Abstract
Reactive oxygen species as potent regulators of leaf development poses special interest for cell expansion.
The conditions under which plants grow greatly fluctuate and require that plants continuously monitor their environment and adjust their developmental program accordingly. Recent advances have indicated a clear and distinct role for reactive oxygen species (ROS) in both environmental stress sensing and guiding plant development. Leaf growth is a flexible process in which the final shape and size of the organ is tailored to the environment. Both during development under controlled conditions as well as during abiotic stress, cell expansion in leaves is in part controlled through the regulation of apoplastic ROS homeostasis. The effect of different ROS types on cell wall properties is well documented, but how plants control apoplastic ROS homeostasis is poorly understood. Furthermore, ROS appear to influence other cellular processes that guide cell expansion, including water uptake and cytoskeleton dynamics. Here, an overview of our current understanding on the role of ROS in leaf cell expansion is given and avenues for future research directions are highlighted.
ROS have long been documented as damaging molecules that especially accumulate during stress in plants. Abiotic stresses, such as drought and salinity, are characterized by an initial growth reduction of leaves and the induction of programmed cell death under prolonged stress conditions (Loggini et al., 1999; Hernández et al., 2001). The notion that alterations in the growth rate of leaves correlate with ROS homeostasis have paved the way for a more fundamental role of ROS in the regulation of plant development. Although during abiotic stress ROS levels rise, this does not necessarily apply to the growth zone of the leaf. For instance, studies on leaf expansion in maize (Zea mays) under saline conditions revealed that not an increase in ROS but a decrease caused retarded leaf growth (Rodríguez et al., 2004). Moreover, exposure of maize to salinity or drought causes an increase in the antioxidant capacity of the leaf and thereby restricts cell expansion (Bernstein et al., 2010; Kravchik and Bernstein, 2013; Avramova et al., 2015). Then again, an increase in ROS levels also can result in a restriction of cell growth under abiotic stress conditions (MacAdam and Grabber, 2002; Simonovicova et al., 2004), indicating that ROS have a dual role in the regulation of cell expansion. Still, ROS homeostasis does not act alone, as abiotic stresses like salinity, drought, or osmotic stress all interfere with the water balance and cause a reduction in cell turgor, which affects the mechanical power of the cell to expand (Schopfer, 2006).
The initial observations under abiotic stress have attracted a broad research interest in the relationship between cell growth and ROS. Nowadays, it is clear that ROS homeostasis does not only control growth under stress conditions but also during plant development. ROS, like hydrogen peroxide (H2O2), hydroxyl radicals (˙OH), and superoxide radicals (O2−), have been implicated in developmental processes and emerged as key signaling molecules in plants (Schmidt and Schippers, 2015). For instance, during root hair and pollen elongation, ROS play fundamental roles in the spatial regulation of polar cell growth (Takeda et al., 2008; Kaya et al., 2014). Still, the role of ROS in the regulation of leaf development remains unclear. The leaf emerges at the flank of the shoot apical meristem and at first grows through active cell division (Beemster et al., 2005; Polyn et al., 2015; Schippers et al., 2016). The final size of the leaf is determined by the subsequent expansion of leaf cells, which accounts for up to 95% of final leaf area. Enlargement of leaf cells is driven by two major regulators: turgor pressure and cell wall dynamics (Gonzalez et al., 2012). In addition, cell expansion requires the synthesis and distribution of new biomaterials to sustain growth.
Developmental, biochemical, and abiotic stress studies have revealed the importance of ROS homeostasis in the apoplast for the regulation of cell expansion (Fry, 1998; Rodríguez et al., 2002; Lu et al., 2014; Avramova et al., 2015). Here, we highlight known and potential roles for ROS in the main processes that control cell enlargement during leaf growth.
ROS IN THE APOPLAST: THE OXIDANT’S PLAYGROUND
Cell expansion is the result of a delicate balance between wall relaxation and wall stiffening (Wolf et al., 2012). The primary cell wall consists out of cellulose, hemicellulose, pectin, and structural proteins (Geisler et al., 2008). In primary cell walls, the cellulose matrix is cross-linked by hemicellulose and pectin molecules, in such a fashion that it provides mechanical strength but still allows for cell expansion. Loosening of the cell wall is in part regulated by two classes of proteins (expansins and xyloglucan endotransglucosylases/hydrolases) that act on the interaction between xyloglucans, the major hemicellulose polymer, and the cellulose network (Park and Cosgrove, 2012). Studies on leaf expansion in maize under saline conditions revealed a decreased O2−-derived ˙OH production in the apoplast and a consequent reduction in leaf growth (Rodríguez et al., 2004). Interestingly, it was found that ˙OH promotes cell elongation by loosening the cell wall through oxidative cleavage of polymers like xyloglucan and pectin (Fry, 1998; Müller et al., 2009). In the presence of transition metals, the Haber-Weiss reaction can convert H2O2 and O2− anions to ˙OH, a reaction that can be performed both nonenzymatically as well as enzymatically (Chen and Schopfer, 1999). Although, H2O2 is a prerequisite for the formation of ˙OH and thereby for oxidative cell wall loosening, a buildup of H2O2 causes cross-linking of the cell wall and restricts elongation growth (Schopfer, 1996). Indeed, during salt, drought, or osmotic stress, an increase in the level of apoplastic H2O2 has been noticed and was linked to the inhibition of leaf growth (Lin and Kao, 2001; Kravchik and Bernstein, 2013; Avramova et al., 2015). These observations argue that the apoplastic balance between ˙OH and H2O2 regulates cell expansion during leaf growth, but how is ROS homeostasis regulated in the apoplast?
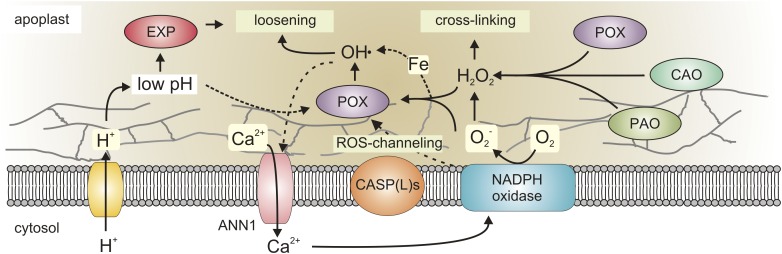
This fundamental question is complex to answer, as large apoplastic and membrane protein families contribute to ROS production (Fig. 1). Among the enzymes that contribute to apoplastic ROS homeostasis are peroxidases (POXs), amine oxidases, oxalate oxidases, and NADPH oxidases (Kärkönen and Kuchitsu, 2015). NADPH oxidases are plasma membrane-located enzymes that, in a Ca2+-dependent manner, produce O2− in the apoplast (Fig. 1). The O2− dismutates into H2O2, which can cause cross-linking of the cell wall or is converted into the cell wall loosening ˙OH (Richards et al., 2015). During biotic stress, NADPH oxidases produce an oxidative burst to eliminate invading pathogens and send stress signals (Suzuki et al., 2011); during abiotic stress, they appear to mainly act as stress signal amplifiers to promote adaptive responses. If NADPH oxidase-derived O2− is required for cell wall stiffening by H2O2, then loss-of-function mutants would be expected to develop more elongated cells. In contrast, mutations in NADPH oxidases have been shown to impair the elongation of root hairs and pollen tubes (Foreman et al., 2003; Takeda et al., 2008; Kaya et al., 2014) and the growth of leaves (Chaouch et al., 2012). Thus, NADPH oxidases are positive regulators of cell growth. So, how can plants control the transition of the NADPH oxidase-derived O2− into ˙OH to promote cell expansion? A recent report suggests that NADPH oxidase-derived ROS is channeled to apoplastic peroxidases during lignification in roots, by a protein scaffold that brings both proteins in close proximity (Fig. 1; Lee et al., 2013). NADPH oxidase/peroxidase scaffolds have evolved in animals as single proteins, the DUOX class of NADPH oxidases. Still, DUOX proteins are absent in plants, indicating a necessity for a flexible protein scaffold. The identified scaffold proteins belong to the CASPARIAN STRIP MEMBRANE DOMAIN PROTEIN (CASP) family, which, in Arabidopsis, has 20 members (Roppolo et al., 2014). Therefore, it would be interesting to determine which CASP-LIKE proteins might regulate ROS channeling from NADPH oxidases during leaf growth.
Figure 1.
Cell wall remodeling and apoplastic ROS homeostasis. ROS have a dual role in regulating cell expansion. NADPH oxidase-derived ROS can both promote as well as restrict cell wall extensibility. H2O2 derived from the dismutation of O2− or produced through the action of cell wall-located POXs, polyamine oxidases (POA), or copper-containing amine oxidase (COA) results in dehydration and cross-linking of cell wall polymers. On the other hand, H2O2 and O2− can act as substrates for the Haber-Weiss reaction or pH-dependent POXs, leading to the production of highly reactive ˙OH. The ˙OH results in cleavage of cell wall polymers and thereby promotes cell wall loosening. The pH-dependent formation of ˙OH by POXs can operate in parallel to the pH-dependent activation of EXPANSINs (EXPs) to stimulate cell growth. ROS channeling from NADPH oxidases to POXs is mediated by CASP-LIKE proteins [CASP(L)s], allowing for control over the balance between H2O2 and ˙OH production. In addition, apoplastic ˙OH provokes activation of the Ca2+ transporter ANN1. The influx of Ca2+ might act as a feed-forward signal, since it can activate NADPH oxidases.
The secreted class III POXs in Arabidopsis (Arabidopsis thaliana) consists of 73 members with largely unexplored biochemical properties (Passardi et al., 2004). Early reports on cell growth revealed an inverse correlation between cell growth and POX activity (Fry, 1986; Goldberg et al., 1987). Also, during abiotic stress, POX activity increases and can cause lignification and rigidification of the cell wall, limiting leaf growth (Heggie et al., 2005; Lee et al., 2007). In Arabidopsis, several POX genes have been linked to the regulation of cell expansion during leaf development. Plants overexpressing POX57 or POX71 show a reduced leaf size due to the accumulation of H2O2 (Lu et al., 2014; Raggi et al., 2015). Interestingly, both POXs only affect cell expansion, but not proliferation, indicating that POXs might be specifically employed during the regulation of cell growth. However, not all apoplastic POXs generate H2O2, some do the opposite while others convert H2O2 and O2− into ˙OH (Chen and Schopfer, 1999; Liszkay et al., 2003). The enzymatic generation of ˙OH in the apoplast by POXs requires a low pH, which can be achieved through the action of auxin (Schopfer et al., 2002). Still, it is not known which of the 73 class III POX proteins contribute to ˙OH formation.
The accumulation of ˙OH in the apoplast activates the atypical Ca2+-permeable ANNEXIN1 (ANN1) channel (Laohavisit et al., 2012). Studies on ANN1 in Arabidopsis indicate that loss-of-function mutants have reduced vegetative growth (Konopka-Postupolska et al., 2009). The influx of Ca2+ might act as an amplification mechanism for the production of ˙OH, as it activates NADPH oxidases (Renew et al., 2005). Intriguingly, ANN1 is a multifunctional protein that also exhibits peroxidase activity (Gorecka et al., 2005). As stated above, a low pH can promote ˙OH production by several apoplastic POXs, suggesting that ANN1 might also contribute to the production of apoplastic ROS. This assumption would be worth to test as it would place the action of ANN1 into a different perspective, allowing for a feed-forward model in which NADPH oxidase-dependent ˙OH production is amplified by the activation of ANN1.
Next to NADPH oxidases and POXs, other enzymes also contribute to ROS homeostasis in the apoplast. Both amine and oxalate oxidases have a major impact on apoplastic H2O2 production (Kärkönen and Kuchitsu, 2015). Two types of amine oxidases, copper-containing oxidases and polyamine oxidases, catalyze the oxidative deamination of di- and polyamines, resulting in the formation of H2O2 (Ghuge et al., 2015). To what extent these enzymes contribute to growth regulation during development still needs to be further explored.
Among the large number of proteins that control ROS homeostasis at the cell wall, several have now been demonstrated to have a role in regulating cell expansion during leaf growth. Still, additional research efforts are needed to understand how a cell manages to produce ROS in a specific and timely manner to control cell growth. The recent discovery of potential protein scaffolds that modulate ROS channeling at the apoplast represent attractive new avenues for future research efforts.
WATER UPTAKE AND TRANSPORT OF H2O2
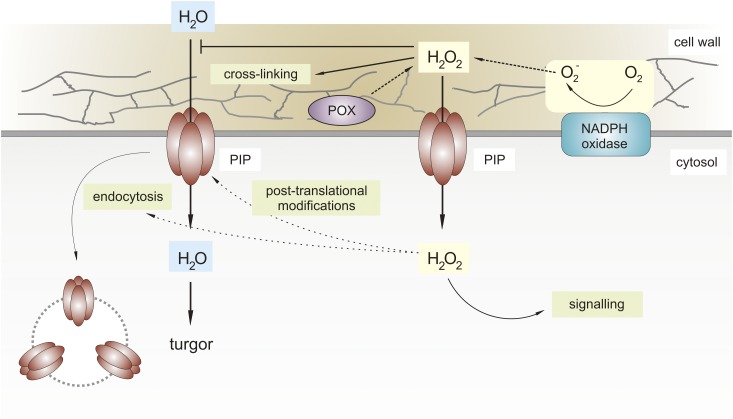
Leaf growth relies on active water uptake through controlled transport across membranes (Steudle and Frensch, 1996). Water uptake by cells from the apoplast is facilitated by aquaporins (AQPs). Interestingly, AQPs contribute to the permeability of lipid membranes for a variety of neutral molecules, which are, next to water itself, carbon dioxide and H2O2 (Bienert and Chaumont, 2014). Thus, movement of apoplastic H2O2 relies on an active transport system, which might serve several functions (Fig. 2). For several AQPs, roles in leaf growth have been demonstrated (Chaumont et al., 1998; Lee et al., 2012). Overexpression of PIP1;2 from Arabidopsis in tobacco (Nicotiana tabacum) speeds up whole-plant growth and increases leaf dry matter accumulation under nonstress conditions. Thus, water transport via AQPs represents a rate-limiting step for growth (Aharon et al., 2003).
Figure 2.
H2O2 modulates water uptake by aquaporins. Turgor pressure-driven cell expansion is controlled by AQPs, which facilitate the transport of H2O across the plasma membrane. Uptake of extracellular H2O2, derived from either NADPH oxidases or apoplastic POXs, across the membrane is regulated by AQPs. In the apoplast, a buildup of H2O2 limits cell growth by cross-linking the cell wall. In addition, the influx of H2O2 might also limit the water uptake as it appears to activate the endocytosis of AQPs, regulating their abundance at the plasma membrane. Thus, apoplastic H2O2 could cause both cell wall stiffening and the inhibition of water uptake.
The transport of H2O2 across membranes via plant AQPs was first demonstrated using yeast (Bienert et al., 2007; Dynowski et al., 2008). Expression of Arabidopsis PIP2;1, PIP2;4, TIP1;1, or TIP1;2 increased intracellular accumulation of H2O2 (Bienert et al., 2007). The comparable dipole moment and molecular diameter of H2O2 and H2O allow these molecules to undergo hydrogen bonds with residues at the inner surface of the AQP pore (Bienert and Chaumont, 2014). Consistently, mutation of a cytosolic His residue within AtPIP2;1 impairs both water transport and H2O2 uptake (Dynowski et al., 2008).
Considering the negative role of H2O2 on cell wall extensibility, it was assumed that H2O2 might also interfere with AQP activity. Indeed, H2O2 treatment of the alga Chara corallina significantly reduced the water permeability of the plasma membrane (Henzler et al., 2004). Interestingly, AQPs contain redox-sensitive Cys residues that form disulphide bonds between PIP monomers, which could be potential targets of H2O2 to deactivate the channel (Bienert et al., 2012). However, mutation of these conserved Cys residues into redox-insensitive residues did not affect the channeling activity of maize PIPs under oxidizing or reducing conditions in oocytes (Bienert et al., 2012). Still, to determine whether this is also the case in plant cells, one should complement specific aqp mutants with their corresponding redox-insensitive forms.
PIPs move into or out of membrane microdomains, allowing a dynamic distribution between the plasma membrane and intracellular compartments to control water permeability of the cell (Boursiac et al., 2008; Wudick et al., 2015). Abiotic stress conditions, which result in increased H2O2 levels, promote PIP endocytosis. Moreover, intracellular accumulation of PIPs in Arabidopsis upon salt stress was prevented in the presence of catalase (Boursiac et al., 2008). Furthermore, a phosphoproteomics study points to oxidative stress-induced posttranslational modifications of PIPs that initiate endocytosis (Prak et al., 2008). Differential phosphorylation of two Ser residues within the C terminus of AtPIP2;1 upon H2O2 treatment correlated with redistribution of AQPs from the plasma membrane to intracellular vesicles (Prak et al., 2008). A lower PIP abundance at the plasma membrane ultimately results in diminished water transport through the membrane (Wudick et al., 2015). Thus, H2O2 not only restricts cell expansion by cross-linking the cell wall, but potentially also reduces water transport across the membrane, by promoting the endocytosis of PIPs (Fig. 2).
ROS AND THE REGULATION OF THE CYTOSKELETON AND VESICLE TRANSPORT DURING EXPANSION
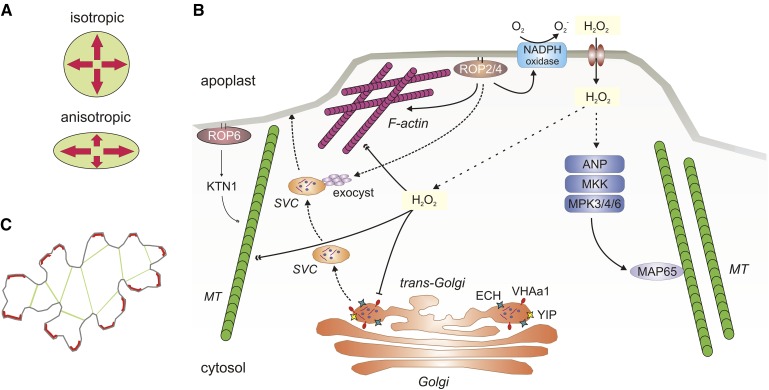
Cell size and shape is controlled by the extension of the cell wall (Smith and Oppenheimer, 2005). The building blocks for cell growth are delivered by Golgi-derived vesicles that are transported to the cell surface through the action of the cytoskeleton. The cytoskeleton allows for controlling the growth direction of cells by guiding the delivery of Golgi-derived vesicles and the cellulose synthase machinery (Geisler et al., 2008). Uniform cell enlargement in all directions is called isotropic expansion (Fig. 3A), while cell growth along a preferred axis, or in a preferred direction, is known as anisotropic expansion (Crowell et al., 2010). During leaf growth, mesophyll cells mainly show isotropic expansion while epidermal cells show mainly anisotropic expansion (Fu et al., 2002). Still, in single cells, both isotropic and anisotropic growth can occur at the same time (Crowell et al., 2010).
Figure 3.
Interplay between ROS, the cytoskeleton, and vesicle transport during expansion growth. A, Isotropic growth implies an equal rate of cell expansion in all direction, whereas anisotropic growth modulates cell shape through differential cell expansion rates. B, Schematic overview of the integration points between ROS and cell growth processes that involve the cytoskeleton and transport of Golgi-derived vesicles. ROP-mediated activation of NADPH oxidases results in an increased level of H2O2. The H2O2 can enter the cell through the action of AQPs and there directly cause depolymerization of either F-actin or MTs. In addition, ROP promotes the exocytosis of trans-Golgi-derived SVCs that contain novel cell wall material. Formation of SVCs relies on the action of ECH, YIP, and the ATPase VHAa1. Accumulation of H2O2 causes oxidation of VHAa1 and subsequent inhibition of vesicle transport. Next to the promotive effect of ROP2/4 on F-actin formation in the outgrowing region of a cell, ROP6 mainly acts through KTN1 on the MT bundling to restrict cellular outgrowth. In addition, MT orientation is controlled by MAP65 proteins, which act downstream of the H2O2-activated MAPK cascade, consisting of ANP, MKK6, MAPK3, MAPK4, and MAPK6.
The importance of microtubules (MTs) and actin filaments in the regulation of cell expansion has been widely demonstrated. For leaf development, ACTIN2 and ACTIN7 also are required for optimal growth (Gilliland et al., 2002) together with numerous tubulin genes (Ishida et al., 2007; Komis et al., 2011). In contrast to its name, the cytoskeleton consists of a highly dynamic and mobile network of protein polymers. MTs and actin filaments exhibit a treadmilling movement caused by a fast rate of net polymerization at the plus end and a slower rate of depolymerization at the minus end (Blanchoin et al., 2010). Interestingly, microtubules and actin filaments are known to be very sensitive to ROS, and emerging evidence suggests that redox cues impact on cytoskeleton dynamics (Livanos et al., 2012). Chemical interference with ROS homeostasis results in the reorientation and replacement of MTs in plants. For instance, NADPH oxidase activity has been shown to modulate MT and actin polymerization upon abiotic stress or treatment with cytoskeletal toxins (Yao et al., 2011; Liu et al., 2012). Interestingly, tolerance of plants toward cold stress is associated with the stability of microtubules (Wang and Nick, 2001). Plants that maintain microtubule stability show cold-resistant leaf expansion (Ahad et al., 2003). Oxidative stress as part of abiotic stress can result in oxidative modifications of tubulin and actin proteins (Fig. 3B; Wang et al., 2012) and reorientation of the cytoskeleton.
Next to a direct effect of H2O2 on cytoskeleton polymers, it also modulates the activity of microtubule/actin-associated proteins (MAPs; Fig. 3B). MAPs are direct targets of MAPK cascades (Komis et al., 2011), of which some have a role in ROS signaling (Schmidt and Schippers, 2015). ARABIDOPSIS NUCLEUS- AND PHRAGMOPLAST-LOCALIZED KINASE1-RELATED PROTEIN KINASE1 (ANP1), ANP2, and ANP3 encode MAP3Ks that are activated by H2O2 and initiate a signaling cascade involving MKK6 and the MAPKs MAPK3, MAPK4, and MAPK6 (Kovtun et al., 2000; Beck et al., 2010). ANPs are required during cytokinesis, but also regulate pavement cell expansion, since anp2anp3 mutants show aberrant epidermal cell morphology (Beck et al., 2010). Notably, downstream the MAPK cascade act two conserved key regulators of cortical MT array organization, the microtubule-associated proteins MAP65-1 and MAP65-2, which both have been implicated in ROS responses (Lucas et al., 2011; Zhu et al., 2013; Livanos et al., 2012). Potentially, H2O2 sensed by the ANP cascade modulates cytoskeleton dynamics during cell proliferation and expansion (Fig. 3B). Additional research efforts are needed to understand the importance of the ANP cascade in ROS signaling during cell growth.
The Golgi is a highly diverse organelle with roles in many different physiological and molecular processes within the plant, including cell growth (Fu et al., 2002). Still, a potential role of the Golgi in oxidative stress responses in plants was presented only recently (Lee et al., 2015). The trans-Golgi network regulates cell expansion by directing vesicle trafficking and targeting (Gendre et al., 2011). Secretion of pectin and hemicellulose, the two major cell wall polysaccharides, is controlled by a trans-Golgi network-localized complex formed by YPT/RAB GTPase Interacting Protein 4a (YIP4a), YIP4b, and ECHIDNA (ECH; Gendre et al., 2013). In addition, the vacuolar H+-ATPase subunit a1 (VHAa1) colocalizes with the ECH/YIP4 complex. As chemical inhibition of VHAa1 activity mimics ech/yip4 mutants, the proteins appear to act in the same pathway (Gendre et al., 2011). Since VHAa1 activity is redox regulated (Tavakoli et al., 2001), it might represent an integration site for ROS within the trafficking network. Treatment of Arabidopsis with H2O2 results in Cys oxidation of VHA subunits (Waszczak et al., 2014), which inactivates their H+-pumping activity (Tavakoli et al., 2001). Thus, oxidative stress might act as a brake on vesicle trafficking, which could hamper cell expansion. The mobile secretory vesicle compartments (SVCs) move toward the cell surface to interact with the exocyst complex (Hála et al., 2008) and deliver cell wall material during growth. Rho of plant (ROP) GTPases, together with their effector proteins, have been shown to interact with exocyst subunits to mediate directed cell growth (Lavy et al., 2007; Uhrig and Hülskamp, 2014). Next to a role for ROP proteins in vesicle trafficking, they have also been implemented in the regulation of actin and MTs during leaf pavement cell morphogenesis (Fu et al., 2002; Oda and Fukuda, 2012). On the one hand, activated ROP2/4 promotes the formation of F-actin networks required for the lateral outgrowth of a lobe (Fu et al., 2005); on the other hand, activation of ROP6 promotes the MT-severing activity of KATANIN1 (KTN1), which results in the reassembly and alignment of MTs to restrict cell growth in the neck regions of the developing pavement cell (Fig. 3, B and C). Furthermore, NADPH oxidases are activated by a direct interaction with ROP proteins, indicating an intimate relation between the regulation of ROS homeostasis and the diverse functions of ROP during cell elongation (Wong et al., 2007). How H2O2 and NADPH oxidase activity regulate cytoskeleton dynamics and vesicle trafficking is poorly understood and requires extensive research efforts in the future.
TRANSCRIPTIONAL REGULATION OF ROS HOMEOSTASIS DURING LEAF EXPANSION
Under nonstress conditions, the final size of plant leaves is predictable due to the strict genetic control of cell proliferation and expansion. Although many transcription factors regulating cell proliferation have been identified (Polyn et al., 2015), those controlling leaf expansion growth are largely lacking (Powell and Lenhard, 2012).
Thus far, only two transcription factors specifically regulating leaf cell expansion were reported: KUODA1 (KUA1) and ZHD5 (Lu et al., 2014; Hong et al., 2011). ZHD5 belongs to the group of zinc finger homeodomain (ZHD) transcription factors and its activity is regulated by a mini zinc finger protein (MIF1). Scanning electron microscopy of epidermal cells of ZHD5 overexpression lines revealed bigger cell sizes compared to the wild type (Hong et al., 2011). Overexpression of the non-DNA binding MIF1 causes a great decrease in leaf area due to the inhibition of ZHD5 (Hong et al., 2011; Hu et al., 2008). Still, the factors causing cell enlargement and the direct targets of ZHD5 are not known (Hong et al., 2011).
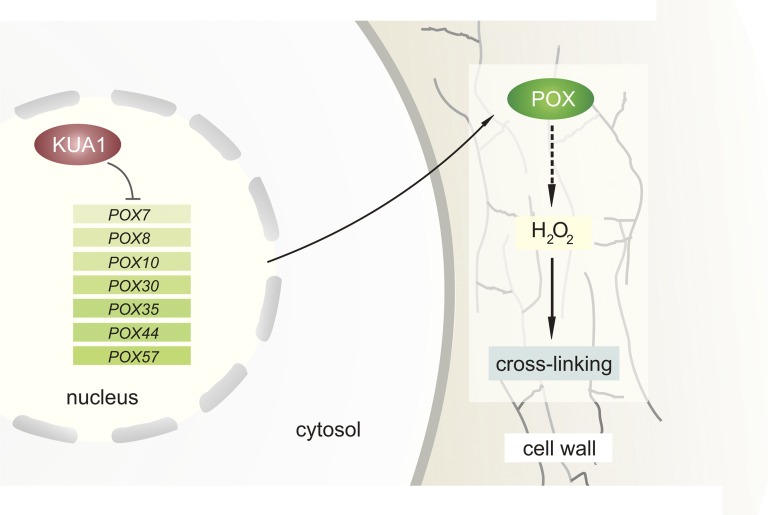
KUA1 is a MYB-like transcription factor that is upregulated during expansion growth of the leaf (Lu et al., 2014). Overexpression of KUA1 results in an increase in leaf cell size compared to the wild type, while kua1 mutants show a decrease in cell size. Detailed analysis revealed that KUA1 negatively regulates POX activity by direct repression of seven POX genes during leaf growth (Lu et al., 2014). As mentioned above, class III POXs can promote cell wall loosening or cross-linking depending on the apoplastic conditions. KUA1-regulated POXs increase apoplastic H2O2 levels, which restricts expansion (Fig. 4). Therefore, KUA1 positively regulates leaf growth by lowering the levels of apoplastic H2O2 and promoting cell wall relaxation (Lu et al., 2014). Still, as H2O2 acts as a precursor for the production of ˙OH, it is likely that the regulation of POX activity within the cell wall is more complex.
Figure 4.
Transcriptional regulation of ROS homeostasis during leaf expansion. Cell expansion is restricted by cell wall stiffening due to the action of H2O2. During cell expansion in Arabidopsis, the levels of apoplastic H2O2 are maintained low through the action of the transcription factor KUA1. KUA1 represses the expression of seven POX genes (POX7, POX8, POX10, POX30, POX35, POX44, and POX57), which produce H2O2 in the apoplast. The repression of the POX genes is crucial for cell wall relaxation and expansion.
Interestingly, in roots, a transcription factor of the bHLH family similarly acts on POX gene expression. UPBEAT (UPB1) is mainly expressed in the root elongation zone and its overexpression causes a decrease in root cell size, but increased cell proliferation, resulting in longer roots compared to the wild type (Tsukagoshi et al., 2010). Among the direct target genes of UPB1, 21 encode POXs, including POX57, which is also targeted by KUA1 in leaves (Tsukagoshi et al., 2010; Lu et al., 2014). While KUA1-regulated POXs promote cell wall stiffening in leaves, the POXs controlled by UPB1 in roots mainly cause cell wall relaxation by either scavenging H2O2 or promoting the formation of ˙OH. In both cases, loss of the transcriptional regulators results in increased H2O2 levels and decreased cell size, indicating that the biochemical action of H2O2 in roots and leaves on cell enlargement is conserved. Notably, UPB1 was shown to act independently from plant hormones, while overexpression of KUA1 is accompanied by an increase in auxin (Kwon et al., 2013). As KUA1 and UPB1 show no homology, it indicates that plants evolved diverse transcriptional regulators to control POXs.
So far, to our knowledge, KUA1 is the first described transcription factor with a clearly defined role in modulating leaf cell expansion through the regulation of ROS homeostasis. In accordance with the successful identification of transcription factors controlling cell proliferation (Polyn et al., 2015), it is expected that future research will reveal additional transcriptional regulators of leaf cell expansion.
CONCLUSIONS AND PERSPECTIVES
Since the initial discovery that ROS play pivotal roles in the regulation of root hair and pollen tube elongation, a picture of the complex interplay between ROS and growth is starting to emerge. That said, here, we have specifically highlighted our current understanding of the regulation of cell expansion by ROS homeostasis during leaf growth. Although the exact molecular details are incomplete, it is clear that ROS are implemented in all processes that control leaf expansion. The modulation of cell wall extensibility and water uptake by ROS are convincing examples of how fine-tuning of ROS signaling modulates growth. Still, our current understanding only hints to a role of ROS in cytoskeleton dynamics and vesicle trafficking (see Outstanding Questions). Nonetheless, the field of ROS-regulated development foresees a fruitful future with many novel molecular mechanisms and transcriptional regulators to be identified that will provide fundamental understanding of the regulation of multicellular development.
Glossary
- ROS
reactive oxygen species
- POX
peroxidase
- AQP
aquaporin
- MT
microtubule
- SVC
secretory vesicle compartment
Footnotes
The work was supported by RWTH Aachen University. A.B.K. is supported by a fellowship from the RWTH Aachen University.
Articles can be viewed without a subscription.
References
- Ahad A, Wolf J, Nick P (2003) Activation-tagged tobacco mutants that are tolerant to antimicrotubular herbicides are cross-resistant to chilling stress. Transgenic Res 12: 615–629 [DOI] [PubMed] [Google Scholar]
- Aharon R, Shahak Y, Wininger S, Bendov R, Kapulnik Y, Galili G (2003) Overexpression of a plasma membrane aquaporin in transgenic tobacco improves plant vigor under favorable growth conditions but not under drought or salt stress. Plant Cell 15: 439–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avramova V, AbdElgawad H, Zhang Z, Fotschki B, Casadevall R, Vergauwen L, Knapen D, Taleisnik E, Guisez Y, Asard H, Beemster GT (2015) Drought induces distinct growth response, protection, and recovery mechanisms in the maize leaf growth zone. Plant Physiol 169: 1382–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck M, Komis G, Müller J, Menzel D, Samaj J (2010) Arabidopsis homologs of nucleus- and phragmoplast-localized kinase 2 and 3 and mitogen-activated protein kinase 4 are essential for microtubule organization. Plant Cell 22: 755–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemster GT, De Veylder L, Vercruysse S, West G, Rombaut D, Van Hummelen P, Galichet A, Gruissem W, Inzé D, Vuylsteke M (2005) Genome-wide analysis of gene expression profiles associated with cell cycle transitions in growing organs of Arabidopsis. Plant Physiol 138: 734–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein N, Shoresh M, Xu Y, Huang B (2010) Involvement of the plant antioxidative response in the differential growth sensitivity to salinity of leaves vs roots during cell development. Free Radic Biol Med 49: 1161–1171 [DOI] [PubMed] [Google Scholar]
- Bienert GP, Møller AL, Kristiansen KA, Schulz A, Møller IM, Schjoerring JK, Jahn TP (2007) Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J Biol Chem 282: 1183–1192 [DOI] [PubMed] [Google Scholar]
- Bienert GP, Cavez D, Besserer A, Berny MC, Gilis D, Rooman M, Chaumont F (2012) A conserved cysteine residue is involved in disulfide bond formation between plant plasma membrane aquaporin monomers. Biochem J 445: 101–111 [DOI] [PubMed] [Google Scholar]
- Bienert GP, Chaumont F (2014) Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim Biophys Acta 1840: 1596–1604 [DOI] [PubMed] [Google Scholar]
- Blanchoin L, Boujemaa-Paterski R, Henty JL, Khurana P, Staiger CJ (2010) Actin dynamics in plant cells: a team effort from multiple proteins orchestrates this very fast-paced game. Curr Opin Plant Biol 13: 714–723 [DOI] [PubMed] [Google Scholar]
- Boursiac Y, Boudet J, Postaire O, Luu DT, Tournaire-Roux C, Maurel C (2008) Stimulus-induced downregulation of root water transport involves reactive oxygen species-activated cell signalling and plasma membrane intrinsic protein internalization. Plant J 56: 207–218 [DOI] [PubMed] [Google Scholar]
- Chaouch S, Queval G, Noctor G (2012) AtRbohF is a crucial modulator of defence-associated metabolism and a key actor in the interplay between intracellular oxidative stress and pathogenesis responses in Arabidopsis. Plant J 69: 613–627 [DOI] [PubMed] [Google Scholar]
- Chaumont F, Barrieu F, Herman EM, Chrispeels MJ (1998) Characterization of a maize tonoplast aquaporin expressed in zones of cell division and elongation. Plant Physiol 117: 1143–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SX, Schopfer P (1999) Hydroxyl-radical production in physiological reactions. A novel function of peroxidase. Eur J Biochem 260: 726–735 [DOI] [PubMed] [Google Scholar]
- Crowell EF, Gonneau M, Vernhettes S, Höfte H (2010) Regulation of anisotropic cell expansion in higher plants. C R Biol 333: 320–324 [DOI] [PubMed] [Google Scholar]
- Dynowski M, Schaaf G, Loque D, Moran O, Ludewig U (2008) Plant plasma membrane water channels conduct the signalling molecule H2O2. Biochem J 414: 53–61 [DOI] [PubMed] [Google Scholar]
- Foreman J, Demidchik V, Bothwell JH, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JD, Davies JM, Dolan L (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422: 442–446 [DOI] [PubMed] [Google Scholar]
- Fry SC. (1986) Cross-linking of matrix polymers in the growing cells of angiosperms. Annu Rev Plant Physiol 37: 165–186 [Google Scholar]
- Fry SC. (1998) Oxidative scission of plant cell wall polysaccharides by ascorbate-induced hydroxyl radicals. Biochem J 332: 507–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Li H, Yang Z (2002) The ROP2 GTPase controls the formation of cortical fine F-actin and the early phase of directional cell expansion during Arabidopsis organogenesis. Plant Cell 14: 777–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Gu Y, Zheng Z, Wasteneys G, Yang Z (2005) Arabidopsis interdigitating cell growth requires two antagonistic pathways with opposing action on cell morphogenesis. Cell 120: 687–700 [DOI] [PubMed] [Google Scholar]
- Geisler DA, Sampathkumar A, Mutwil M, Persson S (2008) Laying down the bricks: logistic aspects of cell wall biosynthesis. Curr Opin Plant Biol 11: 647–652 [DOI] [PubMed] [Google Scholar]
- Gendre D, Oh J, Boutté Y, Best JG, Samuels L, Nilsson R, Uemura T, Marchant A, Bennett MJ, Grebe M, Bhalerao RP (2011) Conserved Arabidopsis ECHIDNA protein mediates trans-Golgi-network trafficking and cell elongation. Proc Natl Acad Sci USA 108: 8048–8053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendre D, McFarlane HE, Johnson E, Mouille G, Sjödin A, Oh J, Levesque-Tremblay G, Watanabe Y, Samuels L, Bhalerao RP (2013) Trans-Golgi network localized ECHIDNA/Ypt interacting protein complex is required for the secretion of cell wall polysaccharides in Arabidopsis. Plant Cell 25: 2633–2646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghuge SA, Carucci A, Rodrigues-Pousada RA, Tisi A, Franchi S, Tavladoraki P, Angelini R, Cona A (2015) The apoplastic copper AMINE OXIDASE1 mediates jasmonic acid-induced protoxylem differentiation in Arabidopsis roots. Plant Physiol 168: 690–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliland LU, Kandasamy MK, Pawloski LC, Meagher RB (2002) Both vegetative and reproductive actin isovariants complement the stunted root hair phenotype of the Arabidopsis act2-1 mutation. Plant Physiol 130: 2199–2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg R, Liberman M, Mathieu C, Pierron M, Catesson AM (1987) Development of epidermal cell wall peroxidases along the mung bean hypocotyl: possible involvement in the cell wall stiffening process. J Exp Bot 38: 1378–1390 [Google Scholar]
- Gonzalez N, Vanhaeren H, Inzé D (2012) Leaf size control: complex coordination of cell division and expansion. Trends Plant Sci 17: 332–340 [DOI] [PubMed] [Google Scholar]
- Gorecka KM, Konopka-Postupolska D, Hennig J, Buchet R, Pikula S (2005) Peroxidase activity of annexin 1 from Arabidopsis thaliana. Biochem Biophys Res Commun 336: 868–875 [DOI] [PubMed] [Google Scholar]
- Hála M, Cole R, Synek L, Drdová E, Pecenková T, Nordheim A, Lamkemeyer T, Madlung J, Hochholdinger F, Fowler JE, Zárský V (2008) An exocyst complex functions in plant cell growth in Arabidopsis and tobacco. Plant Cell 20: 1330–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heggie L, Jansen MA, Burbridge EM, Kavanagh TA, Thorneley RN, Dix PJ (2005) Transgenic tobacco (Nicotiana tabacum L. cv. Samsun-NN) plants over-expressing a synthetic HRP-C gene are altered in growth, development and susceptibility to abiotic stress. Plant Physiol Biochem 43: 1067–1073 [DOI] [PubMed] [Google Scholar]
- Henzler T, Ye Q, Steudle E (2004) Oxidative gating of water channels (aquaporins) in Chara by hydroxyl radicals. Plant Cell Environ 27: 1184–1195 [DOI] [PubMed] [Google Scholar]
- Hernández JA, Ferrer MA, Jiménez A, Barceló AR, Sevilla F (2001) Antioxidant systems and O2.-/H2O2 production in the apoplast of pea leaves. Its relation with salt-induced necrotic lesions in minor veins. Plant Physiol 127: 817–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SY, Kim OK, Kim SG, Yang MS, Park CM (2011) Nuclear import and DNA binding of the ZHD5 transcription factor is modulated by a competitive peptide inhibitor in Arabidopsis. J Biol Chem 286: 1659–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, dePamphilis CW, Ma H (2008) Phylogenetic analysis of the plant-specific zinc finger-homeobox and mini zinc finger gene families. J Integr Plant Biol 50: 1031–1045 [DOI] [PubMed] [Google Scholar]
- Ishida T, Kaneko Y, Iwano M, Hashimoto T (2007) Helical microtubule arrays in a collection of twisting tubulin mutants of Arabidopsis thaliana. Proc Natl Acad Sci USA 104: 8544–8549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya H, Nakajima R, Iwano M, Kanaoka MM, Kimura S, Takeda S, Kawarazaki T, Senzaki E, Hamamura Y, Higashiyama T, et al. (2014) Ca2+-activated reactive oxygen species production by Arabidopsis RbohH and RbohJ is essential for proper pollen tube tip growth. Plant Cell 26: 1069–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kärkönen A, Kuchitsu K (2015) Reactive oxygen species in cell wall metabolism and development in plants. Phytochemistry 112: 22–32 [DOI] [PubMed] [Google Scholar]
- Komis G, Illés P, Beck M, Šamaj J (2011) Microtubules and mitogen-activated protein kinase signalling. Curr Opin Plant Biol 14: 650–657 [DOI] [PubMed] [Google Scholar]
- Konopka-Postupolska D, Clark G, Goch G, Debski J, Floras K, Cantero A, Fijolek B, Roux S, Hennig J (2009) The role of annexin 1 in drought stress in Arabidopsis. Plant Physiol 150: 1394–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovtun Y, Chiu WL, Tena G, Sheen J (2000) Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc Natl Acad Sci USA 97: 2940–2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravchik M, Bernstein N (2013) Effects of salinity on the transcriptome of growing maize leaf cells point at cell-age specificity in the involvement of the antioxidative response in cell growth restriction. BMC Genomics 14: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y, Kim JH, Nguyen HN, Jikumaru Y, Kamiya Y, Hong SW, Lee H (2013) A novel Arabidopsis MYB-like transcription factor, MYBH, regulates hypocotyl elongation by enhancing auxin accumulation. J Exp Bot 64: 3911–3922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laohavisit A, Shang Z, Rubio L, Cuin TA, Véry AA, Wang A, Mortimer JC, Macpherson N, Coxon KM, Battey NH, et al. (2012) Arabidopsis annexin1 mediates the radical-activated plasma membrane Ca²+- and K+-permeable conductance in root cells. Plant Cell 24: 1522–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavy M, Bloch D, Hazak O, Gutman I, Poraty L, Sorek N, Sternberg H, Yalovsky S (2007) A Novel ROP/RAC effector links cell polarity, root-meristem maintenance, and vesicle trafficking. Curr Biol 17: 947–952 [DOI] [PubMed] [Google Scholar]
- Lee BR, Kim KY, Jung WJ, Avice JC, Ourry A, Kim TH (2007) Peroxidases and lignification in relation to the intensity of water-deficit stress in white clover (Trifolium repens L.). J Exp Bot 58: 1271–1279 [DOI] [PubMed] [Google Scholar]
- Lee SH, Chung GC, Jang JY, Ahn SJ, Zwiazek JJ (2012) Overexpression of PIP2;5 aquaporin alleviates effects of low root temperature on cell hydraulic conductivity and growth in Arabidopsis. Plant Physiol 159: 479–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Park HJ, Jung WY, Lee A, Yoon DH, You YN, Kim HS, Kim BG, Ahn JC, Cho HS (2015) OsCYP21-4, a novel Golgi-resident cyclophilin, increases oxidative stress tolerance in rice. Front Plant Sci 6: 797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Rubio MC, Alassimone J, Geldner N (2013) A mechanism for localized lignin deposition in the endodermis. Cell 153: 402–412 [DOI] [PubMed] [Google Scholar]
- Lin CC, Kao CH (2001) Abscisic acid induced changes in cell wall peroxidase activity and hydrogen peroxide level in roots of rice seedlings. Plant Sci 160: 323–329 [DOI] [PubMed] [Google Scholar]
- Liszkay A, Kenk B, Schopfer P (2003) Evidence for the involvement of cell wall peroxidase in the generation of hydroxyl radicals mediating extension growth. Planta 217: 658–667 [DOI] [PubMed] [Google Scholar]
- Liu SG, Zhu DZ, Chen GH, Gao XQ, Zhang XS (2012) Disrupted actin dynamics trigger an increment in the reactive oxygen species levels in the Arabidopsis root under salt stress. Plant Cell Rep 31: 1219–1226 [DOI] [PubMed] [Google Scholar]
- Livanos P, Galatis B, Quader H, Apostolakos P (2012) Disturbance of reactive oxygen species homeostasis induces atypical tubulin polymer formation and affects mitosis in root-tip cells of Triticum turgidum and Arabidopsis thaliana. Cytoskeleton (Hoboken) 69: 1–21 [DOI] [PubMed] [Google Scholar]
- Loggini B, Scartazza A, Brugnoli E, Navari-Izzo F (1999) Antioxidative defense system, pigment composition, and photosynthetic efficiency in two wheat cultivars subjected to drought. Plant Physiol 119: 1091–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D, Wang T, Persson S, Mueller-Roeber B, Schippers JHM (2014) Transcriptional control of ROS homeostasis by KUODA1 regulates cell expansion during leaf development. Nat Commun 5: 3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas JR, Courtney S, Hassfurder M, Dhingra S, Bryant A, Shaw SL (2011) Microtubule-associated proteins MAP65-1 and MAP65-2 positively regulate axial cell growth in etiolated Arabidopsis hypocotyls. Plant Cell 23: 1889–1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAdam JW, Grabber JH (2002) Relationship of growth cessation with the formation of diferulate cross-links and p-coumaroylated lignins in tall fescue leaf blades. Planta 215: 785–793 [DOI] [PubMed] [Google Scholar]
- Müller K, Linkies A, Vreeburg RA, Fry SC, Krieger-Liszkay A, Leubner-Metzger G (2009) In vivo cell wall loosening by hydroxyl radicals during cress seed germination and elongation growth. Plant Physiol 150: 1855–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda Y, Fukuda H (2012) Initiation of cell wall pattern by a Rho- and microtubule-driven symmetry breaking. Science 337: 1333–1336 [DOI] [PubMed] [Google Scholar]
- Park YB, Cosgrove DJ (2012) Changes in cell wall biomechanical properties in the xyloglucan-deficient xxt1/xxt2 mutant of Arabidopsis. Plant Physiol 158: 465–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passardi F, Penel C, Dunand C (2004) Performing the paradoxical: how plant peroxidases modify the cell wall. Trends Plant Sci 9: 534–540 [DOI] [PubMed] [Google Scholar]
- Polyn S, Willems A, De Veylder L (2015) Cell cycle entry, maintenance, and exit during plant development. Curr Opin Plant Biol 23: 1–7 [DOI] [PubMed] [Google Scholar]
- Powell AE, Lenhard M (2012) Control of organ size in plants. Curr Biol 22: R360–R367 [DOI] [PubMed] [Google Scholar]
- Prak S, Hem S, Boudet J, Viennois G, Sommerer N, Rossignol M, Maurel C, Santoni V (2008) Multiple phosphorylations in the C-terminal tail of plant plasma membrane aquaporins: role in subcellular trafficking of AtPIP2;1 in response to salt stress. Mol Cell Proteomics 7: 1019–1030 [DOI] [PubMed] [Google Scholar]
- Raggi S, Ferrarini A, Delledonne M, Dunand C, Ranocha P, De Lorenzo G, Cervone F, Ferrari S (2015) The Arabidopsis class III peroxidase AtPRX71 negatively regulates growth under physiological conditions and in response to cell wall damage. Plant Physiol 169: 2513–2525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renew S, Heyno E, Schopfer P, Liszkay A (2005) Sensitive detection and localization of hydroxyl radical production in cucumber roots and Arabidopsis seedlings by spin trapping electron paramagnetic resonance spectroscopy. Plant J 44: 342–347 [DOI] [PubMed] [Google Scholar]
- Richards SL, Wilkins KA, Swarbreck SM, Anderson AA, Habib N, Smith AG, McAinsh M, Davies JM (2015) The hydroxyl radical in plants: from seed to seed. J Exp Bot 66: 37–46 [DOI] [PubMed] [Google Scholar]
- Rodríguez AA, Córdoba AR, Ortega L, Taleisnik E (2004) Decreased reactive oxygen species concentration in the elongation zone contributes to the reduction in maize leaf growth under salinity. J Exp Bot 55: 1383–1390 [DOI] [PubMed] [Google Scholar]
- Rodríguez AA, Grunberg KA, Taleisnik EL (2002) Reactive oxygen species in the elongation zone of maize leaves are necessary for leaf extension. Plant Physiol 129: 1627–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roppolo D, Boeckmann B, Pfister A, Boutet E, Rubio MC, Dénervaud-Tendon V, Vermeer JE, Gheyselinck J, Xenarios I, Geldner N (2014) Functional and evolutionary analysis of the CASPARIAN STRIP MEMBRANE DOMAIN PROTEIN family. Plant Physiol 165: 1709–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schippers JHM, Foyer CH, van Dongen JT (2016) Redox regulation in shoot growth, SAM maintenance and flowering. Curr Opin Plant Biol 29: 121–128 [DOI] [PubMed] [Google Scholar]
- Schmidt R, Schippers JHM (2015) ROS-mediated redox signaling during cell differentiation in plants. Biochim Biophys Acta 1850: 1497–1508 [DOI] [PubMed] [Google Scholar]
- Schopfer P, Liszkay A, Bechtold M, Frahry G, Wagner A (2002) Evidence that hydroxyl radicals mediate auxin-induced extension growth. Planta 214: 821–828 [DOI] [PubMed] [Google Scholar]
- Schopfer P. (1996) Hydrogen peroxide-mediated cell-wall stiffening in vitro in maize coleoptile. Planta 199: 43–49 [Google Scholar]
- Schopfer P. (2006) Biomechanics of plant growth. Am J Bot 93: 1415–1425 [DOI] [PubMed] [Google Scholar]
- Simonovicova M, Huttova J, Mistrik I, Siroka B, Tamas L (2004) Peroxidase mediated hydrogen peroxide production in barley roots grown under stress conditions. Plant Growth Regul 44: 267–275 [Google Scholar]
- Smith LG, Oppenheimer DG (2005) Spatial control of cell expansion by the plant cytoskeleton. Annu Rev Cell Dev Biol 21: 271–295 [DOI] [PubMed] [Google Scholar]
- Steudle E, Frensch J (1996) Water transport in plants: role of the apoplast. Plant Soil 187: 67–79 [Google Scholar]
- Suzuki N, Miller G, Morales J, Shulaev V, Torres MA, Mittler R (2011) Respiratory burst oxidases: the engines of ROS signaling. Curr Opin Plant Biol 14: 691–699 [DOI] [PubMed] [Google Scholar]
- Takeda S, Gapper C, Kaya H, Bell E, Kuchitsu K, Dolan L (2008) Local positive feedback regulation determines cell shape in root hair cells. Science 319: 1241–1244 [DOI] [PubMed] [Google Scholar]
- Tavakoli N, Kluge C, Golldack D, Mimura T, Dietz KJ (2001) Reversible redox control of plant vacuolar H+-ATPase activity is related to disulfide bridge formation in subunit E as well as subunit A. Plant J 28: 51–59 [DOI] [PubMed] [Google Scholar]
- Tsukagoshi H, Busch W, Benfey PN (2010) Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell 143: 606–616 [DOI] [PubMed] [Google Scholar]
- Uhrig JF, Hülskamp M (2014) Rop GTPases: Polarity and Cell Shape in Plants. eLS, 10.1002/9780470015902.a0023758
- Wang QY, Nick P (2001) Cold acclimation can induce microtubular cold stability in a manner distinct from abscisic acid. Plant Cell Physiol 42: 999–1005 [DOI] [PubMed] [Google Scholar]
- Wang H, Wang S, Lu Y, Alvarez S, Hicks LM, Ge X, Xia Y (2012) Proteomic analysis of early-responsive redox-sensitive proteins in Arabidopsis. J Proteome Res 11: 412–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waszczak C, Akter S, Eeckhout D, Persiau G, Wahni K, Bodra N, Van Molle I, De Smet B, Vertommen D, Gevaert K, et al. (2014) Sulfenome mining in Arabidopsis thaliana. Proc Natl Acad Sci USA 111: 11545–11550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S, Hématy K, Höfte H (2012) Growth control and cell wall signaling in plants. Annu Rev Plant Biol 63: 381–407 [DOI] [PubMed] [Google Scholar]
- Wong HL, Pinontoan R, Hayashi K, Tabata R, Yaeno T, Hasegawa K, Kojima C, Yoshioka H, Iba K, Kawasaki T, Shimamoto K (2007) Regulation of rice NADPH oxidase by binding of Rac GTPase to its N-terminal extension. Plant Cell 19: 4022–4034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wudick MM, Li X, Valentini V, Geldner N, Chory J, Lin J, Maurel C, Luu DT (2015) Subcellular redistribution of root aquaporins induced by hydrogen peroxide. Mol Plant 8: 1103–1114 [DOI] [PubMed] [Google Scholar]
- Yao LL, Zhou Q, Pei BL, Li YZ (2011) Hydrogen peroxide modulates the dynamic microtubule cytoskeleton during the defence responses to Verticillium dahliae toxins in Arabidopsis. Plant Cell Environ 34: 1586–1598 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Zuo M, Liang Y, Jiang M, Zhang J, Scheller HV, Tan M, Zhang A (2013) MAP65-1a positively regulates H2O2 amplification and enhances brassinosteroid-induced antioxidant defence in maize. J Exp Bot 64: 3787–3802 [DOI] [PMC free article] [PubMed] [Google Scholar]