Abstract
In this perspective, we summarize and discuss critical advancements in the study of 4-hydroxy-2-nonenal (4-HNE) as it relates to diseases and clinical complications either caused or exacerbated by oxidative stress. Since its identification in 1980, 4-HNE has been extensively studied with an emphasis on its formation, its role in pathology, and its targets. As a reactive aldehyde, and a product of lipid peroxidation, studies corroborate its ability to disrupt signal transduction and protein activity, as well as induce inflammation and trigger cellular apoptosis in conditions of oxidative stress. Notably, we discuss the role of natural enzymes involved in the regulation of 4-HNE, and how they can be applied to its detoxification in various physiological conditions.
Keywords: 4-HNE, ROS, oxidative stress, ALDH2, mitochondria
oxidative stress is a common complication of numerous pathologies including cancer, diabetes, and Alzheimer’s disease, and is occasionally the outcome of oxygen therapy when administered at concentrations of FiO2 > 0.8 (13, 21). Furthermore, it is well documented and widely known that oxidative stress is a precursor to elevated levels of reactive oxygen species (ROS) (19, 29). Low levels of ROS play a fundamental role in redox signaling, and are endogenously produced primarily in the mitochondria but also in the endoplasmic reticulum, plasma membrane, and peroxisomes (1, 15, 24). Specifically, in the mitochondria, ROS are known to be generated by monoamine oxidase (MAO), complex I, and complex III, and in the cytosol by NADPH oxidase (NOX) and xanthine oxidase (XO) (23, 28, 29). Under normal physiological conditions, ROS mediate several responses including cell differentiation, migration, and proliferation (19). Conversely, elevated levels of ROS serve as a marker for oxidative stress and are associated with lipid peroxidation and imbalance of the redox system (1, 15). Just as ROS are inevitable by-products of oxidative stress, so are secondary intermediates such as 4-hydroxy-2-nonenal (4-HNE) and malondialdehyde (MDA) (27). As a product of lipid peroxidation, 4-HNE is also considered a biomarker of oxidative stress and is identified as one of the most formidable reactive aldehydes (13, 27).
4-HNE is known to be quite reactive; it participates in multiple physiological processes as a nonclassical secondary messenger and readily forms covalent modifications of numerous targets (1, 15, 16). The majority of recent research has made a considerable shift from determining the mechanisms of formation and analysis, to determining mechanisms of 4-HNE detoxification and identification of its protein targets (1). While 4-HNE adduction can result in either cell survival or death based on cell type, the presence of 4-HNE in disease pathology is notable (1). 4-HNE has been associated with diseases including, but not limited to, Alzheimer's disease (AD), Parkinson’s disease (PD), heart disease, atherosclerosis, cancers, diabetes, and acute lung injury (1, 3, 5, 11–13, 20, 22, 27, 33, 34, 37, 39, 41, 42, 46, 48). This is largely due to its ability to form protein adducts via Michael Addition with a vast number of targets. 4-HNE preferentially forms adducts with cysteine residues of thiol-containing proteins, a number of which are proteins involved in redox signaling (39) (Fig. 1). Moreover, its deleterious effects are frequently found to be proportional to the number of adducts formed (13). Markedly, 4-HNE has been shown to affect mitochondria by impairing ATPase activity, disrupting oxygen consumption, and even triggering premature apoptosis via adduct formation with c-Jun NH2-terminal kinase (JNK) (13) (Fig. 1).
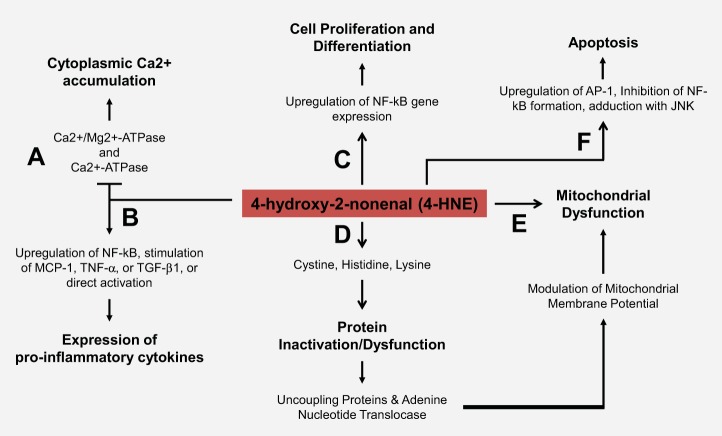
Fig. 1.
4-Hydroxy-2-nonenal (4-HNE) physiological and cytotoxic effects. A: 4-HNE has been shown to cause cytoplasmic Ca2+ accumulation via inhibition of (Ca2+/Mg2+)-ATPase and Ca2+-ATPase. B: 4-HNE was found to induce the expression of proinflammatory cytokines by regulation of nuclear factor-κB (NF-κB), direct activation, and stimulation of monocyte chemotactic protein 1 (MCP-1), tumor necrosis factor-α (TNF-α), and/or transforming growth factor-β1 (TGF-β1). C: 4-HNE is reported to upregulate the expression of NF-κB, which leads to cell proliferation and differentiation. D: typically, 4-HNE facilitates the formation of 1,4-Michael addition adducts with cysteine, lysine, and histidine residues of various targets. These adducts often result in protein inactivation or dysfunction. 4-HNE adduction with uncoupling proteins (UCPs) and adenine nucleotide translocase (ANT) has been shown to serve as a mechanism of physiological regulation of proton leak. This results in a feedback loop that naturally regulates mitochondrial membrane potential but can lead to mitochondrial dysfunction. E: 4-HNE has been shown to affect mitochondria by impairing ATPase activity, disrupting oxygen consumption, and altering membrane fluidity. F: elevated levels of 4-HNE have been shown to trigger premature apoptosis via adduct formation with c-Jun NH2-terminal kinase (JNK), inhibition of NF-κB formation, and upregulation of apoptosis promoting proteins such as activating protein-1 (AP-1).
Although 4-HNE-mediated adduction is typically deleterious, 4-HNE has also been shown to be of physiological relevance. While the mechanism remains to be revealed, studies show that 4-HNE participates in partial mitochondrial uncoupling by increasing proton leak (uptake) via adduction with uncoupling proteins (UCPs) and adenine nucleotide translocase (ANT) (2, 7) (Fig. 1). As mentioned, ROS are naturally produced in the mitochondria. Reports indicate that this ROS production acts as feedback inhibition through 4-HNE-mediated mitochondrial uncoupling, and therefore may also play a role in regulation of redox signaling (2, 7). When mitochondrial membrane potential is high, ROS are produced and result in a production of 4-HNE which in turn leads to a decrease in membrane potential via UCPs and ANT (Fig. 1) (2). Taken together, this relationship suggests that targeting 4-HNE has the potential to be disruptive to natural mechanisms of redox signaling.
As its production pathway is not directly regulated, and its adduction nonenzymatic, some studies consider it a nonclassical secondary messenger (15, 16). 4-HNE modification of SHP-1 [Src homology 2 (SH2) domain] displays characteristics of ubiquitination signaling as opposed to the signaling of a secondary messenger (15, 16). However, many 4-HNE targets are redox-sensitive signaling molecules or their associated upstream regulators, such as thioredoxin (a scavenger of intracellular ROS) and glutathione (15, 16, 39). 4-HNE has also been shown to alter cell signaling by causing cytoplasmic Ca2+ accumulation via inhibition of (Ca2+/Mg2+)-ATPase and Ca2+-ATPase (Fig. 1). More importantly, 4-HNE is known to modify critical molecules such as nuclear factor erythroid 2-related factor 2 (Nrf2), peroxisome-proliferator-activated receptors (PPAR), and mitochondrial aldehyde dehydrogenase 2 (ALDH2). At micromolar concentrations, 4-HNE is reported to upregulate the expression of transcription factors, such as nuclear factor-κB (NF-κB), which in turn regulates genes involved in cell proliferation and differentiation (43). Comparatively, in greater concentrations, 4-HNE has been shown to inhibit formation of NF-κB, and upregulate apoptosis-promoting proteins including activating protein-1 (AP-1) (Fig. 1) (43). Notably, 4-HNE is also a known activator of mitogen-activated protein kinases (MAPK), a group of serine-threonine kinases that include extracellular signal-regulated kinase (ERK), p38MAPK, and c-Jun NH2-terminal kinase (JNK), as well as tyrosine kinase receptors (RTKs), such as epidermal growth factor (EGFR) (1, 15, 16, 27).
Based on the prominent evidence that 4-HNE is a critical component in the pathology of numerous diseases, and due to its short half-life, relative stability, and the availability of 4-HNE detoxifying enzymes, including glutathione-S-transferase (GST), ALDHs, and aldo-keto reductases (AKRs), there has been a substantial increase in studies of its detoxification (13). Here we highlight recent developments in the detoxification and targets of 4-HNE as they relate to the topics of oxidative stress, mitochondrial dysfunction, and disease pathology, while also revisiting the critical features of 4-HNE that contribute to its significance as a clinical target.
Origin, Formation, and Regulation of 4-HNE
In 1980, Esterbauer and colleagues identified 4-HNE as one of the major cytotoxic substances produced by lipid peroxidation (3). From this point forward, a great number of studies focused on the mechanisms of 4-HNE generation and their relevance in various pathophysiological conditions through alteration of cell differentiation and proliferation (43). As discussed before, lipid peroxidation is a discrete outcome of oxidative stress and serves to produce molecules such as 4-HNE (3). When ROS oxidize ω-6 polyunsaturated fatty acids (PUFAs), such as linoleic, γ-linolenic, or arachidonic acid, 4-HNE is produced (13, 39). Once formed, if it is not controlled by natural inhibitors (ALDHs, AKRs, and GSTs), 4-HNE will participate in chemical reactions with multiple targets (7). 4-HNE facilitates the formation of 1,4-Michael addition adducts primarily with cysteine, but also with lysine and histidine in lower frequency (39). Furthermore, 4-HNE is documented to possess a reactive preference towards amino and thiol groups (1, 39, 40).
In normal physiological conditions, 4-HNE displays a half-life of less than 2 minutes and is typically tightly regulated by ALDHs, AKRs, and GSTs (13). 4-HNE-mediated Michael additions occasionally run in the reverse direction, resulting in production of nontoxic molecules: cysteine and glutathione (13, 39). This reaction, termed retro Michael addition, can be catalyzed by the presence of GST (Fig. 2). However, the reaction is not strongly favored in the reverse direction and is susceptible to a number of external factors (39).
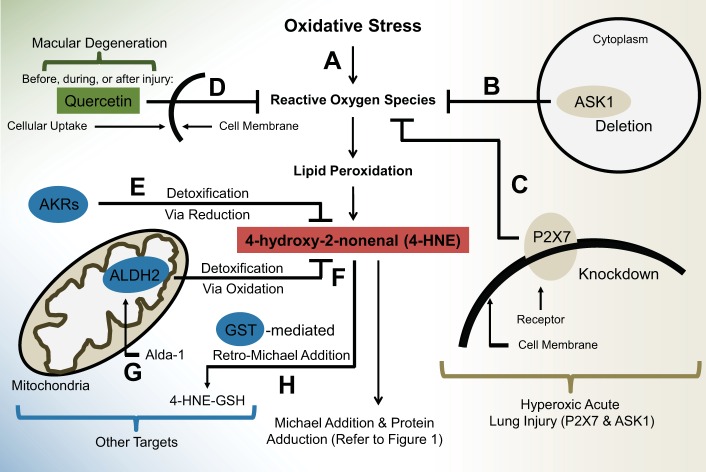
Fig. 2.
Mechanisms of 4-HNE detoxification and inhibition. A: subjection to sources of oxidative stress leads to production of ROS. These reactive species induce lipid peroxidation at the cell membrane, which gives rise to a toxic byproduct: 4-HNE. This cytotoxic aldehyde is then free to form protein adducts with various targets via Michael addition, which often results in protein dysfunction and even cell death. B and C: two molecules were recently identified as possible therapeutic targets for 4-HNE-mediated cellular damage in hyperoxic acute lung injury (HALI). B: in HALI, deletion of ASK1 is associated with a decrease in ROS-mediated damage, which may suggest a role in inhibition of lipid peroxidation by-products. C: knockdown of the P2X7 membrane receptor is shown to inhibit 4-HNE production via mediation of ROS levels. D: quercetin is an experimental chemical compound that has been found to act as an antioxidant, before, during, and after macular degeneration. E–H: AKRs, ALDHs, and GSTs exist as therapeutic targets in many pathologies; their modification of 4-HNE is widely known and beginning to be a target of recent research. E: ARKs detoxify 4-HNE by reducing an active group on the molecule. F: ALDH2 (specifically) is a known cytoprotective molecule that detoxifies 4-HNE in the mitochondria by oxidizing its carbonyl group to form a carboxylic acid. G: Alda-1 is a chemically synthesized small molecule that, when administered, serves as a molecular activator of ALDH2, effectively upregulating ALDH2-mediated detoxification of 4-HNE. H: GST is an enzyme that has been shown to induce retro-Michael addition, or conjugation with 4-HNE, forming 4-HNE derivatives and inactive isoforms.
In the case of lipid peroxides, Nrf2 has been known to upregulate the expression of AKR as a response to oxidative stress (13, 39). In this situation, AKR is capable of reducing the carbonyl group of 4-HNE, effectively detoxifying the molecule (Fig. 2) (39). ALDHs are another prominent inhibitor of 4-HNE. ALDH isoforms have been reported to oxidize the carbonyl group of 4-HNE to result in production of 4-hydroxy-nonenoic acid (Fig. 2) (13, 39). Particularly, ALDH2 and ALDH3 are reported to have the highest efficacy of 4-HNE detoxification, compared with other isoforms, such as class 1 ALDH, which have a narrow effect on 4-HNE cytotoxicity (13, 39). Studies across many fields have examined the role of 4-HNE, its formation, and the mechanisms of its reactions with target molecules, but fewer studies have utilized the knowledge of these natural inhibitors.
New Therapeutic Developments
Since the activities of these 4-HNE detoxifying molecules vary in different diseases, it is a slow process to determine their usefulness as therapeutic strategies. In particular fields, such as lung biology, molecules like ALDH2 are only recently being posited as therapeutic possibilities. For instance, studies of hyperoxic acute lung injury (HALI), a notable complication often associated with treatment of acute lung injury, have made recent advances regarding the remediation of ROS and 4-HNE-mediated damage via regulation or alteration of other molecules such as apoptosis signal-regulating kinase 1 (ASK1) (18) and membrane receptor P2X7 (21).
A recent study reports that deletion of ASK1, a member of the MAP3K family that acts as a proinflammatory molecule, is associated with the prevention of ROS-mediated damage, and therefore may also be associated with the regulation of 4-HNE (Fig. 2) (18). Coinciding with this, another study reports that 4-HNE is capable of activating ASK1, JNK, and caspase-3, which results in Fas-mediated death-inducing signaling complex (DISC)-independent apoptosis, identifying additional evidence that ASK1 deletion may be beneficial (10). Furthermore, another recent study in lung injury identified the membrane receptor P2X7 as a prominent therapeutic target in HALI (21). Knockdown of P2X7 was reported to significantly decrease the expression of lipid peroxidation by-products including 4-HNE and MDA by reducing ROS (Fig. 2) (21). Overall, these therapeutic targets warrant more study in various diseases caused by oxidative stress, as they may be the key to control of the toxic by-products.
Role of 4-HNE in Disease Pathology
4-HNE has been implicated in numerous diseases. Evidence shows that cancerous tissue has greater levels of ROS when compared with noncancerous tissue, which is associated with an increase in 4-HNE levels (33). More importantly, 4-HNE adduction with protein targets in carcinogenic tissue is largely associated with the progression of kidney and colon cancer (48). 4-HNE is known to form adducts with enzymes in the ETC complexes, which in turn results in increased metastasis (48). However, the deleterious effects of 4-HNE is not constrained to cancer.
Some of the most notable implications of 4-HNE toxicity have arisen from studies on neurological diseases, namely Alzheimer's disease (AD) (5) and Parkinson's disease (PD) (41). In AD, 4-HNE has been shown to be toxic to primary hippocampal neuronal cultures and can cause damage to regions of neural tissue (5). Remarkably, in preclinical AD, lipid peroxidation in the hippocampus elevates the levels of 4-HNE, which consequently impedes cell function (5). These reports indicate that protein-bound 4-HNE could not only signal development of AD, but also serve as a target for prevention of disease progression (37). 4-HNE is similarly implicated in PD, and for both diseases 4-HNE is found to target neurons (46). Taken together, attenuation of 4-HNE could limit the progression of neurological damage in AD and PD.
Oxidative stress and 4-HNE have also been extensively studied in heart diseases (34). In atherosclerosis, 4-HNE accumulates in atherosclerotic plaques, exacerbates the inflammatory response by stimulating the release of IL-8 and IL-1β, and can ultimately lead to plaque rupture (22). Evidence shows that 4-HNE is also present during myocardial ischemia-reperfusion, and is correlated with tissue damage (42).
In diabetes, 4-HNE plays a more complicated role, as both a physiological protective response to high glucose, and the familiar cytotoxic apoptosis-, inflammation-inducing reactive aldehyde (12). Much like its role in the redox system, low levels of 4-HNE act as a defense mechanism that effectively triggers an antioxidant response (12). Elevated levels, on the other hand, result in inhibition or inactivation of numerous cellular targets, i.e., membrane proteins and key metabolic enzymes (TCA and ETC) (12). This suggests that, in some diseases, 4-HNE may act as a target for detection, early prevention, and even cessation of disease progression.
Lastly, 4-HNE has been implicated in acute lung injury. Prolonged exposure to oxygen in excess of 0.8 (O2 fractions) has been shown to induce ROS production resulting in development of pulmonary edema and even proliferative fibrosis in animal models (20). 4-HNE remains to be extensively studied in lung injury, but recent reports show that 4-HNE forms adducts with multiple mitochondrial targets, which ultimately results in mitochondrial dysfunction and disruption of cellular bioenergetics (20).
Overall, these are mere highlights of known roles 4-HNE plays in disease progression. By no means does this brief discussion exhaust the involvement of 4-HNE in these diseases, nor does it explore its involvement in more specific diseases. Rather than delve further into the pathological role of 4-HNE, suffice it to say that 4-HNE is a critical mediator in a vast number of serious diseases.
Toxicity of 4-HNE: Cell Types and Molecular Targets
Indeed, there is no shortage of studies determining the toxicity of 4-HNE in various diseases and physiological settings. Because of its reactive character and general targeting of cysteine, lysine, and histidine, 4-HNE has been implicated in the damage of numerous cell types including hepatocytes, cardiomyocytes, retinal pigment epithelial cells, osteosarcoma cells, alveolar epithelial cells, astrocytes, and spermatozoa (4, 6, 14, 26, 30, 31, 45). Additionally, recent studies have reported 4-HNE-mediated cell cycle arrest in the G2/M phase, as well as covalent modification of CDK2 (8, 9). 4-HNE most commonly causes damage to these targets by three primary mechanisms: signaling, protein deactivation, and inflammation (14, 32).
By altering protein structure, 4-HNE plays an active role in altering signal transduction, typically characterized by protein degradation, or upregulation of other proteins including proapoptotic factors (32) (Fig. 1). Furthermore, 4-HNE has been shown to induce deactivation of proteins either by structural change or by modes of inhibition (27). Studies report that 4-HNE acts as a reversible mixed-type inhibitor of ALDH2, one of its primary regulators in normal physiology (27). Participation as a mixed-type inhibitor indicates that 4-HNE can bind to ALDH2, inhibiting it, while also decreasing the affinity for its substrate (27). Physiologically, this is significant because ALDH2 is a natural inhibitor of 4-HNE; thus this interaction acts as another feedback inhibition loop that 4-HNE participates in (along with regulation of membrane uncoupling) (2, 7, 27). When 4-HNE inhibits ALDH2 activity, it results in uninhibited production of 4-HNE, which in turn leads to more protein adducts and greater damage. Lastly, 4-HNE was found to induce the expression of proinflammatory cytokines by regulation of NF-κB and by direct activation (20) (Fig. 1). Reports also indicate that 4-HNE may promote inflammation by stimulating monocyte chemotactic protein 1 (MCP-1), tumor necrosis factor-α (TNF-α), and/or transforming growth factor-β1 (TGF-β1) (13, 47) (Fig. 1). However, 4-HNE is not limited to these interactions, having been reported to alter membrane fluidity in hepatic mitochondria as well as disrupt Na+-K+-ATPase activity (39) (Fig. 1).
4-HNE-Mediated Mitochondrial Dysfunction
While 4-HNE displays targeting of many cellular components, proteins, and cell types, mitochondria are a prominent target of its toxic activity (13, 20). Since the mitochondrial electron transport chain is one of the primary sources of endogenous ROS, it is also one of the more vital cellular components susceptible to 4-HNE attack (13, 25, 30). In normal physiological conditions, the organelle utilizes a variety of mechanisms to tightly regulate ROS (25). Conversely, under conditions of oxidative stress these regulatory mechanisms become overwhelmed in the mitochondria, which leaves the organelle subject to massive damage by ROS and lipid peroxidation by-products (25).
Owing to the importance of mitochondria in cell bioenergetics, 4-HNE-induced mitochondrial dysfunction has been reported as a crucial link in several pathologies including Parkinson's disease, cardiomyopathy, and hyperoxic acute lung injury (13, 20, 30). Despite the elucidation of a few mechanisms of 4-HNE-mediated mitochondrial dysfunction, the key role of this relationship remains to be studied in many fields. Further definition of this relationship could serve as a crucial push towards alleviation of a plethora of diseases related to oxidative stress, especially since mitochondrial ALDH2 is known to be a plausible therapeutic target.
Detoxification of 4-HNE As a Clinically Relevant Topic of Study
As 4-HNE has been implicated in an increasing number of diseases and clinical complications, a handful of studies have directed focus at detoxifying 4-HNE and preserving its targets. Originally, antioxidants were developed in an attempt to curb the effects of ROS and the by-products of the lipid peroxidation that they cause. Unfortunately, ROS are unstable, which, as a result, makes it difficult for antioxidants to target them and reduce the production of secondary intermediates (32). Although some antioxidants display positive results in terms of preventing oxidative injury, they largely produce negative outcomes during clinical trials, which renders their therapeutic potential very limited until issues with delivery and regulation can be improved (17, 36).
Regardless, some instances of antioxidants are still being studied as an option of treatment for specific diseases. For example, one study describes the relevance and clinical applications of the antioxidant quercetin in the prevention of macular degeneration (Fig. 2) (24). Being a flavonoid (a water-soluble plant metabolite), quercetin is abundantly present in many fruits and vegetables and is shown to modulate pathways such as sirtuin-FoxO, NF-κB, and Nrf-2/ARE (14). The aforementioned study shows that ARPE-19 cells exposed to quercetin display enhanced resistance to 4-HNE-mediated damage (24). This cytoprotection is conferred by quercetin regardless of when the antioxidant is added: before, during, or after insult (24). This evidence suggests that the production of lipid peroxidation by-products can be slowed or even reversed, during and after insult. Regrettably, the establishment of this compound as a plausible therapeutic for 4-HNE-mediated injury is in early stages, and while further study of quercetin could substantiate it as a therapeutic target for clinical translation, the majority of research should maintain its focus on the enzymes that naturally play a role in the regulation of 4-HNE.
A Pivotal Shift Towards Study of Therapeutic Strategies
Recent studies have turned to therapeutic targets that regulate or detoxify lipid peroxidation by-products. Many additional, and promising therapeutic targets exist for attenuation of 4-HNE-mediated damage, such as the antioxidant MitoQ, as well as regulation of molecules that induce ROS production such as thrombin (11, 38). Unlike quercetin, MitoQ is an antioxidant that directly targets mitochondria, and recent studies have linked it to alleviation of liver fibrosis (38). On the other hand, thrombin has been strongly implicated in neurological diseases (Alzheimer's disease and Parkinson's disease) and associated with the production of ROS, thereby making it a possible target for control of ROS production (11). While there is an ample number of molecules, chemicals, and compounds that influence with ROS, and therefore modulate 4-HNE, this article will only delve into those that have warranted the most discussion in previous literature.
The majority of previous literature of 4-HNE discusses the therapeutic potential of ALDHs, AKRs, and GSTs (Fig. 2) (13). Highlighting the importance of these studies, recent literature has turned to identify compounds that interact with the aforementioned. A small molecular activator called Alda-1 has been in the spotlight for its controlled activation of ALDH2, an enzyme naturally responsible for the regulation of 4-HNE. There is an increasing number of studies that demonstrate the importance of ALDH2 in physiological conditions and thus also support the importance of small molecules, such as Alda-1, which could prove invaluable in the advancement of therapeutic strategies.
Since the discovery of Alda-1, its mechanisms of action have been greatly studied, but rarely applied (35). Few studies have moved to characterize the benefits of Alda-1, despite the common finding that it confers protection by activating and upregulating the function of ALDH2 (Fig. 2) (14, 17). Indeed, studies identifying the benefits of Alda-1 involvement in disease remediation have only just begun in the fields of cardiac disease, neurological disorders, and particularly lung biology.
Small molecules such as Alda-1 could serve as therapeutics by restoring the naturally efficient regulatory mechanisms of 4-HNE that are debilitated in disease conditions. Recent reports identify that pretreatment of alveolar epithelial cells with Alda-1 confers protection against lung ischemia-reperfusion by detoxification of 4-HNE (14). Additionally, Alda-1 treatment displayed similar results in a study of cerebral ischemia and myocardial infarction, demonstrating that Alda-1-mediated activation of ALDH2 is capable of remediating injury in multiple physiological systems (17, 44). Based on these studies, further elucidation of molecular enzymes such as ALDH2, AKR, and GST, as well as other molecular targets such as ASK1 and P2X7, and the corresponding discovery of molecular activators like Alda-1, could reveal new therapeutic routes for the treatment of multiple diseases complicated by 4-HNE.
Conclusion
As substantial evidence shows, 4-HNE is a critical pathological target in numerous diseases. Each year, new studies identify novel targets of the cytotoxic aldehyde, but comparatively few studies focus on the mechanisms of its regulation. Its characteristic and damaging formation of covalent adducts with various proteins can act as both an inducer of inflammation and apoptosis and a deactivator of key proteins (1, 3, 9, 18, 20, 21, 26, 29, 32, 39, 45). The common consensus of current research is that 4-HNE is a molecule of concern, and that, because of the diversity of its targets, study of its remediation could be applicable to numerous oxidative stress-related injuries, despite the roles it plays in normal physiology. Study of 4-HNE should continue to shift towards identification of mechanisms of 4-HNE control, regulation, and ultimately detoxification. Furthermore, as the studies of antioxidants slowly decline, it is to be expected that new molecular targets and molecules, such as ALDH2 and Alda-1, will be increasingly studied. Considering its relation to oxidative stress, 4-HNE is of notable relevance, especially in the field of lung biology. Yet, despite its implications, very few studies have focused on its attenuation. With its role in mitochondrial dysfunction, future exploration of mitochondrial preservation and 4-HNE detoxification could serve to alleviate diseases such as Parkinson's, Alzheimer's, diabetes, acute lung injury, and cardiovascular diseases (1, 3, 5, 11–13, 20, 22, 27, 33, 34, 37, 39, 41, 42, 46, 48). Broad investigation of 4-HNE and its subcellular targets may also reveal new methods of preserving cell viability and preventing 4-HNE-mediated covalent modification of proteins. Overall, advancements in the management of 4-HNE by enzymes that naturally regulate its formation and activity, as well as novel compounds and antioxidants, have dramatic potential for redefining our understanding and control of numerous oxidative-stress driven pathologies.
GRANTS
N. Kolliputi was funded by the American Heart Association National Scientist Development Grant 09SDG2260957, National Institutes of Health National Heart, Lung, and Blood Institute Grant R01 HL-105932, and the Joy McCann Culverhouse endowment to the Division of Allergy and Immunology.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.B. conception and design of research; M.B. prepared figures; M.B. drafted manuscript; M.B., C.B., R.L., and N.K. edited and revised manuscript; M.B., R.L., and N.K. approved final version of manuscript.
REFERENCES
- 1.Ayala A, Munoz MF, Arguelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev 2014: 360438, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azzu V, Parker N, Brand MD. High membrane potential promotes alkenal-induced mitochondrial uncoupling and influences adenine nucleotide translocase conformation. Biochem J 413: 323–332, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benedetti A, Comporti M, Esterbauer H. Identification of 4-hydroxynonenal as a cytotoxic product originating from the peroxidation of liver microsomal lipids. Biochim Biophys Acta 620: 281–296, 1980. [DOI] [PubMed] [Google Scholar]
- 4.Benedetti E, D'Angelo B, Cristiano L, Di Giacomo E, Fanelli F, Moreno S, Cecconi F, Fidoamore A, Antonosante A, Falcone R, Ippoliti R, Giordano A, Cimini A. Involvement of peroxisome proliferator-activated receptor beta/delta (PPAR beta/delta) in BDNF signaling during aging and in Alzheimer disease: possible role of 4-hydroxynonenal (4-HNE). Cell Cycle 13: 1335–1344, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradley MA, Markesbery WR, Lovell MA. Increased levels of 4-hydroxynonenal and acrolein in the brain in preclinical Alzheimer disease. Free Radic Biol Med 48: 1570–1576, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown JM, Kuhlman C, Terneus MV, Labenski MT, Lamyaithong AB, Ball JG, Lau SS, Valentovic MA. S-adenosyl-l-methionine protection of acetaminophen mediated oxidative stress and identification of hepatic 4-hydroxynonenal protein adducts by mass spectrometry. Toxicol Appl Pharmacol 281: 174–184, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camara AKS, Lesnefsky EJ, Stowe DF. Potential therapeutic benefits of strategies directed to mitochondria. Antioxid Redox Signal 13: 279–347, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camarillo JM, Rose KL, Galligan JJ, Xu S, Marnett LJ. Covalent modification of CDK2 by 4-hydroxynonenal as a mechanism of inhibition of cell cycle progression. Chem Res Toxicol 29: 323–332, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaudhary P, Sharma R, Sahu M, Vishwanatha JK, Awasthi S, Awasthi YC. 4-Hydroxynonenal induces G2/M phase cell cycle arrest by activation of the ataxia telangiectasia mutated and Rad3-related protein (ATR)/checkpoint kinase 1 (Chk1) signaling pathway. J Biol Chem 288: 20532–20546, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaudhary P, Sharma R, Sharma A, Vatsyayan R, Yadav S, Singhal SS, Rauniyar N, Prokai L, Awasthi S, Awasthi YC. Mechanisms of 4-hydroxy-2-nonenal induced pro- and anti-apoptotic signaling. Biochemistry 49: 6263–6275, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Citron BA, Ameenuddin S, Uchida K, Suo WZ, SantaCruz K, Festoff BW. Membrane lipid peroxidation in neurodegeneration: role of thrombin and proteinase-activated receptor-1. Brain Res 1643: 10–17, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen G, Riahi Y, Sunda V, Deplano S, Chatgilialoglu C, Ferreri C, Kaiser N, Sasson S. Signaling properties of 4-hydroxyalkenals formed by lipid peroxidation in diabetes. Free Radic Biol Med 65: 978–987, 2013. [DOI] [PubMed] [Google Scholar]
- 13.Dalleau S, Baradat M, Gueraud F, Huc L. Cell death and diseases related to oxidative stress: 4-hydroxynonenal (HNE) in the balance. Cell Death Differ 20: 1615–1630, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding J, Zhang Q, Luo Q, Ying Y, Liu Y, Li Y, Wei W, Yan F, Zhang H. Alda-1 attenuates lung ischemia-reperfusion injury by reducing 4-hydroxy-2-nonenal in alveolar epithelial cells. Crit Care Med 44: e544–e552, 2016. [DOI] [PubMed] [Google Scholar]
- 15.Forman HJ. Reactive oxygen species and alpha,beta-unsaturated aldehydes as second messengers in signal transduction. Ann NY Acad Sci 1203: 35–44, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forman HJ, Fukuto JM, Miller T, Zhang H, Rinna A, Levy S. The chemistry of cell signaling by reactive oxygen and nitrogen species and 4-hydroxynonenal. Arch Biochem Biophys 477: 183–195, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu SH, Zhang HF, Yang ZB, Li TB, Liu B, Lou Z, Ma QL, Luo XJ, Peng J. Alda-1 reduces cerebral ischemia/reperfusion injury in rat through clearance of reactive aldehydes. Naunyn Schmiedebergs Arch Pharmacol 387: 87–94, 2014. [DOI] [PubMed] [Google Scholar]
- 18.Fukumoto J, Cox R, Fukumoto I, Cho Y, Parthasarathy PT, Galam L, Lockey RF, Kolliputi N. Deletion of ASK1 protects against hyperoxia-induced acute lung injury. PLoS One 11: e0147652, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukumoto J, Fukumoto I, Parthasarathy PT, Cox R, Huynh B, Ramanathan GK, Venugopal RB, Allen-Gipson DS, Lockey RF, Kolliputi N. NLRP3 deletion protects from hyperoxia-induced acute lung injury. Am J Physiol Cell Physiol 305: C182–C189, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galam L, Failla A, Soundararajan R, Lockey RF, Kolliputi N. 4-Hydroxynonenal regulates mitochondrial function in human small airway epithelial cells. Oncotarget 6: 41508–41521, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galam L, Rajan A, Failla A, Soundararajan R, Lockey RF, Kolliputi N. Deletion of P2X7 attenuates hyperoxia-induced acute lung injury via inflammasome suppression. Am J Physiol Lung Cell Mol Physiol 310: L572–L581, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gargiulo S, Gamba P, Testa G, Rossin D, Biasi F, Poli G, Leonarduzzi G. Relation between TLR4/NF-kappaB signaling pathway activation by 27-hydroxycholesterol and 4-hydroxynonenal, and atherosclerotic plaque instability. Aging Cell 14: 569–581, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He WH, Li B, Zhu X, Zhang KH, Li BM, Liu ZJ, Liu GY, Wang J. [The role and mechanism of NADPH oxidase in leptin-induced reactive oxygen species production in hepatic stellate cells]. Zhonghua Gan Zang Bing Za Zhi 18: 849–854, 2010. [DOI] [PubMed] [Google Scholar]
- 24.Hytti M, Piippo N, Salminen A, Honkakoski P, Kaarniranta K, Kauppinen A. Quercetin alleviates 4-hydroxynonenal-induced cytotoxicity and inflammation in ARPE-19 cells. Exp Eye Res 132: 208–215, 2015. [DOI] [PubMed] [Google Scholar]
- 25.Iqbal S, Hood DA. Oxidative stress-induced mitochondrial fragmentation and movement in skeletal muscle myoblasts. Am J Physiol Cell Physiol 306: C1176–C1183, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ji GR, Yu NC, Xue X, Li ZG. 4-Hydroxy-2-nonenal induces apoptosis by inhibiting AKT signaling in human osteosarcoma cells. Sci World J 2014: 873525, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jinsmaa Y, Florang VR, Rees JN, Anderson DG, Strack S, Doorn JA. Products of oxidative stress inhibit aldehyde oxidation and reduction pathways in dopamine catabolism yielding elevated levels of a reactive intermediate. Chem Res Toxicol 22: 835–841, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kai K, Kasa S, Sakamoto M, Aoki N, Watabe G, Yuasa T, Iwaya-Inoue M, Ishibashi Y. Role of reactive oxygen species produced by NADPH oxidase in gibberellin biosynthesis during barley seed germination. Plant Signal Behav 11: e1180492, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee S, Kim SM, Lee RT. Thioredoxin and thioredoxin target proteins: from molecular mechanisms to functional significance. Antioxid Redox Signal 18: 1165–1207, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mali VR, Ning R, Chen J, Yang XP, Xu J, Palaniyandi SS. Impairment of aldehyde dehydrogenase-2 by 4-hydroxy-2-nonenal adduct formation and cardiomyocyte hypertrophy in mice fed a high-fat diet and injected with low-dose streptozotocin. Exp Biol Med (Maywood) 239: 610–618, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moazamian R, Polhemus A, Connaughton H, Fraser B, Whiting S, Gharagozloo P, Aitken RJ. Oxidative stress and human spermatozoa: diagnostic and functional significance of aldehydes generated as a result of lipid peroxidation. Mol Hum Reprod 21: 502–515, 2015. [DOI] [PubMed] [Google Scholar]
- 32.Negre-Salvayre A, Coatrieux C, Ingueneau C, Salvayre R. Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. Br J Pharmacol 153: 6–20, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okamoto K, Toyokuni S, Uchida K, Ogawa O, Takenewa J, Kakehi Y, Kinoshita H, Hattori-Nakakuki Y, Hiai H, Yoshida O. Formation of 8-hydroxy-2′-deoxyguanosine and 4-hydroxy-2-nonenal-modified proteins in human renal-cell carcinoma. Int J Cancer 58: 825–829, 1994. [DOI] [PubMed] [Google Scholar]
- 34.Pashkow FJ. Oxidative stress and inflammation in heart disease: do antioxidants have a role in treatment and/or prevention? Int J Inflamm 2011: 514623, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perez-Miller S, Younus H, Vanam R, Chen CH, Mochly-Rosen D, Hurley TD. Alda-1 is an agonist and chemical chaperone for the common human aldehyde dehydrogenase 2 variant. Nat Struct Mol Biol 17: 159–164, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pisoschi AM, Pop A. The role of antioxidants in the chemistry of oxidative stress: a review. Eur J Med Chem 97: 55–74, 2015. [DOI] [PubMed] [Google Scholar]
- 37.Reed TT, Pierce WM, Markesbery WR, Butterfield DA. Proteomic identification of HNE-bound proteins in early Alzheimer disease: insights into the role of lipid peroxidation in the progression of AD. Brain Res 1274: 66–76, 2009. [DOI] [PubMed] [Google Scholar]
- 38.Rehman H, Liu Q, Krishnasamy Y, Shi Z, Ramshesh VK, Haque K, Schnellmann RG, Murphy MP, Lemasters JJ, Rockey DC, Zhong Z. The mitochondria-targeted antioxidant MitoQ attenuates liver fibrosis in mice. Int J Physiol Pathophysiol Pharmacol 8: 14–27, 2016. [PMC free article] [PubMed] [Google Scholar]
- 39.Schaur RJ, Siems W, Bresgen N, Eckl PM. 4-Hydroxy-nonenal-A bioactive lipid peroxidation product. Biomolecules 5: 2247–2337, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schneider C, Tallman KA, Porter NA, Brash AR. Two distinct pathways of formation of 4-hydroxynonenal. Mechanisms of nonenzymatic transformation of the 9- and 13-hydroperoxides of linoleic acid to 4-hydroxyalkenals. J Biol Chem 276: 20831–20838, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Selley ML. (E)-4-hydroxy-2-nonenal may be involved in the pathogenesis of Parkinson's disease. Free Radic Biol Med 25: 169–174, 1998. [DOI] [PubMed] [Google Scholar]
- 42.Shinmura K, Bolli R, Liu SQ, Tang XL, Kodani E, Xuan YT, Srivastava S, Bhatnagar A. Aldose reductase is an obligatory mediator of the late phase of ischemic preconditioning. Circ Res 91: 240–246, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Shoeb M, Ansari NH, Srivastava SK, Ramana KV. 4-Hydroxynonenal in the pathogenesis and progression of human diseases. Curr Med Chem 21: 230–237, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun L, Ferreira JC, Mochly-Rosen D. ALDH2 activator inhibits increased myocardial infarction injury by nitroglycerin tolerance. Sci Transl Med 3: 107ra111, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vatsyayan R, Chaudhary P, Sharma A, Sharma R, Rao Lelsani PC, Awasthi S, Awasthi YC. Role of 4-hydroxynonenal in epidermal growth factor receptor-mediated signaling in retinal pigment epithelial cells. Exp Eye Res 92: 147–154, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoritaka A, Hattori N, Uchida K, Tanaka M, Stadtman ER, Mizuno Y. Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proc Natl Acad Sci USA 93: 2696–2701, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang XM, Guo L, Huang X, Li QM, Chi MH. 4-hydroxynonenal regulates TNF-alpha gene transcription indirectly via ETS1 and microRNA-29b in human adipocytes induced from adipose tissue-derived stromal cells. Anat Rec (Hoboken) 10: 23371, 2016. [DOI] [PubMed] [Google Scholar]
- 48.Zhong H, Yin H. Role of lipid peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: focusing on mitochondria. Redox Biol 4: 193–199, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]