Abstract
Racial disparities in breast cancer incidence and outcome are a major health care challenge. Patients in the black race group more likely present with an early onset and more aggressive disease. The occurrence of high numbers of macrophages is associated with tumor progression and poor prognosis in solid malignancies. Macrophages are observed in adipose tissues surrounding dead adipocytes in “crown-like structures” (CLS). Here we investigated whether the numbers of CD163+ tumor-associated macrophages (TAMs) and/or CD163+ CLS are associated with patient survival and whether there are significant differences across blacks, non-black Latinas, and Caucasians. Our findings confirm that race is statistically significantly associated with the numbers of TAMs and CLS in breast cancer, and demonstrate that the highest numbers of CD163+ TAM/CLS are found in black breast cancer patients. Our results reveal that the density of CD206 (M2) macrophages is a significant predictor of progression-free survival univariately and is also significant after adjusting for race and for HER2, respectively. We examined whether the high numbers of TAMs detected in tumors from black women were associated with macrophage proliferation, using the Ki-67 nuclear proliferation marker. Our results reveal that TAMs actively divide when in contact with tumor cells. There is a higher ratio of proliferating macrophages in tumors from black patients. These findings suggest that interventions based on targeting TAMs may not only benefit breast cancer patients in general but also serve as an approach to remedy racial disparity resulting in better prognosis patients from minority racial groups.
Keywords: Breast cancer, Race/ethnicities, Macrophages, Crown-like structures, Inflammation
Introduction
Breast cancer is the second leading cause of cancer mortality in women in the United States (US) [1]. Disparities in cancer incidence and outcome due to ethnicity and race-related differences are a major health care challenge [2]. Even after considering socio-economic factors, education and access to health care, African-American (AA) women still exhibit breast cancer health disparities in incidence and outcome [3, 4]. These patients present with a particular pattern of breast cancer pathogenesis, i.e., early onset, higher incidence in younger women, and more aggressive disease with less favorable prognosis. A number of biological differences may account for the nature of this disease in AA as compared to Caucasian (CA) women [5–11]. Some of these differences may be inheritable; inherited predisposition to breast cancer has been shown among AA women [12]. However, whether and how immune inflammatory components in the tumor microenvironment (TME) may correlate with the aggressiveness of breast cancer in AA women remains unclear.
The occurrence of high numbers of macrophages in the TME has been associated with tumor progression and poor tumor prognosis in breast and other solid malignancies [13, 14]. Pro-inflammatory (M1) TAMs can be cytotoxic to tumor cells [15, 16]. However, M1 TAMs can also contribute to tumor initiation through the mutagenic reactive oxygen and nitrogen species they generate [17, 18]. TAMs further impact tumor progression by promoting invasion, angiogenesis, and metastasis when in their immunosuppressive (M2) mode [19–21]. Actually, mixtures of pro-inflammatory M1 and immunosuppressive M2 macrophages co-exist in the TME of advanced mouse and human solid malignancies [22–26]. In contrast, high numbers of M1 macrophages colonize obese adipose tissues, providing obese adipose tissues a pro-inflammatory condition, while M2 macrophages are found in lean adipose tissues [27]. Macrophages are also observed in visceral adipose tissues of obese humans and mice surrounding dying adipocytes, forming particular structures known as “crown-like structures” (CLS) [28]. In the breast TME, macrophages are observed as isolated or grouped TAMs and also in CLS within the breast adipose tissue. Increased numbers of breast CLS have been associated with enhanced aromatase expression/activity and with inflammation in mammary tumors of obese mice and in obese women with breast cancer [29–31]. Aromatase converts androgens to estrogens [32] and promotes ER+ cell growth [33–35]; importantly, ER+ breast cancers are the most prevalent breast malignancies [1].
Using samples from an archival breast cancer tumor bank with cases obtained from 1978 to 1997 at the University of Miami/Jackson Memorial Hospital in Miami, Florida, we previously investigated in a pilot study (30 cases) whether the presence of CD68+ TAMs was an independent prognostic factor in small T1 ER+ breast cancers across three different racial groups [blacks (BL), non-black Latinas (NBLA), and CA women [36]. In Miami, where these cases were recruited and treated, there are many black Islanders, Caribbean, Haitians, South Americans (Brazilians and others), who do not identify themselves as AA; additionally, there are North Africans and South Africans of dark or light black skin color and these also are not AA. This led us to classify the patients from this archival bank on the basis of their race, not necessarily of their ethnicity. We demonstrated that TAM numbers were significantly higher in tumors from BL and NBLA than in CA patients [36]. These findings led us to design the present study, in which we used a larger number of tumors of all types and stages from the same archival bank and a different pan macrophage marker, CD163. CD163 is more sensitive than CD68 in detecting macrophages [37–39]. We also examined whether TAMs and CLS are associated with survival in these racial groups, and whether there are any differences in TAMs proliferation. Furthermore, we aimed to correlate the density and M1/M2 phenotypes of TAMs and also of CLS with clinical pathologic characteristics of the tumors across the three different racial groups.
Materials and methods
Case selection
One hundred fifty (150) consecutive cases (50 BL, 50 NBLA, and 50 CA) of women treated for breast cancer between 1978 and 1997 at Jackson Memorial Hospital (JMH) and University of Miami's Sylvester Comprehensive Cancer Center (UM/SCCC) in Miami, Florida, were selected. Tumors were formalin-fixed paraffin-embedded (FFPE) specimens from the Cancer Center's Tumor Bank Core Facility. Tumors of any size and any ER and PR status were included. Patient inclusion criteria of samples were any stages, any hormonal receptor, and HER2 receptor. These cases were followed up for at least 5 years. Patient exclusion criteria included: the presence of previous cancers, exposure to previous chemotherapy, radio-therapy or hormonal therapy, or presenting bilateral or multifocal breast cancers. Patients were not treated with systemic anti-cancer therapy at the moment of the tumor sample collection in the surgery, and all (if any) systemic therapy was delivered post-surgery. Information concerning patient demographics, clinical characteristics, pathologic reports, and administered treatments was gathered from both UM/Jackson Memorial Hospital's Tumor Registry and medical records and UM/SCCC. The characteristics of patients and their tumors (de-identified) by race are described in Supplementary Table 1. The study was approved by the University of Miami's Institutional Review Board (IRB).
Immunohistochemistry (IHC)
IHC was carried out and assessed as briefly described in the legends of Figs. 1, 2 and 3 and as previously published [36]. Primary antibodies used were CD163 (pan macrophage marker), CD206 (M2 marker), CD40 (M1 marker), and Ki-67 (proliferation marker). Qualitative and quantitative analysis of TAMs and CLS densities was assessed blindly and independently by two investigators (MJ and AMS), including a breast pathologist (MJ). Photomicrographs and measurement of macrophages were conducted blind to patient identity. Macrophage proliferation was independently assessed by a breast pathologist (TAI) and two other study investigators (LS and AMS).
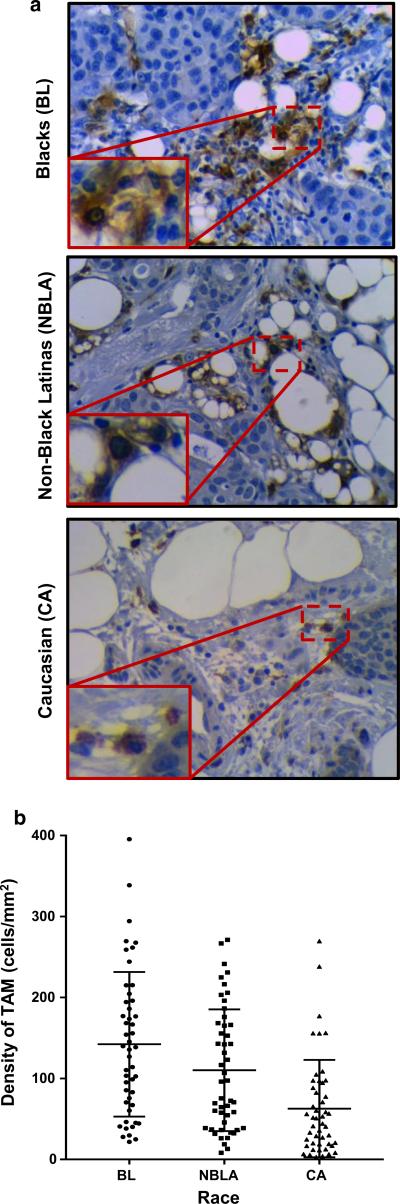
Fig. 1.
TAMs occur at significantly higher densities in breast cancers from blacks. a IHC for CD163 macrophage marker (AbD Serotec, Raleigh, NC, USA) was done in 4 μm sections from FFPE tumor blocks. Sections were deparaffinized in xylene and hydrated in series of graded alcohols (100, 95 and 75 %). Heat-induced antigen retrieval was carried out in water bath (90 °C) in the presence of antigen unmasking solution (Citric Acid Based from Vector Laboratories, Burlingame, CA, USA). Antigen retrieval was followed by one incubation step with Peroxidase blocking reagent (dilution 1:100 of Perdrogen 30 %, Sigma-Aldrich, St. Louis, MO, USA) for 30 min at room temperature, followed by another step of blocking serum (normal horse serum 1.5 %, Vector Laboratories, in PBS) for 20 min at RT. CD163 (1:250) was incubated for 1 h at RT. As a detection system, we used Vectastain Elite ABC kit (Biotin/Avidin System) from Vector Laboratories; slides were counterstained with hematoxylin for 30 s. Pictures (×40) show the different densities of CD163 expression across the three racial groups. b Quantitative (calculated) macrophage density was determined in one tumor section slide per tumor. Density was calculated by dividing the number of CD163+ cells observed using a ×40 lens of a Olympus BX41 microscope by the area of the visual field, calculated as πr2, where the radius of the visual field was determined using a calibration graduated slide. TAMs’ density was calculated for 20 different and randomly selected visual fields in each tissue section, and a final average was determined and reported as the TAMs’ density per each case as cells/mm2. The graph shows TAMs’ density distribution across the three racial groups studied
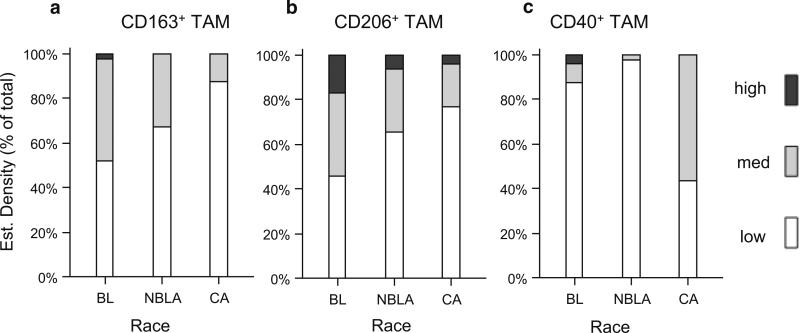
Fig. 2.
Estimated densities of TAMs and their M1/M2 activation phenotypes in breast cancers exhibit different distributions across races. IHC for CD206 (1:50, M2 macrophage marker) and CD40 (1:200, M1 macrophage marker), both from R&D, Minneapolis, MN, was done as in Fig. 1, except Antigen Unmasking Solution High pH from Vector Laboratories was used. After staining, each histological sample was assessed qualitatively for CD163, CD206, and CD40. These estimated densities were assessed by visually scanning the whole tissue slide with a ×10 lens and arbitrarily assigning 1+ (low), 2+ (medium) or 3+ (high) as follows: 1+ = 1–150 cells/mm2; 2+ = 151–300 cells/mm2, and 3+ = >300 cells/mm2. a Estimated density of CD163+ TAMs expressed in % of total patients with low, medium, or high densities per each race. b Estimated density of CD206+ (M2) expressed in % of total patients with low, medium, or high densities per each race. c Estimated density of CD40+ (M1) expressed in % of total patients with low, medium, or high densities per each race
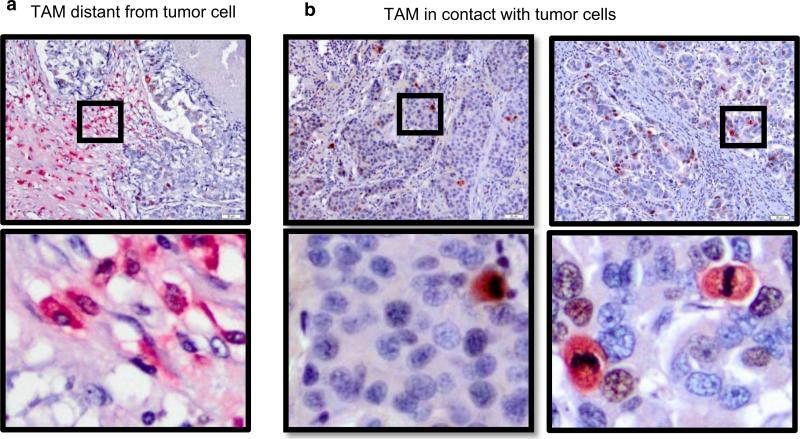
Fig. 3.
TAMs in breast cancers proliferate when in contact with tumor cells. For the analysis of proliferating macrophages (double staining with Ki-67 and CD163), tumor sections were first incubated with Ki-67 (Dako, Carpinteria, CA, USA) for 180 min (1:25 dilution) at RT and developed in brown. Then, the slides were incubated with CD163 (1:250) for 30 min (RT) and developed in red. The slides were then counterstained with hematoxylin for 30 s. As a detection system, Vectastain Elite ABC and ABC-AP Mouse kits (Biotin/AvidinSystem) from Vector Laboratories were used. In this case, breast biopsies were photographed at ×20 using an Olympus BX41 microscope with an Olympus DP15 digital camera. Images were stored in JPEG format, and density was calculated by dividing the number of proliferating TAMs in one image per case by the area observed in each picture of the analyzed tissue, calculated using the reference of 50 μm divisions. Proliferating macrophages were considered the cells simultaneously expressing CD163 (red) and Ki-67 (brown) staining. a TAMs far from the tumor cells marked negative for proliferative activity (CD163+/Ki67−, see inset with amplification); b TAMs in close contact with tumor cells exhibit proliferative activity (CD163+/Ki-67+, see inset with amplification)
Statistical analysis
The clinical data were summarized by mean, standard deviation (SD) for continuous variables, and by frequencies and percentages for categorical variables for overall sample as well as by race (BL, NBLA, CA). Differences in means were tested by either Student's t test or one-way ANOVA with Bonferroni correction for multiple comparisons. Differences in proportions were tested by Chi-square or Fisher's exact test for categorical variables. Overall survival (OS) time is calculated as the elapsed time between the dates of diagnosis and death from any cause or last follow-up for alive patients. Progression-free survival (PFS) time is calculated as the elapsed time between the dates of diagnosis and earliest progression (local recurrence or distant metastasis or death) or last follow-up for patients without progression. Median survival and survival rates for both OS and PFS at 12, 24, 36, 60, 96, and 120 months were calculated by Kaplan–Meier method for all patients as well as by race. Log-rank test was used to test the differences in survival between the groups. Unadjusted and adjusted hazard ratio (HR), its 90 % confidence interval (90 %CI) along with p value were calculated from fitting several univariate and bivariate Cox proportional hazard regression models to identify significant predictors of OS and PFS, respectively. Race, ER, PR, HER2, CD163, CD206, and CD40 included one at a time in the univariate models. The predictors for bivariate models included paired variables between each of conventional breast cancer markers (ER, PR, and HER2) with each of macrophages surface markers (CD163, CD206, and CD40), and between race and each marker (ER, PR, HER2, CD163, CD206, and CD40). Patients with unknown ER or PR were excluded from all regression models. Due to the exploratory nature of the study, we set the type-I error rate as 10 % for all analysis, where p values <0.10 were considered as statistically significant. Statistical analyses were performed by SAS v9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Higher densities of tumor-associated macrophages (TAMs) in breast tumors from black patients compared to non-black Latino and Caucasian patients
Our IHC staining procedures successfully identified CD163+ TAMs in breast tumor sections from the archived samples (Fig. 1a). We calculated the densities of TAMs (cells/mm2) in the different tumors. We found significantly different occurrences of TAMs in breast cancers across these races with BL showing the highest numbers and CA the lowest (Fig. 1b).
As detailed in Supplementary Table-2A, densities of TAMs are significantly different among the three ethnicities, with tumors from BL patients presenting with the highest densities of CD163+ TAMs (mean = 142.21 cells/mm2), followed by tumors from NBLA (110.16 cells/mm2), with tumors from CA showing the lowest TAM densities (62.72 cells/mm2). With Bonferroni multiple comparison analysis, both BL and NBLA tumors exhibited significantly higher TAMs densities than CA (p < 0.0001, Supplementary Table-2A).
Higher densities of immunosuppressive M2 macrophages predominate in breast tumors from black patients compared to non-black Latino and Caucasian patients
To assess the pro-inflammatory (M1) vs. immunosuppressive (M2) profiles of TAMs, CD40+ (M1) and CD206+ (M2) macrophage densities were estimated (Supplementary Table-2B). As shown in Fig. 2a, tumors from BL patients contained the highest estimated densities of CD163+ total TAM (p = 0.0009).
For all three races, the majority of TAMs were immunosuppressive M2, with BL showing the highest densities of M2, followed by NBLA (Fig. 2b; Supplementary Table-2B, p = 0.0238). In contrast, pro-inflammatory CD40 (M1) macrophages were detected at the lowest densities in tumors from NBLA patients (2 %), followed by BL (12.5 %), with CA showing the highest (56.3 %) (Fig. 2c; Supplementary Table-2B, p < 0.0001).
TAMs actively proliferate when in contact with breast tumor cells
TAMs have been recently shown to proliferate [40], thus we investigated the proliferative status of TAMs across different races. We used double staining IHC (CD163+/Ki-67+) to detect dividing TAMs. Ki-67 is a nuclear protein associated with proliferation [41]; during interphase, the Ki-67 antigen can be exclusively detected within the cell nucleus, whereas in mitosis, most of the protein is relocated to the surface of the chromosomes. Therefore, we characterized proliferating macrophages as (a) CD163+ macrophages expressing Ki-67 in the nucleus in the absence of mitotic figures, suggestive of non-mitotic phases of the cell cycle and (b) CD163+ macrophages with mitotic condensed chromatin, indicative of active proliferation. As shown in Fig. 3a, CD163+ TAMs not adjacent to tumor cells are not Ki-67+. However, TAMs in contact with tumor cells in the TME exhibited Ki-67 staining (Fig. 3b).
Higher proliferative capacity for TAMs in breast tumors from black patients compared to non-black Latino and Caucasian patients
To analyze whether any differences in the proliferative capacity of TAMs could be detected across races, we randomly selected 23 cases representatives of the three racial groups studied and blindly assessed TAMs’ proliferation. The majority of the samples with high TAM proliferation activity (CD163+/Ki-67+ cells with or without mitotic chromatin) were tumors from BL patients, with NBLA and CA showing lower proliferative activity (Fig. 4a, b). Supplementary Table-3A shows the calculated densities of TAMs (total, CD163+ and proliferating, CD163+/Ki-67+), as well as the average numbers of CD163+/Ki-67+ proliferating TAMs in the three races within these 23 cases when all CD163+/Ki-67+ TAMs were considered. Supplementary Table-3B is similar to Table-3A except that it shows only actively dividing TAMs, exclusively expressing Ki-67 in mitotic figures. The calculated densities of total number of TAMs (CD163+) and of total dividing TAMs (CD163+/Ki-67+) in these 23 patients are shown in Fig. 4c and d, respectively; Fig. 4e depicts the proliferating ratios of TAMs across the three races, which are significantly different. When all proliferating TAMs are considered (Supplementary Table-3A), the ratios resulting from dividing the density of proliferating TAMs by the density of total number of TAMs (proliferation ratio) are approximately 1:18 (or 0.05) for NBLA, 1:12 (0.091) for CA and 1:10 (0.095) for BL. When the comparison is done considering only actively dividing TAMs (Supplementary Table-3B), the ratios are 1:56 (0.018) for NBLA, 1:59 (0.017) for CA and 1:23 (0.044) for BL, indicating a significantly higher proliferative capacity for TAMs in tumors from BL than in the other two races (p = 0.0353). Importantly, when actively proliferating TAMs were only considered (Supplementary Table-3B), the average numbers of proliferating TAMs as well as the density of proliferating TAMs were all statistically significantly different across the races.
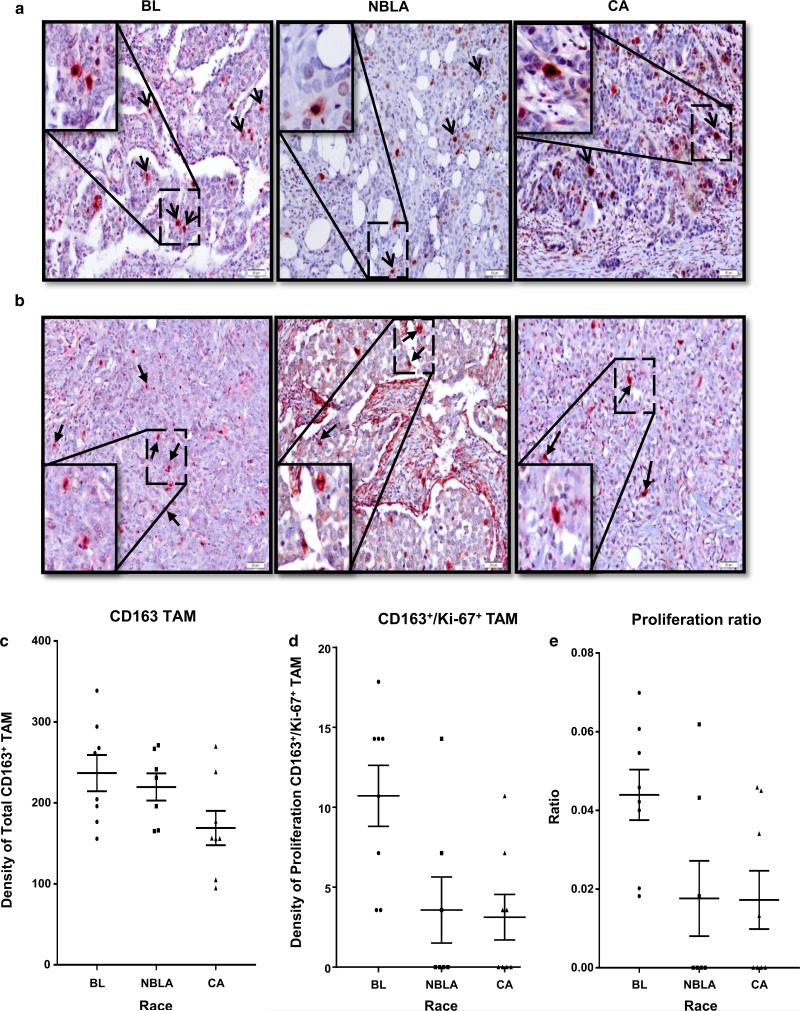
Fig. 4.
Breast cancers from BL patients show the highest numbers of proliferating TAMs. a Double staining (CD163+/Ki-67+) shows proliferative activity in the three racial groups. b Double staining (CD163+/Ki-67+) show proliferative activity with mitotic figures in the three racial groups. c Graph shows the density distribution of total CD163+ TAMs in 23 patients of the three racial groups studied. d Graph shows the density distribution of proliferating CD163+/Ki67+ TAMs in the 23 patients of the three racial groups studied. e TAMs from Black patients exhibit increased proliferating ratios as compared with the other two races
Even within the small group of n = 23 patients used in the TAMs proliferation study, the calculated densities of CD163+ TAMs show distributions similar to those in the large sample of 145 patients (Supplementary Table-2A, p < 0.001), with statistically significant differences across the three racial groups, revealing that CA patients exhibit on average significantly less (mean = 169.0) TAM density than BL (mean = 236.9) (Bonferroni multiple comparison analysis, p = 0.0701) (Supplementary Table-3A, B).
Density of CD163+ TAMs (cells/mm2), average number of cells of CD163/Ki67+, and density of CD163/Ki67+ were summarized by ER, PR, and HER2 status (Supplementary Tables 4–6, respectively). There were no significant differences observed except the mean density of CD163+ (cells/mm2) of ER (p = 0.0269) and PR (p = 0.0722) positive was less than ER and PR negative patients.
Crown-like structures (CLS), like TAMs, are differently expressed in the breast cancer microenvironment across diverse races
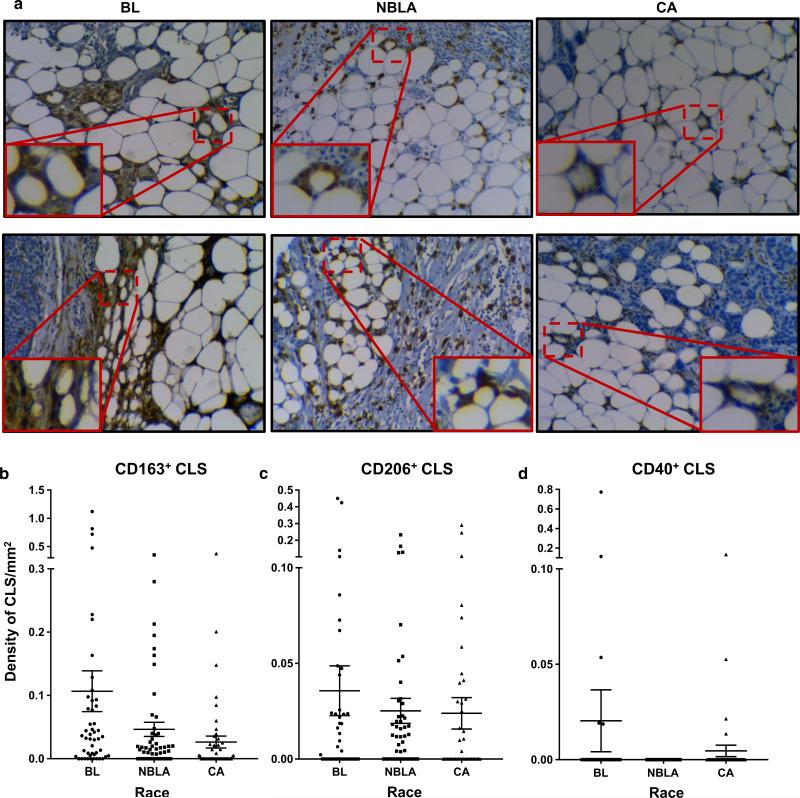
Since the numbers and inflammatory profiles of TAMs present differently in breast cancers across three different racial groups of women, we examined whether macrophages within CLS in the adipose tissues of these tumors would follow a similar expression pattern. To this aim, we inspected the breast adipose tissues in tumors for CD163+ CLS using the same sections analyzed for CD163+ TAMs and calculated the densities of these structures in the different sections. Moreover, the M1 or M2 phenotypes of the macrophages within these CLS were investigated. Supplementary Table-7 reveals that, as expected, the frequency of CLS is much lower than the frequency of TAMs, because CLS are structures only present in the fat tissue of the breast TME, whereas TAMs occur throughout the entire TME. Supplementary Table-7 also shows a signifi-cant difference in the densities of CLS (p = 0.0167) among BL, NBLA, and CA patients, with BL exhibiting significantly higher densities than CA, and NBLA being in between, as happened with TAMs. However, the densities of neither CD40 nor CD206 CLS were significantly different among the three racial groups. Figure 5a shows representative IHC showing staining of CLS (CD163+ macrophages surrounding dying adipocytes) in breast cancers from patients of the three different races. These results are summarized in Fig. 5b, c, and d.
Fig. 5.
Crown-like structure (CLS) occur at significantly higher numbers in breast cancers from Blacks. To calculate the density of CLS, total numbers of CLS present in one slide per tumor sample were counted, and this number was divided by the tumor tissue area (approximately calculated as a rectangle). This was done for CD163, CD206, and CD40 markers in CLS. a IHC results showing CD163 + CLS in the three racial groups (see inset and amplification). b Graph shows density of CD163+ CLS in the three racial groups. c Graph shows density of CD206+ CLS in the three racial groups. d Graph shows density of CD40+ CLS in the three racial groups
Overall survival (OS) and progression-free survival (PFS) analysis
OS and PFS curves were similar across three racial groups (Supplementary Figure 1). However, survival rates for BL patients were lower than NBLA and CA patients for both OS and PFS, and survival rates for NBLA were lower than for CA (Supplementary Table-8).
Surprisingly, densities of CLS with CD40+ macrophages were significant predictors of OS (Table 1). The higher the density of CLS for CD40, the worse the OS was (HR = 12.15; p = 0.058) in univariate analysis, and in bivariate analysis after adjusting the effect of race (HR = 9.14; p = 0.100), PR status (HR = 17.43; p = 0.036), or HER2 status (HR = 13.59; p = 0.047).
Table 1.
Cox regression models for overall survival
| Category | Univariate |
Bivariate |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| With Race |
With ER |
With PR |
With HER2 |
||||||||
| HR (90 % CI) | p | HR (90 % CI) | p | HR (90 % CI) | p | HR (90 % CI) | p | HR (90 % CI) | p | ||
| Qual. Density (CD 163) | 2+/3+ vs 1+ | 1.15 (0.78, 1.70) | 0.549 | 1.04 (0.69, 1.55) | 0.885 | 0.69 (0.43, 1.10) | 0.193 | 0.73 (0.46, 1.14) | 0.248 | 1.07 (0.72, 1.60) | 0.781 |
| Qual. Density(CD206) | 2+/3+ vs 1+ | 1.44 (0.99, 2.09) | 0.111 | 1.27 (0.86, 1.89) | 0.314 | 1.09 (0.72, 1.65) | 0.736 | 1.14 (0.77, 1.71) | 0.584 | 1.38 (0.95, 2.02) | 0.155 |
| Qual. density (CD40) | 2+/1+ vs 0 | 0.97 (0.61, 1.54) | 0.922 | 1.23 (0.73, 2.08) | 0.518 | 0.89 (0.56, 1.41) | 0.680 | 0.90 (0.57, 1.43) | 0.712 | 1.01 (0.63, 1.60) | 0.980 |
| Density of TAMs (CD163) | Density | 1.00 (1.00, 1.00) | 0.195 | 1.00 (1.00, 1.00) | 0.549 | 1.00 (1.00, 1.00) | 0.599 | 1.00 (1.00, 1.00) | 0.740 | 1.00 (1.00, 1.00) | 0.436 |
| Density of CLS (CD163) | Density | 2.97 (0.69, 12.81) | 0.222 | 2.14 (0.46, 9.96) | 0.415 | 1.68 (0.35, 8.01) | 0.584 | 1.99 (0.42, 9.31) | 0.465 | 2.42 (0.54, 10.89) | 0.334 |
| Density of CLS (CD206) | Density | 0.94 (0.05, 17.62) | 0.970 | 0.65 (0.03, 12.58) | 0.809 | 0.32 (0.01, 7.03) | 0.544 | 0.36 (0.02, 7.59) | 0.581 | 0.74 (0.04, 15.55) | 0.872 |
| Density of CLS (CD40) | Density | 12.15 (1.39, 106.49) | 0.058 | 9.14 (1.00, 83.60) | 0.100 | 7.41 (0.80, 68.54) | 0.139 | 17.43 (1.85, 163.75) | 0.036 | 13.59 (1.56, 118.16) | 0.047 |
| ER | Pos vs Neg | 0.52 (0.36, 0.75) | 0.004 | 0.54 (0.37, 0.79) | 0.007 | - | - | - | - | - | - |
| PR | Pos vs Neg | 0.52 (0.36, 0.75) | 0.003 | 0.53 (0.37, 0.78) | 0.006 | - | - | - | - | - | - |
| HER2 | Pos vs Neg | 1.71 (1.00, 2.94) | 0.102 | 1.57 (0.91, 2.72) | 0.177 | - | - | - | - | - | - |
| Ethnicity | BL vs CA | 1.72 (1.07, 2.78) | 0.061 | - | - | - | - | - | - | - | - |
| NBLA vs CA | 1.39 (0.85, 2.26) | 0.267 | - | - | - | - | - | - | - | - | |
To be consisted across models with the same patients, 11 patients with unknown ER or PR were excluded resulted in 134 patients. Bivariate model included two predictors only (predictors in row + predictor in column) and displayed the HR (90 % CI) and p value for row predictor only
HR (90 % CI) hazard ratio and 90% confidence interval
Type-I error rate=10%
The bold values indicate statically significant probabilities
On the other hand, a higher estimated density of M2 TAMs (CD206+) was a predictor for lower PFS (HR = 1.65; p = 0.019) in the univariate model (Table 2). In addition, estimated densities of CD206+ M2 TAMs were significant predictors of PFS after adjusted with race in the bivariate models (HR = 1.55; p = 0.056). Furthermore, a higher density CD206 was associated with worse PFS (HR = 1.59; p = 0.031) after adjusting the effect for HER2 in the bivariate model.
Table 2.
Cox regression models for progression-free survival
| Category | Univariate |
Bivariate |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| With Race |
With ER |
With PR |
With HER2 |
||||||||
| HR (90 % CI) | p | HR (90 % CI) | p | HR (90 % CI) | p | HR (90 % CI) | p | HR (90 % CI) | p | ||
| Qual. density (CD163) | 2+/3+ vs 1+ | 1.20 (0.83, 1.73) | 0.420 | 1.11 (0.76, 1.62) | 0.663 | 0.75 (0.48, 1.16) | 0.273 | 0.81 (0.53, 1.25) | 0.421 | 1.11 (0.76, 1.62) | 0.664 |
| Qual. density (CD206) | 2+/3+ vs 1+ | 1.65 (1.16, 2.35) | 0.019 | 1.55 (1.06, 2.25) | 0.056 | 1.30 (0.88, 1.92) | 0.274 | 1.37 (0.94, 2.01) | 0.170 | 1.59 (1.12, 2.27) | 0.031 |
| Qual. density (CD40) | 2+/1+ vs 0 | 0.89 (0.57, 1.39) | 0.668 | 1.03 (0.62, 1.71) | 0.914 | 0.82 (0.52, 1.28) | 0.455 | 0.84 (0.54, 1.31) | 0.517 | 0.93 (0.59, 1.45) | 0.786 |
| Density of TAMs (CD163) | Density | 1.00 (1.00, 1.00) | 0.161 | 1.00 (1.00, 1.00) | 0.408 | 1.00 (1.00, 1.00) | 0.658 | 1.00 (1.00, 1.00) | 0.919 | 1.00 (1.00, 1.00) | 0.459 |
| Density of CLS (CD163) | Density | 2.88 (0.87, 9.51) | 0.145 | 2.30 (0.66, 8.03) | 0.275 | 1.71 (0.48, 6.12) | 0.486 | 2.12 (0.61, 7.39) | 0.322 | 2.21 (0.65, 7.59) | 0.288 |
| Density of CLS (CD206) | Density | 1.56 (0.13, 18.84) | 0.768 | 1.16 (0.09, 14.28) | 0.924 | 0.56 (0.04, 7.64) | 0.717 | 0.68 (0.05, 8.84) | 0.803 | 1.17 (0.09, 15.35) | 0.922 |
| Density of CLS (CD40) | Density | 5.20 (0.64, 42.25) | 0.196 | 4.12 (0.49, 34.92) | 0.276 | 3.09 (0.36, 26.71) | 0.390 | 6.94 (0.80, 60.40) | 0.141 | 5.87 (0.73, 47.23) | 0.162 |
| ER | Pos vs Neg | 0.52 (0.37, 0.74) | 0.002 | 0.54 (0.38, 0.77) | 0.004 | - | - | - | - | - | - |
| PR | Pos vs Neg | 0.55 (0.39, 0.78) | 0.005 | 0.56 (0.39, 0.80) | 0.007 | - | - | - | - | - | - |
| HER2 | Pos vs Neg | 1.90 (1.15, 3.16) | 0.036 | 1.81 (1.08, 3.02) | 0.057 | - | - | - | - | - | - |
| Ethnicity | BL vs CA | 1.50 (0.97, 2.34) | 0.126 | - | - | - | - | - | - | - | - |
| NBLA vs CA | 1.25 (0.80, 1.96) | 0.411 | - | - | - | - | - | - | - | - | |
To be consisted across models with the same patients, 11 Patients with unknown ER or PR were excluded resulted in 134 patients. Bivariate model included two predictors only (predictors in row + predictor in column) and displayed the HR (90 % CI) and p value for row predictor only
HR (90 % CI) hazard ratio and 90 % confidence interval
The bold values indicate statically significant probabilities
Discussion
Here, we provide evidence that the breast TME of BL women contains significantly higher numbers of TAMs compared to the other two races studied. We show that these are mainly tumor-promoting, M2, immunosuppressive macrophages with higher proliferative capacity, as compared with tumors from NBLA and CA women. Our results offer another potential explanation for the aggressiveness of breast cancers among BL women. To the best of our knowledge, our work is one of the first to report the existence of statistically significant differences in the numbers, densities, activation profiles, and proliferative capacity of TAMs and of CLS among breast cancer patients of different races. We found that tumors were mainly populated by immunosuppressive M2 TAMs (CD206+), as previously reported in other solid malignancies and specifically in breast cancer [42, 43]; M2 TAMs have been associated with tumor invasion, angiogenesis, and metastasis [44–46]. Interestingly, the highest M2 densities were found in tumors from BL women, followed by tumors from NBLA, with CA exhibiting the lowest M2 densities. In contrast, tumors were in general less populated by M1 pro-inflammatory TAMs (CD40+), and interestingly, the highest M1 densities were observed in tumors from CA, followed by tumors from BL, with tumors from NBLA showing the lowest densities of M1 TAMs.
We further showed that tumors from BL patients exhibit significantly higher densities of CLS than those from NBLA, with CA having the lowest densities, paralleling the situation with TAM. Previously, Morris et al. [29] found increased numbers of CLS in breast cancers from obese women. Due to an absence of height information, we were not able to calculate body mass index (BMI). It has been reported that obese individuals have CLS with M1 macrophages in their visceral adipose tissues [27]. In our study, we assessed the inflammatory profiles of macrophages within CLS in the fat portions of the tumor sections, and even though we saw no statistically significant differences across races for both M2 (CD206+) or M1 (CD40+) CLS, we did observe a trend for a prevalence of an M2 immunosuppressive CLS phenotype in all three groups, and a trend that showed a density of CD206+ M2 CLS that decreased from highest in BL to the lowest in CA (BL > NBLA > CA). Therefore, our results suggest that the patients in our study were unlikely to have been obese because a predominance of M2 CLS and not of M1 CLS was observed in all tumors. The high numbers of CLS—and thus, the amount of breast fat inflammation—observed in the breast tumors of BL patients was more likely associated with their biological race rather than potential obesity. Interestingly though, the only tumors showing few M1 CLS were from BL patients. Importantly, the high numbers of CLS in BL parallels the high numbers of immunosuppressive TAMs in the same tumors. Nevertheless, future studies are needed wherein not only races but also particular ethnicities within races and patient's BMI information will be gathered, to corroborate our findings in a larger Florida-based population, as well as the patient population in all states and worldwide.
Macrophages have been considered terminally differentiated cells without proliferative capacity, thought to originate exclusively by replenishment and differentiation from blood monocytes. However, evidence on the diverse ontogeny and proliferative capacity of resident versus inflammatory macrophages has recently emerged [40, 47]. These new data demonstrate the embryonal origin of the majority of tissue resident macrophages with self-renewal capabilities, in contrast to macrophages recruited to the tissues after a pathogenic or damaging insult, which in the majority of the cases seem to differentiate from blood monocytes and lack proliferative capacity [48]. Proliferation of macrophages has now been identified in various settings, such as in nematode-infected tissues, obese adipose tissues, glomerulonephritis, atherosclerosis, AIDS-related dementia, and in a variety of murine tumors, when the resident versus inflammatory origin (ontogeny) of these macrophages is in many cases uncertain [49, 50]. Proliferating macrophages have also been recently recognized in human lymphomas and in breast tumors [51–54].
We identified Ki-67+ nuclei in various stages of the cell cycle in CD163+ TAMs. Interestingly, macrophage proliferation was only observed when they were in direct contact with tumor cells and not when they were in contact with stromal cells of the TME, suggesting that cytokines or other factors produced by tumor cells could be acting in paracrine fashion stimulating macrophage proliferation. Of relevance, when assessing ratios of proliferating vs. total number of TAMs across the three races, our data also strongly demonstrate a significantly higher active (mitotic) proliferation in TAMs from BL tumors compared with TAMs from the other two races.
A recent study on young BL breast cancer patients reports that the prevalence of BRCA mutations among a Florida-based sample of young black women with breast cancer exceeds that previously reported for non-Hispanic white women [55]. However, it is becoming increasingly clear that some of the differences in cancer risk, incidence and survival among individuals of different racial and ethnic backgrounds can be attributed to biological factors other than the inheritance of predisposing tumor suppressor genes [3]. We propose that significant differences in the cellular and molecular components of the TMEs do exist across various races and may contribute to these differences. Future studies are needed to determine if there is a difference in inflammatory gene expression profiles of the TME across these three races with or without obesity. Difference in epigenetic regulation may also exist that contribute to TAM abundance and function. This might also impact the future use of novel immunomodulating agents in patients with different TME and should be considered as potential companion diagnostics in future clinical trials.
In summary, our work demonstrates that breast cancers from BL patients contain significantly higher densities of TAMs and of CLS compared with NBLA and CA. Importantly, numbers of M2 TAMs and M1 CLS are associated with worse survival in all racial groups. Our results also confirm that TAMs do have the capacity to divide in breast cancers, and that they do it when in direct contact with tumor cells. Our work also demonstrates the existence of significant differences in the average numbers and densities of actively proliferating TAMs across races, with TAMs from BL patients proliferating in higher numbers than those in tumors from NBLA and CA. Possibly, significant differences in the cellular and molecular regulation of the TME exist across various races. Further studies are needed to elucidate the particular cytokines, chemokines, and other molecules that predominate in TME of breast cancer patients in the minority race groups. Those molecular differences may account for the particular TAMs’ functional patterns that may contribute to aggressiveness of the tumors. Overall, our findings suggest the potential application of third-generation checkpoint inhibitors [56] to activate macrophage's M1 killing activities in breast cancer immunotherapies, and its possible use as a biological intervention to address breast cancer disparity in minority race groups.
Supplementary Material
Acknowledgments
We would like to extend our thanks to the University of Miami Sylvester Comprehensive Cancer Center's Tissue Bank Core Facility and the Tumor Registry, and particularly to Drs. Consuelo Alvarez and Clara Milikowski without whom this investigation would not have been carried out. This study was funded by the Braman Family Breast Cancer Institute Development Grant from Sylvester Comprehensive Cancer Center at University of Miami Miller School of Medicine and also in part by the NCI/NIH R21CA176055 both to MTK. Research for this article was supported in part by funding to T.A.I from Breast Cancer Research Foundation and Play for P.I.N.K., NIEHS R01-ES024991, Women's Cancer Association of UM and Sylvester Comprehensive Cancer Center; to L.G.S from a supplement to R21CA176055, to ZC from R21CA178675; to MLP from R01CA181115 and to M.P.C. from R01CA157012.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10549-016-3847-3) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Cancer Facts and Figures 2014. American Cancer Society; Atlanta: 2014. [Google Scholar]
- 2.Porter PL, Lund MJ, Lin MG, Yuan X, Liff JM, Flagg EW, Coates RJ, Eley JW. Racial differences in the expression of cell cycle-regulatory proteins in breast carcinoma. Cancer. 2004;100(12):2533–2542. doi: 10.1002/cncr.20279. doi:10.1002/cncr.20279. [DOI] [PubMed] [Google Scholar]
- 3.Henderson BE, Lee NH, Seewaldt V, Shen H. The influence of race and ethnicity on the biology of cancer. Nat Rev Cancer. 2012;12(9):648–653. doi: 10.1038/nrc3341. doi:10.1038/nrc3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kolonel LN, Altshuler D, Henderson BE. The multiethnic cohort study: exploring genes, lifestyle and cancer risk. Nat Rev Cancer. 2004;4(7):519–527. doi: 10.1038/nrc1389. doi:10.1038/nrc1389. [DOI] [PubMed] [Google Scholar]
- 5.Amend K, Hicks D, Ambrosone CB. Breast cancer in African-American women: differences in tumor biology from European-American women. Cancer Res. 2006;66(17):8327–8330. doi: 10.1158/0008-5472.CAN-06-1927. doi:10.1158/0008-5472.CAN-06-1927. [DOI] [PubMed] [Google Scholar]
- 6.Furberg H, Millikan R, Dressler L, Newman B, Geradts J. Tumor characteristics in African American and white women. Breast Cancer Res Treat. 2001;68(1):33–43. doi: 10.1023/a:1017994726207. doi:10.1023/A:1017994726207. [DOI] [PubMed] [Google Scholar]
- 7.Martin DN, Boersma BJ, Yi M, Reimers M, Howe TM, Yfantis HG, Tsai YC, Williams EH, Lee DH, Stephens RM, Weissman AM, Ambs S. Differences in the Tumor Microenvironment between African-American and European-American Breast Cancer Patients. PLoS One. 2009;4(2):e4531. doi: 10.1371/journal.pone.0004531. doi:10.1371/journal.pone.0004531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jatoi I, Becher H, Leake CR. Widening disparity in survival between white and African-American patients with breast carcinoma treated in the U. S. Department of Defense Healthcare system. Cancer. 2003;98(5):894–899. doi: 10.1002/cncr.11604. doi:10.1002/cncr.11604. [DOI] [PubMed] [Google Scholar]
- 9.Newman LA, Griffith KA, Jatoi I, Simon MS, Crowe JP, Colditz GA. Meta-analysis of survival in African American and white American patients with breast cancer: ethnicity compared with socioeconomic status. J Clin Oncol Off J Am Soc Clin Oncol. 2006;24(9):1342–1349. doi: 10.1200/JCO.2005.03.3472. doi:10.1200/JCO.2005.03.3472. [DOI] [PubMed] [Google Scholar]
- 10.Elledge RM, Clark GM, Chamness GC, Osborne CK. Tumor biologic factors and breast cancer prognosis among white, Hispanic, and black women in the United States. J Natl Cancer Inst. 1994;86(9):705–712. doi: 10.1093/jnci/86.9.705. doi:10.1158/0008-5472.CAN-06-1927. [DOI] [PubMed] [Google Scholar]
- 11.Chlebowski RT, Chen Z, Anderson GL, Rohan T, Aragaki A, Lane D, Dolan NC, Paskett ED, McTiernan A, Hubbell FA, Adams-Campbell LL, Prentice R. Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J Natl Cancer Inst. 2005;97(6):439–448. doi: 10.1093/jnci/dji064. doi:10.1093/jnci/dji064. [DOI] [PubMed] [Google Scholar]
- 12.Churpek JE, Walsh T, Zheng Y, Moton Z, Thornton AM, Lee MK, Casadei S, Watts A, Neistadt B, Churpek MM, Huo D, Zvosec C, Liu F, Niu Q, Marquez R, Zhang J, Fackenthal J, King MC, Olopade OI. Inherited predisposition to breast cancer among African American women. Breast Cancer Res Treat. 2015;149(1):31–39. doi: 10.1007/s10549-014-3195-0. doi:10.1007/s10549-014-3195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahmoud SM, Lee AH, Paish EC, Macmillan RD, Ellis IO, Green AR. Tumour-infiltrating macrophages and clinical outcome in breast cancer. J Clin Pathol. 2012;65(2):159–163. doi: 10.1136/jclinpath-2011-200355. doi:10.1136/jclinpath-2011-200355. [DOI] [PubMed] [Google Scholar]
- 14.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196(3):254–265. doi: 10.1002/path.1027. doi:10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 15.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, Torigian DA, O'Dwyer PJ, Vonderheide RH. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331(6024):1612–1616. doi: 10.1126/science.1198443. doi:10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kondo T, Tsunematsu T, Yamada A, Arakaki R, Saito M, Otsuka K, Kujiraoka S, Ushio A, Kurosawa M, Kudo Y, Ishimaru N. Acceleration of tumor growth due to dysfunction in M1 macrophages and enhanced angiogenesis in an animal model of autoimmune disease. Lab Investig J Tech Methods Pathol. 2016;96(4):468–480. doi: 10.1038/labinvest.2015.166. doi:10.1038/labinvest.2015.166. [DOI] [PubMed] [Google Scholar]
- 17.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7(3):211–217. doi: 10.1016/j.ccr.2005.02.013. doi:10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. doi:10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–555. doi: 10.1016/s1471-4906(02)02302-5. doi:10.1016/S1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 20.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4(1):71–78. doi: 10.1038/nrc1256. doi:10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 21.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41(1):49–61. doi: 10.1016/j.immuni.2014.06.010. doi:10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torroella-Kouri M, Ma X, Perry G, Ivanova M, Cejas PJ, Owen JL, Iragavarapu-Charyulu V, Lopez DM. Diminished expression of transcription factors nuclear factor kappaB and CCAAT/enhancer binding protein underlies a novel tumor evasion mechanism affecting macrophages of mammary tumor-bearing mice. Cancer Res. 2005;65(22):10578–10584. doi: 10.1158/0008-5472.CAN-05-0365. doi:10.1158/0008-5472.CAN-05-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torroella-Kouri M, Silvera R, Rodriguez D, Caso R, Shatry A, Opiela S, Ilkovitch D, Schwendener RA, Iragavarapu-Charyulu V, Cardentey Y, Strbo N, Lopez DM. Identification of a subpopulation of macrophages in mammary tumor-bearing mice that are neither M1 nor M2 and are less differentiated. Cancer Res. 2009;69(11):4800–4809. doi: 10.1158/0008-5472.CAN-08-3427. doi:10.1158/0008-5472.CAN-08-3427. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez D, Silvera R, Carrio R, Nadji M, Caso R, Rodriguez G, Iragavarapu-Charyulu V, Torroella-Kouri M. Tumor microenvironment profoundly modifies functional status of macrophages: peritoneal and tumor-associated macrophages are two very different subpopulations. Cell Immunol. 2013;283(1–2):51–60. doi: 10.1016/j.cellimm.2013.06.008. doi:10.1016/j.cellimm.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Torroella-Kouri M, Rodriguez D, Caso R. Alterations in macrophages and monocytes from tumor-bearing mice: evidence of local and systemic immune impairment. Immunol Res. 2013;57(1–3):86–98. doi: 10.1007/s12026-013-8438-3. doi:10.1007/s12026-013-8438-3. [DOI] [PubMed] [Google Scholar]
- 26.Yuan A, Hsiao YJ, Chen HY, Chen HW, Ho CC, Chen YY, Liu YC, Hong TH, Yu SL, Chen JJ, Yang PC. Opposite effects of M1 and M2 macrophage subtypes on lung cancer progression. Sci Rep. 2015;5:14273. doi: 10.1038/srep14273. doi:10.1038/srep14273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Investig. 2007;117(1):175–184. doi: 10.1172/JCI29881. doi:10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46(11):2347–2355. doi: 10.1194/jlr.M500294-JLR200. doi:10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Morris PG, Hudis CA, Giri D, Morrow M, Falcone DJ, Zhou XK, Du B, Brogi E, Crawford CB, Kopelovich L, Subbaramaiah K, Dannenberg AJ. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res (Phila) 2011;4(7):1021–1029. doi: 10.1158/1940-6207.CAPR-11-0110. doi:10.1158/1940-6207.CAPR-11-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subbaramaiah K, Howe LR, Bhardwaj P, Du B, Gravaghi C, Yantiss RK, Zhou XK, Blaho VA, Hla T, Yang P, Kopelovich L, Hudis CA, Dannenberg AJ. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res (Phila) 2011;4(3):329–346. doi: 10.1158/1940-6207.CAPR-10-0381. doi:10.1158/1940-6207.CAPR-10-0381. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Santander AM, Lopez-Ocejo O, Casas O, Agostini T, Sanchez L, Lamas-Basulto E, Carrio R, Cleary MP, Gonzalez-Perez RR, Torroella-Kouri M. Paracrine interactions between adipocytes and tumor cells recruit and modify macrophages to the mammary tumor microenvironment: the role of obesity and inflammation in breast adipose tissue. Cancers. 2015;7(1):143–178. doi: 10.3390/cancers7010143. doi:10.3390/cancers7010143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nelson LR, Bulun SE. Estrogen production and action. J Am Acad Dermatol. 2001;45(3 Suppl):S116–S124. doi: 10.1067/mjd.2001.117432. doi:10.1067/mjd.2001.117432. [DOI] [PubMed] [Google Scholar]
- 33.Fanelli MA, Vargas-Roig LM, Gago FE, Tello O, Lucero De Angelis R, Ciocca DR. Estrogen receptors, progesterone receptors, and cell proliferation in human breast cancer. Breast Cancer Res Treat. 1996;37(3):217–228. doi: 10.1007/BF01806503. doi:10.1007/BF01806503. [DOI] [PubMed] [Google Scholar]
- 34.Tan H, Zhong Y, Pan Z. Autocrine regulation of cell proliferation by estrogen receptor-alpha in estrogen receptor-alpha-positive breast cancer cell lines. BMC Cancer. 2009;9:31. doi: 10.1186/1471-2407-9-31. doi:10.1186/1471-2407-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liao XH, Lu DL, Wang N, Liu LY, Wang Y, Li YQ, Yan TB, Sun XG, Hu P, Zhang TC. Estrogen receptor alpha mediates proliferation of breast cancer MCF-7 cells via a p21/PCNA/E2F1-dependent pathway. FEBS J. 2014;281(3):927–942. doi: 10.1111/febs.12658. doi:10.1111/febs.12658. [DOI] [PubMed] [Google Scholar]
- 36.Carrio R, Koru-Sengul T, Miao F, Gluck S, Lopez O, Selman Y, Alvarez C, Milikowski C, Gomez C, Jorda M, Nadji M, Torroella-Kouri M. Macrophages as independent prognostic factors in small T1 breast cancers. Oncol Rep. 2013;29(1):141–148. doi: 10.3892/or.2012.2088. doi:10.3892/or.2012.2088. [DOI] [PubMed] [Google Scholar]
- 37.Pettersen JS, Fuentes-Duculan J, Suarez-Farinas M, Pierson KC, Pitts-Kiefer A, Fan L, Belkin DA, Wang CQ, Bhuvanendran S, Johnson-Huang LM, Bluth MJ, Krueger JG, Lowes MA, Carucci JA. Tumor-associated macrophages in the cutaneous SCC microenvironment are heterogeneously activated. J Invest Dermatol. 2011;131(6):1322–1330. doi: 10.103/jid.2011.9. doi:10.103/jid.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shabo I, Stal O, Olsson H, Dore S, Svanvik J. Breast cancer expression of CD163, a macrophage scavenger receptor, is related to early distant recurrence and reduced patient survival. Int J Cancer J Int du Cancer. 2008;123(4):780–786. doi: 10.1002/ijc.23527. doi:10.1002/ijc.23527. [DOI] [PubMed] [Google Scholar]
- 39.Lau SK, Chu PG, Weiss LM. CD163: a specific marker of macrophages in paraffin-embedded tissue samples. Am J Clin Pathol. 2004;122(5):794–801. doi: 10.1309/QHD6-YFN8-1KQX-UUH6. doi:10.1309/QHD6-YFN8-1KQX-UUH6. [DOI] [PubMed] [Google Scholar]
- 40.Lahmar Q, Keirsse J, Laoui D, Movahedi K, Van Overmeire E, Van Ginderachter JA. Tissue-resident versus monocyte-derived macrophages in the tumor microenvironment. Biochim Biophys Acta 1865. 2016;1:23–34. doi: 10.1016/j.bbcan.2015.06.009. doi:10.1016/j.bbcan.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 41.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182(3):311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. doi:10.1002/(SICI)1097-4652(200003)182:3\311:AID-JCP1[3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y, Cheng S, Zhang M, Zhen L, Pang D, Zhang Q, Li Z. High-infiltration of tumor-associated macrophages predicts unfavorable clinical outcome for node-negative breast cancer. PLoS One. 2013;8(9):e76147. doi: 10.1371/journal.pone.0076147. doi:10.1371/journal.pone.0076147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Medrek C, Ponten F, Jirstrom K, Leandersson K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer. 2012;12:306. doi: 10.1186/1471-2407-12-306. doi:10.1186/1471-2407-12-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qian BZ, Zhang H, Li J, He T, Yeo EJ, Soong DY, Carragher NO, Munro A, Chang A, Bresnick AR, Lang RA, Pollard JW. FLT1 signaling in metastasis-associated macrophages activates an inflammatory signature that promotes breast cancer metastasis. J Exp Med. 2015;212(9):1433–1448. doi: 10.1084/jem.20141555. doi:10.1084/jem.20141555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pollard JW. Macrophages define the invasive microenvironment in breast cancer. J Leukoc Biol. 2008;84(3):623–630. doi: 10.1189/jlb.1107762. doi:10.1189/jlb.1107762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mantovani A, Locati M. Tumor-associated macrophages as a paradigm of macrophage plasticity, diversity, and polarization: lessons and open questions. Arterioscler Thromb Vasc Biol. 2013;33(7):1478–1483. doi: 10.1161/ATVBAHA.113.300168. doi:10.1161/ATVBAHA.113.300168. [DOI] [PubMed] [Google Scholar]
- 47.Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, Becker CD, See P, Price J, Lucas D, Greter M, Mortha A, Boyer SW, Forsberg EC, Tanaka M, van Rooijen N, Garcia-Sastre A, Stanley ER, Ginhoux F, Frenette PS, Merad M. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38(4):792–804. doi: 10.1016/j.immuni.2013.04.004. doi:10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gordon S, Pluddemann A, Martinez Estrada F. Macrophage heterogeneity in tissues: phenotypic diversity and functions. Immunol Rev. 2014;262(1):36–55. doi: 10.1111/imr.12223. doi:10.1111/imr.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng C, Yang Q, Cao J, Xie N, Liu K, Shou P, Qian F, Wang Y, Shi Y. Local proliferation initiates macrophage accumulation in adipose tissue during obesity. Cell Death Dis. 2016;7:e2167. doi: 10.1038/cddis.2016.54. doi:10.1038/cddis.2016.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lindau A, Hardtner C, Hergeth SP, Blanz KD, Dufner B, Hoppe N, Anto-Michel N, Kornemann J, Zou J, Gerhardt LM, Heidt T, Willecke F, Geis S, Stachon P, Wolf D, Libby P, Swirski FK, Robbins CS, McPheat W, Hawley S, Braddock M, Gilsbach R, Hein L, von Zur Muhlen C, Bode C, Zirlik A, Hilgendorf I. Atheroprotection through SYK inhibition fails in established disease when local macrophage proliferation dominates lesion progression. Basic Res Cardiol. 2016;111(2):20. doi: 10.1007/s00395-016-0535-8. doi:10.1007/s00395-016-0535-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang W, He KF, Yang JG, Ren JG, Sun YF, Zhao JH, Zhao YF. Infiltration of M2-polarized macrophages in infected lymphatic malformations: possible role in disease progression. Br J Dermatol. 2016 doi: 10.1111/bjd.14471. doi:10.1111/bjd.14471. [DOI] [PubMed] [Google Scholar]
- 52.Campbell MJ, Tonlaar NY, Garwood ER, Huo D, Moore DH, Khramtsov AI, Au A, Baehner F, Chen Y, Malaka DO, Lin A, Adeyanju OO, Li S, Gong C, McGrath M, Olopade OI, Esserman LJ. Proliferating macrophages associated with high grade, hormone receptor negative breast cancer and poor clinical outcome. Breast Cancer Res Treat. 2011;128(3):703–711. doi: 10.1007/s10549-010-1154-y. doi:10.1007/s10549-010-1154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Campbell MJ, Wolf D, Mukhtar RA, Tandon V, Yau C, Au A, Baehner F, van't Veer L, Berry D, Esserman LJ. The prognostic implications of macrophages expressing proliferating cell nuclear antigen in breast cancer depend on immune context. PLoS One. 2013;8(10):e79114. doi: 10.1371/journal.pone.0079114. doi:10.1371/journal.pone.0079114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mukhtar RA, Moore AP, Tandon VJ, Nseyo O, Twomey P, Adisa CA, Eleweke N, Au A, Baehner FL, Moore DH, McGrath MS, Olopade OI, Gray JW, Campbell MJ, Esserman LJ. Elevated levels of proliferating and recently migrated tumor-associated macrophages confer increased aggressiveness and worse outcomes in breast cancer. Ann Surg Oncol. 2012;19(12):3979–3986. doi: 10.1245/s10434-012-2415-2. doi:10.1245/s10434-012-2415-2. [DOI] [PubMed] [Google Scholar]
- 55.Pal T, Bonner D, Kim J, Monteiro AN, Kessler L, Royer R, Narod SA, Vadaparampil ST. Early onset breast cancer in a registry-based sample of African-american women: BRCA mutation prevalence, and other personal and system-level clinical characteristics. Breast J. 2013;19(2):189–192. doi: 10.1111/tbj.12083. doi:10.1111/tbj.12083. [DOI] [PubMed] [Google Scholar]
- 56.Kong F, Gao F, Li H, Liu H, Zhang Y, Zheng R, Chen J, Li X, Liu G, Jia Y. CD47: a potential immunotherapy target for eliminating cancer cells. Clin Transl Oncol Off Publ Fed Spanish Oncol Soc Natl Cancer Inst Mexico. 2016 doi: 10.1007/s12094-016-1489-x. doi:10.1007/s12094-016-1489-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.