Abstract
Background: Atrial fibrillation (Afib) patients are at an increased risk of stroke. Patients at moderate to high risk of stroke typically receive antithrombotics, placing them at an increased risk of bleeding. The HAS-BLED tool has been validated in Afib patients receiving warfarin for prediction of major bleeding events. Although HAS-BLED has been researched in patients receiving warfarin, this tool has not been validated with the novel anticoagulant rivaroxaban.
Methods: The trial design was retrospective case-control approved by the Institutional Review Board at University of Tennessee Medical Center. Patients who were identified as having a bleeding event were cross-referenced with a list of patients receiving rivaroxaban. Inclusion criteria were adult patients with atrial fibrillation who were taking rivaroxaban for at least six months, with a CHA2DS2-VASc score greater than or equal to 2 OR CHADS2 score greater than or equal to 1. The primary endpoint is the predictive ability of HAS-BLED as measured through the c-statistic. Secondary endpoints include correlation of HAS-BLED and bleeding risk.
Results: After reviewing 9621 medical records, 15 patients met the inclusion criteria for major bleeding. Ninety patients were randomly selected for inclusion as the matched control group. The predictive ability of HAS-BLED was not statistically significant (c statistic = 0.68; p = 0.07), but did show some diagnostic ability to predict major bleeding events. Patients with major bleeding were more likely to have a history of bleeding and use concomitant antiplatelet agents. There were significantly more patients with a HAS-BLED score greater than or equal to 3 in the patients that experienced a major bleeding event.
Conclusion: HAS-BLED demonstrated some diagnostic ability to predict major bleeding events in patients receiving rivaroxaban but this was not statistically significant due to limited sample size.
Keywords: Atrial Fibrillation, HAS-BLED, Rivaroxaban
Introduction
Atrial fibrillation patients are at a five-fold increased risk of ischemic stroke.[1] With the aging population, the incidence of atrial fibrillation increases annually, putting more patients at risk for stroke. CHADS2 and CHA2DS2-VASc are proven risk assessment tools to determine a patient’s annual risk of ischemic stroke. CHADS2 includes congestive heart failure, hypertension, age greater than 75, diabetes and prior stroke or transient ischemic attack. The 2012 CHEST guidelines recommended anticoagulation in patients with a CHADS2 score greater than or equal to one indicating an annual stroke risk of at least 2.8%.[2] Two years later, CHADS2 was updated to include vascular disease, female sex and age greater than 65; this became the CHADS2-VASc scoring system. The AHA/ACC atrial fibrillation guidelines recommend anticoagulation when a patient’s annual stroke risk is greater than 1.3%, i.e. CHA2DS2-VASc greater than or equal to 2.[3] When determining the best anticoagulation option for these patients, it is imperative to weigh the risk of stroke with the risk of bleeding. Historically, bleeding risk was determined by subjective opinion of a practitioner. The European Heart Survey determined that many patients were denied guideline recommended anticoagulation due to perceived increased bleeding risk, despite no objective evaluation.[4] Insufficient anticoagulation in atrial fibrillation patients leads to a significant increase in stroke risk.[5] Subsequently, several bleeding risk assessment tools were derived for atrial fibrillation patients.
The HAS-BLED bleeding risk assessment tool has been proven to be more clinically useful in predicting major bleeding as compared to other bleeding risk schemas.[6-11] The criteria making up HAS-BLED are listed in table 1. A HAS-BLED score greater than or equal to 3 indicates a high risk of major bleeding. HAS-BLED has been validated in patients with atrial fibrillation receiving warfarin, but there is inadequate evidence in the novel anticoagulants.[7] There is a retrospective review of patients receiving dabigatran which found to have a higher risk of bleeding when the HAS-BLED score was greater or equal to three.[12] To our knowledge, the present study is the first to evaluate HAS-BLED in patients receiving rivaroxaban. This retrospective analysis was designed to identify if the HAS-BLED bleeding risk assessment tool can be used in atrial fibrillation patients receiving rivaroxaban to determine major bleeding risk.
Table 1.
| H | Hypertension | 1 point |
| A | Abnormal renal or liver function | 2 points possible |
| S | Stroke or TIA | 1 point |
| B | Bleeding or predisposition | 1 point |
| L | Labile INR | 1 point |
| E | Age greater than or equal to 65 | 1 point |
| D | Drugs or Alcohol concomitantly | 2 points possible |
Methods
This study was a single center, retrospective case-control study designed to identify the predictive ability of the HAS-BLED bleeding risk assessment tool in patients receiving rivaroxaban. This study was approved by the University of Tennessee Medical Center (UTMC) institutional review board (IRB). For the case patients, adult UTMC patients were eligible for inclusion if they had atrial fibrillation and a major bleeding event while on rivaroxaban. Major bleeding was defined by the following criteria: primary reason for hospitalization as a bleeding event, need for red blood cell transfusion of 1 unit or more or hemoglobin drop of at least 2 g/L.[7] Patients were included if they were also a patient within the University Cardiology electronic medical record (EMR) and had their major bleeding event between October 2011 – October 2014. Once the patients with major bleeding were identified, a control group of atrial fibrillation patients receiving rivaroxaban were matched based on CHADS2 and CHADS2-VASc. These patients were identified by a report generated from the University Cardiology EMR of patients receiving rivaroxaban during the pre-defined timeframe. Patients were excluded if they were pregnant, had another indication for anticoagulation, or if they were missing any information necessary to calculate HAS-BLED, CHADS2, or CHA2DS2-VASc scores. Dosing of rivaroxaban was up to the discretion of the provider.
The primary endpoint was the predictive ability of HAS-BLED for bleeding, as defined by the c-statistic. The secondary endpoints included demographic predictors of bleeding and the predictive ability of CHADS2 and CHA2S2-VASc for major bleeding. Secondary endpoints and baseline characteristics were analyzed with the chi square test, Fisher’s exact test, Student’s t test, and Wilcoxon rank sum test. All p values were two-sided and considered statistically significant if less than 0.05. Statistical analyses were performed using the SPSS Version 21 software (Armonk, NY: IBM Corp.).
Results
Between October 2011 and October 2014, there were 85 patients identified to have experienced a non-traumatic bleeding event and also found in the University Cardiology EMR. Fifteen patients met the inclusion criteria and were included in the analysis. 887 patients were identified as having received rivaroxaban during the study period. Ninety patients were randomly selected as atrial fibrillation patients without bleeding events. The primary reason for exclusion was prior treatment with dabigatran. All control patients received rivaroxaban for at least six months. Baseline characteristics are summarized in table 2. Baseline characteristics were not significantly different regarding age, gender, weight, serum creatinine or rivaroxaban dose. There were significantly more patients with hypertension in the non-bleeding group as compared to the bleeding group (67% vs 89%, p = 0.023). Patients who bled had a significantly lower hemoglobin as compared to the control group (9.46 g/L v. 13.25 g/L p < 0.001).
Table 2. Group characteristics.
| Characteristic | Bleeds (n=15) | No Bleeds (n=90) | P Value |
|---|---|---|---|
| Age in yrs (SD) | 73.3 (11.9) | 72.04 (10.52) | - |
| Gender (% males) | 8 (53) | 43 (47.78) | - |
| Weight in kg (BMI in kg/m2) | 87.2 (30.3) | 88.85 (25.4) | - |
| Serum creatinine in mg/dL (SD) | 1.01 (0.31) | 0.96 (0.32) | - |
| Estimated GFR in mL/min (SD) | 57.79 (21.83) | 65.48 (27.02) | - |
| Antiplatelet use (%) | 11 (73) | 37 (41.11) | 0.02 |
| Hemoglobin (SD) | 9.46 (2.36) | 13.25 (1.96) | <0.001 |
| Hematocrit (SD) | 29.46 (6.84) | 44.34 (5.22) | <0.001 |
| Rivaroxaban dose (%) | - | ||
| 20 mg | 8 (53.33) | 56 (62.22) | |
| 15 mg | 6 (40) | 33 (36.67) | |
| 10 mg | 1 (6.67) | 1 (1.11) | |
| Presence of drug interactions (%) | 5 (33.3) | 18 (20) | - |
| CHADS2 (SD) | 2.07 (1.33) | 2.26 (1) | - |
| CHA2DS2-VASc (SD) | 3.47 (1.13) | 3.62 (1.47) | - |
| Heart failure (%) | 5 (33.33) | 26 (28.89) | - |
| Hypertension (%) | 10 (66.67) | 80 (88.89) | 0.023 |
| Age ≥ 65 years of age (%) | 13 (86.67) | 68 (75.56) | - |
| Age ≥ 75 years of age (%) | 6 (40) | 43 (47.78) | - |
| Diabetes (%) | 4 (26.67) | 26 (28.89) | - |
| Stroke (%) | 3 (20) | 14 (15.56) | - |
| Vascular disease (%) | 0 (0) | 12 (13.33) | - |
| HAS-BLED (SD) | 3.13 (1.18) | 2.58 (0.87) | 0.035 |
| Abnormal renal function (%) | 1 (6.67) | 1 (1.11) | - |
| Abnormal liver function (%) | 0 (0) | 2 (2.22) | - |
| Bleeding history (%) | 4 (26.67) | 5 (5.56) | 0.023 |
| Labile INR (%) | 1 (6.67) | 7 (7.78) | - |
| NSAID use (%) | 3 (20) | 11 (12.2) | - |
| Alcohol use (%) | 1 (6.67) | 8 (8.89) | - |
| HAS-BLED ≥ 3 | 6 (40) | 11 (12.22) | 0.015 |
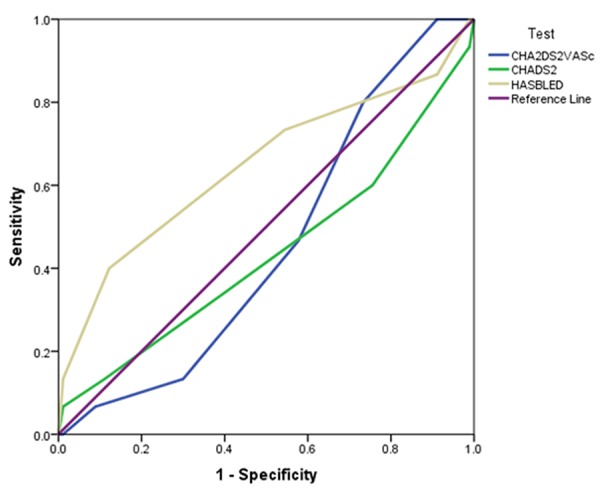
For the primary endpoint, the c-statistic of 0.68 was not statistically significant but this was highly indicative of a type II error (table 3). There were significantly more patients with a HAS-BLED score greater than or equal to three in the bleeding group. There were significantly more patients on an antiplatelet agent in the bleeding group as compared to the non-bleeding control group (73% v. 41.11%; p = 0.02). The average HAS-BLED score was significantly higher in the bleeding group as compared to the non-bleeding group (3.13 [SD 1.18] v. 2.58 [SD 0.87]; p = 0.035). The bleeding group was five-times more likely to have a history of bleeding as compared to the control group (26.67% v. 5.56%; p = 0.023). As compared to CHADS2 and CHA2DS2-VASc, HAS-BLED was the scoring system which showed a trend toward predicting major bleeding events in patients receiving rivaroxaban (figure 1).
Table 3. C-statistics for scoring systems.
| Predictive Ability of Scoring System | C Statistic | P Value |
|---|---|---|
| HAS-BLED | 0.65 | 0.07 |
| CHADS2 | 0.44 | 0.43 |
| CHA2DS2-VASc | 0.45 | 0.57 |
Figure 1. Receiver operator characteristic (ROC) for three scoring systems.
Discussion
Findings from this study indicate the HAS-BLED score for patients on rivaroxaban has some diagnostic ability to predict major bleeding, but this was not statistically significant. The calculated c-statistic in this study was similar and in some cases higher than previously published studies.[6,7] Patients were more likely to have a HAS-BLED score greater than or equal to three in the bleeding population. Based on the results of Lip et al., a HAS-BLED score greater than 3 is indicative of a high risk of bleeding.[6] To our knowledge, this is the first study to evaluate the predictive ability of HAS-BLED in atrial fibrillation patients receiving rivaroxaban.
Major bleeding events were found to be more likely in patients receiving antiplatelet agents or with a history of bleeding. These characteristics are not included in the CHADS2 and CHA2DS2-VASc scoring systems, which limits their predictive abilities as a bleeding risk scoring system. This adds to the growing body of evidence that HAS-BLED should be used in conjunction with stroke assessment tools to guide anticoagulation decisions in patients with atrial fibrillation.
Conclusions
There are several limitations within this study. One of the most apparent limitations is the small sample size. This trial was a single site, retrospective analysis which may not have included major bleeding events at another institution. Future researchers should seek out a larger sample size to further validate the diagnostic ability of the HAS-BLED scoring system to predict major bleeding events. Another limitation is the fact that major bleeding was defined in accordance to the original HAS-BLED definition which is less exclusive as compared to other major bleeding criteria; therefore patients may have been included in this analysis that would have been excluded if a more stringent definition was used. In conclusion, for atrial fibrillation patients receiving rivaroxaban, the HAS-BLED scoring system demonstrated some diagnostic ability to predict major bleeding events but this was not statistically significant due to limited sample size.
Disclosures
None.
References
- 1.Wang Yishen, Bajorek Beata. Safe use of antithrombotics for stroke prevention in atrial fibrillation: consideration of risk assessment tools to support decision-making. Ther Adv Drug Saf. 2014 Feb;5 (1):21–37. doi: 10.1177/2042098613506592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.You John J, Singer Daniel E, Howard Patricia A, Lane Deirdre A, Eckman Mark H, Fang Margaret C, Hylek Elaine M, Schulman Sam, Go Alan S, Hughes Michael, Spencer Frederick A, Manning Warren J, Halperin Jonathan L, Lip Gregory Y H. Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012 Feb;141 (2 Suppl):e531S–75S. doi: 10.1378/chest.11-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.January Craig T, Wann L Samuel, Alpert Joseph S, Calkins Hugh, Cigarroa Joaquin E, Cleveland Joseph C, Conti Jamie B, Ellinor Patrick T, Ezekowitz Michael D, Field Michael E, Murray Katherine T, Sacco Ralph L, Stevenson William G, Tchou Patrick J, Tracy Cynthia M, Yancy Clyde W. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2014 Dec 2;64 (21):e1–76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 4.Nieuwlaat Robby, Capucci Alessandro, Camm A John, Olsson S Bertil, Andresen Dietrich, Davies D Wyn, Cobbe Stuart, Breithardt Günter, Le Heuzey Jean-Yves, Prins Martin H, Lévy Samuel, Crijns Harry J G M. Atrial fibrillation management: a prospective survey in ESC member countries: the Euro Heart Survey on Atrial Fibrillation. Eur. Heart J. 2005 Nov;26 (22):2422–34. doi: 10.1093/eurheartj/ehi505. [DOI] [PubMed] [Google Scholar]
- 5.Wyse D G, Waldo A L, DiMarco J P, Domanski M J, Rosenberg Y, Schron E B, Kellen J C, Greene H L, Mickel M C, Dalquist J E, Corley S D. A comparison of rate control and rhythm control in patients with atrial fibrillation. N. Engl. J. Med. 2002 Dec 5;347 (23):1825–33. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 6.Lip Gregory Y H, Frison Lars, Halperin Jonathan L, Lane Deirdre A. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J. Am. Coll. Cardiol. 2011 Jan 11;57 (2):173–80. doi: 10.1016/j.jacc.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 7.Pisters Ron, Lane Deirdre A, Nieuwlaat Robby, de Vos Cees B, Crijns Harry J G M, Lip Gregory Y H. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010 Nov;138 (5):1093–100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 8.Lip Gregory Y H, Lin Hung-Ju, Hsu Hsiu-Ching, Su Ta-Chen, Chen Ming-Fong, Lee Yuan-Teh, Chien Kuo-Liong. Comparative assessment of the HAS-BLED score with other published bleeding risk scoring schemes, for intracranial haemorrhage risk in a non-atrial fibrillation population: the Chin-Shan Community Cohort Study. Int. J. Cardiol. 2013 Oct 3;168 (3):1832–6. doi: 10.1016/j.ijcard.2012.12.076. [DOI] [PubMed] [Google Scholar]
- 9.Apostolakis S, Lane D A, Buller H, Lip G Y H. Comparison of the CHADS2, CHA2DS2-VASc and HAS-BLED scores for the prediction of clinically relevant bleeding in anticoagulated patients with atrial fibrillation: the AMADEUS trial. Thromb. Haemost. 2013 Nov;110 (5):1074–9. doi: 10.1160/TH13-07-0552. [DOI] [PubMed] [Google Scholar]
- 10.Apostolakis Stavros, Lane Deirdre A, Guo Yutao, Buller Harry, Lip Gregory Y H. Performance of the HEMORR 2 HAGES, ATRIA, and HAS-BLED bleeding risk-prediction scores in nonwarfarin anticoagulated atrial fibrillation patients. J. Am. Coll. Cardiol. 2013 Jan 22;61 (3):386–7. doi: 10.1016/j.jacc.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Roldán Vanessa, Marín Francisco, Manzano-Fernández Sergio, Gallego Pilar, Vílchez Juan Antonio, Valdés Mariano, Vicente Vicente, Lip Gregory Y H. The HAS-BLED score has better prediction accuracy for major bleeding than CHADS2 or CHA2DS2-VASc scores in anticoagulated patients with atrial fibrillation. J. Am. Coll. Cardiol. 2013 Dec 10;62 (23):2199–204. doi: 10.1016/j.jacc.2013.08.1623. [DOI] [PubMed] [Google Scholar]
- 12.Nunez AS, Htun WW, Patel K, Kim J, Fernaine G. Validity of HAS-BLED Score in Prediction of Major Bleeding Risk in Patients with Atrial Fibrillation Taking Warfarin and Dabigatran. Circulation. 2012;126:A15722. [Google Scholar]


