Results from binding assays and paramagnetic relaxation enhancement ssNMR demonstrate that β-expansin EXPB1 binds matrix polysaccharides, predominantly glucuronarabinoxylan, rather than cellulose.
Abstract
The wall-loosening actions of β-expansins are known primarily from studies of EXPB1 extracted from maize (Zea mays) pollen. EXPB1 selectively loosens cell walls (CWs) of grasses, but its specific binding target is unknown. We characterized EXPB1 binding to sequentially extracted maize CWs, finding that the protein primarily binds glucuronoarabinoxylan (GAX), the major matrix polysaccharide in grass CWs. This binding is strongly reduced by salts, indicating that it is predominantly electrostatic in nature. For direct molecular evidence of EXPB1 binding, we conducted solid-state nuclear magnetic resonance experiments using paramagnetic relaxation enhancement (PRE), which is sensitive to distances between unpaired electrons and nuclei. By mixing 13C-enriched maize CWs with EXPB1 functionalized with a Mn2+ tag, we measured Mn2+-induced PRE. Strong 1H and 13C PREs were observed for the carboxyls of GAX, followed by more moderate PREs for carboxyl groups in homogalacturonan and rhamnogalacturonan-I, indicating that EXPB1 preferentially binds GAX. In contrast, no PRE was observed for cellulose, indicating very weak interaction of EXPB1 with cellulose. Dynamics experiments show that EXPB1 changes GAX mobility in a complex manner: the rigid fraction of GAX became more rigid upon EXPB1 binding while the dynamic fraction became more mobile. Combining these data with previous results, we propose that EXPB1 loosens grass CWs by disrupting noncovalent junctions between highly substituted GAX and GAX of low substitution, which binds cellulose. This study provides molecular evidence of β-expansin’s target in grass CWs and demonstrates a new strategy for investigating ligand binding for proteins that are difficult to express heterologously.
Expansins comprise the only group of plant proteins demonstrated to induce stress relaxation and long-term, irreversible extension (creep) of plant cell walls (CWs), which are essential for cell enlargement during plant growth (Cosgrove, 2000, 2016). Two major classes of plant expansins, α-expansins (EXPA) and β-expansins (EXPB), are recognized and both are present as multigene families in all land plants (Li et al., 2002; Sampedro and Cosgrove, 2005; Cosgrove, 2015). Expansin genes are also found in a variety of bacteria, fungi, and other microbes (Darley et al., 2003; Georgelis et al., 2014), acquired by horizontal gene transfer from plants (Nikolaidis et al., 2014). Analysis of BsEXLX1, the expansin from Bacillus subtilis (Kerff et al., 2008; Georgelis et al., 2015), has been particularly insightful for assessing the complex binding properties and functional targeting of this protein within the CW. Such progress was enabled by expression of BsEXLX1 in Escherichia coli, facilitating creation of site-directed mutants (Georgelis et al., 2011, 2012) and 13C, 15N labeling for solid-state NMR (SSNMR) analysis of EXLX1-CW interactions in muro (Wang et al., 2013). Compared with BsEXLX1, plant expansins have stronger wall-loosening activity and different selectivity, so their actions still require further study. Unfortunately, heterologous expression of active plant expansins has been difficult so far; thus, our understanding of their targets of action is based on limited studies with native proteins.
The CW-loosening activity of β-expansin is known primarily through studies of maize (Zea mays) EXPB1, which is abundantly expressed in pollen and is known in the immunology literature as Zea m 1d, a group 1 grass pollen allergen. Phylogenetic analysis shows that EXPB1 is part of a distinctive group of β-expansins that evolved uniquely in the grass family (Poaceae; Sampedro et al., 2015) and serve a biological function that appears to be unique among expansins, which is to facilitate penetration of the pollen tube through the stigma and style, thereby quickening delivery of sperm to the ovule (Valdivia et al., 2007, 2009). The crystal structure of EXPB1 was solved with native protein purified from maize pollen and shows two tightly packed domains forming an elongated ellipsoidal protein with a long, open, presumptive binding surface that spans the two domains (Yennawar et al., 2006).
EXPB1 induces stress relaxation and creep of CWs from grasses but has negligible loosening effect on dicot CWs (Cosgrove et al., 1997; Li et al., 2003; Sampedro et al., 2015). In contrast, α-expansin induces stress relaxation and creep of CWs from both dicots and grasses, in some cases with a greater effect on dicot CWs (McQueen-Mason et al., 1992; Cho and Kende, 1997; Sampedro et al., 2015). These observations led to the proposal (Li et al., 2003) that EXPB1 specifically targets glucuronoarabinoxylan (GAX), which is abundant in grass CWs but is largely absent in primary CWs of dicots (Carpita, 1996; Vogel, 2008).
Consistent with this idea, Tabuchi et al. (2011) found that treatment of maize CWs with EXPB1 induces release (solubilization) of a form of GAX with a high degree of arabinose substitution (hsGAX) as well as smaller amounts of homogalacturonan (HG). Extensive testing of EXPB1 did not detect any enzymatic activities, just as tests for enzymatic activities with α-expansins and bacterial expansins proved to be negative (McQueen-Mason and Cosgrove, 1995; Kerff et al., 2008; Georgelis et al., 2011, 2015). Moreover, EXPB1 caused marked mechanical weakening of grass CWs, as measured in tensile force/extension assays. These actions were likewise specific to grass CWs, as polysaccharide release and wall weakening were not seen when EXPB1 was applied to CWs from a variety of nongrass species, nor have they been found with α-expansins (McQueen-Mason and Cosgrove, 1995; Yuan et al., 2001; Tabuchi et al., 2011) or bacterial expansins (Georgelis et al., 2014). Thus, while EXPB1 action has commonalities with α-expansin action, namely induction of CW creep and stress relaxation, it also has unique features. The molecular details of these unique actions are not well understood, but they apparently entail structural features unique to grass CWs.
We set out in this study to assess EXPB1 binding with specific components of the grass CW. The primary CWs of grasses consist of cellulose (approximately 20–60%), GAX (approximately 20–35%), mixed-linkage glucan (MLG; approximately 5–20%, transient), xyloglucan (XyG, approximately 2–10%), and pectic polysaccharides (approximately 5–10%). The percentages shown here are based on analyses of maize and barley coleoptiles and vary by developmental stage (Carpita, 1996; Carpita et al., 2001; Gibeaut et al., 2005). The most notable differences between dicot and grass CW compositions are the low amount of pectins and XyG and the high content of GAX in grass CWs. GAX varies considerably in arabinose substitution, and this variation correlates with binding to cellulose (Carpita, 1983): hsGAX (Ara: Xyl > 0.6) is weakly held in the CW and may be extracted with calcium chelators and even water, whereas GAX with low substitution (lsGAX, Ara: Xyl of < 0.5) is tightly bound in the CW and requires strong extractants such as 1 to 4 m NaOH for solubilization. GAX may also be oxidatively cross-linked via ferulate esterified to arabinose (Carpita et al., 2001; Buanafina, 2009), but EXPB1 does not break such cross links (Tabuchi et al., 2011).
To assess EXPB1 binding to grass CW polymers, in this study we quantified EXPB1 binding to whole CWs and to sequentially extracted CWs in which more tightly bound matrix polysaccharides were progressively removed. This approach was used previously to assess the binding target of α-expansin in dicot walls (McQueen-Mason and Cosgrove, 1995). We also employed a SSNMR technique, paramagnetic relaxation enhancement (PRE; Solomon, 1955; Bertini et al., 2001), to determine which wall polysaccharides are the closest to EXPB1. Unpaired electrons in paramagnetic ions or organic radicals enhance nuclear-spin T2 and T1 relaxation in a distance-dependent fashion. The PRE effect is sensitive to electron-nuclear distances up to approximately 2 nm (Nadaud et al., 2009; Jaroniec, 2015), which is much longer than the distances measurable from nuclear-spin dipolar couplings. PRE solid-state NMR has been used to refine protein structure (Sengupta et al., 2012, 2015), measure the depth of insertion of membrane proteins in lipid bilayers (Buffy et al., 2003; Su et al., 2012; Marbella et al., 2013; Maltsev et al., 2014), and study metal ion complexation in bacterial CWs (Kern et al., 2010). Here we demonstrate a novel application of PRE NMR, where EXPB1 extracted from natural sources is functionalized with a paramagnetic EDTA-Mn2+ tag to allow the determination of the polysaccharide binding target, without the need for recombinant 13C, 15N-labeled protein.
Both binding assays and PRE SSNMR data indicate that EXPB1 binds primarily to GAX, while binding to cellulose is negligible. More specifically, the PRE SSNMR spectra show that the GlcA residues in GAX experience the strongest PRE by Mn2+-tagged EXPB1, followed by carboxyl groups in pectins. These results represent the first molecular evidence to our knowledge of the β-expansin binding target in grass CWs and offer insights into the loosening mechanism of EXB1.
RESULTS
EXPB1 Binding to Maize CWs
CW binding to EXPB1 was first characterized by depletion isotherms carried out in 20 and 50 mm NaOAc, adjusted to pH 5.5, using CWs isolated from maize silks. We used these CWs because EXPB1 normally interacts with silks in nature (Valdivia et al., 2009). These buffers were chosen because they are commonly used to assay CW creep activity (Li et al., 2003). The isotherms (Fig. 1A) show saturable binding, with approximately 60% greater binding in 20 mm NaOAc. Parallel measurements of CW extension (creep) show higher activity in 20 mm NaOAc than in 50 mm NaOAc (Supplemental Fig. S1). EXPB1 shows strong CW creep activity under conditions corresponding to approximately 1.5 μmol EXPB1 bound per gram CW, that is, at the initial (steep) part of these binding isotherms. The EXPB1-binding capacity of maize CWs thus greatly exceeds the amount of EXPB1 needed for robust CW creep activity.
Figure 1.
EXPB1 binding to maize CW in 20 mm and 50 mm NaOAc. A, Bound EXPB1 as a function of free EXPB1. The binding curves are best-fit to one-site and two-site Langmuir models for low and high concentrations of NaOAc. B and C, Scatchard plots of the binding data with trend lines, confirming the distinct binding patterns under the two conditions.
EXPB1 binding in 50 mm NaOAc fits well to a single-site Langmuir model, whereas a two-site Langmuir model is a better fit for binding in 20 mm NaOAc solution (Fig. 1A). This difference in binding kinetics is also seen in Scatchard plots (Fig. 1, B and C), where a high-affinity binding site (Kd 0.03 μm) is observed in 20 mm NaOAc but not in 50 mm NaOAc. This result indicates that binding properties are sensitive to buffer strength or ionic conditions. Because of the complex nature of the CW, these fitting parameters are best viewed at this point as empirical summaries of binding characteristics without a more specific molecular interpretation.
Additional experiments showed that binding was largely insensitive to pH in the range of 4.0 to 5.5 (Supplemental Fig. S2) but was inhibited >90% by addition of various salts in a concentration-dependent manner (Fig. 2), indicating that EXPB1 binding to the CW is strongly electrostatic. At low added ionic strength (12 mM), Al3+ was appreciably more effective at inhibiting binding than Na+, indicating a partial specificity of Al3+ interference with binding beyond simple ionic strength, but this difference was reduced at higher ionic strengths as binding was strongly inhibited (Fig. 2, inset). There was little difference in the effectiveness of Ca2+ and Mg2+ in inhibiting EXPB1 binding, indicating that the well-known specific interaction of Ca2+ with pectins does not affect EXPB1 binding. Together, these results suggest that EXPB1 binds preferentially to GAX, which is the predominant, weakly acidic polysaccharide in the maize CW (Carpita, 1996; Carpita et al., 2001).
Figure 2.
EXPB1 binding to maize silk CW in 20 mm NaOAc as a function of the ionic strength of added Cl− salts. Inset compares Al3+ and Na+ effects, showing larger attenuation of binding by Al3+ at low ionic strength. Total EXPB1 was 7.8 μmol/g CW. Means ± se of 3 replicates.
To test this inference further, we assessed EXPB1 binding to CWs that were extracted sequentially to first remove weakly bound pectins and hsGAX, then more tightly bound lsGAX, MLG, and XyG. A final trifloroacetic acid (TFA) hydrolysis step removed noncrystalline polysaccharides, leaving crystalline cellulose as the residue. EXPB1 binding to the extracted residues decreased almost linearly in proportion to the loss of material from the CW, shown by the lower gray line in Figure 3A. When expressed per gram of CW residue (Fig. 3A, top line), the loss of EXPB1 binding is found to be the steepest in the later extraction steps after 4 m NaOH, which swells cellulose and extracts tightly bound hemicelluloses, and particularly after TFA hydrolysis of noncrystalline polysaccharides. Thus, we infer that EXPB1 targets both weakly bound and strongly bound matrix polysaccharides, but with a preference toward the latter. Expansin binding to the TFA-hydrolyzed residue is comparatively weak and similar to binding to the commercial cellulose Avicel (Fig. 3; Supplemental Fig. S3). As was the case with unextracted CW, EXPB1 binding to the extracted CW residues was strongly reduced by added salt (Fig. 3B), except for cellulose. Sugar analysis of the CW residues and extracts (Supplemental Table S1) confirms that the first step in the extraction series removes pectins (approximately 60%; predominantly HG and a small amount of rhamnogalacturonan I) and hsGAX (approximately 30%). Sugar composition of the second extract (0.1 m NaOH) is consistent with hsGAX, while the compositions of the 1 and 4 m NaOH extracts are consistent with a mixture of GAX, XyG, and MLG. The composition and extraction patterns for this CW resemble that of previous work on maize coleoptiles (Carpita, 1983; Carpita et al., 2001). These results indicate that EXPB1 binds to both highly substituted and lowly substituted GAX in the grass CW, although additional binding to XyG and MLG cannot be excluded. Despite the strong electrostatic component of EXPB1 binding to the whole CW, substantial binding to pectins is not indicated by these results, because while 60% of the pectin is removed in the first extraction step, the residue retained most of the EXPB1-binding ability.
Figure 3.
EXPB1 binding to sequentially extracted CWs. A, Amount of EXPB1 bound to extracted CW residues, expressed per gram CW residue (black, top curve) or per gram of starting material (gray, bottom curve). B, Reduction of EXPB1 binding by salt (25 mm MgCl2) in the sequentially extracted CW samples. Assayed in 20 mm NaOAc with total EXPB1 of 7.8 μmol per gram CW in A and 6.1 μmol per gram CW in B. Means ± se of three replicates.
To test the idea that CW binding is essential for EXPB1 activity, we assessed the effect of salt on EXPB1-induced CW creep and release of feruoylated GAX from maize CWs (Tabuchi et al., 2011). As shown in Figure 4, A and B, 10 mm CaCl2 strongly inhibited EXPB1-induced creep activity and likewise inhibited release of sugars and 320-nm absorbance, corresponding to feruoylated GAX. It is possible that the calcium inhibition of CW creep was caused by mechanical stiffening of CW by cross-linking of nonesterified HG by calcium. However, 10 mm CaCl2 did not reduce elastic or plastic compliances of the walls, as measured in stress/strain assays (Fig. 4C), excluding a purely mechanical basis for the calcium inhibition of EXPB1 activity. Therefore, we conclude that the salt-induced inhibition of EXPB1 activities results primarily from reduced EXPB1 interaction with the CW. This is also consistent with the reduced CW creep in 50 mm versus 20 mm buffer (Supplemental Fig. S1).
Figure 4.
Inhibition of EXPB1 activities by 10 mm CaCl2. A, Creep of wheat coleoptile walls upon addition of EXPB1 at two concentrations in the presence or absence of 10 mm Ca2+. B, Release of feruoylated GAX (A320) and polysaccharide from 1 mg of maize silk CW after 20 h in 20 mm NaOAc with or without EXPB1 (100 μg/mL) and with or without 10 mm Ca2+. C, Effect of Ca2+ on mechanical compliances of wheat coleoptile walls. In A, curves are means of 8 to 10 replicates; in B and C, the values are means ± 3 and 12 replicates, respectively.
Paramagnetic Tagging of EXPB1
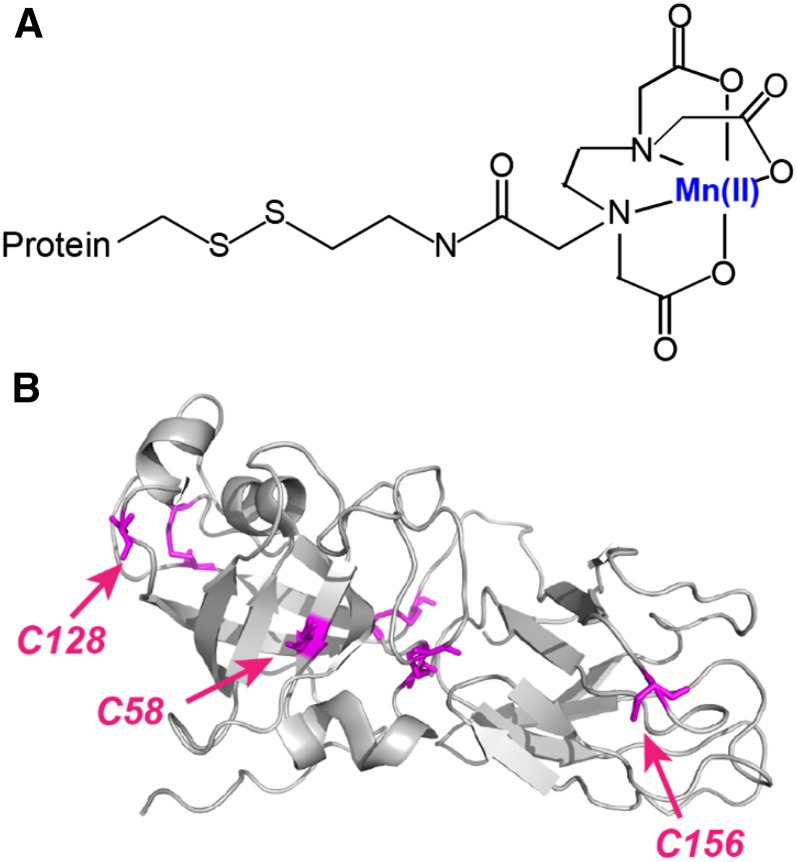
To provide structural evidence of the expansin binding target, we conducted SSNMR PRE experiments. For this purpose, we prepared EDTA-Mn+2-tagged EXPB1 and mixed it with hydrated 13C-labeled maize CWs at a mass ratio of 0.1, corresponding to the mid part of the binding curve in 20 mm NaOAc (approximately 3.6 μmol EXPB1 per gram CW; Fig. 1A). This concentration corresponds to the mid-high range of the activity-concentration curve for EXPB1 and is below its saturation point for wall extension activity (Li et al., 2003). The EDTA-Mn2+ tag was covalently attached to accessible free cysteines on the protein surface (Fig. 5). There are nine cysteines in EXPB1, six of which are involved in three conserved disulfide bonds (Yennawar et al., 2006), leaving three cysteines, C58, C128, and C156, accessible to solvent and potentially capable of binding EDTA-Mn2+. C128 and C156 lie at the two ends of the protein, flanking the putative polysaccharide binding surface, while C58 is located at the bottom of a shallow cleft, close to the presumptive active site of EXPB1 domain 1. C58 is sterically less accessible than C128 and C156, so it may be less reactive with the EDTA tag. Electrospray ionization mass spectrometry (ESI-MS) analysis of the labeled protein showed that the dominant species incorporated two linkers/two Mn2+ ions (Supplemental Fig. S4A), while smaller signals were found for EXPB1 with two linkers/one Mn2+ and three linkers/three Mn2+. Tagging did not alter EXPB1 binding to the CW (Supplemental Fig. S4B), but it partially inhibited CW creep activity (Supplemental Fig. S4D). The reduced activity may indicate partial steric hindrance of EXPB1 action by the EDTA-Mn+2 tags. Because tagging did not appreciably affect bulk binding characteristics, we expect the CW-EXPB1 interactions, as detected by SSNMR, to be similar to those of untagged EXPB1.
Figure 5.
Mn2+ tagging of β-expansin. A, Modification of solvent-accessible Cys residues in proteins using [S-(2-pyridylthio) cysteaminyl] EDTA-metal (Ebright et al., 1992; Ermácora et al., 1992). B, The structure of EXPB1, Zea m 1 (PDB code 2HCZ), showing all nine Cys residues (magenta), among which are three solvent-accessible cysteines (red arrows) that can be modified with EDTA-Mn2+. The other six cysteines form three disulfide bonds.
EXPB1 Targets Matrix Polysaccharides
Figure 6A compares quantitative 13C direct polarization (DP) magic angle spinning (MAS) spectra of maize CWs with Mn2+-tagged or untagged EXPB1. The spectra were measured with a long recycle delay of 25 s, so that the signals of dynamic and rigid polysaccharides are quantitatively represented. Most 13C signals <110 ppm show similar intensities between the two samples, while the carbonyl region shows a clear intensity decrease for the 177-ppm peak in the paramagnetic sample. Spectral deconvolution indicates at least six peaks in this region (Fig. 6, B and C), four of which have narrow linewidths of 0.6 to 1.0 ppm. Based on previous resonance assignment of Arabidopsis (Arabidopsis thaliana) and Brachypodium CWs, we assign the 177-ppm peak to the carboxylate group in the GlcA residues of GAX (Wang et al., 2014), the 176-ppm peak to GalA in pectins, the 174-ppm peak to acetyl in RG-1 and GAX, and the 172-ppm peak to methyl esters in HG (Wang et al., 2012; White et al., 2014; Supplemental Fig. S5). Interestingly, this maize CW carbonyl spectral pattern resembles a composite of the spectra of the Arabidopsis and Brachypodium CWs: both share the GalA and acetyl peaks, but the Arabidopsis spectrum lacks the 177-ppm GlcA peak while the Brachypodium spectrum lacks the 172-ppm methyl ester peak. Thus, the maize CW contains significant amounts of both GAX and pectins. In addition to the well-resolved carbonyl peaks, two broad carbonyl peaks with full width at half maximum of approximately 7 ppm and approximately 2 ppm are necessary to fit the spectra (Supplemental Table S2). We tentatively assign these broad peaks to matrix polysaccharides that undergo segmental motions on the tens to hundreds of kilohertz timescale, which interfere with 1H dipolar decoupling. Our previous correlation and relaxation experiments consistently found partial immobilization of some of the matrix polysaccharides, presumably due to binding to cellulose (Wang et al., 2012; Wang et al., 2014).
Figure 6.
A, Quantitative 13C DP spectra of maize CWs containing EXPB with (red) and without (black) Mn2+ tags. The spectra were measured at 296 K with a 25-s recycle delay. The difference spectrum shows that only carboxylate carbons are dephased by PRE, indicating protein binding. The small peak at 165 ppm is the spinning sideband of the 105-ppm peak. B, Spectral deconvolution of the carbonyl region of the EXPB control spectrum using Dmfit (Massiot et al., 2002). C, Spectral deconvolution of the carbonyl region of the EXPB-Mn2+ spectrum. Deconvolution indicates that the carboxylate linewidth increased from 0.6 ppm in the control sample to 1.0 ppm in the Mn2+ tagged sample, consistent with the PRE effect.
With the distinction of the multiple carbonyl peaks, we can see that the Mn2+-tagged EXPB1 decreased the 177-ppm peak intensity to 57% of the intensity of the diamagnetic sample, with a concomitant increase of the peak line width from 135 Hz (0.6 ppm) to 225 Hz (1.0 ppm; Fig. 6, B and C). Thus, Mn2+-tagged expansin preferentially enhances the T2 relaxation rate of GlcA in GAX. In contrast, the resolved carbonyl peaks of pectins do not show intensity dephasing, indicating that expansin is further from pectins.
Although these quantitative 13C DP spectra reflect the relative concentrations of dynamic and rigid polysaccharides, direct excitation of the 13C magnetization has the disadvantage that PRE occurs on 13C spins, whose low gyromagnetic ratio reduces the upper-limit distance that is measurable. The transverse PRE, 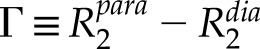 , depends on the electron-nuclear distance r and the nuclear-spin gyromagnetic ratio γn according to the Solomon-Bloembergen equation (Solomon, 1955; Bloembergen, 1957):
, depends on the electron-nuclear distance r and the nuclear-spin gyromagnetic ratio γn according to the Solomon-Bloembergen equation (Solomon, 1955; Bloembergen, 1957):
 |
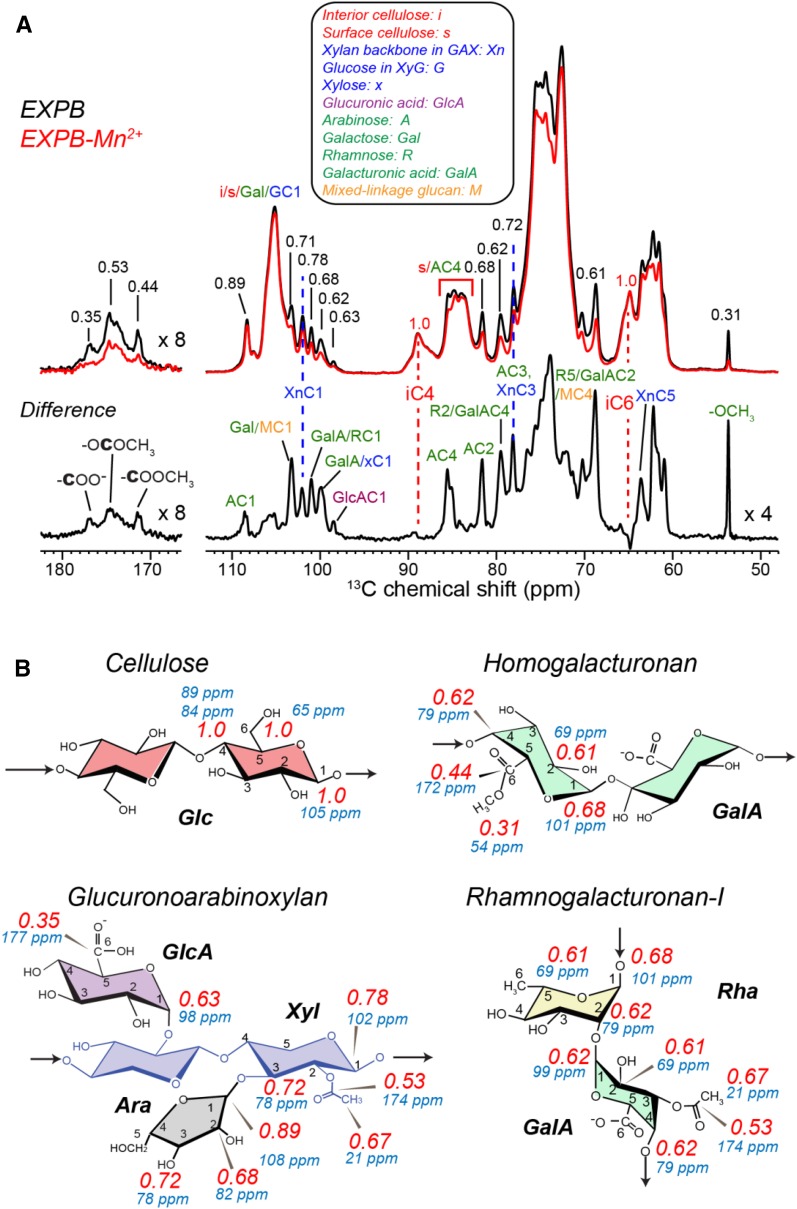
where μ0 is the vacuum permeability, ge is the electron g-factor, μB is the Bohr magneton, S is the electron-spin quantum number, and ωn and ωe denote the nuclear and electron Larmor frequencies, respectively. This equation indicates that the PRE scales with the square of the nuclear-spin gyromagnetic ratio; thus, for the same distances, the 1H PRE should be 16-fold stronger than 13C PRE, or conversely, the same 1H PRE corresponds to 2.5-fold longer distances than 13C PRE. To obtain longer distances, we measured the 13C cross-polarization (CP) spectra (Fig. 7A), where the magnetization originates from 1H spins. Indeed, Figure 7A shows significant intensity dephasing for many peaks in the paramagnetic sample, consistent with the detection of 1H PRE due to Mn2+. Interestingly, the difference spectrum between the two samples shows only sharp GAX and pectin signals while lacking cellulose signals, indicating that EXPB1 preferentially binds matrix polysaccharides. More quantitatively, Figure 7B summarizes the peak height ratios (S/S0) between the EXPB1-Mn2+-bound CW and the diamagnetic EXPB1-bound CW. These S/S0 ratios depend on the average electron-nuclei distances, which take into account potential structural heterogeneities that may cause only a subpopulation of a polysaccharide in the CW to be close to expansin. Cellulose retains full signals in the presence of Mn2+, indicating that it is uniformly far from EXPB1, while GAX and pectins show considerable dephasing, with S/S0 ratios ranging from 0.31 to 0.89. Consistent with the 13C DP spectra, the CP spectra show the lowest S/S0 value at GlcA in GAX, indicating that EXPB1 binds GAX most strongly. However, the 54-ppm methyl ester carbon shows a similarly low S/S0 value of 0.31, indicating that GalA in HG is strongly influenced by Mn2+. Since this peak did not show detectable PRE in the 13C DP spectra (Fig. 6), we conclude that EXPB1 preferentially binds the more rigid fraction of HG, while the mobile pectins, which dominate the quantitative DP spectra, are far from EXPB1.
Figure 7.
A, 13C CP spectra of the EXPB1-containing maize CWs with (red) and without (black) Mn2+. The difference spectrum shows only intensities of matrix polysaccharides, indicating that EXPB1 binds matrix polysaccharides but not cellulose. Assignment abbreviations used in this work are given. B, Intensity ratios (red) of the Mn2+-containing spectrum and the control spectrum. The chemical shifts of the carbons are shown in blue. Cellulose does not show intensity decrease, indicating that it is far from EXPB1. GAX shows moderately low intensity ratios (0.7–0.9) for Xyl and Ara but much lower intensity ratios for GlcA carboxylate (0.35). Pectins also exhibit low intensity ratios of 0.3 to 0.7.
The larger intensity decrease of the 13C CP spectra compared to the DP spectra may not only result from stronger 1H PRE than 13C PRE but may also be influenced by preferential detection of rigid polysaccharides in the CP spectra. In these hydrated CW samples, the mass ratio of EXPB1 to total polysaccharide is about 1:10. Excluding the approximately 25 wt% of cellulose in the CW (Supplemental Table S1), the protein to matrix polysaccharide dry mass ratio is approximately 1:7.5. The 13C CP experiments detect approximately 40% of the matrix polysaccharide signals, thus the CP-detected average protein to matrix polysaccharide mass ratio is approximately 1:3. This relatively high protein concentration makes it possible to detect significant intensity dephasing due to Mn2+ PRE.
To further resolve the 13C signals that are indicative of site-specific Mn2+ PRE, we measured 2D PDSD spectra of the paramagnetic and diamagnetic samples (Fig. 8A). Upon first inspection, the two 2D spectra appear very similar, both dominated by cellulose signals. However, the difference 2D spectrum reveals clear intensity changes only of GAX and pectin peaks (Fig. 8, B and C), while the cellulose peaks show no intensity difference. The 2D spectra also resolved the surface cellulose signals from matrix-polysaccharide signals, for example in the 82- to 86-ppm region, where both arabinose and surface cellulose C4 peaks resonate, and in the 61- to 63-ppm region, where the surface cellulose C6 signal overlaps with the Gal C6, Xn C5, and Ara C5 signals. The improved spectral resolution reveals that the surface cellulose signals such as C4-C1 at (85, 105) ppm and C6-C4 at (62, 85) ppm are absent in the difference spectrum, further confirming that EXPB1 is far from cellulose, including the microfibril surface.
Figure 8.
2D 13C-13C CP-PDSD spectra of EXPB1-containing maize CWs. A, The 100-ms spectra of CWs with EXPB1 (black) and with Mn2+-tagged EXPB1 (red). B, Difference spectrum, showing only matrix polysaccharide signals. Gray horizontal bar indicates the interior cellulose C4 position. C, Representative cross-sections of the 2D PDSD spectra. The diamagnetic (black) and paramagnetic (red) samples show identical cross-sections for cellulose, but the GAX and pectin signals are lower in the paramagnetic sample. Assignment abbreviations are given in Figure 7.
EXPB1 Changes GAX Mobility
To investigate the effect of EXPB1 on polysaccharide mobility, we measured 13C-1H dipolar couplings and C-H order parameters (SCH) with and without EXPB1. The DIPSHIFT experiment detects the amplitudes of motion of C-H bonds on the submicrosecond timescale. The protein-containing CWs show very similar order parameters as protein-free CWs in the quantitative 13C DIPSHIFT experiment (Fig. 9), indicating that the average motional amplitudes of the two samples are the same. However, in the CP-DIPSHIFT experiment, which preferentially detects more rigid molecules, the GAX signals show larger dipolar couplings or order parameters, indicating that the motional amplitudes decrease in the presence of the protein, while cellulose and pectin motions are not affected by protein binding (Fig. 9; Supplemental Fig. S6). Thus, the more rigid portion of GAX is further immobilized by EXPB1, whereas the more mobile portion of GAX increases the motional amplitudes so that the average GAX signals show no net change ± EXPB1.
Figure 9.
13C-1H DIPSHIFT dipolar curves of maize CWs measured with quantitative 13C DP (A) and 1H-13C CP (B). All spectra were measured at 296 K under 7 kHz MAS. The best-fit 13C-1H dipolar couplings and dipolar order parameters are indicated for each panel. CWs without and with EXPB1 are plotted in orange and black, respectively. The DP-DIPSFHIT data were measured using a long recycle delay of 20 s and detect all the molecules in a quantitative manner, while CP-DIPSHIFT preferentially detects the signals of rigid polysaccharides.
DISCUSSION
The binding isotherms and SSNMR data presented here indicate that the main target of EXPB1 binding in maize CWs is GAX. This conclusion is supported by binding studies with sequentially extracted CWs, by inhibition of EXPB1 binding by salts, and by preferential PRE to GlcA residues of GAX. This conclusion is also consistent with the selectivity of EXPB1 action: EXPB1 loosens GAX-containing grass primary CWs but has little effect on dicot CWs, which are rich in GalA-containing pectins but lack GAX (Li et al., 2003; Sampedro et al., 2015; Carpita, 1996). Moreover, the PRE NMR data (Fig. 7) show that EXPB1 does not bind cellulose in whole CW mixtures, even though it binds isolated cellulose in vitro (Supplemental Fig. S3).
EXPB1 binding to GlcA is most likely electrostatic. The protein contains 31 cationic residues (22 Lys and 9 Arg) but only 22 anionic residues (13 Asp and 9 Glu), has a pI of 9.5 (Li et al., 2003), and all cationic residues are located on the protein surface. Thus, in 20 mm NaOAc at pH 5.5, the protein is positively charged, favoring electrostatic interactions with the negatively charged GlcA and GalA residues in matrix polysaccharides.
13C-1H dipolar order parameters indicate that EXPB1 exerts a complex effect on GAX mobility: the rigid portion of GAX became more rigid upon protein binding while the mobile portion became more dynamic. In contrast, cellulose and pectin mobilities were unaffected by the protein: cellulose retained the same near rigid-limit couplings, while pectins exhibited the same low-order parameters. Thus, although PRE of pectins was observed in the 13C CP spectra, EXPB1 did not change the mobilities of pectins, while EXPB1 immobilized some of the GAX. Combined with the fact that the quantitative 13C DP spectra exhibit only 13C PRE to GAX, these data indicate that EXPB1 binds GlcA-rich arabinoxylan more strongly than GalA-rich pectins in grass CWs. Since both GAX and pectins contain negatively charged carboxyl groups, we attribute this difference in EXPB1 affinities to conformational differences between GAX and pectins. EXPB1 has a long, shallow groove formed by highly conserved polar and aromatic residues. This groove is about 47 Å long, spans both EXPB1 domains, and could accommodate a GAX backbone of up to 10 sugar residues (Yennawar et al., 2006). This shallow and relatively flat groove is less likely to fit the helical conformation of HG or the highly branched RG-1. The GAX-EXPB1 binding may be further stabilized by specific interactions between GAX and polar and aromatic residues on the protein surface (Yennawar et al., 2006; Sampedro et al., 2015). Attempts to crystallize a complex of EXPB1 and GAX have so far not been successful (Y. Chen, N. Yennawar, D.J. Cosgrove, unpublished data), so other means to test this hypothesis are needed.
A Model of EXPB1-Mediated CW Loosening
Our results indicate that EXPB1 has negligible interaction with cellulose but binds GAX and changes its mobility, potentially by unlocking GAX-GAX junctions in grass CWs. This new information complements previous data showing that EXPB1 specifically loosens grass CWs and solubilizes hsGAX from maize CWs (Li et al., 2003; Tabuchi et al., 2011). Previous biochemical data indicate that hsGAX displays only weak binding to cellulose (Carpita, 1983), thus it is unlikely to serve as a load-bearing linker directly interconnecting cellulose microfibrils but may act as space-filling interstitial material between cellulose microfibrils (Carpita et al., 2001). Additionally, it could serve a load-bearing role if it bound to matrix polysaccharides that in turn bind tightly to cellulose. Candidate polysaccharides that bind tightly to cellulose include lsGAX, XyG, and MLG (Carpita et al., 2001). We focus on lsGAX because our new data indicate the rigid component of GAX, which is presumably lsGAX, becomes more rigid after EXPB1 treatment. Figure 10 sketches a potential mechanism by which EXPB1 may exert its loosening effects on grass CWs. In this scheme, hsGAX binds to lsGAX, thereby indirectly spanning multiple cellulose microfibrils; we propose that EXPB1 may bind and disrupt the hsGAX-lsGAX junctions. Consistent with this scenario, lsGAX is known to bind to cellulose in vitro (Carpita, 1983; Köhnke et al., 2011; Selig et al., 2015), whereas hsGAX does not. Strong GAX-cellulose cross peaks are seen in 2D SSNMR spectra of Brachypodium, a model grass species, indicating subnanometer contacts between the two polysaccharides (Wang et al., 2014), which is also supported by the presence of a cellulose form that is restructured by interactions with GAX in Brachypodium and XyG in Arabidopsis CWs (Wang and Hong, 2016; Wang et al., 2016). If EXPB1 selectively disrupted noncovalent junctions between hsGAX and lsGAX, as envisioned in Figure 10, it would result in (1) increased mobility and solubilization of hsGAX, (2) decreased mobility of lsGAX, which would now interact more strongly with cellulose, and (3) promotion of CW stress relaxation and extension as a result of removal of the load-bearing hsGAX. These are the known actions of EXPB1 on grass CWs. In this scheme, release of HG would be incidental to solubilization of hsGAX. For EXPB1 acting on grass CWs, these actions would also weaken the middle lamella, which in grasses is composed of GAX and HG (Ishii, 1984), thereby promoting pollen tube penetration between cells of the maize stigma and style (Valdivia et al., 2007, 2009). This model may apply specifically to the clade of β-expansins that include group-1 grass pollen allergens, but additional work is needed to test its applicability to other groups of β-expansins and to other types of GAX-containing CWs (Sampedro et al., 2015).
Figure 10.
Conceptual scheme to account for the known CW-loosening and -binding activities of maize EXPB1. In this limited depiction of the grass CW, lsGAX binds to cellulose surfaces and hsGAX binds to lsGAX but not cellulose. EXPB1 is hypothesized to disrupt the noncovalent junctions (depicted as short black lines) between hsGAX and lsGAX. The result is solubilization of hsGAX and physical weakening of the grass CW.
Helical Conformation of Xylan Backbone of GAX from C4 Chemical Shifts
The 13C chemical shifts of GAX in maize CWs are very similar to those in Brachypodium (Wang et al., 2014). The XnC4 chemical shift is sensitive to conformational changes of glycosidic bonds (Viëtor et al., 2002) and has been used as an indicator of the helical conformation of xylan in a recent SSNMR study (Dupree et al., 2015). MD simulation suggests that xylan in solution adopts a 3-fold helical screw (31-fold, three residues per 360° twist) but is folded as a 2-fold helical screw (21-fold, two residues per 360° twist) once adsorbed onto cellulose surface. The latter should better align with the 21 conformations of glucan chains (Bromley et al., 2013; Busse-Wicher et al., 2014). The typical XnC4 chemical shift is 76 to 78 ppm in solution (Vignon and Gey, 1998; Hollmann et al., 2009; Busse-Wicher et al., 2014) but changes to 79 to 85 ppm in the dry secondary CW of Arabidopsis, indicating a broad distribution of conformations with only a minor population adopting the 31-fold conformation (Dupree et al., 2015). In comparison, in the current maize CW samples, the XnC4 chemical shift is 74 ppm, which is similar to the chemical shifts in solution but different from the chemical shifts of xylan in Arabidopsis secondary CW (Dupree et al., 2015). Thus, the GAX backbone in maize primary CW may adopt a conformation similar to the 3-fold helical conformation in solution, implying that much of the GAX is well solvated and not tightly adsorbed onto cellulose surfaces. Future chemical shift calculations may be useful for correlating the NMR chemical shifts with the detailed conformations of xylan in different CWs (Kubicki et al., 2013, 2014; Watts et al., 2013; Zhao et al., 2014).
MATERIALS AND METHODS
EXPB1 Purification
Maize (Zea mays) pollen was collected in July 2014 at Rock Springs Farm (University Park, PA). EXPB1 was eluted from pollen with 50 mm NaOAc (adjusted to pH 4.5 with glacial acetic acid) containing 100 mm NaCl and fractionated stepwise by cation ion-exchange CM Sepharose (Sigma-Aldrich no. CCF-100) and ENrich (Bio-Rad no. 780-0021) chromatography and further fractionated on a Discover C5 reverse-phase HPLC column (Sigma-Aldrich no. 568422) (Li et al., 2003; Tabuchi et al., 2011).
Silk CW Preparation, Extraction, and Binding
Frozen maize silks (approximately 50 g wet weight) were ground to a fine powder by ball milling, washed sequentially with 1.5% (w/v) SDS and water, and then resuspended in 50 mL 50 mm HEPES buffer, pH 6.8, containing 20 units porcine amylase (Sigma-Aldrich no. A3176) to remove starch and 2 mm NaN3 to inhibit microbial growth. CWs were digested for 20 h at 37°C with constant stirring, then rinsed in water and lyophilized. For some experiments, we sequentially extracted silk CWs (with 50 mm cyclohexane diamine tetraacetic acid, 0.1 m NaOH, 1 m NaOH, and 4 m NaOH) as described (Tabuchi et al., 2011). The residue after 4 m NaOH extraction was hydrolyzed with 2 m TFA at 121°C for 2 h. Wall residues at each step were washed 5× with water before dialysis against water for 48 h to remove salts. The extracted polysaccharides were desalted by membrane filters (3-kD molecular weight cutoff) and lyophilized. Wall/polysaccharide constituents were analyzed by monosaccharide analysis using ion chromatography (Dionex ICS5000 with Carbopac column and pulsed amperometric detection), as described previously (Tabuchi et al., 2011). Various amounts of EXPB1 were bound to 1 mg CW residues in 20 mm NaOAc buffer, pH 5.5, to a total volume of 400 µL. Binding equilibrium was established by shaking at 1,100 rpm for 1 h at 26°C. Residual EXPB1 in the supernatant was determined by Bradford protein assay kit (Thermo). Binding isotherms and Langmuir fits were analyzed with Origin v9.1 curve-fitting software (Microcal).
EXPB1 Function ± Ca2+
Phenolics/Polysaccharide Release
A total of 1 mg nonextracted silk CW was incubated with 100 μg/mL EXPB1 in 500 μL 20 mm NaOAc buffer, pH 5.5, with 5 mm DTT to maintain expansin activity and 2 mm NaN3 to retard microbial growth, and shaken at 1,000 rpm for 20 h ± 10 mm CaCl2. Release of feruoylated GAX into the supernatant was assayed by 320-nm absorbance and the released sugar was measured with the phenol-sulfuric acid method (Dubois et al., 1956).
Mechanical Compliances
Four-day-old etiolated wheat coleoptiles were abraded, heat-inactivated, and incubated in 20 mm NaOAc buffer, pH 5.5, ±10 mm CaCl2 for 30 min and extended twice at 3 mm/min to a target force of 10g. The total, elastic, and plastic compliances were calculated from the slopes of the force/extension curves as detailed by Cosgrove (2011).
Wall Extension (Creep)
Coleoptile walls were prepared as above, clamped into a constant force extensometer at 20g force, and incubated in 20 mm NaOAc buffer, pH 5.5, containing 2 mm DTT for 30 min to establish a stable baseline. The buffer was replaced with same buffer ±10 mm CaCl2. The specimens were incubated for another 30 min before the addition of EXPB1.
Mn2+ Labeling of EXPB1
Linker (thiol-specific disulfide reagent, N-[S-(2-pyridylthio)cysteaminyl]EDTA; Toronto Research Chemicals) was dissolved in 50 mm NaOAc buffer (pH 5.5, filtered through a 0.45-µm syringe filter) to a concentration of 5 mg/mL and 100 µL linker solution (1 µmol) was mixed with 1 µL of 1 m MnCl2 (99.999%; Sigma-Aldrich) in deionized water and incubated at 4°C for 2 h to load Mn2+ onto the EDTA. EXPB1 was purified by reverse-phase HPLC, and 2.8 mg (0.1 µmol) was mixed with linker-Mn2+ solution. The reaction volume was brought to 200 µL by adding 50 mm NaOAc buffer, pH 5.5, and allowed to react overnight at 4°C. Excess linker-Mn2+ was removed by filtration through a 3-kD cutoff membrane. Incorporation of linker-Mn2+ was assessed by ESI-MS (Proteomics and Mass Spectrometry Core Facility, PSU), and creep activity of labeled EXPB1 was assayed by extensometer assay.
13C Uniform Labeling of Maize CWs
Maize seeds were surface sterilized twice (5 min/sterilization) in 50% bleach with 0.05% (v/v) Tween 20 and soaked overnight in sterile water. Endosperms were removed manually and the embryos were cultured in Murashige and Skoog medium (4.3 g MS salts [Sigma-Aldrich], 0.55 g MES·H2O per liter, adjusted to pH 5.7) containing 5 g/L 13C-Glc for 4 d in the dark at 27°C with 130 rpm shaking. Coleoptiles were collected, frozen in liquid nitrogen, and ground to a fine powder with a mortar and a pestle. CWs were prepared as described above for maize silks. Amylase-digested walls were washed with water, then centrifuged at 1,000g for 5 min and 5,000g for 60 min with a 40-µm nylon membrane filter to reduce water content to approximately 75%. A wet mass of 50 mg, which is estimated to contain approximately 12 mg dry mass, was used to bind protein.
EXPB1 and CW Binding
A total of 1.2 mg of paramagnetic Mn2+-tagged EXPB1 and diamagnetic EXPB1 was incubated for approximately 10 h with 50 mg hydrated CW in 300 µL 20 mm NaOAc buffer, pH 5.5, and 2 mm NaN3 at 4°C. The water content of the wall-protein mixture was reduced to approximately 75% by centrifugation. The hydrated CW samples were stored frozen prior to SSNMR analysis.
Solid-State NMR Spectroscopy
All SSNMR experiments were measured on a Bruker 900-MHz (21.1 Tesla) spectrometer using a 3.2-mm MAS probe at 296K. Typical radio-frequency strengths were 62.5 to 80 kHz for 1H and 62.5 kHz for 13C. 13C chemical shifts were reported on the tetramethylsilane scale, using the adamantane CH2 signal at 38.48 ppm as external reference.
1D 13C spectra were measured at 296 K under 13.5-kHz MAS. Initial 13C magnetization was created using either 13C DP or 1 ms 1H-13C CP. Quantitative DP spectra were measured using a recycle delay of 25 s. The difference spectra were obtained by subtracting the spectrum of the paramagnetic sample from that of the diamagnetic sample. A near-unity scaling factor of 0.94 to 1.0 was used to compensate for minor differences in sample amounts. PRE-induced intensity decrease is reported as S/S0, the ratio of the peak height of the 13C spectrum of the paramagnetic sample with the peak height of the diamagnetic sample. The carbonyl region of the quantitative 13C DP spectra was deconvoluted using Dmfit (Massiot et al., 2002). The full widths at half maximum, the peak heights, and the integrals of the deconvoluted peaks are reported in Supplemental Table S2.
2D 1H-driven 13C spin diffusion (PDSD) spectra were measured at 296 K under 13.5-kHz MAS (Meier, 1994; Bardet et al., 1997). Then 1 ms 1H-13C CP was used to create the initial 13C magnetization. The mixing time was 100 ms to observe intramolecular cross peaks (Wang et al., 2015). All CP-PDSD spectra were measured with 64 scans per t1 slice, an acquisition time of 14.5 ms in the t2 dimension, 472 t1 slices acquired in the states fashion at an increment of 35 μs, which give a maximum t1 evolution time of 8.3 ms. Difference 2D spectrum was obtained by subtracting the EXPB1-Mn2+ spectrum from the EXPB1 spectrum without any scaling factor. All 2D spectra were processed using a QSINE window function with a moderate shifted sine bell of 2.5 and were plotted using Topspin parameters of lev0 = 4.5, Toplev = 40, and nlev = 16.
13C-1H dipolar-chemical shift experiments (Munowitz et al., 1981) were measured under 7-kHz MAS at 296K. 1H homonuclear decoupling was achieved using frequency-switched Lee-Goldburg (Bielecki et al., 1989) with a tilted 1H radio-frequency field strength of 98 kHz. The scaling factor is confirmed to be 0.577 using the model tripeptide formyl-Met-Leu-Phe (Hong and Griffin, 1998; Rienstra et al., 2002). Order parameters were obtained by dividing the measured dipolar couplings by the scaled rigid-limit coupling (13.1 kHz).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. CW extension (creep) activity of heat-inactivated wheat coleoptiles in 50 mm v 20 mm sodium acetate buffer.
Supplemental Figure S2. Dependence of EXPB1 binding to maize silk CW as a function of pH.
Supplemental Figure S3. EXPB1 binding to pure cellulose (Avicel).
Supplemental Figure S4. Characterization of Mn2+-tagged EXPB1.
Supplemental Figure S5. Carbonyl region of the 13C spectra of Arabidopsis, Brachypodium, and maize CWs.
Supplemental Figure S6. 13C-1H CP-DIPSHIFT dipolar curves of maize CWs.
Supplemental Table S1. Monosaccharide compositions of CW and extracted polysaccharides after sequential chemical extractions.
Supplemental Table S2. Fitting parameters of the carbonyl signals in 13C quantitative DP spectra.
Supplementary Material
Acknowledgments
We thank Ed Wagner and Dr. Tatiana Laremore for technical assistance.
Glossary
- CP
cross-polarization
- CW
cell wall
- DP
direct polarization
- GAX
glucuronoarabinoxylan
- HG
homogalacturonan
- hsGAX
GAX with a high degree of arabinose substitution
- lsGAX
GAX with low substitution
- MLG
mixed-linkage glucan
- PRE
paramagnetic relaxation enhancement
- SSNMR
solid-state NMR
- TFA
trifloroacetic acid
- XyG
xyloglucan
Footnotes
Articles can be viewed without a subscription.
References
- Bardet M, Emsley L, Vincendon M (1997) Two-dimensional spin-exchange solid-state NMR studies of 13 C-enriched wood. Solid State Nucl Magn Reson 8: 25–32 [DOI] [PubMed] [Google Scholar]
- Bertini I, Luchinat C, Parigi G (2001) Solution NMR of Paramagnetic Molecules: Applications to Metallobiomolecules and Models. Elsevier, Amsterdam, The Netherlands [Google Scholar]
- Bielecki A, Kolbert AC, Levitt MH (1989) Frequency-switched pulse sequences - homonuclear decoupling and dilute spin NMR in solids. Chem Phys Lett 155: 341–346 [Google Scholar]
- Bloembergen N. (1957) Proton relaxation times in paramagnetic solutions. J Chem Phys 27: 572–573 [Google Scholar]
- Bromley JR, Busse-Wicher M, Tryfona T, Mortimer JC, Zhang Z, Brown DM, Dupree P (2013) GUX1 and GUX2 glucuronyltransferases decorate distinct domains of glucuronoxylan with different substitution patterns. Plant J 74: 423–434 [DOI] [PubMed] [Google Scholar]
- Buanafina MM. (2009) Feruloylation in grasses: current and future perspectives. Mol Plant 2: 861–872 [DOI] [PubMed] [Google Scholar]
- Buffy JJ, Hong T, Yamaguchi S, Waring AJ, Lehrer RI, Hong M (2003) Solid-state NMR investigation of the depth of insertion of protegrin-1 in lipid bilayers using paramagnetic Mn2+. Biophys J 85: 2363–2373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse-Wicher M, Gomes TC, Tryfona T, Nikolovski N, Stott K, Grantham NJ, Bolam DN, Skaf MS, Dupree P (2014) The pattern of xylan acetylation suggests xylan may interact with cellulose microfibrils as a twofold helical screw in the secondary plant cell wall of Arabidopsis thaliana. Plant J 79: 492–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita NC. (1983) Hemicellulosic polymers of cell walls of zea coleoptiles. Plant Physiol 72: 515–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita NC. (1996) Structure and biogenesis of the cell walls of grasses. Annu Rev Plant Physiol Plant Mol Biol 47: 445–476 [DOI] [PubMed] [Google Scholar]
- Carpita NC, Defernez M, Findlay K, Wells B, Shoue DA, Catchpole G, Wilson RH, McCann MC (2001) Cell wall architecture of the elongating maize coleoptile. Plant Physiol 127: 551–565 [PMC free article] [PubMed] [Google Scholar]
- Cho HT, Kende H (1997) Expansins in deepwater rice internodes. Plant Physiol 113: 1137–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ. (2000) Loosening of plant cell walls by expansins. Nature 407: 321–326 [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. (2011) Measuring in vitro extensibility of growing plant cell walls. Methods Mol Biol 715: 291–303 [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. (2015) Plant expansins: diversity and interactions with plant cell walls. Curr Opin Plant Biol 25: 162–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ. (2016) Catalysts of plant cell wall loosening. F1000 Res 5: F1000 Faculty Rev-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ, Bedinger P, Durachko DM (1997) Group I allergens of grass pollen as cell wall-loosening agents. Proc Natl Acad Sci USA 94: 6559–6564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darley CP, Li Y, Schaap P, McQueen-Mason SJ (2003) Expression of a family of expansin-like proteins during the development of Dictyostelium discoideum. FEBS Lett 546: 416–418 [DOI] [PubMed] [Google Scholar]
- Dubois M, Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28: 350–356 [Google Scholar]
- Dupree R, Simmons TJ, Mortimer JC, Patel D, Iuga D, Brown SP, Dupree P (2015) Probing the molecular architecture of Arabidopsis thaliana secondary cell walls using two- and three-dimensional (13)C solid state nuclear magnetic resonance spectroscopy. Biochemistry 54: 2335–2345 [DOI] [PubMed] [Google Scholar]
- Ebright YW, Chen Y, Pendergrast PS, Ebright RH (1992) Incorporation of an EDTA-metal complex at a rationally selected site within a protein: application to EDTA-iron DNA affinity cleaving with catabolite gene activator protein (CAP) and Cro. Biochemistry 31: 10664–10670 [DOI] [PubMed] [Google Scholar]
- Ermácora MR, Delfino JM, Cuenoud B, Schepartz A, Fox RO (1992) Conformation-dependent cleavage of staphylococcal nuclease with a disulfide-linked iron chelate. Proc Natl Acad Sci USA 89: 6383–6387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgelis N, Nikolaidis N, Cosgrove DJ (2014) Biochemical analysis of expansin-like proteins from microbes. Carbohydr Polym 100: 17–23 [DOI] [PubMed] [Google Scholar]
- Georgelis N, Nikolaidis N, Cosgrove DJ (2015) Bacterial expansins and related proteins from the world of microbes. Appl Microbiol Biotechnol 99: 3807–3823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgelis N, Tabuchi A, Nikolaidis N, Cosgrove DJ (2011) Structure-function analysis of the bacterial expansin EXLX1. J Biol Chem 286: 16814–16823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgelis N, Yennawar NH, Cosgrove DJ (2012) Structural basis for entropy-driven cellulose binding by a type-A cellulose-binding module (CBM) and bacterial expansin. Proc Natl Acad Sci USA 109: 14830–14835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibeaut DM, Pauly M, Bacic A, Fincher GB (2005) Changes in cell wall polysaccharides in developing barley (Hordeum vulgare) coleoptiles. Planta 221: 729–738 [DOI] [PubMed] [Google Scholar]
- Hollmann J, Elbegzaya N, Pawelzik E, Lindhauer MG (2009) Isolation and characterization of glucuronoarabinoxylans from wheat bran obtained by classical and ultrasound-assisted extraction methods. Qual Assur Saf Crop 1: 231–239 [Google Scholar]
- Hong M, Griffin RG (1998) Resonance assignments for solid peptides by dipolar-mediated 13C/15N correlation solid-state NMR. J Am Chem Soc 120: 7113–7114 [Google Scholar]
- Ishii S. (1984) Cell wall cementing materials of grass leaves. Plant Physiol 76: 959–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaroniec CP. (2015) Structural studies of proteins by paramagnetic solid-state NMR spectroscopy. J Magn Reson 253: 50–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerff F, Amoroso A, Herman R, Sauvage E, Petrella S, Filée P, Charlier P, Joris B, Tabuchi A, Nikolaidis N, et al. (2008) Crystal structure and activity of Bacillus subtilis YoaJ (EXLX1), a bacterial expansin that promotes root colonization. Proc Natl Acad Sci USA 105: 16876–16881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern T, Giffard M, Hediger S, Amoroso A, Giustini C, Bui NK, Joris B, Bougault C, Vollmer W, Simorre JP (2010) Dynamics characterization of fully hydrated bacterial cell walls by solid-state NMR: evidence for cooperative binding of metal ions. J Am Chem Soc 132: 10911–10919 [DOI] [PubMed] [Google Scholar]
- Köhnke T, Ostlund A, Brelid H (2011) Adsorption of arabinoxylan on cellulosic surfaces: influence of degree of substitution and substitution pattern on adsorption characteristics. Biomacromolecules 12: 2633–2641 [DOI] [PubMed] [Google Scholar]
- Kubicki JD, Mohamed MNA, Watts HD (2013) Quantum mechanical modeling of the structures, energetics and spectral properties of I alpha and I beta cellulose. Cellulose 20: 9–23 [Google Scholar]
- Kubicki JD, Watts HD, Zhao Z, Zhong LH (2014) Quantum mechanical calculations on cellulose-water interactions: structures, energetics, vibrational frequencies and NMR chemical shifts for surfaces of I alpha and I beta cellulose. Cellulose 21: 909–926 [Google Scholar]
- Li LC, Bedinger PA, Volk C, Jones AD, Cosgrove DJ (2003) Purification and characterization of four beta-expansins (Zea m 1 isoforms) from maize pollen. Plant Physiol 132: 2073–2085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Darley CP, Ongaro V, Fleming A, Schipper O, Baldauf SL, McQueen-Mason SJ (2002) Plant expansins are a complex multigene family with an ancient evolutionary origin. Plant Physiol 128: 854–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltsev S, Hudson SM, Sahu ID, Liu L, Lorigan GA (2014) Solid-state NMR (31)P paramagnetic relaxation enhancement membrane protein immersion depth measurements. J Phys Chem B 118: 4370–4377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marbella LE, Cho HS, Spence MM (2013) Observing the translocation of a mitochondria-penetrating peptide with solid-state NMR. Biochim Biophys Acta 1828: 1674–1682 [DOI] [PubMed] [Google Scholar]
- Massiot D, Fayon F, Capron M, King I, Le Calve S, Alonso B, Durand JO, Bujoli B, Gan ZH, Hoatson G (2002) Modelling one- and two-dimensional solid-state NMR spectra. Magn Reson Chem 40: 70–76 [Google Scholar]
- McQueen-Mason S, Durachko DM, Cosgrove DJ (1992) Two endogenous proteins that induce cell wall extension in plants. Plant Cell 4: 1425–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen-Mason SJ, Cosgrove DJ (1995) Expansin mode of action on cell walls. Analysis of wall hydrolysis, stress relaxation, and binding. Plant Physiol 107: 87–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier BH. (1994) Polarization transfer and spin diffusion in solid-state NMR. Adv Magn Opt Reson 18: 1–116 [Google Scholar]
- Munowitz MG, Griffin RG, Bodenhausen G, Huang TH (1981) Two-dimensional rotational spin-echo nuclear magnetic-resonance in solids: correlation of chemical-shift and dipolar interactions. J Am Chem Soc 103: 2529–2533 [Google Scholar]
- Nadaud PS, Helmus JJ, Kall SL, Jaroniec CP (2009) Paramagnetic ions enable tuning of nuclear relaxation rates and provide long-range structural restraints in solid-state NMR of proteins. J Am Chem Soc 131: 8108–8120 [DOI] [PubMed] [Google Scholar]
- Nikolaidis N, Doran N, Cosgrove DJ (2014) Plant expansins in bacteria and fungi: evolution by horizontal gene transfer and independent domain fusion. Mol Biol Evol 31: 376–386 [DOI] [PubMed] [Google Scholar]
- Rienstra CM, Tucker-Kellogg L, Jaroniec CP, Hohwy M, Reif B, McMahon MT, Tidor B, Lozano-Pérez T, Griffin RG (2002) De novo determination of peptide structure with solid-state magic-angle spinning NMR spectroscopy. Proc Natl Acad Sci USA 99: 10260–10265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampedro J, Cosgrove DJ (2005) The expansin superfamily. Genome Biol 6: 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampedro J, Guttman M, Li LC, Cosgrove DJ (2015) Evolutionary divergence of β-expansin structure and function in grasses parallels emergence of distinctive primary cell wall traits. Plant J 81: 108–120 [DOI] [PubMed] [Google Scholar]
- Selig MJ, Thygesen LG, Felby C, Master ER (2015) Debranching of soluble wheat arabinoxylan dramatically enhances recalcitrant binding to cellulose. Biotechnol Lett 37: 633–641 [DOI] [PubMed] [Google Scholar]
- Sengupta I, Gao M, Arachchige RJ, Nadaud PS, Cunningham TF, Saxena S, Schwieters CD, Jaroniec CP (2015) Protein structural studies by paramagnetic solid-state NMR spectroscopy aided by a compact cyclen-type Cu(II) binding tag. J Biomol NMR 61: 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta I, Nadaud PS, Helmus JJ, Schwieters CD, Jaroniec CP (2012) Protein fold determined by paramagnetic magic-angle spinning solid-state NMR spectroscopy. Nat Chem 4: 410–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon I. (1955) Relaxation processes in a system of two spins. Phys Rev 99: 559–565 [Google Scholar]
- Su Y, Hu F, Hong M (2012) Paramagnetic Cu(II) for probing membrane protein structure and function: inhibition mechanism of the influenza M2 proton channel. J Am Chem Soc 134: 8693–8702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi A, Li LC, Cosgrove DJ (2011) Matrix solubilization and cell wall weakening by β-expansin (group-1 allergen) from maize pollen. Plant J 68: 546–559 [DOI] [PubMed] [Google Scholar]
- Valdivia ER, Stephenson AG, Durachko DM, Cosgrove D (2009) Class B beta-expansins are needed for pollen separation and stigma penetration. Sex Plant Reprod 22: 141–152 [DOI] [PubMed] [Google Scholar]
- Valdivia ER, Wu Y, Li LC, Cosgrove DJ, Stephenson AG (2007) A group-1 grass pollen allergen influences the outcome of pollen competition in maize. PLoS One 2: e154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viëtor RJ, Newman RH, Ha MA, Apperley DC, Jarvis MC (2002) Conformational features of crystal-surface cellulose from higher plants. Plant J 30: 721–731 [DOI] [PubMed] [Google Scholar]
- Vignon MR, Gey C (1998) Isolation, 1H and 13C NMR studies of (4-O-methyl-D-glucurono)-D-xylans from luffa fruit fibres, jute bast fibres and mucilage of quince tree seeds. Carbohydr Res 307: 107–111 [Google Scholar]
- Vogel J. (2008) Unique aspects of the grass cell wall. Curr Opin Plant Biol 11: 301–307 [DOI] [PubMed] [Google Scholar]
- Wang T, Hong M (2016) Solid-state NMR investigations of cellulose structure and interactions with matrix polysaccharides in plant primary cell walls. J Exp Bot 67: 503–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Park YB, Caporini MA, Rosay M, Zhong L, Cosgrove DJ, Hong M (2013) Sensitivity-enhanced solid-state NMR detection of expansin’s target in plant cell walls. Proc Natl Acad Sci USA 110: 16444–16449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Salazar A, Zabotina OA, Hong M (2014) Structure and dynamics of Brachypodium primary cell wall polysaccharides from two-dimensional 13C solid-state nuclear magnetic resonance spectroscopy. Biochemistry 53: 2840–2854 [DOI] [PubMed] [Google Scholar]
- Wang T, Williams JK, Schmidt-Rohr K, Hong M (2015) Relaxation-compensated difference spin diffusion NMR for detecting 13C-13C long-range correlations in proteins and polysaccharides. J Biomol NMR 61: 97–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Yang H, Kubicki JD, Hong M (2016) Cellulose structural polymorphism in plant primary cell walls investigated by high-field 2D solid-state NMR spectroscopy and density functional theory calculations. Biomacromolecules 17: 2210–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Zabotina O, Hong M (2012) Pectin-cellulose interactions in the Arabidopsis primary cell wall from two-dimensional magic-angle-spinning solid-state nuclear magnetic resonance. Biochemistry 51: 9846–9856 [DOI] [PubMed] [Google Scholar]
- Watts HD, Mohamed MNA, Kubicki JD (2013) A DFT study of vibrational frequencies and 13C NMR chemical shifts of model cellulosic fragments as a function of size. Cellulose 21: 53–70 [Google Scholar]
- White PB, Wang T, Park YB, Cosgrove DJ, Hong M (2014) Water-polysaccharide interactions in the primary cell wall of Arabidopsis thaliana from polarization transfer solid-state NMR. J Am Chem Soc 136: 10399–10409 [DOI] [PubMed] [Google Scholar]
- Yennawar NH, Li LC, Dudzinski DM, Tabuchi A, Cosgrove DJ (2006) Crystal structure and activities of EXPB1 (Zea m 1), a beta-expansin and group-1 pollen allergen from maize. Proc Natl Acad Sci USA 103: 14664–14671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S, Wu Y, Cosgrove DJ (2001) A fungal endoglucanase with plant cell wall extension activity. Plant Physiol 127: 324–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Crespi VH, Kubicki JD, Cosgrove DJ, Zhong LH (2014) Molecular dynamics simulation study of xyloglucan adsorption on cellulose surfaces: effects of surface hydrophobicity and side-chain variation. Cellulose 21: 1025–1039 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.