Abstract
Objective
To identify an early response criterion for predicting ≥5% weight loss with liraglutide 3.0 mg at week 56 and to compare efficacy outcomes in early responders (ERs) and early nonresponders (ENRs).
Methods
Using pooled data from the SCALE Obesity and Prediabetes and SCALE Diabetes trials, weight loss of ≥4% at 16 weeks best predicted ≥5% weight loss after 56 weeks. Weight loss and changes in cardiometabolic risk factors and health‐related quality of life were evaluated in ERs (≥4% weight loss at week 16) and ENRs (<4% weight loss at week 16) completing 56 weeks’ treatment.
Results
Proportions of ERs/ENRs to liraglutide 3.0 mg were 77.3%/22.7% (individuals without type 2 diabetes, T2D) and 62.7%/37.3% (those with T2D). Greater mean weight loss was observed in ERs versus ENRs: 10.8% versus 3.0% (without T2D) and 8.5% versus 3.1% (T2D). In both trials, greater proportions of ERs versus ENRs achieved ≥5%, >10%, and >15% weight loss at week 56 with liraglutide 3.0 mg. Greater improvements in cardiometabolic risk factors and health‐related quality of life scores were observed in ERs versus ENRs.
Conclusions
The early response criterion was clinically useful to identify individuals who would achieve clinically meaningful weight loss at 56 weeks.
Introduction
Managing obesity with pharmacotherapy plus lifestyle intervention can help increase the proportion of people reaching ≥5% weight loss, the regulatory benchmark for clinically meaningful weight loss 1, 2, but use of pharmacotherapy must be balanced against potential adverse effects of treatment and costs. One strategy to increase benefit versus risk in obesity pharmacotherapy is through identification of long‐term weight loss predictors. By stopping drug therapy early in patients unlikely to achieve clinical benefit, clinicians can minimize drug exposure, improve the benefit:risk ratio for the patient 3, and use health resources more effectively. Early weight loss, whether through lifestyle 4, 5, 6, 7 or pharmacotherapy 8, 9, 10, 11, is a good predictor of long‐term weight loss. Indeed, all recently approved weight loss medication labels include “stopping rules” stating when pharmacotherapy should be discontinued if clinically relevant weight loss is not, or is unlikely to be, achieved. However, current labels provide little information on outcomes in those individuals eligible for continued treatment beyond the early milestone; reported results are for all randomized individuals.
Liraglutide is a glucagon‐like peptide‐1 analog with 97% homology to human glucagon‐like peptide‐1, a physiological regulator of appetite 12, 13. Liraglutide at doses up to 1.8 mg once daily (Victoza®, Novo Nordisk, Bagsvaerd, Denmark) has been licensed for glycemic control in type 2 diabetes (T2D) since 2009. More recently, liraglutide 3.0 mg (Saxenda®; Novo Nordisk), as an adjunct to a reduced‐calorie diet and increased physical activity, has been approved for weight management in the USA, EU, and elsewhere.
This article describes how the early treatment criterion that best predicts ≥5% weight loss with liraglutide 3.0 mg at week 56 was identified, based on data from the two largest trials in the SCALE program of phase 3a trials of liraglutide 3.0 mg for weight management. Post hoc analyses are presented of the efficacy and safety results from these trials by early responder status, using this early response criterion.
Methods
Trial design
The design, methods, patient populations, and results of the SCALE Obesity and Prediabetes (NCT01272219) and SCALE Diabetes (NCT01272232) trials were previously published 14, 15. Briefly, in SCALE Obesity and Prediabetes, 3,731 individuals with overweight or obesity and without diabetes (body mass index [BMI] ≥30 kg/m2 or ≥27 kg/m2 with ≥1 obesity‐related comorbidity) were randomized 2:1 to liraglutide 3.0 mg or placebo for 56 weeks 14. In SCALE Diabetes, 846 individuals with BMI ≥27 kg/m2 and T2D were randomized 2:1:1 to liraglutide 3.0 mg, liraglutide 1.8 mg, or placebo for 56 weeks 15. Both trials were double‐blind, placebo‐controlled, multicenter trials, and trial drug was given as adjunct to lifestyle intervention (500 kcal/day deficit diet and physical activity ≥150 min/week). Here, only results for liraglutide 3.0 mg and placebo are reported, referred to as “liraglutide 3.0 mg” or “placebo” hereafter. Liraglutide was initiated at a dose of 0.6 mg and dose‐escalated by 0.6 mg increments weekly to the 3.0 mg treatment dose. This was a forced dose escalation, with the dose at 3.0 mg for all individuals by week 4; investigators could delay dose escalation by 7 days in total.
Determination of early response criterion
We used pooled data from SCALE Obesity and Prediabetes and SCALE Diabetes to determine the optimal treatment time point and weight loss threshold for identifying subjects likely to achieve ≥5% loss of initial body weight after 56 weeks’ treatment. Given the objective of the analysis (predictive value for 1‐year weight loss), only trials of minimum 1‐year duration were eligible. Two of the four phase 3a trials were excluded: SCALE Sleep Apnea because it was a 32‐week trial and SCALE Maintenance because it required individuals to lose ≥5% weight through diet and exercise before randomization; thus their initial weight loss after randomization would not have represented general practice. The pooled analysis was predefined with the FDA before individual trial data became available. For mean weight loss in Table 1, only subjects with body weight measurements at baseline and the specific time point being analyzed (8, 12, 16 weeks) and week 56 contributed to the analysis. For identifying individuals with ≥5% weight loss at week 56, missing data were imputed using last observation carried forward (LOCF). Reasons for choosing these time points are in the Supporting Information.
Table 1.
Positive and negative predictive values for achieving ≥5% weight loss with liraglutide 3.0 mg at 56 weeks using different early response criteria: pooled analysis of the SCALE Obesity and Prediabetes and SCALE Diabetes trials
| Week | Early response criterion (%) | N | Early response, n (%) | Mean week 56 weight change (%) in early responders | Positive predictive value, n (%)a | Early nonresponse, n (%) | Mean week 56 weight change (%) in early nonresponders | Negative predictive value, n (%)b | Correctly predicted, n (%)c |
|---|---|---|---|---|---|---|---|---|---|
| 8 | 3 | 2,653 | 2,035 (76.7) | −10.09 | 1,544 (75.9) | 618 (23.3) | −3.69 | 439 (71.0) | 1,983 (74.7) |
| 4 | 1,644 (62.0) | −11.00 | 1,373 (83.5) | 1,009 (38.0) | −4.53 | 659 (65.3) | 2,032 (76.6) | ||
| 5 | 1,245 (46.9) | −12.10 | 1,096 (88.0) | 1,408 (53.1) | −5.46 | 781 (55.5) | 1,877 (70.8) | ||
| 12 | 3 | 2,578 | 2,084 (80.8) | −9.98 | 1,601 (76.8) | 494 (19.2) | −2.79 | 389 (78.7) | 1,990 (77.2) |
| 4 | 1,821 (70.6) | −10.68 | 1,485 (81.5) | 757 (29.4) | −3.56 | 536 (70.8) | 2,021 (78.4) | ||
| 5 | 1,515 (58.8) | −11.55 | 1,312 (86.6) | 1,063 (41.2) | −4.37 | 669 (62.9) | 1,981 (76.8) | ||
| 16 | 3 | 2,519 | 2,099 (83.3) | −9.95 | 1,609 (76.7) | 420 (16.7) | −2.29 | 338 (80.5) | 1,947 (77.3) |
| 4 | 1,893 (75.1) | −10.46 | 1,541 (81.4) | 626 (24.9) | −3.02 | 476 (76.0) | 2,017 (80.1) | ||
| 5 | 1,637 (65.0) | −11.21 | 1,411 (86.2) | 882 (35.0) | −3.78 | 602 (68.3) | 2,013 (79.9) |
Week 56 response is defined as at least a 5% reduction in body weight. If data were missing for week 56, the last available body weight measurement was used (i.e., missing data were imputed using last observation carried forward). Mean weight loss is based on observed data only.
Positive predictive value is defined as the percentage of early responders who were week 56 responders.
Negative predictive value is defined as the percentage of early nonresponders who were week 56 nonresponders.
Correctly predicted proportion = (number of correctly predicted week 56 responders + number of correctly predicted week 56 nonresponders)/(total number of subjects).
The ability to predict response status after 56 weeks was evaluated by the positive predictive value (PPV; i.e., proportion of subjects with an early response who had ≥5% weight loss after 56 weeks) and the negative predictive value (NPV; i.e., proportion of subjects with an early nonresponse who had <5% weight loss after 56 weeks) for ≥3%, 4%, and 5% weight loss at 8, 12, and 16 weeks. The proportions of “correctly predicted overall” were calculated from PPV and NPV (Table 1). The sensitivity and specificity of these criteria were also evaluated (Supporting Information).
The pooled analysis was repeated for male and female subjects, and the results were also analyzed separately by trial, to ensure that the identified cut point for defining early response would be valid for both sexes and for subjects with or without T2D. A sensitivity analysis was also performed in which missing week 56 responses were imputed as nonresponse, rather than using LOCF.
Post hoc analysis of end points by early response status
Once the optimal early response criterion was identified, subjects were classified as early responders (ERs) or early nonresponders (ENRs). We then assessed efficacy outcomes at week 56 for ERs and ENRs, based on individuals who completed 56 weeks’ treatment. Weight end points were mean change in body weight from baseline and the proportion of patients with a weight loss of ≥5%, >10%, and >15% at week 56. Secondary efficacy outcomes were the changes from baseline to week 56 in HbA1c, fasting plasma glucose, systolic (SBP) and diastolic blood pressure (DBP), BMI, waist circumference, heart rate, fasting lipid profile, and various additional cardiometabolic biomarkers. Changes in the following health‐related quality of life (HRQoL) scores were also evaluated: Impact of Weight on Quality of Life‐Lite (IWQOL‐Lite) total score and physical function score 16 (both trials); and Short Form‐36 (SF‐36) physical component summary score 17 (SCALE Obesity and Prediabetes only).
Efficacy outcomes are reported for ERs and ENRs who completed 56 weeks’ treatment. Safety is reported based on the safety analysis set for ERs and ENRs (i.e., all subjects with data at week 16, regardless of whether they completed 56 weeks’ treatment) (Figure 1). The results are reported by trial for ERs and ENRs to liraglutide 3.0 mg or placebo so that any potential differences in clinical outcomes in patients with T2D would not be masked due to the relatively small size of this study population.
Figure 1.
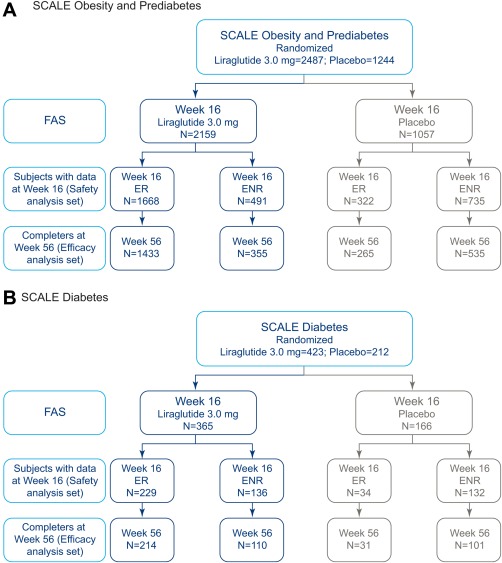
Subject disposition for results of post hoc analyses. Early responders (ERs), individuals who achieved ≥4% weight loss from baseline at 16 weeks; early nonresponders (ENRs), individuals who achieved <4% weight loss from baseline at 16 weeks. Full analysis set (FAS): in order to be defined as ER/ENR at week 16, individuals had to have a fasting body weight measurement at baseline and the week 16 visit. Completers at week 56 were individuals with a body weight measurement at week 56. Only individuals satisfying the respective criteria are shown. [Color figure can be viewed at wileyonlinelibrary.com]
Statistical analysis
For the pooled analysis to assess the optimal response criterion, no covariate adjustments were made. Missing response status after 56 weeks was imputed using LOCF. The observed mean weight loss was plotted during the course of the trial (Figure 2).
Figure 2.
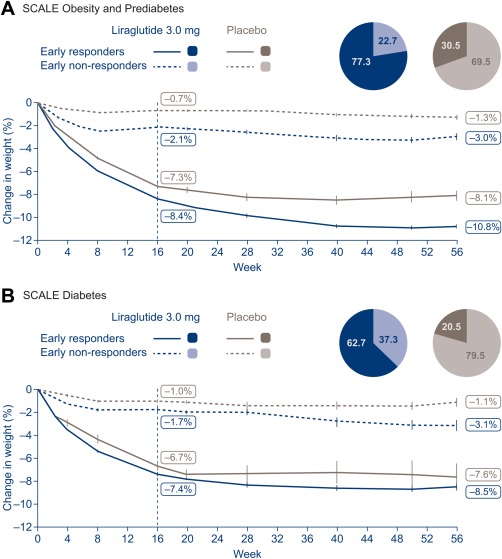
Fasting body weight loss by early responder status. Line graphs are observed means (±SE) for ERs/ENRs who completed 56 weeks. Fasting visit data only. ERs, early responders (individuals who achieved ≥4% weight loss from baseline at 16 weeks); ENRs, early nonresponders (individuals who achieved <4% weight loss from baseline at 16 weeks); SE, standard error. Note that ENRs continued on treatment until week 56; in clinical practice, treatment would cease for ENRs in line with the stopping rule.
Analyses of outcomes at week 56 were performed in trial completers split by ER and ENR status. The analyses of outcomes used the same model as in the individual trials 14, 15. The model included treatment, country, sex, and interaction between BMI strata and prediabetes status as fixed effects, with baseline body weight as covariate. In the SCALE Diabetes ANCOVA model, BMI stratification and prediabetes status was replaced by baseline HbA1c stratification and background medication. Continuous variables were estimated using ANCOVA, as described above; categorical variables were estimated using a logistic regression model with the same fixed effects and covariates as the relevant ANCOVA. Efficacy data are estimated means or estimated proportions; safety data are observed proportions or observed means.
Statistical analyses were conducted using SAS version 9.3 (SAS Institute Inc., Cary, NC). As ERs and ENRs are not randomized populations, differences between them were not quantified or analyzed statistically.
Results
Subject disposition by trial for the individuals covered in these analyses (i.e., those with body weight measurement at baseline and weeks 16 and 56) is shown in Figure 1.
Optimal early response criterion for ≥5% weight loss after 56 weeks
The proportion of subjects treated with liraglutide 3.0 mg who lost ≥3%, ≥4%, or ≥5% weight at 8, 12, and 16 weeks in the pooled analysis of SCALE Obesity and Prediabetes and SCALE Diabetes and associated PPVs and NPVs are shown in Table 1. The sensitivity and specificity of each criterion were also calculated (Supporting Information Table S1).
The analyses showed that ≥4% weight loss at 16 weeks yielded the highest correctly predicted value (80.1%), consistent with high values for both PPV (81.4%) and NPV (76.0%) (Table 1). The criteria based on the 8‐week time point were associated with lower overall correctly predicted values and, in particular, low NPVs, meaning that treatment would have been incorrectly discontinued in a notable number of individuals who would have gone on to achieve ≥5% weight loss after 56 weeks. For example, using NPV for 4% weight loss at 8 weeks, 34.7% (350 of 1,009 subjects identified as ENRs) would have been incorrectly discontinued, versus 24% (150 of 626 subjects) using the 16‐week value (Table 1). The 12‐week time point criteria had PPVs similar to those at the 16‐week time point but comparatively lower NPVs (Table 1). Thus, using NPV for 4% weight loss at 12 weeks, 29.2% (221 of 757 subjects identified as ENRs) would have been incorrectly discontinued, versus 24% (150 of 626 subjects) using the 16‐week value (Table 1). Furthermore, the choice of week 16 meant that individuals would have received treatment‐dose liraglutide 3.0 mg for 12 weeks, consistent with the exposure period generally recommended for other antiobesity medications.
Consistent results and conclusions were reached when the pooled analysis was conducted separately for males and females, or for each trial, and from a sensitivity analysis with missing week 56 responses imputed as nonresponse (Supporting Information Tables S2‐S4).
A separate analysis showed that ≥4% weight loss at 16 weeks was also a good criterion for predicting weight loss with placebo, yielding a high overall correctly predicted value (80.0%), consistent with a reasonably high PPV (66.1%) and NPV (85.8%) (Supporting Information Table S5).
Early responder populations
Among individuals on liraglutide 3.0 mg with a week 16 measurement, 77.3% without T2D and 62.7% with T2D were ERs, and 22.7% and 37.3% were ENRs. The proportions of individuals who were ERs to placebo were much lower than with liraglutide 3.0 mg: 30.5% of individuals without T2D and 20.5% with T2D.
Demographic and other baseline characteristics of all randomized subjects, as well as ERs and ENRs in each trial, are shown in Tables 2 and 3. The “all‐randomized” group included individuals who discontinued the trial before week 16, while the ER/ENR groups did not (by definition they could not be classified as ER or ENR).
Table 2.
Demographics and baseline characteristics of patients by early responder status (SCALE Obesity and Prediabetes trial)
| Liraglutide 3.0 mg | Placebo | |||||
|---|---|---|---|---|---|---|
| All randomized (N = 2,487) | Early responders (N = 1,433) | Early nonresponders (N = 355) | All randomized (N = 1,244) | Early responders (N = 265) | Early nonresponders (N = 535) | |
| Female sex, n (%) | 1957 (78.7) | 1151 (80.3) | 251 (70.7) | 971 (78.1) | 205 (77.4) | 415 (77.6) |
| Mean age, years [SD] | 45.2 [12.1] | 46.4 [11.6] | 45.4 [11.8] | 45.0 [12.0] | 47.0 [11.7] | 45.6 [12.1] |
| Race, n (%) | ||||||
| White | 2107 (84.7) | 1238 (86.4) | 289 (81.4) | 1061 (85.3) | 240 (90.6) | 455 (85.0) |
| Black/African‐American | 242 (9.7) | 126 (8.8) | 41 (11.5) | 114 (9.2) | 15 (5.7) | 51 (9.5) |
| Other | 138 (5.5) | 69 (4.8) | 25 (7.0) | 69 (5.5) | 10 (3.8) | 29 (5.4) |
| Ethnicity, n (%) | ||||||
| Hispanic/Latino | 259 (10.4) | 126 (8.8) | 31 (8.7) | 134 (10.8) | 31 (11.7) | 46 (8.6) |
| Non‐Hispanic/Latino | 2228 (89.6) | 1307 (91.2) | 324 (91.3) | 1110 (89.2) | 234 (88.3) | 489 (91.4) |
| Mean weight, kg [SD] | 106.2 [21.2] | 105.3 [20.4] | 109.8 [23.7] | 106.2 [21.7] | 107.4 [23.6] | 106.7 [22.3] |
| Mean BMI, kg/m2 [SD] | 38.3 [6.4] | 38.1 [6.3] | 38.9 [6.8] | 38.3 [6.3] | 38.7 [7.1] | 38.3 [6.4] |
| Glycemic status, n (%) | ||||||
| Normoglycemic | 959 (38.6) | 528 (36.8) | 151 (42.5) | 487 (39.1) | 100 (37.7) | 196 (36.6) |
| With prediabetes | 1528 (61.4) | 905 (63.2) | 204 (57.5) | 757 (60.9) | 165 (62.3) | 339 (63.4) |
| Mean HbA1c, % points [SD] | 5.6 [0.4] | 5.6 [0.4] | 5.6 [0.4] | 5.6 [0.4] | 5.6 [0.4] | 5.6 [0.4] |
| Mean FPG, mg/dL [SD] | 95.9 [10.6] | 96.1 [10.4] | 97.2 [11.3] | 95.5 [9.8] | 95.7 [9.2] | 95.9 [9.9] |
Early responders, individuals who achieved ≥4% weight loss from baseline at 16 weeks; early nonresponders, individuals who achieved <4% weight loss from baseline at 16 weeks. Based on individuals with a fasting body weight measurement at baseline and week 16 and who completed 56 weeks of treatment. “All randomized” refers to all randomized patients in the overall trial.
BMI, body mass index; FPG, fasting plasma glucose; SD, standard deviation.
Table 3.
Demographics and baseline characteristics of patients by early responder status (SCALE Diabetes trial)
| Liraglutide 3.0 mg | Placebo | |||||
|---|---|---|---|---|---|---|
| All randomized (N = 423) | Early responders (N = 214) | Early nonresponders (N = 110) | All randomized (N = 212) | Early responders (N = 31) | Early nonresponders (N = 101) | |
| Female sex, n (%) | 203 (48.0) | 112 (52.3) | 43 (39.1) | 115 (54.2) | 15 (48.4) | 61 (60.4) |
| Mean age, years [SD] | 55.0 [10.8] | 55.5 [10.1] | 54.1 [9.8] | 54.7 [9.8] | 57.9 [9.4] | 55.7 [8.8] |
| Race, n (%) | ||||||
| White | 353 (83.5) | 184 (86.0) | 86 (78.2) | 175 (82.5) | 27 (87.1) | 83 (82.2) |
| Black/African‐American | 44 (10.4) | 16 (7.5) | 14 (12.7) | 27 (12.7) | 3 (9.7) | 13 (12.9) |
| Other | 26 (6.1) | 14 (6.5) | 10 (9.1) | 10 (4.7) | 1 (3.2) | 5 (5.0) |
| Ethnicity, n (%) | ||||||
| Hispanic/Latino | 46 (10.9) | 19 (8.9) | 15 (13.6) | 24 (11.3) | 3 (9.7) | 9 (8.9) |
| Non‐Hispanic/Latino | 375 (88.7) | 193 (90.2) | 95 (86.4) | 187 (88.2) | 28 (90.3) | 92 (91.1) |
| Mean weight, kg [SD] | 105.7 [21.9] | 106.8 [21.3] | 102.8 [19.5] | 106.5 [21.3] | 109.3 [18.6] | 104.7 [22.1] |
| Mean BMI, kg/m2 [SD] | 37.1 [6.5] | 37.7 [6.5] | 36.1 [6.3] | 37.4 [7.1] | 37.7 [5.7] | 37.4 [7.6] |
| Mean HbA1c, % points [SD] | 7.9 [0.8] | 7.9 [0.8] | 8.0 [0.8] | 7.9 [0.8] | 7.6 [0.5] | 7.8 [0.7] |
| Mean FPG, mg/dL [SD] a | 158.4 [32.8] | 158.5 [34.8] | 157.7 [27.9] | 155.5 [33.0] | 151.4 [33.0] | 149.6 [30.0] |
Overall values are based on the full analysis set (N = 407 and 211, respectively).
Early responders, individuals who achieved ≥4% weight loss from baseline at 16 weeks; early nonresponders, individuals who achieved <4% weight loss from baseline at 16 weeks. Based on individuals with a fasting body weight measurement at baseline and week 16 and who completed 56 weeks of treatment. “All randomized” refers to all randomized patients in the overall trial.
BMI, body mass index; FPG, fasting plasma glucose; SD, standard deviation.
In general, baseline characteristics in the ER and ENR groups appeared similar across treatment groups within each trial. In both liraglutide 3.0 mg and placebo groups, individuals of White origin appeared more prevalent in the ER versus ENR groups, and female sex appeared consistently associated with early response to liraglutide 3.0 mg (see percentages, Supporting Information Table S3) but not to placebo in individuals with and without T2D.
Weight loss at week 56 in ERs and ENRs
In SCALE Obesity and Prediabetes, weight loss in ERs to liraglutide 3.0 mg was 10.8% (11.2 kg) versus 3.0% (3.2 kg) for ENRs (Figure 2A). Similarly, in SCALE Diabetes, ERs had a greater mean weight loss than ENRs (8.5% [9.0 kg] vs. 3.1% [3.2 kg]) at 56 weeks (Figure 2B). ERs to placebo also achieved greater weight loss than ENRs to placebo (Figure 2).
The proportions of ERs and ENRs achieving ≥5%, >10%, and >15% weight loss at week 56 in both trials were always greater for ERs versus ENRs to liraglutide 3.0 mg (Figure 3). The same pattern was observed for placebo, although proportions achieving each category were smaller than for liraglutide 3.0 mg.
Figure 3.
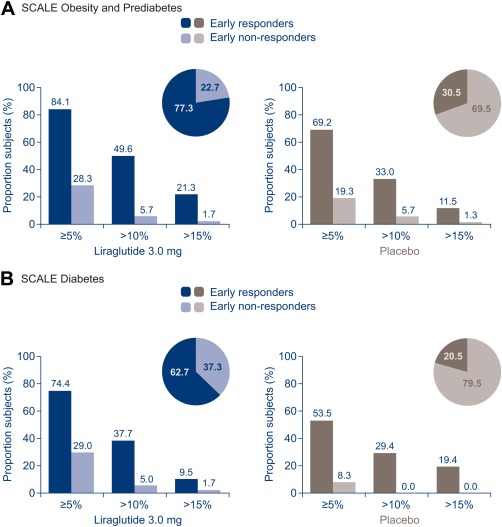
Categorical weight loss at week 56. Proportions of subjects are estimated proportions from a logistic regression model for ERs/ENRs who completed 56 weeks of treatment. ERs, early responders (individuals who achieved ≥4% weight loss from baseline at 16 weeks); ENR, early nonresponders (individuals who achieved <4% weight loss from baseline at 16 weeks).
Secondary end points at week 56 in ERs and ENRs
In SCALE Obesity and Prediabetes, changes in all cardiometabolic biomarkers examined, except heart rate, appeared to be more favorable in ERs than ENRs to liraglutide 3.0 mg, consistent with greater weight loss (Table 4, Supporting Information Table S6a). In particular, greater improvements were observed in ERs versus ENRs to liraglutide 3.0 mg for SBP, HDL cholesterol, LDL cholesterol, total cholesterol, and triglycerides. Most changes were also more favorable in ERs versus ENRs to placebo. With liraglutide 3.0 mg, pulse rate increased by 2.7 beats per minute (bpm) in ERs and 2.6 bpm in ENRs. With placebo, pulse rate changes were −1.2 and +0.2 bpm, respectively. Improvements in the IWQOL‐Lite total score and physical function score and the SF‐36 physical component summary score were reported by all groups, but appeared greater in ERs versus ENRs to both liraglutide 3.0 mg and placebo (Table 4, Supporting Information Table S6a).
Table 4.
Changes from baseline in selected secondary end points (SCALE Obesity and Prediabetes trial)
| Liraglutide 3.0 mg | Placebo | |||||||
|---|---|---|---|---|---|---|---|---|
| Early responders (N = 1,433) | Early nonresponders (N = 355) | Early responders (N = 265) | Early nonresponders (N = 535) | |||||
| Baseline | Change at 56 weeks [SE] | Baseline | Change at 56 weeks [SE] | Baseline | Change at 56 weeks [SE] | Baseline | Change at 56 weeks [SE] | |
| HbA1c (% points) | 5.6 | −0.36 [0.01] | 5.6 | −0.23 [0.01] | 5.6 | −0.17 [0.02] | 5.6 | −0.06 [0.01] |
| FPG (mg/dL) | 96.1 | −8.2 [0.2] | 97.2 | −6.3 [0.5] | 95.7 | −2.3 [0.5] | 95.9 | +0.9 [0.4] |
| SBP (mm Hg) | 123.4 | −5.1 [0.3] | 123.8 | −2.0 [0.6] | 123.0 | −2.3 [0.7] | 124.0 | −1.8 [0.5] |
| DBP (mm Hg) | 78.9 | −3.3 [0.2] | 78.6 | −1.4 [0.4] | 78.3 | −3.0 [0.5] | 79.4 | −2.1 [0.3] |
| Pulse (bpm) | 71.2 | +2.7 [0.2] | 70.8 | +2.6 | 71.0 | −1.2 | 71.0 | +0.2 |
| HDL cholesterol, mg/dL (%) a | 51.8 | +3.9 [0.4] | 52.0 | +0.0 [0.8] | 51.9 | +4.8 [0.9] | 50.9 | −1.0 [0.6] |
| LDL cholesterol, mg/dL (%) a | 111.4 | −3.6 [0.5] | 113.2 | −0.9 [1.1] | 115.6 | −2.0 [1.3] | 111.7 | −1.0 [0.9] |
| Total cholesterol, mg/dL (%) a | 193.0 | −3.2 [0.3] | 196.7 | −1.7 [0.7] | 198.6 | −1.7 [0.8] | 193.9 | −0.9 [0.6] |
| Triglycerides, mg/dL (%) a | 125.2 | −15.3 [0.7] | 129.4 | −7.1 [1.6] | 128.3 | −12.8 [1.8] | 130.3 | −2.5 [1.4] |
| IWQOL-Lite total score | 73.0 | +12.7 | 70.7 | +8.2 | 72.7 | +13.0 | 73.9 | +6.3 |
Baseline value is in mg/dL, and change is presented as relative change.
Early responders, individuals who achieved ≥4% weight loss from baseline at 16 weeks; early nonresponders, individuals who achieved <4% weight loss from baseline at 16 weeks. Based on individuals with a fasting body weight measurement at baseline and week 16 and who completed 56 weeks of treatment. Changes are estimated mean changes from baseline to week 56 from an ANCOVA. Missing values post‐baseline were imputed using last observation carried forward. Additional end points are reported in Supporting Information Table S6a, b.
DBP, diastolic blood pressure; FPG, fasting plasma glucose; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; IWQOL‐Lite, Impact of Weight on Quality of Life‐Lite; SBP, systolic blood pressure.
Similarly in SCALE Diabetes, changes in most cardiometabolic biomarkers and HRQoL scores appeared more favorable in ERs than ENRs (Table 5, Supporting Information Table S6b). Notably, improvements in glycemic markers were observed in both ERs and ENRs to liraglutide 3.0 mg.
Table 5.
Changes from baseline in selected secondary end points (SCALE Diabetes trial)
| Liraglutide 3.0 mg | Placebo | |||||||
|---|---|---|---|---|---|---|---|---|
| Early responders (N = 214) | Early nonresponders (N = 110) | Early responders (N = 31) | Early nonresponders (N = 101) | |||||
| Baseline | Change at 56 weeks [SE] | Baseline | Change at 56 weeks [SE] | Baseline | Change at 56 weeks [SE] | Baseline | Change at 56 weeks [SE] | |
| HbA1c (% points) | 7.9 | −1.60 [0.05] | 8.0 | −1.11 [0.08] | 7.6 | −1.17 [0.14] | 7.7 | −0.30 [0.09] |
| FPG (mg/dL) | 158.5 | −44.2 [2.4] | 157.7 | −30.1 [30.3] | 151.4 | −30.4 [6.2] | 151.4 | −1.9 [3.7] |
| SBP (mm Hg) | 128.4 | −3.3 [0.8] | 129.1 | −1.3 [1.1] | 128.7 | −3.2 [2.1] | 129.8 | +0.7 [1.2] |
| DBP (mm Hg) | 78.5 | −0.6 [0.5] | 79.9 | −1.8 [0.8] | 78.5 | −2.1 [0.4] | 79.3 | −1.0 [0.8] |
| Pulse (bpm) | 74.0 | +1.7 [0.6] | 72.1 | +3.1 [0.8] | 74.3 | −3.8 [1.5] | 73.4 | −1.4 [0.8] |
| HDL cholesterol, mg/dL (%) a | 45.3 | +7.2 [1.0] | 46.2 | +0.7 [1.3] | 43.7 | +7.7 [2.6] | 45.6 | +0.6 [1.3] |
| LDL cholesterol, mg/dL (%) a | 85.1 | +1.6 [1.9] | 91.7 | −0.3 [2.5] | 76.7 | +2.5 [4.9] | 79.8 | +4.1 [2.7] |
| Total cholesterol, mg/dL(%) a | 169.1 | −1.5 [1.0] | 175.6 | −1.1 [1.5] | 159.3 | +0.3 [2.8] | 164.2 | +3.9 [1.6] |
| Triglycerides, mg/dL (%) a | 162.0 | −19.2 [2.1] | 155.8 | −6.9 [3.3] | 163.6 | −12.7 [5.9] | 157.2 | +3.2 [3.8] |
| IWQOL‐Lite total score | 69.7 | +13.2 [0.8] | 79.5 | +7.9 [1.1] | 73.8 | +10.8 [2.1] | 75.9 | +7.2 [1.2] |
Baseline value is in mg/dL, and change is presented as relative change.
Early responders, individuals who achieved ≥4% weight loss from baseline at 16 weeks; early nonresponders, individuals who achieved <4% weight loss from baseline at 16 weeks. Based on individuals with a fasting body weight measurement at baseline and week 16 and who completed 56 weeks of treatment. Changes are estimated mean changes from baseline to week 56 from an ANCOVA. Missing values post‐baseline were imputed using last observation carried forward. Additional end points are reported in Supporting Information Table S6a, b.
DBP, diastolic blood pressure; FPG, fasting plasma glucose; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; IWQOL‐Lite, Impact of Weight on Quality of Life‐Lite; SBP, systolic blood pressure.
Safety
Safety results for the randomized populations in both trials were reported previously 14, 15. The most common adverse events (AEs), occurring more frequently with liraglutide 3.0 mg versus placebo, were gastrointestinal.
An overview of safety results by early responder status is shown in Tables 6 and 7. AEs occurring in ≥5% of individuals treated with liraglutide 3.0 mg and more frequently with liraglutide 3.0 mg than placebo are listed by preferred term in Supporting Information Table S7. AE rates were generally comparable between ERs and ENRs to liraglutide 3.0 mg, and comparable to the overall trial population, except that in SCALE Diabetes, rates of gastrointestinal‐ or appetite‐related AEs were higher in ERs versus ENRs.
Table 6.
Overview of adverse events in the overall trial and in early responders and early nonresponders (SCALE Obesity and Prediabetes trial)
| Liraglutide 3.0 mg | Placebo | |||||
|---|---|---|---|---|---|---|
| All randomized (N = 2,481) | ERs (N = 1,668) | ENRs (N = 491) | All randomized (N = 1,242) | ERs (N = 322) | ENRs (N = 735) | |
| Adverse events | 2,285 (92.1) | 1,559 (93.5) | 453 (92.3) | 1,043 (84.0) | 285 (88.5) | 651 (88.6) |
| Serious adverse events | 154 (6.2) | 106 (6.4) | 24 (4.9) | 62 (5.0) | 21 (6.5) | 33 (4.5) |
| Severe adverse events | 304 (12.3) | 190 (11.4) | 51 (10.4) | 113 (9.1) | 36 (11.2) | 66 (9.0) |
| Fatal | 1 (0.0) | 0 (0.0) | 1 (0.2) | 2 (0.2) | 0 (0.0) | 0 (0.0) |
| Leading to withdrawala | 244 (9.8) | 54 (3.2) | 20 (4.1) | 47 (3.8) | 4 (1.2) | 17 (2.3) |
AE data given as n (% of patients). Safety analysis set (for ERs and ENRs, all subjects with data at week 16).
aWithdrawal rates cover the entire trial period for “All randomized” and week 16 onwards for ERs and ENRs.
ERs, early responders (individuals who achieved ≥4% weight loss from baseline at 16 weeks); ENRs, early nonresponders (individuals who achieved <4% weight loss from baseline at 16 weeks).
Table 7.
Overview of adverse events in the overall trial and in early responders and early nonresponders (SCALE Diabetes trial)
| Liraglutide 3.0 mg | Placebo | |||||
|---|---|---|---|---|---|---|
| All randomized (N = 422)b | ERs (N = 229) | ENRs (N = 136) | All randomized (N = 212) | ERs (N = 34) | ENRs (N = 132) | |
| Adverse events | 392 (92.9) | 222 (96.9) | 124 (91.2) | 182 (85.8) | 32 (94.1) | 120 (90.9) |
| Serious adverse events | 37 (8.8) | 17 (7.4) | 15 (11.0) | 13 (6.1) | 1 (2.9) | 10 (7.6) |
| Severe adverse events | 52 (12.3) | 30 (13.1) | 14 (10.3) | 21 (9.9) | 1 (2.9) | 17 (12.9) |
| Fatal | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Leading to withdrawal a | 39 (9.2) | 7 (3.1) | 6 (4.4) | 7 (3.3) | 0 (0.0) | 3 (2.3) |
AE data given as n (% of patients).
Safety analysis set (for ERs and ENRs, all subjects with data at week 16).
Withdrawal rates cover the entire trial period for “All randomized” and week 16 onwards for ERs and ENRs.
One subject did not receive treatment and was excluded from the safety analysis set for SCALE Diabetes.
ERs, early responders (individuals who achieved ≥4% weight loss from baseline at 16 weeks); ENRs, early nonresponders (individuals who achieved <4% weight loss from baseline at 16 weeks).
In SCALE Obesity and Prediabetes, pancreatitis was uncommon in ERs (<0.1/100 patient‐years’ exposure [PYE]) and ENRs (0.2/100 PYE) to liraglutide 3.0 mg. Gallbladder disorders were more frequent in ERs (2.8% of individuals; 2.9/100 PYE) versus ENRs to liraglutide 3.0 mg (1.4%; 1.9/100 PYE). Psychiatric AEs appeared similar in ER and ENRs.
In SCALE Diabetes, no events of pancreatitis were reported, and there were too few gallbladder‐related and psychiatric AEs to allow any conclusions to be drawn. Documented symptomatic hypoglycemia, defined according to ADA criteria 18, was similar in ERs versus ENRs to liraglutide 3.0 mg (event rates of 79.5/100 PYE and 93.3/100 PYE, respectively).
For ERs and ENRs to placebo, results were as follows: in SCALE Obesity and Prediabetes, no pancreatitis was reported in either ERs or ENRs; gallbladder disorders occurred in 1.6% of ERs (2.2/100 PYE) and 0.5% of ENRs (0.6/100 PYE); and psychiatric AEs appeared similar in ER and ENRs. In SCALE Diabetes, documented hypoglycemia occurred at event rates of 20.5/100 PYE (ERs to placebo) and 31.1/100 PYE (ENRs to placebo).
Discussion
It is well documented that early response to a weight loss intervention can predict long‐term weight loss 8, 9, 10, 11; this is the basis for the stopping rules for all recently approved weight loss medications 19, 20, 21. Weight loss response after 1 to 4 months has been used to predict weight loss at 1 year. Interestingly, in the Look AHEAD study, 1‐ and 2‐month weight loss was associated with weight loss through year 8 among individuals with T2D who received an intensive lifestyle intervention 7.
In order to identify an optimal early response criterion for liraglutide 3.0 mg, we examined weight loss of ≥3%, 4%, or 5% at 8, 12, or 16 weeks in a pooled analysis predefined with the US FDA before trial data became available. Weight loss of ≥4% at week 16 was shown to be the best predictor of ≥5% weight loss at 56 weeks and to be appropriate for individuals with and without T2D and for both genders (Table 1, Supporting Information Table S1). Earlier time points would result in discontinuation of treatment in a significant number of individuals who would indeed achieve ≥5% weight loss after 56 weeks, while later time points would likely have achieved even greater predictive accuracy but were not considered as they would have entailed additional unnecessary exposure in nonresponders. Accordingly, the stopping rule in the USA specifies a weight loss of ≥4% at 16 weeks 22. While health authorities across the world focus on limiting treatment to those who will benefit, their approaches differ slightly. The European Medicines Agency required an early response criterion optimized to exclude individuals who were unlikely to achieve ≥10% weight loss at 56 weeks; this was best fulfilled by weight loss of ≥5% after 16 weeks (data not shown). Accordingly, the European label requires ≥5% weight loss after 12 weeks on the full 3.0 mg dose to qualify for continued treatment 23.
In both trials, higher proportions of subjects were early responders to liraglutide 3.0 mg than to placebo. Early responders to liraglutide achieved greater mean weight loss than early nonresponders and were more likely to achieve ≥5%, >10%, and >15% weight loss at 56 weeks. Greater responses were also seen among early responders versus early nonresponders to placebo; but fewer subjects were early responders to placebo compared with liraglutide 3.0 mg.
The greater weight loss in early responders was accompanied by a trend toward greater improvements in cardiometabolic biomarkers. Decreases in mean SBP and DBP and favorable changes in lipid profile, in particular, are expected effects of weight loss, contributing to a decreased risk of developing cardiovascular disease 24. In SCALE Diabetes, clinically meaningful reductions in HbA1c and fasting plasma glucose were seen in both early responders and early nonresponders to liraglutide, due to its direct glucose‐lowering effect 25; in early responders, this effect appeared further enhanced by the greater weight loss versus early nonresponders. The combination of direct effects of liraglutide on glycemia, as well as further improvement likely mediated by enhanced weight loss in early responders, may be particularly beneficial for slowing progression to T2D and increasing regression to normoglycemia in individuals with prediabetes, which further reduces the risk of conversion to T2D 26.
It has been suggested that, in trials of antiobesity drugs, improvements in feeling and functioning should be measured when validated measures exist 27. Improvements in HRQoL were recorded as assessed by IWQOL‐Lite, a questionnaire developed specifically to evaluate the impact of weight on quality of life (both trials), and SF‐36, a more general HRQoL questionnaire (SCALE Obesity and Prediabetes only). For both liraglutide 3.0 mg and placebo, improvements were greater in early responders than early nonresponders. The changes recorded for early responders (to either intervention) were clinically relevant. (Clinically relevant improvements for an individual are increases of 7.7 to 12 points for IWQOL‐Lite total score, depending on baseline score 28, and ≥2 points for SF‐36 physical component summary score 17.)
The greater improvements observed in early responders, compared with early nonresponders, were generally not accompanied by an increase in AEs: the safety profiles of early responders and early nonresponders were similar within each trial, except that early responders reported higher rates of gastrointestinal disorders in SCALE Diabetes and higher rates of gallbladder disorders in SCALE Obesity and Prediabetes. No new safety signals arose among early responders or early nonresponders.
In pooled analyses of the SCALE trials, women had slightly greater weight loss than men with liraglutide 3.0 mg 29; however, both men and women experienced a clinically meaningful weight loss. Consistent with this, there appeared to be more women than men among early responders to liraglutide 3.0 mg in both trials. Greater weight loss in women may in part be explained by higher plasma liraglutide exposure in women versus men 30.
The comparisons between early responders and early nonresponders must be interpreted with caution as they are not comparisons between randomized groups and were therefore not subjected to significance testing. In the case of the diabetes trial, conclusions are further limited by the low number of individuals in the liraglutide early nonresponder and placebo early responder groups. An additional limitation is the use of a forced dose escalation of liraglutide from 0.6 mg daily to 3.0 mg daily in 0.6 mg increments over 4 weeks (with 1 additional week at investigator's discretion) in the original trials. Also, early nonresponders in these trials were continued on treatment for 56 weeks; in clinical practice their treatment would be discontinued after 16 weeks, and improvements in end points might therefore be smaller than those reported here.
From a clinical perspective, use of the stopping rules should help optimize the use of liraglutide 3.0 mg for weight management. Patients can be informed that, if they respond well during the first 16 weeks, it is likely they will continue to do so. It is reassuring that most AEs were no more frequent in early responders compared with early nonresponders, even with greater weight loss, with the exception of gallbladder disorders, perhaps reinforcing the suggestion that at least part of the increased risk of gallbladder disorders seen in SCALE Obesity and Prediabetes was related to weight loss 14.
Conclusion
Weight loss of ≥4% after 16 weeks of treatment is a strong predictor of a clinically meaningful weight loss at 56 weeks. More individuals without or with T2D were early responders to liraglutide 3.0 mg in combination with lifestyle intervention compared with lifestyle intervention alone.
Among early responders to liraglutide 3.0 mg, greater mean weight loss, greater proportions achieving weight loss thresholds, and generally greater improvements in cardiometabolic risk factors and HRQoL scores were observed compared with early nonresponders.
Supporting information
Supporting Information
Acknowledgments
Medical writing assistance, supported by Novo Nordisk A/S, was provided by Grace Townshend, MSc, of Watermeadow Medical, an Ashfield Company, who wrote the first draft of the manuscript under the guidance of the authors. Statistical analyses were performed by Arne Haahr Andreasen of Novo Nordisk.
Funding agencies: The preparation of this article was supported by Novo Nordisk A/S, which sponsored the trials on which this article is based.
Disclosure: KF received research grants from Orexigen Therapeutics; received research grants, acted as consultant, and attended speakers’ bureaus for Novo Nordisk; received research grants and acted as consultant for Enteromedics; received research grants and attended speakers’ bureaus for Shire; attended speakers' bureaus for Abbott; acted as consultant and attended speakers’ bureaus for Takeda; received research grants, acted as consultant, and attended speakers’ bureaus for Eisai; and acted as consultant for Gelesis, Nazura, and Zafgen. PMO received research grants from Orexigen Therapeutics; received research grants from and attended speakers’ bureaus for Weight Watchers International; received research grants from and attended speakers’ bureaus for Novo Nordisk; attended advisory panels for Fleishman‐Hillard; attended speakers’ bureaus for Vindico CME, Practicing Clinicians Exchange, and Eisai; and attended advisory panels for Medscape CME. MD attended advisory boards, acted as consultant, and attended speakers’ bureaus for Sanofi‐Aventis, Eli Lilly and Company, Merck, Boerhinger Ingelheim, AstraZeneca, Janssen, and Novo Nordisk and attended speakers’ bureaus for Mitsubishi Tanabe Pharma Corp. FG reviewed a proposal for American Pistachio; attended medical advisory boards for Curves; had travel reimbursement for meeting attendance from Diabetes Technology Society; attended editorial board meetings for Diabetic Living; attended scientific advisory boards, acted as consultant for, and holds stock options in MicroBiome Therapeutics; acted as consultant for and holds stock options in Neothetics, Inc.; attended scientific advisory boards for Neurium, GNC, and Pamlab, Inc.; holds stock options in and patent licenses with NeuroQuest, Inc.; attended advisory boards for Novo Nordisk; attended board meetings for Obesity Medicine Society (OMA); attended advisory boards and acted as consultant for Orexigen Therapeutics; acted as medical expert for PlenSat, Inc.; acted as consultant for Synergy Medical Education, AlphaSights, ClearView Healthcare Partners, Eisai, and Embera; attended medical scientific boards for Takeda Pharmaceuticals; attended a GRAS panel for Techenterprises, LLC; acted as faculty consultant for Vindico Medical Education; was a witness for Wilson, Sonsini, Goodrich & Rosati Professional Corp; attended scientific advisory boards for and acted as consultant for Zafgen; is in receipt of research grants from NIH (NIDDK), Novo Nordisk, Hanmi Pharmaceuticals, NIH, and Tufts University; has been in receipt of research grants from American Egg Board, Biologene, Pennington Biomedical Research Foundation, MannKind Corporation, Wright Group, NuMe Health, Orexigen Therapeutics, OmniActive, and PepsiCo; and has two patents pending (WO 2016/033063 A1; PCT/US16/15395). DCWL attended advisory boards and speakers’ bureaus for Amgen, Janssen, and Valeant; attended advisory boards for Roche; attended advisory boards for Shire; is President of the Canadian Association of Bariatric Physicians and Surgeons; is President of Obesity Canada; and received research grants from and attended advisory boards and speakers’ bureaus for AstraZeneca, Boehringer‐Ingelheim, Bristol‐Myers Squibb, Eli Lilly and Company, Merck, and Novo Nordisk. BC, TVS, and CBJ are employees and shareholders of Novo Nordisk. JPHW received research grants from and attended advisory boards and speakers’ bureaus for AstraZeneca, Bristol‐Myers Squibb, and Novo Nordisk; attended advisory panels and speakers’ bureaus for Boehringer Ingelheim, Janssen, and Astellas; attended speakers’ bureaus for Eli Lilly and Company; attended advisory panels for Merck, Sanofi, and Orexigen Therapeutics; and acted as consultant for Pfizer.
Author contributions: The decision to examine the outcomes in early responders and early nonresponders was a joint one by all the authors, based on trials in which all the academic authors were investigators. The statistical analyses were performed by Novo Nordisk. All authors were involved in discussing the results of the analyses, writing the article, and approving the submitted version. The final version of the manuscript was reviewed and approved by the authors.
Clinical trial registration: ClinicalTrials.gov identifiers NCT01272219 and NCT01272232.
References
- 1.Golden J. FDA 2007 Draft Guidance for Industry: Developing Products for Weight Management Endocrinologic and Metabolic Drugs Advisory Committee Meeting. March 28, 2012. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM299133.pdf. Accessed 5 May 2016.
- 2.European Medicines Agency. Guideline on Clinical Evaluation of Medicinal Products used in Weight Control http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/07/WC500170278.pdf. Published 2014 June 26. Accessed 5 May 2016.
- 3. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2015;100:342‐362. [DOI] [PubMed] [Google Scholar]
- 4. Wadden TA, Foster GD, Wang J, et al. Clinical correlates of short‐ and long‐term weight loss. Am J Clin Nutr 1992;56:271S‐274S. [DOI] [PubMed] [Google Scholar]
- 5. Stotland SC, Larocque M. Early treatment response as a predictor of ongoing weight loss in obesity treatment. Br J Health Psychol 2005;10:601‐614. [DOI] [PubMed] [Google Scholar]
- 6. Handjieva‐Darlenska T, Handjiev S, Larsen TM, et al. Initial weight loss on an 800‐kcal diet as a predictor of weight loss success after 8 weeks: the Diogenes study. Eur J Clin Nutr 2010;64:994‐999. [DOI] [PubMed] [Google Scholar]
- 7. Unick JL, Neiberg RH, Hogan PE, et al.; Look AHEAD Research Group. Weight change in the first 2 months of a lifestyle intervention predicts weight changes 8 years later. Obesity (Silver Spring) 2015;23:1353‐1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rissanen A, Lean M, Rössner S, Segal KR, Sjöström L. Predictive value of early weight loss in obesity management with orlistat: an evidence‐based assessment of prescribing guidelines. Int J Obes Relat Metab Disord 2003;27:103‐109. [DOI] [PubMed] [Google Scholar]
- 9. Fujioka K, Plodkowski R, O'Neil PM, Gilder K, Walsh B, Greenway FL. The relationship between early weight loss and weight loss at 1 year with naltrexone ER/bupropion ER combination therapy. Int J Obes (Lond) 2016;40:1369‐1375. [DOI] [PubMed] [Google Scholar]
- 10. Finer N, Ryan DH, Renz CL, Hewkin AC. Prediction of response to sibutramine therapy in obese non‐diabetic and diabetic patients. Diabetes Obes Metab 2006;8:206‐213. [DOI] [PubMed] [Google Scholar]
- 11. Smith SR, O'Neil PM, Astrup A, et al. Early weight loss while on lorcaserin, diet and exercise as a predictor of week 52 weight‐loss outcomes. Obesity (Silver Spring) 2014;22:2137‐2146. [DOI] [PubMed] [Google Scholar]
- 12. Knudsen LB. Liraglutide: the therapeutic promise from animal models. Int J Clin Pract 2010;64:4‐11. [DOI] [PubMed] [Google Scholar]
- 13. Van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WH. Effects of the once‐daily GLP‐1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non‐diabetic adults. Int J Obes (Lond) 2014;38:784‐793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pi‐Sunyer X, Astrup A, Fujioka K, et al. A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. N Engl J Med 2015;373:11‐22. [DOI] [PubMed] [Google Scholar]
- 15. Davies MJ, Bergenstal R, Bode B, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE Diabetes randomized clinical trial. JAMA 2015;314:687‐699. [DOI] [PubMed] [Google Scholar]
- 16. Kolotkin RL, Crosby RD, Kosloski KD, Williams GR. Development of a brief measure to assess quality of life in obesity. Obes Res 2001;9:102‐111. [DOI] [PubMed] [Google Scholar]
- 17. Maruish ME. User's Manual for the SF‐36V2 Health Survey. 3rd ed. Lincoln, RI: Quality Metric; 2011.
- 18. American Diabetes Association. Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care 2005;28:1245‐1249. [DOI] [PubMed] [Google Scholar]
- 19. Eisai Inc . Belviq (Lorcaserin Hydrochloride) Full Prescribing Information http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022529lbl.pdf. Revised June 2012. Accessed 5 May 2016.
- 20. Takeda Pharmaceuticals America, Inc . Contrave (Naltrexone HCl and Bupropion HCl) Extended‐Release Tablets Full Prescribing Information http://general.takedapharm.com/content/file.aspx?filetypecode=CONTRAVEPI&cacheRandomizer=eedcee06-8dad-4cf0-910b-06e13004a474. Revised September 2014. Accessed 5 May 2016.
- 21. Vivus Inc . QSYMIA (Phentermine and Topiramate Extended‐Release Capsules, for Oral Use, CIV). Highlights of Prescribing Information https://qsymia.com/patient/include/media/pdf/prescribing-information.pdf. Revised October 2014. Accessed 5 May 2016.
- 22. Novo Nordisk Inc. Saxenda® (Liraglutide [rDNA Origin] Injection) Full Prescribing Information http://www.novo-pi.com/saxenda.pdf. Revised January 2015. Accessed 5 May 2016.
- 23. European Medicines Agency. Saxenda (Liraglutide 3.0 mg). Summary of Product Characteristics 2015. http://ec.europa.eu/health/documents/community-register/2015/20150323131125/anx_131125_en.pdf. Accessed 5 May 2016.
- 24. Jensen MD, Ryan DH, Donato KA, et al. Guidelines (2013) for managing overweight and obesity in adults. Obesity 2014;22(S2):S1‐S410. [DOI] [PubMed] [Google Scholar]
- 25. Bode B. An overview of the pharmacokinetics, efficacy and safety of liraglutide. Diabetes Res Clin Pract 2012;97:27‐42. [DOI] [PubMed] [Google Scholar]
- 26. Perreault L, Pan Q, Mather KJ, Watson KE, Hamman RF, Kahn SE, Diabetes Prevention Program Research Group. Effect of regression from prediabetes to normal glucose regulation on long‐term reduction in diabetes risk: results from the Diabetes Prevention Program Outcomes Study. Lancet 2012;379:2243‐2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ferguson C, David S, Divine L, et al., George Washington University. Obesity Drug Outcome Measures. A Consensus Report of Considerations Regarding Pharmacologic Intervention Available from: http://publichealth.gwu.edu/pdf/obesitydrugmeasures.pdf Published 2012. Accessed 5 May 2016.
- 28. Crosby RD, Kolotkin R, Williams GR. An integrated method to determine meaningful changes in health‐related quality of life. J Clin Epidemiol 2004;57:1153‐1160. [DOI] [PubMed] [Google Scholar]
- 29. FDA . Liraglutide 3.0 mg for Weight Management, NDA 206‐321. Briefing Document. Endocrinologic and Metabolic Drugs Advisory Committee Meeting http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/endocrinologicandmetabolicdrugsadvisorycommittee/ucm413318.pdf. Published 11 September 2014. Accessed 5 May 2016.
- 30. Wilding JP, Overgaard RV, Jacobsen LV, Jensen CB, le Roux CW. Exposure‐response analyses of liraglutide 3.0 mg for weight management. Diabetes Obes Metab 2016;18:491‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


