Abstract
Women and children in developing countries are often exposed to high levels of air pollution including polycyclic aromatic hydrocarbons (PAHs), which may negatively impact their health, due to household combustion of biomass fuel for cooking and heating. We compared creatinine adjusted hydroxy-PAH (OH-PAH) concentrations in pregnant women in Trujillo, Peru who cook with wood to levels measured in those who cook with kerosene, liquefied petroleum gas or a combination of fuels. Seventy-nine women were recruited for the study between May and July 2004 in the first trimester of their pregnancy. Urine samples were collected from the subjects in the first, second and third trimesters for OH-PAH analyses. The concentrations of the OH-PAHs were compared across the type of fuel used for cooking and pregnancy trimesters. The relationships between OH-PAHs levels in the first trimester and concurrently measured personal exposures to PM2.5, carbon monoxide and nitrogen dioxide together with their indoor and outdoor air concentrations were also investigated. Women cooking with wood or kerosene had the highest creatinine adjusted OH-PAH concentrations compared with those using gas, coal briquette or a combination of fuels. Concentrations of creatinine adjusted 2-hydroxy-fluorene, 3-hydroxy-fluorene, 1-hydroxy-fluorene, 2-hydroxy-phenanthrene and 4-hydroxy-phenanthrene were significantly higher (p<0.05) in women who used wood or kerosene alone compared with women who used liquefied petroleum gas (LPG), coal briquette or a combination of fuels. An increase in the concentrations of creatinine adjusted 9-hydroxy-fluorene, 1-hydroxy-phenanthrene, 2-hydroxy-phenanthrene, 4-hydroxy-phenanthrene and 1-hydroxy-pyrene in the third trimesters was also observed. Weak positive correlation (Spearman correlation coefficient, ρ<0.4; p<0.05) was observed between all first trimester creatinine adjusted OH-PAHs and indoor (kitchen and living room), and personal 48-h TWA PM2.5. Women who cooked exclusively with wood or kerosene had higher creatinine adjusted OH-PAH levels in their urine samples compared to women who cooked with LPG or coal briquette.
Keywords: Indoor air pollution, Biomass, Gas, hydroxyl-substituted polycyclic aromatic, hydrocarbon, Pregnant women, Peru
1. Introduction
Indoor air pollution is a global problem (Zhang and Smith, 2003), and is responsible for 2.6% of the disease burden worldwide and nearly 3.6% in developing countries (Torres-Duque et al., 2008). One of the main sources of indoor air pollution, especially in developing countries, is household combustion of unprocessed biomass fuel. It is estimated that approximately 50% of the world’s population and nearly 90% of households in rural areas of developing countries still rely on wood, dung and crop residues as their source of energy (Torres-Duque et al., 2008); 1.6 million deaths are attributable to exposure to indoor smoke from combustion of biomass fuel each year and it ranks second among all environmental risk factors for the global burden of disease (Naeher et al., 2007; Perez-Padilla et al., 2010).
The most commonly used unprocessed biomass fuel is wood (Torres-Duque et al., 2008). Smoke generated by the combustion of wood and other biomass fuels contains many health damaging pollutants including particulate matter, carbon monoxide, aldehydes, nitrogen and sulfur oxides, and polycyclic aromatic hydrocarbons (PAH) (Joshi et al., 1989; Naeher et al., 2007; Venkataraman et al., 2002; Zelikoff et al., 2002; Zhang and Smith, 2007). Household biomass fuel combustion compared to the use of cleaner fuels such as gas could contribute significantly more to indoor levels of these pollutants, including PAHs (Bhargava et al., 2004; Hamada et al., 1991; Oanh and Dungs, 1999; Viau et al., 2000). The International Agency for Research on Cancer (IARC) classifies some of the individual PAHs as Class II carcinogens, and identifies benzo(a)pyrene as a Class I carcinogen (IARC, 2010; Straif et al., 2005). Additionally, PAHs have been associated with immunotoxicity (Laupeze et al., 2002; Oh et al., 2006).
Women and their young children in developing countries are especially vulnerable to exposure to indoor air pollution as they usually spend more time than men at home. Women are also more likely to do the cooking, and sometimes rely on less clean biomass fuels. Exposure to indoor air pollution due to household combustion of wood and other unprocessed biomass fuel has been associated with reduced birth weight, (Mishra et al., 2004; Pope et al., 2010) acute respiratory infection in children, (Mishra, 2003; Smith et al., 2011) and chronic obstructive pulmonary disease in women (Ekici et al., 2005; Orozco-Levi et al., 2006). The International Agency for Research on Cancer (IARC) has also classified emissions from household combustion of coal as carcinogenic to humans (Group 1 carcinogen) and emissions from household combustion of biomass as probably carcinogenic to humans (Group 2A) (IARC, 2010).
In this study, biomonitoring was used to characterize exposure to PAHs in pregnant women cooking with different fuels in Trujillo, Peru. PAHs are metabolized in the body to form hydroxylated metabolites (OH-PAHs) which are excreted in urine. OH-PAHs have been used as biomarkers of PAHs in different exposure situations (Gündel et al., 2000; Kuusimäki et al., 2004; Li et al., 2008; Toriba and Hayakawa, 2007), and 1-hydroxy-pyrene (1-PYR) has been used in some studies as a biomarker of exposure to PAHs in woodsmoke (Cavanagh et al., 2007; Kato et al., 2004; Viau et al., 2000).
In this study, the effect of the cooking fuel type on the pregnant women’s exposures to PAHs was assessed by measuring urinary 10 OH-PAHs, metabolites of naphthalene, fluorene, phenanthrene and pyrene, throughout the study participants’ gestation period. Additionally, indoor and ambient air, and personal exposure monitoring was done for air pollutants in order to determine if there was a relationship between indoor air quality and PAH metabolite concentrations.
2. Material and methods
This study was part of a larger study conducted by the University of Georgia focusing on the exposure of pregnant women to indoor air pollution. We selected pregnant women because a growing body of research indicates that the human fetus is vulnerable to air pollution (Bell et al., 2007; Bobak, 2000; Brauer et al., 2008; Choi et al., 2008; Makri and Stilianakis, 2008; Mishra et al., 2004; Parker et al., 2005; Šrám et al., 2005).
Each woman recruited for this study provided one urine sample for each trimester of pregnancy for the analysis of OH-PAHs and other biomarkers of exposure and nutrition. All biological samples were handled by local medical personnel trained by the Centers for Disease Control and Prevention’s National Center for Environmental Health laboratory (CDC/NCEH).
2.1. Study location and subject recruitment
The study was conducted in Trujillo, a coastal city of more than 800,000 and the capital of the La Libertad region of Peru. The study participants resided in seven districts within 10 miles of the city of Trujillo: Trujillo, La Esperanza, El Porvenir, Florencia de Mora, Moche, El Milagro, and Alto Trujillo. One hundred women were initially recruited from a target population of non-smoking women residing in Trujillo to participate in a larger indoor air exposure study. Seventy-nine of these women provided urine samples during the first trimester of their pregnancy between April and July of 2004, and were included in the urinary OH-PAH study. The women cooked exclusively with wood, kerosene, coal briquette or liquefied petroleum gas (LPG), or a combination of fuels. The study was approved by Institutional Review Boards from the University of Georgia, the Centers for Disease Control and Prevention (CDC), and health authorities at Trujillo City Hall. Informed consent was obtained from all subjects.
2.2. Questionnaire
Questionnaires, written and administered in Spanish, were used to determine residential characteristics and to obtain information on fuel type, socioeconomic status (SES), age, cooking characteristics such as cooking time and frequency, and possible confounding exposures. SES was determined based on observation of residential characteristics; subjects were grouped into three categories (poor, middle, and affluent). The questionnaires were administered immediately prior to air exposure monitoring in the homes and on the persons of the subjects in the first trimester.
2.3. Urine sample collection
Spot urine samples (50 mL) were collected from each study participant once each trimester. After collection, samples were aliquoted and shipped on dry ice to the CDC for analyses, and were then stored at −70 °C until analyzed. Any personal identifiable information was not available to CDC researchers.
2.4. Air pollution exposure monitoring
As part of the larger indoor air pollution study, measurements of daily ambient and indoor air pollution as well as personal exposure monitoring were conducted and completed within three days before the collection of urine samples in the first trimester. Details of the methodology for the personal exposure, indoor and ambient air monitoring will be presented elsewhere but are provided briefly below. Real-time CO was measured using Dräger Pac III single gas monitors (Draeger Safety Inc., Pittsburgh, PA) outfitted with CO sensors. The personal, indoor and ambient PM2.5, CO and OH-PAHs were analyzed in order to determine potential correlations.
2.4.1. Personal exposure monitoring
Personal exposures to carbon monoxide (CO), nitrogen dioxide (NO2) and particulate matter with an aerodynamic diameter less than or equal to 2.5 micrometers (PM2.5) were monitored over 48-h periods and completed within three days before the collection of urine samples in the first trimester. Time-integrated PM2.5 concentrations were measured gravimetrically. Particles were collected on 37 mm Teflon filters (Pall, 2.0 μm) loaded into Triplex PM2.5 cyclones (BGI, model SCC 1.062), and air was drawn using AirChek 2000 pumps (SKC Inc.) with flow rate set at 1.5 L/min. The weights of the particles were determined gravimetrically using the Cahn C-35 microbalance following the United States Environmental Protection Agency Guidance document (USEPA, 1998). CO was measured in real time using Dräger Pac III single gas monitors (Draeger Safety Inc., Pittsburgh, PA) outfitted with CO sensors. The datalogger of the instrument was set to record CO concentrations every 30 s. Time integrated NO2 personal exposure over the 48-h period was determined using Palmes Tubes. NO2 was determined spectrophotometrically (Milton Roy Company, Spectronic 20D). All the samplers for personal exposure monitoring were placed within the breathing zones of the subjects.
2.4.2. Indoor and ambient air monitoring
Concentrations of PM2.5, CO, and NO2 were measured in the kitchens and living rooms in the homes of the subjects over 48-h periods that were concurrent with the personal exposure monitoring. Ambient air concentrations of PM2.5 and NO2 were also measured over 48-h periods and concurrently with the personal exposure monitoring, on the roof of the City Hall in downtown Trujillo and at the local airport station nine miles outside of Trujillo. Additionally, volatile organic compounds (VOCs) were collected concurrently with other exposure monitoring in the kitchen and in ambient air at the two outdoor locations using passive diffusion stainless tubes (90 mm long, 6.3 mm OD and 5 mm ID, Perkin-Elmer) packed with Tenax™ TA (60/80 mesh, 200 mg) as an adsorbent. The samples were analyzed using an automated thermal desorption system (ATD400) coupled to a Hewlett-Packard model 5890A gas chromatograph/VG model Trio 1 mass spectrometer. Indoor and ambient air monitoring of PM2.5, CO, and NO2 were done using the same instruments and methods described for personal exposure monitoring, except that the monitors were mounted on stationary supports for the duration of measurements.
The indoor and ambient air and personal exposures to PM2.5, CO, NO2 were measured in order to determine their potential correlations with OH-PAH levels in urine.
2.5. Hydroxy-PAH analyses
Urine samples were analyzed for 10 OH-PAHs using gas chromatography/isotope dilution high resolution mass spectrometry (GC-IDHRMS) according to a previously reported method (Li et al., 2006). A mixture of 10 13C-labeled internal standards was spiked into 2-mL urine specimens prior to sample preparation. The methodology for measuring OH-PAH metabolites, present in human urine as glucuronide and/or sulfate conjugates, is based on enzymatic deconjugation of the samples to yield free OH-PAHs, followed by automated liquid-liquid extraction into pentene using the Gilson 215 Liquid Handler (Gilson Inc., Middleton, WI). The sample extracts were thereafter evaporated under a chemical fume hood. Finally, the extracts were re-constituted in toluene and derivatized to yield the trimethylsiloxane derivatives. Analytical determination of the target analytes were performed on a MAT95XL high resolution mass spectrometer (Thermo Scientific, Bremen, Germany) coupled with a 6890 gas chromatography (Agilent Technologies, Palo Alto, CA, USA). The quantification of the 10 target analytes was based on the use of their 13C-labeled internal standards to account for potential losses during samples preparation and instrument variation. Urinary creatinine was measured on a Roche Hitachi 912 Chemistry Analyzer (Hitachi Inc., Pleasanton, CA) by use of the Creatinine Plus Assay (Roche Diagnostics, Indianapolis, IN).
2.6. Statistical analyses
Creatinine adjusted concentrations (the weight of OH-PAH per unit weight of creatinine in urine: ng/g creatinine) of all the urinary OH-PAHs were calculated in order to correct for urine dilution. Statistical significance for all analyses was set at p<0.05. All analyses were done using SAS version 9.1 (Cary, NC).
Repeated measures analysis of covariance, using linear mixed effect models, was used to analyze the differences of OH-PAHs across trimesters and fuel types. The age of the subject, highest grade of education, SES and the district where the subject lived were included as covariates in each model. The term for fuel type-trimester interaction was not significant in any of the models and was dropped. The models allowed a general, unstructured within-subject variance-covariance matrix to allow for possible correlation and non-constant variance among each subject’s repeated measures across the three trimesters. The metabolite concentrations were log transformed before inclusion in the models, and concentrations below the limits of detection (LOD) were calculated as LOD divided by the square root of 2 (Hornung and Reed, 1990). Post-hoc pairwise comparisons of the means across fuel types, and across trimesters were done using Tukey’s honestly significant difference (HSD) procedure (Gravetter and Wallnau, 2008) in order to control for the strong familywise error rate.
The overall geometric mean for each creatinine adjusted OH-PAH was calculated based upon the fitted linear mixed effect model as follows. The model-based estimated log-concentration means for each cooking fuel type and trimester combination were obtained and then averaged over trimester and cooking fuel type to estimate the overall population mean OH-PAH level. This was done by taking an equally weighted average across trimester to estimate an average value over the entire course of pregnancy. However, averaging across cooking fuel types was done in proportion to the sample frequencies of subjects in each cooking fuel category. The resulting average of the log-concentration mean was then exponentiated to produce a geometric mean. This procedure is based upon a single model fit to data from all subjects, and thus produces covariate adjusted geometric means using all available data while avoiding the difficulty of computing separate covariate adjusted means from the data within each fuel type group, some of which are too small for group-specific analyses. Confidence intervals for the OH-PAH means were obtained by exponentiating the endpoints of the corresponding intervals for the averaged log-means.
Partial rank correlation was used to determine whether the first trimester urinary and creatinine adjusted OH-PAHs correlated with personal exposure to PM2.5, CO and NO2, their indoor and outdoor concentrations, and kitchen and ambient air VOC concentrations, and whether the OH-PAHs were correlated with each other. Fuel type, which had a significant effect on the concentrations of eight of the 10 metabolites, was controlled for in the partial correlation analyses.
3. Results
The 21 women who were recruited for the larger indoor air exposure study but did not participate in biomarker study were similar with regards to fuel type and education, but tended to be younger (age: 21.6 years; CLs: 18.9–24.4 years) compared to the women who were also enrolled in the biomarker study (age: 24.9 years; CLs: 23.3–26.6 years). Of the 79 women who enrolled, 64 further provided urine samples in the second trimester and 59 in the third. All the participants in the study were self-reported non-smokers. The average age (mean±SD) of all enrolled women was 26±6 years (range: 14 to 46 years). Most of the subjects (59%) used gas or combinations of fuels including gas for cooking, while 35% of the women uniquely used wood, kerosene or vegetable coal briquettes (Table 1). A majority (84%) of the women cooked at least twice daily with an average (mean±SD) cook time of 84±45 minn (range: 20 to 240 min). The detection frequency was over 95% except for 4-hydroxy-phenanthrene (4-PHE) and 3-hydroxy-fluorene (3-FLU) which were detected in 90% and 88% of the samples respectively.
Table 1.
Number and percentage of subjects stratified by demographic sub-categories. The average age was 26±6 years (range: 14–46).
| Demographic | N (%) |
|---|---|
| Highest education obtained | |
| Primary | 16 (20) |
| Secondary | 46 (58) |
| Superior | 17 (22) |
| Fuel type used for cooking | |
| Gas | 27 (34.1) |
| Wood | 12 (15.2) |
| Kerosene | 4 (5.1) |
| Coal briquette | 11 (13.9) |
| Combo with gas | 19 (24.0) |
| Combo without gas | 4 (5.1) |
| Electric | 1 (1.3) |
| Unknown | 1 (1.3) |
| Socioeconomic status | |
| Poor | 50 (63) |
| Middle | 27 (34) |
| Affluent | 2 (3) |
There were significant differences between cooking fuel types for creatinine adjusted OH-PAHs (Table 2). Women using wood or kerosene had significantly higher levels of creatinine adjusted 2- and 3-FLU and 1-, 2- and 4-PHE compared to women using gas, coal briquette or a combination of fuels. Additionally, the concentrations of creatinine adjusted OH-PAHs were significantly higher in subjects who used only wood as their cooking fuel than women using only gas, except for 1-hydroxy-naphthalene (1-NAP), or combinations of fuels, except for 1-NAP and 9-FLU; while women using only kerosene had significantly higher creatinine adjusted OH-PAHs compared to women using only gas except for 1-NAP and 1-PYR. None of the creatinine adjusted OH-PAHs were significantly different between women using wood and those using kerosene as their cooking fuel. However, only four (5%) subjects in the study used kerosene exclusively as their cooking fuel. Creatinine adjusted concentrations of 2-hydroxy-fluorene (2-FLU), 3-hydroxy-fluorene (3-FLU), 1-hydroxy-phenanthrene (1-PHE), 2-hydroxy-phenanthrene (2-PHE) and 4-hydroxy-phenanthrene (4-PHE) were significantly higher (p<0.05) in women that used wood or kerosene alone compared with women who used gas, coal briquette or combinations of fuels for cooking.
Table 2.
Geometric mean creatinine adjusted hydroxyl-PAH concentrations (ng/g creatinine) by fuel type adjusted for age of the subject, highest grade of education, SES and the district where the subject lived and trimester.
| Fuel | 1-NAP | 2-NAPa | 2-FLUb | 3-FLUb | 9-FLUc | 1-PHEb | 2-PHEb | 3-PHEd | 4-PHEb | 1-PYRe |
|---|---|---|---|---|---|---|---|---|---|---|
| Wood | 8841 | 8068 | 919 | 228 | 1897 | 1236 | 466 | 426 | 239 | 800 |
| UCL1 | 17594 | 14084 | 1427 | 381 | 3008 | 1842 | 731 | 656 | 392 | 1351 |
| LCL2 | 4443 | 4622 | 592 | 136 | 1196 | 829 | 296 | 276 | 146 | 474 |
| Kerosene | 7389 | 7576 | 1056 | 184 | 2874 | 1582 | 575 | 428 | 296 | 626 |
| UCL | 18535 | 15799 | 1882 | 364 | 5288 | 2669 | 1041 | 754 | 568 | 1249 |
| LCL | 2946 | 3633 | 593 | 93 | 1562 | 938 | 318 | 242 | 154 | 313 |
| Fuel combination with gas | 6275 | 5512 | 542 | 137 | 1355 | 737 | 290 | 260 | 131 | 476 |
| UCL | 11701 | 9217 | 816 | 221 | 2070 | 1068 | 440 | 388 | 205 | 766 |
| LCL | 3366 | 3296 | 361 | 85 | 887 | 509 | 191 | 174 | 83 | 295 |
| Fuel Combination without gas | 2441 | 3778 | 446 | 75 | 1142 | 599 | 256 | 176 | 109 | 282 |
| UCL | 6739 | 8496 | 843 | 158 | 2237 | 1066 | 493 | 329 | 223 | 605 |
| LCL | 884 | 1680 | 236 | 35 | 583 | 336 | 133 | 94 | 53 | 131 |
| Gas | 4864 | 3307 | 407 | 101 | 973 | 537 | 234 | 189 | 111 | 324 |
| UCL | 8212 | 5052 | 570 | 149 | 1383 | 728 | 330 | 262 | 161 | 482 |
| LCL | 2881 | 2165 | 291 | 68 | 684 | 397 | 166 | 136 | 76 | 218 |
| Coal briquette | 4412 | 3785 | 532 | 119 | 1373 | 777 | 282 | 236 | 142 | 477 |
| UCL | 9003 | 6750 | 840 | 203 | 2216 | 1176 | 451 | 370 | 237 | 819 |
| LCL | 2162 | 2122 | 337 | 70 | 850 | 513 | 176 | 150 | 85 | 278 |
Statistically significant differences at p=0.05 are lettered.
LCL: lower confidence limit.
UCL — upper confidence limit.
concentrations in wood significantly higher than only gas, coal briquette and combo without gas; and kerosene group significantly higher than only gas.
concentrations in kerosene and wood groups significantly higher than all other groups.
concentration in wood group significantly higher than only gas group; and kerosene group significantly higher than all other groups except wood group.
concentrations in wood group significantly higher than all other groups but not different from kerosene group; but kerosene group significantly higher than only gas, coal briquette and combo without gas and not combo with gas.
concentrations in wood group significantly higher than all other groups except kerosene group; but kerosene not significantly higher than any other group.
There were significant changes and an increase in the creatinine adjusted concentrations of 9-hydroxy-fluorene (9-FLU), 1-PHE, 2-PHE, 4-PHE and 1-hydroxy-pyrene (1-PYR) across the trimesters (Table 3) (p<0.05). The concentrations measured in the third trimester were significantly higher than those measured in the first and second trimesters for creatinine adjusted 9-FLU and 4-PHE, while they were significantly higher than those measured only in the first trimester for creatinine adjusted 1-PHE, 2-PHE and 1-PYR. The creatinine adjusted metabolite concentrations were not significantly affected by age, highest level of education and SES except for creatinine adjusted 1-PYR which was significantly affected by age. The concentrations were also not affected by the frequency of or time spent cooking, and this factor was not included in the final statistical models.
Table 3.
Geometric mean creatinine adjusted hydroxyl-PAH concentrations (ng/g creatinine) by trimester adjusted for age of the subject, highest grade of education, SES and the district where the subject lived and fuel type.
| Trimester | 1-NAP | 2-NAP | 2-FLU | 3-FLU | 9-FLUa,c | 1-PHEa | 2-PHEa | 3-PHE | 4-PHEa,c | 1-PYRa,b |
|---|---|---|---|---|---|---|---|---|---|---|
| First | 5556 | 4961 | 643 | 146 | 1338 | 730 | 284 | 271 | 133 | 381 |
| UCL1 | 9883 | 8027 | 943 | 228 | 1984 | 1038 | 420 | 396 | 203 | 593 |
| LCL2 | 3124 | 3066 | 438 | 94 | 902 | 514 | 192 | 186 | 87 | 245 |
| Second | 4845 | 4912 | 589 | 135 | 1387 | 824 | 316 | 256 | 153 | 469 |
| UCL | 8526 | 7783 | 845 | 206 | 2031 | 1140 | 459 | 366 | 229 | 718 |
| LCL | 2753 | 3100 | 410 | 88 | 947 | 595 | 218 | 180 | 102 | 306 |
| Third | 5444 | 5180 | 596 | 115 | 1809 | 998 | 403 | 276 | 196 | 570 |
| UCL | 9703 | 8378 | 881 | 180 | 2688 | 1417 | 597 | 404 | 300 | 890 |
| LCL | 3054 | 3202 | 404 | 73 | 1218 | 703 | 273 | 189 | 128 | 365 |
Statistically significant differences at p=0.05 are lettered.
third trimester significantly higher than first trimester.
second trimester significantly higher than first trimester.
third trimester significantly higher than second trimester.
LCL: lower confidence limit.
UCL — upper confidence limit.
The average levels of PM2.5, CO and NO2 at the various locations where they were measured during the first trimester are presented in Table 4. Weak positive correlation (Spearman correlation coefficient, ρ<0.4; p<0.05) was observed between all first trimester creatinine adjusted OH-PAHs and indoor (kitchen and living room), and personal 48-h TWA PM2.5. Most of the first trimester creatinine adjusted OH-PAHs were not significantly correlated with outdoor PM2.5 (Table 5). There was also moderate correlation (ρ>0.4 but<0.6; p<0.05) between some of the metabolites in the first trimester and personal exposure to CO as indicated by peak exposure to CO. Non-significant correlation was observed between the metabolites and indoor, outdoor and personal exposure to NO2. Creatinine adjusted OH-PAHs, were highly correlated with each other during each trimester (ρ was mostly>0.7; p<0.05). The results for the first trimester are presented in Table 6.
Table 4.
Kitchen and living room concentrations and personal exposures to CO, NO2 and PM2.5.
| Pollutant | Sampling | N | Average | Standard deviation | Min | Max |
|---|---|---|---|---|---|---|
| CO (48 h) (ppm) | Kitchen | 95 | 3.4 | 6.7 | 0.0 | 46.9 |
| Personal | 98 | 1.1 | 1.7 | 0.0 | 8.0 | |
| Living Room | 90 | 0.6 | 1.3 | 0.0 | 6.5 | |
| NO2 (48 h) (ppb) | Kitchen | 93 | 18.4 | 17.5 | 0.0 | 91.3 |
| Personal | 92 | 10.4 | 8.8 | 0.0 | 48.1 | |
| Living Room | 88 | 9.4 | 8.6 | 0.0 | 45.7 | |
| City Hall | 52 | .7.9 | 5.1 | 1.7 | 21.0 | |
| Airport | 52 | 2.8 | 2.4 | 0.5 | 8.2 | |
| PM2.5 (48 h) (μg/m3) | Kitchen | 97 | 92.1 | 118.8 | 1.4 | 665.2 |
| Personal | 93 | 122.8 | 135.2 | 6.3 | 1101.5 | |
| Living Room | 88 | 48.2 | 44.6 | 1.0 | 414.8 | |
| City Hall | 52 | 29.3 | 9.1 | 15.5 | 50.2 | |
| Airport | 49 | 27.0 | 12.0 | 5.5 | 64.9 |
Table 5.
Spearman correlation coefficients between first trimester creatinine adjusted hydroxy-PAH concentration and PM2.5 48 h time weighed average (μg/m3) and carbon monoxide 48 h time weighed average and maximum concentration (ppm).
| Compound | 1-NAP | 2-NAP | 2-FLU | 3-FLU | 9-FLU | 1-PHE | 2-PHE | 3-PHE | 4-PHE | 1-PYR |
|---|---|---|---|---|---|---|---|---|---|---|
| Particulate matter <2.5 μ (PM2.5) (μg/m3) | ||||||||||
| Kitchen1 | 0.11 | 0.22 | 0.32 | 0.29 | 0.32 | 0.28 | 0.39 | 0.31 | 0.32 | 0.30 |
| Living room1 | 0.17 | 0.30 | 0.35 | 0.30 | 0.40 | 0.31 | 0.46 | 0.36 | 0.40 | 0.36 |
| Personal1 | 0.27 | 0.28 | 0.31 | 0.34 | 0.35 | 0.27 | 0.34 | 0.33 | 0.31 | 0.40 |
| City Hall1 | 0.11 | 0.17 | 0.15 | 0.14 | 0.23 | 0.08 | 0.15 | 0.14 | 0.12 | 0.14 |
| Trujillo Airport1 | 0.03 | 0.26 | 0.16 | 0.08 | 0.19 | 0.06 | 0.21 | 0.18 | 0.15 | 0.16 |
| Carbon monoxide (CO) (ppm) | ||||||||||
| Kitchen1 | 0.14 | 0.04 | 0.21 | 0.21 | 0.16 | 0.26 | 0.26 | 0.19 | 0.25 | 0.21 |
| Kitchen2 | 0.11 | 0.06 | 0.29 | 0.31 | 0.16 | 0.30 | 0.31 | 0.30 | 0.30 | 0.31 |
| Living room1 | 0.01 | 0.03 | 0.11 | 0.07 | 0.22 | 0.17 | 0.18 | 0.16 | 0.17 | 0.16 |
| Living room2 | −0.00 | 0.01 | 0.05 | 0.01 | 0.17 | 0.12 | 0.13 | 0.10 | 0.13 | 0.14 |
| Personal1 | −0.04 | 0.05 | 0.31 | 0.20 | 0.30 | 0.21 | 0.26 | 0.21 | 0.25 | 0.15 |
| Personal2 | 0.25 | 0.32 | 0.51 | 0.50 | 0.48 | 0.46 | 0.48 | 0.46 | 0.46 | 0.36 |
Correlation coefficients that are significant at p=0.05 and are in bold fonts and those significant at p=0.1 (p>0.05 and <0.1) are in bold italicized fonts.
48 h time weighted average concentration of PM2.5 or CO in kitchen, living room, at city hall, Trujillo Airport, and 48 h time weighted average personal exposure to pollutant.
Maximum real time CO concentrations in the kitchen, living room, and maximum real time personal exposure to CO.
Table 6.
Correlation between creatinine adjusted hydroxy-PAHs during the first trimester.
| 1-NAP | 2-NAP | 2-FLU | 3-FLU | 9-FLU | 1-PHE | 2-PHE | 3-PHE | 4-PHE | 1-PYR | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1-NAP | 1.00 | 0.68 | 0.70 | 0.67 | 0.62 | 0.60 | 0.58 | 0.61 | 0.61 | 0.55 |
| 2-NAP | 1.00 | 0.69 | 0.64 | 0.64 | 0.66 | 0.65 | 0.64 | 0.65 | 0.55 | |
| 2-FLU | 1.00 | 0.90 | 0.84 | 0.88 | 0.90 | 0.92 | 0.87 | 0.80 | ||
| 3-FLU | 1.00 | 0.70 | 0.82 | 0.81 | 0.90 | 0.79 | 0.83 | |||
| 9-FLU | 1.00 | 0.82 | 0.90 | 0.84 | 0.90 | 0.76 | ||||
| 1-PHE | 1.00 | 0.89 | 0.89 | 0.86 | 0.82 | |||||
| 2-PHE | 1.00 | 0.94 | 0.94 | 0.85 | ||||||
| 3-PHE | 1.00 | 0.90 | 0.89 | |||||||
| 4-PHE | 1.00 | 0.81 | ||||||||
| 1-PYR | 1.00 |
All correlation coefficients are significant at p=0.05 and are in bold fonts.
4. Discussion
Biomass smoke contains many hazardous pollutants including PAHs which are produced as a result of incomplete combustion. Despite the high prevalence of indoor/residential combustion of biomass for cooking and heating in developing countries where inefficient stoves are often used, there is limited information about exposure to PAHs in these conditions. A few studies have characterized the risk of exposure to PAHs due to residential combustion of different biomass fuels by measuring PAH levels at breathing zone height in cooking environments (Bhargava et al., 2004; Hamada et al., 1991; Oanh and Dungs, 1999; Pandit et al., 2001) and under experimental conditions (Oanh et al., 2002) and report that various PAHs are elevated during the combustion of wood, kerosene, cow dung and coal briquette. Results of biomarker studies have shown that people exposed to smoke from biomass combustion have elevated levels of hydroxy-substituted PAHs in urine (Cavanagh et al., 2007; Kato et al., 2004; Li et al., 2011; Riojas-Rodriguez et al., 2011; Viau et al., 2000), and receive a significant PAH exposure from biomass smoke (Li et al., 2011; Riojas-Rodriguez et al., 2011). Reductions in urinary two- to four-ring OH-PAHs after the installation of improved woodstoves ranged from 19% to 52% in women participating in woodstove intervention programs in the Santiago de Chuco Province in Peru (Li et al., 2011), and from 20% to 42% in women participating in a similar program in the state of Michoacan in Mexico (Riojas-Rodriguez et al., 2011). In this study, we investigated exposure to PAHs among pregnant women who use uniquely one or combinations of fuel types for cooking and heating.
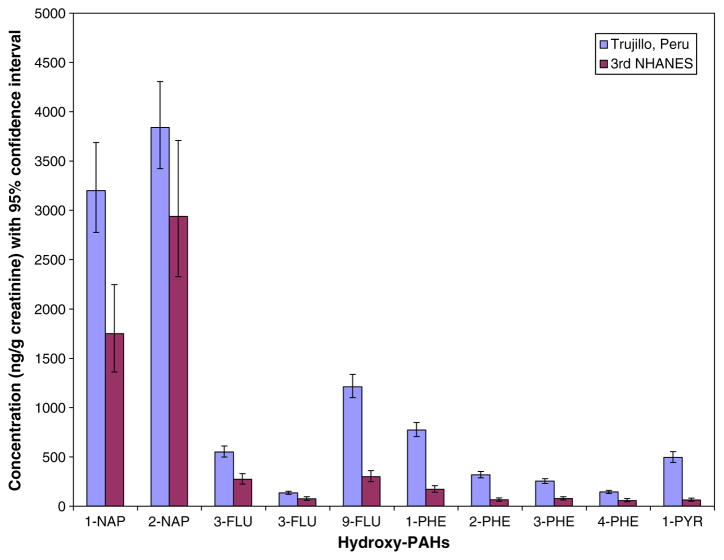
The higher levels of creatinine adjusted OH-PAHs of women in this study compared to those measured among pregnant women in the US population (Fig. 1) indicate higher levels of exposure to PAHs in their environment. Geometric mean creatinine adjusted OH-PAHs for all pregnant women in this study was at least 1.3 times higher than those measured in pregnant women extracted from the Centers for Disease Prevention and Control’s (CDC) 3rd National Health and Nutrition Examination Survey (NHANES) report (NCEH) database, and was up to 8 times higher in the case of 1-PYR. The concentration of 1-PYR (800 ng/g creatinine; CLs: 474, 1351) in women that reported cooking exclusively with wood alone in the study was 12 times higher than for pregnant women in the NHANES database. Although indoor exposure to emissions from combustion of biomass fuel may explain some of the differences compared to concentrations measured in the US population, it is not certain that it is the only contributory factor. Women who used gas alone still had substantially (~5 times; 324 ng/g creatinine; CLs: 218, 482) higher levels compared to the pregnant women in the NHANES study. Other factors such as diet could be important (Falco et al., 2003; Scherer et al., 2000). Pregnant women in this study had higher levels of creatinine adjusted OH-PAHs compared to levels observed in charcoal workers in Brazil (Kato et al., 2004), but are comparable to those observed in non-smoking women in a city in the industrial Ruhr Valley in Germany (Gündel et al., 1996). Concentration of creatinine adjusted 1-PYR was higher in female cooks who worked in a mill in China and cooked with wood, coal briquette or LPG (~2 times higher) (Chen et al., 2007), and in a rural Burundi population exposed to indoor biomass smoke mainly from the combustion of wood (~3.5 times higher) (Viau et al., 2000) compared to the pregnant women in this study. Concentrations of creatinine adjusted two- to four ring OH-PAHs (same as measured in this study) were also higher in women participating in woodstove intervention programs in the Santiago de Chuco Province in Peru: approximately 1.5 to 5 times higher before, and 1.1 to 3.5 times higher after the installation of improved woodstoves (Li et al., 2011). Concentrations were higher by similar magnitudes in women participating in a woodstove intervention program in Mexico (Riojas-Rodriguez et al., 2011).
Fig. 1.
Comparison of geometric mean creatinine adjusted OH-PAHs in pregnant women in Trujillo Peru with pregnant women in the 3rd United States National Health and Nutrition Examination Survey (NHANES) Report.
Creatinine adjusted OH-PAHs were correlated with 48-h TWA indoor and personal exposure to PM2.5 measured in the kitchen and the living room, but not ambient air levels measured at Trujillo city hall and airport. Some of the metabolites in the first trimester also were moderately correlated with peak personal exposure to CO. These significant correlations indicate that exposure to PAHs were associated with indoor sources. Contrary to expectation, there was no association between concentrations of OH-PAHs and the frequency of or time spent cooking. Such associations may have been dampened by other factors including the amount of time spent indoors, individual behaviors during cooking, and the quality of ventilation in the homes. There was also no association between the PAH metabolites and qualitative second hand smoke (SHS) exposure. Only 11% of the participants reported that a smoker lived in their homes, however, only two subjects reported that the smoker smoked one or more cigarettes per day. Hence, it is assumed that the magnitude of SHS exposure is low in our cohort.
The results show that women who used wood or kerosene alone have higher exposure to PAHs. The creatinine adjusted OH-PAHs in their urine were higher than those measured in women who used gas, coal briquette or a combination of fuels. However, there was no difference in metabolite concentrations between women who used wood and those that used kerosene. Other factors such as cooking methods, ventilation and the nature of the food being cooked could affect the amount of PAHs released during cooking (Chen et al., 2007; See et al., 2006; Zhu and Wang, 2003), and these may also partly account for why differences were not observed among women who used gas, coal briquette, or a combination of fuels.
Significant increases in the concentrations of urinary and creatinine adjusted 9-FLU, 1-PYR and the OH-PHEs except 3-PHE were observed from the first trimester to the third trimester. Third trimester concentrations for all five metabolites were significantly higher than those measured in the first trimester. It is unlikely that weather is responsible for this observation. Recruitment for the study and the collection of first trimester urine samples were done during the winter season in Trujillo, Peru when biomass combustion is used for heating and ventilation is reduced to maximize heat retention. The observed increase may reflect less mobility and/or suggest pregnant women spending more time indoors at later stage of pregnancy, and/or possibly a change in cooking habits which may enhance PAH emission and exposure. It is however difficult to explain this trend or determine if it is truly representative of actual exposure across pregnancy stages as only one single urine sample per subject was collected per trimester, and no air monitoring took place during the second or third trimesters.
The lack of fuel type/trimester interaction suggests a uniform increase in exposure during pregnancy across the fuel types. The increase in the metabolite concentrations during gestation may reflect an increase in exposure to PAHs, and may be important because of evidences that pre-natal exposure to PAH could influence birth outcomes and childhood mental development (Choi et al., 2008; Laupeze et al., 2002; Mishra et al., 2004). However, a study based on the collection of urine samples throughout the gestation period, and using a more effective method such as specific gravity adjustment for adjusting for urine dilution, is the only way to verify whether the observed increase represents actual changes in exposure across pregnancy stages. Creatinine adjustment may be inadequate as creatinine excretion is known to increase by 30% during the course of pregnancy (Adibi et al., 2008; Williams, 2005), and could have introduced some errors into the results, especially with regards to the changes of creatinine adjusted concentrations of OH-PAHs across the pregnancy trimesters. We also observed a non-significant increase of about 15% from the first to the second trimester which stabilized in the third trimester in our study subjects. However, creatinine adjustment would only have biased the results away from the increase of creatinine adjusted OH-PAHs across the trimesters. Expectedly, increases of wet weight OH-PAH concentrations (not adjusted for creatinine) across the trimesters were more pronounced than those observed for the creatinine adjusted concentrations. The interaction term between fuel type and pregnancy trimester was not significant for any of the OH-PAHs indicating consistently higher levels across trimesters of hydroxyl substituted phenanthrene and pyrene, 2-FLU and 3-FLU in women cooking with wood compared with those cooking with gas, coal briquette or a combination of fuels.
Other limitations of the study included the small number of subjects per fuel type investigated that were further decreased by subjects who did not complete the 3rd trimester urine collection (n=20). This may have reduced the ability to detect differences between fuel types and across trimester for some of the metabolites, and also the ability to determine the interaction between these two factors. Only one spot urine sample per trimester per subject was used to estimate OH-PAH concentrations in this study. Therefore, exposure misclassification could have occurred due to the relatively high intra-individual variability (time-to-time within-person variation) that is associated with creatinine adjusted OH-PAHs concentrations in urine, which could be due to exposures to different sources of PAHs (Li et al., 2010). Although possible confounding factors such as cooking methods and the nature of the food being cooked were not taken into consideration, the sample was culturally homogenous. Difference in socioeconomic status was small and was adjusted for in the statistical models. Indoor and outdoor air and personal exposure monitoring and collection of questionnaire data were only done once during the first trimester when there was adequate number of researchers in the field. No measurement of ambient and/or personal PAH exposure was done in this study, and comparisons could only be made with related environmental markers but not the pollutants which are direct precursors of OH-PAHs. Measurement of ambient and personal PAH exposure in future studies would improve the characterization of the major sources contributing to PAH exposure in pregnant women.
5. Conclusion
In conclusion, women in this study had elevated levels of creatinine adjusted OH-PAHs in their urine compared to pregnant women in the 3rd National Health and Nutrition Examination Survey in the United States. We found that concentrations of creatinine adjusted OH-PAH levels measured in pregnant women in Peru were significantly affected by the type of fuel they used for cooking. Women who cooked exclusively with wood seemed to have higher exposures to PAHs, as they also had higher concentrations of creatinine adjusted OH-PAHs compared with women who cooked with gas, coal briquette or a combination of fuels. Creatinine adjusted 9-FLU, 1-, 2-, and 4-PHE, and 1-PYR increased significantly from the first to the third trimester. While a real change across trimester in exposure to polycyclic aromatic hydrocarbon may be important for the developing fetus, and reflect changes in the behavior and cooking habits of the mother during pregnancy, results in this study could have been affected by changes in creatinine excretion that normally occur during pregnancy.
Acknowledgments
Role of funding source
The authors thank the International Society of Exposure Science (ISES) [formerly International Society of Exposure Assessment (ISEA)] and the American Chemistry Council (ACC) for funding support for this study through the ISEA Young Investigator Award to Dr. Luke P. Naeher. The organizations were not involved in the study design; collection, analyses and interpretation of data; the writing of this manuscript; nor the decision to submit it to Environment International.
The authors thank Ing. Jorge Murgia and Trujillo City Hall for their invaluable support and assistance with this study. We also thank the women from Trujillo, Peru who participated in this study.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention (CDC). The use of trade names and commercial sources is for identification only and does not constitute endorsement by the US Department of Health and Human Services or CDC.
Conflict of interest statement
The authors declare no conflict of interest.
References
- Adibi JJ, Whyatt RM, Williams PL, Calafat AM, Camann D, Herrick R, et al. Characterization of phthalate exposure among pregnant women assessed by repeat air and urine samples. Environ Health Perspect. 2008;116(4):467–73. doi: 10.1289/ehp.10749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML, Ebisu K, Belanger K. Ambient air pollution and low birth weight in Connecticut and Massachusetts. Environ Health Perspect. 2007;115(7):1118–24. doi: 10.1289/ehp.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava A, Khanna RN, Bhargava SK, Kumar S. Exposure risk to carcinogenic PAHs in indoor-air during biomass combustion whilst cooking in rural India. Atmos Environ. 2004;38(28):4761–7. [Google Scholar]
- Bobak M. Outdoor air pollution, low birth weight, and prematurity. Environ Health Perspect. 2000;108(2):173–6. doi: 10.1289/ehp.00108173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer M, Lencar C, Tamburic L, Koehoorn M, Demers P, Karr C. A cohort study of traffic-related air pollution impacts on birth outcomes. Environ Health Perspect. 2008;116(5):680–6. doi: 10.1289/ehp.10952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JAE, Brown L, Trought K, Kingham S, Epton MJ. Elevated concentrations of 1-hydroxypyrene in schoolchildren during winter in Christchurch, New Zealand. Sci Total Environ. 2007;374(1):51–9. doi: 10.1016/j.scitotenv.2006.11.042. [DOI] [PubMed] [Google Scholar]
- Chen B, Hu Y, Jin T, Zheng L, Wang Q, Shen Y, et al. Higher urinary 1-hydroxypyrene concentration is associated with cooking practice in a Chinese population. Toxicol Lett. 2007;171(3):119–25. doi: 10.1016/j.toxlet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Choi H, Rauh V, Garfinkel R, Tu Y, Perera FP. Prenatal exposure to airborne polycyclic aromatic hydrocarbons and risk of intrauterine growth restriction. Environ Health Perspect. 2008;116(5):658–65. doi: 10.1289/ehp.10958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekici A, Ekici M, Kurtipek E, Akin A, Arslan M, Kara T, et al. Obstructive airway diseases in women exposed to biomass smoke. Environ Res. 2005;99(1):93–8. doi: 10.1016/j.envres.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Falco G, Domingo JL, Llobet JM, Teixido A, Casas C, Muller L. Polycyclic aromatic hydrocarbons in foods: human exposure through the diet in Catalonia, Spain. J Food Prot. 2003;66(12):2325–31. doi: 10.4315/0362-028x-66.12.2325. [174] [DOI] [PubMed] [Google Scholar]
- Gravetter FJ, Wallnau LB. Essentials of statistics for the behavioral science. 6. Belmont: Thomson Wadsworth; 2008. [Google Scholar]
- Gündel J, Mannschreck C, Büttner K, Ewers U, Angerer J. Urinary levels of 1-hydroxypyrene, 1-, 2-, 3-, and 4-hydroxyphenanthrene in females living in an industrial area of Germany. Arch Environ Contam Toxicol. 1996;31(4):585–90. doi: 10.1007/BF00212444. [DOI] [PubMed] [Google Scholar]
- Gündel J, Schaller KH, Angerer J. Occupational exposure to polycyclic aromatic hydrocarbons in a fireproof stone producing plant: biological monitoring of 1-hydroxypyrene, 1-, 2-, 3- and 4-hydroxyphenanthrene, 3-hydroxybenz (a) anthracene and 3-hydroxybenzo (a) pyrene. Int Arch Occup Environ Health. 2000;73(4):270–4. doi: 10.1007/s004200050427. [DOI] [PubMed] [Google Scholar]
- Hamada GS, Kowalski LP, Murata Y, Matsushita H, Matsuki H. Wood stove effects on indoor air quality in Brazilian homes: carcinogens, suspended particulate matter, and nitrogen dioxide analysis. Tokai J Exp Clin Med. 1991;17(3):145–53. [PubMed] [Google Scholar]
- Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5(1):46–51. [Google Scholar]
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 95. Lyon: International Agency for Research on Cancer; 2010. Household use of solid fuels and high-temperature frying. [PMC free article] [PubMed] [Google Scholar]
- Joshi V, Venkataraman C, Ahuja DR. Emissions from burning biofuels in metal cook-stoves. Environ Manage. 1989;13(6):763–72. [Google Scholar]
- Kato M, Loomis D, Brooks LM, Gattas GFJ, Gomes L, Carvalho AB, et al. Urinary biomarkers in charcoal workers exposed to wood smoke in Bahia State, Brazil. Cancer Epidemiol Biomarkers Prev. 2004;13(6):1005–12. [PubMed] [Google Scholar]
- Kuusimäki L, Peltonen Y, Mutanen P, Peltonen K, Savela K. Urinary hydroxy-metabolites of naphthalene, phenanthrene and pyrene as markers of exposure to diesel exhaust. Int Arch Occup Environ Health. 2004;77(1):23–30. doi: 10.1007/s00420-003-0477-y. [DOI] [PubMed] [Google Scholar]
- Laupeze B, Amiot L, Sparfel L, Le Ferrec E, Fauchet R, Fardel O. Polycyclic aromatic hydrocarbons affect functional differentiation and maturation of human monocyte-derived dendritic cells 1. J Immunol. 2002;168(6):2652–8. doi: 10.4049/jimmunol.168.6.2652. [DOI] [PubMed] [Google Scholar]
- Li Z, Romanoff LC, Trinidad DA, Hussain N, Jones RS, Porter EN, et al. Measurement of urinary monohydroxy polycyclic aromatic hydrocarbons using automated liquid–liquid extraction and gas chromatography/isotope dilution high-resolution mass spectrometry. Anal Chem. 2006;78(16):5744–51. doi: 10.1021/ac0606094. [DOI] [PubMed] [Google Scholar]
- Li Z, Sandau CD, Romanoff LC, Caudill SP, Sjodin A, Needham LL, et al. Concentration and profile of 22 urinary polycyclic aromatic hydrocarbon metabolites in the US population. Environ Res. 2008;107(3):320–31. doi: 10.1016/j.envres.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Li Z, Romanoff LC, Lewin MD, Porter EN, Trinidad DA, Needham LL, et al. Variability of urinary concentrations of polycyclic aromatic hydrocarbon metabolite in general population and comparison of spot, first-morning, and 24-h void sampling. J Exposure Sci Environ Epidemiol. 2010;20:526–35. doi: 10.1038/jes.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Sjodin A, Romanoff LC, Horton K, Fitzgerald CL, Eppler A, et al. Evaluation of exposure reduction to indoor air pollution in stove intervention projects in Peru by urinary biomonitoring of polycyclic aromatic hydrocarbon metabolites. Environ Int. 2011;37(7):1157–63. doi: 10.1016/j.envint.2011.03.024. [DOI] [PubMed] [Google Scholar]
- Makri A, Stilianakis NI. Vulnerability to air pollution health effects. Int J Hyg Environ Health. 2008;211(3–4):326–36. doi: 10.1016/j.ijheh.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Mishra V. Indoor air pollution from biomass combustion and acute respiratory illness in preschool age children in Zimbabwe. Int J Epidemiol. 2003;32(5):847–53. doi: 10.1093/ije/dyg240. [DOI] [PubMed] [Google Scholar]
- Mishra V, Dai X, Smith KR, Mika L. Maternal exposure to biomass smoke and reduced birth weight in Zimbabwe. Ann Epidemiol. 2004;14(10):740–7. doi: 10.1016/j.annepidem.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Naeher LP, Brauer M, Lipsett M, Zelikoff JT, Simpson CD, Koenig JQ, et al. Woodsmoke health effects: a review. Inhal Toxicol. 2007;19(1):67–106. doi: 10.1080/08958370600985875. [DOI] [PubMed] [Google Scholar]
- Oanh NTK, Dungs NT. Emission of polycyclic aromatic hydrocarbons and particulate matter from domestic combustion of selected fuels. Environ Sci Technol. 1999;33(16):2703–9. [Google Scholar]
- Oanh NTK, Nghiem LH, Phyu YL. Emission of polycyclic aromatic hydrocarbons, toxicity, and mutagenicity from domestic cooking using sawdust briquettes, wood, and kerosene. Environ Sci Technol. 2002;36(5):833–9. doi: 10.1021/es011060n. [DOI] [PubMed] [Google Scholar]
- Oh E, Im H, Kang HS, Jung W, Won NH, Lee E, et al. Comparison of immunnological and genotoxicological parameters in automobile emission inspectors exposed to polycyclic aromatic hydrocarbons. Environ Toxicol Pharmacol. 2006;21(1):108–17. doi: 10.1016/j.etap.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Orozco-Levi M, Garcia-Aymerich J, Villar J, Ramirez-Sarmiento A, Anto JM, Gea J. Wood smoke exposure and risk of chronic obstructive pulmonary disease. Eur Respir J. 2006;27(3):542–6. doi: 10.1183/09031936.06.00052705. [DOI] [PubMed] [Google Scholar]
- Pandit GG, Srivastava PK, Mohan Rao AM. Monitoring of indoor volatile organic compounds and polycyclic aromatic hydrocarbons arising from kerosene cooking fuel. Sci Total Environ. 2001;279(1–3):159–65. doi: 10.1016/s0048-9697(01)00763-x. [DOI] [PubMed] [Google Scholar]
- Parker JD, Woodruff TJ, Basu R, Schoendorf KC. Air pollution and birth weight among term infants in California. Pediatr. 2005;115(1):121–8. doi: 10.1542/peds.2004-0889. [DOI] [PubMed] [Google Scholar]
- Perez-Padilla R, Schilmann A, Riojas-Rodriguez H. Respiratory health effects of indoor air pollution. Int J Tuberc Lung Dis. 2010;14(9):1079–86. [PubMed] [Google Scholar]
- Pope DP, Mishra V, Thompson L, Siddiqui AR, Rehfuess EA, Weber MM, et al. Risk of low birth weight and stillbirth associated with indoor air pollution from solid fuel use in developing countries. Epidemiol Rev. 2010;32(1):70–81. doi: 10.1093/epirev/mxq005. [DOI] [PubMed] [Google Scholar]
- Riojas-Rodriguez H, Schilmann A, Marron-Mares AT, Masera O, Li Z, Romanoff L, et al. Impact of the improved Patsari biomass stove on urinary polycyclic aromatic hydrocarbons biomarkers and carbon monoxide exposures in rural Mexican women. Environ Health Perspect. 2011;119(9):1301–7. doi: 10.1289/ehp.1002927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer G, Frank S, Riedel K, Meger-Kossien I, Renner T. Biomonitoring of exposure to polycyclic aromatic hydrocarbons of nonoccupationally exposed persons. Cancer Epidemiol Biomarkers Prev. 2000;9(4):373–80. [PubMed] [Google Scholar]
- See SW, Karthikeyan S, Balasubramanian R. Health risk assessment of occupational exposure to particulate-phase polycyclic aromatic hydrocarbons associated with Chinese, Malay and Indian cooking. J Environ Monit. 2006;8(3):369–76. doi: 10.1039/b516173h. [DOI] [PubMed] [Google Scholar]
- Smith KR, McCracken JP, Webber MW, Habil M, Hubbard A, Jenny A, et al. Effect of reduction in household air pollution on childhood pneumonia in Guatemala (RESPIRE): a randomized controlled trial. Lancet. 2011;378:1717–26. doi: 10.1016/S0140-6736(11)60921-5. [DOI] [PubMed] [Google Scholar]
- Šrám RJ, Binková B, Dejmek J, Bobak M. Ambient air pollution and pregnancy outcomes: a review of the literature. Environ Health Perspect. 2005;113(4):375–82. doi: 10.1289/ehp.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straif K, Baan R, Grosse Y, Secretan B, El Ghissassi F, Cogliano V. Carcinogenicity of poly-cyclic aromatic hydrocarbons. Lancet. 2005;6(12):931–2. doi: 10.1016/s1470-2045(05)70458-7. [DOI] [PubMed] [Google Scholar]
- Toriba A, Hayakawa K. Biomarkers of exposure to polycyclic aromatic hydrocarbons and related compounds. J Health Sci. 2007;53(6):631–8. [Google Scholar]
- Torres-Duque C, Maldonado D, Perez-Padilla R, Ezzatti M. Biomass and fuels and respiratory diseases: a review of the evidence. Proc Am Thorac Soc. 2008;5(5):577–90. doi: 10.1513/pats.200707-100RP. [DOI] [PubMed] [Google Scholar]
- USEPA. Quality assurance guidance document; method compendium; field standard operating procedures for the PM2.5 performance evaluation program. Research Triangle Park: United States Environmental Protection Agency; 1998. [Google Scholar]
- Venkataraman C, Negi G, Brata Sardar S, Rastogi R. Size distributions of polycyclic aromatic hydrocarbons in aerosol emissions from biofuel combustion. J Aerosol Sci. 2002;33(3):503–18. [Google Scholar]
- Viau C, Hakizimana G, Bouchard M. Indoor exposure to polycyclic aromatic hydrocarbons and carbon monoxide in traditional houses in Burundi. Int Arch Occup Environ Health. 2000;73(5):331–8. doi: 10.1007/s004209900112. [DOI] [PubMed] [Google Scholar]
- Williams J. Williams obstetrics. New York: McGraw-Hill; 2005. [Google Scholar]
- Zelikoff JT, Chen LC, Cohen MD, Schlesinger RB. The toxicology of inhaled woodsmoke. J Toxicol Environ Health. 2002;5(3):269–82. doi: 10.1080/10937400290070062. [DOI] [PubMed] [Google Scholar]
- Zhang J, Smith KR. Indoor air pollution: a global health concern. Br Med Bull. 2003;68(1):209–25. doi: 10.1093/bmb/ldg029. [DOI] [PubMed] [Google Scholar]
- Zhang JJ, Smith KR. Household air pollution from coal and biomass fuels in China: measurements, health impacts, and interventions. Environ Health Perspect. 2007;115(6):848–55. doi: 10.1289/ehp.9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Wang J. Sources and patterns of polycyclic aromatic hydrocarbons pollution in kitchen air, China. Chemosphere. 2003;50(5):611–8. doi: 10.1016/s0045-6535(02)00668-9. [DOI] [PubMed] [Google Scholar]