Abstract
Vitamin C, or ascorbic acid, both tightens the endothelial permeability barrier in basal cells and also prevents barrier leak induced by inflammatory agents. Barrier tightening by ascorbate in basal endothelial cells requires nitric oxide derived from activation of nitric oxide synthase. Although ascorbate did not affect cyclic AMP levels in our previous study, there remains a question of whether it might activate downstream cyclic AMP-dependent pathways. In this work, we found in both primary and immortalized cultured endothelial cells that ascorbate tightened the endothelial permeability barrier by ∼30%. In human umbilical vein endothelial cells, this occurred at what are likely physiologic intracellular ascorbate concentrations. In so doing, ascorbate decreased measures of oxidative stress and also flattened the cells to increase cell-to-cell contact. Inhibition of downstream cyclic AMP-dependent proteins via protein kinase A did not prevent ascorbate from tightening the endothelial permeability barrier, whereas inhibition of Epac1 did block the ascorbate effect. Although Epac1 was required, its mediator Rap1 was not activated. Furthermore, ascorbate acutely stabilized microtubules during depolymerization induced by colchicine and nocodazole. Over several days in culture, ascorbate also increased the amount of stable acetylated α-tubulin. Microtubule stabilization was further suggested by the finding that ascorbate increased the amount of Epac1 bound to α-tubulin. These results suggest that physiologic ascorbate concentrations tighten the endothelial permeability barrier in unstimulated cells by stabilizing microtubules in a manner downstream of cyclic AMP that might be due both to increasing nitric oxide availability and to scavenging of reactive oxygen or nitrogen species.
Keywords: ascorbate, endothelial permeability, Epac1, paracellular transport, microtubules
in many organs and tissues, the permeability of the endothelial barrier limits passage of albumin and smaller nonprotein molecules from the vascular compartment into the interstitium. Such paracellular transfer occurs within minutes and is regulated largely by the function of adherens junctions between endothelial cells (26, 45). The integrity of these junctions also depends on the structures of the actin and tubulin cytoskeletons within the cell. Typically, a tight endothelial barrier is indicated by a cortical ring of actin at the periphery of the confluent endothelial cells (9). This ring and the permeability barrier are disrupted by various inflammatory agents, including thrombin (12, 14), agonists of the receptor for advanced glycation end products (RAGE) (20, 48), and oxidants such as hydrogen peroxide (17, 41). The endothelial permeability barrier is stabilized in response to increases in intracellular cyclic AMP/cyclic GMP (44), prostaglandin E2 (4), sphingosine 1-phosphate (13), and ascorbic acid. Regarding the latter, we found that ascorbate both acutely tightens the basal endothelial barrier and prevents leakiness due to thrombin (35), oxidized LDL (31), and RAGE activation (34).
Tightening of the basal endothelial permeability barrier by ascorbate requires nitric oxide (NO) (32), which is generated by nitric oxide synthase (eNOS) in response to ascorbate recycling of tetrahydrobiopterin (18, 19). An increase in NO then increases endothelial cell cyclic GMP, which in turn inhibits phosphodiesterase III, slowing degradation of cyclic AMP and allowing it to accumulate (44). In our recent studies (35), ascorbate prevented decreases in cyclic AMP induced by thrombin in human umbilical vein endothelial cells (HUVECs). Although ascorbate loading of basal HUVECs doubled cyclic GMP levels, cyclic AMP was unaffected. In nonendothelial cells, ascorbate loading either decreased cyclic AMP concentrations (5, 24) or increased cyclic AMP only when stimulated by other agents (22, 29, 49). Our failure to see an increase in cyclic AMP due to ascorbate alone in these previous studies could be due to changes in local or compartmental cyclic AMP concentrations too small to detect in measurements of total cellular nucleotide. If so, then one should still expect to see activation of downstream pathways known to be dependent on cyclic AMP. These would include activation of protein kinase A (PKA) or the guanine nucleotide exchange factor Epac1, with subsequent changes in cytoskeletal and especially microtubule configuration (38). To test this hypothesis, we examined the effect of ascorbate loading on these pathways in two types of endothelial cell cultures: primary culture HUVECs and an immortalized mouse brain endothelial cell line, bEnd.3 cells.
MATERIALS AND METHODS
Reagents.
Sigma-Aldrich Chemical (St. Louis, MO) supplied l-ascorbic acid, colchicine, dehydroascorbic acid (DHA), dihydrofluorescin diacetate (DHF-DA), nocodazole, and saponin. Dichlorodihydrofluorescin diacetate (DCFH-DA) was supplied by Cayman Chemical (Ann Arbor, MI). Tocris Bioscience (Bristol, UK) supplied α-[2-(3-chlorophenyl)hydrazinylidene]-5-(1,1-dimethylethyl)-β-oxo-3-isoxazolepropanenitrile (ESI09), N-[2-[[3-(4-bromophenyl)-2-propenyl]amino]ethyl]-5-isoquinolinesulfonamide dihydrochloride (H89), and (9R,10S,12S)-2,3,9,10,11,12-hexahydro-10-hydroxy-9-methyl-1-oxo-9,12-epoxy-1H-diindolo[1,2,3-fg:3′,2′,1′-kl]pyrrolo[3,4-i][1,6]benzodiazocine-10-carboxylic acid, hexyl ester (KT5720). Aobius (Gloucester, MA) provided 4-cyclopentyl-2-[(2,5-dimethylbenzyl)thio]-6-oxo-1,6-dihydropyrimidine-5-carbonitrile (HJC0197). Perkin-Elmer Life and Analytical Sciences (Boston, MA) supplied the [carboxyl-14C]inulin (molecular weight range 5,000-5,500, 2 mCi/g). DHF-DA, DCFH-DA, ESI09, H89, and HJC0197 were dissolved in dimethyl sulfoxide (DMSO) and diluted to a final DMSO concentration of 0.05% before use.
Cell culture.
HUVECs were obtained from ScienCell Research Laboratories (Carlsbad, CA) and cultured in endothelial cell medium (catalog no. 1001) but without antioxidant supplements. This medium contained 5% (vol/vol) fetal bovine serum (catalog no. 0025), 1% (vol/vol) endothelial cell growth supplement (catalog no. 1052), and penicillin/streptomycin (catalog no. 0503). HUVECs were used between passages 3 and 10. Murine brain endothelial cells (bEnd.3) were obtained from American Type Culture Collection (Manassas, VA). They were cultured in Dulbecco's modified Eagle's medium (Life Technologies, Carlsbad, CA) with 10% fetal bovine serum. All cells were cultured at 37 °C in humidified air containing 5% CO2.
Assay of trans-endothelial inulin transfer.
Cells were cultured 4–5 days past confluence in six-well plates on polyethylene terephthalate cell culture inserts (0.4-μm pores at a density of 2 + 0.2 × 106 pores per cm2; Falcon BD Biosciences, Franklin Lakes, NJ) with 1.7 ml of medium in the upper well and 2.8 ml of medium in the lower well. After treatment with agents as indicated, 0.1 μCi of [carboxyl-14C]inulin was added to the upper well and cells were incubated at 37 °C. After 60 min, medium above and below the cells/filter was sampled for liquid scintillation counting of radiolabeled inulin. The permeability of the endothelial cell layer to radiolabeled inulin was calculated as previously described (33) as derived from Ref. 40. The calculated permeability coefficients for [carboxyl-14C]inulin were corrected for the rate of [carboxyl-14C]inulin transfer across filters after removal of cells (16). This accounted for any changes in permeability due to deposition of the matrix laid down by the cells during culture.
Knockdown of PKAα catalytic subunit.
Thirty-six hours before the inulin transfer assay, HUVECs cultured in Transwell filters were exposed to either small interfering RNA (siRNA) to inhibit expression of PKAα catalytic subunit (PKA C-α; catalog no. 6406; Cell Signaling Technology; 10 nM) or control siRNA with fluorescein conjugate (catalog no. 6201; Cell Signaling Technology; 10 nM). siRNA was prepared in serum- and antibiotic-free HUVEC media containing a 1:100 dilution of Lipofectamine RNAiMAX Transfection Reagent (catalog no. 13778075; Thermo Fisher, Waltham, MA) following the manufacturer's protocol. Transfection efficiency was confirmed microscopically by evaluating fluorescein uptake in control siRNA cells.
Assay of ascorbate.
To measure intracellular ascorbate, cells cultured in six-well plates were rinsed twice with Krebs-Ringer HEPES buffer (KRH) to remove extracellular ascorbate. KRH consisted of 20 mM HEPES, 128 mM NaCl, 5.2 mM KCl, 1 mM NaH2PO4, 1.4 mM MgSO4, and 1.4 mM CaCl2 and at pH 7.4. The cells were lysed with 0.1 ml of 25% (wt/vol) metaphosphoric acid, scraped from the plate with a rubber spatula, and treated with 0.35 ml of 0.1 M Na2HPO4 and 0.05 mM EDTA, pH 8.0. The partially neutralized cell lysates were centrifuged for 1 min at 13,000 g, and the supernatant was taken for assay of ascorbate in duplicate by high-performance liquid chromatography as previously described (17). Intracellular ascorbate concentrations were calculated based on the measured intracellular distribution space of 3-O-methylglucose relative to protein: in bEnd.3 cells this was 2.9 μl/mg protein and in HUVECs it was taken as that previously measured in HUVEC-derived EA.hy926 endothelial cells (3.6 μl/mg protein) (23).
DCFH-DA and DHF-DA assays.
HUVECs and bEnd.3 cells were grown to 90% confluence in 96-well black-walled plates. HUVECs were incubated with 10 μM DCFH-DA for 1 h in Dulbecco's phosphate-buffered saline (PBS) with calcium and magnesium, pH 7.0–7.3, after a 1-h treatment with ascorbate or DHA. BEnd.3 cells were incubated with 20 μM DHF-DA for 30 min before being rinsed with KRH and read at time 0 and time 36 min. Fluorescence intensity was measured using 480 nm excitation and 530 nm emission settings on a plate reader (Synergy HT multi-mode microtiter reader; Biotek, Winooski, VT). For HUVECs, final values were obtained by subtracting values of cells not treated with DCFH-DA from those that were. For bEnd.3 cells, the percentage increase in fluorescence per well over time was calculated by using the formula [(Tf − Ti)/Ti × 100], where Tf = fluorescence at time 36 min and Ti = fluorescence at time zero (47).
For fluorescence images, HUVECs were grown on glass-bottom culture dishes coated with poly-l-lysine and fibronectin and probed with 10 μM DCFH-DA for 1 h and the nuclear stain Hoechst 33342 (Life Technologies) for 30 min. 2′,7′-Dichlorofluorescein (DCF) intensity was then observed with an EVOS XL Core fluorescence microscope and camera using a ×20 LWD lens (AMG, Bothell, WA). Identical laser intensity and gain settings were used for comparisons.
Western blot analysis.
Cells that were 90% confluent were lysed with RIPA Buffer (Sigma), and immunoblotting was performed as described previously (20). Briefly, protein yield was quantified using a BCA assay (catalog no. 23225; Pierce Biotechnology, Rockford, IL). Samples with the same protein content were prepared with Laemmli sample buffer containing 5% β-mercaptoethanol and were electrophoresed on a 4–20% SDS-polyacrylamide gel. Proteins were transferred to polyvinylidene difluoride membranes and probed with rabbit anti-Epac1 (Upstate Biotechnology, Lake Placid, NY; 1:3000), mouse anti-α-tubulin (catalog no. 53646; Santa Cruz Biotechnology, Santa Cruz, CA; 1:3,000), mouse anti-acetylated α-tubulin (catalog no. 23950; Santa Cruz Biotechnology; 1:3,000), mouse anti-nitrotyrosine (catalog no. 32757; Santa Cruz Biotechnology; 1:1,000), or rabbit anti-PKAα catalytic subunit (catalog no. 903; Santa Cruz Biotechnology; 1:5,000). Antibody binding was detected with an enhanced chemiluminescence reagent (catalog no. NEL103001EA; Perkin Elmer, Waltham, MA) using 1:5,000 horseradish peroxidase-conjugated secondary antibodies (catalog no. W4011 and W4021; Promega, Madison, WI). Signal intensity was analyzed within a linear range using ImageJ (National Institutes of Health, Bethesda, MD).
Immunoprecipitation.
Active Rap1 was immunoprecipitated using a kit from Cell Signaling Technology (catalog no. 8818; Danvers, MA) following the manufacturer's instructions. α-Tubulin-bound Epac1 was immunoprecipitated by incubating whole cell lysates containing 300 μg total protein with 1:100 anti-α-tubulin (catalog no. 5546; Santa Cruz Biotechnology) rotating overnight at 4°C. Lysates with antibody were then incubated with 10 μg protein A/G agarose beads (catalog no. 2003; Santa Cruz Biotechnology) for 3 h at 4°C with rotation. Beads were washed with RIPA Buffer, eluted with 2 × Laemmli sample buffer containing 5% β-mercaptoethanol, and used for Western blot analysis as described above.
Real-time tubulin depolymerization.
HUVECs were grown on glass-bottom culture dishes coated with poly-l-lysine and fibronectin. Live cells were transfected overnight with 20 μl/ml CellLight Tubulin-GFP, BacMam 2.0 (Life Technologies) and incubated for 30 min with the nuclear stain RedDot1 (Biotium, Hayward, CA; 1:200). Real-time tubulin depolymerization was visualized using a Zeiss LSM 510 META inverted confocal microscope (Carl Zeiss, Oberkochen, Germany) with a ×63/1.40 Plan-Apachromat oil-immersion lens at 37°C in humidified air containing 5% CO2. Zeiss ZEN software was used, and fields were manually focused between time-points.
Fixed-cell microscopy.
HUVECs grown on glass coverslips coated with poly-l-lysine and fibronectin were fixed with 4% formaldehyde for 15 min. Cells were blocked with 10% bovine serum albumin, permeabilized with 0.1% saponin and probed overnight with the following antibodies: mouse anti-acetylated α-tubulin (1:200), rabbit anti-α-tubulin (1:100), and/or goat anti-VE-cadherin alone (catalog no. 6458; Santa Cruz Biotechnology; 1:200). Cells were then incubated for 1 h with 1:500 dilutions of the respective Alexa Fluor 488- and 555-conjugated secondary antibodies (catalog no. A-21422, A-11034, or A-11055; Life Technologies). Nuclei were stained for 30 min with 1:200 RedDot1 or Hoechst 33342.
BEnd.3 cells were sparsely cultured on poly-d-lysine (catalog no. A-003-E; Millipore, MA)-coated glass coverslips. After treatments as noted, cells were fixed (15 min at room temperature) in 4% paraformaldehyde, 0.025% glutaraldehyde, and 0.3% Triton in cytoskeleton buffer [10 mM 2-(N-morpholino)ethanesulfonic acid, 150 mM NaCl, 5 mM ethylene glycol tetraacetic acid, 5 mM glucose, and 5 mM MgCl2, pH 6.1]. Nonspecific binding was blocked using 5% horse serum in PBS and probed with an anti-α-tubulin antibody (catalog no. sc-53646; Santa Cruz Biotechnology; 1:500) for 1 h. An anti-mouse antibody conjugated to fluorescein isothiocyanate (catalog no. sc-2082; Santa Cruz Biotechnology; 1:500) was used as the secondary antibody. Coverslips were mounted on slides using MOWIOL mounting media (cat no. 81381; Sigma-Aldrich). Cells were visualized using an Olympus FV1000 inverted confocal microscope with a ×40/1.3 Plan-Neofluar or ×60/1.45 Plan-Apachromat oil-immersion lens at room temperature with FV10-ASW 1.7 acquisition software (Olympus, Tokyo, Japan) through the use of the Vanderbilt University Medical Center Cell Imaging Shared Resource. Between compared conditions, the same laser intensity and gain settings were used.
Cell size measures.
Cells cultured on glass coverslips were fixed, stained for VE-cadherin, and visualized as described above by a blinded observer. Cross-sectional areas (pixels) of each individual whole cell per field from three to four representative fields per condition were measured by a second blinded observer using ImageJ (1.47v; National Institutes of Health software). Individual cells were used as replicates.
Data analysis.
Results are shown as mean ± SD or SE, as noted. One-way ANOVA with Tukey's post hoc tests and unpaired, two-tailed t-tests were performed as noted using Prism 6 (GraphPad Software, La Jolla, CA). Differences with P < 0.05 were considered significant.
RESULTS
Cellular ascorbate loading decreases endothelial permeability and increases cell size.
HUVECs in culture without added ascorbate typically contain only trace amounts of the vitamin, probably derived from fetal calf serum (Fig. 1A). They can be loaded with either ascorbate via the SVCT2 or with DHA on the GLUT-type transporters. For the latter, DHA is rapidly taken up and reduced to ascorbate within the cells, keeping extracellular ascorbate concentrations low. Since we carried out experiments with both forms of ascorbate, it was necessary to compare intracellular levels and efficacy in tightening the endothelial permeability barrier. As shown in Fig. 1A, loading HUVECs with increasing concentrations of both forms of ascorbate over 90 min resulted in progressive increases in intracellular ascorbate, similar to bEnd.3 cells (34). The curve for ascorbate plateaued, whereas that for DHA was linear. This reflects the divergent Km values for the transporters involved. However, at the loading concentration of 100–150 μM used in the present experiments, intracellular ascorbate concentrations were similar for each form of the vitamin loaded. Changes in endothelial permeability over the same time as assessed by radiolabeled inulin transfer were similar and decreased 25–30% from basal with each form of ascorbate at 118 μM (Fig. 1B). Since extracellular ascorbate concentrations are very low with DHA loading, these results suggest that the intracellular ascorbate concentration is responsible for tightening the endothelial barrier. In subsequent studies we varied the loading form of ascorbate, using DHA in longer incubations, when there might be a risk of confounding effects due to redox cycling of ascorbate with traces of iron in the culture medium.
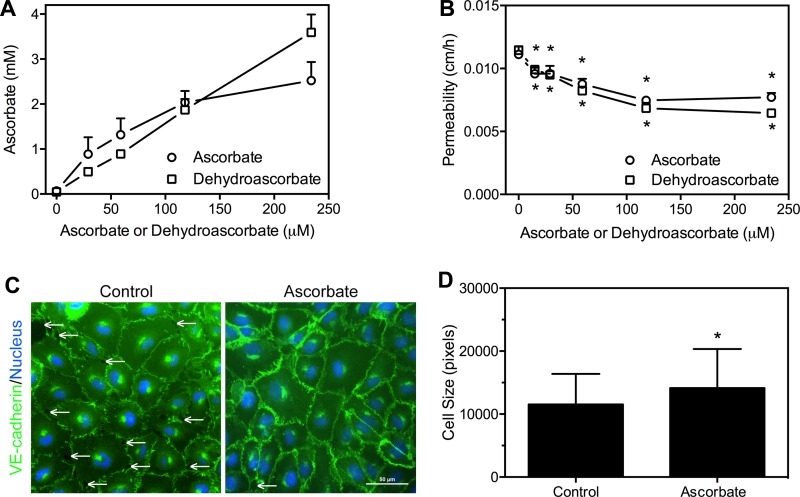
Fig. 1.
Ascorbate loading decreases endothelial barrier permeability and increases cell surface area. A: confluent human umbilical vein endothelial cells (HUVECs) were incubated for 90 min with the indicated concentrations of ascorbate or dehydroascorbic acid (DHA) and taken for assay of ascorbate as described under materials and methods. Results are from 4 plates with each form of ascorbate ± SE. B: HUVECs grown several days past confluence on Transwell filters were treated in the upper or luminal chamber with the indicated concentrations of ascorbate or DHA for 30 min before the 60-min radiolabeled inulin transfer assay. Results are shown from 5 experiments with DHA and 6 with ascorbate ± SE. *P < 0.05, compared with the respective sample not treated with ascorbate or DHA. C: confluent HUVECs on fibronectin-coated glass coverslips were treated overnight with 150 μM DHA, fixed, and stained for VE-cadherin to define cell boundaries as described in materials and methods. Cells were visualized at ×20 as described in materials and methods. Arrows indicate gaps between cells. D: quantification of cell size ± SD from 355 control and 232 DHA-treated cells by a second blinded observer. *P < 0.05, compared with control by nonpaired Student's t-testing.
If ascorbate tightens endothelial barrier permeability by decreasing gaps between the cells, this may be associated with an increase in cell size. To visualize this effect, confluent HUVEC monolayers were fixed and stained for VE-cadherin to define cell boundaries. Cells treated with 150 μM DHA overnight visibly decreased gaps between cells (Fig. 1C, compare number of arrows between conditions). In addition, ascorbate increased overall cell surface area of HUVECs by 23% compared with untreated cells (Fig. 1D). Taken together, the data from Fig. 1 suggest that while intracellular ascorbate is low in cultured HUVEC, loading cells with either form of ascorbate decreases permeability across the endothelial barrier by diminishing gaps between cells and increasing cell surface area.
Ascorbate decreases oxidative stress in cultured endothelial cells.
Another line of endothelial cells experiences mild oxidative stress under standard culture conditions (42). Oxidative stress is well known to cause endothelial barrier dysfunction (30). If the endothelial cells used in this study also experience oxidative stress from basal culture conditions, this may partly explain why ascorbate, a classic antioxidant, promotes their barrier function. To determine whether the endothelial cells used in the current studies generate a basal level of oxidative stress that could be decreased by ascorbate, HUVECs (Fig. 2A) and bEnd.3 (Fig. 2B) cells in 96-well plates were treated with ascorbate or DHA for 1 h and exposed either to DCFH-DA or to DHF-DA, respectively, for another hour. Both of these molecules diffuse into cells and are deacetylated by cellular esterases. With oxidative stress, DCFH is oxidized to DCF and DHF to fluorescein, which can be measured fluorescently. In HUVECs, both ascorbate and DHA at 150 μM lowered oxidative stress to 85% of basal levels (Fig. 2A). In bEnd.3 cells, ascorbate dose dependently decreased oxidative stress, becoming significant at 200 μM ascorbate (Fig. 2B).
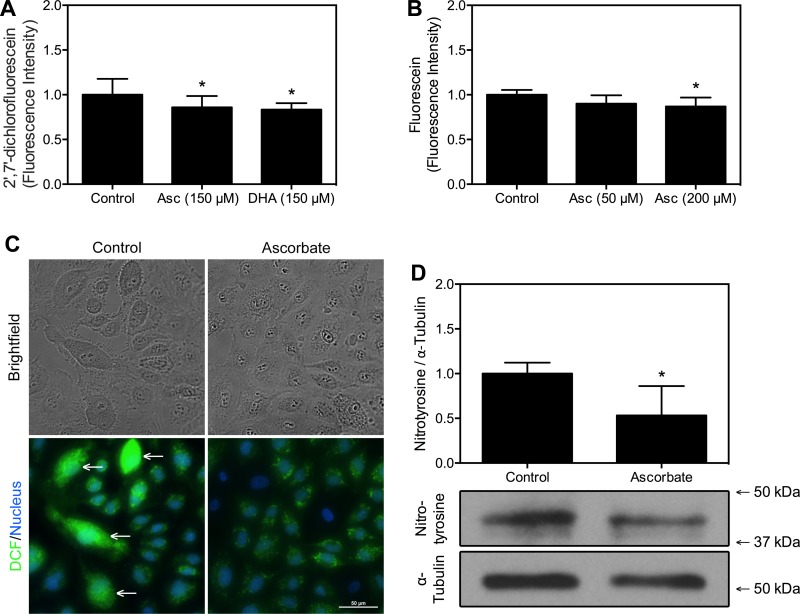
Fig. 2.
Ascorbate decreases oxidative stress markers in endothelial cells. A: HUVECs were exposed to 150 μM ascorbate (Asc), 150 μM DHA, or a media change for 1 h, then to 10 μM DCFH-DA in PBS for 1 h, and then rinsed and incubated in PBS for 1 h. Fluorescence intensity was measured and values were normalized relative to control; n = 24 wells per condition ± SD. B: BEnd.3 cells were exposed to 50 or 200 μM Asc for 30 min, to 20 μM dihydrofluorescin diacetate (DHF-DA) for 30 min, and then washed and incubated in Krebs-Ringer HEPES buffer (KRH). Fluorescence measures were taken at 0 and 36 min, and increase in fluorescence was quantified by subtracting initial from final values, which were then scaled relative to control; n = 7 wells per condition ± SD. C: HUVECs were treated as in A and DCF (green) and nuclei (Hoescht 33342; blue) were visualized as described in materials and methods. The reference bar shown measures 50 μm. White arrows point to discrete cells exhibiting high levels of fluorescence. Images are representative of 4 culture plates per condition. D: HUVECs were cultured for 5 days with 150 μM DHA-containing daily media changes and then lysed and subjected to Western blot analysis as described in materials and methods. The most prominent band formed at 42 kDa and was used for densitometry measures of nitrotyrosine, expressed relative to total α-tubulin; n = 4 lysates per condition. Molecular weight markers are indicated. Numerical results are shown as means ± SD. *P < 0.05, compared with the respective sample not treated with ascorbate or DHA.
To confirm these findings visually, HUVECs were treated with or without 150 μM DHA, exposed to DCFH-DA, and visualized with fluorescence microscopy (Fig. 2C). Despite slight variation in cell numbers per field, untreated HUVECs clearly exhibited more individual cells with high levels of fluorescence (white arrows), likely contributing to the overall higher fluorescence compared with DHA-treated cells.
As a final measure of oxidative stress, Western blot analysis was used to measure nitration of tyrosines in HUVECs (Fig. 2D). Tyrosine nitration is due largely to peroxynitrite, which is formed with the combination of either hydrogen peroxide and nitrite or superoxide and nitric oxide. Peroxynitrite in turn will increase tyrosine nitration (25). HUVECs were cultured for 5 days and treated with media changes or 150 μM DHA each day. Although nitration of tyrosines can occur on any protein containing a tyrosine, the most prominent band occurred at ∼42 kDa, and this was quantified relative to total α-tubulin. Ascorbate loading decreased nitration of this protein by almost 50%. Taken together, the data from Fig. 2 show that acute or chronic ascorbate loading decreases basal oxidative stress in both cell types.
Ascorbate depends on Epac1 but not PKA or Rap1 to tighten the endothelial barrier.
Despite our inability to show a significant change in cyclic AMP in untreated endothelial cells loaded with ascorbate (35), ascorbate may nonetheless activate one or more cyclic AMP-dependent pathways to tighten the endothelial permeability barrier. This could be due to a small or compartmentalized ascorbate-induced change in cyclic AMP through the NO/cyclic GMP pathway. It could also be due to reversal by ascorbate of an undetected decrease in cyclic AMP caused by the observed oxidative stress in basal cells (Fig. 2) (17, 43).
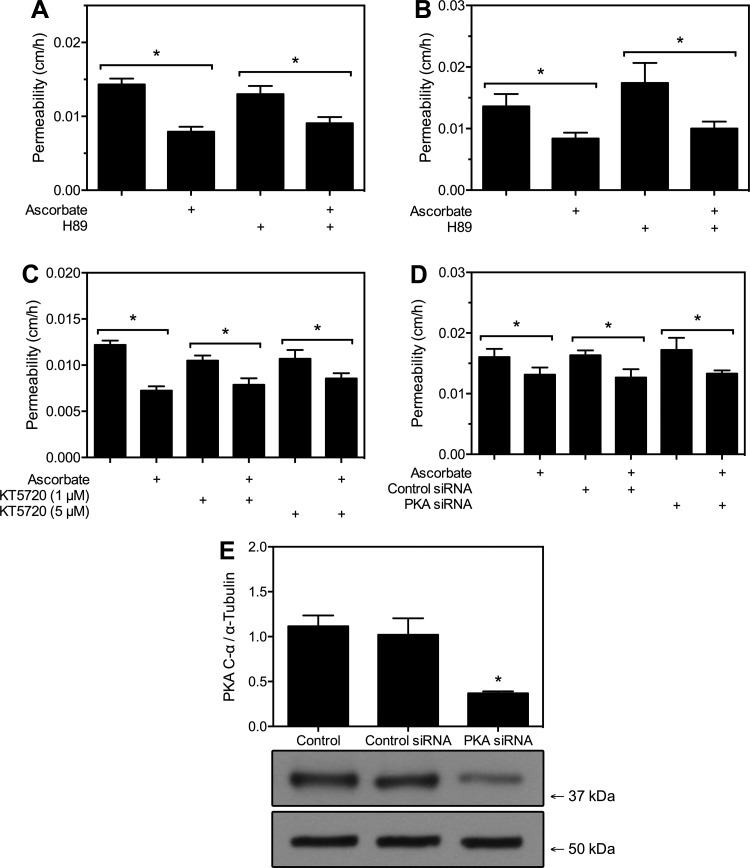
Cyclic AMP signaling is mediated by two canonical downstream effectors, PKA and Epac1 (6). To assess whether ascorbate might tighten endothelial permeability through one or both of these pathways, selective inhibitors of each were employed. First, PKA was inhibited in HUVECs with addition of 10 μM H89 for 20 min before the cells were loaded with 120 μM ascorbate over 30 min followed by the 60 min radiolabeled inulin transfer assay. As shown in Fig. 3A, H89 did not change basal radiolabeled inulin permeability nor did it prevent the decrease in permeability due to ascorbate. Similar results were seen with bEnd.3 cells following the treatment regimen (Fig. 3B). To confirm this finding, HUVECs were treated with the highly specific PKA inhibitor KT5720 in the same fashion (Fig. 3C). At moderate (1 μM) and high (5 μM) doses, KT5720 also failed to prevent barrier stabilization by ascorbate. As a third confirmation of this effect, HUVEC expression of the PKA C-α was knocked down with siRNA before treatment with ascorbate and the permeability assay (Fig. 3D). As with the PKA inhibitor treatments, decrease in the amount of PKA did not affect ascorbate action. Western blot was used to confirm the knockdown of PKA C-α, showing a 67% decrease in expression (Fig. 3E). Failure of PKA C-α knockdown, H89, and KT5270 to blunt the ascorbate effect on permeability suggests that PKA is not involved.
Fig. 3.
Barrier stabilization by ascorbate does not depend on PKA. A–C: postconfluent HUVECs (A and C) or bEnd.3 cells (B) cultured on Transwell filters were treated for 20 min with PKA inhibitors H89 or KT5720 as indicated, followed by a 30-min treatment with 120 μM ascorbate and then the inulin transfer assay. Results are shown ± SE from 5–6 experiments in A–C. D: siRNA knockdown of the catalytic subunit of PKA in HUVECs was performed as described in materials and methods 36 h before treatment with ascorbate and inulin transfer assay. Control siRNA was used for comparison. Results are shown ± SE from 7 experiments. E: Western blot for the catalytic subunit of PKA was performed on HUVECs exposed to PKA C-α siRNA or control siRNA for 36 h, as described in materials and methods. Values are relative to total α-tubulin; n = 3 lysates per condition ± SD. *P < 0.05, between samples under each bar.
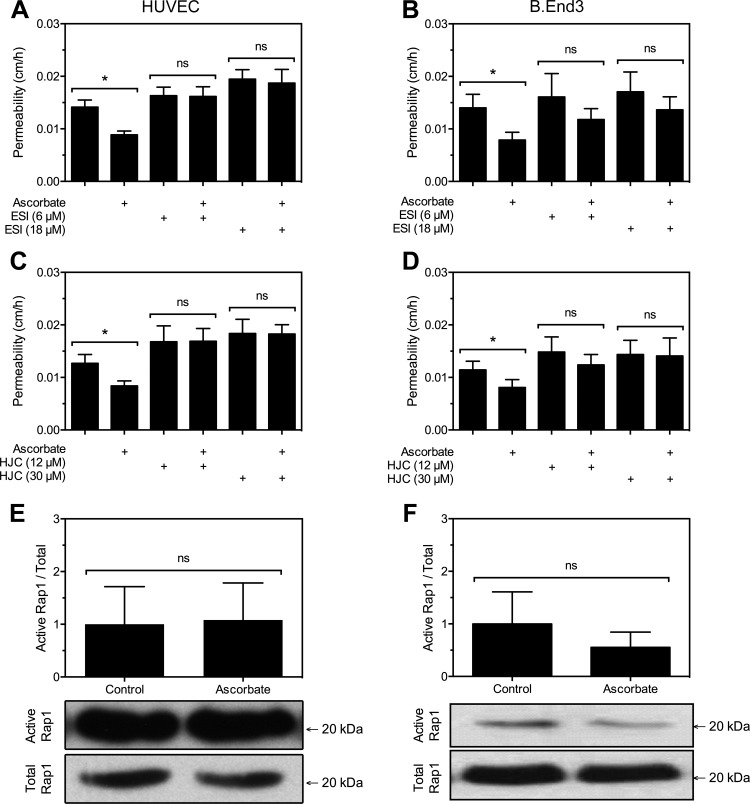
Next, the role of Epac1 in mediating endothelial barrier tightening by ascorbate was investigated with two Epac1 inhibitors. First, HUVECs were treated sequentially as above with 6 or 18 μM ESI09 for 20 min before addition of 120 μM ascorbate followed in 30 min by the radiolabeled inulin transfer assay (Fig. 4A). Increasing concentrations of ESI09 did not affect permeability. However, both concentrations of ESI09 completely prevented ascorbate from decreasing basal permeability. The same treatment of bEnd.3 cells also showed that ESI09 prevented a significant decrease in the ability of ascorbate to tighten the endothelial barrier (Fig. 4B). Second, an analogous experiment using 12 μM and 30 μM concentrations of the Epac1 inhibitor HJC0197 again completely prevented tightening of the endothelial barrier by ascorbate in both HUVECs (Fig. 4C) and bEnd.3 cells (Fig. 4D). Together, these results suggest that Epac1 is required for ascorbate barrier stabilization.
Fig. 4.
Barrier stabilization by ascorbate depends on Epac1 but not Rap1. A–D: postconfluent HUVECs (A and C) or bEnd.3 cells (B and D) cultured on Transwell filters were treated for 20 min with Epac1 inhibitors ESI09 (ESI) or HJC0197 (HJC) as indicated, followed by a 30-min treatment with 120 μM ascorbate and then the inulin transfer assay. Results are shown ± SE from 5–6 experiments in A–D. G–H: confluent HUVECs (E) or bEnd.3 cells (F) were treated for 30 min with 100 μM ascorbate and taken for assay of GTP-bound (active) and total Rap1 by immunoprecipitation and Western blotting as described in materials and methods. Molecular mass markers are indicated. Results are shown ± SD from 4–8 experiments. *P < 0.05, between samples under each bar; ns, no significance.
Epac1 classically activates the small GTPase Rap1 that is associated with many functions of cyclic AMP, including endothelial barrier stability (6, 35). Since Rap1 is active when bound to GTP, the GTP-bound protein was isolated in a pull-down assay. Western blotting was then used to detect GTP-bound Rap1 vs. total Rap1. Ascorbate treatment for 60 min at 100 μM did not significantly affect the amount of active Rap1 in HUVECs (Fig. 4E) or bEnd.3 cells (Fig. 4F). Combined, the data from Figs. 3 and 4 show that ascorbate stabilized the endothelial barrier through a Rap1-independent mechanism that requires Epac1 and does not involve PKA.
Ascorbate decreases drug-induced depolymerization of tubulin.
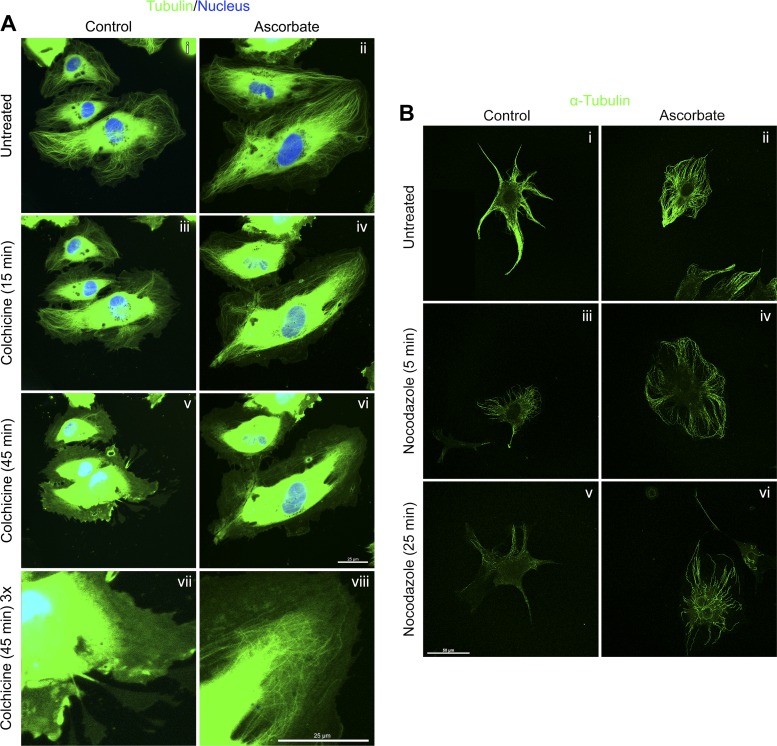
Epac1 can work independently of Rap1 to stabilize the endothelium by associating with tubulin and promoting microtubule polymerization (38). We hypothesized that ascorbate might affect tubulin via Epac1 to tighten the endothelial barrier. To test this in HUVECs, cells grown with or without 150 μM DHA for 5 days were transfected with GFP-tagged tubulin and treated with the microtubule depolymerizing agent colchicine (Fig. 5A). The greatest depolymerizing effect in live cells was seen 45 min after the addition of colchicine. Whereas both control and ascorbate-treated cells showed depolymerization, at 45 min microtubules were visibly better maintained in cells loaded with ascorbate. This is best shown at three times the magnification in Fig. 5, Avii and Aviii. Of note, overexposure of the tubulin signal in the center of cells was necessary to visualize individual microtubules in the cell periphery after colchicine treatment. However, it is the peripheral microtubules that are critical to endothelial barrier function (1).
Fig. 5.
Ascorbate delays drug-induced microtubule depolymerization. A: subconfluent HUVECs grown with or without daily addition of 150 μM DHA for 5 days were transfected with CellLight Tubulin-GFP overnight and then exposed to RedDot1 nuclear counterstain (blue) for 30 min. The fields were first visualized at ×63 using a Zeiss LSM 510 META inverted confocal microscope (i and ii). Plates were then treated with 2.5 μM colchicine and the same fields were visualized after 15 min (iii and iv) and 45 min (v and vi). The bottom images (vii and viii) represent a digital ×3 magnification of the panel above. B: subconfluent bEnd.3 cells were treated with a media change or 100 μM ascorbate for 1 h. Nocodazole (10 μM) was added for 0 min (i and ii), 5 min (iii and iv), or 25 min (v and vi). Cells were then fixed and stained for α-tubulin as described in materials and methods. Images were captured with an Olympus FV1000 inverted confocal microscope at ×60. All images are representative of several fields per condition, and size of field is indicated with scale bars.
To further establish ascorbate's protection of microtubules, bEnd.3 cells treated with 100 μM ascorbate for 1 h were then exposed to 10 μM of the microtubule depolymerizing agent nocodazole for 5 or 25 min, fixed, and stained for α-tubulin (Fig. 5B). While the microtubule amounts and patterns differ in the two cell types studied, nocodazole depolymerized microtubules in control bEnd.3 cells and ascorbate visibly preserved the microtubule network. In particular, ascorbate seems to selectively preserve microtubules extending to the periphery of the cell, associated with endothelial stability, over central microtubule stability (Fig. 5, Bv and Bvi). Together, these experiments implicate ascorbate as a protector of peripheral microtubules, which supports a role for Epac1 in promoting endothelial barrier integrity.
Ascorbate promotes acetylation of α-tubulin in HUVECs.
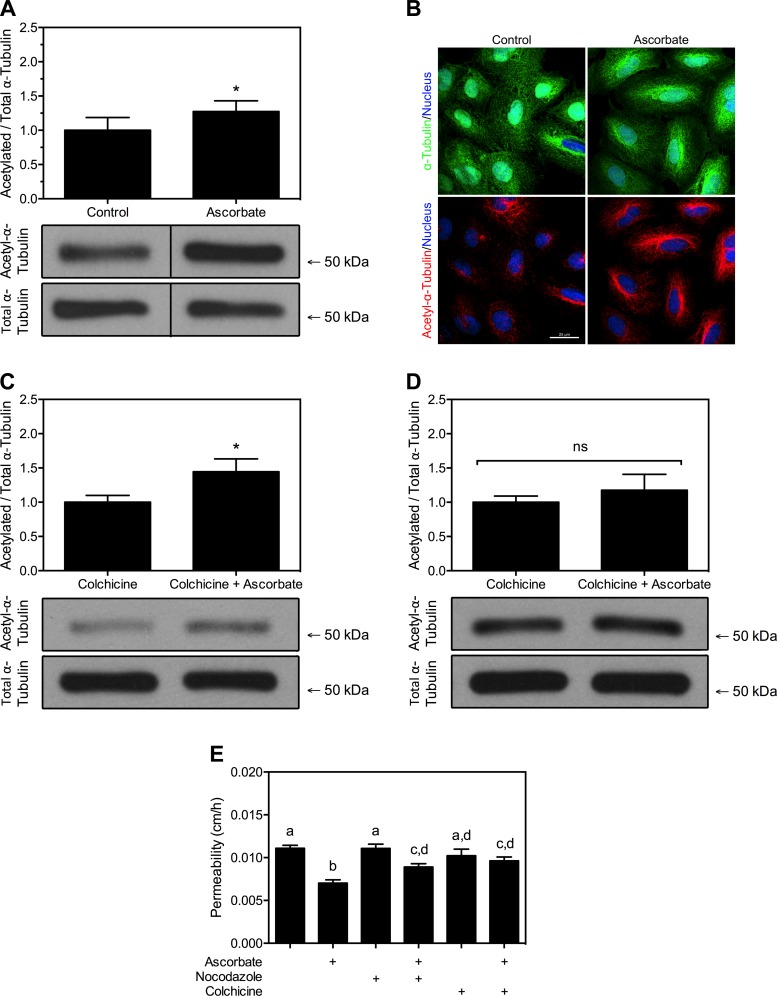
A well-characterized effect of microtubule polymerizing agents is their ability to increase acetylation of α-tubulin, a hallmark of stable microtubules (37). To test if ascorbate stabilizes microtubules by increasing α-tubulin acetylation, the levels in HUVECs were measured after ascorbate loading. HUVECs were cultured for 5 days and treated with a media change or 150 μM DHA each day. Cells were then lysed and subjected to Western blot for acetyl-α-tubulin vs. total α-tubulin (Fig. 6A). Ascorbate increased α-tubulin acetylation significantly by 27% compared with control cells.
Fig. 6.
Ascorbate increases acetylated α-tubulin in HUVECs. A: HUVECs cultured with or without daily addition of 150 μM DHA for 5 days were lysed and probed for either total or acetylated α-tubulin via Western blot analysis, as described in materials and methods. B: HUVECs grown on coverslips were treated with DHA as described in A and then fixed and probed for total (green) and acetylated (red) α-tubulin with RedDot1 nuclear counterstain (blue). Images were captured with an Olympus FV1000 inverted confocal microscope at ×40 and are representative of at least 10 fields per condition. C: 150 μM DHA was added to HUVECs for 60 min and then 2.5 μM colchicine was added for 30 min. Western blot was performed as in A. D: 2.5 μM colchicine was added to HUVECs for 30 min and then 150 μM DHA was added for 60 min. Western blot was performed as in A. All Western blots had n = 4–5 samples per condition and are expressed as means ± SD. *P < 0.05; ns, no significance. Molecular mass markers are indicated. E: postconfluent HUVECs on Transwell filters were treated for 60 min with ascorbate and then for 30 min with 10 μM nocodazole or 2.5 μM colchicine. Cells were then subjected to an inulin transfer assay; n = 10 per condition. Bars not having the same lowercase letters are different at P < 0.05.
To confirm the increase in acetylated tubulin due to ascorbate in HUVECs, cells were grown with or without DHA in the same fashion, fixed and probed for acetylated and total α-tubulin and then visualized via fluorescence microscopy (Fig. 6B). With the use of the same laser intensity and gain settings, ascorbate visibly increased acetylated α-tubulin relative to control, while total α-tubulin remained the same between conditions. In addition, ascorbate seemed to promote the extension of acetylated α-tubulin to the periphery of the cell, whereas control HUVECs exhibited largely perinuclear localization of acetyl-α-tubulin.
Next, to provide a quantitative counterpart to the data from Fig. 5, α-tubulin acetylation was measured in HUVECs under microtubule-depolymerizing conditions. In HUVECs treated for 60 min with DHA and then exposed to 2.5 μM colchicine for 30 min, ascorbate was observed to significantly protect the cells from a 34% drop in acetyl-α-tubulin (Fig. 6C). Since ascorbate protects microtubules from depolymerization, the next question was whether ascorbate could reverse microtubule depolymerization after it had already occurred. To test this, colchicine was added to HUVECs first for 30 min before the addition of DHA for an additional 60 min (Fig. 6D). No statistical difference was noted when DHA was added last, suggesting that ascorbate's role in protecting the endothelial cell takes place before depolymerization occurs.
As a final confirmation that the interaction between ascorbate and microtubules affects endothelial permeability, inulin transfer assays were performed with nocodazole and colchicine (Fig. 6E). Neither agent independently affected basal permeability (compare bars 1, 3, and 5). However, both agents blunted ascorbate from fully stabilizing the endothelial layer (compare bars 2, 4, and 6). These data indicate that ascorbate's ability to protect against microtubule destabilizing agents may not be enough to fully rescue the endothelium, which is consistent with the only-partial rescue of microtubules in the data from Fig. 5. Importantly, these data suggest that under basal conditions, ascorbate works in a manner that requires an intact microtubule network. This finding emphasizes the importance of microtubule stabilization by ascorbate as a means by which the vitamin promotes endothelial integrity.
Ascorbate enhances association of Epac1 with α-tubulin in HUVECs.
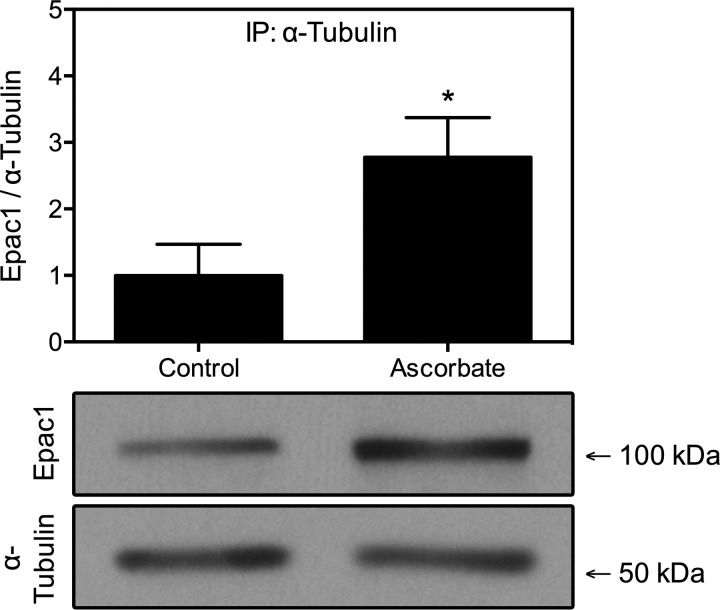
The Rap1-independent mechanism by which Epac1 stabilizes microtubules involves the direct association of Epac1 with α-tubulin (38). If ascorbate increases microtubule stabilization through Epac1, then it should enhance this protein association. To test this, HUVECs were treated with a media change or 150 μM DHA twice, 24 h and then 2 h before cell lysis. Epac1 was immunoprecipitated from lysates, and Western blot analysis was used to detect α-tubulin. Ascorbate significantly increased the association of Epac1 with α-tubulin by >250% (Fig. 7). Immunofluorescence studies were performed to detect colocalization of Epac1 with α-tubulin, but differences were visually unapparent due to the large amount of diffuse, soluble Epac1 in both treatment conditions (results not shown).
Fig. 7.
Ascorbate increases binding of Epac1 to α-tubulin. HUVECs treated with 150 μM DHA at 24 and 2 h before lysis were immunoprecipitated with anti-α-tubulin antibody as described in materials and methods. Immunoprecipitates were subjected to Western blot analysis to detect Epac1. A second anti-α-tubulin antibody from a different host species was used to probe immunoprecipitated lysates for α-tubulin. Densitometry measures of Epac1/total α-tubulin are expressed as means ± SD. *P < 0.05 (paired Student's t-test).
DISCUSSION
The studies presented here show a novel mechanism by which ascorbate decreases endothelial barrier permeability through Epac1. Both reduced and oxidized ascorbate tightened the basal endothelial permeability barrier by ∼30% in HUVECs and bEnd.3 cells. Barrier tightening was half-maximal at ∼1 mM intracellular ascorbate in HUVECs and complete at concentrations of 2 mM and greater. Loading cells to these concentrations in the present studies occurred at extracellular ascorbate concentrations in the upper physiologic plasma range of 60–120 μM. Together, these results suggest that the endothelial permeability barrier tightening is a physiologic response to ascorbate.
Ascorbate appears to tighten the basal endothelial permeability barrier by at least two mechanisms. The first may be due to the antioxidant function of ascorbate. Human aortic endothelial cells under presumably basal culture conditions show significant increases in oxidative stress markers, which are decreased by ascorbate loading of the cells (42). We also found that ascorbate loading of both HUVECs and bEnd.3 cells modestly decreased oxidation of reduced fluorescein derivatives and decreased nitrotyrosine formation in HUVECs. Since increases in reactive species cause leaks in the endothelial permeability barrier (28, 51), it is possible that ascorbate acted as a primary antioxidant and scavenge reactive oxygen or nitrogen species (i.e., superoxide or peroxynitrite, respectively). The molecular mechanism by which oxidative stress increases endothelial permeability is likely multifactorial, since changes in both intracellular calcium (39) and cyclic AMP (17) have been implicated. Regarding the latter as a likely second mechanism, the present data suggest that, just as with thrombin (35), a cyclic AMP-dependent pathway is responsible for tightening of the endothelial permeability barrier by ascorbate.
Previous studies from our laboratory showed that tightening of the permeability barrier in unstimulated endothelial cells required NO generated by eNOS (32). More recently, we found that ascorbate doubled cellular cyclic GMP concentrations in untreated HUVECs (35). Although an increase in cyclic GMP should increase cyclic AMP through inhibition of phosphodiesterase III, this was not observed (35). Nonetheless, the current results suggest that a cyclic AMP-dependent pathway does in fact help tighten the basal endothelial permeability after ascorbate loading. Cyclic AMP is well known to decrease permeability in endothelial cells (3, 11, 36). This can occur by two distinct pathways. First, activation of PKA by cyclic AMP initiates a well-established signaling cascade that ultimately stabilizes the cytoskeleton and adhesive structures that hold cells together (50). Second, cyclic AMP directly activates the guanine-nucleotide exchange factor Epac1, which then activates the small GTPase Rap1 (10). The latter initiates downstream signaling cascades to rearrange the cytoskeleton and increase adhesion protein interactions between cells (21).
Our finding that the PKA inhibition failed to affect ascorbate-induced barrier tightening in both endothelial cell types suggests that the PKA pathway has minimal involvement in the ascorbate effect. Rather, our results for the first time reported support activation of an Epac1-dependent pathway in the barrier tightening effect of ascorbate. Epac1 is the primary isoform expressed in endothelial cells and is intimately involved in cell adhesion (15, 16, 27, 38). In the current study, inhibition of Epac1 by ESI09 (2) and HJC0197 (8) prevented ascorbate-dependent barrier tightening in HUVECs and partially did so in bEnd.3 cells. However, as when Epac1 was specifically activated by 8-pCPT-2′-O-Me-cyclic AMP (38), there was no associated activation of Rap1. This could be explained by data showing that the cytoskeleton and microtubules in particular sequester Epac1, which in turn prevents it from binding and activating Rap1.
Ascorbate increased acetylated α-tubulin and partially inhibited microtubule depolymerization by nocodazole and colchicine. Both effects are associated with increased microtubule stabilization (37). Importantly, this is not the first time ascorbate has been shown to promote microtubule assembly. Clinical data have long shown an improvement of microtubule polymerization with the addition of ascorbate in the function of polymorphonuclear leukocytes (46), particularly in Chediak-Higashi Syndrome where microtubule assembly is impaired (7). Enhanced microtubule assembly in these cells improved cell shape and restored physiologic function, but the molecular mechanism was not understood. Our findings similarly conclude a role for ascorbate in enhancing the microtubule network in endothelial cells and newly suggest this as a function of Epac1 binding α-tubulin. Epac1 activation by 8-pCPT-2′-O-Me-cyclic AMP (38) both increased Epac1 binding to α-tubulin and enhanced peripheral microtubule elongation. Although one might expect microtubule stabilization to affect cortical actin and its association with VE-cadherin, in confluent HUVECs this was not evident by qualitative immunocytochemistry (35). Evidence that ascorbate did actually modify the cortical actin ring is our finding that it increased endothelial surface area, which would close gaps between the cells. Together, these findings suggest that ascorbate tightens the endothelial barrier by stabilizing the microtubule infrastructure required for barrier maintenance.
In conclusion, the proximal mechanisms by which ascorbate tightens the basal endothelial permeability barrier depend on its ability to preserve NO and possibly to scavenge reactive oxygen and nitrogen species as previously shown. The final pathway involves activation of Epac1, leading to its binding to microtubules and their stabilization. Given that physiologic ascorbate concentrations tighten the endothelial permeability barrier, persons with low ascorbate levels might be subject to increased capillary leakage.
GRANTS
This work was supported by National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-050435 and T32-DK-007061, and by the Cell Culture Core of the Vanderbilt Diabetes Research and Training Center (DK-020593). Microscope image acquisition was performed in part through the use of the Vanderbilt University Medical Center Cell Imaging Shared Resource (supported by National Institutes of Health Grants CA-68485, DK-20593, DK-58404, DK-59637, and EY-08126).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
W.H.P., E.M.R., and J.M.M. conception and design of research; W.H.P., E.M.R., Z.-c.Q., and M.R.H. performed experiments; W.H.P., E.M.R., M.R.H., and J.M.M. analyzed data; W.H.P., E.M.R., M.R.H., and J.M.M. interpreted results of experiments; W.H.P., E.M.R., and J.M.M. prepared figures; W.H.P., E.M.R., and J.M.M. drafted manuscript; W.H.P., E.M.R., and J.M.M. edited and revised manuscript; W.H.P., E.M.R., Z.-c.Q., M.R.H., and J.M.M. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address of E. M. Rhea: Department of Medicine, University of Washington, Seattle, WA 98195-5852.
REFERENCES
- 1.Alieva IB, Zemskov EA, Smurova KM, Kaverina IN, Verin AD. The leading role of microtubules in endothelial barrier dysfunction: disassembly of peripheral microtubules leaves behind the cytoskeletal reorganization. J Cell Biochem 114: 2258–2272, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almahariq M, Tsalkova T, Mei FC, Chen H, Zhou J, Sastry SK, Schwede F, Cheng X. A novel EPAC-specific inhibitor suppresses pancreatic cancer cell migration and invasion. Mol Pharmacol 83: 122–128, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birukova AA, Burdette D, Moldobaeva N, Xing J, Fu P, Birukov KG. Rac GTPase is a hub for protein kinase A and Epac signaling in endothelial barrier protection by cAMP. Microvasc Res 79: 128–138, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birukova AA, Zagranichnaya T, Fu P, Alekseeva E, Chen W, Jacobson JR, Birukov KG. Prostaglandins PGE(2) and PGI(2) promote endothelial barrier enhancement via PKA- and Epac1/Rap1-dependent Rac activation. Exp Cell Res 313: 2504–2520, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bordignon B, Mones S, Rahman F, Chiron J, Peiretti F, Vidal N, Fontes M. A derivative of ascorbic acid modulates cAMP production. Biochem Biophys Res Commun 439: 137–141, 2013. [DOI] [PubMed] [Google Scholar]
- 6.Borland G, Smith BO, Yarwood SJ. EPAC proteins transduce diverse cellular actions of cAMP. Br J Pharmacol 158: 70–86, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boxer LA, Albertini DF, Baehner RL, Oliver JM. Impaired microtubule assembly and polymorphonuclear leucocyte function in the Chediak-Higashi syndrome correctable by ascorbic acid. Br J Haematol 43: 207–213, 1979. [DOI] [PubMed] [Google Scholar]
- 8.Chen H, Tsalkova T, Mei FC, Hu Y, Cheng X, Zhou J. 5-Cyano-6-oxo-1,6-dihydro-pyrimidines as potent antagonists targeting exchange proteins directly activated by cAMP. Bioorg Med Chem Lett 22: 4038–4043, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cullere X, Shaw SK, Andersson L, Hirahashi J, Luscinskas FW, Mayadas TN. Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood 105: 1950–1955, 2005. [DOI] [PubMed] [Google Scholar]
- 10.de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396: 474–477, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Deli MA, Dehouck MP, Abraham CS, Cecchelli R, Joo F. Penetration of small molecular weight substances through cultured bovine brain capillary endothelial cell monolayers: the early effects of cyclic adenosine 3′,5′-monophosphate. Exp Physiol 80: 675–678, 1995. [DOI] [PubMed] [Google Scholar]
- 12.Draijer R, Atsma DE, van der LA, van Hinsbergh VW. cGMP and nitric oxide modulate thrombin-induced endothelial permeability. Regulation via different pathways in human aortic and umbilical vein endothelial cells. Circ Res 76: 199–208, 1995. [DOI] [PubMed] [Google Scholar]
- 13.Dudek SM, Jacobson JR, Chiang ET, Birukov KG, Wang P, Zhan X, Garcia JG. Pulmonary endothelial cell barrier enhancement by sphingosine 1-phosphate: roles for cortactin and myosin light chain kinase. J Biol Chem 279: 24692–24700, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Ehringer WD, Edwards MJ, Miller FN. Mechanisms of alpha-thrombin, histamine, and bradykinin induced endothelial permeability. J Cell Physiol 167: 562–569, 1996. [DOI] [PubMed] [Google Scholar]
- 15.Fukuhara S, Sakurai A, Sano H, Yamagishi A, Somekawa S, Takakura N, Saito Y, Kangawa K, Mochizuki N. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol Cell Biol 25: 136–146, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gloerich M, Bos JL. Epac: defining a new mechanism for cAMP action. Annu Rev Pharmacol Toxicol 50: 355–375, 2010. [DOI] [PubMed] [Google Scholar]
- 17.Hastie LE, Patton WF, Hechtman HB, Shepro D. H2O2-induced filamin redistribution in endothelial cells is modulated by the cyclic AMP-dependent protein kinase pathway. J Cell Physiol 172: 373–381, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Heller R, Münscher-Paulig F, Gräbner R, Till U. l-ascorbic acid potentiates nitric oxide synthesis in endothelial cells. J Biol Chem 274: 8254–8260, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Heller R, Unbehaun A, Schellenberg B, Mayer B, Werner-Felmayer G, Werner ER. l-ascorbic acid potentiates endothelial nitric oxide synthesis via a chemical stabilization of tetrahydrobiopterin. J Biol Chem 276: 40–47, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Hirose A, Tanikawa T, Mori H, Okada Y, Tanaka Y. Advanced glycation end products increase endothelial permeability through the RAGE/Rho signaling pathway. FEBS Lett 584: 61–66, 2010. [DOI] [PubMed] [Google Scholar]
- 21.Horai S, Nakagawa S, Tanaka K, Morofuji Y, Couraud PO, Deli MA, Ozawa M, Niwa M. Cilostazol strengthens barrier integrity in brain endothelial cells. Cell Mol Neurobiol 33: 291–307, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang W, Yang Z, Lee D, Copolov DL, Lim AT. Ascorbic acid enhances forskolin-induced cyclic AMP production and pro-ANF mRNA expression of hypothalamic neurons in culture. Endocrinology 132: 2271–2273, 1993. [DOI] [PubMed] [Google Scholar]
- 23.Jones W, Li X, Perriott LM, Whitesell RR, May JM. Uptake, recycling, and antioxidant functions of α-lipoic acid in endothelial cells. Free Radic Biol Med 33: 83–93, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Kaya F, Belin S, Bourgeois P, Micaleff J, Blin O, Fontes M. Ascorbic acid inhibits PMP22 expression by reducing cAMP levels. Neuromuscul Disord 17: 248–253, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Knepler JL Jr, Taher LN, Gupta MP, Patterson C, Pavalko F, Ober MD, and Hart CM. Peroxynitrite causes endothelial cell monolayer barrier dysfunction. Am J Physiol Cell Physiol 281: C1064–C1075, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Komarova Y, Malik AB. Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu Rev Physiol 72: 463–493, 2010. [DOI] [PubMed] [Google Scholar]
- 27.Kooistra MR, Corada M, Dejana E, Bos JL. Epac1 regulates integrity of endothelial cell junctions through VE-cadherin. FEBS Lett 579: 4966–4972, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Lee HS, Namkoong K, Kim DH, Kim KJ, Cheong YH, Kim SS, Lee WB, Kim KY. Hydrogen peroxide-induced alterations of tight junction proteins in bovine brain microvascular endothelial cells. Microvasc Res 68: 231–238, 2004. [DOI] [PubMed] [Google Scholar]
- 29.López-Lluch G, Burón MI, Alcaín FJ, Quesada JM, Navas P. Redox regulation of cAMP levels by ascorbate in 1,25-dihydroxy-vitamin D3-induced differentiation of HL-60. Biochem J 331: 21–27, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lum H, Roebuck KA. Oxidant stress and endothelial cell dysfunction. Am J Physiol Cell Physiol 280: C719–C741, 2001. [DOI] [PubMed] [Google Scholar]
- 31.May JM, Qu ZC. Ascorbic acid prevents increased endothelial permeability caused by oxidized low density lipoprotein. Free Radic Res 44: 1359–1368, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.May JM, Qu ZC. Nitric oxide mediates tightening of the endothelial barrier by ascorbic acid. Biochem Biophys Res Commun 404: 701–705, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.May JM, Qu ZC, Qiao H. Transfer of ascorbic acid across the vascular endothelium: mechanism and self-regulation. Am J Physiol Cell Physiol 297: C169–C178, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meredith ME, Qu ZC, May JM. Ascorbate reverses high glucose- and RAGE-induced leak of the endothelial permeability barrier. Biochem Biophys Res Commun 445: 30–35, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parker WH, Qu ZC, May JM. Intracellular ascorbate prevents endothelial barrier permeabilization by thrombin. J Biol Chem 290: 21486–21497, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patterson CE, Lum H, Schaphorst KL, Verin AD, Garcia JG. Regulation of endothelial barrier function by the cAMP-dependent protein kinase. Endothelium 7: 287–308, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Piperno G, LeDizet M, Chang XJ. Microtubules containing acetylated alpha-tubulin in mammalian cells in culture. J Cell Biol 104: 289–302, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sehrawat S, Cullere X, Patel S, Italiano J Jr, Mayadas TN. Role of Epac1, an exchange factor for Rap GTPases, in endothelial microtubule dynamics and barrier function. Mol Biol Cell 19: 1261–1270, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shasby DM, Lind SE, Shasby SS, Goldsmith JC, Hunninghake GW. Reversible oxidant-induced increases in albumin transfer across cultured endothelium: alterations in cell shape and calcium homeostasis. Blood 65: 605–614, 1985. [PubMed] [Google Scholar]
- 40.Siflinger-Birnboim A, del Vecchio PJ, Cooper JA, Blumenstock FA, Shepard JM, Malik AB. Molecular sieving characteristics of the cultured endothelial monolayer. J Cell Physiol 132: 111–117, 1987. [DOI] [PubMed] [Google Scholar]
- 41.Siflinger-Birnboim A, Lum H, del Vecchio PJ, Malik AB. Involvement of Ca2+ in the H2O2-induced increase in endothelial permeability. Am J Physiol Lung Cell Mol Physiol 270: L973–L978, 1996. [DOI] [PubMed] [Google Scholar]
- 42.Smith AR, Visioli F, Hagen TM. Vitamin C matters: increased oxidative stress in cultured human aortic endothelial cells without supplemental ascorbic acid. FASEB J 16: 1102–1104, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Suttorp N, Weber U, Welsch T, Schudt C. Role of phosphodiesterases in the regulation of endothelial permeability in vitro. J Clin Invest 91: 1421–1428, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Hinsbergh WM. Endothelial permeability for macromolecules. Mechanistic aspects of pathophysiological modulation. Arterioscler Thromb Vasc Biol 17: 1018–1023, 1997. [DOI] [PubMed] [Google Scholar]
- 45.Vandenbroucke E, Mehta D, Minshall R, Malik AB. Regulation of endothelial junctional permeability. Ann NY Acad Sci 1123: 134–145, 2008. [DOI] [PubMed] [Google Scholar]
- 46.Vogel RI, Lamster IB, Wechsler SA, Macedo B, Hartley LJ, Macedo JA. The effects of megadoses of ascorbic acid on PMN chemotaxis and experimental gingivitis. J Periodontol 57: 472–479, 1986. [DOI] [PubMed] [Google Scholar]
- 47.Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med 27: 612–616, 1999. [DOI] [PubMed] [Google Scholar]
- 48.Warboys CM, Toh HB, Fraser PA. Role of NADPH oxidase in retinal microvascular permeability increase by RAGE activation. Invest Ophthalmol Vis Sci 50: 1319–1328, 2009. [DOI] [PubMed] [Google Scholar]
- 49.Yang Z, Copolov DL, Lim AT. Ascorbic acid augments the adenylyl cyclase-cAMP system mediated POMC mRNA expression and beta-endorphin secretion from hypothalamic neurons in culture. Brain Res 706: 243–248, 1996. [DOI] [PubMed] [Google Scholar]
- 50.Yuan SY. Protein kinase signaling in the modulation of microvascular permeability. Vascul Pharmacol 39: 213–223, 2002. [DOI] [PubMed] [Google Scholar]
- 51.Zhao Y, Davis HW. Hydrogen peroxide-induced cytoskeletal rearrangement in cultured pulmonary endothelial cells. J Cell Physiol 174: 370–379, 1998. [DOI] [PubMed] [Google Scholar]