pituitary adenylate cyclase-activating polypeptide (PACAP) exerts a variety of physiological effects in the nervous, endocrine, gastrointestinal, immune, and cardiovascular systems. For example, it is a neurotrophic factor; it modulates synaptic transmission and cellular excitability; it controls metabolic function/energy homeostasis; and it plays key roles in the coordinated physiological response to stress (5, 6, 10). The two closely related PACAP peptides (a 38 amino acid peptide and a C-terminal truncated 27 amino acid peptide) show similar pharmacology/physiology mediated through a family of three G protein-coupled receptors (GPCRs) (3). The PAC1 receptor is selectively activated by PACAP, whereas VPAC1 and VPAC2 are also activated by VIP (vasoactive intestinal peptide). PAC1 can couple to both Gs and Gq type G proteins and recruit their respective canonical downstream signaling pathways (adenylate cyclase/cAMP/PKA, or phospholipase C/IP3/DAG/PKC). Adding to this complexity, signaling endosomes might also contribute to the effects of PACAP/PAC1 (4). Originally, β-arrestin-dependent endocytosis of GPCRs was thought to terminate signaling and the receptor was then either recycled to the plasma membrane for reuse or targeted for degradation. Now it is clear that, at least in some cases, the endosomal receptors/β-arrestin can serve as a platform to recruit a second wave of signaling via cAMP and/or MAP kinase pathways (1, 9). In this issue of American Journal of Physiology-Cell Physiology, Tompkins et al. (8) provide evidence that PAC1 receptors activate MEK/ERK via signaling endosomes to enhance NaV1.7 voltage-gated sodium channels and neuronal excitability in guinea pig cardiac ganglia. This insight expands the already complex repertoire of signaling pathways by which PACAP controls neuronal function.
PACAP is a cotransmitter in both sympathetic and parasympathetic preganglionic cholinergic nerves. The Parsons lab (e.g., Tompkins et al.) use a guinea pig atrial whole mount preparation which enables both immunohistochemical and electrophysiological (intracellular recording) investigation of the intact, innervated neurons in the intracardiac ganglia. Both exogenously applied and endogenously released PACAP acts via PAC1 receptors to increase excitability of the postganglionic cardiac neurons; under control conditions most neurons respond to a depolarizing current step with one or a few action potentials, but following exposure to PACAP the same stimulus evoked repeated action potentials (i.e., conversion from a phasic to tonic firing pattern). This enhanced excitability involves Gs/cAMP signaling pathways, extracellular calcium entry, enhancement of low voltage-activated (T-type) Ca2+ channels, and enhancement of a hyperpolarization-activated nonselective cation channel current (Ih). However, direct activation of adenylate cyclase/cAMP by forskolin did not fully recapitulate the effects of PACAP and, along with other work, this suggested that additional signaling pathways (MEK/ERK) were recruited by PAC1.
Tompkins et al. first confirmed that PACAP led to an increase in phosphorylated ERK (pERK) in cardiac ganglia in a time-dependent (5-20 min) and concentration-dependent (1–25 nM) manner. Notably, the increase in pERK was dramatically reduced when the preparation was treated at ambient room temperature rather than 37°C, consistent with endosomal PAC1 signaling. The MEK inhibitor PD98059 blocked the increase in pERK and markedly suppressed the increase in neuronal excitability produced by PACAP. However, it did not prevent the enhancement of T-type Ca2+ channels or Ih, which led the authors to consider other ion channel targets of MEK/ERK. After ruling out a role for Kv4.2 potassium channels, they focused on voltage-gated sodium channels. NaV1.7 channels are phosphorylated by ERK in sensory neurons (7), and Tompkins et al. found the cardiac ganglia express both NaV1.7 and NaV1.3 channels. Consequently, the authors probed the involvement of NaV1.7 using a recently reported selective inhibitor of this channel, PF-04856264. While block of NaV1.7 had no effect on basal action potential properties, it did reduce the increase in excitability produced by PACAP. On the basis of the current paper and a body of previous work the authors conclude that PACAP targets multiple signaling pathways and ion channels to increase neuronal excitability (Fig. 1).
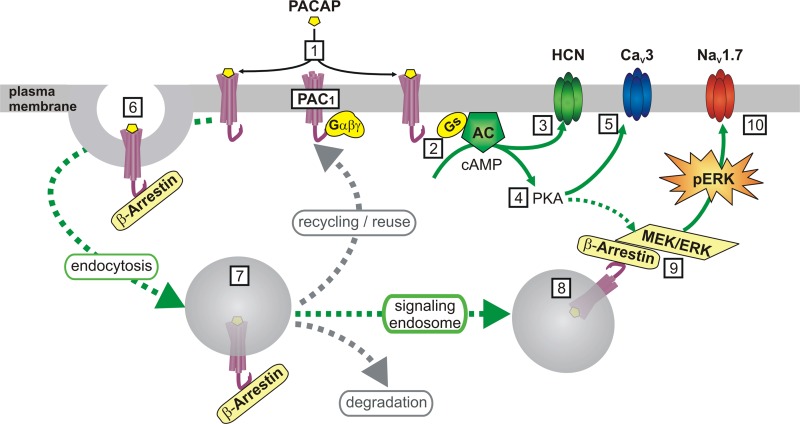
Fig. 1.
Schematic representation of the pathways leading from PACAP/PAC1 receptors to enhanced excitability of cardiac neurons. 1) PACAP released from preganglionic cholinergic nerve terminals binds to PAC1 receptors on the cardiac neurons and initiates signaling through canonical G protein-coupled pathways and via signaling endosomes. 2) Activation of Gs-type G proteins stimulates cAMP production by adenylate cyclase (AC); 3) cAMP enhances activity of the Ih current mediated by HCN channels, and hence excitability; 4) cAMP activates PKA; 5) PKA phosphorylates CaV3 Ca2+channels to enhance T-type Ca2+ current and hence excitability; 6) interaction with β-arrestin triggers clathrin-mediated endocytosis of PAC1 receptors; 7) endosomal PAC1 can be recycled to the plasma membrane for reuse, targeted for degradation, or form signaling endosomes; 8) PAC1/β-arrestin forms a scaffold to recruit the MEK/ERK signaling cascade. cAMP and/or PKA might also facilitate MEK/ERK signaling; 9) MEK leads to an increase in phosphorylated ERK (pERK); 10) pERK is proposed to phosphorylate NaV1.7 sodium channels to shift gating properties and enhance excitability.
Several interesting questions remain. Notably, the authors did not actually demonstrate modulation of sodium channel currents by PACAP. This will require future experiments with isolated cells that permit better voltage-clamp than the whole mount preparation used in this study. Nevertheless, the gating properties of NaV1.7 (slow closed-state inactivation) make it an attractive candidate to function at near threshold potentials and thus control membrane excitability, as demonstrated in sensory neurons (2). Moreover, MEK/ERK signaling shifts the gating of NaV1.7 in sensory neurons, so it will be interesting to see whether the proposed paradigm of PAC1/ERK signaling from endosomes also controls the excitability of sensory or sympathetic neurons where PAC1 and NaV1.7 are also expressed. The temporal dynamics of PACAP signaling within the cardiac ganglia (and other sites) is also of interest. Neuropeptides are typically thought to be released from large dense core vesicles upon strong presynaptic stimulation. Presumably activation of Gs/cAMP/PKA at the cell surface will occur initially, but the proposed role for signaling endosomes adds another dimension by potentially recruiting a second wave of distinct signaling events (MEK/ERK). Perhaps this prolongs the impact of PACAP, and it is worth noting that nuclear pERK was also increased by PACAP in the cardiac ganglia, raising the possibility that transcriptional regulation could lead to longer-term changes in postganglionic neuron function. The temperature dependence of the signaling endosome pathway is also noteworthy, as it raises the possibility that this form of GPCR signaling might be more widespread than currently appreciated due to experiments being performed at ambient room temperature. Overall, the paper by Tompkins et al. adds some interesting new twists to the map of pathways leading from PACAP to neuronal excitability.
GRANTS
This work was supported in part by National Institute of Neurological Disorders and Stroke (NINDS) Grant R21 NS081492.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
K.P.C. prepared figure; K.P.C. drafted manuscript; K.P.C. edited and revised manuscript; K.P.C. approved final version of manuscript.
REFERENCES
- 1.Calebiro D, Nikolaev VO, Persani L, Lohse MJ. Signaling by internalized G-protein-coupled receptors. Trends Pharmacol Sci 31: 221–228, 2010. [DOI] [PubMed] [Google Scholar]
- 2.Dib-Hajj SD, Yang Y, Black JA, Waxman SG. The Na(V)1.7 sodium channel: from molecule to man. Nat Rev Neurosci 14: 49–62, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Harmar AJ, Fahrenkrug J, Gozes I, Laburthe M, May V, Pisegna JR, Vaudry D, Vaudry H, Waschek JA, Said SI. Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: IUPHAR review 1. Br J Pharmacol 166: 4–17, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merriam LA, Baran CN, Girard BM, Hardwick JC, May V, Parsons RL. Pituitary adenylate cyclase 1 receptor internalization and endosomal signaling mediate the pituitary adenylate cyclase activating polypeptide-induced increase in guinea pig cardiac neuron excitability. J Neurosci 33: 4614–4622, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudecki AP, Gray SL. PACAP in the defense of energy homeostasis. Trends Endocrinol Metab 27: 620–632, 2016. [DOI] [PubMed] [Google Scholar]
- 6.Smith CB, Eiden LE. Is PACAP the major neurotransmitter for stress transduction at the adrenomedullary synapse? J Mol Neurosci 48: 403–412, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stamboulian S, Choi JS, Ahn HS, Chang YW, Tyrrell L, Black JA, Waxman SG, Dib-Hajj SD. ERK1/2 mitogen-activated protein kinase phosphorylates sodium channel Na(v)1.7 and alters its gating properties. J Neurosci 30: 1637–1647, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tompkins JD, Clason TA, Hardwick JC, Girard BM, Merriam LA, May V, Parsons RL. Activation of MEK/ERK signaling contributes to the PACAP-induced increase in guinea pig cardiac neuron excitability. Am J Physiol Cell Physiol. doi: 10.1152/ajpcell.00164.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsvetanova NG, Irannejad R, von Zastrow M. G protein-coupled receptor (GPCR) signaling via heterotrimeric G proteins from endosomes. J Biol Chem 290: 6689–6696, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, Fournier A, Chow BK, Hashimoto H, Galas L, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev 61: 283–357, 2009. [DOI] [PubMed] [Google Scholar]