Abstract
First characterized in neuronal tissues, the multifunctional calcium/calmodulin-dependent protein kinase II (CaMKII) is a key signaling component in several mammalian biological systems. Its unique capacity to integrate various Ca2+ signals into different specific outcomes is a precious asset to excitable and nonexcitable cells. Numerous studies have reported roles and mechanisms involving CaMKII in brain and heart tissues. However, corresponding functions in vascular cell types (endothelium and vascular smooth muscle cells) remained largely unexplored until recently. Investigation of the intracellular Ca2+ dynamics, their impact on vascular cell function, the regulatory processes involved and more recently the spatially restricted oscillatory Ca2+ signals and microdomains triggered significant interest towards proteins like CaMKII. Heteromultimerization of CaMKII isoforms (four isoforms and several splice variants) expands this kinase's peculiar capacity to decipher Ca2+ signals and initiate specific signaling processes, and thus controlling cellular functions. The physiological functions that rely on CaMKII are unsurprisingly diverse, ranging from regulating contractile state and cellular proliferation to Ca2+ homeostasis and cellular permeability. This review will focus on emerging evidence of CaMKII as an essential component of the vascular system, with a focus on the kinase isoform/splice variants and cellular system studied.
Keywords: CaMKII, calcium signaling, endothelium, vascular smooth muscle
seminal work from bennett et al. (13) described a unique protein isolated from brain extracts, calcium/calmodulin-dependent protein kinase II (CaMKII) (13). The following years were marked by intensive research on this protein in neurons, driven by the high abundance and significant role of the kinase. Indeed, CaMKII is crucial for long-term memory processes through its capacity to integrate intracellular calcium (Ca2+) oscillations (for review see refs. 91, 119, 180). A tissue-specific distribution of the four mammalian CaMKII isoforms (α, β, δ, and γ) was initially established, with CaMKIIα and β being restricted to brain while δ and γ are expressed in peripheral tissues (104, 168). Additional work led to the identification of CaMKII as an important regulator of cardiac gene expression (168) and intracellular Ca2+ dynamics (183), further evidenced by its involvement in cardiac arrhythmia and hypertrophy (for review see refs. 110, 149, 162). More recently, a growing body of evidence in the vascular smooth muscle cell (VSMC) literature suggests a crucial involvement of CaMKII in the regulation of vascular functions. Indeed, VSMC migration, proliferation, and hypertrophy (28, 41, 87, 89, 143) as well as VSMC-dependent regulation of vascular tone were shown to be controlled by CaMKII (76, 135). CaMKII functions in endothelial cells (ECs) remained, however, largely unexplored despite significant potential involvement of the kinase. Recent work shows that CaMKII regulates crucial endothelial functions, including vascular permeability and nitric oxide (NO) production (18, 32, 68, 78, 117). In light of the rapidly changing and sometimes divergent nature of the literature on vascular CaMKII, this review will highlight exciting findings and future perspectives for the multifunctional kinase in vascular smooth muscle and endothelial cells.
The Blueprint
The unique capacity of CaMKII to integrate various Ca2+ signals into specific outcomes involves a complex series of autoregulatory processes attributable to the serine/threonine kinase's particular structure. Of the four different isoforms of CaMKII characterized in mammals, CaMKIIα and β were first identified in brain and, until recently, were considered as brain specific while CaMKIIδ and γ were found in a broader range of tissues (168). A common organization of functional domains (e.g., N-terminal catalytic domain, central regulatory domain, C-terminal association domain) is shared amongst the four isoforms (90, 168). The association domain, which is unique to CaMKII within the CaM Kinase family, permits a multimeric conformation (or association) of 6 to 12 subunits and is the core of CaMKII's ability to decode different types of Ca2+ oscillations (Fig. 1A; for review, see ref. 163). CaMKII oligomerization is not restricted to identical isoforms (74). Heteromultimerization is actually a key feature of the enzyme, allowing a sophisticated detection of Ca2+ signals and the associated response, seen as a capacity to finely tune Ca2+-based intracellular signaling. However, to date the only heteromultimeric complexes to be detected in vivo comprise CaMKIIα/β: initially observed in neurons (22), the presence of CaMKIIα/β complexes was recently demonstrated in native endothelium (169). The inclusion of different isoforms and/or splice variants can convey unique properties to the CaMKII oligomer. For example, CaMKIIβ binds F-actin, an ability not shared by other CaMKII isoforms, which can be useful to other subunits through heteromultimerization (145). Similarly, including splice variants can result in distinct subcellular distributions, as evidenced by the nuclear distribution of CaMKIIδB compared with the sarcoplasmic reticulum restricted localization of CaMKIIδC (109). Therefore, CaMKII heteromultimerization expands the already broad range of interlinked signaling pathways into an amazing web of possibilities.
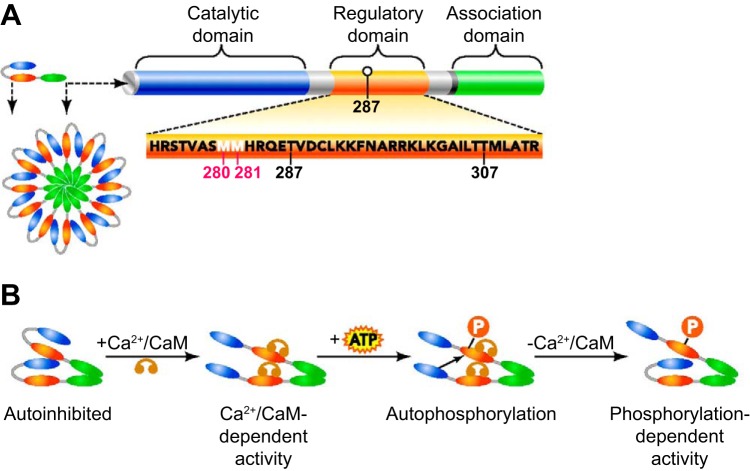
Fig. 1.
Structure and activation of CaMKII. A: each CaMKII monomer consists of a catalytic domain (N-terminal), a regulatory domain and an association domain (C-terminal). The association domain allows CaMKII to form a multimer of 6- to 12 monomers. Autophosphorylation sites (287 and 307) and residues sensitive to oxidation (280 and 281) are conserved among CaMKII isoforms and allow posttranscriptional modulation of the enzyme. B: mechanism of activation of CaMKII. Binding of Ca2+/CaM complex to a CaMKII monomer triggers a conformational change, and activates the subunit (Ca2+/CaM dependent-activity). The activated (CaM-bound) subunit phosphorylates an adjacent CaM-bound subunit which becomes active in a Ca2+/CaM independent-manner. [From Couchonnal and Anderson (30).]
Initiation of CaMKII activation, common to all CaMKII isoforms, requires an increase in intracellular Ca2+ levels. In resting conditions, the catalytic activity of the enzyme is inhibited by the binding of the autoinhibitory sequence of the regulatory domain to the catalytic domain. As the first step leading to CaMKII activation, in response to an increase in intracellular Ca2+, Ca2+/calmodulin (Ca2+/CaM) complexes form and bind to a subunit of CaMKII within the oligomeric complex (“Ca2+/CaM-dependent activation”). This disrupts the association between the regulatory autoinhibitory sequence and the catalytic domain, thus relieving inhibition and allowing phosphorylation of serine/threonine residues (reviewed in ref. 70). Such activity, being Ca2+/CaM dependent, would closely parallel the subsequent decline in intracellular Ca2+ concentration and the resulting dissociation of the Ca2+/CaM complex from the CaMKII subunit. The unique feature of CaMKII resides largely in the ability of activated subunits within the oligomer to undergo trans-autophosphorylation (138). Activated CaMKII subunits can phosphorylate threonine 286 residue (Thr286 for CaMKIIα; Thr287 for β/δ/γ) of an adjacent and already activated subunit (bound to Ca2+/CaM) (20, 141). The activation of phosphorylated CaMKII subunits will persist even upon Ca2+/CaM dissociation, a state called “Ca2+/CaM independent” or “autonomous” activation (Fig. 1B)(94, 142). Autophosphorylation at Thr286 also decreases (1,000-fold) the rate of Ca2+/CaM dissociation (“CaM-trapping”) from CaMKII subunits, allowing the enzyme to remain active longer. In addition, a subsequent elevation in intracellular Ca2+ level will increase both the extent and duration of activation of a partly active CaMKII multimer (i.e., more active subunits) (130). This heteromultimeric structure combined with the ability of trans-autophosphorylation to augment and prolong activation allows CaMKII to integrate divers forms of Ca2+ signaling (in frequency and amplitude) by converting a wide range of inputs into specific outcomes. The extent and duration of CaMKII activation is also influenced by the rate of dephosphorylation by protein serine/threonine phosphatases such as PP1 (17, 157), PP2A (64, 157), and PP2C (48).
Although less studied, autophosphorylation at threonine 305/306 (Thr305/306) also impacts on CaMKII activity; however, Thr305/306 phosphorylation is preceded by Thr286 phosphorylation (56, 125). In addition, autophosphorylation at Thr253 has been reported from in vivo studies (38) and is thought to be responsible for the targeting of CaMKII to neuronal postsynaptic densities (108). Modulation of prolonged CaMKII activity is not limited to autophosphorylation processes, as the enzyme is also sensitive to cellular redox state (27, 43), a potentially very important feature for an enzyme expressed in vascular cells and thus exposed to various degrees of oxidative stress. Anderson's group showed that increased levels of reactive oxygen species (ROS) is associated with the oxidation of methionine residues (Met281/282) of CaMKIIδ, which may be functionally analogous to autophosphorylation at Thr286/287 (43). Indeed, Met281/282 residues lie within the CaMKII regulatory domain and their oxidation prevents the regulatory domain from associating with the catalytic domain, thus maintaining CaMKII in an active conformation. This paired methionine motif is conserved in CaMKIIβ, δ, and γ isoforms, while the first methionine is replaced by a cysteine in CaMKIIα. As with methionine, cysteine residues are susceptible to oxidation. However, both ROS- and autophosphorylation-dependent mechanisms require binding of Ca2+/CaM to the regulatory domain for initial activation of a subunit (67, 122).
The Origins
The literature contains many reviews discussing the expression, subcellular distribution mechanisms of activation, function, and regulation of neuronal and cardiac CaMKII (21, 42, 54, 56, 61, 65, 152, 153). Accordingly, this section includes only a brief overview of CaMKII in these tissues as an introduction to its biological and cellular functions.
CaMKII was initially characterized in brain tissue as a Ca2+-dependent kinase controlling presynaptic neurotransmitter release, neuronal excitability, and postsynaptic regulation of learning and memory through long-term potentiation (LTP) (21, 57, 65, 70, 152). Indeed, upon synaptic stimulation, CaMKII converts transient Ca2+ signals into LTP, a sustained response (57, 92). NMDAR (51, 84, 158) and AMPAR receptors, two glutamate-gated ion channels, are the main targets for CaMKII regulation of LTP. CaMKII phosphorylation of the AMPAR GluR1 subunit increases single-channel conduction (10, 34, 100, 154, 165), and is essential to LTP since transgenic mice lacking the corresponding phosphorylation site are characterized by a deficiency in LTP (10, 100, 195). CaMKII also regulates the trafficking and assembly of proteins such as GluA2 subunits within AMPAR, thus modulating Ca2+ permeation of the channels (97). Furthermore, the importance of CaMKII in learning and memory has been confirmed by reports of altered associated cognitive functions in mice deficient in CaMKIIα (147) or expressing CaMKIIα-Thr286Ala, which cannot undergo phosphorylation at position 286 (52).
Neuronal excitability is also linked to intracellular Ca2+ levels, and CaMKII has been suggested to modulate neuronal Ca2+ influx. CaMKII phosphorylates voltage-gated L-type calcium channels (Cav2.1) when expressed heterologously in HEK or neuronal cell lines (72, 99). CaMKII is also a major player in cardiac intracellular Ca2+ homeostasis (21, 30, 45, 148, 164), as confirmed by studies of cardiovascular pathologies (30, 42). CaMKII regulates Ca2+ influx in cardiomyocytes by modulating Cav1.2 channels and the associated Ca2+ current (16, 194). Furthermore, McCarron et al. (103) showed that inhibition of CaMKII activity prevented the increase in Cav1.2 current elicited by Ca2+ in smooth muscle cells. In addition to Ca2+ influx, CaMKII also modulates the release of Ca2+ from intracellular stores. Phosphorylation of ryanodine receptors (RyR2) by CaMKII and oxidized-CaMKII was initially reported to stimulate sarcoplasmic reticulum (SR) Ca2+ release (31, 63, 164, 184, 186), although Yang et al. (2007) found that RyR2 phosphorylation by CaMKII reduces Ca2+ release and intracellular Ca2+ dynamics, including Ca2+ sparks and waves (191). Alternatively, CaMKII accelerates cytoplasmic Ca2+ cycling by enhancing SR Ca2+ uptake. Indeed, phosphorylation of proteins involved in refilling SR stores such as the sarco/endoplasmic reticulum Ca2+ ATPase pump (SERCA) (188, 189) or phospholamban (PLB) (148, 183) by CaMKII is associated with an increased SR Ca2+ uptake (176, 190). SR Ca2+ regulation appears to be dependent on αKAP, a CaMKII anchoring protein, allowing targeting of the kinase to SR in skeletal myocytes (11). CaMKII-αKAP interaction enhances the capacity of CaMKII to phosphorylate SR proteins such as RyR (55, 177, 186), SERCA (188, 189), and phospholamban (148, 183), and thereby, regulate myocyte Ca2+ homeostasis. Analogous anchoring proteins might very well be involved in directing the subcellular distribution of CaMKII in striated and nonstriated myocytes. Inositol 1,4,5-trisphosphate (IP3) receptor type 2 (IP3R2)-dependent increases in nucleoplasmic Ca2+ are reduced by CaMKII-mediated phosphorylation at Ser150, which reduces the open probability of the IP3R Ca2+ channel (9, 102). Hence, by directly phosphorylating Ca2+ channels or their accessory proteins, CaMKII is able to regulate both Ca2+ homeostasis and the dynamics of transient Ca2+ signaling events.
Excitable cells are essentially dependent on their membrane potential, and minor changes in their electrophysiological properties can result in significant outcomes. The first evidence that CaMKII may regulate cardiac membrane potential was the demonstration that CaMKII inhibitors autocamtide-2 and KN-63 prevent spontaneous depolarization in sinoatrial node cells (175). CaMKII positively modulates sodium voltage-gated channel (Nav1.5), an essential component of cardiac EC coupling, resulting in an increased spontaneous activity (192). Still controversial, the actual CaMKII phosphorylation site in Nav1.5 requires further investigation (3, 71). The functional expression of cardiac small conductance Ca2+-activated potassium channels (KCa2.x) is also increased by CaMKII, leading to arrhythmia in ventricular hypertrophy (111). In addition to AMPAR and NMDAR channels, CaMKII also modulates other neuronal ion channels. The open probability of large conductance Ca2+ activated potassium (K+) channels (BKca) is increased by CaMKII in neurons. Indeed, van Welie et al. (172) reported a 50% increase in BKca channel open probability when activated CaMKII was included in the patch pipette. Phosphorylation by CaMKII shifted the voltage dependency of BKca towards more negative membrane potentials, resulting in increased channel activity at resting membrane potentials, membrane hyperpolarization, and diminished neuronal excitability (172). Heterologous expression systems have also been used to study the effects of CaMKII activation on ion channels found in neurons and cardiomyocytes, such as the voltage-gated potassium channel (Kv) largely expressed in cardiomyocytes and dendrites of hippocampal pyramidal neurons. Cotransfection of COS cells with Kv4.2 and constitutively active CaMKII resulted in increased channel trafficking to the plasma membrane (173). Regulation of Kv4.2 by CaMKII is not limited to channel trafficking, as electrophysiological studies in rat cardiomyocytes have shown an acceleration of Ito,fast (Kv4.2-Kv4.3 complexes) inactivation, potentially occurring through CaMKII-Kv4.3 interactions (29). Hence, there is accumulating evidence showing direct regulation of membrane potential by CaMKII in excitable cells of cardiac and neuronal origin.
The control of gene expression by CaMKII, another hallmark of the significant role CaMKII plays in regulating key cellular functions, occurs mostly via two main pathways. Through phosphorylation of histone deacetylase (HDAC) in cardiomyocytes and other cell types, CaMKII stimulates the export of HDAC from the nucleus, allowing gene expression (5). This process is involved in CaMKII-induced cardiac hypertrophy (4, 95). Merlen et al. (107) recently showed that nuclear type B endothelin receptors activate CaMKII through IP3R-dependent Ca2+ release in cardiomyocytes and might lead to HDAC phosphorylation. Additionally, the transcription factor “cAMP responsive element binding protein” (CREB) has been identified as a CaMKII target. However, regulation of CREB by CaMKII is complex and involves phosphorylation at two different sites having opposing effects. Although phosphorylation by CaMKII at Ser142 blocks CREB activation, phosphorylation at Ser133 by CaMKII or PKA activates CREB (161). In addition, phosphorylation at Ser142 prevents phosphorylation at Ser133 from activating CREB. The negative effect of Ser142 phosphorylation appears to be dominant, as constitutively active CaMKII only activates CREB when Ser142 was replaced with alanine (161).
Decades of research on CaMKII in cardiac and neuronal tissue have led to significant advancements in our understanding of Ca2+-dependent regulation of many cellular processes. More recently the vascular field has become interested in the role of CaMKII, as there exist vascular counterparts to several of the cellular functions controlled by neuronal/cardiac CaMKII that play critical roles in cardiovascular homeostasis.
The Cardiovascular Sequel: Vascular Smooth Muscle
The main physiological function of vascular smooth muscle cells is to develop force through contraction to maintain appropriate levels of vascular tone. Despite the major influence of vascular endothelial cells, vascular smooth muscle function is essentially dependent on intrinsic mechanisms. These mechanisms, such as those regulating myocyte contraction, are sophisticated. Moreover, processes guiding VSMC proliferation and migration are also critical as they have been associated with vascular pathologies such as atherosclerosis or hypertension (reviewed in refs. 26, 137). Similar to neurons and cardiomyocytes, CaMKII appears to modulate several VSMC functions (Table 1). VSMCs show a variety of intracellular Ca2+ signals with distinct kinetics, frequencies, and subcellular origin (44, 121), providing a wide range of potential sources for CaMKII activation with specific intracellular localization.
Table 1.
CaMKII in smooth muscle cells
| Isoform | Cell Type | Culture Condition | Target | Effect | Function | Ref. |
|---|---|---|---|---|---|---|
| CaMKIIδ2 | Rat thoracic aortic SMC | Fresh + NC P3-P10 | Increase cell cycle progression | Positively regulates proliferation | (66) | |
| CaMKII | Rat thoracic aortic SMC | NC P7-P14 | Positively regulates proliferation | (126) | ||
| CaMKIIδ2 | Rat aortic SMC | NC P3-P10 | Positively regulates proliferation | (127) | ||
| CaMKIIδ | Mouse aortic SMC/entire mouse carotid | NC P4-P10 | AKT/Mdm2/p53/p21 pathway | Downregulation of p21 | Positively regulates proliferation | (89) |
| CaMKIIδ | Rat thoracic aortic SMC | NC P4-P8/NC P3-P9 | Raf1 | Complex with ERK, translocation nucleus | Positively regulates proliferation | (28, 106) |
| CaMKIIδ | Rat aortic SMC | NC P3-P7 | CREB | Inhibits CREB activity | Positively regulates proliferation | (93) |
| CaMKIIδ | Mouse aortic SMC/entire mouse carotid | NC P4-P10 | MMP9 | Increase MMP9 activity | Positively regulates migration | (143) |
| CaMKII/CaMKIIδ | Rat thoracic aortic SMC/human aortic VSMC | NC P5-P14/NC P3-P10 | ERK | Activated by adhesion, increase ERK phosphorylation | Positively regulates migration | (14, 96) |
| CaMKII | Second-order mouse mesenteric SMC | Fresh | Cavβ3 subunit/PLB | Increase Ca2+ entry, increase SR Ca2+ load | (128) | |
| CaMKII | Rabbit pulmonary/coronary SMC | Fresh/Fresh | Decrease Cl− current | (53, 82) | ||
| CaMKIIγ | Ferret thoracic aorta (± endothelium) | N/A | MLCK/LC20 | Increase MLCK/LC20 phosphorylation | Positively regulates SMC contraction | (76) |
| CaMKIIγ-G2 | Ferret thoracic aorta (± endothelium)/ferret aortic SMC | Fresh | LC20 | Increase LC20 phosphorylation, CaMKII plasma membrane recruitment after depolarization | Positively regulates SMC contraction | (101) |
| CaMKII | Rat thoracic aortic SMC/rat aortic SMC | NC P4-P8/NC P3-P9 | NOS2 | Resting CaMKII in complex with NOS2/NOS2 trafficking | Production or inhibition of NOS2 activity | (35, 73) |
| CaMKIIδ2 | Rat aortic SMC | NC P4-P10 | Increase VSMC hypertrophy | (87) | ||
| CaMKII/CaMKIIα | Rabbit aortic SMC/human aortic SMC | NC P2-P10/NC P4-P10/C (ATTC) P-NS | MEK/PLA2 | Increasing MEK/PLA2 activity, translocation of MEK/PLA2/CaMKII complex to the nucleus, AA production | (113–115) |
Migration and proliferation.
The relationship between CaMKII and VSMC proliferation and migration has been extensively studied and still garners significant interest. Indeed, vasculogenesis and pathological neointimal proliferation are physiologically relevant and enticed research in this field. Interestingly, the expression pattern of CaMKII is linked to myocyte phenotype. Indeed, primary/differentiated VSMCs express primarily CaMKIIγ whereas CaMKIIδ expression is higher in proliferative/cultured VSMCs (66). Platelet-derived growth factor (PDGF) triggers both a rapid activation of CaMKII and CaMKII-dependent rise in intracellular Ca2+ in cultured proliferative VSMCs but not in quiescent myocytes (126). This cytoplasmic increase in Ca2+ and CaMKII activation is important for VSMC migration and proliferation: inhibition of CaMKII with KN-62 (126) or KN-93 (127) almost abolishes PDGF-induced myocyte migration. In a carotid injury model, medial hyperplasia correlates with an increase in the expression of CaMKIIδ (≈43%) but not of CaMKIIγ. Accordingly, neointimal proliferation of VSMCs is significantly lower in CaMKIIδ−/− mice compared with wild-type mice (89). The involvement of CaMKIIδ in pathological vascular remodeling can also be extended further, as knockout mice are also protected against structural changes to the external elastic laminae (EEL) following carotid injury (143).
Agonist-induced, CaMKIIδ-dependent proliferation and migration of VSMCs is not limited to PGDF. Indeed, cultured VSMCs from CaMKIIδ−/− mice showed similarly reduced migration in the presence of other growth factors (e.g., FBS, TNF-α) but also in the absence of stimulation (143). Accordingly, overexpression of CaMKIIδ rescued VSMC migration (143). Transgenic expression of an oxidant-resistant form of CaMKII (methionine-to-valine mutation) did not affect VSMC proliferation in the carotid ligation injury model, suggesting that ROS-dependent activation of CaMKII is required (199). Despite conflicting reports obtained using cultured cells (66, 106, 127), CaMKIIδ is still considered as a major regulator of VSMC proliferation and migration.
Cell cycle progression.
The modulation of VSMC proliferation by CaMKII is a consequence of its role in cell cycle control. Indeed, CaMKII regulates the expression of cyclin-dependent kinase 2 (Cdk2) and cyclin E (89), critical cell cycle activators that promote the transition from G1 to S phase (146). Li et al. (89) showed that the increase in Cdk2 and cyclin E expression in a carotid ligation injury model was prevented by knocking out CaMKIIδ. Moreover, Cdk2 and cyclin E activity is regulated by the cell cycle inhibitor p21, and p21 expression is higher in carotids from CaMKIIδ−/− mice that underwent carotid ligation injury than in ligated arteries from wild-type mice. Therefore, the expression pattern of cell cycle regulators appear to be influenced by CaMKIIδ, as the absence of this kinase correlates with a decrease in VSMC proliferation and cell cycle arrest (89). A detailed signaling pathway encompassing AKT/Mdm2/p53 was further elucidated by Li et al. (89), and CaMKII was shown to stimulate AKT activation in VSMCs (85). This leads to high levels of Mdm2 phosphorylation at the AKT-specific Ser166 site (198) and the ensuing p53 degradation in WT mice. The loss of p53 significantly decreases p21 expression. In CaMKIIδ−/− VSMCs, activation of the AKT/Mdm2/p53 pathway is attenuated, p21 expression increases, and proliferation is thus reduced (89).
Crucial to cell cycle control through the sustained expression of proteins such as cyclin D1 (182, 185), the ERK pathway is also targeted by CaMKII. CaMKIIδ is required for ERK activation, since its suppression is associated with lower ERK activation (106). A direct interaction between ERK and CaMKII was demonstrated by coimmunoprecipitation assays that also showed that formation of an ERK-CaMKII complex was prevented by either U0216, which inhibits ERK activation, or KN-93 (28). The binding of Raf1 with CaMKII precedes the formation of ERK-CaMKII complexes (28). Indeed, the interaction of CaMKII/Raf1 with ERK allows ERK to phosphorylate CaMKII, leading to nuclear translocation of the macromolecular complex and alterations in transcription.
CREB activation is strongly associated with inhibition of VSMC proliferation. Expressing a constitutively active CREB in VSMCs eliminates FBS-induced myocyte proliferation and decreases the expression of growth factors and growth factor receptors such as the PDGF receptor (77). The role of CaMKII in modulating the cell cycle in VSMCs via CREB was investigated by Singer's group, following up on initial reports of CREB inhibition by CaMKII-mediated phosphorylation at Ser142 (161). Thrombin- and ionomycin-induced increases in CREB phosphorylation at Ser142 were significantly lower in cells exposed to KN-93 or siRNA targeting CaMKII (93). In summary, CaMKII strongly stimulates VSMC proliferation through inhibition of CREB activity.
Interaction with the extracellular matrix.
CaMKII modulation of VSMC migration includes degradation of the extracellular matrix or reduced cellular adhesion. Matrix metalloproteinases (MMPs) are a family of proteases involved in extracellular matrix breakdown or remodeling. Using rat cardiac fibroblasts, Zhang et al. (197) provided the first evidence that CaMKII regulates MMP expression in the cardiovascular system. A similar relationship exists in smooth muscle (143). Upon stimulation with TNF-α, MMP9 activity, immunoreactivity, and mRNA levels were markedly lower in serum collected from cultured CaMKIIδ−/− VSMCs relative to wild-type cells (143). These results suggest that CaMKII regulates MMP9 activity at the level of its transcription or translation; however, the authors suggest that CaMKII regulates MMP9 posttranscriptionally by increasing the stability of its mRNA. This study established that CaMKII may significantly influence cell migration through extracellular matrix breakdown.
Cellular interactions with the extracellular matrix, including focal adhesions, are critical for cell migration. Activating integrin signaling triggers Ca2+ transients in VSMCs (139) that are thought to activate Ca2+-dependent proteins involved in cell migration. Integrin-dependent CaMKII activation was first investigated with PDGF-induced migration of smooth muscle cells (14). In vitro, inhibition of the vitronectin receptor, an integrin found in VSMCs, decreases PGDF-induced CaMKII activation twofold. This led to the suggestion that inhibition of vitronectin receptors suppresses IP3 production and the corresponding changes in intracellular Ca2+ (14). However, Lu et al. (96) reported the activation of CaMKII by cellular adhesion through an integrin-independent mechanism. Although no evidence of a direct interaction was shown, subsequent phosphorylation of ERK can be blocked by KN-93 or shRNA targeting CaMKIIδ. This study, along with work from Cipolletta et al. (28), illustrates the convoluted pathways and reciprocal relationships involving CaMKII in the control of VSMC proliferation and migration.
Smooth muscle contractility.
The primary role as a regulator of vascular tone is conferred to vascular smooth muscle cell by its contractile capacity and is of utmost physiological relevance. In contrast to studies of migration and proliferation, investigation of the involvement of CaMKII in modulation of vascular tone is mainly limited to native cells, since cultured cells are generally in a proliferative state. Despite a significant body of evidence establishing the regulation of cardiomyocyte contraction by CaMKII, its role in regulation of VSMC contraction and vascular tone is less well characterized.
Contraction of VSMCs is essentially, but not exclusively, dependent on Ca2+ homeostasis (for review see ref. 181) and relies primarily on Ca2+ entry through Cav1.2 and to a lesser extent on other mechanisms including IP3R-dependent Ca2+ release from the SR (for review see ref. 62). Investigation of the modulation of Ca2+ homeostasis in VSMCs by CaMKII, as described above in neurons or in cardiomyocytes, turned out to be more complex than first expected. Indeed, the most commonly used CaMKII inhibitor, KN-93, appeared to have nonspecific effects on smooth muscle Ca2+ and K+ channels (50, 81, 133), indicating that data obtained using KN-93 in VSMCs requires careful interpretation. Early work by McCarron et al. (103) showed that CaMKII phosphorylates Cav1.2 in smooth muscle cells. More recently, Grumbach's group investigated the role of CaMKII in the regulation of Ca2+ homeostasis using TG-SM-CaMKIIN, a transgenic mouse line with the VSMC-specific expression of a CaMKII inhibitory peptide, CaMKIIN (128). Prasad et al. (128) reported a reduction of CaMKII activity (−65%), which correlates with an alteration of myocyte electrophysiological properties. Phosphorylation of the Cavβ3 subunit was lower in VSMCs from TG-SM-CaMKIIN mice, resulting in a decreased angiotensin II (ANGII)-induced Ca2+ entry. Moreover, SR Ca2+ loading was also diminished by the expression of CaMKIIN, correlating with a 50% reduction of PLB phosphorylation (Thr17) and hence, a greater inhibition of SERCA (128).
Regulation of VSMC membrane potential is an efficient approach to modulate VSMC contraction, owing to the strong voltage-dependency of Ca2+ channel opening. The inward rectifier K+ channel (Kir) is a strong modulator of VSMC membrane potential level. Activation by extracellular K+ leads to VSMC hyperpolarization and vasodilatation (40, 196). Despite the absence of a reported relationship between CaMKII and Kir, a recent study in primarily cultured bovine pulmonary arterial endothelial cells (PAEC) shows that CaMKII is able to regulate Ikr current (132). One could then speculate that VSMC Kir is also modulated by CaMKII. In addition to potassium currents, the resting membrane potential of native smooth muscle cells is partly regulated by chloride currents and Ca2+-activated chloride channels (ClCa) (for review see refs. 23, 80). Leblanc's group was the first to investigate the role of CaMKII in the modulation of ClCa channels in VSMCs (53, 82). Inhibition of CaMKII with KN-93 or autocamtide-2-related inhibitory peptide (AIP) resulted in a larger ClCa current in VSMCs from rabbit pulmonary (53) and coronary arteries (82). Moreover, the intracellular Ca2+ rise evoked by ionomycin triggered translocation of CaMKII to the plasma membrane, showing that CaMKII can modulate ion channels upon activation (82). Acting as a negative feedback mechanism following an increase in intracellular Ca2+, activated CaMKII reduces chloride currents and thereby contributes to membrane hyperpolarization and regulation of VSMC contractility, as shown in intestinal smooth muscle cell (58, 79).
CaMKII activation alters myofibrillar function in VSMCs. Decreased CaMKIIγ expression or pharmacological inhibition of CaMKII activity diminishes contraction in endothelium-denuded ferret aortae in response to high KCl (76). Interestingly, the CaMKII isoform identified in this study, CaMKIIγ, is different from the isoform generally considered (CaMKIIδ). Although Kim et al. (76) did not demonstrate a direct association between CaMKIIγ and myofibrillar proteins, myosin light-chain kinase (MLCK) activity and subsequent myosin light-chain (LC20) phosphorylation were decreased following inhibition of CaMKIIγ. Rokolya and Singer (135) observed similar effects of CaMKII inhibition on contractile force in porcine carotid arteries. Further investigation of the relationship between CaMKII and the contractile apparatus by Morgan's group led to the identification of CaMKIIγ-G2, a novel CaMKII variant expressed in ferret aortic myocytes. This variant is characterized by the presence of two SH3 domains, important for cytoskeleton interactions, within its association domain. In accordance with their previous work, these authors showed that a knockdown of CaMKIIγ-G2 results in a significant decrease in KCl-induced force development and LC20 phosphorylation (101), indicating that CaMKIIγ-G2 modulates myofibrillar activation in VSMCs. Moreover, the interaction of CaMKII with the cytoskeleton upon depolarization was also assessed. Under resting conditions, CaMKIIγ-G2 colocalizes with vimentin and α-actinin, but not actin, in regions described as cytoplasmic dense bodies. However, smooth muscle depolarization triggers the translocation and recruitment of CaMKIIγ-G2 to the cell periphery (cortical dense plaque) (101). This translocation to the plasmalemmal membrane allows CaMKIIγ-G2 to regulate Ca2+ channels. Hence, localization or translocation of CaMKII plays an important role in the ability of these kinases to regulate a multitude of cellular processes.
Impaired vascular homeostasis due to dysfunctional or damaged endothelium can be partly compensated for by modification of the VSMC phenotype. For example, an inflammatory response can promote the expression of inducible nitric oxide synthase (iNOS; NOS2), which generates large amount of NO, a potent vasodilator, anti-inflammatory, and antioxidant agent (for review see refs. 59, 75). NOS2 regulation (expression, subcellular localization, or activity) is complex and still not fully understood. Recent studies implicate CaMKII as a posttranscriptional regulator via modulation of NOS2 trafficking. Increased NOS2 expression is observed following exposure to TNF-α, IL-1β (73), or lipopolysaccharides (LPS) (35). VSMCs exposed to cytokines showed significant colocalization and association of NOS2 and CaMKIIδ2. However, if ionomycin is applied to cytokine-stimulated myocytes NOS2-CaMKIIδ2 complexes are not observed and the subcellular distribution of NOS2 is altered. Corroborative results are obtained if cytokine- (73) or LPS-exposed (35) VSMCs are preincubated with KN-93. Interestingly, these studies emphasize the role of CaMKII as a scaffolding protein, thereby modulating NOS2 activity. Although further investigation is required, inactivated CaMKII also appears to associate with NOS2 in an inhibitory complex with a specific subcellular localization.
CaMKII modulates other signaling pathways involved in vascular homeostasis. Ca2+ influx in VSMCs evoked by ANGII leads to phospholipase A2 (PLA2) activation (47). Interestingly, PLA2 modulates VSMC contractility through the release of arachidonic acid (AA), a precursor for prostacyclin synthesis. Similarly, norepinephrine (NE)-induced Ca2+ influx leads to the CaMKII-dependent activation and translocation of PLA2 to the nucleus in rabbit aortic VSMCs (114). Indeed, CaMKII interacts with cytosolic PLA2 in vitro and phosphorylates PLA2 at Ser515 both in vitro and in intact VSMCs (115). CaMKII activation precedes mitogen-activated protein kinase kinase (MEK) activation, which is involved in PLA2 activation. CaMKII inhibition also abolishes the release of AA induced by NE, demonstrating the critical role of CaMKII (114). Several AA metabolites, such as hydroxy-eicosatetraenoic acids (HETEs), are vasoactive and involved in a positive feedback loop on AA production. For example, CaMKII stimulates the production of HETEs, which activates ERK1/2, therein increasing PLA2 activation and AA production (113).
Pathological hypertrophy.
Vascular disorders such as hypertension have been associated with VSMCs hypertrophy, particularly with the increased cell size (≈35% greater) observed in spontaneous hypertensive rats in comparison to their normotensive counterparts (120). ANGII is a well-described vasoactive endogenous agent with a strong hypertrophic influence (for review see ref. 136). Reports of CaMKII involvement in cardiac hypertrophy led Grumbach's group to investigate similar roles for vascular CaMKII in response to ANGII. They and others showed that the blood pressure rise induced by ANGII infusion was blunted by inhibition of CaMKII with KN-93 (87). Furthermore, ANGII-induced VSMC hypertrophy was reduced by adenoviral transfection of CaMKIIN. A corresponding exacerbation of VSMC hypertrophy was found in myocytes overexpressing CaMKII (87, 116). Although current literature is limited to ANGII-induced hypertrophy, CaMKII might also be involved in myocyte hypertrophy in response to other stimuli. Furthermore, the mechanisms involved remain unidentified and require further study.
The Last Frontier: Endothelial Cells
The endothelium is an active and essential component in cardiovascular hemostasis. Intracellular Ca2+, the crux of endothelial signaling and functions, is tightly regulated but the role of CaMKII as an effector or regulator has only recently gathered attention. As in VSMCs, distinct patterns of Ca2+ oscillation have been identified in endothelial cells that differ in their dynamic characteristics as well as source of Ca2+, illustrating the potential for CaMKII in modulating endothelial functions (83, 156, 160). However, conflicting data have been reported, presumably because of differences in the type of endothelial cells used. Indeed, early studies in ECs were largely performed in cultured ECs, notorious for their altered phenotype. Modifications in the protein expression pattern by the culture process and passage number can thus result in signaling pathway adaptation. However, when taken together, findings from various cell lines strengthen the conclusions from cultured ECs studies. Similarly, results obtained using freshly harvested tissue or cells are limited to the respective species unless confirmed in human or across other species. Nonetheless, growing interest in the function of endothelial CaMKII can be noted and is summarized in the following sections as well as in Table 2.
Table 2.
CaMKII in endothelial cells
| Isoform | Cell Type | Culture Condition | Target | Effect | Function | Ref. |
|---|---|---|---|---|---|---|
| CaMKII | Bovine pulmonary artery EC | NC P3-P5 | Ikr | Increase Ikr current and protein expression | (132) | |
| CaMKII | Bovine pulmonary artery EC | NC P4-P10 | Filamin/F-actin/gap junction | Filamin translocation/F-actin rearrangement/gap junction formation | (178) | |
| CaMKII | Bovine pulmonary artery EC | C (ATTC) P19-P24 | Increase vascular permeability | (19) | ||
| CaMKII/CaMKIIα | Bovine pulmonary artery EC | C (ATTC) P19-P24 | ERK | ERK activation | Involve in cell barrier dysfunction | (18) |
| CaMKIIδ6 | Human umbilical vein EC | C (Cascade biologics) P3- P12 | RhoA | RhoA activation leading to ROCK activation | Hyperpermeability | (179) |
| CaMKII | Rat pulmonary microvascular EC/rat pulmonary artery EC/bovine pulmonary EC | NC P-NS/NC P5-P9/NC P4-P10 | Positively regulates EC migration | (105, 178) | ||
| CaMKII | Bovine retinal EC | NC P1-P2 | AKT | AKT phosphorylation following VEGF stimulation | Positively regulates EC migration | (8) |
| CaMKII | Calf pulmonary artery EC | C (ATTC) P15-P21 | IP3R/I mechanism | Decreasing Ca2+ store release/Favor Ca2+ entry | (1, 2) | |
| CaMKIIα/CaMKIIβ | Mouse mesentery artery | N/A | IP3R | Decreases IP3R Ca2+ release | (169) | |
| CaMKII | Macaque choroid-retinal EC (RF/6A) | C (CAS) P3-P4 | Fas/JNK | Increase mitochondrial membrane potential/increase cytochrome C release | Positively regulates EC apoptosis | (88) |
| CaMKIIα | Porcine aortic EC | NS P29-P30 | NOS3 | Increase NOS3 activity | Positively regulates NO production | (140) |
| CaMKII | Human umbilical vein EC/bovine aortic EC | C (Cell system) P-NS/NC P3-P5 | NOS3 mRNA | Increase NOS3 mRNA level | Positively regulates NO production | (24, 25, 86) |
| CaMKII | Rat aorta/human umbilical vein EC/bovine aortic EC | NC P1/N/A/NC P6-P10 | NOS3 | Increase NOS3 activity (PSer1177) | Positively regulates NO production | (46, 78, 123) |
| CaMKIIα/β | Native mouse mesenteric EC | N/A | NOS3 | Increasing NO production | Positively regulates NO production | (27) |
| CaMKII | Human umbilical vein EC/bovine pulmonary artery EC | NC P1/NC P4-P10 | NOS3 | Regulates NOS3 localization | (46, 178) |
Endothelial CaMKII expression pattern.
Most studies looking at CaMKII expression in ECs were focusing on the δ and γ isoforms, based on early work suggesting that expression of CaMKIIα and β is restricted to neuronal tissue. Indeed, Wang et al. (179) probed for CaMKII isoforms in different cultured ECs lines, including human umbilical vascular EC (HUVEC), bovine arterial EC (BAEC), and human dermal microvascular EC (HDMEC). Using a pan-CaMKII antibody, a single band obtained on Western blots (50-kDa) led them to conclude that CaMKIIδ is the endothelial isoform. However, a similar single ≈50-kDa band detected in PAEC was attributed to CaMKIIα (140). Since the predicted molecular weights for CaMKIIα and CaMKIIδ are similar, the use of pan-CaMKII antibodies in immunoblots does not provide sufficient information to discriminate between CaMKIIα and δ. Furthermore, transcripts for CaMKIIγb, γc,and δ2 variants have been detected in BCEC4 cells (7). In contrast, only CaMKIIα was detected by in situ hybridization and Western blot in primary cultured rat brain EC, although CaMKIIδ and γ isoforms were not sought (33). More recently, CaMKIIα, β and δ, but not CaMKIIγ, were found in native endothelial cells from mouse mesenteric arteries (27). Although CaMKIIδ and γ appear to be expressed in smooth muscle cells from the same arteries, CaMKIIα and β were not detected in myocytes. Confocal imaging showed that CaMKIIα and β shared similar intracellular distribution in situ, with clusters found within endothelial-smooth muscle communication structures called myoendothelial projections (MEPs). Both isoforms were also found to be in close proximity and might form heteromultimers (169). The subcellular localization of endothelial CaMKIIδ was distinct, with the enzyme being mainly found at the plasma membrane and in the Golgi (27). Such specific spatial distribution suggests that endothelial CaMKII isoforms might play different roles, being activated by different stimuli and/or having distinct targets and functions. Indeed, CaMKIIα and β were reported to translocate to MEPs upon activation of endothelial Ca2+ signaling (27) while CaMKIIδ did not.
Cell permeability.
Endothelial permeability is tightly regulated: a leaky endothelial barrier can have deleterious outcomes, but may be required for appropriate physiological functions such as inflammatory processes (for review see ref. 193). The endothelial barrier is passive and relies on tightness of cell-to-cell junctions, which are modulated in inflammation to allow access of immune cells to the tissue. Incubation of bovine pulmonary arterial EC (BPAEC) with a CaMKII inhibitory peptide decreased bradykinin-induced cytoskeletal rearrangement (translocation of cytosolic filamin to cell the membrane and modification of F-actin). These mechanisms are thought to increase vascular permeability and appear to be modulated by CaMKII (178). Moreover, intercellular communications through gap junctions are dependent on the identity of the connexin subunits forming the hemichannels. Recent in vitro work showed that CaMKII could directly modulate connexin 43, one of the three endothelial connexins (connexin 37, 40 and 43; for review see ref. 32, 69). Bradykinin-induced gap junction association also occurs through a CaMKII-dependent pathway (178). However, additional in vivo investigations are required to better understand role of CaMKII in modulating cell-to-cell communication.
The intracellular signaling regulating transendothelial permeability also appears to involve CaMKII. Previously shown to activate CaMKII (33), thrombin, a major modulator of vascular permeability, was used to elucidate the relationship between CaMKII and EC permeability (19). CaMKII activity in BAECs is increased in the presence of thrombin and endothelial permeability (monitored by albumin clearance and transendothelial electrical resistance measurement) is significantly impaired in the presence of KN-93 (19). Accordingly, CaMKII autophosphorylation at Thr287, used as an index of CaMKII activation, is increased in HUVEC cells exposed to thrombin (179). Therefore, thrombin activation of CaMKII correlates with increased endothelial permeability. From a mechanistic perspective, CaMKII modulation of vascular permeability appears to be linked to the ERK1/2 pathway. As reported in VSMCs, KN-93 significantly impairs ERK activation in ECs with a concomitant loss of actin stress fibers (18). The CaMKII isoform involved might, however, be different from VSMCs since transfection of HUVEC cells with CaMKIIδ-targeted siRNA did not prevent ERK activation. Interestingly, a critical determinant in thrombin-induced hyperpermeability, the RhoA pathway, is significantly inhibited by CaMKIIδ silencing (179). Since RhoA is an important player for regulation of VSMC contractility (for review see refs. 129, 155, 187), one can speculate that CaMKII may be analogously involved in the control of endothelial permeability through RhoA. The elucidation of the roles other CaMKII isoforms play in the control of endothelial permeability is an interesting avenue and could lead to identification of pharmacological targets for specific signaling pathways.
Migration and proliferation.
Both physiological and pathological angiogenesis begins with EC migration and proliferation (124, 144). Plexiform lesions found in patients with pulmonary arterial hypertension (PAH) result from pathological and disorganized cellular proliferation and migration within the arterial lumen (171). Plexiform lesions appear to be enriched in fibronectin, an extracellular matrix component that can act as chemoattractant (105), and thus promote ECs migration. Moreover, thrombin-stimulated migration of pulmonary microvascular ECs (PMVECs) grown on fibronectin can be abolished by inhibition of protease-activated receptor 1 (PAR1). As KN-93 prevents the thrombin/fibronectin-induced EC migration, one or more subtypes of CaMKII are involved (105). These studies suggest that endothelial CaMKII might be an interesting target to limit the formation of plexiform lesions in PAH.
The retina is an established model for the study of angiogenesis, and the role of CaMKII regulating EC migration has been studied in the retinal vasculature. Vascular endothelial growth factor (VEGF) was shown to raise intracellular Ca2+ levels in bovine retinal ECs (BRECs) (8). A twofold increase in AKT activity was also reported in response to VEGF. Although the link between AKT activation and intracellular Ca2+ has not been established in the context of the retinal endothelium, CaMKII is an interesting prospect. Indeed, AKT phosphorylation was significantly diminished by KN-93 in VEGF-treated BRECs (8), similarly to VSMCs. Further evidence of a role of CaMKII in EC migration was provided when KN-62 was shown to reduce BPAEC migration in a wound-healing assay by 30–40% (178).
Calcium dynamics.
Essential to virtually every endothelial function, numerous facets of endothelial Ca2+ dynamics have been scrutinized. Indeed, Ca2+ ions are an essential cofactor to basically every endothelial function (37, 112, 166). CaMKII is linked to Ca2+ homeostasis in cardiomyocytes and VSMCs but also in vascular endothelium. For example, the role of CaMKII in the ATP-dependent increase in intracellular Ca2+ was fully described in calf pulmonary arterial ECs (CPAECs) (1, 2). ATP is known to stimulate both the release of Ca2+ from intracellular Ca2+ stores and capacitative Ca2+ entry (CCE; also known as store-operated Ca2+ entry; SOCE) in ECs. Inhibition of CaMKII altered the morphology of ATP-induced Ca2+ transients by decreasing the sustained phase without affecting the Ca2+ peak amplitude. However, exposure of CPAECs to KN-93 alone (in the absence of ATP) triggered a similar Ca2+ transient, even in the absence of extracellular Ca2+. The KN-93-sensitive Ca2+ rise thus originates from intracellular stores. Although nonspecific effects of KN-93 were not ruled out, it was proposed that inhibition of CaMKII could evoke a Ca2+ transient through IP3R in the endoplasmic reticulum. Accordingly, inhibition of IP3R receptors led to a 91% decrease in KN-93-induced Ca2+ transients (1). Moreover, endothelial CaMKII is involved in a regulatory feedback loop where the kinase can be activated by local increases in Ca2+ arising from intracellular stores (27, 169) in native endothelial cells from mesenteric resistance arteries. Upon activation, CaMKII appears to modulate intracellular Ca2+ store content through inhibition of IP3R activity. Recent advances on local endothelial Ca2+ signaling (6, 83, 156, 160) and subcellular compartmentalization of intracellular pathways offer a renewed perspective on the physiological specificity of otherwise broad pathways. Indeed, Ca2+ signals within MEP called Ca2+ pulsars activate and recruit CaMKIIα and β to these endothelial cellular projections where the kinase can regulate the activity of other proteins (27, 169). Noteworthy, CaMKII activity in resting endothelium (in the absence of agonist) is sufficient to modulate intracellular Ca2+, since inhibition of CaMKII with KN-93 stimulated Ca2+ pulsars in mesenteric arteries. Further studies showed that CaMKII controls local Ca2+ dynamics and ER Ca2+ levels in native endothelium through inhibition of IP3R (169). In addition to modulation of Ca2+ stores, work on cultured CPAECs showed that CaMKII stimulates CCE (1) in a similar fashion to what has been reported in Xenopus oocytes and skeletal muscle cells (98, 174). Modulation of CCE by CaMKII has also been reported in VSMCs, highlighting the ubiquitous role of CaMKII in regulating Ca2+ signals (134). In summary, the reciprocal relationship between CaMKII activation and intracellular Ca2+ dynamics is definitely convoluted: the high level of sophistication in CaMKII signaling that is becoming apparent allows for the fine-tuning of critical cellular pathways and functions.
Pathological levels of cytoplasmic Ca2+, as provoked by hyperglycemia, are deleterious to endothelial cells and can trigger apoptosis. Exposure to high glucose levels has been used to explore the involvement of CaMKII in EC apoptosis (15, 170). Incubation of RF/6A cells (a cell line derived from macaque choroid-retinal ECs) in hyperglycemic milieu for 96 hours significantly increased apoptosis. Moreover, intracellular Ca2+ release and CaMKII activation were stimulated by increasing glucose levels from 5.5 mM (normal glucose) to 30 mM. Interestingly, increased CaMKII activity did not coincide with changes in enzyme expression. The impact of CaMKII activity on EC apoptosis was demonstrated as a significant reduction of hyperglycemia-induced apoptosis when cells were treated with KN-93 (88). The mechanism involves the modulation of mitochondrial membrane potential and cytochrome c release by CaMKII and, possibly, the fas/JNK cascade (88) similar to macrophages (167).
Nitric oxide regulation.
Nitric oxide, the most potent endogenous endothelium-derived vasodilator, is produced primarily by endothelial nitric oxide synthase (eNOS: NOS3) in ECs. Ca2+ ions, in complex with CaM, are essential cofactors for NOS3 activation. Therefore, CaMKII through regulation of endothelial intracellular Ca2+ signaling could indirectly modulate NOS3 activity and NO production. In addition to activation by Ca2+, posttranslational processes including phosphorylation have been shown to elevate NOS3 activity (for review see refs. 39, 131). Indeed, acute application of the Ca2+ ionophore A23187 triggered NO production and release in PAECs in a CaMKII-dependent fashion (140).
CaMKII can modulate the activity of proteins such as NOS3 by various means, including transcription. In HUVECs, the histamine-dependent increase in NOS3 mRNA levels was sensitive to CaMKII inhibition but independent of PKC, JAK2, MAPK, and PI3K, suggesting that the CaMKII stimulates NOS3 expression (86). Exogenous application of hydrogen peroxide (H2O2) to BAECs increased CaMKII activation (24). NOS3 mRNA levels were also higher in cells exposed to H2O2, an effect that was attenuated (≈−77%) by CaMKII inhibition. Although the precise mechanism involved in the regulation of NOS3 expression has yet to be determined, a CaMKII-dependent increase in the half-life of NOS3 mRNA has been excluded (24). In contrast to the normal dynamic nature of the endothelium's physiological environment in vivo, conditions in culture are relatively stagnant, without flow and shear stress. Indeed, blood flow continuously exerts a non-stationary force on vascular walls. The impact of oscillatory shear stress on the NOS3-CaMKII interaction was therefore investigated. As expected, a CaMKII-dependent increase in NOS3 expression and NO production was observed in cells exposed to oscillatory shear stress (25).
Phosphorylation of NOS3 is also a common regulatory process not restricted to CaMKII as consensus sites for other kinases (AKT, AMPK, and PKA) have been reported. Phosphorylation of NOS3 at Ser1177 significantly increases NOS3 activity as does substituting Ser1177 with a “phosphomimetic” acidic amino acid (36). Seminal work on CaMKII-dependent phosphorylation of the NOS family was performed on NOS1 (nNOS) in brain tissue (117). Later, NOS3 phosphorylation by CaMKII was demonstrated in HUVECs (46) and rat aortic endothelial cells (78). NOS3 phosphorylation is also strongly altered by CaMKII inhibition in BAECs (123). Endothelial MEPs appear to be enriched in NOS3 (159), whereas CaMKII was shown to translocate upon activation of local Ca2+ signaling (27). Phosphorylated NOS3 (Ser1177) immunoreactivity within MEPs was also decreased upon inhibition of CaMKII (27). Furthermore, Ca2+ pulsar-dependent NO production, monitored using the fluorescent NO-sensitive dye DAF-FM, was reduced by CaMKII inhibition, suggesting that CaMKII activation serves to amplify Ca2+-dependent NO production (27). Akt is also able to phosphorylate NOS3 at Ser1177 (49); hence, as CaMKII can also modulate Akt activation, the effect of CaMKII on NOS3 activity may be either direct or mediated via a CaMKII-AKT-NOS3 pathway.
The subcellular localization of NOS3 may also be regulated by CaMKII. Indeed, while membrane-bound NOS3 is inactive, CaMKII activation results in translocation of NOS3 to the cytoplasm (178). The direct interaction of CaMKII with NOS3 may be responsible for this translocation. In support of this, immunoprecipitation assays show increased association of CaMKII with NOS3 in HUVECs stimulated with bradykinin (46). Hence, CaMKII may function as both regulator and targeting subunit for NOS3.
Vascular CaMKII: Now and Then
As detailed in this review, CaMKII is involved in a wide range of vascular functions (Fig. 2; nonexhaustive representation of the relationships presented in this review), which might explain the growing interest in this enzyme. In light of the complexity arising from oligomerization and the large number of distinct CaMKII variants, additional roles for CaMKII, possibly of interest in vascular cells, likely remain to be identified. As mentioned above, evidence suggests that CaMKII can modulate proteins independently of its kinase activity, serving as an anchoring, targeting, or structural partner. Research into these aspects of CaMKII signaling and their ability to modulate vascular function will expand our understanding of the physiological roles of the CaMKII family.
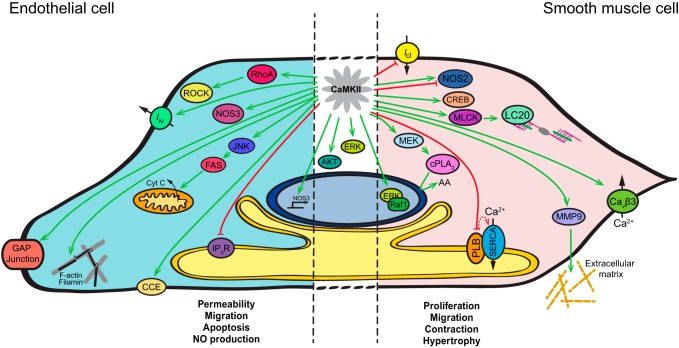
Fig. 2.
CaMKII signaling targets in vascular cells. Schematic summary of CaMKII's targets in endothelial cells (left, blue), smooth muscle cells (right, pink) or shared by both vascular cell types (middle). ROCK, Rho-associated protein kinase; NOS3, nitric oxide synthase 3; JNK, c-Jun NH2-terminal kinase; Cyt c: cytochrome c; IP3R, inositol 1,4,5-trisphosphate receptor; CCE, capacitative calcium entry; ERK, extracellular signal-regulated kinase; Icl, chloride current; NOS2, nitric oxide synthase 2; CREB, cAMP response element-binding protein; MLCK, myosin light chain kinase; LC20, myosin light chain; MEK, mitogen-activated protein kinase kinase; PLA2, phospholipase A2; AA, arachidonic acid; PLB, phospholamban; SERCA, sarco/endoplasmic reticulum Ca2+-ATPase; MMP9, matrix metalloproteinase 9; Cavβ3, voltage-dependent calcium channel, subunit-β3.
Among the various functions controlled by CaMKII, Ca2+ homeostasis is intriguing as it allows the enzyme to regulate its own activation. However, the dogma by which CaMKII is activated by global rise in intracellular Ca2+ might shift according to the recent developments in local Ca2+ signaling, especially in endothelial cells. Indeed, as a consequence of its oligomeric structure, CaMKII has the unique property of being modulated by Ca2+ signal amplitude and frequency. Therefore, slight modification in intracellular Ca2+ dynamics might have a profound effect on CaMKII-dependent events. It seems then reasonable to speculate that Ca2+ microdomains might experience distinct patterns of CaMKII activation and, as a consequence, highly localized changes in CaMKII-dependent functions.
The role of CaMKII has largely been studied using pharmacological inhibitors such as KN-93 or KN-62. However, although KN-93 is a relatively specific inhibitor for CaMKII, it has been shown to alter VSMC Cav1.2 channel activity. This important Ca2+ channel may directly influence CaMKII activation as well as CaMKII-independent pathways (50). Moreover, KN-93 has been shown to inhibit Kv channels (81, 133) and, more recently, IKr current (60). Some in vitro studies have also employed molecular tools, including siRNA or AIP expression, in addition to pharmacological inhibitors. Unfortunately, use of these tools cannot seamlessly be extended to in vivo investigations and their use is often restricted to cell cultures. Alternatively, knockout and transgenic animals could be developed. For example, CaMKIIδ knockout mice are commercially available. A transgenic mouse expressing a CaMKII peptide inhibitor specifically in VSMCs has also been used, as reported in this review. However, there are currently no mouse models (knockout or transgenic) available where endothelial CaMKII isoforms (systemic or endothelial specific) are targeted.
Regulation of CaMKII activity is generally expected to involve phosphorylation and/or oxidation as discussed previously although a vascular counterpart to cardiac oxidized-CaMKII remains to be established. Subcellular targeting and translocation are also relevant properties. The anchoring of CaMKII to different cell membranes has been suggested to be important in the formation of signalosomes that include CaMKII and a Ca2+ source or CaMKII target protein. Emerging evidence on Ca2+ microdomains could then be of interest, as targeting specific CaMKII complexes to Ca2+ microdomains would allow for spatial and temporal localization of CaMKII activation and hence CaMKII-mediated cellular functions. Indeed, endothelial KCa2.x and KCa3.1 channels have a distinct intracellular distribution and might be differentially regulated by heterogeneously distributed CaMKII. Studies in skeletal muscle (11, 12) or cardiomyocytes (150, 151) suggest that membrane targeting requires αKAP, a unique CaMKII subunit featuring transmembrane and CaMKII association domains, but lacking a catalytic domain (12). Thus, heteromultimerization of CaMKII isoforms with this subunit may result in targeting of CaMKII complexes to a specific subcellular region such as the SR (11, 150), nucleus (118) or plasma membrane. However, the possible expression and potential role for an analogous vascular membrane-anchoring CaMKII subunit remains to be determined.
Most of the investigations discussed in this review have been performed in cultured ECs and VSMCs and have significantly improved our understanding of the roles of CaMKII in the cardiovascular system. In situ investigations are still required to confirm the findings from cultured cells as discrepancies may occur, as evidenced by distinct differences in the pattern of CaMKII isoform expression in native versus culture ECs. Moreover, it is now clear that CaMKII, and, possibly, isoform-specific targeting, represents an interesting therapeutic avenue for treatment of vascular disease such as hypertension.
GRANTS
This work was supported by Montreal Heart Institute (F. Toussaint and C. Charbel), Fonds de recherche du Québec - Santé (F. Toussaint and J. Ledoux), Société Québécoise D'hypertension Artérielle (F. Toussaint), and Heart and Stroke Foundation of Canada (J. Ledoux and B. G. Allen).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
F.T. prepared figures; F.T., C.C., and J.L. drafted manuscript; F.T., C.C., B.G.A., and J.L. edited and revised manuscript; F.T., C.C., B.G.A., and J.L. approved final version of manuscript.
Glossary
- AIP
Autocamtide-2-related inhibitory peptide
- AMPAR
α-Amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor
- AMPK
5′-AMP-activated protein kinase
- ANGII
Angiotensin II
- AA
Arachidonic acid
- BAEC
Bovine arterial endothelial cell
- BKCa
Large conductance Ca2+-activated K+ channels
- BPAEC
Bovine pulmonary arterial endothelial cell
- BREC
Bovine retinal endothelial cell
- Ca2+
Calcium
- Ca2+/CaM
Calcium/calmodulin complex
- CaM
Calmodulin
- CaMKII
Calcium/calmodulin-dependent protein kinase II
- Cav1.2
Voltage-gated L-type calcium channel
- CCE
Capacitative calcium entry
- Cdk2
Cyclin-dependent kinase 2
- Clca
Ca2+-activated chloride channels
- CPAEC
Calf pulmonary arterial endothelial cell
- CREB
cAMP response element-binding protein
- Cyt c
Cytochrome c
- EC
Endothelial cell
- EEL
External elastic laminae
- HDMEC
Human dermal microvascular endothelial cell
- ERK
Extracellular signal-regulated kinase
- FBS
Fetal bovine serum
- H2O2
Hydrogen peroxide
- HDAC
Histone deacetylase
- HDMEC
Human dermal microvascular endothelial cell
- HETEs
Hydroxyeicosatetraenoic acids
- HUVEC
Human umbilical vascular endothelial cell
- Icl
Chloride current
- iNOS/NOS2
Inductible nitric oxide synthase
- IL-1β
Interleukin-1β
- IP3
Inositol 1,4,5-trisphosphate
- IP3R
Inositol 1,4,5-trisphosphate receptor
- JAK2
Janus kinase 2
- JNK
c-Jun NH2-terminal kinase
- K+
Potassium
- KCa2.x
Small conductance Ca2+-activated potassium channels
- Kir
Inward rectifying K+ channel
- KN-62
4-[(2S)-2-[(5-isoquinolinylsulfonyl)methylamino]-3-oxo-3-(4-phenyl-1-piperazinyl)propyl] phenyl isoquinolinesulfonic acid ester
- KN-93
N-[2-[[[3-(4-Chlorophenyl)-2-propenyl]methylamino]methyl]phenyl]-N-(2 hydroxyethyl)-4-methoxybenzenesulfonamide
- Kv
Voltage-gated potassium channel
- LC20
Myosin light chain
- LPS
Lipopolysaccharides
- LTP
Long-term potentiation
- MAPK
Mitogen-activated protein kinase
- MEP
Myoendothelial projection
- MEK
Mitogen-activated protein kinase kinase
- Met
Methionine
- MLCK
Myosin light chain kinase
- MMP
Matrix metalloproteinase
- Nav1.5
Sodium voltage-gated channel
- NE
Norepinephrine
- NMDAR
N-methyl-d-aspartate receptor
- nNOS/NOS1
Neuronal nitric oxide synthase
- NO
Nitric oxide
- PAEC
Pulmonary arterial endothelial cell
- PAH
Pulmonary arterial hypertension
- PAR1
Protease-activated receptor 1
- PDGF
Platelet-derived growth factor
- PI3K
Phosphoinositide 3-kinase
- PKA
Protein kinase A
- PKC
Protein kinase C
- PLA2
Phospholipase A2
- PLB
Phospholamban
- PP1
Protein phosphatase 1
- PP2A
Protein phosphatase 2
- PP2C
Protein phosphatase 2C
- ROCK
Rho-associated protein kinase
- ROS
Reactive oxygen species
- RyR
Ryoanodine receptor
- Ser
Serine
- SERCA
Sarco/endoplasmic reticulum Ca2+-ATPase pump
- SMC
Smooth muscle cell
- SR
Sarcoplasmic reticulum
- Thr
Threonine
- TNF-α
Tumor necrosis factor-α
- VEGF
Vascular endothelial growth factor
- VSMC
Vascular smooth muscle cell
REFERENCES
- 1.Aromolaran AA, Blatter LA. Modulation of intracellular Ca2+ release and capacitative Ca2+ entry by CaMKII inhibitors in bovine vascular endothelial cells. Am J Physiol Cell Physiol 289: C1426–C1436, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Aromolaran AS, Zima AV, Blatter LA. Role of glycolytically generated ATP for CaMKII-mediated regulation of intracellular Ca2+ signaling in bovine vascular endothelial cells. Am J Physiol Cell Physiol 293: C106–C118, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Ashpole NM, Herren AW, Ginsburg KS, Brogan JD, Johnson DE, Cummins TR, Bers DM, Hudmon A. Ca2+/calmodulin-dependent protein kinase II (CaMKII) regulates cardiac sodium channel NaV1.5 gating by multiple phosphorylation sites. J Biol Chem 287: 19856–19869, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Backs J, Backs T, Bezprozvannaya S, McKinsey TA, Olson EN. Histone deacetylase 5 acquires calcium/calmodulin-dependent kinase II responsiveness by oligomerization with histone deacetylase 4. Mol Cell Biol 28: 3437–3445, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Backs J, Song K, Bezprozvannaya S, Chang S, Olson EN. CaM kinase II selectively signals to histone deacetylase 4 during cardiomyocyte hypertrophy. J Clin Invest 116: 1853–1864, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bagher P, Beleznai T, Kansui Y, Mitchell R, Garland CJ, Dora KA. Low intravascular pressure activates endothelial cell TRPV4 channels, local Ca2+ events, and IKCa channels, reducing arteriolar tone. Proc Natl Acad Sci USA 109: 18174–18179, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balla Z, Hoch B, Karczewski P, Blasig IE. Calcium/calmodulin-dependent protein kinase IIdelta 2 and gamma isoforms regulate potassium currents of rat brain capillary endothelial cells under hypoxic conditions. J Biol Chem 277: 21306–21314, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Banumathi E, O'Connor A, Gurunathan S, Simpson DA, McGeown JG, Curtis TM. VEGF-induced retinal angiogenic signaling is critically dependent on Ca2+ signaling by Ca2+/calmodulin-dependent protein kinase II. Invest Ophthalmol Vis Sci 52: 3103–3111, 2011. [DOI] [PubMed] [Google Scholar]
- 9.Bare DJ, Kettlun CS, Liang M, Bers DM, Mignery GA. Cardiac type 2 inositol 1,4,5-trisphosphate receptor: interaction and modulation by calcium/calmodulin-dependent protein kinase II. J Biol Chem 280: 15912–15920, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Barria A, Derkach V, Soderling T. Identification of the Ca2+/calmodulin-dependent protein kinase II regulatory phosphorylation site in the alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate-type glutamate receptor. J Biol Chem 272: 32727–32730, 1997. [DOI] [PubMed] [Google Scholar]
- 11.Bayer KU, Harbers K, Schulman H. alphaKAP is an anchoring protein for a novel CaM kinase II isoform in skeletal muscle. EMBO J 17: 5598–5605, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bayer KU, Lohler J, Harbers K. An alternative, nonkinase product of the brain-specifically expressed Ca2+/calmodulin-dependent kinase II alpha isoform gene in skeletal muscle. Mol Cell Biol 16: 29–36, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennett MK, Erondu NE, Kennedy MB. Purification and characterization of a calmodulin-dependent protein kinase that is highly concentrated in brain. J Biol Chem 258: 12735–12744, 1983. [PubMed] [Google Scholar]
- 14.Bilato C, Curto KA, Monticone RE, Pauly RR, White AJ, Crow MT. The inhibition of vascular smooth muscle cell migration by peptide and antibody antagonists of the alphavbeta3 integrin complex is reversed by activated calcium/calmodulin- dependent protein kinase II. J Clin Invest 100: 693–704, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bishara NB, Ding H. Glucose enhances expression of TRPC1 and calcium entry in endothelial cells. Am J Physiol Heart Circ Physiol 298: H171–H178, 2010. [DOI] [PubMed] [Google Scholar]
- 16.Blaich A, Welling A, Fischer S, Wegener JW, Kostner K, Hofmann F, Moosmang S. Facilitation of murine cardiac L-type Cav1.2 channel is modulated by calmodulin kinase II-dependent phosphorylation of S1512 and S1570. Proc Natl Acad Sci USA 107: 10285–10289, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blitzer RD, Connor JH, Brown GP, Wong T, Shenolikar S, Iyengar R, Landau EM. Gating of CaMKII by cAMP-regulated protein phosphatase activity during LTP. Science 280: 1940–1942, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Borbiev T, Verin AD, Birukova A, Liu F, Crow MT, Garcia JG. Role of CaM kinase II and ERK activation in thrombin-induced endothelial cell barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 285: L43–L54, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Borbiev T, Verin AD, Shi S, Liu F, Garcia JG. Regulation of endothelial cell barrier function by calcium/calmodulin-dependent protein kinase II. Am J Physiol Lung Cell Mol Physiol 280: L983–L990, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Bradshaw JM, Hudmon A, Schulman H. Chemical quenched flow kinetic studies indicate an intraholoenzyme autophosphorylation mechanism for Ca2+/calmodulin-dependent protein kinase II. J Biol Chem 277: 20991–20998, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Braun AP, Schulman H. The multifunctional calcium/calmodulin-dependent protein kinase: from form to function. Annu Rev Physiol 57: 417–445, 1995. [DOI] [PubMed] [Google Scholar]
- 22.Brocke L, Chiang LW, Wagner PD, Schulman H. Functional implications of the subunit composition of neuronal CaM kinase II. J Biol Chem 274: 22713–22722, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Bulley S, Jaggar JH. Cl− channels in smooth muscle cells. Pflügers Arch 466: 861–872, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai H, Davis ME, Drummond GR, Harrison DG. Induction of endothelial NO synthase by hydrogen peroxide via a Ca2+/calmodulin-dependent protein kinase II/janus kinase 2-dependent pathway. Arterioscler Thromb Vasc Biol 21: 1571–1576, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Cai H, McNally JS, Weber M, Harrison DG. Oscillatory shear stress upregulation of endothelial nitric oxide synthase requires intracellular hydrogen peroxide and CaMKII. J Mol Cell Cardiol 37: 121–125, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Cao Y, Li H, Mu FT, Ebisui O, Funder JW, Liu JP. Telomerase activation causes vascular smooth muscle cell proliferation in genetic hypertension. FASEB J 16: 96–98, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Charbel C, Toussaint F, Béziau D, Gillis MA, Blanchette A, Mamarbachi M, Bousette N, Comtois P, Ledoux J. Functional activation of endothelial CaMKII by Ca2+ microdomains. Sci Adv. Accepted: 2015. [Google Scholar]
- 28.Cipolletta E, Monaco S, Maione AS, Vitiello L, Campiglia P, Pastore L, Franchini C, Novellino E, Limongelli V, Bayer KU, Means AR, Rossi G, Trimarco B, Iaccarino G, Illario M. Calmodulin-dependent kinase II mediates vascular smooth muscle cell proliferation and is potentiated by extracellular signal regulated kinase. Endocrinology 151: 2747–2759, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colinas O, Gallego M, Setien R, Lopez-Lopez JR, Perez-Garcia MT, Casis O. Differential modulation of Kv4.2 and Kv4.3 channels by calmodulin-dependent protein kinase II in rat cardiac myocytes. Am J Physiol Heart Circ Physiol 291: H1978–H1987, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Couchonnal LF, Anderson ME. The role of calmodulin kinase II in myocardial physiology and disease. Physiology (Bethesda) 23: 151–159, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Currie S, Loughrey CM, Craig MA, Smith GL. Calcium/calmodulin-dependent protein kinase IIdelta associates with the ryanodine receptor complex and regulates channel function in rabbit heart. Biochem J 377: 357–366, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Wit C, Griffith TM. Connexins and gap junctions in the EDHF phenomenon and conducted vasomotor responses. Pflügers Arch 459: 897–914, 2010. [DOI] [PubMed] [Google Scholar]
- 33.Deli MA, Joo F, Krizbai I, Lengyel I, Nunzi MG, Wolff JR. Calcium/calmodulin-stimulated protein kinase II is present in primary cultures of cerebral endothelial cells. J Neurochem 60: 1960–1963, 1993. [DOI] [PubMed] [Google Scholar]
- 34.Derkach V, Barria A, Soderling TR. Ca2+/calmodulin-kinase II enhances channel conductance of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors. Proc Natl Acad Sci USA 96: 3269–3274, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Pietro N, Di Tomo P, Di Silvestre S, Giardinelli A, Pipino C, Morabito C, Formoso G, Mariggio MA, Pandolfi A. Increased iNOS activity in vascular smooth muscle cells from diabetic rats: Potential role of Ca2+/calmodulin-dependent protein kinase II delta 2 (CaMKIIdelta(2)). Atherosclerosis 226: 88–94, 2013. [DOI] [PubMed] [Google Scholar]
- 36.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399: 601–605, 1999. [DOI] [PubMed] [Google Scholar]
- 37.Dora KA, Garland CJ. Linking hyperpolarization to endothelial cell calcium events in arterioles. Microcirculation 20: 248–256, 2013. [DOI] [PubMed] [Google Scholar]
- 38.Dosemeci A, Gollop N, Jaffe H. Identification of a major autophosphorylation site on postsynaptic density-associated Ca2+/calmodulin-dependent protein kinase. J Biol Chem 269: 31330–31333, 1994. [PubMed] [Google Scholar]
- 39.Dudzinski DM, Michel T. Life history of eNOS: partners and pathways. Cardiovasc Res 75: 247–260, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature 396: 269–272, 1998. [DOI] [PubMed] [Google Scholar]
- 41.EROB, Ma X, Simard T, Pourdjabbar A, Hibbert B. Pathogenesis of neointima formation following vascular injury. Cardiovasc Hematol Disord Drug Targets 11: 30–39, 2011. [DOI] [PubMed] [Google Scholar]
- 42.Erickson JR, He BJ, Grumbach IM, Anderson ME. CaMKII in the cardiovascular system: sensing redox states. Physiol Rev 91: 889–915, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O'Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJ, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell 133: 462–474, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fedoryak OD, Searls Y, Smirnova IV, Burns DM, Stehno-Bittel L. Spontaneous Ca2+ oscillations in subcellular compartments of vascular smooth muscle cells rely on different Ca2+ pools. Cell Res 14: 379–388, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Ferris CD, Huganir RL, Bredt DS, Cameron AM, Snyder SH. Inositol trisphosphate receptor: phosphorylation by protein kinase C and calcium calmodulin-dependent protein kinases in reconstituted lipid vesicles. Proc Natl Acad Sci USA 88: 2232–2235, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fleming I, Fisslthaler B, Dimmeler S, Kemp BE, Busse R. Phosphorylation of Thr(495) regulates Ca2+/calmodulin-dependent endothelial nitric oxide synthase activity. Circ Res 88: E68–E75, 2001. [DOI] [PubMed] [Google Scholar]
- 47.Freeman EJ, Ruehr ML, Dorman RV. ANG II-induced translocation of cytosolic PLA2 to the nucleus in vascular smooth muscle cells. Am J Physiol Cell Physiol 274: C282–C288, 1998. [DOI] [PubMed] [Google Scholar]
- 48.Fukunaga K, Kobayashi T, Tamura S, Miyamoto E. Dephosphorylation of autophosphorylated Ca2+/calmodulin-dependent protein kinase II by protein phosphatase 2C. J Biol Chem 268: 133–137, 1993. [PubMed] [Google Scholar]
- 49.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 399: 597–601, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao L, Blair LA, Marshall J. CaMKII-independent effects of KN93 and its inactive analog KN92: reversible inhibition of L-type calcium channels. Biochem Biophys Res Commun 345: 1606–1610, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Gardoni F, Caputi A, Cimino M, Pastorino L, Cattabeni F, Di Luca M. Calcium/calmodulin-dependent protein kinase II is associated with NR2A/B subunits of NMDA receptor in postsynaptic densities. J Neurochem 71: 1733–1741, 1998. [DOI] [PubMed] [Google Scholar]
- 52.Giese KP, Fedorov NB, Filipkowski RK, Silva AJ. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science 279: 870–873, 1998. [DOI] [PubMed] [Google Scholar]
- 53.Greenwood IA, Ledoux J, Leblanc N. Differential regulation of Ca2+-activated Cl− currents in rabbit arterial and portal vein smooth muscle cells by Ca2+-calmodulin-dependent kinase. J Physiol 534: 395–408, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Griffith LC, Lu CS, Sun XX. CaMKII, an enzyme on the move: regulation of temporospatial localization. Mol Interv 3: 386–403, 2003. [DOI] [PubMed] [Google Scholar]
- 55.Hain J, Onoue H, Mayrleitner M, Fleischer S, Schindler H. Phosphorylation modulates the function of the calcium release channel of sarcoplasmic reticulum from cardiac muscle. J Biol Chem 270: 2074–2081, 1995. [DOI] [PubMed] [Google Scholar]
- 56.Hanson PI, Schulman H. Inhibitory autophosphorylation of multifunctional Ca2+/calmodulin-dependent protein kinase analyzed by site-directed mutagenesis. J Biol Chem 267: 17216–17224, 1992. [PubMed] [Google Scholar]
- 57.Hanson PI, Schulman H. Neuronal Ca2+/calmodulin-dependent protein kinases. Annu Rev Biochem 61: 559–601, 1992. [DOI] [PubMed] [Google Scholar]
- 58.He XD, Goyal RK. CaMKII inhibition hyperpolarizes membrane and blocks nitrergic IJP by closing a Cl− conductance in intestinal smooth muscle. Am J Physiol Gastrointest Liver Physiol 303: G240–G246, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hecker M, Cattaruzza M, Wagner AH. Regulation of inducible nitric oxide synthase gene expression in vascular smooth muscle cells. Gen Pharmacol 32: 9–16, 1999. [DOI] [PubMed] [Google Scholar]
- 60.Hegyi B, Chen-Izu Y, Jian Z, Shimkunas R, Izu LT, Banyasz T. KN-93 inhibits IKr in mammalian cardiomyocytes. J Mol Cell Cardiol 89: 173–176, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heist EK, Schulman H. The role of Ca2+/calmodulin-dependent protein kinases within the nucleus. Cell Calcium 23: 103–114, 1998. [DOI] [PubMed] [Google Scholar]
- 62.Hill-Eubanks DC, Werner ME, Heppner TJ, Nelson MT. Calcium signaling in smooth muscle. Cold Spring Harb Perspect Biol 3: a004549, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ho HT, Liu B, Snyder JS, Lou Q, Brundage EA, Velez-Cortes F, Wang H, Ziolo MT, Anderson ME, Sen CK, Wehrens XH, Fedorov VV, Biesiadecki BJ, Hund TJ, Gyorke S. Ryanodine receptor phosphorylation by oxidized CaMKII contributes to the cardiotoxic effects of cardiac glycosides. Cardiovasc Res 101: 165–174, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoffman A, Carpenter H, Kahl R, Watt LF, Dickson PW, Rostas JA, Verrills NM, Skelding KA. Dephosphorylation of CaMKII at T253 controls the metaphase-anaphase transition. Cell Signal 26: 748–756, 2014. [DOI] [PubMed] [Google Scholar]
- 65.Hook SS, Means AR. Ca2+/CaM-dependent kinases: from activation to function. Annu Rev Pharmacol Toxicol 41: 471–505, 2001. [DOI] [PubMed] [Google Scholar]
- 66.House SJ, Ginnan RG, Armstrong SE, Singer HA. Calcium/calmodulin-dependent protein kinase II-delta isoform regulation of vascular smooth muscle cell proliferation. Am J Physiol Cell Physiol 292: C2276–C2287, 2007. [DOI] [PubMed] [Google Scholar]
- 67.Howe CJ, Lahair MM, McCubrey JA, Franklin RA. Redox regulation of the calcium/calmodulin-dependent protein kinases. J Biol Chem 279: 44573–44581, 2004. [DOI] [PubMed] [Google Scholar]
- 68.Huang CC, Hsu KS. Activation of NMDA receptors reduces metabotropic glutamate receptor-induced long-term depression in the nucleus accumbens via a CaMKII-dependent mechanism. Neuropharmacology 63: 1298–1307, 2012. [DOI] [PubMed] [Google Scholar]
- 69.Huang RY, Laing JG, Kanter EM, Berthoud VM, Bao M, Rohrs HW, Townsend RR, Yamada KA. Identification of CaMKII phosphorylation sites in Connexin43 by high-resolution mass spectrometry. J Proteome Res 10: 1098–1109, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hudmon A, Schulman H. Structure-function of the multifunctional Ca2+/calmodulin-dependent protein kinase II. Biochem J 364: 593–611, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hund TJ, Koval OM, Li J, Wright PJ, Qian L, Snyder JS, Gudmundsson H, Kline CF, Davidson NP, Cardona N, Rasband MN, Anderson ME, Mohler PJ. A beta(IV)-spectrin/CaMKII signaling complex is essential for membrane excitability in mice. J Clin Invest 120: 3508–3519, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jiang X, Lautermilch NJ, Watari H, Westenbroek RE, Scheuer T, Catterall WA. Modulation of CaV2.1 channels by Ca2+/calmodulin-dependent protein kinase II bound to the C-terminal domain. Proc Natl Acad Sci USA 105: 341–346, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jones RJ, Jourd'heuil D, Salerno JC, Smith SM, Singer HA. iNOS regulation by calcium/calmodulin-dependent protein kinase II in vascular smooth muscle. Am J Physiol Heart Circ Physiol 292: H2634–H2642, 2007. [DOI] [PubMed] [Google Scholar]
- 74.Kanaseki T, Ikeuchi Y, Sugiura H, Yamauchi T. Structural features of Ca2+/calmodulin-dependent protein kinase II revealed by electron microscopy. J Cell Biol 115: 1049–1060, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kibbe M, Billiar T, Tzeng E. Inducible nitric oxide synthase and vascular injury. Cardiovasc Res 43: 650–657, 1999. [DOI] [PubMed] [Google Scholar]
- 76.Kim I, Je HD, Gallant C, Zhan Q, Riper DV, Badwey JA, Singer HA, Morgan KG. Ca2+-calmodulin-dependent protein kinase II-dependent activation of contractility in ferret aorta. J Physiol 526: 367–374, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Klemm DJ, Watson PA, Frid MG, Dempsey EC, Schaack J, Colton LA, Nesterova A, Stenmark KR, Reusch JE. cAMP response element-binding protein content is a molecular determinant of smooth muscle cell proliferation and migration. J Biol Chem 276: 46132–46141, 2001. [DOI] [PubMed] [Google Scholar]
- 78.Kobayashi T, Nemoto S, Ishida K, Taguchi K, Matsumoto T, Kamata K. Involvement of CaM kinase II in the impairment of endothelial function and eNOS activity in aortas of Type 2 diabetic rats. Clin Sci (Lond) 123: 375–386, 2012. [DOI] [PubMed] [Google Scholar]
- 79.Koh SD, Perrino BA, Hatton WJ, Kenyon JL, Sanders KM. Novel regulation of the A-type K+ current in murine proximal colon by calcium-calmodulin-dependent protein kinase II. J Physiol 517: 75–84, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Large WA, Wang Q. Characteristics and physiological role of the Ca2+-activated Cl− conductance in smooth muscle. Am J Physiol Cell Physiol 271: C435–C454, 1996. [DOI] [PubMed] [Google Scholar]
- 81.Ledoux J, Chartier D, Leblanc N. Inhibitors of calmodulin-dependent protein kinase are nonspecific blockers of voltage-dependent K+ channels in vascular myocytes. J Pharmacol Exp Ther 290: 1165–1174, 1999. [PubMed] [Google Scholar]
- 82.Ledoux J, Greenwood I, Villeneuve LR, Leblanc N. Modulation of Ca2+-dependent Cl− channels by calcineurin in rabbit coronary arterial myocytes. J Physiol 552: 701–714, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ledoux J, Taylor MS, Bonev AD, Hannah RM, Solodushko V, Shui B, Tallini Y, Kotlikoff MI, Nelson MT. Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proc Natl Acad Sci USA 105: 9627–9632, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Leonard AS, Lim IA, Hemsworth DE, Horne MC, Hell JW. Calcium/calmodulin-dependent protein kinase II is associated with the N-methyl-d-aspartate receptor. Proc Natl Acad Sci USA 96: 3239–3244, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li F, Malik KU. Angiotensin II-induced Akt activation is mediated by metabolites of arachidonic acid generated by CaMKII-stimulated Ca2+-dependent phospholipase A2. Am J Physiol Heart Circ Physiol 288: H2306–H2316, 2005. [DOI] [PubMed] [Google Scholar]
- 86.Li H, Burkhardt C, Heinrich UR, Brausch I, Xia N, Forstermann U. Histamine upregulates gene expression of endothelial nitric oxide synthase in human vascular endothelial cells. Circulation 107: 2348–2354, 2003. [DOI] [PubMed] [Google Scholar]
- 87.Li H, Li W, Gupta AK, Mohler PJ, Anderson ME, Grumbach IM. Calmodulin kinase II is required for angiotensin II-mediated vascular smooth muscle hypertrophy. Am J Physiol Heart Circ Physiol 298: H688–H698, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li J, Wang P, Yu S, Zheng Z, Xu X. Calcium entry mediates hyperglycemia-induced apoptosis through Ca2+/calmodulin-dependent kinase II in retinal capillary endothelial cells. Mol Vis 18: 2371–2379, 2012. [PMC free article] [PubMed] [Google Scholar]
- 89.Li W, Li H, Sanders PN, Mohler PJ, Backs J, Olson EN, Anderson ME, Grumbach IM. The multifunctional Ca2+/calmodulin-dependent kinase II delta (CaMKIIdelta) controls neointima formation after carotid ligation and vascular smooth muscle cell proliferation through cell cycle regulation by p21. J Biol Chem 286: 7990–7999, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lin CR, Kapiloff MS, Durgerian S, Tatemoto K, Russo AF, Hanson P, Schulman H, Rosenfeld MG. Molecular cloning of a brain-specific calcium/calmodulin-dependent protein kinase. Proc Natl Acad Sci USA 84: 5962–5966, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lisman J, Yasuda R, Raghavachari S. Mechanisms of CaMKII action in long-term potentiation. Nat Rev Neurosci 13: 169–182, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu XB, Murray KD. Neuronal excitability and calcium/calmodulin-dependent protein kinase type II: location, location, location. Epilepsia 53, Suppl 1: 45–52, 2012. [DOI] [PubMed] [Google Scholar]
- 93.Liu Y, Sun LY, Singer DV, Ginnan R, Singer HA. CaMKIIdelta-dependent inhibition of cAMP-response element-binding protein activity in vascular smooth muscle. J Biol Chem 288: 33519–33529, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lou LL, Lloyd SJ, Schulman H. Activation of the multifunctional Ca2+/calmodulin-dependent protein kinase by autophosphorylation: ATP modulates production of an autonomous enzyme. Proc Natl Acad Sci USA 83: 9497–9501, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lu J, McKinsey TA, Nicol RL, Olson EN. Signal-dependent activation of the MEF2 transcription factor by dissociation from histone deacetylases. Proc Natl Acad Sci USA 97: 4070–4075, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lu KK, Armstrong SE, Ginnan R, Singer HA. Adhesion-dependent activation of CaMKII and regulation of ERK activation in vascular smooth muscle. Am J Physiol Cell Physiol 289: C1343–C1350, 2005. [DOI] [PubMed] [Google Scholar]
- 97.Lu W, Khatri L, Ziff EB. Trafficking of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPA) receptor subunit GluA2 from the endoplasmic reticulum is stimulated by a complex containing Ca2+/calmodulin-activated kinase II (CaMKII) and PICK1 protein and by release of Ca2+ from internal stores. J Biol Chem 289: 19218–19230, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Machaca K. Ca2+-calmodulin-dependent protein kinase II potentiates store-operated Ca2+ current. J Biol Chem 278: 33730–33737, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Magupalli VG, Mochida S, Yan J, Jiang X, Westenbroek RE, Nairn AC, Scheuer T, Catterall WA. Ca2+-independent activation of Ca2+/calmodulin-dependent protein kinase II bound to the C-terminal domain of CaV2.1 calcium channels. J Biol Chem 288: 4637–4648, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mammen AL, Kameyama K, Roche KW, Huganir RL. Phosphorylation of the alpha-amino-3-hydroxy-5-methylisoxazole4-propionic acid receptor GluR1 subunit by calcium/calmodulin-dependent kinase II. J Biol Chem 272: 32528–32533, 1997. [DOI] [PubMed] [Google Scholar]
- 101.Marganski WA, Gangopadhyay SS, Je HD, Gallant C, Morgan KG. Targeting of a novel Ca2+/calmodulin-dependent protein kinase II is essential for extracellular signal-regulated kinase-mediated signaling in differentiated smooth muscle cells. Circ Res 97: 541–549, 2005. [DOI] [PubMed] [Google Scholar]
- 102.Maxwell JT, Natesan S, Mignery GA. Modulation of inositol 1,4,5-trisphosphate receptor type 2 channel activity by Ca2+/calmodulin-dependent protein kinase II (CaMKII)-mediated phosphorylation. J Biol Chem 287: 39419–39428, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McCarron JG, McGeown JG, Reardon S, Ikebe M, Fay FS, Walsh JV Jr. Calcium-dependent enhancement of calcium current in smooth muscle by calmodulin-dependent protein kinase II. Nature 357: 74–77, 1992. [DOI] [PubMed] [Google Scholar]
- 104.McGuinness TL, Lai Y, Greengard P. Ca2+/calmodulin-dependent protein kinase II. Isozymic forms from rat forebrain and cerebellum. J Biol Chem 260: 1696–1704, 1985. [PubMed] [Google Scholar]
- 105.Meoli DF, White RJ. Thrombin induces fibronectin-specific migration of pulmonary microvascular endothelial cells: requirement of calcium/calmodulin-dependent protein kinase II. Am J Physiol Lung Cell Mol Physiol 297: L706–L714, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mercure MZ, Ginnan R, Singer HA. CaM kinase II-δ2 dependent regulation of vascular smooth muscle cell polarization and migration. Am J Physiol Cell Physiol 294: C1465–C1475, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Merlen C, Farhat N, Luo X, Chatenet D, Tadevosyan A, Villeneuve LR, Gillis MA, Nattel S, Thorin E, Fournier A, Allen BG. Intracrine endothelin signaling evokes IP3-dependent increases in nucleoplasmic Ca2+ in adult cardiac myocytes. J Mol Cell Cardiol 62: 189–202, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Migues PV, Lehmann IT, Fluechter L, Cammarota M, Gurd JW, Sim AT, Dickson PW, Rostas JA. Phosphorylation of CaMKII at Thr253 occurs in vivo and enhances binding to isolated postsynaptic densities. J Neurochem 98: 289–299, 2006. [DOI] [PubMed] [Google Scholar]
- 109.Mishra S, Gray CB, Miyamoto S, Bers DM, Brown JH. Location matters: clarifying the concept of nuclear and cytosolic CaMKII subtypes. Circ Res 109: 1354–1362, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mishra S, Ling H, Grimm M, Zhang T, Bers DM, Brown JH. Cardiac hypertrophy and heart failure development through Gq and CaM kinase II signaling. J Cardiovasc Pharmacol 56: 598–603, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mizukami K, Yokoshiki H, Mitsuyama H, Watanabe M, Tenma T, Takada S, Tsutsui H. Small-conductance Ca2+-activated K+ current is upregulated via the phosphorylation of CaMKII in cardiac hypertrophy from spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 309: H1066–H1074, 2015. [DOI] [PubMed] [Google Scholar]
- 112.Munaron L, Scianna M. Multilevel complexity of calcium signaling: Modeling angiogenesis. World J Biol Chem 3: 121–126, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Muthalif MM, Benter IF, Karzoun N, Fatima S, Harper J, Uddin MR, Malik KU. 20-Hydroxyeicosatetraenoic acid mediates calcium/calmodulin-dependent protein kinase II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. Proc Natl Acad Sci USA 95: 12701–12706, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Muthalif MM, Benter IF, Uddin MR, Malik KU. Calcium/calmodulin-dependent protein kinase IIalpha mediates activation of mitogen-activated protein kinase and cytosolic phospholipase A2 in norepinephrine-induced arachidonic acid release in rabbit aortic smooth muscle cells. J Biol Chem 271: 30149–30157, 1996. [DOI] [PubMed] [Google Scholar]
- 115.Muthalif MM, Hefner Y, Canaan S, Harper J, Zhou H, Parmentier JH, Aebersold R, Gelb MH, Malik KU. Functional interaction of calcium-/calmodulin-dependent protein kinase II and cytosolic phospholipase A(2). J Biol Chem 276: 39653–39660, 2001. [DOI] [PubMed] [Google Scholar]
- 116.Muthalif MM, Karzoun NA, Benter IF, Gaber L, Ljuca F, Uddin MR, Khandekar Z, Estes A, Malik KU. Functional significance of activation of calcium/calmodulin-dependent protein kinase II in angiotensin II–induced vascular hyperplasia and hypertension. Hypertension 39: 704–709, 2002. [DOI] [PubMed] [Google Scholar]
- 117.Nakane M, Mitchell J, Forstermann U, Murad F. Phosphorylation by calcium calmodulin-dependent protein kinase II and protein kinase C modulates the activity of nitric oxide synthase. Biochem Biophys Res Commun 180: 1396–1402, 1991. [DOI] [PubMed] [Google Scholar]
- 118.O'Leary H, Sui X, Lin PJ, Volpe P, Bayer KU. Nuclear targeting of the CaMKII anchoring protein alphaKAP is regulated by alternative splicing and protein kinases. Brain Res 1086: 17–26, 2006. [DOI] [PubMed] [Google Scholar]
- 119.Okamoto K, Bosch M, Hayashi Y. The roles of CaMKII and F-actin in the structural plasticity of dendritic spines: a potential molecular identity of a synaptic tag? Physiology (Bethesda) 24: 357–366, 2009. [DOI] [PubMed] [Google Scholar]
- 120.Owens GK, Rabinovitch PS, Schwartz SM. Smooth muscle cell hypertrophy versus hyperplasia in hypertension. Proc Natl Acad Sci USA 78: 7759–7763, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pabelick CM, Sieck GC, Prakash YS. Invited review: significance of spatial and temporal heterogeneity of calcium transients in smooth muscle. J Appl Physiol (1985) 91: 488–496, 2001. [DOI] [PubMed] [Google Scholar]
- 122.Palomeque J, Rueda OV, Sapia L, Valverde CA, Salas M, Petroff MV, Mattiazzi A. Angiotensin II-induced oxidative stress resets the Ca2+ dependence of Ca2+-calmodulin protein kinase II and promotes a death pathway conserved across different species. Circ Res 105: 1204–1212, 2009. [DOI] [PubMed] [Google Scholar]
- 123.Park JH, Lee S, Cho DH, Park YM, Kang DH, Jo I. Far-infrared radiation acutely increases nitric oxide production by increasing Ca2+ mobilization and Ca2+/calmodulin-dependent protein kinase II-mediated phosphorylation of endothelial nitric oxide synthase at serine 1179. Biochem Biophys Res Commun 436: 601–606, 2013. [DOI] [PubMed] [Google Scholar]
- 124.Patan S. Vasculogenesis and angiogenesis. Cancer Treat Res 117: 3–32, 2004. [DOI] [PubMed] [Google Scholar]
- 125.Patton BL, Miller SG, Kennedy MB. Activation of type II calcium/calmodulin-dependent protein kinase by Ca2+/calmodulin is inhibited by autophosphorylation of threonine within the calmodulin-binding domain. J Biol Chem 265: 11204–11212, 1990. [PubMed] [Google Scholar]
- 126.Pauly RR, Bilato C, Sollott SJ, Monticone R, Kelly PT, Lakatta EG, Crow MT. Role of calcium/calmodulin-dependent protein kinase II in the regulation of vascular smooth muscle cell migration. Circulation 91: 1107–1115, 1995. [DOI] [PubMed] [Google Scholar]
- 127.Pfleiderer PJ, Lu KK, Crow MT, Keller RS, Singer HA. Modulation of vascular smooth muscle cell migration by calcium/calmodulin-dependent protein kinase II-δ2. Am J Physiol Cell Physiol 286: C1238–C1245, 2004. [DOI] [PubMed] [Google Scholar]
- 128.Prasad AM, Nuno DW, Koval OM, Ketsawatsomkron P, Li W, Li H, Shen FY, Joiner ML, Kutschke W, Weiss RM, Sigmund CD, Anderson ME, Lamping KG, Grumbach IM. Differential control of calcium homeostasis and vascular reactivity by Ca2+/calmodulin-dependent kinase II. Hypertension 62: 434–441, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Puetz S, Lubomirov LT, Pfitzer G. Regulation of smooth muscle contraction by small GTPases. Physiology (Bethesda) 24: 342–356, 2009. [DOI] [PubMed] [Google Scholar]
- 130.Putkey JA, Waxham MN. A peptide model for calmodulin trapping by calcium/calmodulin-dependent protein kinase II. J Biol Chem 271: 29619–29623, 1996. [DOI] [PubMed] [Google Scholar]
- 131.Qian J, Fulton D. Post-translational regulation of endothelial nitric oxide synthase in vascular endothelium. Front Physiol 4: 347, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Qu L, Yu L, Wang Y, Jin X, Zhang Q, Lu P, Yu X, Zhong W, Zheng X, Cui N, Jiang C, Zhu D. Inward rectifier K+ currents are regulated by CaMKII in endothelial cells of primarily cultured bovine pulmonary arteries. PLoS One 10: e0145508, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rezazadeh S, Claydon TW, Fedida D. KN-93 (2-[N-(2-hydroxyethyl)]-N-(4-methoxybenzenesulfonyl)]amino-N-(4-chlorocinnamyl)-N-methylbenzylamine), a calcium/calmodulin-dependent protein kinase II inhibitor, is a direct extracellular blocker of voltage-gated potassium channels. J Pharmacol Exp Ther 317: 292–299, 2006. [DOI] [PubMed] [Google Scholar]
- 134.Rodriguez-Moyano M, Diaz I, Dionisio N, Zhang X, Avila-Medina J, Calderon-Sanchez E, Trebak M, Rosado JA, Ordonez A, Smani T. Urotensin-II promotes vascular smooth muscle cell proliferation through store-operated calcium entry and EGFR transactivation. Cardiovasc Res 100: 297–306, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rokolya A, Singer HA. Inhibition of CaM kinase II activation and force maintenance by KN-93 in arterial smooth muscle. Am J Physiol Cell Physiol 278: C537–C545, 2000. [DOI] [PubMed] [Google Scholar]
- 136.Rosendorff C. The renin-angiotensin system and vascular hypertrophy. J Am Coll Cardiol 28: 803–812, 1996. [DOI] [PubMed] [Google Scholar]
- 137.Rudijanto A. The role of vascular smooth muscle cells on the pathogenesis of atherosclerosis. Acta Med Indones 39: 86–93, 2007. [PubMed] [Google Scholar]
- 138.Saitoh T, Schwartz JH. Phosphorylation-dependent subcellular translocation of a Ca2+/calmodulin-dependent protein kinase produces an autonomous enzyme in Aplysia neurons. J Cell Biol 100: 835–842, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Scherberich A, Campos-Toimil M, Ronde P, Takeda K, Beretz A. Migration of human vascular smooth muscle cells involves serum-dependent repeated cytosolic calcium transients. J Cell Sci 113: 653–662, 2000. [DOI] [PubMed] [Google Scholar]
- 140.Schneider JC, El Kebir D, Chereau C, Lanone S, Huang XL, De Buys Roessingh AS, Mercier JC, Dall'Ava-Santucci J, Dinh-Xuan AT. Involvement of Ca2+/calmodulin-dependent protein kinase II in endothelial NO production and endothelium-dependent relaxation. Am J Physiol Heart Circ Physiol 284: H2311–H2319, 2003. [DOI] [PubMed] [Google Scholar]
- 141.Schworer CM, Colbran RJ, Keefer JR, Soderling TR. Ca2+/calmodulin-dependent protein kinase II. Identification of a regulatory autophosphorylation site adjacent to the inhibitory and calmodulin-binding domains. J Biol Chem 263: 13486–13489, 1988. [PubMed] [Google Scholar]
- 142.Schworer CM, Colbran RJ, Soderling TR. Reversible generation of a Ca2+-independent form of Ca2+(calmodulin)-dependent protein kinase II by an autophosphorylation mechanism. J Biol Chem 261: 8581–8584, 1986. [PubMed] [Google Scholar]
- 143.Scott JA, Xie L, Li H, Li W, He JB, Sanders PN, Carter AB, Backs J, Anderson ME, Grumbach IM. The multifunctional Ca2+/calmodulin-dependent kinase II regulates vascular smooth muscle migration through matrix metalloproteinase 9. Am J Physiol Heart Circ Physiol 302: H1953–H1964, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Senger DR, Davis GE. Angiogenesis. Cold Spring Harb Perspect Biol 3: a005090, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shen K, Meyer T. Dynamic control of CaMKII translocation and localization in hippocampal neurons by NMDA receptor stimulation. Science 284: 162–166, 1999. [DOI] [PubMed] [Google Scholar]
- 146.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev 13: 1501–1512, 1999. [DOI] [PubMed] [Google Scholar]
- 147.Silva AJ, Stevens CF, Tonegawa S, Wang Y. Deficient hippocampal long-term potentiation in alpha-calcium-calmodulin kinase II mutant mice. Science 257: 201–206, 1992. [DOI] [PubMed] [Google Scholar]
- 148.Simmerman HK, Collins JH, Theibert JL, Wegener AD, Jones LR. Sequence analysis of phospholamban. Identification of phosphorylation sites and two major structural domains. J Biol Chem 261: 13333–13341, 1986. [PubMed] [Google Scholar]
- 149.Singh MV, Anderson ME. Is CaMKII a link between inflammation and hypertrophy in heart? J Mol Med (Berl) 89: 537–543, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Singh P, Leddy JJ, Chatzis GJ, Salih M, Tuana BS. Alternative splicing generates a CaM kinase IIbeta isoform in myocardium that targets the sarcoplasmic reticulum through a putative alphaKAP and regulates GAPDH. Mol Cell Biochem 270: 215–221, 2005. [DOI] [PubMed] [Google Scholar]
- 151.Singh P, Salih M, Tuana BS. Alpha-kinase anchoring protein alphaKAP interacts with SERCA2A to spatially position Ca2+/calmodulin-dependent protein kinase II and modulate phospholamban phosphorylation. J Biol Chem 284: 28212–28221, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Soderling TR. Calcium-dependent protein kinases in learning and memory. Adv Second Messenger Phosphoprotein Res 30: 175–189, 1995. [DOI] [PubMed] [Google Scholar]
- 153.Soderling TR, Chang B, Brickey D. Cellular signaling through multifunctional Ca2+/calmodulin-dependent protein kinase II. J Biol Chem 276: 3719–3722, 2001. [DOI] [PubMed] [Google Scholar]
- 154.Soderling TR, Derkach VA. Postsynaptic protein phosphorylation and LTP. Trends Neurosci 23: 75–80, 2000. [DOI] [PubMed] [Google Scholar]
- 155.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83: 1325–1358, 2003. [DOI] [PubMed] [Google Scholar]
- 156.Sonkusare SK, Bonev AD, Ledoux J, Liedtke W, Kotlikoff MI, Heppner TJ, Hill-Eubanks DC, Nelson MT. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science 336: 597–601, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Strack S, Barban MA, Wadzinski BE, Colbran RJ. Differential inactivation of postsynaptic density-associated and soluble Ca2+/calmodulin-dependent protein kinase II by protein phosphatases 1 and 2A. J Neurochem 68: 2119–2128, 1997. [DOI] [PubMed] [Google Scholar]
- 158.Strack S, Colbran RJ. Autophosphorylation-dependent targeting of calcium/calmodulin-dependent protein kinase II by the NR2B subunit of the N-methyl-d-aspartate receptor. J Biol Chem 273: 20689–20692, 1998. [DOI] [PubMed] [Google Scholar]
- 159.Straub AC, Billaud M, Johnstone SR, Best AK, Yemen S, Dwyer ST, Looft-Wilson R, Lysiak JJ, Gaston B, Palmer L, Isakson BE. Compartmentalized connexin 43 s-nitrosylation/denitrosylation regulates heterocellular communication in the vessel wall. Arterioscler Thromb Vasc Biol 31: 399–407, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sullivan MN, Gonzales AL, Pires PW, Bruhl A, Leo MD, Li W, Oulidi A, Boop FA, Feng Y, Jaggar JH, Welsh DG, Earley S. Localized TRPA1 channel Ca2+ signals stimulated by reactive oxygen species promote cerebral artery dilation. Sci Signal 8: ra2, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sun P, Enslen H, Myung PS, Maurer RA. Differential activation of CREB by Ca2+/calmodulin-dependent protein kinases type II and type IV involves phosphorylation of a site that negatively regulates activity. Genes Dev 8: 2527–2539, 1994. [DOI] [PubMed] [Google Scholar]
- 162.Swaminathan PD, Purohit A, Hund TJ, Anderson ME. Calmodulin-dependent protein kinase II: linking heart failure and arrhythmias. Circ Res 110: 1661–1677, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Swulius MT, Waxham MN. Ca2+/calmodulin-dependent protein kinases. Cell Mol Life Sci 65: 2637–2657, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Takasago T, Imagawa T, Furukawa K, Ogurusu T, Shigekawa M. Regulation of the cardiac ryanodine receptor by protein kinase-dependent phosphorylation. J Biochem 109: 163–170, 1991. [DOI] [PubMed] [Google Scholar]
- 165.Tan SE, Wenthold RJ, Soderling TR. Phosphorylation of AMPA-type glutamate receptors by calcium/calmodulin-dependent protein kinase II and protein kinase C in cultured hippocampal neurons. J Neurosci 14: 1123–1129, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Taylor MS, Francis M, Qian X, Solodushko V. Dynamic Ca2+ signal modalities in the vascular endothelium. Microcirculation 19: 423–429, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Timmins JM, Ozcan L, Seimon TA, Li G, Malagelada C, Backs J, Backs T, Bassel-Duby R, Olson EN, Anderson ME, Tabas I. Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. J Clin Invest 119: 2925–2941, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tobimatsu T, Fujisawa H. Tissue-specific expression of four types of rat calmodulin-dependent protein kinase II mRNAs. J Biol Chem 264: 17907–17912, 1989. [PubMed] [Google Scholar]
- 169.Toussaint F, Charbel C, Blanchette A, Ledoux J. CaMKII regulates intracellular Ca2+ dynamics in native endothelial cells. Cell Calcium 58: 275–285, 2015. [DOI] [PubMed] [Google Scholar]
- 170.Trudeau K, Molina AJ, Guo W, Roy S. High glucose disrupts mitochondrial morphology in retinal endothelial cells: implications for diabetic retinopathy. Am J Pathol 177: 447–455, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tuder RM, Groves B, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol 144: 275–285, 1994. [PMC free article] [PubMed] [Google Scholar]
- 172.van Welie I, du Lac S. Bidirectional control of BK channel open probability by CAMKII and PKC in medial vestibular nucleus neurons. J Neurophysiol 105: 1651–1659, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Varga AW, Yuan LL, Anderson AE, Schrader LA, Wu GY, Gatchel JR, Johnston D, Sweatt JD. Calcium-calmodulin-dependent kinase II modulates Kv4.2 channel expression and upregulates neuronal A-type potassium currents. J Neurosci 24: 3643–3654, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Vazquez G, de Boland AR, Boland RL. Involvement of calmodulin in 1alpha,25-dihydroxyvitamin D3 stimulation of store-operated Ca2+ influx in skeletal muscle cells. J Biol Chem 275: 16134–16138, 2000. [DOI] [PubMed] [Google Scholar]
- 175.Vinogradova TM, Zhou YY, Bogdanov KY, Yang D, Kuschel M, Cheng H, Xiao RP. Sinoatrial node pacemaker activity requires Ca2+/calmodulin-dependent protein kinase II activation. Circ Res 87: 760–767, 2000. [DOI] [PubMed] [Google Scholar]
- 176.Voss J, Jones LR, Thomas DD. The physical mechanism of calcium pump regulation in the heart. Biophys J 67: 190–196, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wang J, Best PM. Inactivation of the sarcoplasmic reticulum calcium channel by protein kinase. Nature 359: 739–741, 1992. [DOI] [PubMed] [Google Scholar]
- 178.Wang Q, Patton WF, Hechtman HB, Shepro D. A novel anti-inflammatory peptide inhibits endothelial cell cytoskeletal rearrangement, nitric oxide synthase translocation, and paracellular permeability increases. J Cell Physiol 172: 171–182, 1997. [DOI] [PubMed] [Google Scholar]
- 179.Wang Z, Ginnan R, Abdullaev IF, Trebak M, Vincent PA, Singer HA. Calcium/calmodulin-dependent protein kinase II delta 6 (CaMKIIdelta6) and RhoA involvement in thrombin-induced endothelial barrier dysfunction. J Biol Chem 285: 21303–21312, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wayman GA, Lee YS, Tokumitsu H, Silva AJ, Soderling TR. Calmodulin-kinases: modulators of neuronal development and plasticity. Neuron 59: 914–931, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Webb RC. Smooth muscle contraction and relaxation. Adv Physiol Educ 27: 201–206, 2003. [DOI] [PubMed] [Google Scholar]
- 182.Weber JD, Raben DM, Phillips PJ, Baldassare JJ. Sustained activation of extracellular-signal-regulated kinase 1 (ERK1) is required for the continued expression of cyclin D1 in G1 phase. Biochem J 326: 61–68, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wegener AD, Simmerman HK, Lindemann JP, Jones LR. Phospholamban phosphorylation in intact ventricles. Phosphorylation of serine 16 and threonine 17 in response to beta-adrenergic stimulation. J Biol Chem 264: 11468–11474, 1989. [PubMed] [Google Scholar]
- 184.Wehrens XH, Lehnart SE, Reiken SR, Marks AR. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ Res 94: e61–70, 2004. [DOI] [PubMed] [Google Scholar]
- 185.Welsh CF, Roovers K, Villanueva J, Liu Y, Schwartz MA, Assoian RK. Timing of cyclin D1 expression within G1 phase is controlled by Rho. Nat Cell Biol 3: 950–957, 2001. [DOI] [PubMed] [Google Scholar]
- 186.Witcher DR, Kovacs RJ, Schulman H, Cefali DC, Jones LR. Unique phosphorylation site on the cardiac ryanodine receptor regulates calcium channel activity. J Biol Chem 266: 11144–11152, 1991. [PubMed] [Google Scholar]
- 187.Wynne BM, Chiao CW, Webb RC. Vascular smooth muscle cell signaling mechanisms for contraction to angiotensin ii and endothelin-1. J Am Soc Hypertens 3: 84–95, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Xu A, Hawkins C, Narayanan N. Phosphorylation and activation of the Ca2+-pumping ATPase of cardiac sarcoplasmic reticulum by Ca2+/calmodulin-dependent protein kinase. J Biol Chem 268: 8394–8397, 1993. [PubMed] [Google Scholar]
- 189.Xu A, Narayanan N. Ca2+/calmodulin-dependent phosphorylation of the Ca2+-ATPase, uncoupled from phospholamban, stimulates Ca2+-pumping in native cardiac sarcoplasmic reticulum. Biochem Biophys Res Commun 258: 66–72, 1999. [DOI] [PubMed] [Google Scholar]
- 190.Xu ZC, Kirchberger MA. Modulation by polyelectrolytes of canine cardiac microsomal calcium uptake and the possible relationship to phospholamban. J Biol Chem 264: 16644–16651, 1989. [PubMed] [Google Scholar]
- 191.Yang D, Zhu WZ, Xiao B, Brochet DX, Chen SR, Lakatta EG, Xiao RP, Cheng H. Ca2+/calmodulin kinase II-dependent phosphorylation of ryanodine receptors suppresses Ca2+ sparks and Ca2+ waves in cardiac myocytes. Circ Res 100: 399–407, 2007. [DOI] [PubMed] [Google Scholar]
- 192.Yoon JY, Ho WK, Kim ST, Cho H. Constitutive CaMKII activity regulates Na+ channel in rat ventricular myocytes. J Mol Cell Cardiol 47: 475–484, 2009. [DOI] [PubMed] [Google Scholar]
- 193.Yuan SY, Shen Q, Rigor RR, Wu MH. Neutrophil transmigration, focal adhesion kinase and endothelial barrier function. Microvasc Res 83: 82–88, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yuan W, Bers DM. Ca-dependent facilitation of cardiac Ca current is due to Ca-calmodulin-dependent protein kinase. Am J Physiol Heart Circ Physiol 267: H982–H993, 1994. [DOI] [PubMed] [Google Scholar]
- 195.Zamanillo D, Sprengel R, Hvalby O, Jensen V, Burnashev N, Rozov A, Kaiser KM, Koster HJ, Borchardt T, Worley P, Lubke J, Frotscher M, Kelly PH, Sommer B, Andersen P, Seeburg PH, Sakmann B. Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science 284: 1805–1811, 1999. [DOI] [PubMed] [Google Scholar]
- 196.Zaritsky JJ, Eckman DM, Wellman GC, Nelson MT, Schwarz TL. Targeted disruption of Kir2.1 and Kir22 genes reveals the essential role of the inwardly rectifying K+ current in K+-mediated vasodilation. Circ Res 87: 160–166, 2000. [DOI] [PubMed] [Google Scholar]
- 197.Zhang W, Chen DQ, Qi F, Wang J, Xiao WY, Zhu WZ. Inhibition of calcium-calmodulin-dependent kinase II suppresses cardiac fibroblast proliferation and extracellular matrix secretion. J Cardiovasc Pharmacol 55: 96–105, 2010. [DOI] [PubMed] [Google Scholar]
- 198.Zhou BP, Liao Y, Xia W, Zou Y, Spohn B, Hung MC. HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat Cell Biol 3: 973–982, 2001. [DOI] [PubMed] [Google Scholar]
- 199.Zhu LJ, Klutho PJ, Scott JA, Xie L, Luczak ED, Dibbern ME, Prasad AM, Jaffer OA, Venema AN, Nguyen EK, Guan X, Anderson ME, Grumbach IM. Oxidative activation of the Ca2+/calmodulin-dependent protein kinase II (CaMKII) regulates vascular smooth muscle migration and apoptosis. Vascul Pharmacol 60: 75–83, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]