Abstract
Anoctamin-1 (ANO1) is a Ca2+-activated Cl− channel expressed in many types of cells. Splice variants of ANO1 have been shown to influence the biophysical properties of conductance. It has been suggested that several new antagonists of ANO1 with relatively high affinity and selectivity might be useful for experimental and, potentially, therapeutic purposes. We investigated the effects of intracellular Ca2+ concentration ([Ca2+]i) at 100-1,000 nM, a concentration range that might be achieved in cells during physiological activation of ANO1 channels, on blockade of ANO1 channels expressed in HEK-293 cells. Whole cell and excised patch configurations of the patch-clamp technique were used to perform tests on a variety of naturally occurring splice variants of ANO1. Blockade of ANO1 currents with aminophenylthiazole (T16Ainh-A01) was highly dependent on [Ca2+]i. Increasing [Ca2+]i reduced the potency of this blocker. Similar Ca2+-dependent effects were also observed with benzbromarone. Experiments on excised, inside-out patches showed that the diminished potency of the blockers caused by intracellular Ca2+ might involve a competitive interaction for a common binding site or repulsion of the blocking drugs by electrostatic forces at the cytoplasmic surface of the channels. The degree of interaction between the channel blockers and [Ca2+]i depends on the splice variant expressed. These experiments demonstrate that the efficacy of ANO1 antagonists depends on [Ca2+]i, suggesting a need for caution when ANO1 blockers are used to determine the role of ANO1 in physiological functions and in their use as therapeutic agents.
Keywords: Ca2+-activated Cl− channels, ion channel, anion channel, Cl− channel blocker
many cell types express anion channels in their plasma membranes, and intracellular Ca2+ activates prominent members of this class of channels. In 2008 a gene encoding a transmembrane protein with previously unknown function, Tmem16a, was found to encode a Ca2+-activated Cl− channel (CaCC) conductance (6, 31, 38, 47). The channels responsible are known as ANO1, and the revised gene name is Ano1. ANO1 channels are activated by cytosolic Ca2+, with a voltage-sensitive EC50 in the low micromolar range (46). The binding site(s) and mechanism of Ca2+ activation have not been determined decisively. Although some investigators have suggested that Ca2+ activation occurs through Ca2+ binding to calmodulin (CaM) (18, 44), others have argued against this idea; because exogenous CaM did not increase the open probability of ANO1 in excised patches, overexpression of Ca2+-insensitive CaM did not affect ANO1 currents, CaM did not coimmunoprecipitate with ANO1 protein (50), and purified ANO1 protein is sufficient to mediate CaCC currents (40). As for native CaCCs (1, 2, 19), the conductance shows strong outward rectification at submicromolar intracellular Ca2+ concentration ([Ca2+]i), and this rectification is reduced or lost when [Ca2+]i exceeds micromolar concentrations (10, 46, 48). Several splice variants of ANO1 channels of functional significance have been identified, and cells are likely to express a variety of isoforms (6, 10, 11, 14, 25, 29, 36).
Pharmacological blockers are potentially useful for testing the physiological role of ANO1 channels in various cells and tissues. Unfortunately, many of the traditional CaCC blockers, such as niflumic acid (NFA), anthracene-9-carboxylic acid (A9C), and others, are nonspecific. More recently, so-called second-generation blockers were developed using high-throughput screening methods (9, 27). For example, tannic acid and penta-1,2,3,4,6-O-galloyl-β-d-glucose, natural products found in red wine and tea, were shown to be potent and relatively specific inhibitors of ANO1 channels. In another study, ∼110,000 compounds were screened, and an aminophenylthiazole, T16Ainh-A01, was identified as the most potent blocker of ANO1 channels (IC50 = 1 μM) (28). Testing this compound on various epithelial tissues led to the conclusion that ANO1 channels carry a large proportion of Cl− current in salivary gland epithelium and serve as a minor charge carrier in intestinal and airway epithelia. Because of their important role in many excitable and nonexcitable cells, the development of potent and selective ANO1 blockers might be useful for treating several diseases, including gastrointestinal motility dysfunction and diarrhea, asthma, hypertension, cardiac arrhythmias, and several forms of cancer. Therefore, more information is needed about the effects and mechanism(s) of action of these antagonists.
Current ideas on the pacemaker activity of interstitial cells of Cajal (ICC) are that the CaCC, encoded by Ano1, is responsible for localized spontaneous transient inward currents that cause membrane depolarization and activation of a whole cell CaCC current that generates electrical slow waves (37, 39, 51, 52). In previous studies, we observed differences in the potency of Cl− channel blockers in pacemaker activity in the small bowel and stomach, which contrasted with the observation that slow waves were absent in these organs in mice with genetic deactivation of Ano1 (17, 39). These studies also revealed an oddity in the pharmacological actions of CaCC blockers. Slow-wave frequency was reduced at relatively low concentrations of the blockers, but the amplitude and duration of the slow waves did not decrease until higher concentrations of the blockers were applied. These data suggest that spontaneous transient inward currents (the basic events driving pacemaker activity) may be more sensitive to CaCC antagonists than slow waves; however, both events appear to be due to the same conductance. One explanation for this observation is that blockade of CaCC by channel antagonists might be sensitive to the level of Ca2+ activating the conductance. We tested this hypothesis in the present study by examining the effects of CaCC blockers on cells dialyzed with [Ca2+]i commonly used in studies of ANO1 channels expressed in HEK-293 cells (100–500 nM) and higher [Ca2+]i (up to 1,000 nM). We also examined whether alternative splicing has an impact on the pharmacology of ANO1 channels, a property that could confer differential activity of Cl− channel blockers in different cells.
MATERIALS AND METHODS
Generation of mKate2- and green fluorescent protein-tagged mAno1 fusion proteins.
An expressed sequence tag (IMAGE Consortium cDNA clone no. 30547439) homologous to Ano1 (A variant) from mouse (Ano1) was subcloned into pcDNA3.1 (Invitrogen) using standard molecular biological techniques. For expression in mammalian cells, Ano1 was ligated into the pmKate2-N vector (Evrogen, Moscow, Russia), which encodes the far-red fluorescent protein (RFP) mKate2. The Ano1 stop codon was removed, and EcoRI and ApaI restriction sites were placed at the termini by PCR, so that the Ano1 transcript was inserted in-frame, resulting in a plasmid coding for a COOH-terminal mKate2-ANO1-A fusion protein. To generate the Ano1-AB, -AC, and -AD variants that contain the alternative exons 6b, 13, and 15, respectively, we used the QuickChange XL site-directed mutagenesis kit (Agilent Technologies) to insert the 66-nucleotide “B” segment, the 12-nucleotide “C” segment, or the 78-nucleotide “D” segment into the Ano1 coding region.
The sequence corresponding to mouse Ano1 (GenBank accession no. NM_178642.5) was recently updated to include the alternative exon 13 and an additional 114 nucleotides at the 5′ end of exon 1. The additional sequence includes a methionine 58 amino acids upstream of the original start codon. To incorporate this new start codon, 28 nucleotides were inserted between the EcoRI site and the mouse Ano1 5′ end that is included in the Ano1 variants mentioned above. This insertion, which we refer to as Ano1-AC*, was accomplished using the QuickChange XL site-directed mutagenesis kit (Agilent Technologies). For expression in mammalian cells, Ano1-AC* was subcloned in-frame into pAcGFP1-N1 vector (Clontech Laboratories), resulting in a plasmid coding for a COOH-terminal green fluorescent protein (GFP)-ANO1-AC* fusion protein. All plasmids were sequenced at the Nevada Genomics Centre to confirm insertions of the alternative segments.
Cell culture and transient transfection.
Human embryonic kidney (HEK)-293 cells (American Type Culture Collection, Manassas, VA) were used for functional expression of Ano1 variants. HEK-293 cells were maintained in DMEM (Gibco) with FBS [10% (vol/vol); Gibco], penicillin-streptomycin [1% (vol/vol); Gibco], and GlutaMAX [1% (vol/vol); Gibco]. HEK-293 cells were seeded in 12-well plates for transient transfection. On the following day, plasmid DNA (0.5 μg/well) containing mouse Ano1 splice variants (A, AB, AC, and AD) tagged with RFP were transfected into cells using FuGENE 6 transfection reagent (1.5 μl/well; Promega, Madison, WI). Expression of A, AB, AC, and AD variants of ANO1 was monitored by mKate2 expression. Expression of ANO1-AC* was monitored by GFP expression.
Electrophysiological experiments.
Cells were demounted and used for patch-clamp recordings 24–48 h after transfection. Whole cell patch-clamp recordings were performed using an Axopatch 200B amplifier and pClamp 9.0 software (Axon Instruments, Union City, CA) at room temperature (22 ± 2°C). Whole cell currents were obtained by application of step pulses from −80 to +70 mV in 10-mV increments from a holding potential of −80 mV with 500 nM and 1 μM free Ca2+-containing pipette solution for ANO1-A, ANO1-AB, and ANO1-AD and 100 and 200 nM free Ca2+-containing pipette solution for ANO1-AC and ANO1-AC*. The CaCC blocker T16Ainh-A01 (10 and 30 μM) was tested for its ability to block currents arising from each splice variant of ANO1. Benzbromarone (1 and 10 μM) was also tested on ANO1-AC and AC* variants.
Excised patches (inside-out configuration) were obtained by pulling membrane patches off cells in 0 mM Ca2+ solution (ANO1-AC) and voltage-clamped. Under these conditions, low (100 nM) Ca2+ failed to elicit currents under voltage clamp. Therefore, 200 and 500 nM Ca2+ solutions were applied in the bath solution via a rapid-perfusion system (SF-77B, Warner Instruments), which switched the Ca2+ concentration near the cytoplasmic surface of the channels in ∼140 ms. Current responses to voltage ramps (−80 to 80 mV) applied at 2-s intervals from a holding potential of −80 mV were recorded. The external solution for whole cell recordings had the following composition (mM): 150 NMDG, 150 HCl, 2 CaCl2, 1 MgCl2, and 10 HEPES, with pH adjusted to 7.4 with NMDG. The composition of the pipette solution for whole cell recordings and bath solution for single-channel recordings is described in Table 1. MAXCHELATOR (http://maxchelator.stanford.edu) was used to calculate free Ca2+ concentration. Pipettes with a resistance of 4–6 MΩ were used for patch-clamp recordings. Data were filtered at 2 kHz with an eight-pole Bessel filter for whole cell experiments and at 1 kHz for experiments on excised patches. All data were digitized and acquired using pClamp software (Clampex 10.0.0.61, Axon Instruments) and analyzed using Clampfit (v9.02, Axon Instruments) and GraphPad Prism (version 5.0, GraphPad Software, San Diego, CA).
Table 1.
Composition of pipette solutions for whole cell experiments and bath solutions for experiments on excised patches
| Solutions (Free Ca2+) |
|||||
|---|---|---|---|---|---|
| 0 mM | 100 nM | 200 nM | 500 nM | 1 μM | |
| NMDG, mM | 150 | 147.7 | 144.3 | 140.4 | 138.5 |
| HCl, mM | 150 | 147.7 | 144.3 | 140.4 | 138.5 |
| EGTA, mM | 10 | 10 | 10 | 10 | 10 |
| CaCl2, mM | 0 | 4.1 | 5.9 | 7.8 | 8.8 |
| HEPES, mM | 10 | 10 | 10 | 10 | 10 |
All solutions were adjusted to pH 7.2 with NMDG.
Statistical analysis.
Data were tabulated and are presented as means ± SE; n represents the number of cells on which specific protocols were performed. In whole cell experiments, results from experiments using 10 μM T16Ainh-A01 or 1 μM benzbromarone (which produced ∼50% block of ANO1 currents) were analyzed by unpaired Student's t-test. In experiments using excised patches in which two concentrations of Ca2+ were applied to the same patch, paired t-tests were used to compare differences. P < 0.05 was considered statistically significant.
RESULTS
Effect of Ca2+ concentration on block of the A variant of ANO1.
Only small “leak” currents were resolved in cells dialyzed with 0 mM Ca2+ pipette solution (38). When HEK-293 cells transfected with ANO1-A were dialyzed with 500 nM Ca2+, the currents (Fig. 1A) and the current-voltage relationship (Fig. 1D) showed a strong outward rectification and time-dependent activation at positive potentials. The rectification index, which is the ratio of the maximal current measured at +70 mV to that measured at −80 mV [I+70/I-80; described previously by Ferrera et al. (10)] was 11.6 ± 2.2 (Fig. 2A). The time-dependent index, which is the ratio of the maximal current measured at the end of the pulse to that measured at the beginning of the pulse at +70 mV (Iend/Ibeginning) was 5.2 ± 0.4 (Fig. 2B) based on the report of Ferrera et al. (10). When the Ca2+ concentration in the pipette solution was increased to 1 μM, currents were enhanced at both negative and positive potentials (Fig. 1, E and H). However, the increase in current was larger at negative than at positive potentials, resulting in a reduced rectification index (4.0 ± 0.5; Fig. 2A). The initial current at +70 mV elicited by 1 μM Ca2+ was much larger than that elicited by 500 nM Ca2+, resulting in a smaller time-dependent index (1.6 ± 0.1; Fig. 2B). These results show that elevated [Ca2+]i reduced outward rectification and time-dependent activation of ANO1-A.
Fig. 1.
Effect of Ca2+ concentration on T16Ainh-A01 block of anoctamin 1 (ANO1)-A currents. Top: schematic diagram illustrating ANO1-A splice variant. Currents were evoked by application of voltage steps from −80 to +70 mV in 10-mV increments from a holding potential of −80 mV. A–C: currents elicited with 500 nM Ca2+ in the pipette solution. E–G: currents elicited with 1 μM Ca2+ in the pipette solution. B and F: currents elicited by the same voltage-clamp protocol 5 min after addition of 10 μM T16Ainh-A01. C and G: currents recorded 5 min after addition of 30 μM T16Ainh-A01. D and H: current-voltage (I-V) relationships normalized as the ratio of the maximum current measured at each voltage to the maximum current measured at +70 mV (I/Imax). Results from 5 experiments are displayed as means ± SE.
Fig. 2.

Effect of Ca2+ concentration ([Ca2+]) on rectification index and time-dependent index. A: rectification index was determined as the ratio of the current measured at +70 mV to the current measured at −80 mV (I+70/I-80). B: time-dependent index (Iend/Ibeginning) expressed as the ratio of the maximum current measured at the end of the pulse to the current at the beginning of the pulse at +70 mV. Results obtained with 500 nM and 1 μM Ca2+ (A, AB, and AD variants) and 100 and 200 nM Ca2+ (AC and AC* variant) were compared statistically: **P < 0.01; ***P < 0.001. #P < 0.05 vs. AC. Results from 5 or 6 experiments are displayed as means ± SE.
The effects of T16Ainh-A01 were tested on currents elicited in cells dialyzed with 500 nM or 1 μM Ca2+. T16Ainh-A01 (10 and 30 μM) inhibited ANO1-A currents in cells dialyzed with 500 nM Ca2+ (Fig. 1, A–D), but the degree of inhibition of ANO1-A currents was reduced in cells dialyzed with 1 μM Ca2+ (Fig. 1, E–H). For example, at −60 mV, T16Ainh-A01 (10 μM) inhibited ANO1-A currents by 56 ± 12.8% in cells dialyzed with 500 nM Ca2+ (n = 5) but only 20 ± 6.9% in cells dialyzed with 1 μM Ca2+ (P < 0.05, n = 5).
Effect of Ca2+ concentration on block of the AB variant of ANO1.
The current traces (Fig. 3A) and current-voltage relationship (Fig. 3D) elicited from cells with ANO1-AB and 500 nM Ca2+ in the pipette solution also showed strong outward rectification and time-dependent activation at positive potentials. The rectification index for these currents was 9.2 ± 1.2 (Fig. 2A), and the time-dependent index at +70 mV was 4.9 ± 0.6 (Fig. 2B). When the Ca2+ concentration in the pipette solution was increased to 1 μM, the currents increased more at negative than at positive potentials (Fig. 3, E and H). Thus the rectification index (i.e., 6.3 ± 1.0) and the time-dependent index (i.e., 4.6 ± 0.6) at +70 mV were not significantly different from those at 500 nM Ca2+. These data suggest that the rectification index and time-dependent activation of ANO1-AB are independent of [Ca2+]i.
Fig. 3.
Effect of Ca2+ concentration on T16Ainh-A01 block of ANO1-AB currents. Top: schematic diagram illustrating ANO1-AB splice variant. Currents were evoked with the voltage-clamp protocol described in Fig. 1 legend. A–C: currents elicited with 500 nM Ca2+ in the pipette solution. E–G: currents elicited with 1 μM Ca2+ in the pipette solution. B and F: currents recorded 5 min after addition of 10 μM T16Ainh-A01. C and G: currents recorded 5 min after addition of 30 μM T16Ainh-A01. D and H: current-voltage relationships normalized as the ratio of the maximum current measured at each voltage to the maximum current measured at +70 mV. Results from 5 experiments are displayed as means ± SE.
The effects of T16Ainh-A01 were tested on currents elicited in cells dialyzed with 500 nM or 1 μM Ca2+. T16Ainh-A01 (10 and 30 μM) inhibited ANO1-B currents in cells dialyzed with 500 nM Ca2+ (Fig. 3, A–D). As with ANO1-A currents, there was a trend toward reduction in the inhibition of ANO1-B currents when cells were dialyzed with 1 μM Ca2+ (Fig. 3, E–H); however, with the small currents elicited at −60 mV, the difference in block of ANO1 by T16Ainh-A01 (10 μM) was 64 ± 4.2% of control currents in cells dialyzed with 500 nM Ca2+ (n = 5) and 49 ± 13.0% of control currents in cells dialyzed with 1 μM Ca2+ (n = 5). This difference did not reach the level of significance (P < 0.05) in unpaired t-tests.
Effect of Ca2+ concentration on block of the AC variants of ANO1.
The AC variant was shown to display three- to fourfold greater sensitivity than the ABC variant of Ano1 (10). This suggested that the presence of the B variant decreases the Ca2+ sensitivity of the channel. Because of the increased Ca2+ sensitivity of the AC variant, we examined the pharmacological profile of AC variants using pipette solutions with lower Ca2+ concentrations (100–200 nM). The longer version of AC (designated AC*) was also examined in these studies.
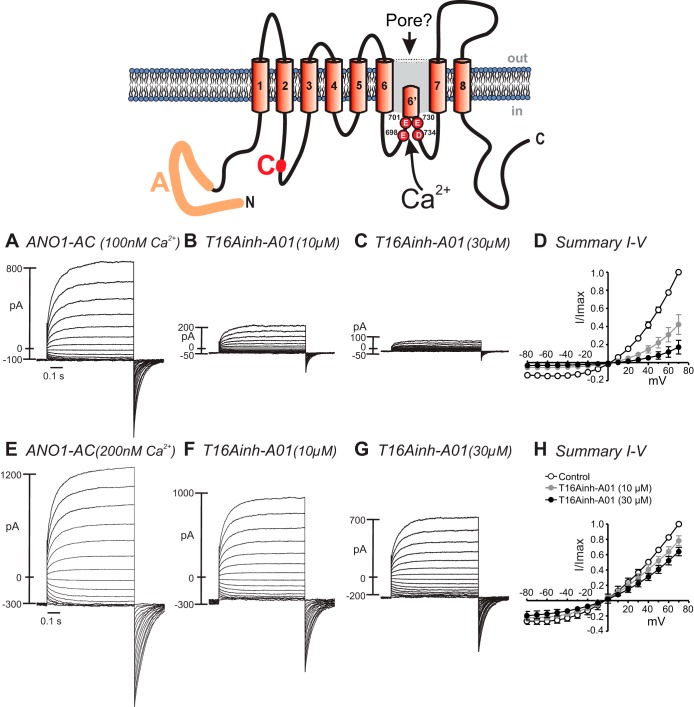
ANO1-AC and ANO1-AC* showed strong outward rectification and time-dependent activation at positive potentials in cells dialyzed with 100 nM Ca2+ (Fig. 4, A and D, and Fig. 5, A and D). The rectification index of ANO1-AC was 9.6 ± 1.1 (Fig. 2A), and the time-dependent index at +70 mV was 5.2 ± 0.3 (Fig. 2B). The rectification index of ANO1-AC* was slightly, but not significantly, reduced (7.4 ± 0.6; Fig. 2A); however, the time-dependent index of ANO1-AC* was reduced significantly (to 4.0 ± 0.4; Fig. 2B) compared with ANO1-AC. When the Ca2+ concentration was increased to 200 nM in cells with ANO1-AC, the increase in current was greater at negative than at positive potentials. Thus the rectification index was reduced (4.3 ± 0.4; Fig. 2A). The initial current at +70 mV elicited by 200 nM Ca2+ was larger than that elicited by 100 nM Ca2+, resulting in a decreased time-dependent index (2.8 ± 0.2; Fig. 2B). Rectification and time-dependent indexes were reduced (to 3.8 ± 0.2 and 2.2 ± 0.2, respectively) when cells with ANO1-AC* were dialyzed with 200 nM Ca2+. Thus, increasing [Ca2+]i reduced outward rectification and time-dependent activation of ANO1-AC and ANO1-AC* in a similar manner.
Fig. 4.
Effect of Ca2+ concentration on T16Ainh-A01 block of ANO1-AC currents. Top: schematic diagram illustrating ANO1-AC splice variant. Currents were evoked with the voltage-clamp protocol described in Fig. 1 legend. A–C: currents elicited with 100 nM Ca2+ in the pipette solution. E–G: currents elicited with 200 nM Ca2+ in the pipette solution. B and F: currents recorded 5 min after addition of 10 μM T16Ainh-A01. C and G: currents recorded 5 min after addition of 30 μM T16Ainh-A01. D and H: current-voltage relationships normalized as the ratio of the maximum current measured at each voltage to the maximum current measured at +70 mV. Results from 6 experiments are displayed as means ± SE.
Fig. 5.
Effect of Ca2+ concentration on T16Ainh-A01 block of ANO1-AC* currents. Top: schematic diagram illustrating ANO1-AC* splice variant. Currents were evoked with the voltage-clamp protocol described in Fig. 1 legend. A–C: currents elicited with 100 nM Ca2+ in the pipette solution. E–G: currents elicited with 200 nM Ca2+ in the pipette solution. B and F: currents recorded 5 min after addition of 10 μM T16Ainh-A01. C and G: currents recorded 5 min after addition of 30 μM T16Ainh-A01. D and H: current-voltage relationships normalized as the ratio of the maximum current measured at each voltage to the maximum current measured at +70 mV. Results from 6 experiments are displayed as means ± SE.
The effects of T16Ainh-A01 were tested on currents elicited in cells dialyzed with 100 or 200 nM Ca2+. T16Ainh-A01 (10 and 30 μM) inhibited ANO1-AC currents in cells dialyzed with 500 nM Ca2+ (Fig. 4, A–D), and the degree of inhibition of ANO1-AC currents was reduced in cells dialyzed with 200 nM Ca2+ (Fig. 4, E–H). For example, at −60 mV, T16Ainh-A01 (10 μM) inhibited ANO1-AC currents by 63 ± 3.2% in cells dialyzed with 100 nM Ca2+ (n = 6) but only by 20 ± 3.2% in cells dialyzed with 200 nM Ca2+ (P < 0.05, n = 6).
Cells expressing ANO1-AC* displayed similar Ca2+ dependency for the block of ANO1 currents by T16Ainh-A01. T16Ainh-A01 (10 and 30 μM) inhibited ANO1-AC* currents in cells dialyzed with 100 nM Ca2+ (Fig. 5, A–D), but the degree of inhibition of ANO1-AC* currents was reduced in cells dialyzed with 200 nM Ca2+ (Fig. 5, E–H). For example, at −60 mV, T16Ainh-A01 (10 μM) inhibited ANO1-AC* currents by 48 ± 7.2% in cells dialyzed with 100 nM Ca2+ (n = 6) but only by 12 ± 7.0% in cells dialyzed with 200 nM Ca2+ (P < 0.01, n = 6).
Since AC variants are the most highly expressed in ICC (16), we also tested another third-generation ANO1 blocker, benzbromarone. ANO1-AC currents were significantly reduced by benzbromarone (1 and 10 μM) in cells dialyzed with 100 nM (Fig. 6, A–D ) and 200 nM (Fig. 6, E–H) Ca2+. At −60 mV, benzbromarone (1 μM) inhibited ANO1-AC currents by 56 ± 2.8% (100 nM Ca2+) and 27 ± 3.0% (200 nM Ca2+) of control currents (P < 0.001). In cells expressing ANO1-AC*, currents were significantly reduced by benzbromarone (1 and 10 μM) in cells dialyzed with 100 nM (Fig. 6, I–L) and 200 nM (Fig. 6, M–P) Ca2+. At −60 mV, benzbromarone (1 μM) inhibited ANO1-AC* currents by 56 ± 2.8% (100 nM Ca2+, n = 5) and 27 ± 3.0% (200 nM Ca2+, n = 5) of control currents (P < 0.001).
Fig. 6.
Effect of Ca2+ concentration on benzbromarone block of ANO1-AC and ANO1-AC* currents. Currents were evoked with the voltage-clamp protocol described in Fig. 1 legend. A–C and I–K: ANO1-AC and ANO1-AC* currents elicited with 100 nM Ca2+ in the pipette solution. E–G and M–O: ANO1-AC and ANO1-AC* currents elicited with 200 nM Ca2+ in the pipette solution. B, F, J, and N: ANO1-AC and ANO1-AC* currents recorded 5 min after addition of 1 μM benzbromarone. C, G, K, and O: currents recorded 5 min after addition of 10 μM benzbromarone. D, H, L, and P: current-voltage relationships normalized as the ratio of the maximum current measured at each voltage to the maximum current measured at +70 mV. Results from 5 experiments are displayed as means ± SE.
Effect of Ca2+ concentration on block of the AD variant of ANO1.
The current responses and current-voltage relationship for cells expressing ANO1-AD and dialyzed with 500 nM Ca2+ showed outward rectification and time-dependent activation at positive potentials (Fig. 7, A and D). The rectification index was 8.0 ± 0.7 (Fig. 2A), and the time-dependent index at +70 mV was 3.3 ± 0.2 (Fig. 2B). ANO1-AD currents were enhanced by dialysis with 1 μM Ca2+ (Fig. 7E), and the increase in currents was greater at negative than at positive potentials, decreasing the rectification index to 4.4 ± 0.7 (Fig. 2A). The initial current at +70 mV was increased at 1 μM Ca2+, resulting in a reduction of the time-dependent index to 2.3 ± 0.2 (Fig. 2B).
Fig. 7.
Effect of Ca2+ concentration on T16Ainh-A01 block of ANO1-AD currents. Top: schematic diagram illustrating ANO1-AD splice variant. Currents were evoked with the voltage-clamp protocol described in Fig. 1 legend. A–C: currents elicited with 500 nM Ca2+ in the pipette solution. E–G: currents elicited with 1 μM Ca2+ in the pipette solution. B and F: currents recorded 5 min after addition of 10 μM T16Ainh-A01. C and G: currents recorded 5 min after addition of 30 μM T16Ainh-A01. D and H: current-voltage relationships normalized as the ratio of the maximum current measured at each voltage to the maximum current measured at +70 mV. Results from 5 experiments are displayed as means ± SE.
The effects of T16Ainh-A01 were tested on currents elicited in cells dialyzed with 500 nM or 1 μM Ca2+. T16Ainh-A01 (10 and 30 μM) inhibited ANO1-D currents in cells dialyzed with 500 nM Ca2+ (Fig. 7, A–D). As with ANO1-A and ANO1-AC splice variants, there was a trend toward reduction in the inhibition of ANO1-D currents when cells were dialyzed with 1 μM Ca2+ (Fig. 7, E–H); however, with the small currents elicited at −60 mV, the difference in block of ANO1-AD by T16Ainh-A01 (10 μM) was 39 ± 5.4% of control currents in cells dialyzed with 500 nM Ca2+ (n = 5) and 27 ± 19.4% of control currents in cells dialyzed with 1 μM Ca2+ (n = 5). This difference did not reach the level of significance (P < 0.05) in unpaired t-tests.
Effect of ANO1 blockers on ANO1-AC currents at low and high Ca2+ concentrations in excised patches.
Excised patch (inside-out configuration) recordings were performed to test whether ANO1 blockers can exert their effect when applied from the internal surface of the channels and, if so, whether their inhibitory activity is influenced by internal Ca2+. Inside-out patches were created in 0 mM Ca2+ solution from HEK-293 cells expressing ANO1-AC. The bath solution (cytoplasmic surface) was changed from 0 mM to 100 nM Ca2+, as used in whole cell experiments to evoke ANO1-AC currents. However, 100 nM Ca2+ did not produce appreciable current (not shown). When the Ca2+ concentration was increased to 200 nM, currents were elicited (Figs. 8A and 9A) and the current-voltage relationship to ramp potentials (Figs. 8C and 9C) showed a strong outward rectification. ANO1-AC currents were inhibited to 28.5 ± 5.5% at −80 mV and to 29.9 ± 6.7% at +80 mV by 30 μM T16Ainh-A01 (Fig. 8, A–D) and to 8.3 ± 2.5% at −80 mV and 10.5 ± 3.6% at +80 mV by 10 μM benzbromarone (Fig. 9, A–D). When Ca2+ concentration was increased to 500 nM, currents were increased at both negative and positive potentials and the current-voltage relationship (Figs. 8G and 9G) was more linear. These currents were inhibited to 67.7 ± 10.3% at −80 mV and 60.6 ± 10.3% at +80 mV by 30 μM T16Ainh-A01 (Fig. 8, F–H) and 37.8 ± 7.6% at −80 mV and 29.7 ± 4.8% at +80 mV by 10 μM benzbromarone (Fig. 9, F–H). These results show that the higher Ca2+ concentration enhanced currents but cytoplasmic Ca2+ decreased the blocking effects of T16Ainh-A01 and benzbromarone on ANO1-AC currents, as observed in whole cell experiments.
Fig. 8.
Responses of ANO1-AC currents to T16Ainh-A01 in inside-out patches. With symmetrical Cl− (150/150 mM) in the pipette and bath solutions, membrane patches were excised into the inside-out configuration. Currents were evoked by application of ramp potentials from −80 to +80 mV at 2-s intervals from a holding potential of −80 mV. A and E: current responses upon switching bath Ca2+ (i.e., cytoplasmic side of the membrane patches) from 0 mM to 200 or 500 nM. B and F: 30 μM T16Ainh-A01 was applied 5 min before the bath solution was switched to 200 or 500 nM Ca2+. Effects of changing Ca2+ concentration were noted within 2 s. C and G: current-voltage relationships for effects of 30 μM T16Ainh-A01 on ANO1-AC currents with 200 and 500 nM Ca2+, respectively. D and H: summarized data showing average effects of T16Ainh-A01 on ANO1-AC currents evoked by 200 and 500 nM Ca2+, respectively. *P < 0.05; ***P < 0.001 vs. control (before T16Ainh-A01). Results from 5 experiments are displayed as means ± SE.
Fig. 9.
Responses of ANO1-AC currents to benzbromarone in inside-out patches. With symmetrical Cl− (150/150 mM) in the pipette and bath solutions, membrane patches were excised into the inside-out configuration. Currents were evoked by application of ramp potentials from −80 to +80 mV at 2-s intervals from a holding potential of −80 mV. A and E: current responses upon switching bath Ca2+ (i.e., cytoplasmic side of the membrane patches) from 0 mM to 200 or 500 nM. B and F: 10 μM benzbromarone was applied 5 min before bath solution was switched to 200 or 500 nM Ca2+. Effects of changing Ca2+ concentration were noted within 2 s. C and G: current-voltage relationships for effects of 10 μM benzbromarone on ANO1-AC currents with 200 and 500 nM Ca2+, respectively. D and H: summarized data showing average effects of benzbromarone on ANO1-AC currents evoked by 200 and 500 nM Ca2+, respectively. *P < 0.05; ***P < 0.001 vs. control (before benzbromarone). Results from 5 experiments are displayed as means ± SE.
DISCUSSION
This study shows that block of several ANO1 splice variants by the newer-generation blockers T16Ainh-A01 and benzbromarone is affected by the concentration of Ca2+ facing the cytoplasmic side of the channels. Increasing [Ca2+]i within the physiological range likely to activate channels reduced the potency of these blockers in whole cell and excised patch experiments. Moreover, our data provide evidence that alternatively spliced exons of Ano1 can affect the pharmacological profiles of ANO1 blockers, depending on the magnitude of the intracellular Ca2+ signal activating the conductance. The physiological and pathophysiological significance of these findings and the potential molecular mechanism involved in these observations are discussed.
Bradley et al. (4) recently compared the effects of the classical blockers NFA and A9C with the effects of two newer-generation CaCC blockers, CaCCinh-A01 (9, 26) and T16Ainh-A01 (9, 26), on ANO1 channels expressed in HEK-293 cells (summarized in Ref. 37). The voltage dependence of block of native CaCCs by NFA and A9C (reviewed in Refs. 12, 13, 20, and 21) and their distinctive and complex paradoxical stimulatory properties (increased currents at negative potentials by NFA and potent transient stimulation following washout of NFA and large increase in tail current amplitude by A9C) on CaCCs in vascular myocytes (22, 32, 33) were also observed with expressed ANO1 channels (5). Bradley et al. (4) showed that T16Ainh-A01 and CaCCinh-A01 produced high-affinity block of ANO1 (IC50 ≈ 1–2 μM) and that the block was voltage-independent, as reported earlier for T16Ainh-A01 (26) and confirmed in the present study. A more recent study by Reyes et al. (35) on Xenopus ANO1 (xANO1) proposed that T16Ainh-A01 most likely interacts with a binding site located within the outer pore at ∼0.25 of the distance across the membrane electric field. Interestingly, reverse charge mutations (R-to-E or K-to-E) of two binding sites that altered anion permeation, selectivity, and block by DIDS and A9C of xANO1 had no influence on the T16Ainh-A01-induced inhibition of the channel, suggesting that T16Ainh-A01 interacts with one or more distinct binding sites. One important aspect not investigated by either group (4, 35) was the possibility that block of ANO1 by these compounds varies with [Ca2+]i (they used 500 nM and 1 μM) and the nature of alternatively spliced exons translated into forming the pore-forming subunit of ANO1.
Our results show that raising [Ca2+]i from 100 to 1,000 nM significantly attenuated the blocking effectiveness of T16Ainh-A01 and benzbromarone (15) on ANO1 currents. This effect varied considerably, depending on the specific splice variants of ANO1 tested (see below). The implications of these observations are that determinations of the contributions of ANO1 to physiological processes using pharmacological agents could be underestimated, depending on 1) the profile of splice variants expressed and 2) the magnitude and characteristics of the Ca2+ signals triggering activation of ANO1 channels.
There are at least three possible mechanisms that could explain our results. Elevating [Ca2+]i has a profound influence on ANO1 gating, attenuating the steepness of outward rectification, as evidenced by the decreased rectification index, and accelerating and decelerating time-dependent activation and deactivation, respectively. The conformational changes conferring these altered biophysical properties of ANO1 could allosterically reduce the affinity of blockers for the channel.
Consistent with the existence of subconductance states in native CaCCs (8, 34, 43), current models of anion permeation through ANO1 channels suggest that the shape and dimension of the pore are flexible (5, 45). A second possibility to explain our results may be that a rise in [Ca2+]i triggers an increase in open probability that is accompanied by a partial collapse of the pore, yielding to subconductance states that would reduce access of the blocker to the conduction pathway. Piper and Large (34) identified three subconductance states of unitary CaCCs recorded in rabbit pulmonary artery smooth muscle cells: 1.2, 1.8, and 3.5 pS. While only the 1.8- and 3.5-pS channels were observed in inside-out patches exposed to [Ca2+]i of 50 nM+, the 1.2- and 1.8-pS channels predominated in excised patches exposed to [Ca2+]i of 1 μM. Whether the conduction pathway of expressed ANO1 exhibits similar properties is unknown, but, if confirmed, this could explain why organic blockers, especially large organic blockers, such as T16Ainh-A01 and benzbromarone, are less potent inhibitors when [Ca2+]i is elevated.
Whether the opening of ANO1 is directly triggered by a rise in [Ca2+]i (42, 46, 48, 49) or has an obligatory requirement for an accessory Ca2+-binding protein such as CaM (41, 44) or another unknown protein is controversial. Recent studies provided strong evidence against the hypothesis of Ca2+-CaM acting as an obligatory ANO1 activator (42, 49, 50). Two independent groups identified four critical acidic amino acid residues forming a Ca2+-binding pocket located between transmembrane domains 5 and 7 (42, 48). These residues (E698, E701, E730, and D734 of mouse ANO1) were shown to play an important role in Ca2+ sensing and channel activation, with the opening of ANO1 coordinated by a direct cooperative interaction of internal Ca2+ with the ANO1 protein (42, 48). A third possibility to explain our observations is that internal Ca2+ ions may sterically obstruct or electrostatically repulse blockers from a binding site near the pore entrance located outside the transmembrane electric field, since the block by both compounds was voltage-independent. Consistent with this hypothesis was the finding that both T16Ainh-A01 and benzbromarone inhibited ANO1 currents when applied from the internal side of inside-out patches; block from the internal side of the membrane by benzbromarone has been documented previously (15). This is also true for NFA and A9C (45). Current kinetic models of CaCC gating have proposed that two (2, 7), three (1, 19), or four [2 per monomer in the proposed homomeric dimer structure (5)] Ca2+ ions are necessary to activate channels; therefore, a higher state of Ca2+ occupancy at higher [Ca2+]i may limit binding of the blockers within the pore through a competitive electrostatic interaction or steric hindrance. Regardless of the exact location and mechanism of activation of ANO1 by internal Ca2+, our data demonstrate that higher [Ca2+]i diminished the ability of two potent and structurally distinct ANO1 blockers to inhibit CaCC currents when applied from either side of the membrane.
Four alternatively spliced exons, a, b, c, and d, were described in seminal studies reporting the properties of ANO1 (6), and different biophysical properties were attributed to different splice variants (6, 10, 11, 23, 46). With the discovery of additional alternatively spliced exons (29), it is clear that posttranscriptional and posttranslational regulation of Ano1 is more complex than originally thought. Additionally, in many species, including mouse and human, there is an additional region or exon upstream of the first exon (exon 0 in the human and mouse genes) that enhanced ANO1 expression (24). In the mouse, exon 0 encodes for an additional 57 amino acids located just upstream of splice variant a. Our data showed that, with [Ca2+]i of 1 μM, T16Ainh-A01 inhibited the various ANO1 isoforms with the following order of potency: ab > ad > a. With [Ca2+]i of 500 nM, block was more potent with these isoforms and displayed the following order of potency: a ≈ ab > ad. These differences may be, at least in part, attributed to significant differences in Ca2+ and/or voltage sensitivity. The inclusion of splice variant b was shown to reduce the Ca2+ sensitivity of ANO1 by nearly fourfold (10). The properties of the peptide segment encoded by splice variant d have not been characterized in detail. One study showed that its inclusion slows current activation and deactivation while having no effect on current magnitude when elicited with 500 nM intracellular Ca2+ (23). Significant differences were noted in the Ca2+ dependence of ac variants (termed AC and AC* in our study), and we confirmed that AC is likely to be the most Ca2+-sensitive variant of ANO1 (6, 10, 11, 23, 46). In our experiments, expression of the longer form of AC (AC*; including exon 0), which was nearly identical to AC in Ca2+ and voltage sensitivity and kinetics, was blocked by T16Inh-A01 in cells dialyzed with 100 nM intracellular Ca2+, but the block was nearly abolished at 200 nM intracellular Ca2+. These data suggest that differences in splice variant expression in different tissues or cells or remodeling of the pattern of splice variant expression in a disease state could affect whether pharmacological blocking agents have significant effects on the currents mediated by ANO1. This might impact the interpretation of whether ANO1 is involved in physiological or pathophysiological functions and complicate the use of ANO1 blockers as therapeutic agents.
Observations in the literature support the concept that the differential effects of ANO1 blockers reported here may vary with the nature and amplitude of the Ca2+ signal events triggering ANO1 and the exact molecular profile of alternatively spliced exons expressed in different tissues. First, on the basis of the effects of T16Ainh-A01 (and CaCCinh-A01) and partial Ano1 knockdown by siRNA on CaCC conductance in different types of epithelial cells, Namkung et al. (26) concluded that ANO1 represents only a minor component of the CaCC conductance activated by agonists in human intestinal and bronchial epithelial cell lines, whereas it was a major contributor to the CaCC current in salivary gland cells. When determining the efficacy of T16Ainh-A01 to block whole cell currents in all cell types, the authors used a relatively low [Ca2+]i to elicit CaCC currents (275 nM), which invariably led to potent inhibition (>90% block with 10 μM), an observation consistent with our findings for block of many splice variants. However, T16Ainh-A01 inhibited only a fraction of the initial transient current and produced little effect on the sustained short-circuit current evoked by ATP or UTP in airway and intestinal cells. In contrast, T16Ainh-A01 produced strong inhibition of the short-circuit current evoked by ATP in salivary gland cells. One important difference was that while the purinergic response in intestinal and airway cells was composed of a transient (T16Ainh-A01-sensitive) and a sustained (T16Ainh-A01-insensitive) phase, the response to ATP in salivary gland cells was only transient. In light of our data and the fact that another group had confirmed an important role for ANO1 in Ca2+-dependent Cl− movements across intestinal and airway epithelial cell membranes by using Ano1-knockout mice (30), it is possible that the lack of sensitivity of some epithelia could be related to differences in [Ca2+]i attained during agonist responses or differences in splice variant profiles.
An interesting anomaly we observed that prompted the current study was the observation that low concentrations of DIDS and NFA decreased the frequency of pacemaker activity in ICC in murine, nonhuman primate, and human small intestine but caused negligible effects on the amplitude and duration of electrical slow waves until high concentrations of the blockers were applied (16). Yet, slow waves were absent in gastric and intestinal muscles of Ano1−/− mice (16), demonstrating that ANO1 is an important contributor to pacemaker currents. Here again, the apparent differences between pharmacological responses and gene deactivation data might be attributable to differences between the amplitude of the Ca2+ transient necessary to initiate the pacemaker (ANO1) current and that required to sustain the open state of ANO1 channels during the plateau phase of the slow wave. By such a mechanism, it would be possible for a lower concentration of ANO1 blockers to reduce the frequency of pacemaker activity, but once an event was initiated and [Ca2+]i near ANO1 channels rose to higher levels, the effectiveness of the blockers might decrease. Such a scenario would create the illusion, from pharmacological studies, that initiation of pacemaker activity is due to an inward current mediated by ANO1, but the sustained depolarization during the plateau phase of slow waves is due to another conductance.
Summary and conclusions.
This study provides evidence that [Ca2+]i, in the range employed for physiological activation of ANO1, affects the ability of T16Ainh-A01 and benzbromarone, two recently identified channel antagonists, to block ANO1 channels. Although not studied in detail, a similar observation was recently reported for the ANO1 blocker CaCCinh-A01 (3). Our results also show that the interactions between blockers and ANO1 channels depend on the nature of the splice variants expressed in a given cell. A significant body of experiments will be necessary to determine the exact molecular mechanisms involved in these observations. The experimental approaches required to answer such questions and data interpretation may not be as trivial as it may seem, since the molecules investigated here are highly hydrophobic and may interact with both the hydrophilic conduction pathway and the hydrophobic transmembrane domains of the protein and lipid environment (45).
Taken together, our data emphasize the need for caution when using pharmacological tests to determine the role of ANO1 in physiological functions. An important goal for future experiments will be to investigate the consequences of the mechanism we have described in native cells by comparing the effects of ANO1 blockers with those produced by gene knockdown.
GRANTS
This project was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant P01 DK-41315 to K. M. Sanders and S. D. Koh and a Research Enhancement Grant from the University of Nevada, Reno, to N. Leblanc.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.S.S., K.O., H.Z., N.L., S.D.K., and K.M.S. developed the concept and designed the research; T.S.S., K.O., H.Z., and N.J.Y. performed the experiments; T.S.S., H.Z., N.J.Y., S.D.K., and K.M.S. analyzed the data; T.S.S., K.O., H.Z., N.L., S.D.K., and K.M.S. interpreted the results of the experiments; T.S.S. and H.Z. prepared the figures; T.S.S., N.L., S.D.K., and K.M.S. drafted the manuscript; T.S.S., K.O., H.Z., N.L., S.D.K., and K.M.S. edited and revised the manuscript; T.S.S., K.O., H.Z., N.J.Y., N.L., S.D.K., and K.M.S. approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors are grateful to Nancy Horowitz for help with cell cultures.
REFERENCES
- 1.Angermann JE, Sanguinetti AR, Kenyon JL, Leblanc N, Greenwood IA. Mechanism of the inhibition of Ca2+-activated Cl− currents by phosphorylation in pulmonary arterial smooth muscle cells. J Gen Physiol 128: 73–87, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arreola J, Melvin JE, Begenisich T. Activation of calcium-dependent chloride channels in rat parotid acinar cells. J Gen Physiol 108: 35–47, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bill A, Popa MO, van Diepen MT, Gutierrez A, Lilley S, Velkova M, Acheson K, Choudhury H, Renaud NA, Auld DS, Gosling M, Groot-Kormelink PJ, Gaither LA. Variomics screen identifies the re-entrant loop of the calcium-activated chloride channel ANO1 that facilitates channel activation. J Biol Chem 290: 889–903, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradley E, Fedigan S, Webb T, Hollywood MA, Thornbury KD, McHale NG, Sergeant GP. Pharmacological characterization of TMEM16A currents. Channels (Austin) 8: 308–320, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunner JD, Lim NK, Schenck S, Duerst A, Dutzler R. X-ray structure of a calcium-activated TMEM16 lipid scramblase. Nature 516: 207–212, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 322: 590–594, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Contreras-Vite JA, Cruz-Rangel S, De Jesús-Pérez JJ, Figueroa IA, Rodríguez-Menchaca AA, Pérez-Cornejo P, Hartzell HC, Arreola J. Revealing the activation pathway for TMEM16A chloride channels from macroscopic currents and kinetic models. Pflügers Arch 468: 1241–1257, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis AJ, Shi J, Pritchard HA, Chadha PS, Leblanc N, Vasilikostas G, Yao Z, Verkman AS, Albert AP, Greenwood IA. Potent vasorelaxant activity of the TMEM16A inhibitor T16A(inh)-A01. Br J Pharmacol 168: 773–784, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De La Fuente R, Namkung W, Mills A, Verkman AS. Small-molecule screen identifies inhibitors of a human intestinal calcium-activated chloride channel. Mol Pharmacol 73: 758–768, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Ferrera L, Caputo A, Ubby I, Bussani E, Zegarra-Moran O, Ravazzolo R, Pagani F, Galietta LJ. Regulation of TMEM16A chloride channel properties by alternative splicing. J Biol Chem 284: 33360–33368, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrera L, Scudieri P, Sondo E, Caputo A, Caci E, Zegarra-Moran O, Ravazzolo R, Galietta LJ. A minimal isoform of the TMEM16A protein associated with chloride channel activity. Biochim Biophys Acta 1818: 2214–2223, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frings S, Reuter D, Kleene SJ. Neuronal Ca2+-activated Cl− channels. Homing in on an elusive channel species. Prog Neurobiol 60: 247–289, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Hartzell C, Putzier I, Arreola J. Calcium-activated chloride channels. Annu Rev Physiol 67: 719–758, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Hartzell HC, Yu K, Xiao Q, Chien LT, Qu Z. Anoctamin/TMEM16 family members are Ca2+-activated Cl− channels. J Physiol 587: 2127–2139, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang F, Zhang H, Wu M, Yang H, Kudo M, Peters CJ, Woodruff PG, Solberg OD, Donne ML, Huang X, Sheppard D, Fahy JV, Wolters PJ, Hogan BL, Finkbeiner WE, Li M, Jan YN, Jan LY, Rock JR. Calcium-activated chloride channel TMEM16A modulates mucin secretion and airway smooth muscle contraction. Proc Natl Acad Sci USA 109: 16354–16359, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hwang SJ, Blair PJ, Britton FC, O'Driscoll KE, Hennig G, Bayguinov YR, Rock JR, Harfe BD, Sanders KM, Ward SM. Expression of anoctamin 1/TMEM16A by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. J Physiol 587: 4887–4904, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang SJ, Blair PJ, Britton FC, O'Driscoll KE, Hennig G, Bayguinov YR, Rock JR, Harfe BD, Sanders KM, Ward SM. Expression of anoctamin 1/TMEM16A by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. J Physiol 587: 4887–4904, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung J, Nam JH, Park HW, Oh U, Yoon JH, Lee MG. Dynamic modulation of ANO1/TMEM16A HCO3− permeability by Ca2+/calmodulin. Proc Natl Acad Sci USA 110: 360–365, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuruma A, Hartzell HC. Bimodal control of a Ca2+-activated Cl− channel by different Ca2+ signals. J Gen Physiol 115: 59–80, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Large WA, Wang Q. Characteristics and physiological role of the Ca2+-activated Cl− conductance in smooth muscle. Am J Physiol Cell Physiol 271: C435–C454, 1996. [DOI] [PubMed] [Google Scholar]
- 21.Leblanc N, Ledoux J, Saleh S, Sanguinetti A, Angermann J, O'Driscoll K, Britton F, Perrino BA, Greenwood IA. Regulation of calcium-activated chloride channels in smooth muscle cells: a complex picture is emerging. Can J Physiol Pharmacol 83: 541–556, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Ledoux J, Greenwood IA, Leblanc N. Dynamics of Ca2+-dependent Cl− channel modulation by niflumic acid in rabbit coronary arterial myocytes. Mol Pharmacol 67: 163–173, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Mazzone A, Bernard CE, Strege PR, Beyder A, Galietta LJ, Pasricha PJ, Rae JL, Parkman HP, Linden DR, Szurszewski JH, Ordog T, Gibbons SJ, Farrugia G. Altered expression of Ano1 variants in human diabetic gastroparesis. J Biol Chem 286: 13393–13403, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazzone A, Gibbons SJ, Bernard CE, Nowsheen S, Middha S, Almada LL, Ordog T, Kendrick ML, Reid Lombardo K, Shen KR, Galietta LJ, Fernandez-Zapico ME, Farrugia G. Identification and characterization of a novel promoter for the human ANO1 gene regulated by the transcription factor signal transducer and activator of transcription 6 (STAT6). FASEB J 29: 152–163, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milenkovic VM, Krejcova S, Reichhart N, Wagner A, Strauss O. Interaction of bestrophin-1 and Ca2+ channel β-subunits: identification of new binding domains on the bestrophin-1 C-terminus. PLos One 6: e19364, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Namkung W, Phuan PW, Verkman AS. TMEM16A inhibitors reveal TMEM16A as a minor component of calcium-activated chloride channel conductance in airway and intestinal epithelial cells. J Biol Chem 286: 2365–2374, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Namkung W, Thiagarajah JR, Phuan PW, Verkman AS. Inhibition of Ca2+-activated Cl− channels by gallotannins as a possible molecular basis for health benefits of red wine and green tea. FASEB J 24: 4178–4186, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Namkung W, Yao Z, Finkbeiner WE, Verkman AS. Small-molecule activators of TMEM16A, a calcium-activated chloride channel, stimulate epithelial chloride secretion and intestinal contraction. FASEB J 25: 4048–4062, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Driscoll KE, Pipe RA, Britton FC. Increased complexity of Tmem16a/Anoctamin 1 transcript alternative splicing. BMC Mol Biol 12: 35, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ousingsawat J, Martins JR, Schreiber R, Rock JR, Harfe BD, Kunzelmann K. Loss of TMEM16A causes a defect in epithelial Ca2+ dependent chloride transport. J Biol Chem 284: 28698–28703, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pedemonte N, Galietta LJ. Structure and function of TMEM16 proteins (anoctamins). Physiol Rev 94: 419–459, 2014. [DOI] [PubMed] [Google Scholar]
- 32.Piper AS, Greenwood IA. Anomalous effect of anthracene-9-carboxylic acid on calcium-activated chloride currents in rabbit pulmonary artery smooth muscle cells. Br J Pharmacol 138: 31–38, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piper AS, Greenwood IA, Large WA. Dual effect of blocking agents on Ca2+-activated Cl− currents in rabbit pulmonary artery smooth muscle cells. J Physiol 539: 119–131, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piper AS, Large WA. Multiple conductance states of single Ca2+-activated Cl− channels in rabbit pulmonary artery smooth muscle cells. J Physiol 547: 181–196, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reyes JP, Huanosta-Gutiérrez A, López-Rodríguez A, Martínez-Torres A. Study of permeation and blocker binding in TMEM16A calcium-activated chloride channels. Channels (Austin) 9: 88–95, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rock JR, Harfe BD. Expression of TMEM16 paralogs during murine embryogenesis. Dev Dyn 237: 2566–2574, 2008. [DOI] [PubMed] [Google Scholar]
- 37.Sanders KM, O'Driscoll K, Leblanc N. Pharmacological properties of native CaCCs and TMEM16A. Channels (Austin) 8: 473–474, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 134: 1019–1029, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh RD, Gibbons SJ, Saravanaperumal SA, Du P, Hennig GW, Eisenman ST, Mazzone A, Hayashi Y, Cao C, Stoltz GJ, Ordog T, Rock JR, Harfe BD, Szurszewski JH, Farrugia G. Ano1, a Ca2+-activated Cl− channel, coordinates contractility in mouse intestine by Ca2+ transient coordination between interstitial cells of Cajal. J Physiol 592: 4051–4068, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Terashima H, Picollo A, Accardi A. Purified TMEM16A is sufficient to form Ca2+-activated Cl− channels. Proc Natl Acad Sci USA 110: 19354–19359, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tian Y, Kongsuphol P, Hug M, Ousingsawat J, Witzgall R, Schreiber R, Kunzelmann K. Calmodulin-dependent activation of the epithelial calcium-dependent chloride channel TMEM16A. FASEB J 25: 1058–1068, 2011. [DOI] [PubMed] [Google Scholar]
- 42.Tien J, Peters CJ, Wong XM, Cheng T, Jan YN, Jan LY, Yang H. A comprehensive search for calcium binding sites critical for TMEM16A calcium-activated chloride channel activity. Elife 30: 3, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Renterghem C, Lazdunski M. Endothelin and vasopressin activate low conductance chloride channels in aortic smooth muscle cells. Pflügers Arch 425: 156–163, 1993. [DOI] [PubMed] [Google Scholar]
- 44.Vocke K, Dauner K, Hahn A, Ulbrich A, Broecker J, Keller S, Frings S, Mohrlen F. Calmodulin-dependent activation and inactivation of anoctamin calcium-gated chloride channels. J Gen Physiol 142: 381–404, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whitlock JM, Hartzell HC. A pore idea: the ion conduction pathway of TMEM16/ANO proteins is composed partly of lipid. Pflügers Arch 468: 455–473, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiao Q, Yu K, Perez-Cornejo P, Cui Y, Arreola J, Hartzell HC. Voltage- and calcium-dependent gating of TMEM16A/Ano1 chloride channels are physically coupled by the first intracellular loop. Proc Natl Acad Sci USA 108: 8891–8896, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 455: 1210–1215, 2008. [DOI] [PubMed] [Google Scholar]
- 48.Yu K, Duran C, Qu Z, Cui YY, Hartzell HC. Explaining calcium-dependent gating of anoctamin-1 chloride channels requires a revised topology. Circ Res 110: 990–999, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu K, Zhu J, Qu Z, Cui YY, Hartzell HC. Activation of the Ano1 (TMEM16A) chloride channel by calcium is not mediated by calmodulin. J Gen Physiol 143: 253–267, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu Y, Kuan AS, Chen TY. Calcium-calmodulin does not alter the anion permeability of the mouse TMEM16A calcium-activated chloride channel. J Gen Physiol 144: 115–124, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu MH, Kim TW, Ro S, Yan W, Ward SM, Koh SD, Sanders KM. A Ca2+-activated Cl− conductance in interstitial cells of Cajal linked to slow wave currents and pacemaker activity. J Physiol 587: 4905–4918, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu MH, Sung IK, Zheng H, Sung TS, Britton FC, O'Driscoll K, Koh SD, Sanders KM. Muscarinic activation of Ca2+-activated Cl− current in interstitial cells of Cajal. J Physiol 589: 4565–4582, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]