal long midline scans. These scans were selected due to their previously documented reliability 13. The images were de‐identified and scored by experienced readers following a defined scoring protocol. Images from the baseline and week 24 visits (or from the early termination visit if the patient discontinued the study) were read by 2 of 4 independent readers (including authors GSK, MJN, and JRG), and the scores from the 2 readers were averaged. Images from visits at weeks 4, 8, 12, 16, and 20 were scored by a single reader. The same reader read all of the images for a particular patient. Each reader was assigned an equal number of scans, so that reading was equally distributed.
A bilateral 5‐joint semiquantitative system incorporating OMERACT definitions 7 for pathology was used to measure synovial vascularity by PD with possible scores ranging from 0 to 3, where 0 indicates “normal” and 3 indicates “marked” (PD signals in ≥50% of the synovial area). Bilateral 5‐joint composite scores (16 views) were summed from the scores for MCP2, MCP3, and MCP5 (dorsal and volar views) and MTP5 and the wrist (dorsal view only); thus, from 16 views, total synovial vascularity scores could range from 0 to 48. Bilateral 3‐joint composite scores (10 views) were summed from the scores for MCP2 and MCP3 (dorsal and volar views) and the wrist (dorsal view only). The following US outcomes were assessed: mean synovial vascularity score at baseline, 4, 8, 12, 16, 20, and 24 weeks at individual joints as well as 5‐joint and 3‐joint composite scores, and changes in synovial vascularity from baseline to week 24. Disease activity was assessed by the DAS28‐CRP (grouped into scores of <2.6 [disease control], scores of 2.6 to <3.2, scores of 3.2 to <5.1, and scores of ≥5.1), and the Simplified Disease Activity Index (SDAI) (with scores of ≤3.3 indicating remission).
Statistical analysis
Using data pooled from patients in both MTX dosage groups, this post hoc study analyzed the relationship between US findings and clinical findings. Data are reported for the observed patient population. Patients with baseline and week 24 US images were divided into subgroups based on DAS28‐CRP (<2.6, 2.6 to <3.2, 3.2 to <5.1, or ≥5.1) to compare week 24 mean synovial vascularity scores and changes in mean synovial vascularity scores from baseline to week 24. The numbers of joints with a synovial vascularity score of ≥2 were also assessed to exclude possible interference from background.
Pearson's correlation coefficients were calculated to assess the correlation of 5‐joint and 3‐joint scores, as well as disease activity measures, such as the swollen joint count in 66 joints (SJC66), tender joint count in 68 joints (TJC68), CRP, DAS28‐CRP, patient's global assessment of disease activity, physician's global assessment, and the disability index of the Health Assessment Questionnaire, with synovial vascularity scores at week 24. An additional analysis was undertaken comparing swelling and tenderness in the same 10 joints examined by US in patients with a DAS28‐CRP of <2.6 at week 24, or who were in remission according the SDAI (score of ≤3.3) at week 24. These joints were routinely assessed as part of the SJC66 and TJC68.
To assess intrareader reliability, baseline and week 24 images for 10 patients were rescored by the same rheumatologists. To assess interreader reliability, baseline and week 24 images for 20 patients were scored by all 4 rheumatologists. Intraclass correlation coefficients (ICCs) were calculated using Shrout's method 14. An ICC of 0 to 0.2 indicates poor agreement, 0.3 to 0.4 indicates fair agreement, 0.5 to 0.6 indicates moderate agreement, 0.7 to 0.8 indicates strong agreement, and >0.8 indicates almost perfect agreement.
RESULTS
Demographic and baseline characteristics of the patients
Patients enrolled in the study had an average age of 54.8 years, an average disease duration of 5.3 years, and high disease activity, with a mean DAS28‐CRP of 5.8 (12) (Table 1). High baseline SJC66 and TJC68 scores indicated joint disease activity. Of the 5 joints assessed, at baseline the wrist had the highest PD activity, with a mean vascularity score of 1.6 (95% confidence interval [95% CI] 1.5, 1.8), followed by MCP2 (mean vascularity score 1.5 [95% CI 1.2, 1.7]), MCP3 (mean vascularity score 1.2 [95% CI 1.0, 1.4]), MCP5 (mean vascularity score 0.9 [95% CI 0.7, 1.1]), and MTP5 (mean vascularity score 0.5 [95% CI 0.4, 0.6]) (Figure 1A). PD activity at the wrist was significantly higher than that at MTP5 (P < 0.05).
Table 1.
Baseline demographic and disease characteristics of the 309 patients with RAa
| Age, years | 54.8 ± 12.1 |
| Sex, no. (%) female | 231 (74.8) |
| Race, % white/African American | 81.9/11.3 |
| RA duration, years | 5.3 ± 7.6 |
| TJC68 | 31.2 ± 17.0 |
| SJC66 | 18.3 ± 10.9 |
| TJC28 | 16.2 ± 7.2 |
| SJC28 | 12.2 ± 5.7 |
| Physician's global assessment of disease activity (100‐mm VAS) | 61.3 ± 20.1 |
| Patient's global assessment of disease activity (100‐mm VAS) | 63.8 ± 22.8 |
| Patient's global assessment of pain (100‐mm VAS) | 66.8 ± 20.9 |
| CRP, mg/liter | 14.6 ± 21.3 |
| Anti‐CCP positive, no. (%)b | 217 (71.6) |
| RF positive, no. (%)c | 210 (68.9) |
| DAS28‐CRP | 5.8 ± 0.9 |
| CDAI | 40.9 ± 12.9 |
| SDAI | 42.4 ± 13.5 |
| HAQ DI | 1.5 ± 0.6 |
| Synovial vascularity score (range 0–48) | 5.8 ± 5.4 |
| Synovial hypertrophy score (range 0–48) | 33.7 ± 7.3 |
Except where indicated otherwise, values are the mean ± SD. RA = rheumatoid arthritis; TJC68 = tender joint count in 68 joints; SJC66 = swollen joint count in 66 joints; VAS = visual analog scale; CRP = C‐reactive protein; DAS28‐CRP = Disease Activity Score in 28 joints using the CRP; CDAI = Clinical Disease Activity Index; SDAI = Simplified Disease Activity Index; HAQ DI = Health Assessment Questionnaire disability index.
Data on anti–cyclic citrullinated protein (anti‐CCP) status were missing for 6 patients.
Data on rheumatoid factor (RF) status were missing for 4 patients.
Figure 1.

A, Mean synovial vascularity score for metacarpophalangeal joint 2 (MCP2), MCP3, MCP5, metatarsophalangeal joint 5 (MTP5), and the wrist in patients with rheumatoid arthritis at baseline and at weeks 4, 8, 12, 16, 20, and 24 of treatment with adalimumab plus methotrexate. B, Percentage of total change at each joint from baseline to week 24 accounted for by change in dorsal and volar views. C, Percentage of joints with a synovial vascularity score of ≥2 at baseline and week 24. Bilateral dorsal (dorsal left [Dor‐L] and dorsal right) and volar (volar left [Vol‐L] and volar right) scoring was performed for MCP2, MCP3, and MCP5; only dorsal scoring was performed for MTP5 and the wrist. Scores are from the primary reader. Analysis was based on as‐observed cases. ∗ = P < 0.05; ∗∗ = P < 0.01; ∗∗∗ = P < 0.001 versus week 24, by chi‐square test. D, Percentage of patients with a synovial vascularity score of ≤1 at baseline and week 24. ∗ = P < 0.05 by chi‐square test.
Changes in synovial vascularity at individual joints after 24 weeks of treatment
Of the 5 joints assessed, the greatest improvement in mean synovial vascularity score from baseline to week 24 was observed in MCP2 (mean change from baseline −0.5 [95% CI −0.7, −0.3]; P < 0.05), followed by MCP3 (mean change −0.4 [95% CI −0.6, −0.2]; P < 0.05) and the wrist (mean change −0.4 [95% CI −0.5, −0.2]; P < 0.05) (Figure 1A). Smaller improvements were observed in MCP5 (mean change −0.2 [95% CI −0.4, −0.1]; P < 0.05) and MTP5 (mean change −0.2 [95% CI −0.3, −0.1]; P < 0.05). For MCP2, the change in the dorsal and volar scores accounted for ∼58% and ∼42%, respectively, of the total change in synovial vascularity score from baseline to week 24 (Figure 1B). For MCP3 and MCP5, most of the change in synovial vascularity scores from baseline to week 24 was accounted for by changes in the dorsal score (∼82% and ∼92%, respectively). For a majority of views at each joint, the percentage of joints with a synovial vascularity score of ≥2 decreased from baseline to week 24 (Figure 1C). The total percentage of joints with a synovial vascularity score of ≥2 also decreased from baseline to week 24. For MCP2, MCP3, and MCP5, for which both dorsal and volar views were obtained, fair agreement was seen between the views (Table 2). The proportion of patients with a total synovial vascularity score from all views of ≤1 increased from baseline (148 of 309 [47.9%]) to week 24 (160 of 269 [59.5%]) (P < 0.05) (Figure 1D).
Table 2.
Agreement between dorsal and volar views of the joints examineda
| Joint | Kappa (95% CI) |
|---|---|
| All 3 joints | 0.44 (0.39, 0.49) |
| MCP2 | 0.47 (0.39, 0.55) |
| MCP3 | 0.37 (0.28, 0.46) |
| MCP5 | 0.45 (0.35, 0.55) |
95% CI = 95% confidence interval; MCP2 = metacarpophalangeal joint 2.
Relationship between clinical disease activity and changes in synovial vascularity score
Composite 5‐joint synovial vascularity scores were compared in subgroups of patients according to the DAS28‐CRP after 24 weeks of treatment with ADA plus MTX. At week 24, 46 patients had a DAS28‐CRP of <2.6, 45 patients had a DAS28‐CRP of 2.6 to <3.2, 132 patients had a DAS28‐CRP of 3.2 to <5.1, and 47 patients had a DAS28‐CRP of ≥5.1 (Figure 2A). For each of the subgroups, regardless of the week 24 DAS28‐CRP, numerical decreases were observed in the mean 5‐joint synovial vascularity score from baseline to week 24. For all of the subgroups, the largest decreases in the 5‐joint synovial vascularity score occurred in the first 4 weeks after treatment. The smallest decrease in the 5‐joint synovial vascularity score (−1) was observed in the group with the most severe disease activity at week 24, with a DAS28‐CRP of ≥5.1. Interestingly, the largest decrease in the 5‐joint composite synovial vascularity score (−2.1) was observed in the group with a week 24 DAS28‐CRP of 2.6 to <3.2.
Figure 2.
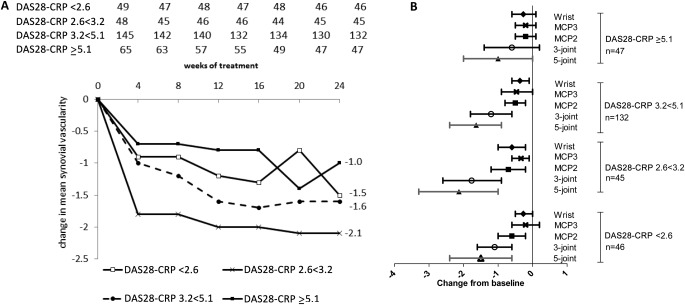
Changes in mean synovial vascularity scores in patients with rheumatoid arthritis over 24 weeks of treatment. A, Changes in mean 5‐joint composite synovial vascularity scores over 24 weeks of treatment. Patients were divided into subgroups based on Disease Activity Score in 28 joints using the C‐reactive protein level (DAS28‐CRP). Values at the top are the number of patients in each DAS28‐CRP group at each time point. Bilateral 5‐joint scores for as‐observed cases are shown. Scores are from primary reader results. Scores at baseline and week 24 are the average of those from 2 readers. B, Changes in synovial vascularity scores from baseline to week 24. Patients were divided into subgroups based on week 24 DAS28‐CRP. Symbols show the mean; horizontal lines indicate the 95% confidence interval. MCP3 = metacarpophalangeal joint 3.
Synovial vascularity scores from the 3 most responsive joints, MCP2, MCP3, and the wrist, were summed to derive a 3‐joint score. At 24 weeks, the 5‐joint and 3‐joint composite mean vascularity scores were lowest in patients with the lowest disease activity (DAS28‐CRP <2.6) and increased steadily with higher levels of disease activity, although the confidence intervals overlapped between the subgroups (Table 3). Vascularity scores at MCP2, MCP3, and the wrist showed similar trends when analyzed separately on the basis of disease activity. Subgroups of patients in whom better disease control was achieved after 24 weeks of treatment also had numerically greater decreases from baseline to week 24 in mean 5‐joint and 3‐joint synovial vascularity scores, although the confidence intervals overlapped between the groups (Figure 2B). For each subgroup, the week 24 mean 5‐joint and 3‐joint composite scores were highly correlated (ρ > 0.9, P < 0.0001). The changes in the 5‐joint and 3‐joint scores from baseline to week 24 were also highly correlated (ρ > 0.9, P < 0.0001).
Table 3.
Week 24 synovial vascularity scoresa
| Joints assessed | DAS28‐CRP <2.6 (n = 46) | DAS28‐CRP 2.6 to <3.2 (n = 45) | DAS28‐CRP 3.2 to <5.1 (n = 132) | DAS28‐CRP ≥5.1 (n = 47) |
|---|---|---|---|---|
| 5‐joint scoreb | 3.2 (2.1, 4.3) | 3.5 (2.2, 4.7) | 4.4 (3.6, 5.1) | 4.9 (3.1, 6.6) |
| 3‐joint scorec | 2.3 (1.5, 3.0) | 2.6 (1.8, 3.4) | 3.2 (2.7, 3.7) | 3.6 (2.3, 4.9) |
| MCP2d | 0.7 (0.3, 1.1) | 0.8 (0.4, 1.1) | 1.0 (0.7, 1.2) | 1.2 (0.6, 1.7) |
| MCP3d | 0.6 (0.3, 0.8) | 0.7 (0.4, 1.0) | 1.0 (0.7, 1.2) | 1.1 (0.5, 1.6) |
| Wriste | 1.0 (0.7, 1.3) | 1.1 (0.8, 1.5) | 1.3 (1.1, 1.5) | 1.3 (1.0, 1.7) |
Analysis was based on as‐observed cases. Scores are the average of those from 2 readers. Values are the mean (95% confidence interval). DAS28‐CRP = Disease Activity Score in 28 joints using the C‐reactive protein level.
Bilateral dorsal and volar scoring were performed for metacarpophalangeal joint 2 (MCP2), MCP3, and MCP5; only dorsal scoring was performed for metatarsophalangeal joint 5 and the wrist.
Bilateral dorsal and volar scoring were performed for MCP2 and MCP3; only bilateral dorsal scoring was performed for the wrist.
Bilateral dorsal and volar scoring were performed.
Only bilateral dorsal scoring was performed.
US activity in patients in clinical remission
After 24 weeks of treatment, 46 (15%) of 309 patients in the study had a DAS28‐CRP of <2.6, with a mean synovial vascularity score of 3.2. Only 19 (6.1%) of 309 patients were in remission according to the more stringent SDAI criteria, with a mean synovial vascularity score of 2.6. For the 10 joints evaluated, 32 (70%) of 46 patients with a DAS28‐CRP of <2.6 had at least 1 joint with a synovial vascularity score of ≥1, while 15 patients (33%) had at least 1 swollen joint, and only 7 patients (15%) had at least 1 tender joint. Fourteen (30.4%) of 46 patients had at least 1 joint with a synovial vascularity score of ≥2. Eleven (58%) of 19 patients in remission according to SDAI had at least 1 joint with a synovial vascularity score of ≥1. None of these patients had swollen joints, and only 1 (5.3%) of 19 had at least 1 tender joint. Six (31.6%) of the 19 patients had at least 1 joint with a synovial vascularity score of ≥2.
Overall, a poor correlation (ρ < 0.2) was observed between synovial vascularity score and DAS28‐CRP, SJC66, SJC28, TJC68, TJC28, Clinical Disease Activity Index, SDAI, physician's global assessment, patient's global assessment of pain, and disease duration. The change from baseline to week 24 in synovial vascularity score was poorly correlated (ρ = 0.184) with the corresponding changes in DAS28‐CRP or SDAI.
Interreader and intrareader reliability
The intrareader ICC was observed to be moderate to almost perfect (0.6 to 0.9) at week 0 for the 4 readers, although there was less agreement at week 24 (Table 4). The interreader ICC was strong to almost perfect at week 0 for 4 of the 6 pairs of readers, but at week 24, the agreement between the pairs of readers was poor to moderate for 4 of the 6 pairs of readers.
Table 4.
Intrareader and interreader ICCs for synovial vascularity at weeks 0 and 24a
| Reader | Week 0 | Week 24 |
|---|---|---|
| Intrareader ICC (95% CI) | ||
| 1 | 0.6 (0, 0.88) | 0.66 (0.1, 0.9) |
| 2 | 0.94 (0.78, 0.98) | 0.86 (0.52, 0.96) |
| 3 | 0.68 (0.13, 0.91) | 0.46 (−0.19, 0.83) |
| 4 | 0.93 (0.74, 0.98) | 0.02 (−0.59, 0.62) |
| Interreader ICC (95% CI) | ||
| 1 and 2 | 0.60 (−0.02, 0.84) | 0.12 (−1.22, 0.65) |
| 1 and 3 | 0.95 (0.87, 0.98) | 0.78 (0.45, 0.91) |
| 1 and 4 | 0.86 (0.65, 0.94) | 0.23 (−0.93, 0.70) |
| 2 and 3 | 0.63 (0.06, 0.85) | −0.04 (−1.63, 0.59) |
| 2 and 4 | 0.76 (0.38, 0.9) | 0.47 (−0.33, 0.79) |
| 3 and 4 | 0.89 (0.73, 0.96) | 0.59 (−0.03, 0.84) |
ICC = intraclass coefficient; 95% CI = 95% confidence interval.
DISCUSSION
MUSICA was the first large trial to use US to assess ongoing changes in joint disease activity in patients with RA in whom ADA treatment was initiated in combination with MTX. This post hoc analysis of PDUS data from MUSICA explored composite 5‐joint and 3‐joint scores for the assessment of synovial vascularity in small joints of the hand and foot over 24 weeks of treatment. Both the 5‐joint and 3‐joint synovial vascularity scores showed improvement in response to treatment with ADA plus MTX. The improvements in synovial vascularity scores paralleled observed improvements in clinical disease status. PDUS detected signs of subclinical joint disease activity in 58% and 70% of the patients in whom clinical disease control was achieved, as indicated by an SDAI of ≤3.3 or a DAS28‐CRP of <2.6, respectively, after 24 weeks of treatment. After 24 weeks of treatment with ADA plus MTX, at many views at each joint the percentage of joints with a synovial vascularity score of ≥2 decreased, and the number of patients with a total synovial vascularity score of ≤1 from all views increased.
It is important to identify joints that accurately reflect levels of inflammation and response to treatment. Another consideration is the minimum “core” number of joints to be assessed, which must balance the convenience of examining fewer joints with the need to obtain adequate information about the current disease state. Scores using 78, 44, 12, and 7 joints have previously been described and compared (4,8–11). In the present study, the summed 5‐joint scores were sensitive to change, showing improvements in synovial vascularity in most patients, irrespective of the disease state reached at week 24. A rapid improvement in synovial vascularity was observed for all joints in the first 4 weeks after initiation of treatment with ADA. The 5‐joint score was reflective of disease state and response to treatment, because patients who had lower disease activity at 24 weeks had numerically lower mean 5‐joint synovial vascularity scores, and achieved numerically greater improvements from baseline in mean 5‐joint synovial vascularity scores, than those with higher disease activity at 24 weeks. Similarly, the 3‐joint score, which included scores from the 3 most responsive joints, was as responsive as the 5‐joint score; patients with lower disease activity after 24 weeks of treatment had lower mean 3‐joint scores and greater decreases from baseline in mean 3‐joint score.
These parallel trends in the 5‐ and 3‐joint scores, along with their high correlation, suggest that the 3‐joint score may be as informative as the 5‐joint score. Interestingly, the joints included in the 3‐joint score, MCP2, MCP3, and the wrist, were included in the reduced 44‐, 28‐, 12‐, and 7‐joint scores, whereas MCP5 and MTP5 were absent from some reduced joint scores. The responsiveness at MCP2, MCP3, and the wrist individually, as well as part of the 3‐joint score, suggests that these may be good “sentinel” joints. This is also supported by the findings of Ceponis et al 9. In joints for which both dorsal and volar views were obtained (MCP2, MCP3, and MCP5), the PD signal for the dorsal view accounted for most of the total PD signal at that joint (58%, 82%, and 92% for MCP2, MCP3, and MCP5, respectively), suggesting that the dorsal view may be more informative than the volar view. Moreover, there was fair agreement between the dorsal and volar views at a given joint. The use of reduced joint counts is supported by the results of Hammer and Kvien 15, who demonstrated that reduced 7‐joint combinations were as sensitive to change upon treatment with biologic agents as the more extensive joint counts.
The change in the 5‐joint synovial vascularity score from baseline to week 24 for the group of patients with a DAS28‐CRP of 2.6 to <3.2 was greater than that for the group with a DAS28‐CRP of <2.6, possibly due to the amount of vascularized pannus accurately detected by PD or because patients with clinical disease control might have had less well established pannus, and hence been more responsive to treatment compared with those with more active disease. The discrepancy could also be because DAS28‐CRP may be less sensitive to detecting differences, and the DAS28‐CRP at week 24 for the various groups are fairly similar. PDUS detected joint vascular activity in >50% of the patients in whom clinical disease control was achieved at week 24, as assessed by DAS28‐CRP or SDAI. Approximately 30% of the patients with clinical disease control had at least 1 joint with a synovial vascularity score of ≥2. Other studies have also reported detection of joint vascularity by US in patients in clinical remission 1, 2, 16, 17.
Among MUSICA patients in whom clinical disease control was achieved, and who had positive PDUS activity, only a few had swollen or tender joints, suggesting that PDUS was more sensitive than clinical examination for detecting joint inflammatory activity. Indeed, poor correlations were observed between synovial vascularity score and SJC28, TJC28, SJC66, and TJC68, and also between synovial vascularity score and SJC10 or TJC10 at the same joints (Kaeley GS, et al: unpublished observations). A poor correlation of synovial vascularity scores with other disease characteristics has also been reported recently in analyses from an open‐label trial of abatacept, which found no correlations between changes in DAS28 scores and PDUS 18. One longitudinal study showed a significant correlation between time‐integrated measurements PDUS and DAS28 over 1 year 19, 20. In that study, the patients had early RA and the first US measurement was obtained at the time of starting DMARD treatment. In contrast, MUSICA patients had been treated with MTX for at least 12 weeks prior to entering the study. A contributing factor to poor correlations between clinical and US assessments here may be the presence of long‐standing disease or chronic changes in the joints. In a study of patients with moderate RA, Mandl et al 21 demonstrated that there was better intraobserver agreement for PDUS than for clinical assessment of synovitis in 11 different combinations of joints.
Overall, the dynamics of change in clinical scores and synovial vascularity scores were similar, with the greatest improvement observed by the 4‐week visit and maintained to the end of the study (Kaeley GS, et al: unpublished observations). Synovial hypertrophy scores were less responsive, showing very little change over the entire 24‐week period.
That the synovial vascularity scores were low despite high disease activity in MUSICA patients may be because of a partial treatment effect of corticosteroids and MTX. A previous study 1 showed an association between residual US scores and radiographic joint deterioration in almost 20% of RA patients in clinical remission after treatment with conventional DMARDs. While there is limited evidence regarding the significance of residual synovitis in RA patients treated with biologic agents in clinical remission, small studies by Fukae et al 22 and Yoshimi et al 23 suggest that persistent PD activity in patients who are in remission or have low disease activity may be associated with the progression of erosions. Additionally, Iwamoto et al 24 recently showed that patients with clinical disease control who had residual PD activity were at an increased risk of disease flare upon discontinuation of treatment.
For synovial vascularity, intrareader agreement was moderate to very strong at baseline and at week 24. Interreader agreements were better at baseline than at week 24. The variability underscores the importance of standardized operator and reader techniques. The study was conducted over a period of more than 2 years. The interreader drift is an important observation with implications for future studies to ensure that the readers continue to read within given tolerances, perhaps by including standard test scans at regular intervals or an adjudication process for errant scoring. This caveat of the present study may undermine the validity of the results. Nevertheless, this was the first large US trial that used central reading, and important lessons were learned regarding ensuring consistent and reliable reading.
Our study had some limitations since the analyses were post hoc. Compared with patients in some other US studies, patients in this study had low synovial vascularity scores at baseline, possibly due to the fact that they had been receiving MTX and corticosteroids for at least 12 weeks before the baseline visit. This may have caused difficulties in detection, and in the ability to discern differences in scores that were already low. The low synovial vascularity scores at baseline may also reflect the presence of more synovial hypertrophy than synovial vascularity. The choice of the Esaote MyLab 5 machine balanced the consideration of sensitivity with that of cost feasible for a clinical setting; the machine settings were empirically optimized to maximize sensitivity, and notably, the equipment demonstrated fair sensitivity in detecting small vessel and low flow states.
There was also no comparator arm to determine the degree of variation in the absence of treatment. While the 5‐ and 3‐joint synovial vascularity scores appear to have good responsiveness and include joints that are commonly affected in RA, both scores include only small joints. Although the 3‐joint score performed comparably to the 5‐joint score in this cohort, the 3 joints were chosen based on the results for the 5‐joint score. It is therefore important to validate the performance of the 3‐joint score in other cohorts. Finally, patients in this data set have established RA. It would be interesting to determine how these reduced scores perform in patients with less established disease, and how these scores perform in comparison to the more extensive joint scores.
In conclusion, our analysis used data from the MUSICA trial, which controlled for variations in machine settings, scanning protocols, and scoring of US images, and enrolled a large number of patients. Our results suggest that PDUS can add value to clinical assessment of joint disease in patients with RA by detecting changes in joint disease activity. Notably, PDUS also detected evidence of synovial vascularity in patients in whom clinical disease control was achieved, which may have implications for preventing further joint damage. In the clinic, the increased ability to accurately assess disease state will enable the adjustment of treatment to improve outcomes for patients.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Kaeley had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Kaeley, Wells, Kupper.
Acquisition of data
Kaeley, Nishio, Goyal.
Analysis and interpretation of data
Kaeley, Goyal, MacCarter, Chen, Kupper, Kalabic.
ROLE OF THE STUDY SPONSOR
AbbVie funded the study, contributed to its design, and was involved in the collection, analysis, and interpretation of data and the writing, review, and approval of the manuscript for publication. All authors contributed to the development of content. All authors and AbbVie reviewed and approved the manuscript. The authors maintained control over the final content. AbbVie provided statistical support (performed by Shufang Liu, PhD) and medical writing assistance (performed by Naina Barretto, PhD).
Supported by AbbVie Deutschland.
Drs. Kaeley, Nishio, Goyal, MacCarter, and Wells have received consulting fees, speaking fees, and/or honoraria from AbbVie (less than $10,000 each). Drs. Chen, Kupper, and Kalabic own stock or stock options in AbbVie.
REFERENCES
- 1. Brown AK, Conaghan PG, Karim Z, Quinn MA, Ikeda K, Peterfy CG, et al. An explanation for the apparent dissociation between clinical remission and continued structural deterioration in rheumatoid arthritis. Arthritis Rheum 2008;58:2958–67. [DOI] [PubMed] [Google Scholar]
- 2. Brown AK, Quinn MA, Karim Z, Conaghan PG, Peterfy CG, Hensor E, et al. Presence of significant synovitis in rheumatoid arthritis patients with disease‐modifying antirheumatic drug‐induced clinical remission: evidence from an imaging study may explain structural progression. Arthritis Rheum 2006;54:3761–73. [DOI] [PubMed] [Google Scholar]
- 3. Hama M, Sugiyama Y, Tsuchida N, Kunishita Y, Kishimoto D, Kamiyama R, et al. PD signal detected by ultrasonography relates to joint destruction in rheumatoid arthritis under biologics therapy in real world [abstract]. Arthritis Rheumatol 2014;66 Suppl:S53 URL: http://acrabstracts.org/abstract/pd-signal-detected-by-ultrasonography-relates-to-joint-destruction-in-rheumatoid-arthritis-under-biologics-therapy-in-real-world/. [Google Scholar]
- 4. Carotti M, Salaffi F, Morbiducci J, Ciapetti A, Bartolucci L, Gasparini S, et al. Colour Doppler ultrasonography evaluation of vascularization in the wrist and finger joints in rheumatoid arthritis patients and healthy subjects. Eur J Radiol 2012;81:1834–8. [DOI] [PubMed] [Google Scholar]
- 5. Terslev L, Torp‐Pedersen S, Qvistgaard E, Kristoffersen H, Rogind H, Danneskiold‐Samsoe B, et al. Effects of treatment with etanercept (Enbrel, TNRF:Fc) on rheumatoid arthritis evaluated by Doppler ultrasonography. Ann Rheum Dis 2003;62:178–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dougados M, Devauchelle‐Pensec V, Ferlet JF, Jousse‐Joulin S, D'Agostino MA, Backhaus M, et al. The ability of synovitis to predict structural damage in rheumatoid arthritis: a comparative study between clinical examination and ultrasound. Ann Rheum Dis 2013;72:665–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Backhaus M, Burmester GR, Gerber T, Grassi W, Machold KP, Swen WA, et al. Guidelines for musculoskeletal ultrasound in rheumatology. Ann Rheum Dis 2001;60:641–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Naredo E, Rodriguez M, Campos C, Rodriguez‐Heredia JM, Medina JA, Giner E, et al. Validity, reproducibility, and responsiveness of a twelve‐joint simplified power Doppler ultrasonographic assessment of joint inflammation in rheumatoid arthritis. Arthritis Rheum 2008;59:515–22. [DOI] [PubMed] [Google Scholar]
- 9. Ceponis A, Onishi M, Bluestein HG, Kalunian K, Townsend J, Kavanaugh A. Utility of the ultrasound examination of the hand and wrist joints in the management of established rheumatoid arthritis. Arthritis Care Res (Hoboken) 2014;66:236–44. [DOI] [PubMed] [Google Scholar]
- 10. Hammer HB, Sveinsson M, Kongtorp AK, Kvien TK. A 78‐joints ultrasonographic assessment is associated with clinical assessments and is highly responsive to improvement in a longitudinal study of patients with rheumatoid arthritis starting adalimumab treatment. Ann Rheum Dis 2010;69:1349–51. [DOI] [PubMed] [Google Scholar]
- 11. Backhaus M, Ohrndorf S, Kellner H, Strunk J, Backhaus TM, Hartung W, et al. Evaluation of a novel 7‐joint ultrasound score in daily rheumatologic practice: a pilot project. Arthritis Rheum 2009;61:1194–201. [DOI] [PubMed] [Google Scholar]
- 12. Kaeley GS, Evangelisto AM, Nishio MJ, Goss SL, Liu S, Kalabic J, et al. Methotrexate dosage reduction upon adalimumab initiation: clinical and ultrasonographic outcomes from the randomized noninferiority MUSICA trial. J Rheumatol 2016;43:1480–9. [DOI] [PubMed] [Google Scholar]
- 13. Dopazo Gonzalez N, Ten Cate DF, Swen WA, Mera Varela A, Insua Vilarino SA, Perez‐Pampin E, et al. The most reliable probe position in the ultrasonographic examination of the wrist in rheumatoid arthritis. Clin Exp Rheumatol 2012;30:566–9. [PubMed] [Google Scholar]
- 14. Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull 1979;86:420–8. [DOI] [PubMed] [Google Scholar]
- 15. Hammer HB, Kvien TK. Comparisons of 7‐ to 78‐joint ultrasonography scores: all different joint combinations show equal response to adalimumab treatment in patients with rheumatoid arthritis. Arthritis Res Ther 2011;13:R78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Balsa A, de Miguel E, Castillo C, Peiteado D, Martin‐Mola E. Superiority of SDAI over DAS‐28 in assessment of remission in rheumatoid arthritis patients using power Doppler ultrasonography as a gold standard. Rheumatology (Oxford) 2010;49:683–90. [DOI] [PubMed] [Google Scholar]
- 17. Naredo E, Valor L, de la Torre I, Martinez‐Barrio J, Hinojosa M, Aramburu F, et al. Ultrasound joint inflammation in rheumatoid arthritis in clinical remission: how many and which joints should be assessed? Arthritis Care Res (Hoboken) 2013;65:512–7. [DOI] [PubMed] [Google Scholar]
- 18. D'Agostino MA, Wakefield RJ, Berner‐Hammer H, Vittecoq O, Filippou G, Balint P, et al., on behalf of the OMERACT‐EULAR‐Ultrasound Task Force . Value of ultrasonography as a marker of early response to abatacept in patients with rheumatoid arthritis and an inadequate response to methotrexate: results from the APPRAISE study. Ann Rheum Dis 2016;75:1763–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zufferey P, Brulhart L, Tamborrini G, Finckh A, Scherer A, Moller B, et al. Ultrasound evaluation of synovitis in RA: correlation with clinical disease activity and sensitivity to change in an observational cohort study. Joint Bone Spine 2014;81:222–7. [DOI] [PubMed] [Google Scholar]
- 20. Naredo E, Collado P, Cruz A, Palop MJ, Cabero F, Richi P, et al. Longitudinal power Doppler ultrasonographic assessment of joint inflammatory activity in early rheumatoid arthritis: predictive value in disease activity and radiologic progression. Arthritis Rheum 2007;57:116–24. [DOI] [PubMed] [Google Scholar]
- 21. Mandl P, Balint PV, Brault Y, Backhaus M, D'Agostino MA, Grassi W, et al. Metrologic properties of ultrasound versus clinical evaluation of synovitis in rheumatoid arthritis: results of a multicenter, randomized study. Arthritis Rheum 2012;64:1272–82. [DOI] [PubMed] [Google Scholar]
- 22. Fukae J, Isobe M, Kitano A, Henmi M, Sakamoto F, Narita A, et al. Positive synovial vascularity in patients with low disease activity indicates smouldering inflammation leading to joint damage in rheumatoid arthritis: time‐integrated joint inflammation estimated by synovial vascularity in each finger joint. Rheumatology (Oxford) 2013;52:523–8. [DOI] [PubMed] [Google Scholar]
- 23. Yoshimi R, Hama M, Takase K, Ihata A, Kishimoto D, Terauchi K, et al. Ultrasonography is a potent tool for the prediction of progressive joint destruction during clinical remission of rheumatoid arthritis. Mod Rheumatol 2013;23:456–65. [DOI] [PubMed] [Google Scholar]
- 24. Iwamoto T, Ikeda K, Hosokawa J, Yamagata M, Tanaka S, Norimoto A, et al. Prediction of relapse after discontinuation of biologic agents by ultrasonographic assessment in patients with rheumatoid arthritis in clinical remission: high predictive values of total gray‐scale and power Doppler scores that represent residual synovial inflammation before discontinuation. Arthritis Care Res (Hoboken) 2014;66:1576–81. [DOI] [PubMed] [Google Scholar]


