Abstract
Catecholaminergic polymorphic ventricular tachycardia (CPVT), an inherited arrhythmia often leading to sudden cardiac death in children and young adults, is characterized by polymorphic/bidirectional ventricular tachycardia induced by adrenergic stimulation associated with emotionally stress or physical exercise. There are two forms of CPVT:
1. CPVT1 is caused by mutations in the RYR2 gene, encoding for ryanodine receptor type 2. CPVT1 is the most common form of CPVT in the population, and is inherited by a dominant mechanism.
2. CPVT2 is caused by mutations in the CASQ2 gene, encoding for cardiac calsequestrin 2 and is inherited by recessive mechanism.
Patient-specific induced Pluripotent Stem Cells (iPSC) have the ability to differentiate into cardiomyocytes carrying the patient's genome including CPVT-linked mutations and expressing the disease phenotype in vitro at the cellular level. The potency for in vitro modeling using iPSC-derived cardiomyocytes (iPSC-CMs) has been exploited to investigate a variety of inherited diseases including cardiac arrhythmias such as CPVT.
In this review we attempted to cover the majority of CPVT patient specific iPSC research studies previously published. CPVT patient-specific iPSC model enables the in vitro investigation of the molecular and cellular disease-mechanisms by the means of electrophysiologycal and Ca+2 imaging methodologies. Furthermore, this in vitro model allows the screening of various antiarrhythmic drugs, specifically for each patient, also known as "personalized medicine".
Keywords: Induced Pluripotent Stem Cell, Cardiomyocytes, Catecholaminergic Polymorphic Ventricular Tachycardia (CPVT), Personalized Medicine, Arrhythmia
Introduction
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is an inherited arrhythmia which may lead to syncope and sudden cardiac death in children and young adults. The arrhythmia is characterized by polymorphic/bidirectional ventricular tachycardia induced by adrenergic stimulation associated with emotional stress or physical exercise.[1-4] CPVT is manifested in one of two forms depending on the mutated gene.
CPVT1 is caused by mutations in the RYR2 gene encoding the ryanodine receptor type 2, it is inherited by a dominant mechanism and constitutes 70% of the CPVT cases.
CPVT2 is caused by mutations in the CASQ2 gene encoding for cardiac calsequestrin 2,[5-8] it is inherited by a dominant mechanism.
There are residual cases of CPVT that are caused by mutations either in TRDN, encoding for triadin or by mutations in CALM1, encoding for calmodulin; these residual CPVT cases are inherited by a recessive/dominant mechanism, respectively.[10,11] RYR2, a homotetramer comprised of 4 pore-forming monomers, regulates Ca2+-induced Ca2+ release process (CICR) during excitation-contraction (EC) coupling by acting as a Ca2+ channel in the sarcoplasmic reticulum (SR) membrane.[9,12,13] CASQ2 also regulates SR Ca2+ release by acting as a buffering factor with a high-capacity low-affinity Ca2+ binding function.[5,8,9,12,13] RYR2 and CASQ2 are both involved in the EC coupling, operating as regulators of the Ca2+ handling machinery in cardiomyocytes.[6,14,15] In both CPVT1 and CPVT2, the mutations cause spontaneous SR Ca2+ release leading to intracellular Ca2+ overload, which in turn can generate delayed afterdepolarizations (DADs). If these spontaneous oscillatory depolarizations reach the activation threshold, triggered arrhythmia occur, and can culminate into lethal ventricular fibrillation.[6] High intracellular Ca2+ level which may result in triggered activity is triggered by one of two mechanisms:
RYR2 mutations resulting in a gain-of-function cause increased Ca2+ sensitivity of RYR2, and thus leads to increased probability and duration of the open state of ryanodine receptor channel, which in turn leads to SR Ca2+ leak.
CASQ2 mutations decrease the binding efficiency of CASQ2 to SR Ca2+, thus leading to SR Ca2+ leak from to the cytoplasm.[1,8,16-20]
There are two mechanisms by which high intracellular Ca2+ levels are reduced: (1) sarcoplasmic endoplasmic reticulum calcium (SERCA) pumps Ca2+ back into the SR. (2) Activation of sodium–calcium exchanger (NCX) that extrudes Ca2+ to the extracellular space and generates a net inward current (so called transient inward current) leading to membrane depolarization and DADs.
Modeling CPVT in a Dish with Induced Pluripotent Stem Cells (iPSCs)
Despite tremendously high mortality rate in CPVT patients (about 35%) the mechanisms of action are not completely clear and the current treatments are limited. Regardless of their mutation, CPVT patients are treated with insufficient efficiency, mostly with β-blockers; hence, patients still experience life-threatening episodes which may require implantable cardioverter defibrillators (ICD).[6,22,23] Therefore, research models for drug screening of new therapeutic agents are encouraged. The recent technology of iPSC offers an innovative approach to study the molecular mechanism of CPVT and to test specific drug targets.
Embryonic stem cells (ESC) derived from the inner cell mass of a blastocyst, are pluripotent stem cells carrying the ability of self-renewal and differentiation into each one of the three germ layers. In 2006, Yamanaka and Takahashi have discovered a series of essential embryonic genes (OCT4, SOX2, KLF4, c-MYC) that if introduced into a mature fully-differentiated somatic cell, reboot a reprogramming process resulting in the generation of iPSCs. Several research groups have exploited this emerged technology to generate iPSCs for the investigation of inherited diseases such as neurodegenerative, metabolic and cardiac syndromes.[24,25] Particularly, in the field of inherited arrhythmias several groups[5,6,33,39] have demonstrated the ability of mutated iPSC-CMs to recapitulate the clinical symptoms, and to serve as a superb in vitro cell model for testing effective antiarrhythmic drugs. Additionally, the iPSC technology has been successfully employed to investigate dilated cardiomyopathy,[34,35] hypertrophic cardiomyopathy[34,36,37] and other channelopathies such as long QT syndrome as well as CPVT.[1,5,6,16,33,34,38-40]
CPVT patient-specific iPSCs carry the ability of differentiating into cardiomyocytes while holding the patient's genome, including CPVT-linked mutation, thereby CPVT-iPSC-CMs express the disease phenotype in vitro at cellular levels.[24-30] CPVT patients carry different mutations in RYR2 or in CASQ2 genes that lead to different impaired protein-activities while causing for resembling clinical phenotypes. Despite the resembling phenotype in CPVT patients, the involved mechanism in each patient requires unique treatment for each specific mutation. iPSC-CMs benefit as in vitro model for investigation of each mechanism and allows the patient-specific personalized drug screening.
This review paper aims to cover most of the published investigations in the field of iPSC-CMs associated with CVPT by shedding light on the known mechanisms of CPVT, and on the explored pharmacological modalities that are suitable for patients carrying various CPVT mutations.
Cell Origin and Reprogramming Method
Different methods have been employed by research groups for cell origin production and reprogramming. While most research groups have used fibroblasts for the infection of pluripotent markers in order to generate iPSCs, Binah’s group was the first to produce CPVT-iPSCs from patient’s hair keratinocytes. Whereas most (9/11) have harnessed Yamanaka’s 4 pluripotent factors: OCT4, SOX2, KLF4 and c-MYC or a reduced combination (OCT4, SOX2 and KLF4), Priori’s group has reprogrammed somatic cells using an altered factor-set: OCT4, SOX2, NANOG and LIN-28 as described before by Yu et al.[41] Despite the different methods used for reprogramming, all groups reported that the CPVT iPSC-CM recapitulated the disease specific phenotype.
Cardiomyocytes Differentiation
To date, there are several known differentiation methods towards the cardiac linage. In the described scientific investigations 2 major methods can be found:
Embryoid body (EB) – spontaneous differentiation.
Differentiation on murine visceral endoderm-like cell line (END2).
The EB spontaneous differentiation method includes detachment of iPSCs colonies by Collagenase type IV and suspension growth for a period of 7 days prior to gelatin coated plating as described before;[42] in contrast, differentiation with END2 cell line requires 3 weeks of co-culture before separation. There is a time difference for cardiac differentiation in each method affecting the cardiomyocytes maturity and thus may create electrophysiological variability. With respect to CPVT research, the new standard of cardiac directed differentiation method was not yet introduced. In the directed method, the differentiation is achieved either by small molecules modulating the activity of GSK-3β and Wnt signaling pathway or by inducible β-catenin shRNA.[43] The directed differentiation does not only provide a more uniform cardiac population, but also affects the cardiomyocytes maturity. As shown before, iPSC-CMs have a more developed SR while achieved by directed compared to spontaneous differentiation;[44,45] accordingly affecting age range selection for electrophysiological/EC-coupling experiments. It will be of great interest to observe CPVT results from iPSC-CMs differentiated by a directed method that is calibrated to generate mature and enriched cardiac population.
CPVT-iPSC-CMs Recapitulate Disease Specific Phenotypes
Several groups have explored CPVT phenotypes in patient-specific iPSC-CMs by the following commonly used electrophysiology and Ca+2-contraction imaging methods:
Patch Clamp
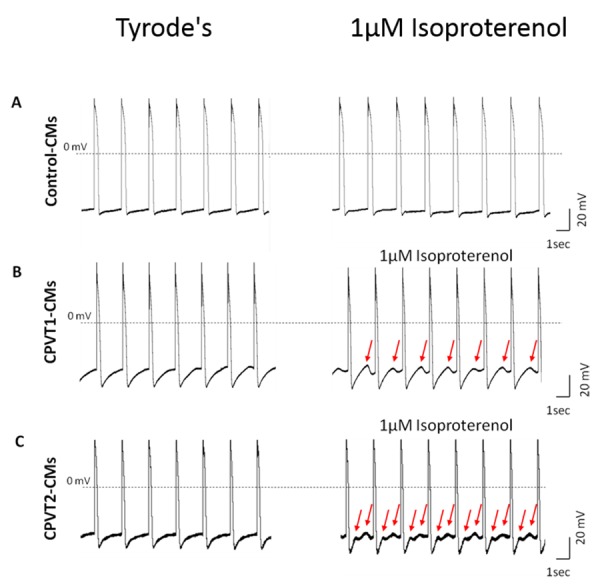
Action potential recordings, by whole cell patch clamp configuration, have shown that CPVT-iPSC-CMs present DADs, early afterdepolarizations (EADs – during phase 2,3), oscillatory prepotentials and triggered arrhythmia (TA). Arrhythmias have been catecholaminergic-linked and increased during exposure to isoproterenol (a β-adrenergic agonist). While in control-cells, isoproterenol had caused positive chronotropic effect; in CPVT-CMs, it led to negative chronotropic effect and to the appearance of DADs and TA (Fig. 1). [5,16,38,39,46] Most groups have reported on similar electrical features (Maximum diastolic potential, action potential amplitude and maximal rate of depolarization (dV/dtmax)) between CPVT-CMs and control-cells.[5,6,16,39] DADs and TA have been also generated by exposure of CPVT-iPSC-CMs to forskolin (an adenylyl cyclase activator) and by the end of a pacing-train, immediately after the last pulse. Thapsigargin – SERCA inhibitor, which causes for depletion of intracellular Ca2+ stores, has reduced the appearance of DADs post pacing or during the exposure to forskolin. This phenomenon suggests for another involved mechanism in the generation of DADs: store-overload-induced Ca2+ release (SOICR). SOICR manifests the necessity to reach a certain threshold of the SR over-load for spontaneous release via RYR2 through depolarization-independent SR release.[39] It was also demonstrated that DADs may prevent the next emerging action potential, thus decreasing the cardiomyocytes beating rate, or may reach action potential threshold and initiate triggered activity. In addition to DADs, oscillatory pre-potentials were observed during action potential recordings. Unlike DADs, oscillatory pre-potentials appear during late diastolic depolarization, last for longer period and its amplitude grow progressively until reaching the action potential threshold.[5,47,48]
Figure 1. Isoproterenol induced DADs in CPVT1 and CPVT2 iPSC-CM. Representative action potentials recordings from healthy control (A), CPVT1 (B) and CPVT2 (C) iPSC-CM before and after exposure to 1µM isoproterenol with 0.5 Hz external pacing. Red arrows indicate on isoproterenol-induced DADs in CPVT iPSC-CMs.
Calcium Handling Measurements
CICR mechanism is the key regulator of Ca2+ handling in cardiac muscle and is responsible for the EC coupling process. Researchers in this field measure Ca2+ transients either by Fura-2 or Flou-4; regardless the transient-recording method, all experiments have reported on a deranged Ca2+ handling machinery seen in CPVT-iPSC-CMs: multiple peaks, oscillations, varying amplitude and local Ca2+ release events interspersed within beats.[1,5,38,46] Abnormalities have been manifested in base line of CPVT-iPSC-CMs Ca2+ measurements, and increased in response to adrenergic stimulation, such as isoproterenol exposure. Ca2+ handling abnormalities may cause arrhythmias, expressed as electrophysiological abnormalities such as DADs, EADs or oscillatory prepotentials that eventually lead to dissimilar beat-to-beat intervals and TA; these electrophysiological abnormalities are mirrored and explained by Ca2+ and contraction measurements.[34] It was also indicated that Ca2+ handling abnormalities increase in exposure to isoproterenol or forskolin and could be reversed by addition of a beta-blocker (propranolol or metoprolol).[5,39,46] Studies have shown that in caffeine-induced calcium release experiments, mutated cells had lower SR Ca2+ load in base line and during adrenergic stimulation; these results represent the enhanced average open period of mutated RYR2.[16,38,40,46] Specifically in CPVT2, CASQ2-mutated cells exhibit marked diastolic [Ca2+]i rise in response to adrenergic stimulation, likely to be due to decreased binding efficiency of Ca2+ by CASQ2.[5] Screening for antiarrhythmic treatments in CPVT-iPSC-CMs could be well studied by combining Ca2+ transient measurement, contraction and electrophysiological investigation.
Antiarrhythmic Pharmacological Candidates for CPVT Treatment
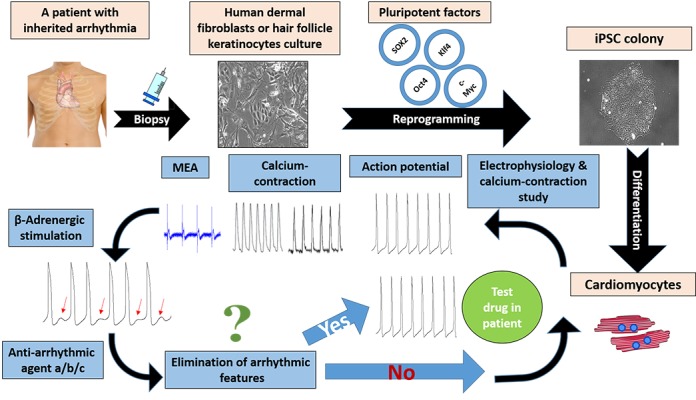
Studies suggested different antiarrhythmic agents for CPVT treatment, depending on the specific point mutations in RYR2 or CASQ2 and on its location. RYR2 is comprised of more than 100 exons and contains about 5000 amino acids, leading to a variety of mutations that can be found in this gene, including point mutations and deletions with varying locations from the C-terminal to the N-terminal of ryanodine receptor. It is clearly understood that mutations in different locations have dissimilar effects on protein dysfunction, resulting in the necessity of different agents to interact with different parts of RYR2 in order to compensate for function loss. The emerging field of personalized medicine acknowledges the importance of patient-specific drug screening and could be thoroughly employed using iPSC-CMs as in vitro model, as demonstrated in Fig. 2. Some progress has been done with few drug candidates linked to specific RYR2 mutations, as proof of concept, presenting the feasibility of this approach.
Figure 2. Representation of the personalized medicine concept in CPVT investigation. The process begins with obtaining skin or hair follicles biopsy from patient followed by reprograming procedure to generate iPSC cell-line holding the ability of differentiating into functional cardiomyocytes. To evaluate the effect of different anti-arrhythmic drugs on patient specific iPSC-CMs the scheme continues with electrophysiology and calcium-contraction study, involving external β-adrenergic stimulation resulting in arrhythmia represented as DADs. Subsequently the model allows the assessment of different anti-arrhythmic drugs for elimination of in vitro arrhythmias. Prominent agents should be tested for their effect within the patient.
Dantrolene
Dantrolene, a muscle relaxant is used as a treatment for malignant hyperthermia, caused by skeletal ryanodine receptor (RYR1) mutations.[49] Datrolene stabilizes the closed state of RYR1 and hence its therapeutic effect; it has also been demonstrated to interact with RYR2.[50] In order to explore its therapeutic effects on CPVT phenotype caused by RYR2 mutation, researchers have generated iPSC-CMs from CPVT1 patient with an autosomal dominant missense RYR2 mutation: S406L. RYR2 dislocation due to its mutation has been excluded by comparing immunefluorescent stainings of CPVT1 and healthy control iPSC-CMs. As expected, increased diastolic Ca2+ levels have been induced by isoproterenol in CPVT-iPSC-CMs, but not in control cells. Furthermore, SR Ca2+ load has been increased in response to isoproterenol in control iPSC-CMs, but not in mutated iPSC-CMs. The investigators have concluded that during chatecholamine stimulation, the increased luminal Ca2+ caused by S406L mutation is due to diastolic Ca2+ leak from the SR. It has been proposed that S406L increases Ca2+ sensitivity of RYR2 and produces diastolic spontaneous activity due to a lower threshold. Ca2+ leak by hyperactive RYR2 under catecholaminergic stress elevated diastolic Ca2+ and decreased SR Ca2+ load in CPVT-iPSC-CMs. Diastolic Ca2+ leak can generate arrhythmia by NCX activation, causing for depolarizing currents, represented as DADs.[51] DADs and TA have been observed in 89% of mutated cells in comparison to 34% in control cells. Stress-induced diastolic Ca2+ leak from the SR via RYR2 could be explained by one of 2 mechanisms:
RYR2 mutations destroy essential inter-domain interactions that stabilize the closed state.[52,53]
RYR2 mutations abolish significant interactions with its modulating proteins.
It has been shown that S406L mutation is located in an inter-domain position, hence the validity of the first mechanism for this RYR2 mutation. Binding of dantrolene to the N-terminal end of skeletal and cardiac RYRs has restored normal Ca2+ handling in CPVT-iPSC-CMs during basal and chatecholamine-stimulation conditions. Furthermore, dantrolene has eliminated the appearance of DADs and TA, and has reestablished the wild-type phenotype, supporting the hypothesis that abnormal inter domain interactions are involved in this RYR2 mutation. Dantrolene has been proved as effective for S406L mutation; probably by restoring essential inter domain interactions within RYR2. To determine its effect on different mutations, the process should be repeated for each unique mutation. In a clinical study for dantrolene-treatment in CPVT1 patients, it markedly reduced arrhythmias in a subgroup of patients, depending on the mutation site within the RYR2 protein.[1] Dantrolene has antiarrhythmic effect only on patients with mutations in the N-terminal or in the central region of the protein; however, it has no antiarrhythmic effect on CPVT-iPSC-CMs with mutations in the trans-membrane region. The researchers disclaimed that exceptional cases have been observed and that the antiarrhythmic effect of dantrolene could not be well determined by the mutation location solely, illustrating the significance of patient-specific drug screening model for CPVT.[1] Furthermore, the results of this research on human subjects, alongside with patient-specific iPSC-CMs, indicate on similar dantrolene effect both in the clinical setting and in the corresponding in vitro model; these findings support the feasibility of in vitro drug screening while avoiding unnecessary side effects in the patient. Dantrolene binding site is a part of the domain switch region, implying for the involved mechanism in restoring defective unzipping and in stabilizing inter-domain interactions, eventually leading to inhibition of Ca2+ leak.[1,5,14,38,54,55]
Flecainide
Flecainide is categorized as class Ic antiarrhythmic drug and may contribute to the most common yet insufficient CPVT beta-blocker treatments. Its contributing effect originates, probably, from the reduction in the availability of membrane sodium channels and the increase of TA threshold[16,38,56,57] or from the blockade of RYR2 in the SR membrane, which decreasing its opening probability.[16,38,58-60] It eliminated DADs and TA in CPVT-iPSC-CMs.[16]
β-Blockers
A side from commonly used β-blockers and the regarding in vitro studies (such as: metoprolol and bisoprolol), the development of new modalities and the patient-specific in vitro screening of existing antiarrhythmic drugs is encouraged before it could be introduced to a CPVT patient. VK-II-86, a synthetic analog of carvedilol, acts as β-blockers and had prevented stress-induced arrhythmia in CPVT mice model, yet has not been tested on human CPVT-iPSC-CMs.[38]
CaMKII Inhibitors
The inhibition of Ca2+/calmodulin-dependent serine–threonine protein kinase II (CaMKII), which phosphorylates main components of calcium handling machinery, has been shown to prevent β adrenergic-induced arrhythmias. KN-93 (2-[N- (2-hydroxyethyl)]-N-(4methoxybenzenesulfonyl)]-amino-N-(4-chlorocinnamyl)-N-methylbenzylamine), a CaMKII inhibitor has prevented arrhythmogenic features in CPVT-iPSC-CMs.[6] It has eliminated isoproterenol-induced DADs and has restored normal calcium handling within cardiomyocytes. In 3D contracting EBs of CPVT-iPSC-CMs multiple sites have been observed for the initiation of calcium transients that, later on, have collided during propagation. KN-93 treatment has resulted in a single initiation site similarly to healthy control-iPSC-CMs.[6]
Conclusions
iPSC-CMs generated from CPVT patients recapitulate the disease specific phenotypes and by this, act as an applicable model for the investigation of key feature phenotypes as well as for the disease mechanism. The most promising ability of this model is with screening and developing of new modalities for patient specific personalized pharmacological treatments. We believe that in the following future, research of iPSC-CMs from CPVT patient, taking the advantages of this well-established model, will give new meaning for the bench to bed-side transition. Using patient specific iPSC-CMs the screening of existing alongside to newly developed pharmacological-agents will spare unnecessary exhausting and risky exploration within the patient’s body. The most prominent drugs to be screened are those previously proven effective in CPVT-mice models such as: ranolazine and propafenone.[61] The currently most used therapeutic treatments including pharmacological β-blockers, implantable cardioverter-defibrillators (ICD) and left cardiac sympathetic denervation must be improved by the developing of new modalities in order to minimize the events of ICD-firing, resuscitation and mortality in CPVT patients.
Disclosures
None.
References
- 1.Penttinen Kirsi, Swan Heikki, Vanninen Sari, Paavola Jere, Lahtinen Annukka M, Kontula Kimmo, Aalto-Setälä Katriina. Antiarrhythmic Effects of Dantrolene in Patients with Catecholaminergic Polymorphic Ventricular Tachycardia and Replication of the Responses Using iPSC Models. PLoS ONE. 2015;10 (5) doi: 10.1371/journal.pone.0125366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leenhardt A, Lucet V, Denjoy I, Grau F, Ngoc D D, Coumel P. Catecholaminergic polymorphic ventricular tachycardia in children. A 7-year follow-up of 21 patients. Circulation. 1995 Mar 1;91 (5):1512–9. doi: 10.1161/01.cir.91.5.1512. [DOI] [PubMed] [Google Scholar]
- 3.Swan H, Piippo K, Viitasalo M, Heikkilä P, Paavonen T, Kainulainen K, Kere J, Keto P, Kontula K, Toivonen L. Arrhythmic disorder mapped to chromosome 1q42-q43 causes malignant polymorphic ventricular tachycardia in structurally normal hearts. J. Am. Coll. Cardiol. 1999 Dec;34 (7):2035–42. doi: 10.1016/s0735-1097(99)00461-1. [DOI] [PubMed] [Google Scholar]
- 4.Coumel P, Fidelle J, Lucet V, Attuel P BY. Catecholaminergic-induced severe ventricular arrhythmias with adams-stokes syndrome in children: Report of four cases. Br Hear. J. 1978;40:28–37. [Google Scholar]
- 5.Novak Atara, Barad Lili, Zeevi-Levin Naama, Shick Revital, Shtrichman Ronit, Lorber Avraham, Itskovitz-Eldor Joseph, Binah Ofer. Cardiomyocytes generated from CPVTD307H patients are arrhythmogenic in response to β-adrenergic stimulation. J. Cell. Mol. Med. 2012 Mar;16 (3):468–82. doi: 10.1111/j.1582-4934.2011.01476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Pasquale E, Lodola F, Miragoli M, Denegri M, Avelino-Cruz J E, Buonocore M, Nakahama H, Portararo P, Bloise R, Napolitano C, Condorelli G, Priori S G. CaMKII inhibition rectifies arrhythmic phenotype in a patient-specific model of catecholaminergic polymorphic ventricular tachycardia. Cell Death Dis. 2013;4 doi: 10.1038/cddis.2013.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lahat Hadas, Pras Elon, Eldar Michael. A missense mutation in CASQ2 is associated with autosomal recessive catecholamine-induced polymorphic ventricular tachycardia in Bedouin families from Israel. Ann. Med. 2004;36 Suppl 1 ():87–91. doi: 10.1080/17431380410032517. [DOI] [PubMed] [Google Scholar]
- 8.Priori S G, Napolitano C, Tiso N, Memmi M, Vignati G, Bloise R, Sorrentino V, Danieli G A. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001 Jan 16;103 (2):196–200. doi: 10.1161/01.cir.103.2.196. [DOI] [PubMed] [Google Scholar]
- 9.Novak Atara, Lorber Avraham, Itskovitz-Eldor Joseph, Binah Ofer. Modeling Catecholaminergic Polymorphic Ventricular Tachycardia using Induced Pluripotent Stem Cell-derived Cardiomyocytes. Rambam Maimonides Med J. 2012 Jul;3 (3) doi: 10.5041/RMMJ.10086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roux-Buisson Nathalie, Cacheux Marine, Fourest-Lieuvin Anne, Fauconnier Jeremy, Brocard Julie, Denjoy Isabelle, Durand Philippe, Guicheney Pascale, Kyndt Florence, Leenhardt Antoine, Le Marec Hervé, Lucet Vincent, Mabo Philippe, Probst Vincent, Monnier Nicole, Ray Pierre F, Santoni Elodie, Trémeaux Pauline, Lacampagne Alain, Fauré Julien, Lunardi Joël, Marty Isabelle. Absence of triadin, a protein of the calcium release complex, is responsible for cardiac arrhythmia with sudden death in human. Hum. Mol. Genet. 2012 Jun 15;21 (12):2759–67. doi: 10.1093/hmg/dds104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nyegaard Mette, Overgaard Michael T, Søndergaard Mads T, Vranas Marta, Behr Elijah R, Hildebrandt Lasse L, Lund Jacob, Hedley Paula L, Camm A John, Wettrell Göran, Fosdal Inger, Christiansen Michael, Børglum Anders D. Mutations in calmodulin cause ventricular tachycardia and sudden cardiac death. Am. J. Hum. Genet. 2012 Oct 5;91 (4):703–12. doi: 10.1016/j.ajhg.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am. J. Physiol. 1983 Jul;245 (1):C1–14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- 13.Bers Donald M. Cardiac excitation-contraction coupling. Nature. 2002 Jan 10;415 (6868):198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 14.Marx Steven O, Marks Andrew R. Dysfunctional ryanodine receptors in the heart: new insights into complex cardiovascular diseases. J. Mol. Cell. Cardiol. 2013 May;58:225–31. doi: 10.1016/j.yjmcc.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venetucci Luigi, Denegri Marco, Napolitano Carlo, Priori Silvia G. Inherited calcium channelopathies in the pathophysiology of arrhythmias. Nat Rev Cardiol. 2012 Oct;9 (10):561–75. doi: 10.1038/nrcardio.2012.93. [DOI] [PubMed] [Google Scholar]
- 16.Kujala Kirsi, Paavola Jere, Lahti Anna, Larsson Kim, Pekkanen-Mattila Mari, Viitasalo Matti, Lahtinen Annukka M, Toivonen Lauri, Kontula Kimmo, Swan Heikki, Laine Mika, Silvennoinen Olli, Aalto-Setälä Katriina. Cell model of catecholaminergic polymorphic ventricular tachycardia reveals early and delayed afterdepolarizations. PLoS ONE. 2012;7 (9) doi: 10.1371/journal.pone.0044660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leenhardt Antoine, Denjoy Isabelle, Guicheney Pascale. Catecholaminergic polymorphic ventricular tachycardia. Circ Arrhythm Electrophysiol. 2012 Oct;5 (5):1044–52. doi: 10.1161/CIRCEP.111.962027. [DOI] [PubMed] [Google Scholar]
- 18.van der Werf Christian, Wilde Arthur A M. Catecholaminergic polymorphic ventricular tachycardia: from bench to bedside. Heart. 2013 Apr;99 (7):497–504. doi: 10.1136/heartjnl-2012-302033. [DOI] [PubMed] [Google Scholar]
- 19.Laitinen P J, Brown K M, Piippo K, Swan H, Devaney J M, Brahmbhatt B, Donarum E A, Marino M, Tiso N, Viitasalo M, Toivonen L, Stephan D A, Kontula K. Mutations of the cardiac ryanodine receptor (RyR2) gene in familial polymorphic ventricular tachycardia. Circulation. 2001 Jan 30;103 (4):485–90. doi: 10.1161/01.cir.103.4.485. [DOI] [PubMed] [Google Scholar]
- 20.Priori Silvia G, Chen S R Wayne. Inherited dysfunction of sarcoplasmic reticulum Ca2+ handling and arrhythmogenesis. Circ. Res. 2011 Apr 1;108 (7):871–83. doi: 10.1161/CIRCRESAHA.110.226845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bers Donald M. Cardiac ryanodine receptor phosphorylation: target sites and functional consequences. Biochem. J. 2006 May 15;396 (1):e1–3. doi: 10.1042/BJ20060377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Priori Silvia G, Napolitano Carlo, Memmi Mirella, Colombi Barbara, Drago Fabrizio, Gasparini Maurizio, DeSimone Luciano, Coltorti Fernando, Bloise Raffaella, Keegan Roberto, Cruz Filho Fernando E S, Vignati Gabriele, Benatar Abraham, DeLogu Angelica. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2002 Jul 2;106 (1):69–74. doi: 10.1161/01.cir.0000020013.73106.d8. [DOI] [PubMed] [Google Scholar]
- 23.Cerrone Marina, Cummings Samori, Alansari Tarek, Priori Silvia G. A clinical approach to inherited arrhythmias. Circ Cardiovasc Genet. 2012 Oct 1;5 (5):581–90. doi: 10.1161/CIRCGENETICS.110.959429. [DOI] [PubMed] [Google Scholar]
- 24.Jaenisch Rudolf, Young Richard. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell. 2008 Feb 22;132 (4):567–82. doi: 10.1016/j.cell.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans M J, Kaufman M H. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981 Jul 9;292 (5819):154–6. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 26.Thomson J A, Itskovitz-Eldor J, Shapiro S S, Waknitz M A, Swiergiel J J, Marshall V S, Jones J M. Embryonic stem cell lines derived from human blastocysts. Science. 1998 Nov 6;282 (5391):1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 27.GURDON J B. The transplantation of nuclei between two species of Xenopus. Dev. Biol. 1962 Aug;5:68–83. doi: 10.1016/0012-1606(62)90004-0. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi Kazutoshi, Tanabe Koji, Ohnuki Mari, Narita Megumi, Ichisaka Tomoko, Tomoda Kiichiro, Yamanaka Shinya. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007 Nov 30;131 (5):861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi Kazutoshi, Yamanaka Shinya. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006 Aug 25;126 (4):663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 30.Priori Silvia G, Napolitano Carlo, Di Pasquale Elisa, Condorelli Gianluigi. Induced pluripotent stem cell-derived cardiomyocytes in studies of inherited arrhythmias. J. Clin. Invest. 2013 Jan;123 (1):84–91. doi: 10.1172/JCI62838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soldner Frank, Hockemeyer Dirk, Beard Caroline, Gao Qing, Bell George W, Cook Elizabeth G, Hargus Gunnar, Blak Alexandra, Cooper Oliver, Mitalipova Maisam, Isacson Ole, Jaenisch Rudolf. Parkinson's disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009 Mar 6;136 (5):964–77. doi: 10.1016/j.cell.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maehr René, Chen Shuibing, Snitow Melinda, Ludwig Thomas, Yagasaki Lisa, Goland Robin, Leibel Rudolph L, Melton Douglas A. Generation of pluripotent stem cells from patients with type 1 diabetes. Proc. Natl. Acad. Sci. U.S.A. 2009 Sep 15;106 (37):15768–73. doi: 10.1073/pnas.0906894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jung Christian B, Moretti Alessandra, Mederos y Schnitzler Michael, Iop Laura, Storch Ursula, Bellin Milena, Dorn Tatjana, Ruppenthal Sandra, Pfeiffer Sarah, Goedel Alexander, Dirschinger Ralf J, Seyfarth Melchior, Lam Jason T, Sinnecker Daniel, Gudermann Thomas, Lipp Peter, Laugwitz Karl-Ludwig. Dantrolene rescues arrhythmogenic RYR2 defect in a patient-specific stem cell model of catecholaminergic polymorphic ventricular tachycardia. EMBO Mol Med. 2012 Mar;4 (3):180–91. doi: 10.1002/emmm.201100194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Penttinen Kirsi, Siirtola Harri, Àvalos-Salguero Jorge, Vainio Tiina, Juhola Martti, Aalto-Setälä Katriina. Novel Analysis Software for Detecting and Classifying Ca2+ Transient Abnormalities in Stem Cell-Derived Cardiomyocytes. PLoS ONE. 2015;10 (8) doi: 10.1371/journal.pone.0135806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun Ning, Yazawa Masayuki, Liu Jianwei, Han Leng, Sanchez-Freire Veronica, Abilez Oscar J, Navarrete Enrique G, Hu Shijun, Wang Li, Lee Andrew, Pavlovic Aleksandra, Lin Shin, Chen Rui, Hajjar Roger J, Snyder Michael P, Dolmetsch Ricardo E, Butte Manish J, Ashley Euan A, Longaker Michael T, Robbins Robert C, Wu Joseph C. Patient-specific induced pluripotent stem cells as a model for familial dilated cardiomyopathy. Sci Transl Med. 2012 Apr 18;4 (130) doi: 10.1126/scitranslmed.3003552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lan Feng, Lee Andrew S, Liang Ping, Sanchez-Freire Veronica, Nguyen Patricia K, Wang Li, Han Leng, Yen Michelle, Wang Yongming, Sun Ning, Abilez Oscar J, Hu Shijun, Ebert Antje D, Navarrete Enrique G, Simmons Chelsey S, Wheeler Matthew, Pruitt Beth, Lewis Richard, Yamaguchi Yoshinori, Ashley Euan A, Bers Donald M, Robbins Robert C, Longaker Michael T, Wu Joseph C. Abnormal calcium handling properties underlie familial hypertrophic cardiomyopathy pathology in patient-specific induced pluripotent stem cells. Cell Stem Cell. 2013 Jan 3;12 (1):101–13. doi: 10.1016/j.stem.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han Lu, Li Yang, Tchao Jason, Kaplan Aaron D, Lin Bo, Li You, Mich-Basso Jocelyn, Lis Agnieszka, Hassan Narmeen, London Barry, Bett Glenna C L, Tobita Kimimasa, Rasmusson Randall L, Yang Lei. Study familial hypertrophic cardiomyopathy using patient-specific induced pluripotent stem cells. Cardiovasc. Res. 2014 Nov 1;104 (2):258–69. doi: 10.1093/cvr/cvu205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fatima Azra, Xu Guoxing, Shao Kaifeng, Papadopoulos Symeon, Lehmann Martin, Arnáiz-Cot Juan J, Rosa Angelo O, Nguemo Filomain, Matzkies Matthias, Dittmann Sven, Stone Susannah L, Linke Matthias, Zechner Ulrich, Beyer Vera, Hennies Hans Christian, Rosenkranz Stephan, Klauke Baerbel, Parwani Abdul S, Haverkamp Wilhelm, Pfitzer Gabriele, Farr Martin, Cleemann Lars, Morad Martin, Milting Hendrik, Hescheler Juergen, Saric Tomo. In vitro modeling of ryanodine receptor 2 dysfunction using human induced pluripotent stem cells. Cell. Physiol. Biochem. 2011;28 (4):579–92. doi: 10.1159/000335753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Itzhaki Ilanit, Maizels Leonid, Huber Irit, Gepstein Amira, Arbel Gil, Caspi Oren, Miller Liron, Belhassen Bernard, Nof Eyal, Glikson Michael, Gepstein Lior. Modeling of catecholaminergic polymorphic ventricular tachycardia with patient-specific human-induced pluripotent stem cells. J. Am. Coll. Cardiol. 2012 Sep 11;60 (11):990–1000. doi: 10.1016/j.jacc.2012.02.066. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X-H, Haviland S, Wei H, Sarić T, Fatima A, Hescheler J, Cleemann L, Morad M. Ca2+ signaling in human induced pluripotent stem cell-derived cardiomyocytes (iPS-CM) from normal and catecholaminergic polymorphic ventricular tachycardia (CPVT)-afflicted subjects. Cell Calcium. 2013 Aug;54 (2):57–70. doi: 10.1016/j.ceca.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu Junying, Vodyanik Maxim A, Smuga-Otto Kim, Antosiewicz-Bourget Jessica, Frane Jennifer L, Tian Shulan, Nie Jeff, Jonsdottir Gudrun A, Ruotti Victor, Stewart Ron, Slukvin Igor I, Thomson James A. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007 Dec 21;318 (5858):1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 42.Itskovitz-Eldor J, Schuldiner M, Karsenti D, Eden A, Yanuka O, Amit M, Soreq H, Benvenisty N. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol. Med. 2000 Feb;6 (2):88–95. [PMC free article] [PubMed] [Google Scholar]
- 43.Lian Xiaojun, Zhang Jianhua, Azarin Samira M, Zhu Kexian, Hazeltine Laurie B, Bao Xiaoping, Hsiao Cheston, Kamp Timothy J, Palecek Sean P. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat Protoc. 2013 Jan;8 (1):162–75. doi: 10.1038/nprot.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Batalov Ivan, Feinberg Adam W. Differentiation of Cardiomyocytes from Human Pluripotent Stem Cells Using Monolayer Culture. Biomark Insights. 2015;10 (Suppl 1):71–6. doi: 10.4137/BMI.S20050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hwang Hyun Seok, Kryshtal Dmytro O, Feaster T K, Sánchez-Freire Verónica, Zhang Jianhua, Kamp Timothy J, Hong Charles C, Wu Joseph C, Knollmann Björn C. Comparable calcium handling of human iPSC-derived cardiomyocytes generated by multiple laboratories. J. Mol. Cell. Cardiol. 2015 Aug;85:79–88. doi: 10.1016/j.yjmcc.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Novak Atara, Barad Lili, Lorber Avraham, Gherghiceanu Mihaela, Reiter Irina, Eisen Binyamin, Eldor Liron, Itskovitz-Eldor Joseph, Eldar Michael, Arad Michael, Binah Ofer. Functional abnormalities in iPSC-derived cardiomyocytes generated from CPVT1 and CPVT2 patients carrying ryanodine or calsequestrin mutations. J. Cell. Mol. Med. 2015 Aug;19 (8):2006–18. doi: 10.1111/jcmm.12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Catanzaro John N, Nett Michael P, Rota Marcello, Vassalle Mario. On the mechanisms underlying diastolic voltage oscillations in the sinoatrial node. J Electrocardiol. 2006 Jul;39 (3):342. doi: 10.1016/j.jelectrocard.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 48.Kim E M, Choy Y, Vassalle M. Mechanisms of suppression and initiation of pacemaker activity in guinea-pig sino-atrial node superfused in high [K+]o. J. Mol. Cell. Cardiol. 1997 May;29 (5):1433–45. doi: 10.1006/jmcc.1997.0382. [DOI] [PubMed] [Google Scholar]
- 49.Kobayashi Shigeki, Yano Masafumi, Suetomi Takeshi, Ono Makoto, Tateishi Hiroki, Mochizuki Mamoru, Xu Xiaojuan, Uchinoumi Hitoshi, Okuda Shinichi, Yamamoto Takeshi, Koseki Noritaka, Kyushiki Hiroyuki, Ikemoto Noriaki, Matsuzaki Masunori. Dantrolene, a therapeutic agent for malignant hyperthermia, markedly improves the function of failing cardiomyocytes by stabilizing interdomain interactions within the ryanodine receptor. J. Am. Coll. Cardiol. 2009 May 26;53 (21):1993–2005. doi: 10.1016/j.jacc.2009.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paul-Pletzer Kalanethee, Yamamoto Takeshi, Bhat Manjunatha B, Ma Jianjie, Ikemoto Noriaki, Jimenez Leslie S, Morimoto Hiromi, Williams Philip G, Parness Jerome. Identification of a dantrolene-binding sequence on the skeletal muscle ryanodine receptor. J. Biol. Chem. 2002 Sep 20;277 (38):34918–23. doi: 10.1074/jbc.M205487200. [DOI] [PubMed] [Google Scholar]
- 51.Schlotthauer K, Bers D M. Sarcoplasmic reticulum Ca(2+) release causes myocyte depolarization. Underlying mechanism and threshold for triggered action potentials. Circ. Res. 2000 Oct 27;87 (9):774–80. doi: 10.1161/01.res.87.9.774. [DOI] [PubMed] [Google Scholar]
- 52.George Christopher H, Jundi Hala, Thomas N Lowri, Fry Debra L, Lai F Anthony. Ryanodine receptors and ventricular arrhythmias: emerging trends in mutations, mechanisms and therapies. J. Mol. Cell. Cardiol. 2007 Jan;42 (1):34–50. doi: 10.1016/j.yjmcc.2006.08.115. [DOI] [PubMed] [Google Scholar]
- 53.Ikemoto N, Yamamoto T. Postulated role of inter-domain interaction within the ryanodine receptor in Ca(2+) channel regulation. Trends Cardiovasc. Med. 2000 Oct;10 (7):310–6. doi: 10.1016/s1050-1738(01)00067-6. [DOI] [PubMed] [Google Scholar]
- 54.Liu Nian, Napolitano Carlo, Venetucci Luigi A, Priori Silvia G. Flecainide and antiarrhythmic effects in a mouse model of catecholaminergic polymorphic ventricular tachycardia. Trends Cardiovasc. Med. 2012 Feb;22 (2):35–9. doi: 10.1016/j.tcm.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 55.Sullivan S, Egli D, Akutsu H, Melton DA, Eggan K, Cowan C. Derivation of human embryonic stem cells. In: eds. 2007:35–51. [Google Scholar]
- 56.Liu Nian, Denegri Marco, Ruan Yanfei, Avelino-Cruz José Everardo, Perissi Andrea, Negri Sara, Napolitano Carlo, Coetzee William A, Boyden Penelope A, Priori Silvia G. Short communication: flecainide exerts an antiarrhythmic effect in a mouse model of catecholaminergic polymorphic ventricular tachycardia by increasing the threshold for triggered activity. Circ. Res. 2011 Jul 22;109 (3):291–5. doi: 10.1161/CIRCRESAHA.111.247338. [DOI] [PubMed] [Google Scholar]
- 57.Parikh Ashish, Mantravadi Rajkumar, Kozhevnikov Dmitry, Roche Michael A, Ye Yanping, Owen Laura J, Puglisi Jose Luis, Abramson Jonathan J, Salama Guy. Ranolazine stabilizes cardiac ryanodine receptors: a novel mechanism for the suppression of early afterdepolarization and torsades de pointes in long QT type 2. Heart Rhythm. 2012 Jun;9 (6):953–60. doi: 10.1016/j.hrthm.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Watanabe Hiroshi, Chopra Nagesh, Laver Derek, Hwang Hyun Seok, Davies Sean S, Roach Daniel E, Duff Henry J, Roden Dan M, Wilde Arthur A M, Knollmann Björn C. Flecainide prevents catecholaminergic polymorphic ventricular tachycardia in mice and humans. Nat. Med. 2009 Apr;15 (4):380–3. doi: 10.1038/nm.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Volders P G, Kulcśar A, Vos M A, Sipido K R, Wellens H J, Lazzara R, Szabo B. Similarities between early and delayed afterdepolarizations induced by isoproterenol in canine ventricular myocytes. Cardiovasc. Res. 1997 May;34 (2):348–59. doi: 10.1016/s0008-6363(96)00270-2. [DOI] [PubMed] [Google Scholar]
- 60.Spencer C Ian, Sham James S K. Effects of Na+/Ca2+ exchange induced by SR Ca2+ release on action potentials and afterdepolarizations in guinea pig ventricular myocytes. Am. J. Physiol. Heart Circ. Physiol. 2003 Dec;285 (6):H2552–62. doi: 10.1152/ajpheart.00274.2003. [DOI] [PubMed] [Google Scholar]
- 61.Hwang Hyun Seok, Hasdemir Can, Laver Derek, Mehra Divya, Turhan Kutsal, Faggioni Michela, Yin Huiyong, Knollmann Björn C. Inhibition of cardiac Ca2+ release channels (RyR2) determines efficacy of class I antiarrhythmic drugs in catecholaminergic polymorphic ventricular tachycardia. Circ Arrhythm Electrophysiol. 2011 Apr;4 (2):128–35. doi: 10.1161/CIRCEP.110.959916. [DOI] [PMC free article] [PubMed] [Google Scholar]


