The expression of OsFRDL4 encoding an Al-induced citrate transporter is enhanced by a 1.2-kb insertion in the promoter region in rice.
Abstract
High aluminum (Al) tolerance of rice (Oryza sativa) is controlled by multiple tolerance genes, but the regulatory mechanisms underlying the differential expression of these genes are poorly understood. Here, we investigated the factors regulating the expression of OsFRDL4, a gene encoding a citrate efflux transporter involved in Al-induced citrate secretion from the roots. Analysis with chromosome segment substitution lines derived from cv Nipponbare (high OsFRDL4 expression) and cv Kasalath (low OsFRDL4 expression) revealed that the differential expression of OsFRDL4 is responsible for the quantitative trait locus for Al tolerance detected previously on chromosome 1. Comparison of the OsFRDL4 gene structure in cv Nipponbare and cv Kasalath showed that there was no difference in the position of the transcriptional start site, but a 1.2-kb insertion showing high similarity to the solo long terminal repeat of the retrotransposon was found in the promoter region of OsFRDL4 in cv Nipponbare. This insertion showed higher promoter activity and contained nine cis-acting elements for ALUMINUM RESISTANCE TRANSCRIPTION FACTOR1 (ART1). However, this insertion did not alter the spatial expression or cellular localization of OsFRDL4. Furthermore, this insertion was found in most japonica varieties but was largely absent from indica varieties or wild rice species. These results indicate that the 1.2-kb insertion in the OsFRDL4 promoter region in japonica subspecies is responsible for their higher expression level of OsFRDL4 due to the increased number of cis-acting elements of ART1. Our results also suggest that this insertion event happened at the initial stage of domestication of japonica subspecies.
The tolerance to aluminum (Al) toxicity differs widely with the plant species. Among small-grain cereal crops, rice (Oryza sativa) is the most Al-tolerant species (Foy, 1988; Famoso et al., 2011). Recent studies have identified a number of Al-tolerance genes involved in high Al tolerance in japonica subspecies (Ma et al., 2014). ALUMINUM RESISTANCE TRANSCRIPTION FACTOR1 (ART1), a C2H2 zinc finger-type transcription factor, was identified as a key regulator for Al tolerance in rice (Yamaji et al., 2009). ART1 regulates at least 32 genes by binding to the core cis-acting element [GGN(T/g/a/C)V(C/A/g)S(C/G)] in the promoter of these genes (Tsutsui et al., 2011). Functional characterization of several ART1-regulated genes showed that they underlie a diverse range of tolerance mechanisms. For example, a bacterial-type ATP-binding cassette transporter formed by STAR1-STAR2 transports UDP-Glc and is implicated in cell wall modification (Huang et al., 2009). Knockout of STAR1 or STAR2 significantly decreased Al tolerance in rice (Huang et al., 2009). This tolerance mechanism also was observed in other species such as Arabidopsis (Arabidopsis thaliana; Huang et al., 2010), suggesting that Al tolerance mediated by STAR1-STAR2 is a common Al-tolerance mechanism in plants. On the other hand, NRAMP ALUMINUM TRANSPORTER1 (NRAT1) and OsALS1 encode an Al transporter localized at the plasma membrane and tonoplast, respectively. NRAT1 transports trivalent Al into the cells (Xia et al., 2010) for subsequent sequestration of Al into the vacuoles by OsALS1 (Huang et al., 2012a). In addition, the magnesium transporter gene OsMGT1 for magnesium uptake (Chen et al., 2012), a gene encoding the small Cys-rich peptide OsCDT3 (Xia et al., 2013), and the citrate transporter gene OsFRDL4 involved in Al-induced secretion of citrate (Yokosho et al., 2011) also were demonstrated to contribute to high Al tolerance in rice. Recently, two other ART1-regulated genes, OsFRDL2 encoding a citrate transporter and OsEXPA10 encoding an expansin for cell elongation in the root tips, were found to be specifically induced by Al, but their contribution to Al tolerance in rice is minor (Che et al., 2016; Yokosho et al., 2016).
On the other hand, there also is a large variation of Al tolerance among different subpopulations of rice. The relative degree of Al tolerance is in the order temperate japonica > tropical japonica > aromatic > indica = aus (Famoso et al., 2011). A number of quantitative trait loci (QTL) for differential Al tolerance have been reported using different populations (Wu et al., 2000; Nguyen et al., 2001, 2002, 2003; Ma et al., 2002; Famoso et al., 2011), but most of these QTL genes have not been identified. Two recent studies showed that both different expression of NRAT1 and transport activity of NRAT1 are responsible for the QTL for Al tolerance detected on chromosome 2 in rice (Li et al., 2014; Xia et al., 2014). There was a good correlation between NRAT1 expression and relative root elongation under Al stress in different rice varieties (Xia et al., 2014). Although the exact mechanisms for the different expression of NRAT1 is still unknown, five single-nucleotide polymorphisms in the NRAT1 promoter unique to the sensitive aus line might be involved in the regulation of NRAT1 expression (Li et al., 2014). By contrast, four single-nucleotide polymorphisms in the coding region caused missense mutations, resulting in decreased Al transport activity of NRAT1 protein in Al-sensitive aus lines (Li et al., 2014). On the other hand, a QTL for Al tolerance on chromosome 1 was detected in five studies (Wu et al., 2000; Nguyen et al., 2001, 2002, 2003; Ma et al., 2002). The position of this QTL is flanked by OsFRDL4 (Os01g0919100; Wu et al., 2000; Nguyen et al., 2001; Ma et al., 2002; Yokosho et al., 2011). In fact, there was a good correlation between the expression level of OsFRDL4 and Al tolerance (Yokosho et al., 2011). However, it has not been demonstrated whether differential OsFRDL4 expression is responsible for the QTL detected on chromosome 1. Furthermore, the mechanisms underlying the differential expression of OsFRDL4 are not understood.
In this study, we used chromosome segment substitution lines (CSSLs) to test whether OsFRDL4 is responsible for the QTL for Al tolerance on chromosome 1. Furthermore, we examined the mechanisms underlying the differential expression of OsFRDL4 and found that a 1.2-kb insertion in the promoter region of OsFRDL4 plays an important role in enhancing the expression of OsFRDL4. Investigation of this insertion in cultivated rice varieties from the world core collection and wild rice species revealed that this insertion event might have occurred at the early stage of rice domestication.
RESULTS
OsFRDL4 Is Responsible for the QTL for Al Tolerance on Chromosome 1
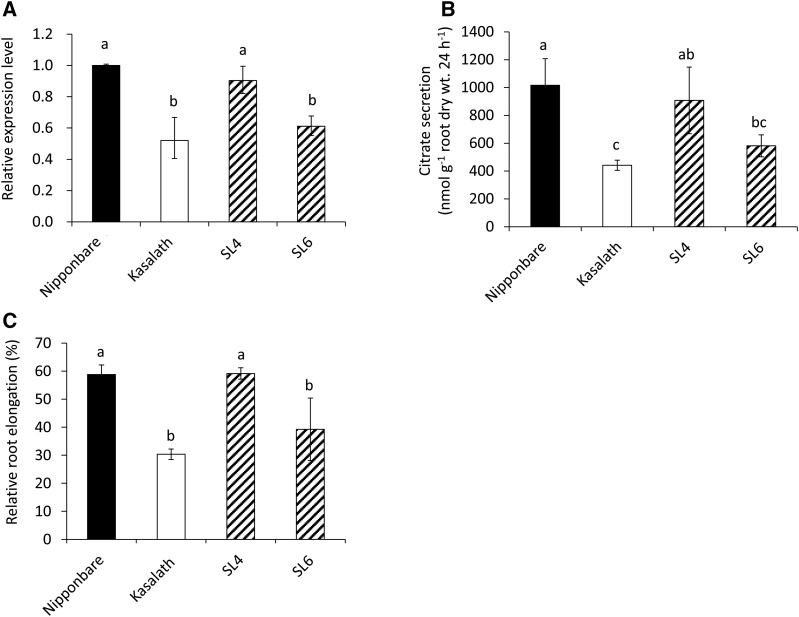
To examine whether OsFRDL4 is responsible for the QTL for Al tolerance detected previously on chromosome 1 (Wu et al., 2000; Nguyen et al., 2001; Ma et al., 2002), we obtained two CSSLs that contained the OsFRDL4 allele from cv Kasalath (SL6) or not (SL4 as a negative control; http://www.rgrc.dna.affrc.go.jp/data/NK-SL54-20030430.pdf; Supplemental Fig. S1). Similar to a previous study (Yokosho et al., 2011), the expression level of OsFRDL4 was two times higher in cv Nipponbare than in cv Kasalath after the roots were exposed to 30 μm Al for 24 h (Fig. 1). Substitution of the OsFRDL4 allele from cv Nipponbare with that from cv Kasalath (SL6) resulted in decreased expression of OsFRDL4 to a level similar to that of cv Kasalath (Fig. 1A), whereas substitution of other regions (SL4) did not affect the expression of OsFRDL4 (Fig. 1A). The Al-induced citrate secretion in SL6 was decreased compared with cv Nipponbare (Fig. 1B) but showed a level similar to that of cv Kasalath. The root elongation in cv Nipponbare and SL4 was inhibited by 40%, but that in cv Kasalath and SL6 was inhibited by 70% and 60%, respectively (Fig. 1C). These results indicate that differential OsFRDL4 expression results in a genotypic difference in Al-induced citrate secretion as well as Al tolerance between cv Nipponbare and cv Kasalath. Accordingly, OsFRDL4 is likely to be responsible for the QTL detected on chromosome 1.
Figure 1.
Expression, citrate secretion, and Al tolerance in two CSSLs. A, Relative expression levels of OsFRDL4 in cv Nipponbare, cv Kasalath, and two CSSLs (SL4 and SL6 carrying cv Nipponbare and cv Kasalath alleles of OsFRDL4, respectively). Five-day-old seedlings were exposed to 30 μm Al (pH 4.5) for 24 h, and the root tips (0–1 cm) were excised for RNA extraction and expression determination by quantitative RT-PCR. Expression levels relative to that in cv Nipponbare roots are shown. Histone H3 was used as an internal control. B, Comparison of Al-induced citrate secretion. Root exudates were collected for 24 h from 14-d-old seedlings exposed to 30 μm Al (pH 4.5). C, Al tolerance. Five-day-old seedlings were exposed to 30 μm Al (pH 4.5) or not for 24 h. Root length was measured before and after treatment, and relative root elongation is shown. Data are means ± sd of three (A and B) or 10 (C) biological replicates. Means with different letters are significantly different (P < 0.05 by Tukey’s test).
Comparison of the Transcriptional Start Site and Promoter Sequence of OsFRDL4 in cv Nipponbare and cv Kasalath
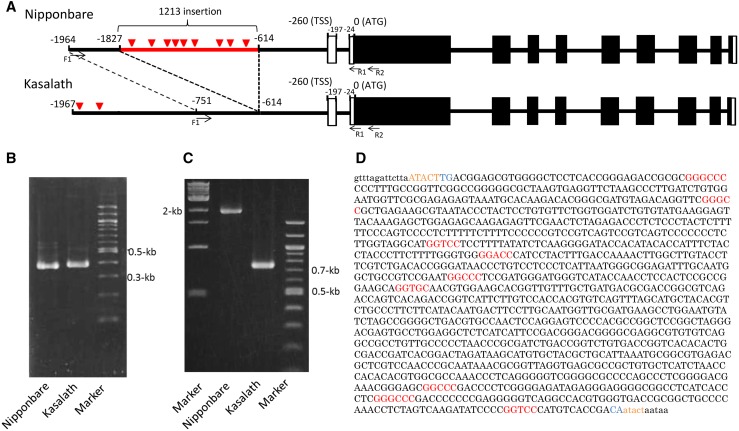
To investigate the mechanisms underlying the genotypic difference in OsFRDL4 expression in cv Nipponbare and cv Kasalath, we first compared the position(s) of the transcriptional start site (TSS) of OsFRDL4 in roots exposed to Al using an oligo-capped 5′ RACE. As a result, there was no difference in the position of the TSS or the splicing pattern (Fig. 2).
Figure 2.
Comparison of the OsFRDL4 promoter sequences in cv Nipponbare and cv Kasalath. A, Schematic presentation of the genomic structures of OsFRDL4 in cv Nipponbare and cv Kasalath. Black lines show introns, black boxes show exons of open reading frames, and white boxes show exons of the untranslated region. The red line shows the cv Nipponbare-specific insertion, and red triangles show ART1 cis-acting elements. B, An agarose gel showing the 5′ RACE PCR products. The OsFRDL4 gene-specific primer (R2) shown in A and an RNA oligo primer were used to amplify the OsFRDL4 5′ untranslated region. C, An agarose gel showing PCR products of the upstream region of OsFRDL4 in cv Nipponbare and cv Kasalath. Primers (F1 and R1) shown in A in the OsFRDL4 upstream region were used. D, Sequence of the 1.2-kb insertion in the OsFRDL4 promoter. Lowercase letters show the common sequence in cv Nipponbare and cv Kasalath, and uppercase letters show the insertion sequence in cv Nipponbare. Orange letters show target site duplications, blue letters show short inverted dinucleotides, and red letters show the ART1 cis-acting element sequence GGN(T/g/a/C)V(C/A/g)S(C/G).
Next, we compared the sequence of the OsFRDL4 promoter region in cv Nipponbare and cv Kasalath. Analysis with PCR using the same primer pairs generated a single fragment in each with a different size (751 bp in cv Kasalath versus 1,964 bp in cv Nipponbare; Fig. 2C). Sequence analysis of these fragments revealed that there was an insertion of 1,213 bp between −615 and −1,828 bp from the translational start site in cv Nipponbare but not in cv Kasalath (Fig. 2A). A BLAST search revealed that this 1.2-kb insertion was annotated to be a long terminal repeat (LTR)-like sequence. The LTR retrotransposons have direct LTRs that can range from a few hundred base pairs to several kilobases (Kumar and Bennetzen, 1999; Vitte et al., 2007). LTRs are terminated by a short inverted dinucleotide, usually 5′-TG-3′ and 5′-CA-3′ (Kumar and Bennetzen, 1999). The 1.2-kb insertion sequence found upstream of OsFRDL4 has 5′-TG-3′ and 5′-CA-3′ and target site duplication (ATACT; Fig. 2D). A full-length LTR retrotransposon encodes proteins for transposition and contains LTRs at both termini. However, this 1.2-kb insertion does not encode any protein and it contains only a single LTR. Since this single LTR is flanked by TSD, it is a solo-LTR, which is derived from intraelement recombination of an intact LTR retrotransposon. Within the solo-LTR sequence, there are nine cis-acting elements of ART1 (Fig. 2, A and D). On the other hand, there were two cis-acting elements of ART1 in the upstream region of cv Kasalath OsFRDL4 (Fig. 2A).
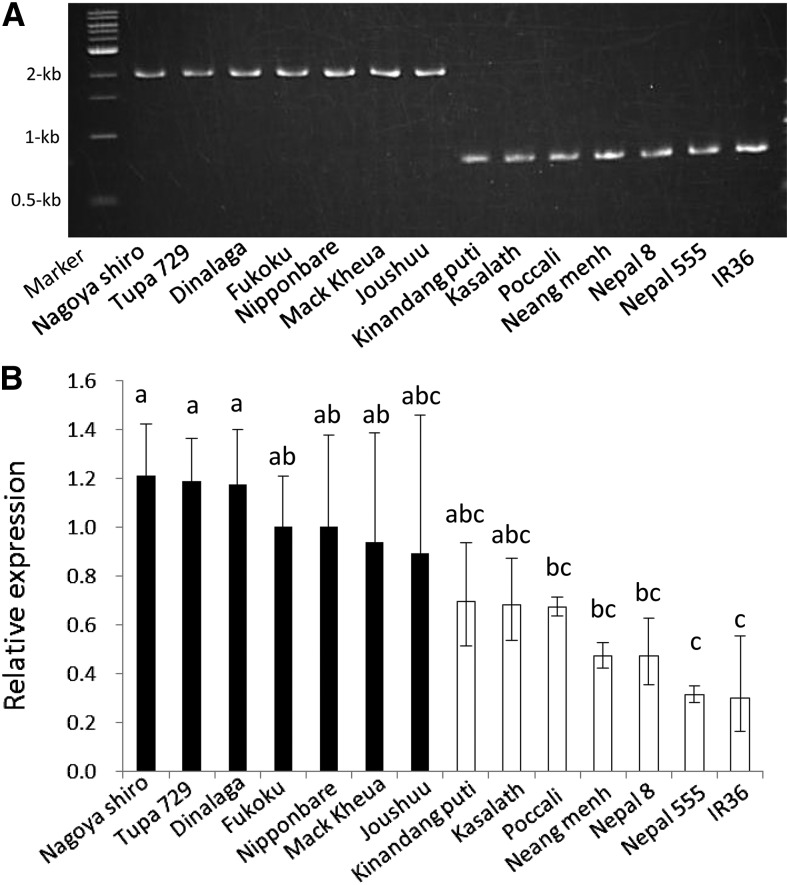
To associate this insertion with OsFRDL4 expression, we randomly selected 14 rice varieties for the insertion and expression analysis. The results indicated that all seven varieties with the insertion showed elevated expression levels of OsFRDL4 compared with those without the insertion (Fig. 3).
Figure 3.
Comparison of the OsFRDL4 promoter genotype and OsFRDL4 expression in different cultivars. A, An agarose gel showing PCR products of the upstream region of OsFRDL4 in 14 cultivars (Nagoya shiro, Tupa 729, Dinalaga, Fukoku, Nipponbare, Mack kheua, Joushuu, Kinandang puti, Kasalath, Poccali, Neang menh, Nepal 8, Nepal 555, and IR36). Primers (F1 and R1) shown in Figure 2A in the OsFRDL4 upstream region were used for PCR analysis. B, Relative expression level of OsFRDL4 in 14 rice varieties. Five-day-old seedlings were exposed to 30 μm Al (pH 4.5) for 24 h. RNA was extracted from the root tips (0–1 cm). Expression levels relative to that in cv Nipponbare roots are shown. Histone H3 was used as an internal control. Data are means ± sd of three biological replicates. Means with different letters are significantly different (P < 0.05 by Tukey’s test).
The 1.2-kb Insertion Increased the Promoter Activity of OsFRDL4
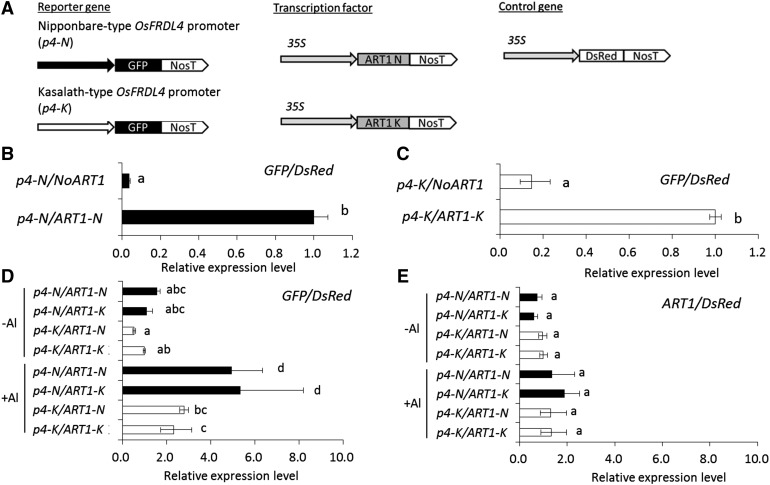
To examine the role of the 1.2-kb insertion found in cv Nipponbare, we compared the promoter activity of OsFRDL4 in cv Nipponbare and cv Kasalath using a transient assay in cultured tobacco (Nicotiana tabacum) cells (BY-2). We amplified the promoter region of OsFRDL4 from cv Nipponbare (−1,964 bp upstream from the start codon) containing the insertion region and cv Kasalath (−1,967 bp upstream from the start codon) and fused them with GFP as a reporter gene (Fig. 4). Since OsFRDL4 was regulated by ART1, we also amplified ART1 by PCR from cv Nipponbare and cv Kasalath. There was no difference in the ART1 expression between cv Nipponbare and cv Kasalath (Supplemental Fig. S2). The ART1 sequence of cv Nipponbare showed seven amino acid substitutions and seven insertion or deletion regions compared with that of cv Kasalath (Supplemental Fig. S2B). When ART1 driven by the cauliflower mosaic virus (CaMV) 35S promoter was coexpressed with the OsFRDL4 promoter fused with GFP in the protoplasts of cultured tobacco cells, ART1 activated the expression of the OsFRDL4 promoter in both cv Nipponbare and cv Kasalath in the presence of Al (Fig. 4, B and C).
Figure 4.
Promoter activity assay in tobacco cultured cell protoplasts. A, Schematic diagram of the reporter, transcriptional factor, and internal control plasmids used in transient expression analysis. N, cv Nipponbare type (−1,964 bp upstream from the start codon); K, cv Kasalath type (−1,967 bp upstream from the start codon). B and C, Expression levels of the reporter GFP gene under the control of the cv Nipponbare-type OsFRDL4 promoter (p4-N; B) or the cv Kasalath-type promoter (p4-K; C) with or without the transcription factor gene (ART1) in the presence of Al. The transformed protoplasts were incubated in Murashige and Skoog medium for 17 h and then exposed to 100 μm AlCl3 for 4 h. D and E, Expression levels of the reporter GFP gene (D) and ART1 (E) under the control of p4-N or p4-K with ART1-N and ART1-K in the presence or absence of Al. The transformed protoplasts were incubated in Murashige and Skoog medium for 17 h and then exposed to 0 or 100 μm AlCl3 for 4 h. The expression levels were determined by quantitative RT-PCR. The relative expression of GFP or ART1 was normalized by DsRed as an internal control. Data are means ± sd of three (B and C) and four (D and E) biological replicates. Means with different letters are significantly different (P < 0.05 by Tukey’s test).
To normalize the expression of the reporter gene, we used the red fluorescent protein gene DsRed monomer driven by the CaMV 35S promoter as an internal control (Fig. 4A). In the absence of Al, coexpression of the OsFRDL4 promoter and ART1 showed low promoter activity, and there was no difference in the activity between cv Nipponbare and cv Kasalath (Fig. 4D). By contrast, in the presence of Al, the promoter activity increased significantly (Fig. 4D). Furthermore, the OsFRDL4 promoter from cv Nipponbare showed higher activity than that from cv Kasalath coexpressed with ART1 either from cv Nipponbare or from cv Kasalath (Fig. 4D), indicating that the difference in the ART1 sequence did not affect its function. On the other hand, there was no difference in the ART1 expression level between cv Nipponbare-type and cv Kasalath-type ART1 sequences (Fig. 4E). These results indicate that ART1 interacts with the cis-acting elements of the OsFRDL4 promoter and that the transcriptional activation potential is enhanced by the repetition of the cis-acting element in the 1.2-kb insertion.
Neither Spatial Expression of OsFRDL4 nor Cellular Localization Was Affected by the Insertion
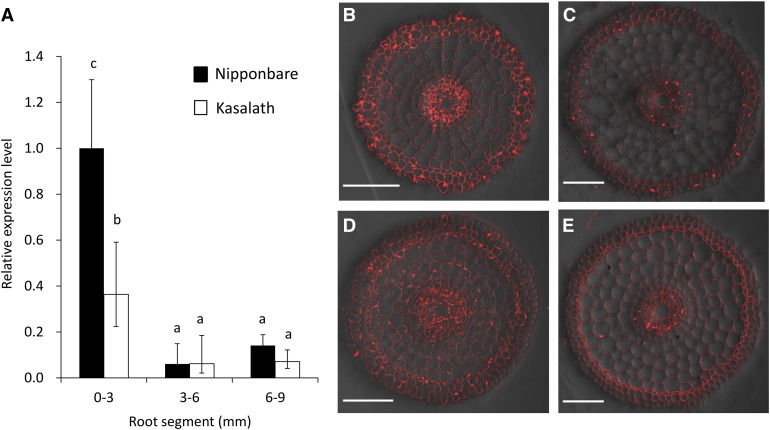
In barley (Hordeum vulgare), a 1-kb insertion upstream of HvAACT1, a major Al tolerance gene, altered the localization of this gene expression from the xylem parenchyma of basal roots to the epidermis of root tips and also increased the expression of HvAACT1 in the root tips (Fujii et al., 2012). To investigate the effect of the 1.2-kb insertion in the promoter region of OsFRDL4 on its spatial expression pattern, we compared the expression of OsFRDL4 in different root segments of cv Nipponbare and cv Kasalath. In both cultivars, Al-induced expression of OsFRDL4 was higher in the root tips (0–3 mm) than in the mature root zones (greater than 3 mm; Fig. 5). The expression was higher in cv Nipponbare than in cv Kasalath only in the root tips (Fig. 5A).
Figure 5.
Comparison of the spatial expression of OsFRDL4 and the cellular localization of OsFRDL4 in cv Nipponbare and cv Kasalath. A, Spatial expression of OsFRDL4 in roots. Different root segments (0–3, 3–6, and 6–9 mm) of both cv Nipponbare and cv Kasalath were excised from the roots exposed to a 0.5 mm CaCl2 solution (pH 4.5) containing 30 μm AlCl3 for 12 h. The expression level was determined by quantitative real-time RT-PCR. Expression levels relative to the cv Nipponbare root tip (0–3 mm) with Al exposure are shown. Histone H3 was used as an internal control. Data are means ± sd of three biological replicates. Different letters indicate significant differences (P < 0.05 by Tukey’s test). B to E, Tissue specificity localization of the OsFRDL4 protein in root tip (3 mm; B and D) and basal root (15 mm; C and E) of cv Nipponbare (B and C) and cv Kasalath (D and E). Immunostaining with anti-OsFRDL4 antibody was performed with roots exposed to 30 μm Al (pH 4.5) for 12 h. Bars = 100 µm.
The cellular localization of OsFRDL4 also was compared in the root tips and mature root zones in cv Nipponbare and cv Kasalath by immunostaining with an anti-OsFRDL4 antibody. OsFRDL4 protein was localized in all root cells, and the signal was stronger in the root tip than in the mature root zone in both cultivars (Fig. 5, B–E). These results indicate that the 1.2-kb insertion did not alter the spatial expression pattern or cellular localization but enhanced the expression of OsFRDL4 in rice.
Distribution of the 1.2-kb Insertion in World Cultivated Rice Core Collection and Wild Rice Species
To link the insertion event with rice domestication, we investigated the 1.2-kb insertion in 68 cultivated rice plants from the world core collection, including 52 indica and 16 japonica varieties, and six wild rice species (Oryza rufipogon, Oryza barthii, Oryza glumaepatula, Oryza meridionalis, Oryza australiensis, and Oryza punctata). Among the japonica varieties tested, all varieties except one (cv Tima) had the 1.2-kb insertion in the OsFRDL4 promoter (Table I; Supplemental Table S1). By contrast, among the indica varieties tested, 47 varieties did not contain the insertion, but five (Local basmati, Padikuning, Bingala, Lebed, and Basilanon) had the insertion in the promotor region (Table I; Supplemental Table S1). No insertion in the promoter region of OsFRDL4 was found in any wild rice species with different genome types (Table I; Supplemental Table S2).
Table I. Distribution of the 1.2-kb insertion in cultivated rice from the world core collection and wild rice species.
The insertion was investigated by PCR.
| Subspecies | Sample No. | Presence of the 1.2-kb Insertion | |
|---|---|---|---|
| − |
+ |
||
| indica | 52 | 47 | 5 |
| japonica | 16 | 1 | 15 |
| Total | 68 | 48 | 20 |
| Wild rice | 6 | 6 | 0 |
Cultivated rice, which is classified as an AA genome diploid, is considered to have been domesticated from Oryza rufipogon thousands of years ago (Khush, 1997; Cheng et al., 2003). Furthermore, a recent study showed that the cultivated rice japonica subspecies was first domesticated from a specific population of O. rufipogon (Or-III; Huang et al., 2012b). In addition to W630 (Or-I) used for the above investigation of the insertion, we also investigated whether the insertion was present in other O. rufipogon accessions and Oryza nivara, which is an annual allied species of O. rufipogon, using public short-read genomic sequence data sets (SRX809753, DRA000419, ERA125029, SRX367231, SRX367229, SRX367232, DRR000348, SRX367236, DRR001186, and SRX367233). The genome sequences of an O. nivara accession and nine O. rufipogon accessions, W0593 (Or-III), W1943 (Or-III), W1807 (Or-III), W1294 (Or-III), W2003 (Or-III), W0106 (Or-I), W0630 (Or-I), W1976 (Or-II), and W0120 (Or-II), were mapped on the cv Nipponbare genome sequence by Bowtie 2. A gene corresponding to OsFRDL4 was present in all O. nivara and O. rufipogon accessions at the same locus. Although sequence fragments very similar to the 1.2-kb insertion also were found in all O. nivara and O. rufipogon accessions due to the wide distribution of LTR retrotransposon-like repetitive sequence in their genomes, no sequence fragments flanked by the promoter region of a FRDL4-like gene were found. This result indicates that the 1.2-kb insertion is not present in the promoters of all wild rice accessions (Supplemental Fig. S4).
To test whether the insertion in exceptional cultivated rice varieties also is associated with the expression of OsFRDL4 (Table I; Supplemental Table S1), we compared the expression of OsFRDL4 and Al-induced citrate secretion among these lines. Irrespective of indica or japonica varieties, the presence of the insertion in the promoter region resulted in increased expression of OsFRDL4 and higher Al-induced secretion of citrate (Supplemental Fig. S3).
DISCUSSION
OsFRDL4 encodes a plasma membrane-localized efflux transporter of citrate, which is localized in all root cells (Yokosho et al., 2011; Fig. 5). It mediates the Al-induced secretion of citrate from the roots in rice. Although the secretion amount of organic acid anions was smaller in rice compared with other cereal crops such as rye (Secale cereale) and wheat (Triticum aestivum), OsFRDL4-mediated secretion of citrate partially contributes to the high Al tolerance in rice because knockout of this gene decreased Al tolerance, especially at high Al concentrations (Yokosho et al., 2011). Different from other Al tolerance genes in rice, the expression of OsFRDL4 in the absence of Al was very low (Yokosho et al., 2011), but its expression was induced greatly upon exposure to Al. Furthermore, there was a genotypic difference in the Al-induced expression of OsFRDL4 (Yokosho et al., 2011). In this study, we found that this differential expression of OsFRDL4 is responsible for the QTL for Al tolerance detected on chromosome 1 in previous studies (Wu et al., 2000; Nguyen et al., 2001; Ma et al., 2002; Fig. 1). Furthermore, we found that an insertion of 1.2 kb in the promoter region of OsFRDL4 plays an important role in regulating the expression of OsFRDL4 (Figs. 2 and 3).
The insertion identified in this study is a solo-LTR derived from a full-length LTR retrotransposon (Fig. 2). The insertion of transposons usually increases the level of methylation, resulting in decreased expression levels of flanking genes (He et al., 2011). However, in our study, we found that a 1.2-kb insertion enhanced the expression of OsFRDL4 (Figs. 2 and 3). Furthermore, all varieties carrying this insertion are associated with an enhanced expression of OsFRDL4. These results indicate that the expression of OsFRDL4 is controlled genetically but not epigenetically. Plant genomes harbor high numbers of retrotransposons compared with other organisms (Flavell, 1986; SanMiguel et al., 1996). LTR retrotransposons constitute at least 22% of the cultivated rice genome (Ma et al., 2004). A database search revealed that there are many sequences similar to the 1.2-kb insertion of the OsFRDL4 promoter in the rice genome. For example, upstream (−2 kb) of Os01g0772200, Os06g0547201, 02g0188600, and Os06g0196600, similar transposon-like sequences (greater than 90% identity) also were found. It would be interesting to examine whether their gene expression also is enhanced by the insertion, like that of OsFRDL4.
In the 1.2-kb insertion identified in this study, there are nine cis-acting elements for ART1 (Fig. 2). ART1 is a C2H2 zinc finger transcription factor for Al response and regulates the expression of at least 32 genes by binding to the cis-acting element GGN(T/g/a/C)V(C/A/g)S(C/G) in the promoter region (Yamaji et al., 2009; Tsutsui et al., 2011). Our transient assay showed that this insertion enhanced the expression of the reporter gene when coexpressed with ART1 (Fig. 4). However, this insertion did not alter the spatial expression pattern and localization of OsFRDL4 (Fig. 5). This is different from the insertion upstream of HvAACT1, a close homolog of OsFRDL4, in barley (Fujii et al., 2012), which alters both expression and localization.
Recently, several studies have revealed the acquisition processes of higher expression of Al-tolerance genes in several cereal crops. For example, in sorghum (Sorghum bicolor), Tourist-like miniature inverted-repeat transposable elements in the promoter region of SbMATE were suggested to be involved in regulating the expression of this gene (Magalhaes et al., 2007). In barley, a 1-kb insertion (CACTA-like transposon) in the 5′ untranscribed region of HvAACT1 enhances its expression in Al-tolerant accessions (Fujii et al., 2012). The higher expression level of TaMATE1B in several Brazilian wheat lines was found to be associated with the presence of a Sukkula-like transposable element (11.1 kb) in its promoter (Tovkach et al., 2013). Furthermore, higher expression of TaALMT1 (a malate transporter gene) in Al-tolerant genotypes of wheat is associated with a series of cis-mutations in the promoter region (Ryan et al., 2009). In maize (Zea mays), Al-tolerant cultivars have three functional copies of ZmMATE1 in the genome, which are identical and part of a tandem triplication (Maron et al., 2013). This copy number variation is associated with both the gene expression of ZmMATE1 and Al tolerance. All these changes probably happened during the expansion of the cultivated area of these crops to an acidic soil environment. In contrast, in Yorkshire fog (Holcus lanatus), the enhanced expression of HlALMT1 involved in malate secretion in an Al-tolerant accession was caused by the increasing number of ART1 cis-acting elements in its promoter (Chen et al., 2013). However, unlike in cereal crops, this increase is not derived from transposon insertion events but by nucleotide substitutions, suggesting its long-term evolutionary history. This study showed a novel acquisition mechanism of Al tolerance with an intermediate time scale conserved at the subspecies level in rice.
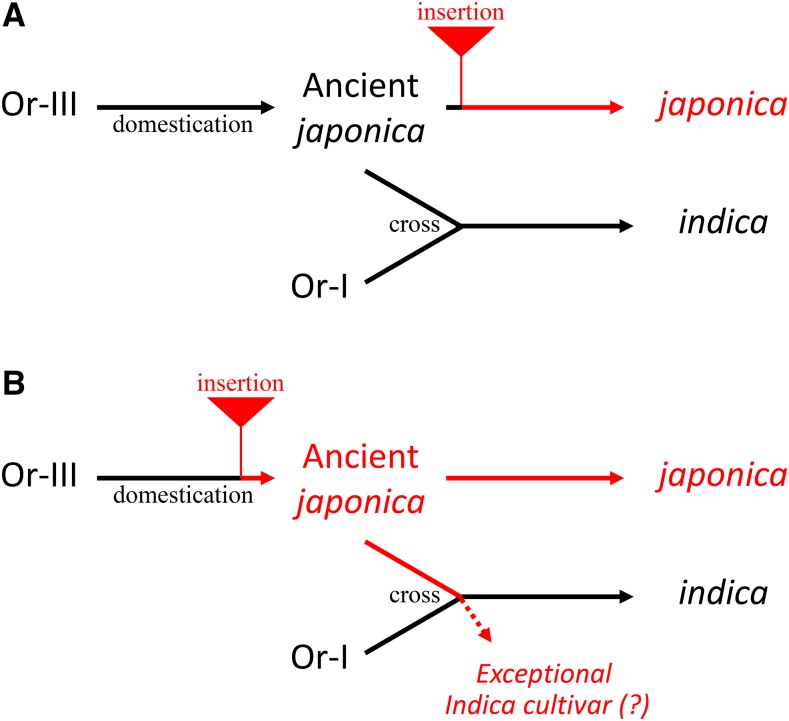
Investigation of world core collection rice and wild rice accessions showed that the 1.2-kb insertion was observed in most japonica varieties but not in indica and wild rice accessions (Table I; Supplemental Table S1). The cultivated rice japonica subspecies was first domesticated from a specific population of O. rufipogon (Or-III) around the middle area of the Pearl River in southern China, and the cultivated rice indica subspecies was developed subsequently from crosses between japonica rice and local O. rufipogon (Or-I) as the initial cultivars spread into southeast and south Asia (Huang et al., 2012b). We found no 1.2-kb insertion in the upstream region of OsFRDL4 orthologs in all three subtypes of O. rufipogon and O. nivara (Table I; Supplemental Table S2; Supplemental Fig. S4). This suggests that the 1.2-kb insertion occurred at an early stage of japonica domestication (Fig. 6). This is different from other crops, such as wheat, sorghum, and barley. In those species, after domestication, the Al-tolerance mechanisms were developed or reinforced during the adaptation to acid soil areas (Magalhaes et al., 2007; Fujii et al., 2012; Tovkach et al., 2013). One possible explanation is that barley originated from the Fertile Crescent in the Middle East, which is an alkaline soil area, and had to acquire Al tolerance by enhancing HvAACT1 expression for adaptation to acid soils when cultivation was expanded to those areas (Fujii et al., 2012). In wheat, the higher TaMATE1B expression in several Brazilian lines that originated from Portuguese landraces was associated with a Sukkula-like transposon (Tovkach et al., 2013; Garcia Oliveira et al., 2014). This insertion is supposed to be the result of adaption to the acid soil in Brazil. By contrast, the japonica rice subspecies was first domesticated from a population of O. rufipogon around the middle area of the Pearl River in southern China (Huang et al., 2012b), which is an acid soil area. Therefore, additional improvement of Al tolerance by enhancing OsFRDL4 expression might be important but not a trait necessary for the cultivation of rice.
Figure 6.
Schematic presentation of the 1.2-kb insertion in association with rice domestication. The 1.2-kb insertion event occurred before (A) or after (B) the domestication of ancient japonica varieties. Insertion in some exceptional indica varieties might have occurred through outcrosses with ancient japonica during the domestication of indica rice.
There are some exceptional varieties in japonica and indica rice (Table I). However, the 1.2-kb insertion also enhanced the expression of OsFRDL4 irrespective of japonica or indica background (Supplemental Fig. S3). Some exceptional indica varieties might be outcrossed with japonica varieties during the domestication of indica rice (Fig. 6B).
In conclusion, we identified a 1.2-kb insertion in the promoter region of OsFRDL4 that is required to enhance the expression of OsFRDL4 in japonica varieties due to the increased number of ART1 cis-acting elements. Our results also suggest that this insertion event occurred at the initial stage of domestication of japonica varieties.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Two rice (Oryza sativa) CSSLs (SL4 and SL6) were obtained from the Rice Genome Resource Center. In SL4 as a control, the segment from marker R2159 to C1370 (87.9–123.5 centimorgan of chromosome 1) was substituted by the cv Kasalath segment in the cv Nipponbare background (http://www.rgrc.dna.affrc.go.jp/data/NK-SL54-20030430.pdf; Supplemental Fig. S1). By contrast, in SL6, the segment from marker C86 to C112 (146.7–181.1 centimorgan of chromosome 1) containing OsFRDL4 was substituted by the cv Kasalath segment in the cv Nipponbare background (http://www.rgrc.dna.affrc.go.jp/data/NK-SL54-20030430.pdf; Supplemental Fig. S1).
A total of 68 varieties of cultivated rice, including 52 indica and 16 japonica varieties, were provided by the National Agriculture and Food Research Organization (http://www.naro.affrc.go.jp/; Supplemental Table S1). The wild rice species Oryza rufipogon (W0630), Oryza barthii (W0720), Oryza glumaepatula (W1169), Oryza meridionalis (W1627), Oryza australiensis (W0008), and Oryza punctata (W1514) were obtained from the National Institute of Genetics. The details for these materials are described in Supplemental Tables S1 and S2.
Seeds were soaked in tap water overnight at 30°C in the dark and then transferred to a net floating on 0.5 mm CaCl2 (pH 5.6) in a 1.5- or 20-L plastic container. After 5 d, the seedlings were used for expression analysis and Al tolerance test as described below. For the Al-induced citrate secretion experiment, seedlings were transferred to a 1.5-L plastic pot containing one-half-strength Kimura B solution in a glasshouse at 25°C to 30°C under natural light. The nutrient solution contained the macronutrients (NH4)2SO4 (0.18 mm), MgSO4·7H2O (0.27 mm), KNO3 (0.09 mm), Ca(NO3)2·4H2O (0.18 mm), and KH2PO4 (0.09 mm) and the micronutrients MnCl2·4H2O (0.5 μm), H3BO3 (3 μm), (NH4)6Mo7O24·4H2O (1 μm), ZnSO4·7H2O (0.4 μm), CuSO4·5H2O (0.2 μm), and Fe-EDTA (20 μm). The pH of this solution was adjusted to 5.6, and the nutrient solution was renewed every 2 d.
Measurement of Relative Root Elongation
Rice seedlings (5 d old) were exposed to 0.5 mm CaCl2 containing 0 or 30 μm Al (pH 4.5) at 25°C for 24 h. Root length was measured with a ruler before and after the exposure. Relative root elongation was calculated as follows: (root elongation with Al)/(root elongation without Al) × 100. Seven to 10 roots were measured for each treatment.
Determination of Al-Induced Citrate Secretion
For the collection of root exudates, rice seedlings (14 d old) prepared as above were exposed to a 0.5 mm CaCl2 (pH 4.5) solution overnight and then exposed to a 0.5 mm CaCl2 (pH 4.5) solution containing 30 μm Al. After 24 h, the root exudates were collected and concentrated according to Yokosho et al. (2011). After the residue was redissolved in 1 mL of Milli-Q water, the concentration of organic acids was analyzed by an enzymatic method according to Delhaize et al. (1993).
Identification of the 1.2-kb Insertion in the OsFRDL4 Promoter
The sequences of the OsFRDL4 upstream region in cv Nipponbare and cv Kasalath were compared using PCR primers designed based on the cv Nipponbare genome sequence (IRGSP-1.0): 5′-GGTACCGCCCGAGTCACTCGCATATGAGTA-3′ (forward) and 5′-TACTAGTGCAGGTGTTGCTTAGCTCGTCGAT-3′ (reverse). The fragments were amplified from cv Nipponbare and cv Kasalath genomic DNA by PCR using KOD-FX (Toyobo) for 35 cycles. The PCR products were separated by electrophoresis on a 1% agarose gel using 0.5× Tris-borate/EDTA buffer stained with ethidium bromide. These fragments were purified by the Quick Step2 PCR Purification Kit (Edge Bio) and then sequenced using an ABI PRISM 310 genetic analyzer and BigDye Terminator version 3.1 cycle sequencing kit (Applied Biosystems). The alignment was performed by GENETYX version 13.0.4 (https://www.genetyx.co.jp/).
RNA Isolation and Gene Expression Analysis
For spatial expression analysis, after the roots of both cv Nipponbare and cv Kasalath were exposed to 30 μm AlCl3 (pH 4.5) for 12 h, different root segments (0–3, 3–6, and 6–9 mm from the apex) were excised, immediately frozen in liquid nitrogen, and stored at −80°C until use. The expression level of OsFRDL4 in CSSLs or different rice cultivars was examined by exposing 5-d-old seedlings to a 0.5 mm CaCl2 (pH 4.5) solution containing 30 µm AlCl3 for 24 h and then excising the root tips (0–1 cm). Total RNA was extracted using the RNeasy Mini Kit (Qiagen) according to the manufacturer’s protocol. Total RNA (1 μg) was used for first-strand cDNA synthesis using a ReverTra Ace (Toyobo) according to the procedures described by the manufacturer. Expression was determined with Sso Fast EvaGreen Supermix (Bio-Rad) on CFX384 (Bio-Rad). Primers used were 5′-CGTCATCAGCACCATCCACAG-3′ (forward) and 5′-TCATTTGCGAAGAAACTTCCACG-3′ (reverse) for OsFRDL4, 5′-CAGTGCTTCTCGTGGGTCTT-3′ (forward) and 5′-CCTGTGCGTGAAGAACCACT-3′ (reverse) for ART1, and 5′-AGTTTGGTCGCTCTCGATTTCG-3′ (forward) and 5′-TCAACAAGTTGACCACGTCACG-3′ (reverse) for Histone H3. Expression data were normalized with the expression level of Histone H3 by the ΔΔCt method. In expression analysis of several exceptional rice varieties, Actin was used as an internal standard because the efficiency of amplification of Histone H3 in cv Tima was lower than in other cultivars. The primers used were 5′-GACTCTGGTGATGGTGTCAGC-3′ (forward) and 5′-GGCTGGAAGAGGACCTCAGG-3′ (reverse). All experiments were conducted with three biological replicates.
Immunostaining of OsFRDL4 Protein in cv Nipponbare and cv Kasalath
Roots of cv Nipponbare and cv Kasalath exposed to a 0.5 mm CaCl2 solution with or without 30 μm Al (pH 4.5) for 12 h were used for immunostaining. An antibody against OsFRDL4 was described before (Yokosho et al., 2011). Immunostaining was performed using the root tip (3 mm from the root tip) and mature root zone (15 mm) as described previously (Yamaji and Ma, 2007). Fluorescence from the secondary antibody (Alexa Fluor 555 goat anti-rabbit IgG; Molecular Probes, available from Invitrogen) was observed with a confocal laser scanning microscope (LSM700; Carl Zeiss).
Identification of the TSS
To determine the TSS of OsFRDL4, total RNA from the roots of both cv Nipponbare and cv Kasalath exposed to 30 μm AlCl3 (pH 4.5) for 6 h was isolated with the RNeasy Plant Mini Kit (Qiagen). 5′ RACE was performed with the GeneRacer Kit (Invitrogen) according to Fujii et al. (2012). The 5′ ends of OsFRDL4 cDNA were amplified by PCR with the GeneRacer primer and a gene-specific primer pair, 5′-GGACACTGACATGGACTGAAGGAGTAGAAA-3′ (forward) and 5′-GCGTTCAGCACGAACAGATGCAG-3′ (reverse). The PCR products were separated by electrophoresis on a 2% agarose gel using 0.5× Tris-borate/EDTA buffer stained with ethidium bromide.
Transient Promoter Assay in Protoplasts
The promoter regions of OsFRDL4 were amplified from the genomic DNA from cv Nipponbare and cv Kasalath and then inserted upstream of a region comprising GFP as a reporter gene and the NOS terminator in pBluescript vector (Stratagene). The primers used for the amplification and introduction of restriction sites were 5′-GGTACCGCCCGAGTCACTCGCATATGAGTA-3′ (forward) and 5′-TACTAGTGCAGGTGTTGCTTAGCTCGTCGAT-3′ (reverse) for OsFRDL4-N (cv Nipponbare allele) and 5′-AGAATTCGGTTTATAATTTGCTACCGTTTT-3′ (forward) and 5′-TACTAGTGCAGGTGTTGCTTAGCTCGTCGAT-3′ (reverse) for OsFRDL4-K (cv Kasalath allele).
The open reading frame of ART1 from cv Nipponbare and cv Kasalath cDNA was amplified by PCR and cloned between the CaMV 35S promoter and the NOS terminator in pBluescript vector using primers 5′-ACTGTCGACATGGACCGCGGCAAGAAT-3′ (forward) and 5′-TAATGCGGCCGCCGGTACATCTGAAATTAT-3′ (reverse). DsRed was used as an internal standard as constructed by Tsutsui et al. (2011). Tobacco (Nicotiana tabacum) cultured cells (BY-2) were cultured for 4 d in 50 mL of modified Linsmaier and Skoog (1965) medium. For the isolation of protoplasts, BY-2 cells were cultured in enzyme mix solution (1% cellulase Onozuka RS, 0.1% macerozime R-10, 0.1% pectoryase, 20 mm MES-KOH, 20 mm CaCl2, and 400 mm mannitol, pH 5.6) with shaking at a low speed (20 rpm) in the dark at 28°C. After 6 h, the digested cells were filtered through a KimWipe to a Falcon tube, followed by centrifugation at 100g for 5 min. After the supernatant was removed, the pellet was resuspended in 10 mL of washing solution (10 mm MES-KOH, 20 mm CaCl2, and 400 mm mannitol, pH 5.6) and washed twice with the washing solution. The protoplasts were finally collected by centrifugation at 100g for 5 min and resuspended in a magnesium solution (10 mm MES-KOH, 30 mm MgCl2, and 400 mm mannitol, pH 5.6).
The reporter plasmid (pOsFRDL4-GFP), transcriptional factor plasmid (ART1), and internal control plasmid (DsRed) were cotransformed into protoplasts by the polyethylene glycol method as described by Tsutsui et al. (2011). The transformed protoplasts were incubated in Murashige and Skoog medium (0.22% [w/v] Murashige and Skoog salts, 400 mm mannitol, and 10 mm MES-KOH, pH 5.6) in the dark for 17 h at 25°C, followed by exposure to 0 or 100 μm AlCl3. After 4 h, the protoplasts were collected by centrifugation at 100g for 5 min, and samples of the pellet were frozen in liquid nitrogen for RNA extraction. RNA extraction and cDNA synthesis were as described above. Quantitative real-time RT-PCR was performed using specific primers 5′-AGGAGCGCACCATCTTCTTCAA-3′ (forward) and 5′-GCTGTTGTAGTTGTACTCCAGC-3′ (reverse) for GFP, 5′-GGACAACACCGAGGACGTCATC-3′ (forward) and 5′-CGCCCTTGGTCACCTGCAGCTT-3′ (reverse) for DsRed, and 5′-CAGTGCTTCTCGTGGGTCTT-3′ (forward) and 5′-CCTGTGCGTGAAGAACCACT-3′ (reverse) for ART1.
Investigation of the 1.2-kb Insertion in World Core Collection Rice and Wild Rice
The presence of the 1.2-kb insertion in the promoter region of OsFRDL4 was investigated in a total of 68 world core collection rice plants (https://www.gene.affrc.go.jp/databases-core_collections.php) and six accessions of wild rice (Supplemental Tables S1 and S2). Leaf (1 cm) samples were taken from each variety and stored at −30°C until use. After the leaf was homogenized with a Tissue Lyser II (Qiagen), DNA was extracted according to the method described by Komatsuda et al. (1998). To determine the presence of the 1.2-kb insertion in the OsFRDL4 promoter region, we performed PCR using the primers described above.
The short-read genomic sequences from paired-end sequencing data sets of one Oryza nivara and nine O. rufipogon accessions, W0593 (Or-III), W1943 (Or-III), W1807 (Or-III), W1294 (Or-III), W2003 (Or-III), W0106 (Or-I), W0630 (Or-I), W1976 (Or-II), and W0120 (Or-II), were obtained from the sequence-read archive (SRX809753, DRA000419, ERA125029, SRX367231, SRX367229, DRR000348, SRX367232, SRX367236, DRR001186, and SRX367233; http://trace.ddbj.nig.ac.jp/dra/index.html). The genomic sequences of O. nivara and O. rufipogon were mapped on the cv Nipponbare genome sequence (IRGSP-1.0) by Bowtie 2 (Langmead and Salzberg, 2012) using the Galaxy/NAAC server (https://galaxy.dna.affrc.go.jp/nias/static/register_en.html). Paired-end parameters were set as minimum insert size of zero and maximum insert size of 400. Default settings were used for other parameters. The mapping sequence was shown with Integrative Genomics Viewer (Robinson et al., 2011).
Statistical Analysis
The analysis of significance was performed by Student’s t test using Excel or Tukey’s test using the statistical analysis software MEPHAS (http://www.gen-info.osaka-u.ac.jp/MEPHAS/). Differences at P < 0.05 were considered as significant and highly significant, respectively.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Schematic presentation of CSSLs on chromosome 1.
Supplemental Figure S2. Comparison of ART1 expression and amino acid sequences in cv Nipponbare and cv Kasalath.
Supplemental Figure S3. Expression of OsFRDL4, citrate secretion, and Al tolerance in several exceptional rice varieties.
Supplemental Figure S4. Comparison of genome sequences including the OsFRDL4 region (5,671 bp) in cv Nipponbare and wild rice (O. nivara and O. rufipogon).
Supplemental Table S1. Presence of the 1.2-kb insertion in the promoter of OsFRDL4 in the world rice core collection.
Supplemental Table S2. Presence of the 1.2-kb insertion in the promoter of OsFRDL4 in wild rice species.
Supplementary Material
Acknowledgments
We thank the Rice Resource Center in Tsukuba for proving CSSL seeds and the National Institute of Genetics for seeds of the wild rice accessions.
Glossary
- QTL
quantitative trait locus
- CSSL
chromosome segment substitution line
- TSS
transcriptional start site
- LTR
long terminal repeat
- CaMV
cauliflower mosaic virus
Footnotes
This work was supported by a Grant-in-Aid for Specially Promoted Research from the Japan Society for the Promotion of Science (grant no. 16H06296 to J.F.M.).
Articles can be viewed without a subscription.
References
- Che J, Yamaji N, Shen RF, Ma JF (2016) An Al-inducible expansin gene, OsEXPA10 is involved in root cell elongation of rice. Plant J, 10.1111/tpj.13237 [DOI] [PubMed] [Google Scholar]
- Chen ZC, Yamaji N, Motoyama R, Nagamura Y, Ma JF (2012) Up-regulation of a magnesium transporter gene OsMGT1 is required for conferring aluminum tolerance in rice. Plant Physiol 159: 1624–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZC, Yokosho K, Kashino M, Zhao FJ, Yamaji N, Ma JF (2013) Adaptation to acidic soil is achieved by increased numbers of cis-acting elements regulating ALMT1 expression in Holcus lanatus. Plant J 76: 10–23 [DOI] [PubMed] [Google Scholar]
- Cheng C, Motohashi R, Tsuchimoto S, Fukuta Y, Ohtsubo H, Ohtsubo E (2003) Polyphyletic origin of cultivated rice: based on the interspersion pattern of SINEs. Mol Biol Evol 20: 67–75 [DOI] [PubMed] [Google Scholar]
- Delhaize E, Ryan PR, Randall PJ (1993) Aluminum tolerance in wheat (Triticum aestivum L.). II. Aluminum-stimulated excretion of malic acid from root apices. Plant Physiol 103: 695–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famoso AN, Zhao K, Clark RT, Tung CW, Wright MH, Bustamante C, Kochian LV, McCouch SR (2011) Genetic architecture of aluminum tolerance in rice (Oryza sativa) determined through genome-wide association analysis and QTL mapping. PLoS Genet 7: e1002221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell RB. (1986) Repetitive DNA and chromosome evolution in plants. Philos Trans R Soc Lond B Biol Sci 312: 227–242 [DOI] [PubMed] [Google Scholar]
- Foy CD. (1988) Plant adaptation to acid, aluminum-toxic soils. Commun Soil Sci Plant Anal 19: 959–987 [Google Scholar]
- Fujii M, Yokosho K, Yamaji N, Saisho D, Yamane M, Takahashi H, Sato K, Nakazono M, Ma JF (2012) Acquisition of aluminium tolerance by modification of a single gene in barley. Nat Commun 3: 713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Oliveira AL, Martins-Lopes P, Tolrá R, Poschenrieder C, Tarquis M, Guedes-Pinto H, Benito C (2014) Molecular characterization of the citrate transporter gene TaMATE1 and expression analysis of upstream genes involved in organic acid transport under Al stress in bread wheat (Triticum aestivum). Physiol Plant 152: 441–452 [DOI] [PubMed] [Google Scholar]
- He XJ, Chen T, Zhu JK (2011) Regulation and function of DNA methylation in plants and animals. Cell Res 21: 442–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CF, Yamaji N, Chen Z, Ma JF (2012a) A tonoplast-localized half-size ABC transporter is required for internal detoxification of aluminum in rice. Plant J 69: 857–867 [DOI] [PubMed] [Google Scholar]
- Huang CF, Yamaji N, Ma JF (2010) Knockout of a bacterial-type ATP-binding cassette transporter gene, AtSTAR1, results in increased aluminum sensitivity in Arabidopsis. Plant Physiol 153: 1669–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CF, Yamaji N, Mitani N, Yano M, Nagamura Y, Ma JF (2009) A bacterial-type ABC transporter is involved in aluminum tolerance in rice. Plant Cell 21: 655–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Kurata N, Wei X, Wang ZX, Wang A, Zhao Q, Zhao Y, Liu K, Lu H, Li W, et al. (2012b) A map of rice genome variation reveals the origin of cultivated rice. Nature 490: 497–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khush GS. (1997) Origin, dispersal, cultivation and variation of rice. Plant Mol Biol 35: 25–34 [PubMed] [Google Scholar]
- Komatsuda T, Nakamura I, Takaiwa F, Oka S (1998) Development of STS markers closely linked to the vrs1 locus in barley, Hordeum vulgare. Genome 41: 680–685 [Google Scholar]
- Kumar A, Bennetzen JL (1999) Plant retrotransposons. Annu Rev Genet 33: 479–532 [DOI] [PubMed] [Google Scholar]
- Langmead B, Salzberg SL (2012) Fast gapped-read alignment with Bowtie 2. Nat Methods 9: 357–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JY, Liu J, Dong D, Jia X, McCouch SR, Kochian LV (2014) Natural variation underlies alterations in Nramp aluminum transporter (NRAT1) expression and function that play a key role in rice aluminum tolerance. Proc Natl Acad Sci USA 111: 6503–6508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsmaier EM, Skoog F (1965) Organic growth factor requirements of tobacco tissue cultures. Physiol Plant 18: 100–127 [Google Scholar]
- Ma J, Devos KM, Bennetzen JL (2004) Analyses of LTR-retrotransposon structures reveal recent and rapid genomic DNA loss in rice. Genome Res 14: 860–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF, Chen ZF, Shen RF (2014) Molecular mechanisms of Al tolerance in gramineous plants. Plant Soil 381: 1–12 [Google Scholar]
- Ma JF, Shen R, Zhao Z, Wissuwa M, Takeuchi Y, Ebitani T, Yano M (2002) Response of rice to Al stress and identification of quantitative trait loci for Al tolerance. Plant Cell Physiol 43: 652–659 [DOI] [PubMed] [Google Scholar]
- Magalhaes JV, Liu J, Guimarães CT, Lana UG, Alves VM, Wang YH, Schaffert RE, Hoekenga OA, Piñeros MA, Shaff JE, et al. (2007) A gene in the multidrug and toxic compound extrusion (MATE) family confers aluminum tolerance in sorghum. Nat Genet 39: 1156–1161 [DOI] [PubMed] [Google Scholar]
- Maron LG, Guimarães CT, Kirst M, Albert PS, Birchler JA, Bradbury PJ, Buckler ES, Coluccio AE, Danilova TV, Kudrna D, et al. (2013) Aluminum tolerance in maize is associated with higher MATE1 gene copy number. Proc Natl Acad Sci USA 110: 5241–5246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen BD, Brar DS, Bui BC, Nguyen TV, Pham LN, Nguyen HT (2003) Identification and mapping of the QTL for aluminum tolerance introgressed from the new source, Oryza rufipogon Griff., into indica rice (Oryza sativa L.). Theor Appl Genet 106: 583–593 [DOI] [PubMed] [Google Scholar]
- Nguyen VT, Burow MD, Nguyen HT, Le BT, Le TD, Paterson AH (2001) Molecular mapping of genes conferring aluminum tolerance in rice (Oryza sativa L.). Theor Appl Genet 102: 1002–1010 [Google Scholar]
- Nguyen VT, Nguyen BD, Sarkarung S, Martinez C, Paterson AH, Nguyen HT (2002) Mapping of genes controlling aluminum tolerance in rice: comparison of different genetic backgrounds. Mol Genet Genomics 267: 772–780 [DOI] [PubMed] [Google Scholar]
- Robinson JT, Thorvaldsdóttir H, Winckler W, Guttman M, Lander ES, Getz G, Mesirov JP (2011) Integrative genomics viewer. Nat Biotechnol 29: 24–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PR, Raman H, Gupta S, Horst WJ, Delhaize E (2009) A second mechanism for aluminum resistance in wheat relies on the constitutive efflux of citrate from roots. Plant Physiol 149: 340–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SanMiguel P, Tikhonov A, Jin YK, Motchoulskaia N, Zakharov D, Melake-Berhan A, Springer PS, Edwards KJ, Lee M, Avramova Z, et al. (1996) Nested retrotransposons in the intergenic regions of the maize genome. Science 274: 765–768 [DOI] [PubMed] [Google Scholar]
- Tovkach A, Ryan PR, Richardson AE, Lewis DC, Rathjen TM, Ramesh S, Tyerman SD, Delhaize E (2013) Transposon-mediated alteration of TaMATE1B expression in wheat confers constitutive citrate efflux from root apices. Plant Physiol 161: 880–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui T, Yamaji N, Ma JF (2011) Identification of a cis-acting element of ART1, a C2H2-type zinc-finger transcription factor for aluminum tolerance in rice. Plant Physiol 156: 925–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitte C, Panaud O, Quesneville H (2007) LTR retrotransposons in rice (Oryza sativa, L.): recent burst amplifications followed by rapid DNA loss. BMC Genomics 8: 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Liao CY, Hu B, Yi KK, Jin WZ, Ni JJ, He C (2000) QTLs and epistasis for aluminum tolerance in rice (Oryza sativa L.) at different seedling stages. Theor Appl Genet 100: 1295–1303 [Google Scholar]
- Xia J, Yamaji N, Che J, Shen RF, Ma JF (2014) Differential expression of Nrat1 is responsible for Al-tolerance QTL on chromosome 2 in rice. J Exp Bot 65: 4297–4304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Yamaji N, Kasai T, Ma JF (2010) Plasma membrane-localized transporter for aluminum in rice. Proc Natl Acad Sci USA 107: 18381–18385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Yamaji N, Ma JF (2013) A plasma membrane-localized small peptide is involved in rice aluminum tolerance. Plant J 76: 345–355 [DOI] [PubMed] [Google Scholar]
- Yamaji N, Huang CF, Nagao S, Yano M, Sato Y, Nagamura Y, Ma JF (2009) A zinc finger transcription factor ART1 regulates multiple genes implicated in aluminum tolerance in rice. Plant Cell 21: 3339–3349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaji N, Ma JF (2007) Spatial distribution and temporal variation of the rice silicon transporter Lsi1. Plant Physiol 143: 1306–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokosho K, Yamaji N, Fujii-Kashino M, Ma JF (2016) Functional analysis of a MATE gene OsFRDL2 revealed its involvement in Al-induced secretion of citrate, but a lower contribution to Al tolerance in rice. Plant Cell Physiol 57: 976–985 [DOI] [PubMed] [Google Scholar]
- Yokosho K, Yamaji N, Ma JF (2011) An Al-inducible MATE gene is involved in external detoxification of Al in rice. Plant J 68: 1061–1069 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.