Unlike animals, plants produce their own B vitamins but may nonetheless suffer vitamin deficiencies under stress conditions, with wide-ranging metabolic consequences.
Abstract
B vitamins are the precursors of essential metabolic cofactors but are prone to destruction under stress conditions. It is therefore a priori reasonable that stressed plants suffer B vitamin deficiencies and that certain stress symptoms are metabolic knock-on effects of these deficiencies. Given the logic of these arguments, and the existence of data to support them, it is a shock to realize that the roles of B vitamins in plant abiotic stress have had minimal attention in the literature (100-fold less than hormones) and continue to be overlooked. In this article, we therefore aim to explain the connections among B vitamins, enzyme cofactors, and stress conditions in plants. We first outline the chemistry and biochemistry of B vitamins and explore the concept of vitamin deficiency with the help of information from mammals. We then summarize classical and recent evidence for stress-induced vitamin deficiencies and for plant responses that counter these deficiencies. Lastly, we consider potential implications for agriculture.
The great pioneers of modern plant physiology pointed out that B vitamins have much in common with hormones and even classified some vitamins as plant hormones (i.e. plant growth regulators in today’s terms; Went et al., 1938; Bonner and Bonner, 1948; Thimann, 1963). In plants, as in animals, B vitamins and hormones are biologically active in minute amounts, are transported, and lead to similarly profound consequences when deficient (Bonner and Bonner, 1948). B vitamins and hormones might consequently be expected to have received comparable research attention. This has broadly been the case in biomedical research since vitamins and hormones were first discovered (Kohler, 1975). It has almost never been the case in any area of plant research, including abiotic stress. Thus, in the Arabidopsis (Arabidopsis thaliana) abiotic stress literature, articles involving hormones outnumber those involving B vitamins by a factor of 100 (Supplemental Table S1).
This stunning disparity suggests two things about past and present thinking in the abiotic stress field. First, it indicates a prevalent default assumption that the whole of B vitamin metabolism always and everywhere continues to work well in stressed plants and so can be safely ignored. Second, it signals a lack of attention to the possibility that stress-induced defects in B vitamin metabolism can be intermediate causes of system-wide plant stress responses. Neither the default assumption nor the inattention is reasonable a priori and they are not justified by the available evidence.

This review sets out to show that B vitamin deficiency is a simple, probable, but overlooked scenario in abiotic stress responses. We begin by providing key background information on the chemical and metabolic lability of B vitamins and the cofactors derived from them and on the concept of vitamin deficiency derived from work in animals. We then revisit classical studies that point clearly to roles for B vitamins in abiotic stress, but are no longer in the modern canon, and assess recent evidence for such roles. Next, we outline how plants combat stress-induced B vitamin deficiencies. Lastly, we consider how understanding stress-induced vitamin deficiency could inform crop breeding and management.
Two points should be made at the outset. First, there has been much research on the B vitamin contents of food plants and on B vitamin synthesis and metabolism in plants. However, the main, if not sole, driver for this valuable work has been plants as vitamin sources for humans rather than plants as vitamin sources for themselves (Fitzpatrick et al., 2012; Gerdes et al., 2012). This article takes the complementary position that “plants need their vitamins too” (Smith et al., 2007). Second, research on vitamin C (ascorbate) shows that plant stress biology does not invariably sideline vitamins (Foyer and Noctor, 2011). This article argues that B vitamins deserve as much attention as vitamin C.
CHEMICAL AND METABOLIC LABILITY: WHY BAD THINGS HAPPEN TO GOOD VITAMINS
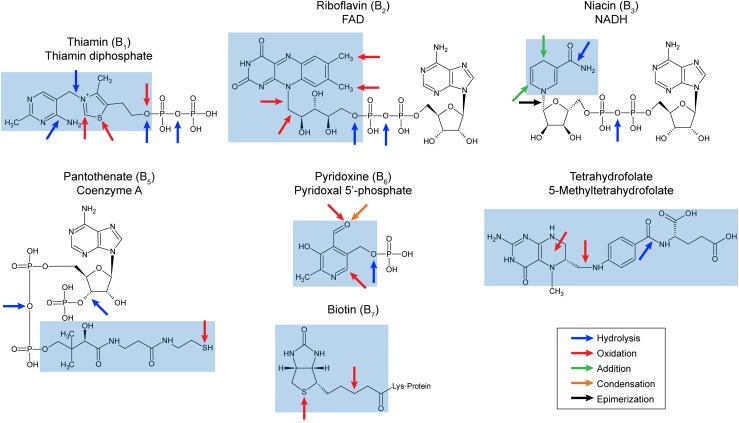
B vitamins are indispensable because they are the metabolic precursors of essential cofactors. Figure 1 shows the seven B vitamins found in plants, along with the cofactors to which they are converted. In brief: Thiamin (vitamin B1) is converted to thiamin diphosphate; riboflavin (vitamin B2) to FMN and FAD; niacin (vitamin B3) to NAD(P)+; pantothenate (vitamin B5) to coenzyme A and the prosthetic group of acyl carrier protein; pyridoxine (vitamin B6) to pyridoxal 5′-phosphate; biotin (vitamin B8) to the biotinyl side chain of enzymes; and tetrahydrofolate (historically termed vitamin B9) to various one-carbon substituted folates and their polyglutamylated derivatives.
Figure 1.
Structures and damage reactions of the seven B vitamins found in plants and of representative cofactors derived from them. The vitamin moieties are highlighted in blue. Note that folates generally have a short γ-linked poly-Glu chain attached to the glutamyl moiety. Color-coded arrows show the site and nature of spontaneous chemical or enzymatic damage reactions that each vitamin/cofactor can undergo in vivo. The damage reactions involved are documented in Table I.
Cofactors function by participating in biochemical reactions, e.g. thiamin diphosphate forms covalent complexes with carbonyl substrates, FAD and NAD(P)+ become reduced as substrates are oxidized, and coenzyme A forms thioesters with acyl groups. Cofactors and their vitamin precursors are thus chemically reactive by nature. Their chemically reactive groups are therefore prone to undergo spontaneous side-reactions such as oxidation, hydrolysis, racemization, or addition that damage or destroy the molecule, and stress conditions promote such side-reactions (Piedrafita et al., 2015). For example, most abiotic stresses cause accumulation of reactive oxygen species (You and Chan, 2015) that can inflict oxidative damage on every compound in Figure 1. Similarly, temperature extremes, high light levels, pH excursions, and stress-driven accumulations of metabolites with which cofactors react all directly accelerate diverse types of spontaneous cofactor damage (Treadwell and Metzler, 1972; Baggott, 2000; Mills et al., 2006; Marbaix et al., 2011). Abiotic stresses can also promote cofactor damage indirectly by altering compartmentation (Akhtar et al., 2010; Mohammadi et al., 2012) by inducing enzymes that break down cofactors (Rapala-Kozik et al., 2008; Higa et al., 2012) and by creating harsh cellular conditions in which enzymes that normally act on other substrates become more promiscuous (Piedrafita et al., 2015) and mistakenly attack cofactors.
Figure 1 uses color-coded arrows to show the site and nature of damage reactions that vitamins and cofactors can undergo in physiological conditions, and Table I catalogs the reactions corresponding to the arrows. Note that Figure 1 is bristling with arrows, which helps explain why most of the B vitamins/cofactors made it into a “Top 30” list of damage-prone metabolites (Lerma-Ortiz et al., 2016) and why all organisms have dedicated systems to deal with vitamin/cofactor damage (Linster et al., 2013). “Bad things happen” to B vitamins and cofactors because these compounds are basically chemical and metabolic “accidents waiting to happen.” Also, unlike many other reactive metabolites, cofactors are end-products, not short-lived intermediates that are quickly converted to something else. The longer an end-product lives, the higher the probability of its getting damaged at some point.
Table I. Chemical and enzymatic damage reactions of B vitamins and the corresponding cofactors.
| Vitamin/Cofactor | Reactions | C/Ea | Referencesb |
|---|---|---|---|
| Thiamin (vitamin B1)/thiamin diphosphate | Pyrimidine ring deamination | C | Windheuser and Higuchi (1962) |
| Thiazole ring oxidation | C,E | Bunik et al. (2007); Dwivedi and Arnold (1973) | |
| Thiazole ring breakdown | C | Jenkins et al. (2007) | |
| Oxidation to thiamin acetate | E | Dalvi et al. (1974) | |
| Hydrolysis to thiazole + pyrimidine | E | Jurgenson et al. (2009) | |
| Dephosphorylation | E | Rapala-Kozik et al. (2009) | |
| Thiamin triphosphate formation | E | Linster et al. (2013) | |
| Riboflavin (vitamin B2)/FMN, FAD | (Photo)oxidative flavin ring loss | C | Choe et al. (2005) |
| (Di)phosphate bond hydrolysis | E | Ogawa et al. (2008); Rawat et al. (2011) | |
| 7α- and 8α-hydroxylation | E | Ohkawa et al. (1983) | |
| Cyclic FMN formation from FAD | C,E | Pinto et al. (1999); Sánchez-Moreno et al. (2009) | |
| Niacin (vitamin B3)/NAD(P)(H) | Hydration of nicotinamide ring | C,E | Marbaix et al., (2011) |
| Epimerization of β- to α-NAD(P)H | C | Oppenheimer and Kaplan (1975) | |
| Hydrolytic loss of nicotinamide ring | E | Everse et al. (1975) | |
| Diphosphate bond hydrolysis | E | Ogawa et al. (2008) | |
| Nicotinamide ring addition reactions | C | Everse et al. (1971) | |
| Pantothenate (vitamin B5)/coenzyme A, ACPc | Oxidations of the thiol group | C | Huang et al. (2016) |
| Diphosphate bond hydrolysis | E | Ogawa et al. (2008) | |
| Hydrolysis of the 3′ phosphate group | E | Paizs et al. (2008) | |
| Pyridoxine (vitamin B6)/pyridoxal 5′-phosphate | Aldehyde group oxidation | C/E | Gerdes et al. (2012) |
| Aldehyde group condensations | C | Dalling et al. (1976) | |
| Dephosphorylation | E | Gerdes et al. (2012) | |
| 6-Hydroxylation | C | Tadera et al. (1986) | |
| Biotin (vitamin B8)/biotinylated enzymes | Oxidation to biotin sulfoxide | C | Melville (1954) |
| Side chain β-oxidation | E | Izumi et al. (1973) | |
| Tetrahydrofolate/C1-substituted folates | Oxidative cleavage of C9-N10 bond | C | Gregory (1989) |
| Pteridine ring oxidation | C/E | Noiriel et al. (2007) | |
| 5-Formyltetrahydrofolate formation | C/E | Baggott (2000); Goyer et al. (2005) | |
| γ-Glutamyl bond hydrolysis | E | Orsomando et al. (2005); Bozzo et al. (2008) |
C, chemical (i.e. nonenzymatic, spontaneous) reaction; E, enzymatic reaction or side-reaction.
Certain reactions have so far been reported only from animals or microbes.
ACP, acyl carrier protein, which has a bound 4'-phosphopantetheinyl prosthetic group derived from coenzyme A.
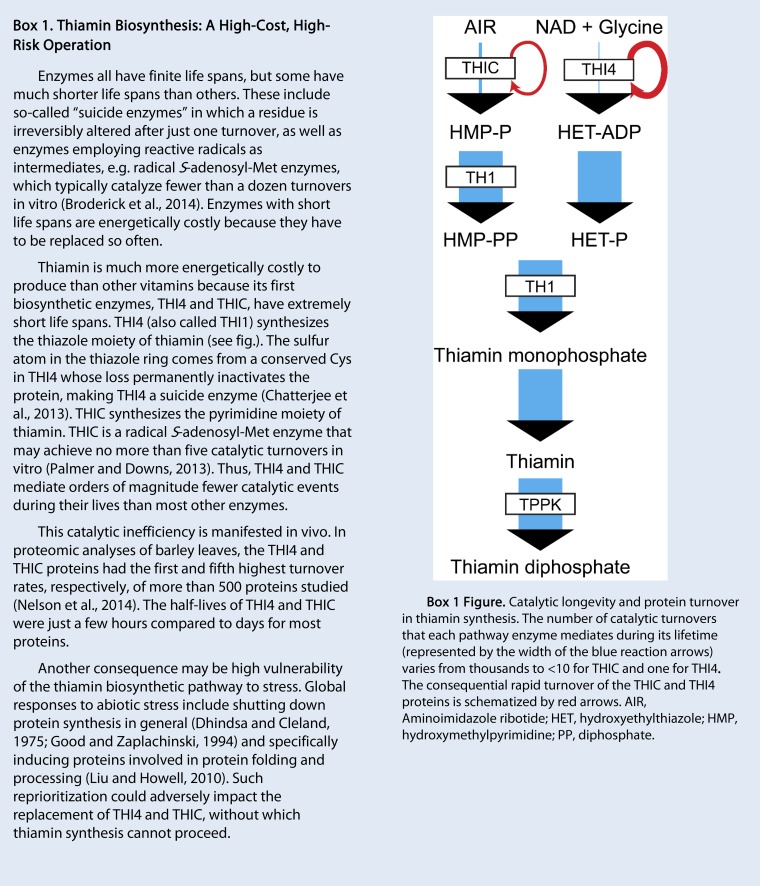
Thiamin diphosphate deserves special mention for metabolic lability because, unlike other cofactors, it can be damaged during the catalytic cycle (catalysis-induced inactivation; McCourt et al., 2006). Oxygen-dependent side-reactions in enzyme active sites convert the thiamin diphosphate cofactor to an inactive thiazolone derivative (Sümegi and Alkonyi, 1983; Bunik et al., 2007). Such use-dependent damage explains why thiamin is the only B vitamin whose dietary requirement in animals is proportional to nonfat energy intake (McCourt et al., 2006). More generally, the damage reactions undergone by all B vitamins account for their dietary requirements; if vitamins were not continuously damaged, they would last forever and not need to be constantly resupplied. In this context, it is noteworthy that the whole-body half-life of thiamin (and its phosphates) in humans is one to two orders of magnitude shorter than those of other B vitamins for which estimates are available, as follows: thiamin, 9.5 to 18.5 d (Ariaey-Nejad et al., 1970); folates, 60 to 150 d (Gregory and Quinlivan, 2002); vitamin B6, ∼500 d (Coburn, 1990); vitamin B12 (absent from plants), 480 to 1284 d (Hall, 1964).
WHAT ACTUALLY CONSTITUTES B VITAMIN DEFICIENCY?
The concept of vitamin deficiency comes from human and animal nutrition; it refers to the consequences of a shortfall in the dietary supply of a specific vitamin. Because plants make their own B vitamins, and indeed are the ultimate source of most of the B vitamins consumed by animals, the concept of “B vitamin deficiency” might seem inapplicable to plants, and hence irrelevant. But, as we will show, this concept is actually very relevant to plants.
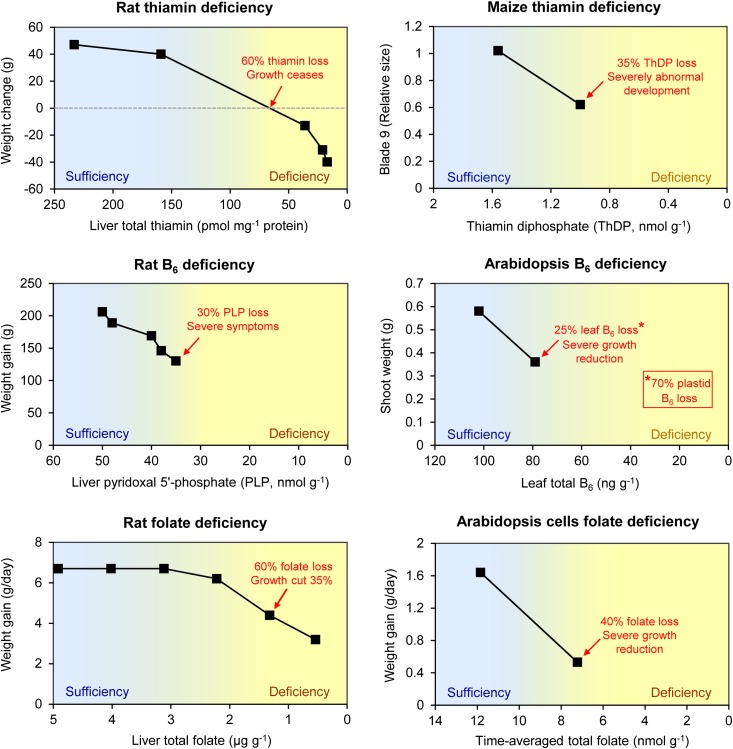
Key questions about B vitamin nutrition in animals are: What dietary intake of each vitamin is needed for optimal growth and health? Also, what happens when the supply of a vitamin falls below the optimal level? Both questions are often answered by measuring weight gain in young animals given various vitamin doses in the diet and by tracking levels of the vitamin and its corresponding cofactor in tissues and organs. Figure 2 (left half) summarizes data from such studies, in which various levels of thiamin, pyridoxine, or folate were supplied to rats whose growth and liver cofactor levels were monitored. Two points that emerge from these data are as follows: (1) There is a continuum rather than a sharp divide between vitamin sufficiency and deficiency, and (2) the cofactor level in liver does not have to fall much before growth is substantially impacted (as are development, metabolic functions, and behavior; data not shown), and declines of 30 to 60% from optimal are devastating. Animals therefore operate their vitamin and cofactor systems with rather narrow margins of safety.
Figure 2.
Comparing growth responses to deficiencies of thiamin, B6, or folate in rats versus plants. The decline from vitamin sufficiency to deficiency is approximated by a color gradient. For rats, deficiencies were obtained by varying dietary vitamin content; liver was used to assess vitamin status. For plants, the experiments involved vitamin deficient mutants (blk-1 for thiamin in maize; pdx1.3 for B6 in Arabidopsis) or cell cultures treated with an antifolate drug for folate in Arabidopsis. Whole plants, leaves, or cells were used to assess vitamin status. Data sources: rat thiamin (Rains et al., 1997), rat B6 (Mackey et al., 2003; Scheer et al., 2005), rat folate (Clifford et al., 1993), maize thiamin (Woodward et al., 2010), Arabidopsis B6 (Rueschhoff et al., 2013), and Arabidopsis folate (Loizeau et al., 2008).
The same appears to be true of plants. Nutritional trials analogous to those above have not yet been conducted using totally vitamin-deficient mutants. However, mutant plants partially deficient in thiamin or vitamin B6 have been studied (Woodward et al., 2010; Rueschhoff et al., 2013), as have cultured cells depleted in folate using a reasonably specific antifolate drug (Loizeau et al., 2008; Fig. 2, right half). Although these experiments produced a single deficient state, not a range as in rats, the data clearly suggest that a 25 to 40% loss of cofactor leads to severe consequences. To summarize: The red zone on the B vitamin fuel gauge seems to be in roughly the same place in plants and animals and is quite close to the full mark.
A subsidiary concept within vitamin deficiency is “functional vitamin deficiency,” wherein vitamin and cofactor measurements need not show marked depletion but strong metabolic disturbances nevertheless ensue. A prime example is vitamin B12 (absent from plants), whose deficiency in humans is better diagnosed by its metabolic consequences (elevated levels of methylmalonic acid and homo-Cys) than by measuring B12 itself (Stabler et al., 1996). Another example is folate, for which, in contrast to B12, deficiency causes elevation only of homo-Cys and not of methylmalonic acid (Green, 2008). Functional deficiencies of other B vitamins in humans generally affect enzymes having the weakest affinity for their cofactor relative to the cofactor’s intracellular concentration, as is the case for vitamin B6 (Ueland et al., 2015). However, the hierarchy of biochemical reactions most susceptible to deficiency of B vitamins has not been fully clarified.
Functional vitamin deficiencies also can arise when the deficiency occurs in a particular (inaccessible) tissue or compartment but does not affect the whole organism, or at least the part convenient for assessing vitamin or cofactor status. Such a situation could develop in roots for thiamin and niacin because roots of certain species import these vitamins from shoots (Bonner and Bonner, 1948). Intracellular deficiencies are also possible given that B vitamins and cofactors are synthesized and used in different compartments. For example, thiamin diphosphate is made in the cytosol but used mainly in plastids and mitochondria (Rapala-Kozik et al., 2012), and vitamin B6 is made in the cytosol but used throughout the cell (Fitzpatrick, 2011). In this connection, note that the plastids of the vitamin B6 deficient Arabidopsis mutant in Figure 2 were far more severely deficient in B6 than the leaf as a whole (Rueschhoff et al., 2013) and that a thiamin-requiring tobacco (Nicotiana sylvestris) mutant appeared to suffer primarily from thiamin deficiency in chloroplasts (McHale et al., 1988).
EVIDENCE FOR STRESS-INDUCED B VITAMIN DEFICIENCY
The previous sections explained that B vitamins and cofactors are chemically and metabolically unstable, that stresses potentially make them even more unstable, and that modest falls in cofactor levels slow growth. We might therefore predict that (1) abiotic stresses cause vitamin and cofactor deficiencies, (2) the deficiencies degrade plant performance, and (3) supplementing stressed plants with the deficient vitamin(s) improves performance. Evidence from both classical and modern work indicates that all these things happen.
Classical Evidence
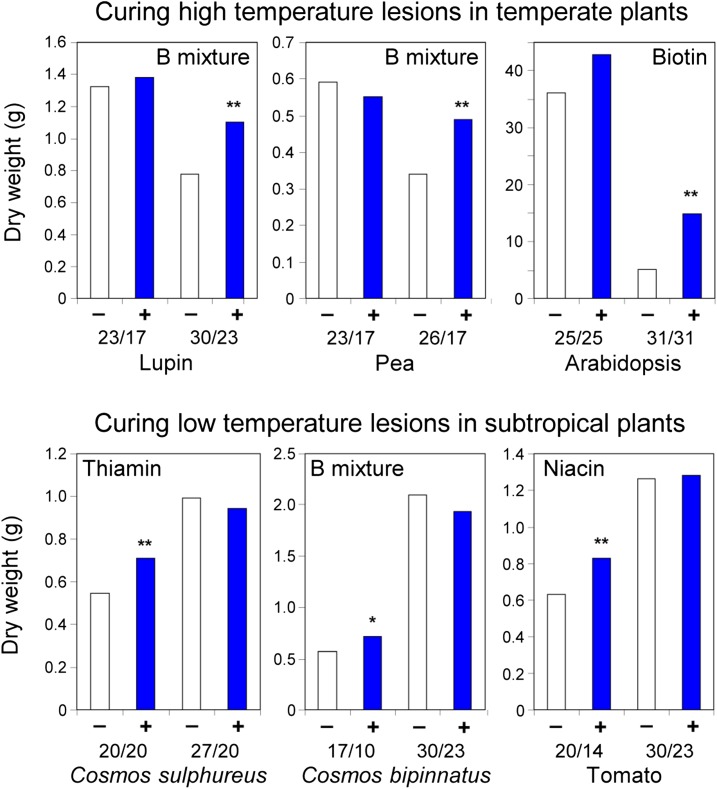
The classical evidence was considered in a short 1957 article with the evocative title “The Chemical Cure of Climatic Lesions” (Bonner, 1957). The idea captured in the title was that negative effects of “climatic lesions” (i.e. physicochemical environmental stresses) could be “cured” by applying vitamins or other essential metabolites, singly or in mixtures. “Chemical cures” by B vitamins were reported for various plants and various abiotic stresses during the 1940s to 1960s; typical data are shown in Figure 3. In each case, applying a vitamin or vitamin mixture promoted growth in unfavorable conditions but not in favorable ones. These results are de facto confirmation that stress can lead to functional B vitamin deficiency. Importantly, the Arabidopsis work in Figure 3 gave stress-induced vitamin deficiency a genetic basis by defining ecotypic differences attributable to one or a few genes (Langridge and Griffing, 1959). This is the pattern expected if the enzymes that use a particular cofactor bind to that cofactor with different strengths. When the vitamin starts to run out and cofactor levels fall, the weakest binder loses activity first, and small allelic differences in the affinity of this enzyme for the cofactor become critical determinants of performance of the organism as a whole (Guenther et al., 1999).
Figure 3.
Classical data on the chemical cure of high- or low-temperature growth lesions. Day/night temperatures are in °C. +, Vitamin(s) added; –, no vitamin; differences significant at *P < 0.05 and **P < 0.01. The data are for species of the ornamental plant Cosmos (Bonner, 1943), for Arabidopsis (Langridge and Griffing, 1959), and for other species (Ketellapper, 1963).
Modern Evidence
Although the exogenous application approach (“spray and pray”) has fallen out of fashion, it can be perfectly valid, and beneficial effects of applying B vitamins, particularly thiamin, to stressed crop species continue to be reported, e.g. for salinized wheat (Triticum aestivum), sunflower (Helianthus annuus), and maize (Zea mays; Al-Hakimi and Hamada, 2001; Sayed and Gadallah, 2002; Tuna et al., 2013; Kaya et al., 2015). Also, a rigorous Arabidopsis study showed that thiamin enhances tolerance to oxidative stress imposed by paraquat (Tunc-Ozdemir et al., 2009). Such protective effects of thiamin on stressed plants are generally interpreted in terms of the antioxidant properties of thiamin and its diphosphate (Lukienko et al., 2000), but the in vivo relevance of these properties has been questioned (Lesgards et al., 2005), and it is not clear that the observed protection is due to direct antioxidant effects (Asensi-Fabado and Munné-Bosch, 2010). The experimental data are equally compatible with the protection being due to relief of a functional deficiency of thiamin diphosphate and the consequent restoration of normal metabolic fluxes. Although bias from prior positive reports may be at work, it is interesting that thiamin features so prominently in the literature on vitamin application, given that thiamin and thiamin diphosphate are particularly labile in vivo (see above). It is also interesting in the light of the extremely high energetic cost and inefficiency of thiamin biosynthesis (Box 1), to which we will return later.
Further modern support for stress-induced deficiency of four different B vitamins has come from molecular genetics, as follows. (1) Pyridoxine: The Arabidopsis SOS4 (SALT OVERLY SENSITIVE4) gene for pyridoxal kinase was cloned via the salt-sensitive phenotype of sos4 mutants, which was reverted by pyridoxine (Shi et al., 2002), a textbook “cure” of a stress lesion. Arabidopsis mutants in the PDX1.3 gene for pyridoxal 5′-phosphate synthase were found to be hypersensitive to salt, osmotic, and oxidative stress (Chen and Xiong, 2005; Titiz et al., 2006). (2) Pantothenate/coenzyme A: The Arabidopsis HAL3A (HALOTOLERANCE DETERMINANT 3A) gene, first identified for its relation to stress tolerance (Espinosa-Ruiz et al., 1999), was shown to code for a key enzyme in pantothenate conversion to coenzyme A (Kupke et al., 2001), and HAL3A overexpression increased salt tolerance (Espinosa-Ruiz et al., 1999; Yonamine et al., 2004). (3) Folate: Ablation of the Arabidopsis gene encoding a cytosolic folate synthesis enzyme (HPPK/DHPS) specifically impacted salt and oxidative stress resistance at germination (Storozhenko et al., 2007; Navarrete et al., 2012). (4) Riboflavin: The Arabidopsis phs1 (PHOTOSENSITIVE1) mutant, first identified by its sensitivity to high-light stress (Ouyang et al., 2010), was found to lack a functional domain of the riboflavin biosynthesis enzyme PyrR (Hasnain et al., 2013; Frelin et al., 2015). Note the pattern here: Either a gene identified via a stress-sensitive phenotype turned out to be a B vitamin or cofactor synthesis enzyme or ablating a known B vitamin/cofactor enzyme caused stress sensitivity.
COUNTERING STRESS-INDUCED B VITAMIN DEFICIENCY
Conceptually, resource deficiencies can be countered by (1) maintaining and then mobilizing reserves, (2) obtaining more of the resource, (3) repairing or recycling it, or (4) using less of it. Plants certainly deploy strategies 2 and 3 to confront B vitamin deficiency and potentially deploy the others.
Strategy 1: Storing and Mobilizing B Vitamins and Precursors
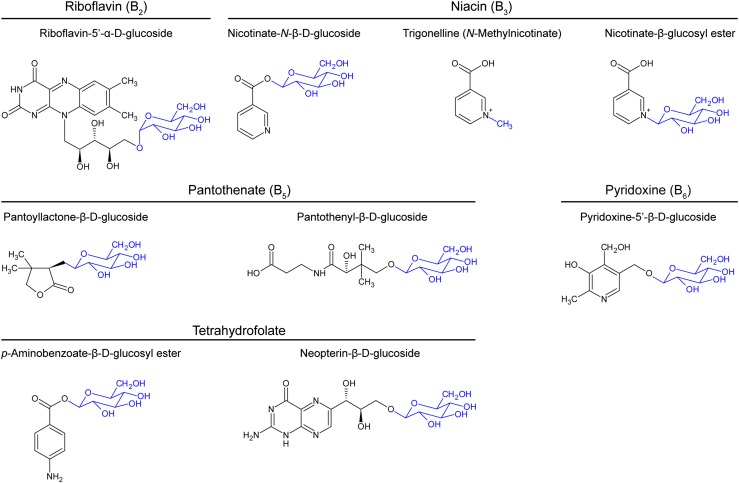
Storage forms of B vitamins have had little attention in the plant literature, in sharp contrast to storage forms of hormones (Ludwig-Müller, 2011; Piotrowska and Bajguz, 2011). Storage forms of vitamins and vitamin precursors, mainly glucosides and Glc esters, nevertheless exist and some can reach higher concentrations than the corresponding free forms (Gregory, 1998). The only B vitamin for which no storage forms have been reported is thiamin. Storage forms have not been analyzed in abiotic stress studies, perhaps because they are not commercially available as standards. Unfortunately, this means that the contribution of storage forms to B vitamin homeostasis during stress remains unresolved; however, this contribution could be large. The main storage forms are shown in Figure 4 and described below, starting with the two vitamins (B6 and B3) whose storage forms are reported to be more abundant than the free forms in certain species and tissues.
Figure 4.
Structures of conjugated forms of B vitamins and their precursors that occur in plants. The molecules to which the vitamins and precursors are conjugated are colored blue.
Pyridoxine (B6)
The 5′-β-d-glucoside of pyridoxine, and other glycosides linked via the 4- or 5-hydroxymethyl groups, can contribute up to 75% of the total vitamin B6 in plant tissues (Gregory, 1998), i.e. they can be the largest item in the B6 budget. A glucosyltransferase responsible for pyridoxine-5′-β-d-glucoside synthesis has been detected in pea (Pisum sativum; Tadera et al., 1982); enzymatic hydrolysis of the glucoside has been shown in vitro (Yasumoto et al., 1976), so it can presumably be mobilized in vivo.
Niacin (B3)
Nicotinic acid-N-β-d-glucoside and N-methylnicotinate (trigonelline) are widespread in plants and can be major metabolites of nicotinamide and nicotinate (Matsui et al., 2007; Ashihara et al., 2010); more complex glycosides also occur (Gregory, 1998). Enzymes that would allow the N-glucoside and trigonelline to act as mobilizable storage forms have been characterized (Upmeier et al., 1988; Shimizu and Mazzafera, 2000; Mizuno et al., 2014). Brassicaceae contain the β-d-glucosyl ester of nicotinic acid, which almost certainly acts as a mobilizable storage form (Li et al., 2015).
Pantothenate (B5)
Pantothenate 4'-O-β-d-glucoside has been isolated from tomato fruit (Solanum lycopersicum; Amachi et al., 1971) and is most likely present in a wide range of species and tissues (Yoshizumi and Amachi, 1969). Glycosides of the lactone form of pantoate, the immediate precursor of pantothenate (pantoyllactone-β-d-glucopyranoside and pantoyllactone primeveroside) occur in rice (Oryza sativa) seedlings, the levels in coleoptile tissue being in the millimolar range (Menegus et al., 2002). These pantoate derivatives seem not to have been sought in any plant besides rice; they could conceivably be widespread.
Tetrahydrofolate Precursors
The precursor p-aminobenzoate was converted to its β-d-glucosyl ester by all tissues and species tested and was found to be the major endogenous form of p-aminobenzoate (Quinlivan et al., 2003). The esterification reaction is reversible and the ester is stored in vacuoles (Eudes et al., 2008). Glycosides (probably β-d-glucosides) of the tetrahydrofolate precursors neopterin and monapterin were found in tomato fruit engineered to overproduce pterins (Díaz de la Garza et al., 2004); it is not known whether such glycosides occur naturally or whether they can be mobilized.
Riboflavin (B2)
The alpha and beta forms of riboflavin-5′-d-glucoside have been found in germinating barley (Hordeum vulgare) seedlings supplied with riboflavin (Suzuki and Uchida, 1983). As with the pterin glycosides above, it is it is not known whether riboflavin glucosides occur naturally or whether they can be mobilized.
Biotin (B7)
While no small-molecule conjugates of biotin have been reported, a biotinyl protein that represents >90% of the total protein-bound biotin has been characterized and cloned from pea seeds (Dehaye et al., 1997) and is conserved among higher plants (Gerdes et al., 2012).
Strategy 2: Increasing B Vitamin Supply
Many analyses of gene and protein expression have indicated that abiotic stresses up-regulate flux through some but not all B vitamin biosynthesis pathways and studies of the effects of stress on the levels of vitamins and cofactors tend to support this inference. As is usual in stress research, differences in experimental design (the species and tissue used, the timing, duration, and severity of stress, and the analysis methods applied, i.e. blots/microarrays/RNA-seq, etc.) preclude meta-analysis of all the data. Below, we therefore focus first on a high-throughput data set, the AtGenExpress global stress expression data set (available at http://jsp.weigelworld.org/expviz/expviz.jsp), supplemented with broadly comparable data from several independent Arabidopsis stress studies (Supplemental Table S2). We then highlight illustrative articles on pyridoxine and thiamin.
Note that even when a vitamin biosynthetic pathway is clearly upregulated in response to stress, stress-induced vitamin or cofactor deficiency may still occur for several reasons. First, activating biosynthetic gene expression may fail to increase vitamin production due to downstream constraints, e.g. the energy cost of making THI4 protein for thiamin biosynthesis (see below and Box 1). Second, as noted above, a high vitamin or cofactor status, as measured in whole organs, tissues, or cells, can obscure functional deficiencies within subcompartments. Lastly, conversion of vitamins to cofactors may become limiting, so that increases in vitamin levels do not always result in higher cofactor levels. Cofactor levels can consequently fall even when free vitamin levels rise, and cofactor levels, not vitamin levels, determine metabolic outcomes.
Arabidopsis Gene Expression
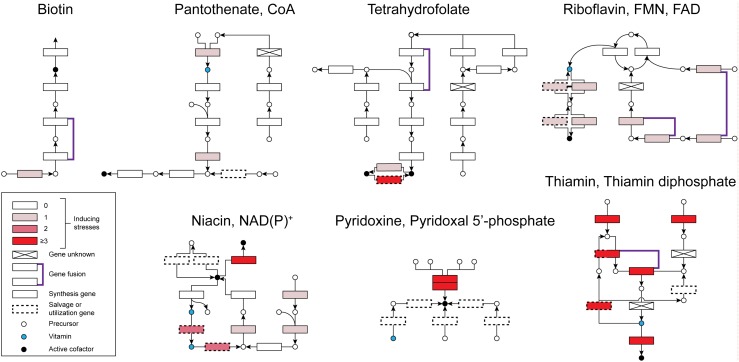
The stresses used for the AtGenExpress global stress expression data set (heat, cold, drought, salt, high osmolarity, UV-B light, and wounding) were essentially single-regime shock treatments, lasting 24 h or less, given to Arabidopsis seedlings (Kilian et al., 2007); the protocols in the other studies listed in Supplemental Table S2 were quite similar. The transcriptional responses observed in these experiments therefore necessarily only included part of the plant’s full repertoire. These responses nonetheless follow the coherent pattern seen in Figure 5, a bird’s-eye schematic of B vitamin synthesis and salvage pathways in which enzymes are represented by rectangles. The color density of each enzyme is proportional to the number of different stresses that induce the corresponding gene by 2-fold or more. The pathways are arranged in order of prevalence of gene induction. At one extreme (biotin, pantothenate/CoA, and tetrahydrofolate pathways), just one or two biosynthetic genes are induced by a single stress, whereas at the other extreme (vitamin B6 and thiamin pathways), all the known biosynthetic genes are induced by at least three stresses. Except for B6, the prevalence of induction of salvage genes basically tracks that of biosynthetic genes. This overview of gene-level data thus indicates that certain B vitamin biosynthesis pathways, particularly those for B6 and thiamin, are upregulated by several abiotic stresses and that others, such as the tetrahydrofolate pathway, are largely not. Vitamin/cofactor analysis data and protein-level data for vitamin B6 and thiamin are consistent with this picture, as discussed next.
Figure 5.
Stress induction of B vitamin and cofactor synthesis and salvage genes in Arabidopsis. Synthesis and salvage pathways for each vitamin are schematized as by Gerdes et al. (2012) except for the omission of compartmentation and the addition of two thiamin salvage reactions (Yazdani et al., 2013; Zallot et al., 2014). Circles are metabolites, arrows are reactions, and rectangles are enzymes. The full pathway schemes, which include enzyme and metabolite names, are available at http://pubseed.theseed.org/seedviewer.cgi?page=PlantGateway. Enzymes whose genes are induced at least 2-fold by 0, 1, 2, or ≥3 different abiotic stresses (in shoots or roots) are color-coded white and three shades of red, respectively. Gene expression data were taken from AtGenExpress and literature summarized in Supplemental Table S2.
Vitamin B6
Pyridoxal 5′-phosphate is made by the pyridoxal synthase complex, which consists of two proteins, PDX1 and PDX2 (the two red-colored boxes in the pyridoxal 5′-phosphate section of Fig. 5). Arabidopsis has three PDX1 homologs, of which PDX1.1 and PDX1.3 are active enzymes and PDX1.2 is a positive regulator (Titiz et al., 2006; Moccand et al., 2014). Paralleling the stress induction of Arabidopsis PDX genes (Fig. 5; Supplemental Table S2), PDX1.3 protein accumulated and the total B6 level increased by 60% in response to UV-B exposure (Ristilä et al., 2011); total B6 also increased by 60% in response to heat stress (Moccand et al., 2014). Levels of pyridoxal 5′-phosphate and free pyridoxal or pyridoxine likewise increased by about 2-fold in tobacco exposed to chilling, UV radiation, osmotic stress, oxidative stress, or drought (Huang et al., 2013).
Thiamin
Thiamin is synthesized by a pathway that is very energetically expensive because the thiazole moiety is made by a suicide enzyme (THI4), and the pyrimidine moiety is made by a radical SAM (S-adenosyl-Met) enzyme (THIC) that probably lasts for only a few catalytic cycles (Box 1). Paralleling the stress induction of Arabidopsis THI4, THIC, and other thiamin synthesis genes (Fig. 5; Supplemental Table S2), the level of total thiamin and of its components (thiamin mono- and diphosphates and free thiamin) increased by up to 2-fold in Arabidopsis seedlings subjected to cold, osmotic, salinity, or oxidative stress (Tunc-Ozdemir et al., 2009). Similarly, total thiamin increased by up to 60% in Arabidopsis seedlings exposed to salt, osmotic, or oxidative stress, with free thiamin contributing as much or more than thiamin diphosphate to the increase (Rapala-Kozik et al., 2012). Also similarly, in maize seedlings subjected to water, salt, or oxidative stress, total thiamin increased by 70 to 150%, due to increases in free thiamin and thiamin monophosphate (Rapala-Kozik et al., 2008). However, in this case levels of the cofactor thiamin diphosphate stayed constant or fell by up to 30%, which is quite enough to cause severe deficiency symptoms (Fig. 2) and illustrates the point made above about cofactor levels falling even though free vitamin levels rise.
Given the suicide nature of the THI4 enzyme, it is striking that THI4 was the most highly or second most highly induced gene in cotton (Gossypium hirsutum) seedlings exposed to cold, drought, salt, or high pH stress; the induction of THI4 relative to unstressed controls ranged from 14- to 44-fold (Zhu et al., 2013). It is equally striking that the THI4 protein showed, among all proteins analyzed, the second largest increase in expression (3.2-fold) in heat-stressed poplar leaves (Ferreira et al., 2006) and the third largest increase in expression (3.7-fold) in heat-stressed wild rice leaves (Scafaro et al., 2010).
Strategy 3: Repairing or Recycling Damaged B Vitamins and Cofactors
Like other organisms, plants have enzyme systems that deal with some of the chemical and enzymatic damage reactions shown in Figure 1 (Linster et al., 2013). These enzyme systems may repair damaged vitamins and cofactors, i.e. directly restore them to the original state, or salvage parts of damaged molecules for reuse in biosynthesis. As vitamin and cofactor repair (Hanson et al., 2016) and recycling (Gerdes et al., 2012) were recently reviewed, we focus here on three cases where the repair or recycling-related activity appears to be upregulated by stress and not simply constitutive.
Thiamin Salvage Hydrolase TenA_E
TenA_E mediates two successive steps in the recovery of the pyrimidine moiety from thiamin damaged in the thiazole moiety (Zallot et al., 2014). The Arabidopsis gene encoding TenA_E is upregulated by several abiotic stresses, in common with thiamin synthesis genes (Fig. 5; Supplemental Table S2). The Arabidopsis gene specifying the thiazole salvage enzyme ThiM (Yazdani et al., 2013) is not upregulated (Fig. 5), which fits with the thiazole moiety of thiamin being more prone to irreversible damage than the pyrimidine moiety, and hence not as recyclable (Zallot et al., 2014).
Thiamin Diphosphate-Related Nudix Hydrolases
These enzymes (NUDT20 and NUDT24 in Arabidopsis) are diphosphatases whose preferred substrates are damaged, and toxic, forms of thiamin diphosphate, including the previously mentioned thiazolone form generated by oxygen-dependent side-reactions (Goyer et al., 2013). The diphosphatase reaction is both a detoxification step and the first step in salvage of the pyrimidine moiety. The Arabidopsis genes (which are not distinguished by microarrays) are induced between 2- and 5-fold by salt, drought, and osmotic stress, and probably also oxidative stress (Kilian et al., 2007; Goyer et al., 2013).
NAD(P)+ Salvage Module
Two enzymes mediating consecutive steps in NAD(P)+ salvage (nicotinamidase and nicotinate phosphoribosyltransferase) are each induced by two stresses in Arabidopsis (Fig. 5), as is a transporter (NiaP) for nicotinate, the intermediate that these enzymes share (Kilian et al., 2007). Arabidopsis NiaP also transports trigonelline (Fig. 4) (Jeanguenin et al., 2012). NiaP is probably located in the plasma membrane (Jeanguenin et al., 2012), suggesting that nicotinate salvaged in one location could be reused for NAD(P)+ synthesis in another. This would be consistent with the classical evidence for inter-organ exchange of nicotinate (Bonner and Bonner, 1948).
Strategy 4: Decreasing Demand for B Vitamins
Metabolism can sometimes be rerouted to bypass certain cofactor-dependent steps, as in the phosphate starvation response, in which alternative bypass pathways of cytosolic glycolysis are upregulated to spare scarce adenylates and phosphate (Plaxton and Tran, 2011). There are as yet no cases of full-blown rerouting in response to vitamin deficiency, although two studies provide indirect evidence for redirection of fluxes that may be, at least in part, active responses. In the first study, tobacco leaves overexpressing transketolase, a thiamin diphosphate-dependent enzyme, became moderately thiamin diphosphate-deficient and showed reduced isoprenoid synthesis via the thiamin diphosphate-dependent enzyme 1-deoxy-d-xylulose 5-phosphate synthase (DXS); this reduction was associated with lower DXS transcript levels (Khozaei et al., 2015). In the second study, a drastic, rapid-onset tetrahydrofolate deficiency in Arabidopsis cells treated with antifolate drugs led to initial depletion of Met and S-adenosyl-Met pools, reflecting reduction in the tetrahydrofolate-dependent flux of one-carbon units to Met as one-carbon fluxes were adaptively reprioritized in favor of nucleotides (Loizeau et al., 2008). Surprisingly, the Met and S-adenosyl-Met pools, and by inference the associated fluxes, subsequently returned to normal, in part via a conserved adaptive mechanism, triggered by tetrahydrofolate deficiency, that involves proteolytic removal of the N-terminal regulatory region of the Met synthesis enzyme cystathionine γ-synthase (Loizeau et al., 2008).
AGRICULTURAL IMPLICATIONS
As Abraham Blum has emphasized (Blum, 2014), humility and caution are needed when trying to predict agricultural benefits by extrapolating from the responses of seedlings of Arabidopsis or other species to short, sharp stresses in growth chambers (which describes most of the experiments covered above). With this warning in mind, what might stress-induced B vitamin and cofactor deficiency imply for crop breeding and management? The primary implication is that breeding for enhanced vitamin content (biofortification), by transgenic or other approaches, could pay off in terms of stress resistance as well as human nutrition (Fitzpatrick et al., 2012). If stress indeed predisposes to deficiency, a clear prescription for stress resistance breeding would be to “keep your cofactors safe.”
The two B vitamins most connected with stress responses, vitamin B6 and thiamin (Fig. 5), are targets of transgenic biofortification efforts in Arabidopsis (Raschke et al., 2011; Pourcel et al., 2013; Vanderschuren et al., 2013; Dong et al., 2015), and stress tests have been run on the engineered plants, albeit mainly with plantlets cultured on agar medium containing Suc (i.e. not photosynthesizing normally). The total B6 content of leaf tissue was boosted up to 4-fold and included large increases in the cofactor pyridoxal 5′-phosphate and pyridoxine-5′-β-d-glucoside (Raschke et al., 2011). The total thiamin content of leaf tissue was boosted up to 3.4-fold, contributed by up to 2-fold increases in the cofactor thiamin diphosphate and up to 6-fold increases in thiamin (Dong et al., 2015). The B6-biofortified plants were larger than controls and more resistant to paraquat-imposed oxidative stress; no other stress data were reported (Raschke et al., 2011). The thiamin-fortified plants showed no change in resistance to salt, cold, osmotic, drought, or oxidative stress (Dong et al., 2015). To summarize: These first biofortification results for B6 and thiamin are inconclusive and merit follow-up.
Stress-induced B vitamin deficiency also has implications for crop management (Plaut et al., 2013). Reported benefits of vitamin applications are cited above; such applications are most likely to be useful in practice if they can be given as seed-priming treatments (Al-Hakimi and Hamada, 2001), i.e. applied to seed before sowing. Such seed treatments are cost-efficient (Tanou et al., 2012).
FUTURE PERSPECTIVES
The preceding overview of work relating B vitamins to abiotic stress raises five issues that are listed in the “Outstanding Questions.” Each issue is developed briefly below.
Itemizing Stress-Relevant Enzyme Reactions
Although various enzymes and genes for repair or recycling of damaged vitamins and cofactors are known, this is still not the case for about half of the 30 damage reactions in Figure 1. While the products of some of these reactions may have no possible fate besides complete degradation, comparative biochemistry suggests that others may well be reclaimed in plants; examples include biotin sulfoxide and chain-shortened biotin (Linster et al., 2013), pyridoxine and nicotinamide N-oxides (Sakuragi and Kummerow, 1960; Shibata et al., 1991), and riboflavin-4',5′-phosphate (cyclic FMN; Fraiz et al., 1998). We also don’t know most of the enzymes or genes for the synthesis and mobilization of the vitamin conjugates in Figure 4, and it is not even clear that we have fully inventoried the conjugates that plants can make. Notably, conjugated forms of thiamin and its pyrimidine and thiazole precursors have not been reported from plants, but they seem not to have been sought.
Graduating to Crops and Realistic Stress
As noted previously, most of the modern data linking abiotic stress with B vitamins and cofactors comes from young Arabidopsis plants and unrealistic stress protocols. This is a generic weakness of molecular-level stress research, and the resulting disconnect from agriculture goes a long way toward explaining why molecular work has had a very limited impact on breeding for stress environments (Blum, 2014). It is consequently important to extend stress/vitamin work from Arabidopsis to good genetic model crops such as tomato, canola (Brassica napus), maize, and rice and to adopt stress protocols that mimic field stresses to plants beyond the seedling stage.
Diagnosing Functional Deficiencies
Vitamin deficiencies, particularly functional ones affecting only certain cells or compartments, may be better diagnosed from characteristic changes in metabolite profiles than by directly measuring vitamin levels, as discussed above for vitamin B12 deficiency in humans (Stabler et al., 1996). Given the possibility that direct vitamin measurements can fail to detect subtle but physiologically critical functional deficiencies, and the high cost and low-throughput nature of many vitamin assays, indirect assessment of plant vitamin and cofactor status via metabolomics is appealing. Confirming or disconfirming the validity of this approach in plants, as in humans (Stabler et al., 2013), will require prior metabolic profiling studies of vitamin-deficient mutants. An alternative, indirect, high-throughput way to detect functional vitamin deficiencies may be via characteristic transcriptome changes, analogous to the induction of specific genes by mineral nutrient deficiencies (Nikiforova et al., 2003; Zheng et al., 2009). Furthermore, transcriptome analysis could uncover mechanisms that reroute metabolism to mitigate the effects of vitamin deficiency (see above). Again, evaluation of these possibilities will require studies of vitamin-deficient mutants.

Genotypic Variation
Although there is natural genotypic variation in vitamin content (Hanson and Gregory, 2011; Fitzpatrick et al., 2012), it would be impractical to use vitamin content as a selection criterion to develop experimental populations due to the issues of assay cost and efficacy just outlined. On the other hand, were high-throughput indirect methods to assess deficiency available (see above), it would be feasible to assess vitamin/cofactor status in populations subjected to realistic stresses and then to work back via genome-wide association studies to genes governing vitamin/cofactor status. Once identified, such genes, which would presumably include known and novel genes with repair/recycling, synthesis, storage, or bypass functions, could be used to explore a “keep your cofactors safe” breeding strategy for abiotic stress resistance.
Is Thiamin an Achilles’ Heel in Stress Metabolism?
A thread running through the whole of this review is that thiamin and thiamin diphosphate are not like the other B vitamins and cofactors. Thiamin diphosphate may turn over exceptionally fast, at least in part because of its unusual feature of catalytic inactivation. Thiamin is energetically costly to make due to the suicidal nature of one of its biosynthetic enzymes and the suicidal tendency of another. Because thiamin synthesis depends absolutely on concurrent protein synthesis, it is vulnerable to stress in a way that other B vitamins are not. Unlike most other B vitamins, thiamin and its precursors lack known storage forms. Thiamin synthesis and salvage genes are particularly highly stress-regulated in Arabidopsis seedlings. Lastly, thiamin is more prominent than other B vitamins in the “spray and pray” literature. Taken together, these features suggest that thiamin could be an Achilles’ heel, a crucial weak point, in plant stress metabolism.
Supplemental Data
The following supplemental materials are available.
Supplemental Table S1. Analysis of Arabidopsis stress literature based on PubMed search terms.
Supplemental Table S2. Stress-induced B vitamin/cofactor synthesis and salvage genes in Arabidopsis.
Supplementary Material
Acknowledgments
We apologize to the authors of relevant studies that could not be cited here due to page limitations. We thank Dr. Claudia Lerma Ortiz for a helpful critique of a draft of the review.
Footnotes
Articles can be viewed without a subscription.
References
- Akhtar TA, Orsomando G, Mehrshahi P, Lara-Núñez A, Bennett MJ, Gregory JF III, Hanson AD (2010) A central role for gamma-glutamyl hydrolases in plant folate homeostasis. Plant J 64: 256–266 [DOI] [PubMed] [Google Scholar]
- Al-Hakimi A, Hamada AM (2001) Counteraction of salinity stress on wheat plants by grain soaking in ascorbic acid, thiamin or sodium salicylate. Biol Plant 44: 253–261 [Google Scholar]
- Amachi T, Imamoto S, Yoshizumi H (1971) A growth factor for malo-lactic fermentation bacteria. Part II. Structure and synthesis of a novel pantothenic acid derivative isolated from tomato juice. Agric Biol Chem 35: 1222–1230 [Google Scholar]
- Ariaey-Nejad MR, Balaghi M, Baker EM, Sauberlich HE (1970) Thiamin metabolism in man. Am J Clin Nutr 23: 764–778 [DOI] [PubMed] [Google Scholar]
- Asensi-Fabado MA, Munné-Bosch S (2010) Vitamins in plants: occurrence, biosynthesis and antioxidant function. Trends Plant Sci 15: 582–592 [DOI] [PubMed] [Google Scholar]
- Ashihara H, Yin Y, Deng WW, Watanabe S (2010) Pyridine salvage and nicotinic acid conjugate synthesis in leaves of mangrove species. Phytochemistry 71: 47–53 [DOI] [PubMed] [Google Scholar]
- Baggott JE. (2000) Hydrolysis of 5,10-methenyltetrahydrofolate to 5-formyltetrahydrofolate at pH 2.5 to 4.5. Biochemistry 39: 14647–14653 [DOI] [PubMed] [Google Scholar]
- Blum A. (2014) Genomics for drought resistance – getting down to earth. Funct Plant Biol 41: 1191–1198 [DOI] [PubMed] [Google Scholar]
- Bonner J. (1943) Effects of application of thiamin to Cosmos. Bot Gaz 104: 475–479 [Google Scholar]
- Bonner J. (1957) The chemical cure of climatic lesions. Eng Sci 20: 28–30 [Google Scholar]
- Bonner J, Bonner H (1948) The B vitamins as plant hormones. Vitam Horm 6: 225–275 [Google Scholar]
- Bozzo GG, Basset GJ, Naponelli V, Noiriel A, Gregory JF III, Hanson AD (2008) Characterization of the folate salvage enzyme p-aminobenzoylglutamate hydrolase in plants. Phytochemistry 69: 29–37 [DOI] [PubMed] [Google Scholar]
- Broderick JB, Duffus BR, Duschene KS, Shepard EM (2014) Radical S-adenosylmethionine enzymes. Chem Rev 114: 4229–4317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunik VI, Schloss JV, Pinto JT, Gibson GE, Cooper AJ (2007) Enzyme-catalyzed side reactions with molecular oxygen may contribute to cell signaling and neurodegenerative diseases. Neurochem Res 32: 871–891 [DOI] [PubMed] [Google Scholar]
- Chatterjee A, Abeydeera ND, Bale S, Pai PJ, Dorrestein PC, Russell DH, Ealick SE, Begley TP (2011) Saccharomyces cerevisiae THI4p is a suicide thiamine thiazole synthase. Nature 478: 542–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Xiong L (2005) Pyridoxine is required for post-embryonic root development and tolerance to osmotic and oxidative stresses. Plant J 44: 396–408 [DOI] [PubMed] [Google Scholar]
- Choe E, Huang R, Min DB (2005) Chemical reactions and stability of riboflavin in foods. J Food Sci 70: R28–R36 [Google Scholar]
- Clifford AJ, Bills ND, Peerson JM, Müller HG, Burk GE, Rich KD (1993) A depletion-repletion folate bioassay based on growth and tissue folate concentrations of rats. J Nutr 123: 926–932 [DOI] [PubMed] [Google Scholar]
- Coburn SP. (1990) Location and turnover of vitamin B6 pools and vitamin B6 requirements of humans. Ann N Y Acad Sci 585: 76–85 [DOI] [PubMed] [Google Scholar]
- Dalling DK, Grant DM, Horton WJ, Sagers RD (1976) Carbon 13 NMR study of nonenzymatic reactions of pyridoxal 5′-phosphate with selected amino acids and of related reactions. J Biol Chem 251: 7661–7668 [PubMed] [Google Scholar]
- Dalvi RR, Sauberlich HE, Neal RA (1974) An examination of the metabolism of thiamin by rat liver alcohol dehydrogenase. J Nutr 104: 1476–1483 [DOI] [PubMed] [Google Scholar]
- Dehaye L, Duval M, Viguier D, Yaxley J, Job D (1997) Cloning and expression of the pea gene encoding SBP65, a seed-specific biotinylated protein. Plant Mol Biol 35: 605–621 [DOI] [PubMed] [Google Scholar]
- Dhindsa RS, Cleland RE (1975) Water stress and protein synthesis: I. Differential inhibition of protein synthesis. Plant Physiol 55: 778–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz de la Garza R, Quinlivan EP, Klaus SM, Basset GJ, Gregory JF III, Hanson AD (2004) Folate biofortification in tomatoes by engineering the pteridine branch of folate synthesis. Proc Natl Acad Sci USA 101: 13720–13725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W, Stockwell VO, Goyer A (2015) Enhancement of thiamin content in Arabidopsis thaliana by metabolic engineering. Plant Cell Physiol 56: 2285–2296 [DOI] [PubMed] [Google Scholar]
- Dwivedi BK, Arnold RG (1973) Chemistry of thiamine degradation in food products and model systems: a review. J Agric Food Chem 21: 54–60 [DOI] [PubMed] [Google Scholar]
- Espinosa-Ruiz A, Bellés JM, Serrano R, Culiáñez-MacIà FA (1999) Arabidopsis thaliana AtHAL3: a flavoprotein related to salt and osmotic tolerance and plant growth. Plant J 20: 529–539 [DOI] [PubMed] [Google Scholar]
- Eudes A, Bozzo GG, Waller JC, Naponelli V, Lim EK, Bowles DJ, Gregory JF III, Hanson AD (2008) Metabolism of the folate precursor p-aminobenzoate in plants: glucose ester formation and vacuolar storage. J Biol Chem 283: 15451–15459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everse J, Cooper Zoll E, Kahan L, Kaplan NO (1971) Addition products of diphosphopyridine nucleotides with substrates of pyridine nucleotide-linked dehydrogenases. Bioorg Chem 1: 207–233 [Google Scholar]
- Everse J, Everse KE, Kaplan NO (1975) The pyridine nucleosidases from Bacillus subtilis and Neurospora crassa. Isolation and structural properties. Arch Biochem Biophys 169: 702–713 [DOI] [PubMed] [Google Scholar]
- Ferreira S, Hjernø K, Larsen M, Wingsle G, Larsen P, Fey S, Roepstorff P, Salomé Pais M (2006) Proteome profiling of Populus euphratica Oliv. upon heat stress. Ann Bot (Lond) 98: 361–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick TB. (2011) Vitamin B6 in plants: more than meets the eye. Adv Bot Res 59: 1–38 [Google Scholar]
- Fitzpatrick TB, Basset GJ, Borel P, Carrari F, DellaPenna D, Fraser PD, Hellmann H, Osorio S, Rothan C, Valpuesta V, Caris-Veyrat C, Fernie AR (2012) Vitamin deficiencies in humans: can plant science help? Plant Cell 24: 395–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Noctor G (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155: 2–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraiz FJ, Pinto RM, Costas MJ, Aavalos M, Canales J, Cabezas A, Cameselle JC (1998) Enzymic formation of riboflavin 4′,5′-cyclic phosphate from FAD: evidence for a specific low-Km FMN cyclase in rat liver1. Biochem J 330: 881–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frelin O, Huang L, Hasnain G, Jeffryes JG, Ziemak MJ, Rocca JR, Wang B, Rice J, Roje S, Yurgel SN, et al. (2015) A directed-overflow and damage-control N-glycosidase in riboflavin biosynthesis. Biochem J 466: 137–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes S, Lerma-Ortiz C, Frelin O, Seaver SM, Henry CS, de Crécy-Lagard V, Hanson AD (2012) Plant B vitamin pathways and their compartmentation: a guide for the perplexed. J Exp Bot 63: 5379–5395 [DOI] [PubMed] [Google Scholar]
- Good AG, Zaplachinski ST (1994) The effects of drought stress on free amino acid accumulation and protein synthesis in Brassica napus. Physiol Plant 90: 9–14 [Google Scholar]
- Goyer A, Collakova E, Díaz de la Garza R, Quinlivan EP, Williamson J, Gregory JF III, Shachar-Hill Y, Hanson AD (2005) 5-Formyltetrahydrofolate is an inhibitory but well tolerated metabolite in Arabidopsis leaves. J Biol Chem 280: 26137–26142 [DOI] [PubMed] [Google Scholar]
- Goyer A, Hasnain G, Frelin O, Ralat MA, Gregory JF III, Hanson AD (2013) A cross-kingdom Nudix enzyme that pre-empts damage in thiamin metabolism. Biochem J 454: 533–542 [DOI] [PubMed] [Google Scholar]
- Green R. (2008) Indicators for assessing folate and vitamin B12 status and for monitoring the efficacy of intervention strategies. Food Nutr Bull (Suppl) 29: S52–S63 [DOI] [PubMed] [Google Scholar]
- Gregory JF., III (1989) Chemical and nutritional aspects of folate research: analytical procedures, methods of folate synthesis, stability, and bioavailability of dietary folates. Adv Food Nutr Res 33: 1–101 [DOI] [PubMed] [Google Scholar]
- Gregory JF., III (1998) Nutritional Properties and significance of vitamin glycosides. Annu Rev Nutr 18: 277–296 [DOI] [PubMed] [Google Scholar]
- Gregory JF III, Quinlivan EP (2002) In vivo kinetics of folate metabolism. Annu Rev Nutr 22: 199–220 [DOI] [PubMed] [Google Scholar]
- Guenther BD, Sheppard CA, Tran P, Rozen R, Matthews RG, Ludwig ML (1999) The structure and properties of methylenetetrahydrofolate reductase from Escherichia coli suggest how folate ameliorates human hyperhomocysteinemia. Nat Struct Biol 6: 359–365 [DOI] [PubMed] [Google Scholar]
- Hall CA. (1964) Long term excretion of Co57-vitamin B12 and turnover within plasma. Am J Clin Nutr 14: 156–162 [DOI] [PubMed] [Google Scholar]
- Hanson AD, Gregory JF III (2011) Folate biosynthesis, turnover, and transport in plants. Annu Rev Plant Biol 62: 105–125 [DOI] [PubMed] [Google Scholar]
- Hanson AD, Henry CS, Fiehn O, de Crécy-Lagard V (2016) Metabolite damage and metabolite damage control in plants. Annu Rev Plant Biol 67: 131–152 [DOI] [PubMed] [Google Scholar]
- Hasnain G, Frelin O, Roje S, Ellens KW, Ali K, Guan JC, Garrett TJ, de Crécy-Lagard V, Gregory JF III, McCarty DR, Hanson AD (2013) Identification and characterization of the missing pyrimidine reductase in the plant riboflavin biosynthesis pathway. Plant Physiol 161: 48–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higa A, Khandakar J, Mori Y, Kitamura Y (2012) Increased de novo riboflavin synthesis and hydrolysis of FMN are involved in riboflavin secretion from Hyoscyamus albus hairy roots under iron deficiency. Plant Physiol Biochem 58: 166–173 [DOI] [PubMed] [Google Scholar]
- Huang L, Khusnutdinova A, Nocek B, Brown G, Xu X, Cui H, Petit P, Flick R, Zallot R, Balmant K, et al. (2016) A family of metal-dependent phosphatases implicated in metabolite damage-control. Nat Chem Biol 12: 621–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Zhang J, Wang L, Huang L (2013) Effect of abiotic stress on the abundance of different vitamin B6 vitamers in tobacco plants. Plant Physiol Biochem 66: 63–67 [DOI] [PubMed] [Google Scholar]
- Izumi Y, Tani Y, Ogata K (1973) The conversion of bisnorbiotin and bisnordethiobiotin to biotin and dethiobiotin, respectively, by microorganisms. Biochim Biophys Acta 326: 485–488 [DOI] [PubMed] [Google Scholar]
- Jeanguenin L, Lara-Núñez A, Rodionov DA, Osterman AL, Komarova NY, Rentsch D, Gregory JF III, Hanson AD (2012) Comparative genomics and functional analysis of the NiaP family uncover nicotinate transporters from bacteria, plants, and mammals. Funct Integr Genomics 12: 25–34 [DOI] [PubMed] [Google Scholar]
- Jenkins AH, Schyns G, Potot S, Sun G, Begley TP (2007) A new thiamin salvage pathway. Nat Chem Biol 3: 492–497 [DOI] [PubMed] [Google Scholar]
- Jurgenson CT, Begley TP, Ealick SE (2009) The structural and biochemical foundations of thiamin biosynthesis. Annu Rev Biochem 78: 569–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya C, Ashraf M, Sonmez O, Tuna AL, Polat T, Aydemir S (2015) Exogenous application of thiamin promotes growth and antioxidative defense system at initial phases of development in salt-stressed plants of two maize cultivars differing in salinity tolerance. Acta Physiol Plant 37: 1741 [Google Scholar]
- Ketellapper HJ. (1963) Temperature-induced chemical defects in higher plants. Plant Physiol 38: 175–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khozaei M, Fisk S, Lawson T, Gibon Y, Sulpice R, Stitt M, Lefebvre SC, Raines CA (2015) Overexpression of plastid transketolase in tobacco results in a thiamine auxotrophic phenotype. Plant Cell 27: 432–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian J, Whitehead D, Horak J, Wanke D, Weinl S, Batistic O, D’Angelo C, Bornberg-Bauer E, Kudla J, Harter K (2007) The AtGenExpress global stress expression data set: protocols, evaluation and model data analysis of UV-B light, drought and cold stress responses. Plant J 50: 347–363 [DOI] [PubMed] [Google Scholar]
- Kohler RE. (1975) The history of biochemistry: a survey. J Hist Biol 8: 275–318 [DOI] [PubMed] [Google Scholar]
- Kupke T, Hernandez-Acosta P, Steinbacher S, Culianez-Macia FA (2001) Arabidopsis thaliana flavoprotein AtHAL3a catalyzes the decarboxylation of 4′-Phosphopantothenoylcysteine to 4′-phosphopantetheine, a key step in coenzyme A biosynthesis. J Biol Chem 276: 19190–19196 [DOI] [PubMed] [Google Scholar]
- Langridge J, Griffing B (1959) A study of high temperature lesions in Arabidopsis thaliana. Aust J Biol Sci 12: 117–135 [Google Scholar]
- Lerma-Ortiz C, Jeffryes JG, Cooper AJ, Niehaus TD, Thamm AM, Frelin O, Aunins T, Fiehn O, de Crécy-Lagard V, Henry CS, Hanson AD (2016) ‘Nothing of chemistry disappears in biology’: the Top 30 damage-prone endogenous metabolites. Biochem Soc Trans 44: 961–971 [DOI] [PubMed] [Google Scholar]
- Lesgards J-F, Lehucher-Michel M-P, Vidal N, Prost M, Stocker P (2005) Assessment of antioxidative activity of lipid- and water-soluble vitamins in human whole blood. Comparative analysis between a biological test and chemical methods. Int J Vitam Nutr Res 75: 11–18 [DOI] [PubMed] [Google Scholar]
- Li W, Zhang F, Chang Y, Zhao T, Schranz ME, Wang G (2015) Nicotinate O-glucosylation is an evolutionarily metabolic trait important for seed germination under stress conditions in Arabidopsis thaliana. Plant Cell 27: 1907–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linster CL, Van Schaftingen E, Hanson AD (2013) Metabolite damage and its repair or pre-emption. Nat Chem Biol 9: 72–80 [DOI] [PubMed] [Google Scholar]
- Liu JX, Howell SH (2010) Endoplasmic reticulum protein quality control and its relationship to environmental stress responses in plants. Plant Cell 22: 2930–2942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loizeau K, De Brouwer V, Gambonnet B, Yu A, Renou JP, Van Der Straeten D, Lambert WE, Rébeillé F, Ravanel S (2008) A genome-wide and metabolic analysis determined the adaptive response of Arabidopsis cells to folate depletion induced by methotrexate. Plant Physiol 148: 2083–2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig-Müller J. (2011) Auxin conjugates: their role for plant development and in the evolution of land plants. J Exp Bot 62: 1757–1773 [DOI] [PubMed] [Google Scholar]
- Lukienko PI, Mel’nichenko NG, Zverinskii IV, Zabrodskaya SV (2000) Antioxidant properties of thiamine. Bull Exp Biol Med 130: 874–876 [PubMed] [Google Scholar]
- Mackey AD, Lieu SO, Carman C, Gregory JF III (2003) Hydrolytic activity toward pyridoxine-5′-beta-D-glucoside in rat intestinal mucosa is not increased by vitamin B-6 deficiency: effect of basal diet composition and pyridoxine intake. J Nutr 133: 1362–1367 [DOI] [PubMed] [Google Scholar]
- Marbaix AY, Noël G, Detroux AM, Vertommen D, Van Schaftingen E, Linster CL (2011) Extremely conserved ATP- or ADP-dependent enzymatic system for nicotinamide nucleotide repair. J Biol Chem 286: 41246–41252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui A, Yin Y, Yamanaka K, Iwasaki M, Ashihara H (2007) Metabolic fate of nicotinamide in higher plants. Physiol Plant 131: 191–200 [DOI] [PubMed] [Google Scholar]
- McCourt JA, Nixon PF, Duggleby RG (2006) Thiamin nutrition and catalysis-induced instability of thiamin diphosphate. Br J Nutr 96: 636–638 [PubMed] [Google Scholar]
- McHale NA, Hanson KR, Zelitch I (1988) A nuclear mutation in Nicotiana sylvestris causing a thiamine-reversible defect in synthesis of chloroplast pigments. Plant Physiol 88: 930–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melville DB. (1954) Biotin sulfoxide. J Biol Chem 208: 495–501 [PubMed] [Google Scholar]
- Menegus F, Lilliu I, Scaglioni L (2002) A unique pantoyllactone glycoside system is activated in rice seedlings developing aerobically in the dark. Physiol Plant 116: 299–307 [Google Scholar]
- Mills PB, Struys E, Jakobs C, Plecko B, Baxter P, Baumgartner M, Willemsen MA, Omran H, Tacke U, Uhlenberg B, Weschke B, Clayton PT (2006) Mutations in antiquitin in individuals with pyridoxine-dependent seizures. Nat Med 12: 307–309 [DOI] [PubMed] [Google Scholar]
- Mizuno K, Matsuzaki M, Kanazawa S, Tokiwano T, Yoshizawa Y, Kato M (2014) Conversion of nicotinic acid to trigonelline is catalyzed by N-methyltransferase belonged to motif B′ methyltransferase family in Coffea arabica. Biochem Biophys Res Commun 452: 1060–1066 [DOI] [PubMed] [Google Scholar]
- Moccand C, Boycheva S, Surriabre P, Tambasco-Studart M, Raschke M, Kaufmann M, Fitzpatrick TB (2014) The pseudoenzyme PDX1.2 boosts vitamin B6 biosynthesis under heat and oxidative stress in Arabidopsis. J Biol Chem 289: 8203–8216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi PP, Moieni A, Hiraga S, Komatsu S (2012) Organ-specific proteomic analysis of drought-stressed soybean seedlings. J Proteomics 75: 1906–1923 [DOI] [PubMed] [Google Scholar]
- Navarrete O, Van Daele J, Stove C, Lambert W, Van Der Straeten D, Storozhenko S (2012) A folate independent role for cytosolic HPPK/DHPS upon stress in Arabidopsis thaliana. Phytochemistry 73: 23–33 [DOI] [PubMed] [Google Scholar]
- Nelson CJ, Alexova R, Jacoby RP, Millar AH (2014) Proteins with high turnover rate in barley leaves estimated by proteome analysis combined with in planta isotope labeling. Plant Physiol 166: 91–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikiforova V, Freitag J, Kempa S, Adamik M, Hesse H, Hoefgen R (2003) Transcriptome analysis of sulfur depletion in Arabidopsis thaliana: interlacing of biosynthetic pathways provides response specificity. Plant J 33: 633–650 [DOI] [PubMed] [Google Scholar]
- Noiriel A, Naponelli V, Gregory JF III, Hanson AD (2007) Pterin and folate salvage. Plants and Escherichia coli lack capacity to reduce oxidized pterins. Plant Physiol 143: 1101–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Yoshimura K, Miyake H, Ishikawa K, Ito D, Tanabe N, Shigeoka S (2008) Molecular characterization of organelle-type Nudix hydrolases in Arabidopsis. Plant Physiol 148: 1412–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K (1983) New metabolites of riboflavin appear in human urine. J Biol Chem 258: 5623–5628 [PubMed] [Google Scholar]
- Oppenheimer NJ, Kaplan NO (1975) The alpha beta epimerization of reduced nicotinamide adenine dinucleotide. Arch Biochem Biophys 166: 526–535 [DOI] [PubMed] [Google Scholar]
- Orsomando G, de la Garza RD, Green BJ, Peng M, Rea PA, Ryan TJ, Gregory JF III, Hanson AD (2005) Plant gamma-glutamyl hydrolases and folate polyglutamates: characterization, compartmentation, and co-occurrence in vacuoles. J Biol Chem 280: 28877–28884 [DOI] [PubMed] [Google Scholar]
- Ouyang M, Ma J, Zou M, Guo J, Wang L, Lu C, Zhang L (2010) The photosensitive phs1 mutant is impaired in the riboflavin biogenesis pathway. J Plant Physiol 167: 1466–1476 [DOI] [PubMed] [Google Scholar]
- Paizs C, Diemer T, Rétey J (2008) The putative coenzyme B12-dependent methylmalonyl-CoA mutase from potatoes is a phosphatase. Bioorg Chem 36: 261–264 [DOI] [PubMed] [Google Scholar]
- Palmer LD, Downs DM (2013) The thiamine biosynthetic enzyme ThiC catalyzes multiple turnovers and is inhibited by S-adenosylmethionine (AdoMet) metabolites. J Biol Chem 288: 30693–30699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedrafita G, Keller MA, Ralser M (2015) The impact of non-enzymatic reactions and enzyme promiscuity on cellular metabolism during (oxidative) stress conditions. Biomolecules 5: 2101–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrowska A, Bajguz A (2011) Conjugates of abscisic acid, brassinosteroids, ethylene, gibberellins, and jasmonates. Phytochemistry 72: 2097–2112 [DOI] [PubMed] [Google Scholar]
- Pinto RM, Fraiz FJ, Cabezas A, Avalos M, Canales J, Costas MJ, Cameselle JC (1999) Preparation of riboflavin 4′,5′-cyclic phosphate by incubation of flavin-adenine dinucleotide with Mn2+ in the absence of riboflavin 5′-phosphate cyclase. Anal Biochem 268: 409–411 [DOI] [PubMed] [Google Scholar]
- Plaut Z, Edelstein M, Ben-Hur M (2013) Overcoming salinity barriers to crop production using traditional methods. Crit Rev Plant Sci 32: 250–291 [Google Scholar]
- Plaxton WC, Tran HT (2011) Metabolic adaptations of phosphate-starved plants. Plant Physiol 156: 1006–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourcel L, Moulin M, Fitzpatrick TB (2013) Examining strategies to facilitate vitamin B1 biofortification of plants by genetic engineering. Front Plant Sci 4: 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlivan EP, Roje S, Basset G, Shachar-Hill Y, Gregory JF III, Hanson AD (2003) The folate precursor p-aminobenzoate is reversibly converted to its glucose ester in the plant cytosol. J Biol Chem 278: 20731–20737 [DOI] [PubMed] [Google Scholar]
- Rains TM, Emmert JL, Baker DH, Shay NF (1997) Minimum thiamin requirement of weanling Sprague-Dawley outbred rats. J Nutr 127: 167–170 [DOI] [PubMed] [Google Scholar]
- Rapala-Kozik M, Gołda A, Kujda M (2009) Enzymes that control the thiamine diphosphate pool in plant tissues. Properties of thiamine pyrophosphokinase and thiamine-(di)phosphate phosphatase purified from Zea mays seedlings. Plant Physiol Biochem 47: 237–242 [DOI] [PubMed] [Google Scholar]
- Rapala-Kozik M, Kowalska E, Ostrowska K (2008) Modulation of thiamine metabolism in Zea mays seedlings under conditions of abiotic stress. J Exp Bot 59: 4133–4143 [DOI] [PubMed] [Google Scholar]
- Rapala-Kozik M, Wolak N, Kujda M, Banas AK (2012) The upregulation of thiamine (vitamin B1) biosynthesis in Arabidopsis thaliana seedlings under salt and osmotic stress conditions is mediated by abscisic acid at the early stages of this stress response. BMC Plant Biol 12: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschke M, Boycheva S, Crèvecoeur M, Nunes-Nesi A, Witt S, Fernie AR, Amrhein N, Fitzpatrick TB (2011) Enhanced levels of vitamin B(6) increase aerial organ size and positively affect stress tolerance in Arabidopsis. Plant J 66: 414–432 [DOI] [PubMed] [Google Scholar]
- Rawat R, Sandoval FJ, Wei Z, Winkler R, Roje S (2011) An FMN hydrolase of the haloacid dehalogenase superfamily is active in plant chloroplasts. J Biol Chem 286: 42091–42098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristilä M, Strid H, Eriksson LA, Strid A, Sävenstrand H (2011) The role of the pyridoxine (vitamin B6) biosynthesis enzyme PDX1 in ultraviolet-B radiation responses in plants. Plant Physiol Biochem 49: 284–292 [DOI] [PubMed] [Google Scholar]
- Rueschhoff EE, Gillikin JW, Sederoff HW, Daub ME (2013) The SOS4 pyridoxal kinase is required for maintenance of vitamin B6-mediated processes in chloroplasts. Plant Physiol Biochem 63: 281–291 [DOI] [PubMed] [Google Scholar]
- Sakuragi T, Kummerow FA (1960) Utilization of pyridoxine N-oxide rats. Proc Soc Exp Biol Med 103: 185–188 [DOI] [PubMed] [Google Scholar]
- Sánchez-Moreno I, Iturrate L, Martín-Hoyos R, Jimeno ML, Mena M, Bastida A, García-Junceda E (2009) From kinase to cyclase: an unusual example of catalytic promiscuity modulated by metal switching. ChemBioChem 10: 225–229 [DOI] [PubMed] [Google Scholar]
- Sayed SA, Gadallah MA (2002) Effects of shoot and root application of thiamin on salt-stressed sunflower plants. Plant Growth Regul 36: 71–80 [Google Scholar]
- Scafaro AP, Haynes PA, Atwell BJ (2010) Physiological and molecular changes in Oryza meridionalis Ng., a heat-tolerant species of wild rice. J Exp Bot 61: 191–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer JB, Mackey AD, Gregory JF III (2005) Activities of hepatic cytosolic and mitochondrial forms of serine hydroxymethyltransferase and hepatic glycine concentration are affected by vitamin B-6 intake in rats. J Nutr 135: 233–238 [DOI] [PubMed] [Google Scholar]
- Shi H, Xiong L, Stevenson B, Lu T, Zhu JK (2002) The Arabidopsis salt overly sensitive 4 mutants uncover a critical role for vitamin B6 in plant salt tolerance. Plant Cell 14: 575–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Mori Y, Onodera M (1991) Nutritional efficiency of nicotinamide N-oxide and N’-methylnicotinamide as niacin in rats. Agric Biol Chem 55: 2591–2597 [Google Scholar]
- Shimizu MM, Mazzafera P (2000) A role for trigonelline during imbibition and germination of coffee seeds. Plant Biol 2: 605–611 [Google Scholar]
- Smith AG, Croft MT, Moulin M, Webb ME (2007) Plants need their vitamins too. Curr Opin Plant Biol 10: 266–275 [DOI] [PubMed] [Google Scholar]
- Stabler SP, Korson M, Jethva R, Allen RH, Kraus JP, Spector EB, Wagner C, Mudd SH (2013) Metabolic profiling of total homocysteine and related compounds in hyperhomocysteinemia: utility and limitations in diagnosing the cause of puzzling thrombophilia in a family. JIMD Rep 11: 149–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabler SP, Lindenbaum J, Allen RH (1996) The use of homocysteine and other metabolites in the specific diagnosis of vitamin B-12 deficiency. J Nutr (4 Suppl) 126: 1266S–1272S [DOI] [PubMed] [Google Scholar]
- Storozhenko S, Navarrete O, Ravanel S, De Brouwer V, Chaerle P, Zhang GF, Bastien O, Lambert W, Rébeillé F, Van Der Straeten D (2007) Cytosolic hydroxymethyldihydropterin pyrophosphokinase/dihydropteroate synthase from Arabidopsis thaliana: a specific role in early development and stress response. J Biol Chem 282: 10749–10761 [DOI] [PubMed] [Google Scholar]
- Sümegi B, Alkonyi I (1983) Paracatalytic inactivation of pig heart pyruvate dehydrogenase complex. Arch Biochem Biophys 223: 417–424 [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Uchida K (1983) Formation of riboflavin glucosides from riboflavin in germinating barley seeds. Agric Biol Chem 47: 1145–1147 [Google Scholar]
- Tadera K, Arima M, Yoshino S, Yagi F, Kobayashi A (1986) Conversion of pyridoxine into 6-hydroxypyridoxine by food components, especially ascorbic acid. J Nutr Sci Vitaminol (Tokyo) 32: 267–277 [DOI] [PubMed] [Google Scholar]
- Tadera K, Yagi F, Kobayashi A (1982) Specificity of a particulate glucosyltransferase in seedlings of Pisum sativum L. which catalyzes the formation of 5′-O-(beta-D-glucopyranosyl)pyridoxine. J Nutr Sci Vitaminol (Tokyo) 28: 359–366 [DOI] [PubMed] [Google Scholar]
- Tanou G, Fotopoulos V, Molassiotis A (2012) Priming against environmental challenges and proteomics in plants: Update and agricultural perspectives. Front Plant Sci 3: 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimann KV. (1963) Plant growth substances; past, present and future. Annu Rev Plant Physiol 14: 1–19 [Google Scholar]
- Titiz O, Tambasco-Studart M, Warzych E, Apel K, Amrhein N, Laloi C, Fitzpatrick TB (2006) PDX1 is essential for vitamin B6 biosynthesis, development and stress tolerance in Arabidopsis. Plant J 48: 933–946 [DOI] [PubMed] [Google Scholar]
- Treadwell GE, Metzler DE (1972) Photoconversion of riboflavin to lumichrome in plant tissues. Plant Physiol 49: 991–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuna AL, Kaya C, Altunlu H, Ashraf M (2013) Mitigation effects of non-enzymatic antioxidants in maize (Zea mays L.) plants under salinity stress. Aust J Crop Sci 7: 1181–1188 [Google Scholar]
- Tunc-Ozdemir M, Miller G, Song L, Kim J, Sodek A, Koussevitzky S, Misra AN, Mittler R, Shintani D (2009) Thiamin confers enhanced tolerance to oxidative stress in Arabidopsis. Plant Physiol 151: 421–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueland PM, Ulvik A, Rios-Avila L, Midttun Ø, Gregory JF (2015) Direct and functional biomarkers of vitamin B6 status. Annu Rev Nutr 35: 33–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upmeier B, Thomzik JE, Wolfgang Barz W (1988) Enzymatic studies on the reversible synthesis of nicotinic acid-N-glucoside in heterotrophic parsley cell suspension cultures. Z Naturforsch C 43: 835–842 [Google Scholar]
- Vanderschuren H, Boycheva S, Li KT, Szydlowski N, Gruissem W, Fitzpatrick TB (2013) Strategies for vitamin B6 biofortification of plants: a dual role as a micronutrient and a stress protectant. Front Plant Sci 4: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Went FW, Bonner J, Warner GC (1938) Aneurin and the rooting of cuttings. Science 87: 170–171 [DOI] [PubMed] [Google Scholar]
- Windheuser JJ, Higuchi T (1962) Kinetics of thiamine hydrolysis. J Pharm Sci 51: 354–364 [DOI] [PubMed] [Google Scholar]
- Woodward JB, Abeydeera ND, Paul D, Phillips K, Rapala-Kozik M, Freeling M, Begley TP, Ealick SE, McSteen P, Scanlon MJ (2010) A maize thiamine auxotroph is defective in shoot meristem maintenance. Plant Cell 22: 3305–3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasumoto K, Iwami K, Tsuji H, Okada J, Mitsuda H (1976) Bound forms of vitamin B6 in cereals and seeds. Vitamins 50: 327–333 [Google Scholar]
- Yazdani M, Zallot R, Tunc-Ozdemir M, de Crécy-Lagard V, Shintani DK, Hanson AD (2013) Identification of the thiamin salvage enzyme thiazole kinase in Arabidopsis and maize. Phytochemistry 94: 68–73 [DOI] [PubMed] [Google Scholar]
- Yonamine I, Yoshida K, Kido K, Nakagawa A, Nakayama H, Shinmyo A (2004) Overexpression of NtHAL3 genes confers increased levels of proline biosynthesis and the enhancement of salt tolerance in cultured tobacco cells. J Exp Bot 55: 387–395 [DOI] [PubMed] [Google Scholar]
- Yoshizumi H, Amachi T (1969) Studies on the bacteria isolated from wine. Part IV. Distribution of the growth factor for a bacterium inducing malo-lactic fermentation. Agric Biol Chem 33: 18–24 [Google Scholar]
- You J, Chan Z (2015) ROS Regulation during abiotic stress responses in crop plants. Front Plant Sci 6: 1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallot R, Yazdani M, Goyer A, Ziemak MJ, Guan JC, McCarty DR, de Crécy-Lagard V, Gerdes S, Garrett TJ, Benach J, et al. (2014) Salvage of the thiamin pyrimidine moiety by plant TenA proteins lacking an active-site cysteine. Biochem J 463: 145–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Huang F, Narsai R, Wu J, Giraud E, He F, Cheng L, Wang F, Wu P, Whelan J, Shou H (2009) Physiological and transcriptome analysis of iron and phosphorus interaction in rice seedlings. Plant Physiol 151: 262–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YN, Shi DQ, Ruan MB, Zhang LL, Meng ZH, Liu J, Yang WC (2013) Transcriptome analysis reveals crosstalk of responsive genes to multiple abiotic stresses in cotton (Gossypium hirsutum L.). PLoS One 8: e80218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.