Abstract
Defects in the outer blood-retinal barrier have significant impact on the pathogenesis of diabetic retinopathy and macular edema. However, the detailed mechanisms involved remain largely unknown. This is, in part, attributed to the lack of suitable animal and cell culture models, including those of mouse origin. We recently reported a method for the culture of retinal pigment epithelial (RPE) cells from wild-type and transgenic mice. The RPE cells are responsible for maintaining the integrity of the outer blood-retinal barrier whose dysfunction during diabetes has a significant impact on vision. Here we determined the impact of high glucose on the function of RPE cells. We showed that high glucose conditions resulted in enhanced migration and increased the level of oxidative stress in RPE cells, but minimally impacted their rate of proliferation and apoptosis. High glucose also minimally affected the cell-matrix and cell-cell interactions of RPE cells. However, the expression of integrins and extracellular matrix proteins including pigment epithelium-derived factor (PEDF) were altered under high glucose conditions. Incubation of RPE cells with the antioxidant N-acetylcysteine under high glucose conditions restored normal migration and PEDF expression. These cells also exhibited increased nuclear localization of the antioxidant transcription factor Nrf2 and ZO-1, reduced levels of β-catenin and phagocytic activity, and minimal effect on production of vascular endothelial growth factor, inflammatory cytokines, and Akt, MAPK, and Src signaling pathways. Thus high glucose conditions promote RPE cell migration through increased oxidative stress and expression of PEDF without a significant effect on the rate of proliferation and apoptosis.
Keywords: diabetes, outer retinal barrier, diabetic retinopathy, cell signaling, apoptosis
diabetic retinopathy (dr) is one of the most common microvascular complications of diabetes leading to vision loss (26). Retinal microaneurysms, hemorrhages and macular edema, and retinal neovascularization are the major clinical manifestations of chronic diabetes resulting in visual impairment (6). Despite extensive laboratory and clinical investigation in DR, the underlying cause(s) still remains elusive. A range of hyperglycemia-linked pathways have been associated with initiation and progression of this major complication of diabetes. In fact, hyperglycemia has been indicated as a key initiator of complications associated with retinal neurovascular damage during diabetes. High glucose may induce activation and dysregulation of various metabolic pathways, which potentially contribute to increased oxidative stress, inflammatory responses, angiogenesis, and reduced survival of vascular cell and production of protective or regenerating factors (1, 4, 10). However, the cell autonomous impact of hyperglycemia on various ocular cells remains largely unexplored.
Glucose is the major source of energy for retinal metabolism (8, 65). Glucose transport to the retina, as the most active metabolic tissue in the eye, is processed via the central retinal artery to the inner retina and choroid located between sclera and retinal pigment epithelium (RPE) to the outer retina (8, 35, 65). The anatomical position of RPE plays a crucial role in glucose delivery in the eye (64). The RPE is actively involved in the uptake of different nutrients, including glucose, retinol, fatty acids, and ascorbic acid, from the blood and regulates the transport of these nutrients to the photoreceptors (70, 73). The RPE cells provide 60 to 80% of retinal glucose via their high-capacity delivery system, indicating the RPE as an important source for the high glucose needs of the retina (16). How high glucose may impact these RPE cell functions remains a significant interest.
RPE cells are involved in defensive immune mechanisms of macula, phagocytosis of photoreceptor outer segments, maintenance of the ocular angiogenic balance, oxidative stress responses, photoreceptor renewal, and preservation of their phototransduction (41, 44, 70). Failure of RPE cell function is associated with major ocular clinical alterations, including retinal degeneration and irreversible vision loss (44, 81). Although the unique role of the RPE in the maintenance of photoreceptor excitability, angiogenic balance, and the retinal microenvironment has been demonstrated in many studies, the effects of diabetes on RPE cell function has begun to receive well-deserved attention. In the past few years a number of studies have attempted to examine the impact of high glucose conditions and vascular endothelial growth factor (VEGF) on human RPE cell function with contradictory results (2, 40, 78). These results are influenced by many factors, including stage and age of RPE cells among others (62).
Metabolic changes affected by diabetes have been observed in preretinopathy RPE cells. These tissue-specific alterations have been postulated to result in RPE cell impairments that potentially affect retinal health and photoreceptor activity (16). However, the underlying mechanisms influenced by high glucose that contribute to abnormal RPE cell function still remain elusive and need further investigation. High glucose conditions are shown to induce apoptosis of RPE cells and compromise their barrier permeability or have no effect on apoptosis and enhance their barrier function (2, 50, 78). Thus better models with well-defined characteristics are needed to delineate how high glucose impacts RPE cell function.
Although rodents do not develop a full spectrum of human pathologies associated with chronic diabetes, they have been very useful in delineating the detailed mechanisms of early nonproliferative changes associated with diabetes. Mouse models of diabetes provide a unique opportunity to determine the impact of various genes and their products in these pathologies. There are very limited studies determining the impact of diabetes on various RPE cell functions. In this study we examined the potential effects of high glucose conditions on mouse RPE cell function, which may contribute to pathogenesis and progression of diabetic retinopathy. We demonstrated that high glucose conditions greatly impacted RPE cell migration through increased oxidative stress and expression of PEDF, with minimal impact on their basal rate of proliferation and apoptosis and their inflammatory state.
MATERIALS AND METHODS
Experimental animals.
All the experiments were conducted in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Institutional Animal Care and Use Committee of the University of Wisconsin School of Medicine and Public Health. Immortomice expressing a temperature-sensitive simian virus (SV) 40 large T antigen were obtained from Charles River Laboratories (Wilmington, MA).
Isolation and culture of RPE cells.
RPE cells were isolated form one litter (6 or 7 pups) of 4-wk-old wild-type and PEDF-deficient (PEDF−/−) Immortomice as previously described (23). RPE cells were maintained in DMEM containing 10% FBS, 2 mM l-glutamine, 100 μg/ml streptomycin, 100 U/ml penicillin, and murine recombinant INF-γ (R&D Systems, Minneapolis, MN) at 44 U/ml in 60-mm tissue culture plates coated with 1% gelatin, and incubated at 33°C with 5% CO2. For all experiments, unless stated otherwise, RPE cells were maintained in normal glucose (NG) (5.7 mM d-glucose), high glucose (HG) (40.7 mM d-glucose), or osmolarity control (NG+L-Glu) (5.7 mM d-glucose and 35 mM l-glucose) for 5 days, as previously described (34, 68). The purity of cultures was confirmed by indirect immunofluorescence and FACS analysis using RPE cell specific markers, including RPE65 and bestrophin (>98%). Primary human fetal RPE cells were obtained from Lonza BioSciences (Anaheim, CA) and maintained in DMEM: F12 (3:1) containing 10% FBS, 1× B27, 100 μg/ml streptomycin, 100 U/ml penicillin at 37°C with 5% CO2 under different glucose conditions, as described above. For all experiments, cells were allowed to from a monolayer where individual cells make physical contacts without cell overgrowth and dome formation (5 days), and all experiments were performed in 3- to 7-day-old cultures as reported for rat RPE cells (52). All cells were maintained in growth medium with different glucose levels for 5 days prior to each experiment, and medium was changed every other day. At least two different isolations of RPE cells were used in these studies.
Scratch wound assays.
Cells (1 × 106) maintained under various glucose conditions were plated in 60-mm tissue culture dishes and allowed to reach confluent (2–3 days). Plates were wounded using a 1-ml micropipette tip, washed twice with growth medium to remove detached cells, and fed with growth medium containing 1 μM 5-fluorouracil (Sigma) to block cell proliferation under various glucose conditions. Wound closure was monitored by phase microscopy at different time points (0, 24, 48 h), and images were captured in digital format. The migrated distance as percentage of total distance was determined for quantitative assessment of data as previously described (19).
Transwell migration assays.
Cell migration was also determined by transwell migration assays. Costar transwell inserts (8-μm pore size, 6.5-mm membrane, Lowell, MA) were coated with PBS containing fibronectin (2 μg/ml) on the bottom side at 4°C overnight. After washing with PBS, inserts were blocked in PBS containing 1% BSA for 1 h at room temperature and rinsed with PBS. Cells were trypsinized and resuspended in serum-free DMEM medium with different glucose concentrations. PDGF-BB (10 ng/ml) was also used as a positive control and inducer of cell migration. Cells (1 × 105) in 0.1 ml of serum-free medium were added to the top of inserts. The inserts were placed in a 24-well plate containing 0.5 ml of serum-free medium and incubated for 4 h at 37°C. Following incubation, cells were fixed with 2% paraformaldehyde for 10 min at room temperature, stained with hematoxylin and eosin, and membranes were mounted on a slide with cells facing down. The number of cells migrated through the membrane under various glucose conditions was determined by counting 10 high-power fields (×200). Each experiment was done in triplicate and repeated twice.
Cell proliferation assays.
Cells (1 × 104) were plated in multiple sets of gelatin-coated 60-mm tissue culture plates under different glucose conditions. Cells were maintained under different glucose conditions and fed every other day for the duration of experiment. Number of cells was determined by counting every other day, on days not fed, in triplicate for 2 wk. The rate of DNA synthesis was also assessed with Click-It EDU Alexa Flour 488, as recommended by the supplier (Life Technologies, Carlsbad, CA). The assay measures DNA synthesis by using 5-ethynyl-2′-deoxyuridine (EdU), a nucleotide analog of thymidine. The percentage of cells undergoing active DNA synthesis was determined by FACScan caliber flow cytometer (Becton Dickinson).
Apoptosis assays.
The rate of apoptosis was determined by TdT-dUPT terminal nick-end labeling (TUNEL) to assess the percentage of cells undergoing apoptotic cell death under various glucose conditions. TUNEL staining was performed with Click-iT-TUNEL Alexa Flour imaging assay, as recommended by the supplier (Life Technologies). Positive apoptotic cells were counted in 10 high-power fields (×200) and calculated as percentage of total cell number. Alternatively, cleaved caspase-3 staining was utilized, and the percentage of positive cells were similarly determined. Cells (1 × 105) were plated on fibronectin-coated 4-well chamber slides (5 μg/ml in serum-free medium) and maintained under various glucose conditions. Cells were then rinsed with PBS, fixed with cold acetone for 10 min on ice, and permeabilized with PBS containing 0.1% Triton X-100 for 12 min at room temperature. Samples were rinsed with PBS and blocked with PBS containing 1% BSA at 37°C for 30 min. Following incubation, cells were washed once with PBS and incubated with cleaved caspase-3 (Alexa flour 555 conjugate, Cell Signaling) at 37°C for 40 min. The antibody was prepared in PBS containing 1% BSA. Cells were then washed four times with PBS, mounted, examined with a fluorescent microscope (Carl Zeiss, Germany), and images were captured in digital format. All samples were prepared in triplicate and repeated twice.
Western blot analysis.
Cells (1 × 106) that were maintained under different glucose conditions were plated in 60-mm tissue culture dishes and allowed to reach ∼90% confluence (2–3 days). Cells were washed with serum-free DMEM medium and incubated in growth medium without serum under different glucose conditions for 2 days. The conditioned medium (CM) was collected, centrifuged at 500 × g for 5 min to remove cell debris, and stored at −80°C for further analysis. Cell lysates were also prepared using 100 μl of lysis buffer [50 mM HEPES, pH 7.5, 1 mM MgCl2, 1 mM CaCl2, 100 mM NaCl and 0.1 mM EDAT with 1% NP-40, 1% Triton X-100, and protease inhibitor cocktail (Roche Biochemicals, Mannheim, Germany)]. BCA protein assay (Bio-Rad, Hercules, CA) was used to determine protein concentration. Samples (50-μg proteins) were mixed with appropriate amount of 6x SDS sample buffer and analyzed by 4–20% SDS-PAGE (Invitrogen). Proteins were transferred to nitrocellulose membrane and blocked in TBS containing 0.05% Tween 20 (TBST) with 5% skim milk for 1 h at room temperature.
Membranes were incubated with primary antibody for 2 h at room temperature, washed with TBST [TBS; Tris-buffered saline (20 mM Tris, pH 7.6 and 150 mM NaCl) and 0.05% Tween 20], and incubated with appropriate horseradish peroxidase-conjugated secondary antibody (1:10,000, Jackson ImmunoResearch) for 1 h at room temperature. The following antibodies were used: anti-fibronectin (SC-9068), anti-Nrf2 (SC-13032), anti-COX1 (SC-1752), anti-COX2 (SC-1745), anti-c-Src (SC-8056), anti-AQP1 (SC-20810), anti-GADD 153 (SC-7351), anti-STAT3 (SC-7179), anti-pSTAT3 (SC-8059) (Santa Cruz Biotechnology), anti-TSP1 (A 6. 1, Neo Markers, Fermont, CA), anti-PEDF, anti-SPARC, anti-MFG-E8, anti-PEDFR, anti-periostin (OSF-2), anti-opticin, anti-osteopontin (OPN) (R&D System), anti-tenascin-C (AB19013), anti-Collagen IV (AB756P) (Millipore), and anti-PDI, anti-Bcl-2, anti-Bim, anti-Bax, anti-HO1, anti-pSRC, anti-pP38, anti-P38, anti-pAkt, anti-Akt, anti-pERK, anti-pPDGF-Rβ and anti-ERK (Cell Signaling), anti-ZO-1 (Life Technologies), anti-β-catenin, anti-N-cadherin, anti-P120 (BD Bioscience), anti-angiopoietin-like 4, anti-PEDF laminin receptor, anti-claudin-1 (Abcam), and anti-β-actin (Thermo Fisher) were used at dilutions recommended by the supplier. The proteins were visualized with enhanced chemoluminescence reagent (GE Bioscience, Piscataway, NJ). The mean band intensities were determined with Image J 1.46a (National Institutes of Health, Bethesda, MD) and compared with appropriate control samples.
Cell adhesion assays.
Cell adhesion assay was conducted by using 96-well plates (Nunc Immunoplate Maxisorp, Fisher Scientific) coated with different concentration of collagen I, collagen IV, vitronectin, and fibronectin (BD Biosciences), diluted in TBS (50 μl/well) containing 2 mM CaCl2 and 2 mM MgCl2 (Ca/Mg), and incubated at 4°C overnight. Plates were rinsed four times with TBS containing Ca/Mg (200 μl/well), blocked with TBS with Ca/Mg containing 1% BSA (200 μl/well) at room temperature for 1 h. Cells maintained under various glucose conditions were collected from tissue culture plates by using 2 ml of dissociation solution (2 mM EDTA, 0.05% BSA in TBS), rinsed with TBS, and resuspended in cell binding buffer (150 mM NaCl, 20 mM HEPES, 4 mg/ml BSA, pH 7.4) at ∼5 × 105 cells/ml. The coated plates were washed with TBS containing Ca/Mg incubated with equal an amount (50 μl/well) of cell suspension and TBS with Ca/Mg for 2 h at 37°C. Following incubation, plates were washed with 200 μl TBS with Ca/Mg to remove nonadherent cells. The number of adherent cells was quantified by measuring intracellular acid phosphatase activity as previously described (59, 58). Adherent cells were lysed with 100 μl of lysis buffer (50 mM sodium acetate pH 5.0, 1% Triton X-100, 6 mg/ml p-nitrophenyl phosphate) and incubated at 4°C overnight. Following incubation, 50 μl of stopping solution (1 M NaOH) was added to neutralize the reaction. The absorbance was determined at 405 nm with a microplate reader. The assays were performed in triplicate and repeated twice.
FACS analysis.
The RPE cells cultured under different glucose concentrations were rinsed with PBS containing 0.04% EDTA and incubated with 1.5 ml of cell dissociation solution (2 mM EDTA, 0.05% BSA in TBS). Cells were then washed, collected from plates with DMEM containing 10% FBS, centrifuged and blocked in 0.5 ml of TBS containing 1% goat serum for 20 min on ice. Cells were then pelleted and incubated with 0.5 ml TBS with 1% BSA containing specific primary antibodies on ice for 30 min. The following antibodies were used: anti-bestrophin (MAB 5466), anti-VCAM-1 (CBL1300), anti-β3 (MAB 1957), anti-β2 (MABT42), anti-αvβ3 (MAB 1976Z), anti-α5β1 (MAB 1999), anti-α2 (AB1936), anti-α3 (AB1920), anti-α5 (AB1921), anti-αv integrins (MAB 1930) (Millipore, Billerica, MA), anti-ICAM-1 (SC-1511), anti-β8 (SC-25714), anti-β5 (SC-5401) (Santa Cruz Biotechnology), anti-ICAM-2, anti-VEGF receptor-1 (VEGF-R1), anti-VEGF-R2 (R&D Systems), anti-PDGF-Rα, and anti-PDGF-Rβ, anti-CD47 (eBioscience, San Diego, CA), anti-α1 (BD Bioscience), anti-FAS, and anti-FASL (Enzo Life Science); antibodies were used at dilutions recommended by the supplier. Cells were then rinsed twice with TBS containing 1% BSA and incubated with appropriate FITC-conjugated secondary antibody (Pierce, Rockford, IL) prepared in TBS containing 1% BSA for 30 min on ice. Following incubation, cells were washed twice with TBS containing 1% BSA, resuspended in 0.5 ml of TBS with 1% BSA and analyzed by a FACScan caliber flow cytometer (Becton Dickinson, Franklin Lakes, NJ). These experiments were repeated twice by using two different isolations of RPE cells with similar results. The mean fluorescent intensity is indicated for each sample.
Indirect immunofluorescence assays.
Cells (1 × 105) maintained under various glucose conditions were plated on fibronectin-coated 4-well chamber slides (5 μg/ml in serum-free medium) and allowed to reach confluence (1–2 days). Cells were then rinsed with phosphate buffered saline (PBS, pH 7.4), fixed with cold acetone for 10 min on ice, and permeabilized with PBS containing 0.1% Triton X-100 for 12 min at room temperature. Samples were rinsed with PBS and blocked with PBS containing 1% BSA at 37°C for 30 min. Following incubation, cells were washed once with PBS and incubated with specific primary antibodies at 37°C for 40 min. The primary antibodies were anti-ZO-1 (Life Technologies), anti-β-catenin, and anti-N-cadherin (BD Bioscience) prepared in PBS containing 1% BSA. Following incubation, cells were washed with PBS and incubated with specific Cy3-conjugated secondary antibodies (1:800, Jackson ImmunoResearch) in PBS containing 1% BSA at 37°C for 40 min. Cells were then washed four times with PBS, mounted, examined with a fluorescent microscope (Carl Zeiss), and images were captured in digital format. The mean fluorescence intensity was determined from intensities of multiple cells (50 cells). Three independent experiments were performed.
Reactive oxygen species assessments.
The level of cellular reactive oxygen species was measured by dihydroethidium staining (DHE). DHE is a blue weak fluorescent dye, which upon oxidation by superoxide forms the red fluorescent ethidium that interacts with DNA in the nucleus. Cells (3 × 104) were plated on fibronectin-coated chamber slides (5 μg/ml, BD Bioscience) and incubated for 5 days in growth medium under various glucose conditions. Following incubation, cells were exposed to 10 μM DHE for 15–20 min, washed with growth medium, and incubated with growth medium twice for 30 min each. Fluorescent intensity was detected with a fluorescence microscope (Carl Zeiss), and images were captured in digital format. For quantitative analysis, images were analyzed with Image J software (National Institutes of Health). The mean fluorescence intensity was determined from intensities of multiple cells (100 cells). Three independent experiments were performed.
Wholemount staining.
RPE-choroid-sclera complex were prepared form 4-mo-old wild-type and Akita/+ diabetic mice by removing the retina and cutting the optic nerve from the posterior portion of the eyes. The remaining cup was washed with PBS three times for 10 min and fixed in 4% PFA in PBS for 2 h. Following the fixation, samples were blocked in blocking solution [50% FCS, 20% normal goat serum (NGS) in PBS containing 0.01% Triton X-100] at room temperature for 1 h. Samples were washed with PBS three times for 10 min each and incubated with primary antibodies, including anti-HNE (Cell Signaling) and anti-ZO-1 (Life Technologies) in PBS containing 20% FCS, 20% NGS, and 0.01% TX-100 overnight at 4°C. Samples were washed with PBS and incubated with specific Cy3-conjugated secondary antibody (1:800, Jackson ImmunoResearch) in PBS containing 20% FCS, 20% NGS, and 0.01% TX-100 at room temperature for 2–4 h. Following incubation, samples were washed five times with PBS (5 min), cut to flatten the samples, and examined with a fluorescence microscope (Carl Zeiss). The images were captured in digital format.
RNA purification and real-time qPCR analysis.
The total RNA from RPE cells under various glucose conditions was extracted with a mirVana PARIS Kit (Invitrogen). The cDNA synthesis was performed with 1 μg of total RNA and a Sprint RT Complete-Double PrePrimed Kit (Clontech, Mountain View, CA). One microliter of each cDNA (dilution 1:10) was used as a template in qPCR assays, performed in triplicate of three biological replicates on Mastercycler Realplex (Eppendorf, Hauppauge, NY) by using the SYBR-Green qPCR Premix (Clontech). Amplification parameters were as follows: 95°C for 2 min, 40 cycles of amplification (95°C for 15 s, 60°C for 40 s), dissociation curve step (95°C for 15 s, 60°C for 15 s, and 95°C for 15 s). Primer sequences for different inflammatory cytokines were TNF-α 5′-ACCGTCAGCCGATTTGCTAT-3′ (forward) and TNF-α 5′-TTGACGGCAGAGAGGAG GTT-3′ (reverse), IL-18 5′-AAGAA AATGGA GACCTGGAATCAG-3′ (forward) and IL-18 5′-ATTCCGTATTACTGC GGTTGTACA-3′ (reverse), MCP-1 5′-GTCTGTGCTGACCCC AAGAAG-3′ (forward) and MCP-1 5′-TGGTTCC GATCCAGGTTTTTA-3′ (reverse), and RANTES 5′-GCCCACGTCAA GGA GTATTTCT-3′ (forward) and RANTES 5′-CAAACA CGACTGCAAGATTGGA-3′ (reverse), IL-1β 5′-GTTCCCATTAGACAACTGCACTACA-3′ (forward), and 5′-CCGACA GCACGAGGCTTTT-3′(reverse), VEGF 5′-GGAGAGCAGAAGT CCCATGA-3′ (forward) and 5′-ACTCCAGGGCTTCATCGTTA-3′ (reveres), IL-6 5′-CAACCACGGCCTTCCCTACT-3′ (forward), and 5′-TTGGGAGTGGTATCCTCTGTGA-3′(reverse), PEDFR 5′-AATCTCTACCGCCTCTCGAA-3′ (forward), and 5′-TTGGTTCAGT AGGCCATTCC-3′(reverse) and PEDF laminin R 5′-CCATCGAGAATCCT GCTGAC-3′ (forward), and 5′-GGCTGCTTGGATCTGGTTAG-3′(reverse).
Standard curves were generated from known quantities for each of the target genes of linearized plasmid DNA. Ten times dilution series were used for each known target, which were amplified with SYBR-Green qPCR. The linear regression line for a nanogram of DNA was determined from relative fluorescent units at a threshold fluorescence value (Ct) to quantify gene targets from cell extracts by comparing the relative fluorescent units at the Ct to the standard curve, normalized by the simultaneous amplification of RpL13A, a housekeeping gene. The following primers for RpL13A 5′-TCTCAAGGTTGTTCGGCTGAA-3′ (forward) and RpL13A 5′-CCAGACG CCCCAGGTA-3′ (reverse) were used.
Phagocytosis assays.
Phagocytosis activity was assayed with the unique pHrodo-based system (Life technologies) based on the acidification of the particles as they are ingested. pHrodo dye is a fluorogenic dye that greatly increases in fluorescence intensity when the pH becomes more acidic. RPE cells cultured under different glucose conditions in 35-mm culture plates were loaded with DMEM medium with various glucose concentration containing pHrodo green Escherichia coli BioParticles conjugates and incubated for different time points (5 and 24 h). Following incubation, cells were rinsed with PBS containing 0.04% EDTA and incubated with 1.5 ml of cell dissociation solution. Cells were then washed, collected from plates with DMEM containing 10% FBS, washed twice with PBS, resuspended in 0.5 ml of PBS, and analyzed by a FACScan caliber flow cytometer (Becton Dickinson).
Capillary morphogenesis assays.
Tissue culture plates (35 mm) were coated with 0.5 ml of Matrigel (10 mg/ml, BD Biosciences) and incubated at 37°C for at least 30 min to allow the Matrigel to harden. Mouse choroidal endothelial cells (ChEC) were prepared and maintained as previously described by us (45). The ChEC were removed by trypsin-EDTA and washed with DMEM containing 10% FBS. Cells (1 × 105 cells/ml) were resuspended in CM collected form RPE cells cultured under different glucose conditions. Cells (2 ml) were applied to the Matrigel-coated plates, incubated at 37°C, and photographed after 14 h with a Nikon microscope in a digital format. For quantitative assessment of the data, the mean number of branch points was determined by counting the branch points in five high-power fields (×100).
Ex vivo sprouting of RPE-choroid complex.
Choroidal explants were prepared and cultured as described previously (42), with some modifications. Briefly, 4-mo-old wild-type and Akita/+ diabetic mice were killed and eyes were enucleated, washed three times, and kept in ice-cold DMEM medium. Attached tissues to the outer surface of the eyeball (blood vessels and fatty and connective tissues) were shaved in ice-cold DMEM medium and under the dissecting microscope. The cornea, lens, and corpus vitreum were removed before the intermediate segment containing the sclera, choroid, RPE, and the retina were dissected along the whole circumference. The retinal layer was removed before the sclera; choroid and the RPE were sectioned into 0.5- to 1.0-mm pieces. These pieces were finally transferred to 35-mm culture dishes coated with 0.3 ml of Matrigel (10 mg/ml, BD Biosciences) and allowed to harden. Tissue preparations were transferred into a 37°C cell culture incubator without medium for 20 min. Endothelial cell growth medium was then added (2 ml/dish) and incubated at 37°C with 5% CO2 for 8–10 days. Explants were fed every other day. After 8–10 days, preparations were fixed in 4% PFA for 30 min at room temperature and washed three times in PBS before they were imaged with a Nikon microscope. Ten explants per eye were prepared and cultured in a single dish, and at least three mice per genotype were used. The area of sprouting was assessed with ImageJ software.
VEGF level measurements.
The levels of VEGF produced by RPE cells were determined with a Mouse VEGF Immunoassay Kit (R&D Systems). Cells (1 × 106) were plated in 60-mm tissue culture dishes and allowed to reach 90% confluence (2–3 days). The cells were washed with serum-free DMEM and incubated with 2 ml of growth medium under different glucose conditions without serum for 2 days. The CM containing different glucose concentrations were collected, centrifuged at 400 g for 5 min to remove cell debris, and used for VEGF measurements as recommended by the supplier.
Statistical analysis.
Statistical differences between control and treated samples were evaluated with Graphpad Prism software (La Jolla, CA) according to the Tukey multiple comparison test with P < 0.05 considered significant.
RESULTS
RPE cells were more migratory under high glucose conditions.
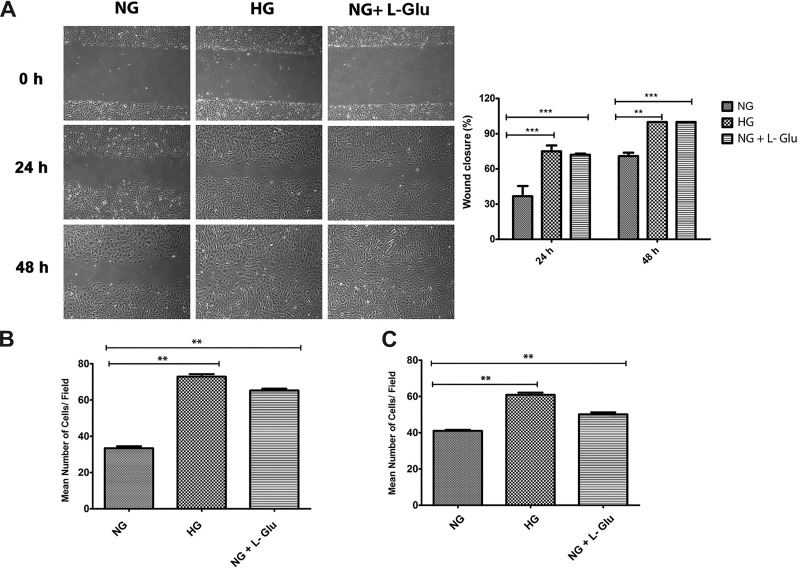
Migration and proliferation of RPE cells is associated with a number of pathological conditions, including proliferative vitreoretinopathy, retinal degeneration and detachment, and breakdown of the outer retinal blood barrier (53). The impact of high glucose on RPE cell migration was determined by scratch wound assay under different glucose conditions. A significant increase in wound closure was observed in RPE cells in high glucose compared with normal glucose. The quantitative assessment of the data is shown in Fig. 1A. Similar results were observed by using a transwell migration assay (Fig. 1B). To investigate whether our results are comparable in human RPE cells, we performed similar assays using early passage primary human fetal RPE cells. High glucose resulted in faster wound closure in human RPE cells (not shown). Similar results were also observed in transwell migration assays (Fig. 1C). l-glucose is an analog of glucose, which is not transported into the cell, and was used as an osmolarity control (80). We also observed a significant increase in migration of RPE cells under osmotic stress. Thus increased osmotic stress contributes to increased migration of RPE cells under high glucose conditions.
Fig. 1.
Increased migration of RPE cells in high glucose conditions. A: a scratch wound assay of mouse RPE cells cultured under different glucose conditions was used to determine their migration activity. Wound closure was monitored by phase microscopy, and digital images were captured at indicated time points. The degree of migration was assessed by determining the percent wound closure (**P < 0.01, ***P < 0.001, n = 3). B: migration of mouse RPE cells in different glucose conditions using a transwell assay. Please note a significant increase in the migration of RPE cells cultured in high glucose and osmotic stress conditions compared with normal glucose (**P < 0.01, n = 3). C: migration of human RPE cells incubated under different glucose conditions using a transwell assay. Please note high glucose and osmotic stress similarly resulted in a significant increase in the migration of human RPE cells (**P < 0.01, n = 3).
High glucose conditions minimally affected RPE cells proliferation and apoptosis.
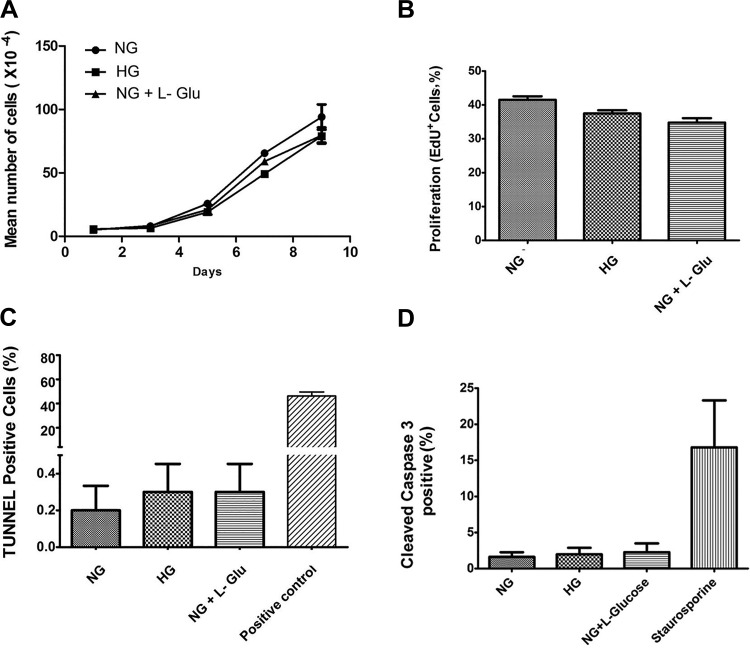
RPE cell integrity plays a crucial role in the proper function of outer retina (9, 62, 63). To determine the effect of high glucose on RPE cell proliferation, we determined the growth rate of RPE cells under various glucose conditions by counting the number of cells for 2 wk. High glucose conditions did not significantly affect the rate of RPE cell proliferation compared with normal glucose conditions (Fig. 2A). These results were consistent with the similar rate of DNA synthesis observed under different glucose conditions. The percentage of cells undergoing active DNA synthesis was determined by using EdU labeling, a synthetic nucleotide analog. The rate of DNA synthesis in RPE cells was not significantly affected under different glucose conditions (Fig. 2B).
Fig. 2.
High glucose conditions minimally affected the proliferation and apoptosis of RPE cells. A: the rate of RPE cell proliferation incubated under various glucose conditions was determined by counting the number of cells as detailed in materials and methods. High glucose conditions did not affect the proliferation rate of RPE cells compared with controls. B: the rate of DNA synthesis in RPE cells under various glucose conditions was determined by EdU labeling. No significant changes in the rate of DNA synthesis was observed in RPE cells under high glucose conditions compared with controls. C: the rate of apoptosis in RPE cells cultured in different glucose conditions was determined by TdT-dUTP terminal nick-end labeling (TUNNEL) assay. Please note no significant differences were observed in the apoptosis of RPE cells in different glucose conditions (P > 0.05, n = 3). D: similar results were observed by determining the percentage of cells that were positive for cleaved caspase-3 in different glucose conditions (P > 0.05, n = 3).
The increased rate of apoptosis in RPE cells plays a major role in the development and progression of different retinal degenerative diseases (9). We next determined whether high glucose conditions affected the rate of apoptosis in RPE cells. TUNEL staining was used to determine the percentage of RPE cells undergoing apoptosis under different glucose conditions. A similar rate of apoptosis was observed in RPE cells cultured under different glucose conditions (Fig. 2C). We also examined whether the expression of Bcl-2 family members including Bcl-2, Bim, and Bax were affected under different glucose conditions. Consistent with our TUNEL results, different glucose conditions did not affect the level of Bcl-2, Bim, and Bax expression in these cells (not shown). Similar results were observed by determining the percentage of RPE cells that are positive for cleaved, activated caspase-3 staining under different glucose conditions (Fig. 2D). Thus high glucose conditions minimally affected the rate of RPE cell proliferation and apoptosis.
High glucose conditions minimally affected the adhesion of RPE cells.
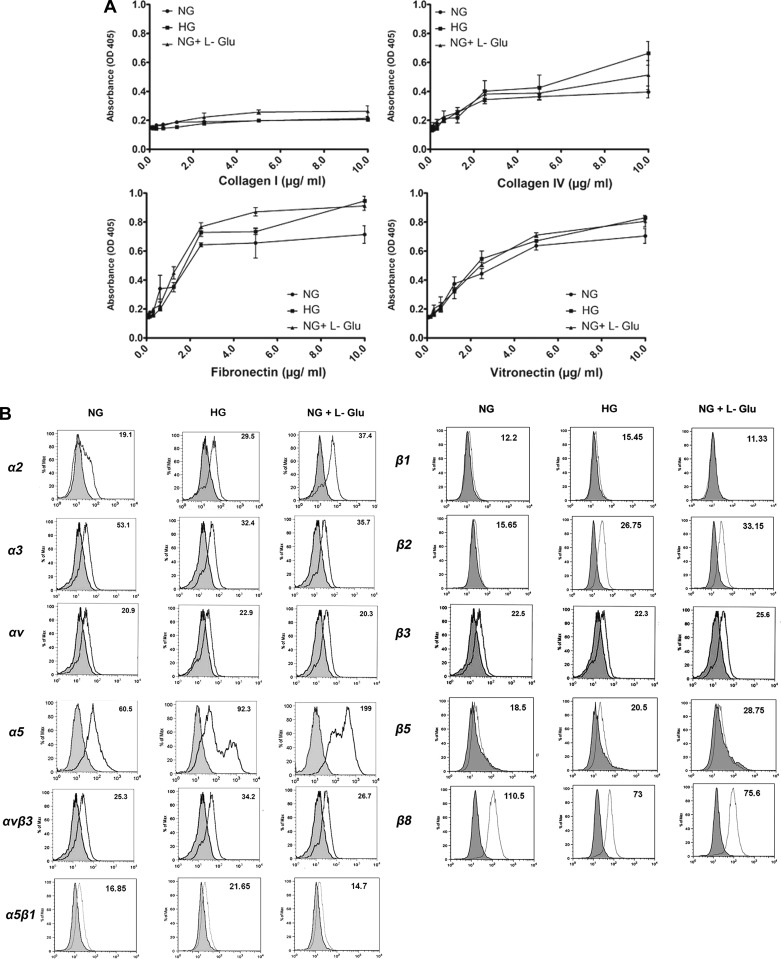
Altered migration of RPE cells under high glucose conditions suggested potential alterations in their adhesion properties. We next examined the adhesion of RPE cells to various extracellular matrix (ECM) proteins. RPE cells cultured under different glucose conditions exhibited a similar adhesion to collagen I, collagen IV, fibronectin, and vitronectin compared with cells cultured under normal glucose condition (Fig. 3A). Thus RPE cell adhesion to various ECM proteins was not affected under high glucose conditions.
Fig. 3.
Adhesion and expression of integrins in RPE cells cultured in different glucose conditions. A: adhesion of RPE cells to collagen IV, collagen I, vitronectin, and fibronectin was determined as described in materials and methods. Please note similar adhesion of RPE cells to collagen I, collagen IV, fibronectin, and vitronectin in different glucose conditions B: expression of integrins in RPE cells. The expression of α1, α2, α3, α5, αv, β1, β2, β3, β5, β8, α5β1, and αvβ3 integrins was determined by FACS analysis as described in materials and methods. The α1 expression was not detected in RPE cells under various glucose conditions (not shown). The expression level of αvβ3 and α5, as well as β1, β2, and β5, and α5β1 were increased, while that of α3 and β8 was decreased in high glucose conditions. Although some of these changes (αvβ3, α5β1, and β1) were observed in high glucose, other changes (α5, β2, β5, and β8) were also observed under osmotic stress. These experiments were repeated with two different isolations of RPE cells with similar results.
Integrins mediate both cell-cell and cell-matrix interactions.
We next determined the expression level of different integrins under various glucose conditions by FACS analysis (Fig. 3B). RPE cells had undetectable levels of α1 integrin (not shown). While expression of α2, α5, β2, and β5 was increased in high glucose and osmotic stress, the expression of α3 and β8 was decreased. Similar expression levels for αv, β1, and β3 were observed under different glucose conditions. However, the level αvβ3 and α5β1 integrins were increased in high glucose. Although these changes in integrin expression minimally affected the adhesion of RPE cells to various ECM proteins, they may contribute to the altered signaling pathways that may impact RPE cell migration, including those affecting cell-cell adhesions.
Increased ZO-1 nuclear localization in high glucose.
Adherent junctions play a crucial role in cell-cell adhesion, a process which is involved in maintaining tissue integrity and invasiveness (7). Altered migration of RPE cells under high glucose conditions may suggest altered cell-cell interactions. We next determined the localization and expression of junctional protein complexes by indirect immunofluorescence staining (Fig. 4, A–D) and Western blot analysis (Fig. 4E). The β-catenin/Wnt signaling pathway has been implicated as a major pathway associated with several fibrotic diseases, including proliferative vitreoretinopathy (76). In addition, β-catenin has an important role in maintaining tissue integrity and normal morphology. Here we did not observe any changes in junctional localization of β-catenin under different glucose conditions (Fig. 4A). However, high glucose resulted in decreased expression of β-catenin in RPE cells compared with normal glucose or osmotic stress (Fig. 4E). N-cadherin is another important junctional protein which is considered a dominant cadherin in RPE cells, with crucial roles in localization at cell-cell junctions, cell migration, and photoreceptor survival. High glucose conditions did not affect N-cadherin expression and junctional localization in RPE cells (Fig. 4, A and E).
Fig. 4.
The cellular localization and expression of junctional proteins. A: the localization of ZO-1, N-cadherin, and β-catenin was determined by immunofluorescence staining. Mouse RPE cells were plated on fibronectin-coated (2 μg/ml) chamber slides in different glucose conditions and stained with specific antibodies as detailed in materials and methods. No staining was observed in the absence of primary antibody (not shown). Please note reduced junctional localization of ZO-1 as well as its increased nuclear localization in RPE cells in high glucose. B: the localization of ZO-1 in human RPE cells. Similar to mouse RPE cells, high glucose resulted in increased nuclear localization of ZO-1 in human RPE cells. C: Wholemount staining of RPE layer in RPE-choroid tissues prepared from wild-type (WT) (nondiabetic) and Akita/+ (diabetic) mice. Please note increased ZO-1 nuclear staining in RPE-choroid tissues from Akita/+ mice compared with wild-type mice. These experiments were repeated with eyes from five mice with similar results. Control is staining with no primary antibody. D: the quantitative assessment of A and B. **P < 0.01, ***P < 0.001. E: Western blot analysis of junctional proteins. Total cell lysates were prepared from RPE cells in different glucose conditions and analyzed for expression of ZO-1, N-cadherin, β-catenin, P120-catenin, and β-actin. Please note a significant decrease in expression of β-catenin in RPE cells cultured in high glucose. There were no significant changes in the levels of N-cadherin, ZO-1, and P-120 catenin under different glucose conditions. The β-actin was used for loading control (*P < 0.05, n = 3).
Tight junction complexes as dynamic structures modulate paracellular diffusion and permeability, polarity, and cell proliferation. Appropriate barrier function of the RPE cells is greatly dependent on the proper formation of tight junctions. ZO-1 plays a major role in the formation and stabilization of tight junctions (27, 74). RPE cells cultured in high glucose exhibited increased nuclear localization of ZO-1 compared with normal glucose or osmotic stress (Fig. 4, A and D). Similar results were observed in human RPE cells and in vivo in RPE-choroid flatmounts prepared from Akita/+ diabetic mice with 3 mo of diabetes (Fig. 4, B–D). However, the total ZO-1 level was not affected in RPE cells under different glucose conditions (Fig. 4E). Expression of p120-catenin was similarly observed in RPE cells under different glucose conditions (Fig. 4E). We also did not observe any significant changes in the level of claudin-1 under high glucose conditions compared with normal glucose (not shown). Thus our results indicated that high glucose conditions did not have a significant effect on the level of junctional proteins in RPE cells, but resulted in increased ZO-1 nuclear localization in both mouse and human RPE cells.
High glucose conditions resulted in altered production of ECM proteins.
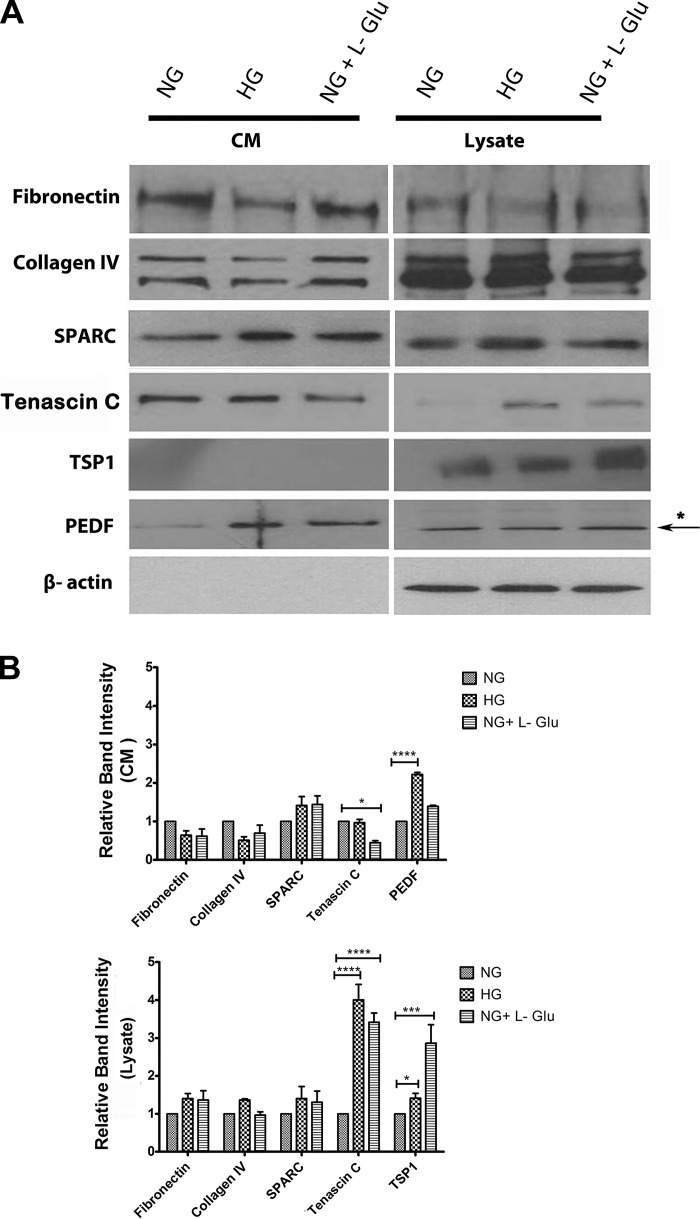
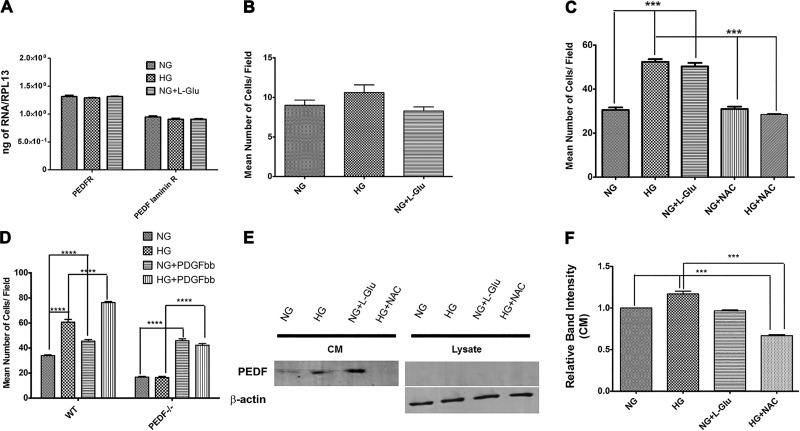
Altered production of ECM proteins is associated with various biological events, including developmental and disease processes. The altered migration of RPE cells under high glucose conditions suggested potential changes in the production of various ECM proteins. We next examined the level of ECM proteins including fibronectin, collagen IV, tenascin C, SPARC, TSP1, and PEDF in RPE cells (Fig. 5). RPE cells expressed increased levels of cell-associated tenascin C under high glucose conditions compared with normal glucose. In contrast, the extracellular level of tenascin C was not affected in high glucose (Fig. 5B). The level of cell-associated TSP1 was also increased under high glucose conditions compared with normal glucose. In addition, the extracellular level of PEDF was significantly increased in high glucose compared with normal glucose or osmotic stress. PEDF was not detectable in cell lysates. The arrow in Fig. 5A indicates a nonspecific band in a blot of cell lysates for PEDF. We observed no significant changes in the expression of fibronectin, collagen IV, and SPARC (Fig. 5) or periostin, opticin, and osteopontin (not shown) under different glucose conditions.
Fig. 5.
Altered expression of ECM proteins in RPE cells. A: RPE cells were plated on gelatin-coated 60-mm dishes in different glucose conditions for 5 days and then were incubated for 48 h in serum-free growth medium in different glucose conditions. The conditioned medium (CM) and cell lysates were prepared for Western blot analysis of different ECM proteins as described in materials and methods. The expression of fibronectin, collagen IV, tenascin C, TSP1, PEDF, and SPARC were determined using specific antibodies. The β-actin was used as a loading control for cell lysates. Please note a significant increase in the level of tenascin C and TSP1 in cell lysates in high glucose and osmotic stress compared with normal glucose. B: the quantitative assessment of the data (*P < 0.05, ***P < 0.001, and ****P < 0.0001, n = 3). Only the level of PEDF was significantly increased in conditioned medium of RPE cells in high glucose. We also examined the levels of periostin, opticin, and osteopontin and there were no significant changes (not shown).
High glucose resulted in increased oxidative stress in RPE cells.
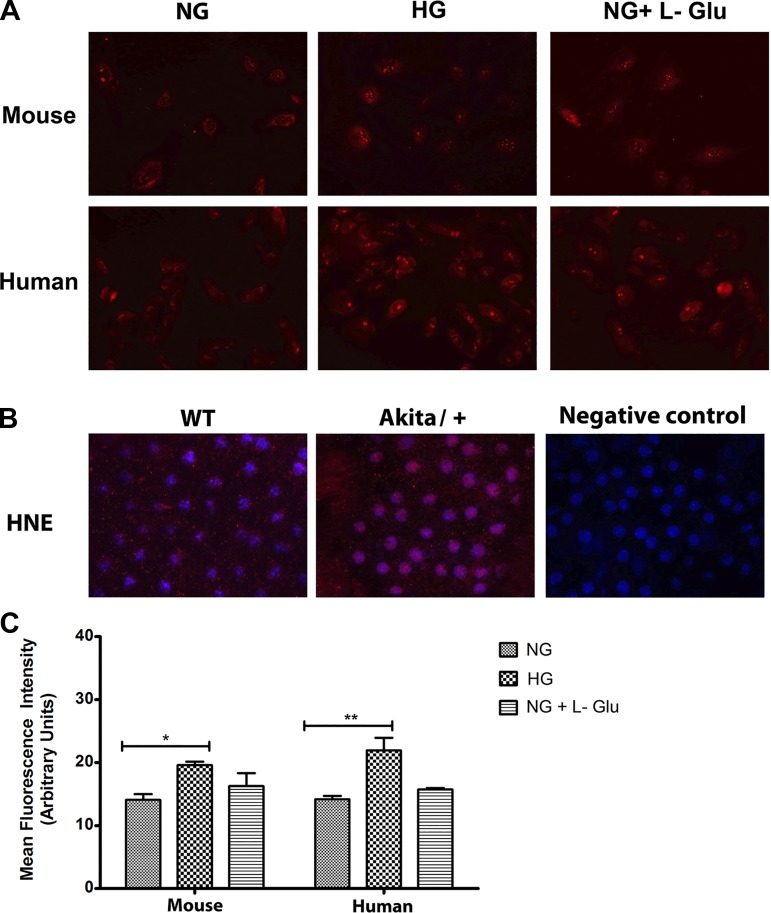
Oxidative stress acts as key initiator of inflammation and is considered a major contributor to various vascular diseases and their subsequent pathologies (5, 7, 20). Increased oxidative stress in RPE cells due to absence of oxidative defense has been indicated as a crucial factor in promoting ocular neovascularization and retinal degeneration (41). Using DHE staining, we examined ROS production at basal level and following incubation under different glucose conditions. RPE cells cultured in high glucose demonstrated more fluorescent intensity compared with normal glucose or osmotic stress (Fig. 6A). Similar results were observed in human RPE cells (Fig. 6A). The quantitative assessments of the data are shown in Fig. 6C.
Fig. 6.
Increased oxidative stress in RPE cells in high glucose. A: the level of oxidative stress in mouse and human RPE cells was assessed by dihydroethidium staining. A significant increase in the level of ROS was detected in high glucose in both mouse and human RPE cells compared with normal glucose or osmotic stress. B: Wholemount staining of RPE-choroid tissues prepared from wild-type and Akita/+ mice with antibody to 4-hydroxy-2-nanonal (HNE). Please note increased HNE staining in RPE-choroid tissues from Akita/+ mice compared with wild-type mice. These experiments were repeated with two different isolations of RPE cells and eyes from five different mice with similar results. C: the quantitative assessment of data in A (*P < 0.05 and **P < 0.01, n = 3).
To further confirm our results in vivo, we investigated whether RPE cells from diabetic mice exhibit an increase in intracellular accumulation of reactive oxygen species (ROS) in vivo. By using wholemount RPE/choroid complexes prepared from wild-type and Akita/+ mice (3 mo of diabetes), the level of oxidative state was determined by 4-hydroxynonenal (4-HNE) staining. The 4-HNE is a key reactive by-product of lipid peroxidation and an indicator of oxidative stress (82). The staining of RPE/choroid complex from Akita/+ mice exhibited increased 4-HNE staining compared with wild-type mice, indicating increased oxidative stress under diabetic conditions (Fig. 6B).
Restoration of normal migration and PEDF expression in RPE cells incubated with N-acetylcysteine under high glucose conditions.
A general increase in the oxidative state of RPE cells is previously shown to result in their increased migration (36). However, the detailed mechanisms involved remain unknown. Here we showed that high glucose resulted in a significant increase in oxidative stress and expression of PEDF in RPE cells compared with normal glucose or osmotic control. We also recently showed an important role for PEDF expression in RPE cell migration. We showed that RPE cells prepared from PEDF−/− mice are less migratory compared with wild-type PEDF+/+ cells, and reexpression of PEDF was sufficient to restore the migratory activity of PEDF−/− RPE cells (23).
PEDF conveys its biological actions through binding to cell surface receptors, including the PEDF receptor (PEDFR; ATGL) or laminin receptor (LR) (31). Here we showed that RPE cells express the PEDF receptors (PEDFR and LR), and their expression was not influenced under different glucose conditions (Fig. 7A). We also showed wild-type RPE cells produce increased levels of PEDF in high glucose, and they are more migratory. We next asked whether high glucose conditions affect the migration of PEDF−/− RPE cells. Figure 7B shows that incubation of PEDF−/− RPE cells under various glucose conditions minimally affected their migration compared with normal glucose. Thus PEDF expression is essential for enhanced migration of RPE cells under high glucose conditions. To determine whether the increased oxidative stress, induced by high glucose contributes to the enhanced migration of RPE cells, we next incubated RPE cells with the antioxidant N-acetylcysteine (NAC) under different glucose conditions and assessed migration in a transwell assay. Figure 7C shows that incubation of RPE cells with NAC under high glucose conditions restored their migration to that observed in normal glucose. Incubation of RPE cells with NAC under normal glucose condition had no significant effect on their migration. PEDF−/− RPE cells are less migratory than wild-type cells. Figure 7D shows that PEDF−/− RPE cells are less migratory compared with wild-type cells, and this is independent of high glucose. However, PDGF-BB, a promigratory factor, similarly promoted the migration of wild-type and PEDF−/− RPE cells independent of high glucose conditions. Thus the enhanced migration of wild-type cells in high glucose is dependent on expression of PEDF, whereas PDGF-mediated migration is not. We next asked whether increased oxidative stress under high glucose conditions is responsible for increased expression of PEDF. Figure 7, E and F, shows incubation of RPE cells with NAC in high glucose significantly reduced PEDF levels compared with normal glucose. Thus our results support a role for increased oxidative stress and increased expression of PEDF under high glucose in enhanced migration of RPE cells.
Fig. 7.
Incubation of RPE cells with NAC restored their migration in high glucose conditions. A: expression of PEDF receptors in RPE cells cultured in different glucose conditions. RPE cells expressed both the PEDFR and laminin R receptor without any changes in their expression in different glucose condition. B: migration of PEDF−/− RPE cells in different glucose conditions using a transwell assay. Please note there is no significant difference in the migration of PEDF−/− RPE cells in high glucose conditions. C: migration of wild-type RPE cells incubated under various glucose conditions using a transwell assay. Please note a significant increase in migration of RPE cells in high glucose conditions that was restored to normal glucose level in the presence of NAC (***P < 0.001, n = 3). Unconditioned high glucose medium or normal glucose conditioned medium with NAC had no effect on migration of RPE cells. D: migration of wild-type and PEDF−/− RPE cells in response to high glucose with or without PDGF-BB. Please note PEDF−/− RPE cells are less migratory compared with wild-type cells regardless of the glucose conditions. In addition, PDGF-BB enhanced the migration of wild-type and PEDF−/− RPE cells regardless of glucose conditions. E and F: expression of PEDF in RPE cells in different glucose conditions. Please note a significant increase in the level of PEDF in RPE cells in high glucose that was reduced to near normal levels in the presence of NAC. (***P < 0.001, n = 3).
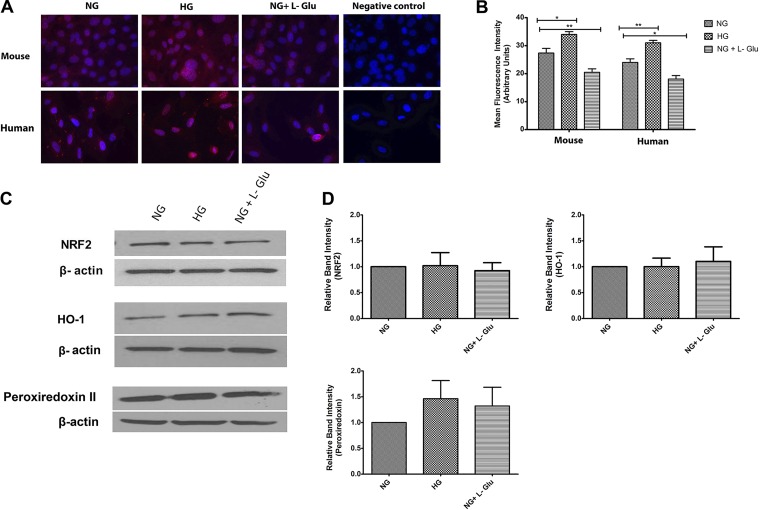
Increased nuclear localization of Nrf2 in RPE cells in high glucose.
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a transcription factor which modulates transcription of ROS-sensitive genes under oxidative stress conditions. Under oxidative stress, Nrf2 translocates into the nucleus and promotes the transcription of genes involved in the protective mechanisms against oxidative stress (48). Increased ROS generation by RPE cells in high glucose suggested possible altered Nrf2 localization and activity. We next examined nuclear localization of Nrf2 by immunofluorescence staining. High glucose resulted in increased nuclear localization of Nrf2 in both mouse and human RPE cells compared with normal glucose and osmotic stress (Fig. 8A). Quantitative assessment of the signal intensity in the nucleus is shown in Fig. 8B.
Fig. 8.
Cellular localization and expression of NRF2 and its downstream target genes Ho-1 and Prdx2. A: the localization of NRF2 was determined by immunofluorescence staining. Mouse and human RPE cells were plated on fibronectin-coated chamber slides in different glucose conditions and stained with specific antibodies as detailed in materials and methods. No staining was observed in the absence of primary antibody (negative control). Please note increased nuclear localization of NRF2 in both human and mouse RPE cells in high glucose. B: the quantitative assessment of the data (*P < 0.05 and **P < 0.01, n = 3). C: the expression of NRF2, HO-1, and peroxiredoxin II was determined by using specific antibodies. The β-actin was used as a loading control. D: the quantitative assessment of data (P > 0.05, n = 3).
To determine the increased transcriptional activity of Nrf2, we next determined the effect of high glucose on expression of heme oxygenase-1 (HO-1) and peroxiredoxin-2 (Prdx2), the two known downstream effectors of Nrf2, in RPE cells. HO-1 is an enzyme which contributes to stress response through anti-inflammatory, antiapoptosis and antiproliferative effects, and its induction is protective in diabetic retinopathy (22). Prdx2 is a cellular peroxidase that decreases H2O2 and inhibits inactivation of redox-sensitive signaling pathways (38). Western blot analysis demonstrated that the total level of Nrf2, HO-1, and Prdx2 was not significantly affected under high glucose conditions (Fig. 8, C and D). Thus nuclear localization of Nrf2 in RPE cells under high glucose conditions was not associated with a significant increased expression of its target genes.
Expression of inflammatory mediators in RPE cells.
Increased oxidative stress in RPE cells in high glucose may contribute to the inflammatory processes associated with diabetes. We next examined the production of inflammatory mediators in RPE cells under different glucose conditions. Figure 9, A–F, shows no significant changes in the expression of IL-18, RANTES, TNF-α, monocyte chemotactic protein-1 (MCP-1), IL-6, and IL-1β under high glucose conditions compared with normal glucose. Thus exposure of RPE cells to high glucose does not result in increased production of inflammatory mediators.
Fig. 9.
Expression of inflammatory mediators in RPE cells cultured in different glucose conditions. A–F: the expression of various inflammatory mediators (IL-18, RANTES, TNF-α, MCP-1, IL-6, and IL-1β) was assessed by quantitative real-time PCR using RNA prepared from RPE cells in different glucose conditions as described in materials and methods. No significant changes in the expression of inflammatory mediators were observed in REP cells cultured in different glucose (P > 0.05, n = 3). G: expression of various proteins with a proinflammatory role. Total cell lysates were prepared from RPE cells in different glucose conditions and analyzed by Western blotting for expression of MFG-E8, PDI, COX-1, COX-2, and PAR-3. The β-actin was used for loading control. H: the quantitative assessment of data. There were no significant differences in the level of these proteins in different glucose conditions, (P > 0.05, n = 3).
Milk fat globule-EGF factor 8 (MFG-E8) plays an important role in the clearance of photoreceptor outer segments shed in the retina through ligation of αvβ5 integrin (17, 24, 57). In addition, MFG-E8 is also involved in adaptive immunity, angiogenesis, and apoptotic cell clearance (17). MFG-E8 protein levels were determined by Western blot analysis. We did not observe significant changes in the expression of MFG-E8 in RPE cells under different glucose conditions (Fig. 9, G and H). We also examined protein disulfide isomerase (PDI) expression in RPE cells. PDI has a major role in cell viability, which plays a crucial function during protein folding (30). Protein aggregation and endoplasmic reticulum stress in human RPE cell is associated with decreased expression of PDI in these cells. Similar expression of PDI was observed under different glucose conditions (Fig. 9, G and H).
We next examined the expression of cyclooxygenase-1 (COX-1) and COX-2, proinflammatory markers, under different glucose conditions. In RPE cells, induced COX-2 expression upon phagocytosis of rod outer segments results in production of VEGF and appropriate function and survival of choriocapillaris (33). High glucose conditions did not affect the expression of COX-1 and COX-2 (Fig. 9, G and H). Partitioning defective-3 (PAR-3) is differentially regulated during inflammation and is a major regulator of cell polarity. Upregulation of PAR-3 has been reported in acute inflammation (15). Here we did not observe any changes in the expression level of PAR-3 under high glucose conditions (Fig. 9, G and H). Collectively, our results suggest that the incubation of RPE cells under high glucose conditions does not affect the expression of COX-1 or COX-2 and/or promote an inflammatory state.
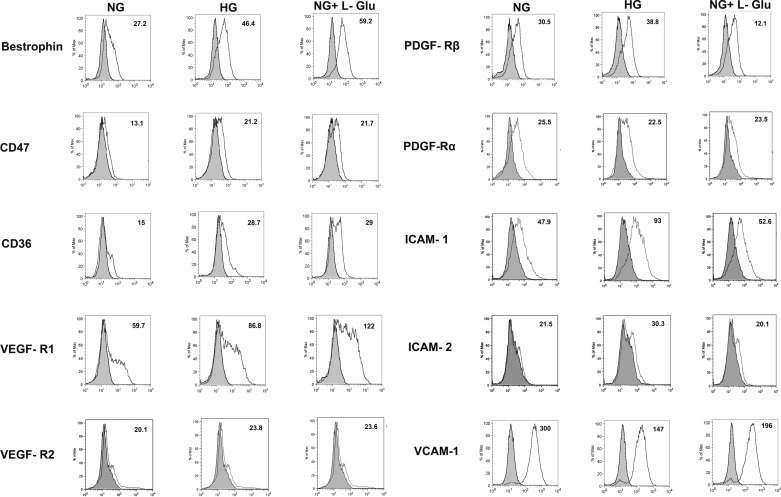
Altered expression of various RPE cell markers under high glucose conditions.
Altered expression of other RPE cell markers, including ICAM-1, VCAM-1, and VEGF receptors, has been indicated as an underlying factor in the development and progress of vascular retinopathies. We next analyzed the impact of high glucose conditions on expression of these RPE cell markers by FACS analysis. The increased expression level of bestrophin was observed in RPE cells under high glucose conditions (Fig. 10). Bestrophin as a specific RPE cell marker plays a critical role in store-operated calcium entry in RPE cells (29). The expression of other markers, including CD36 and CD47 (32, 43, 60), VEGF-R1 (3, 11), and ICAM-1 (18, 46), was also dramatically increased in RPE cells under high glucose conditions compared with normal glucose. We also determined VEGF-R2 and ICAM-2 expression, which were nearly undetectable in RPE cells (Fig. 10). However, a modest increase in expression of ICAM-2 was observed in high glucose compared with normal glucose or osmotic stress.
Fig. 10.
Expression of RPE cell markers. The RPE cells cultured in different glucose conditions were examined for expression of bestrophin, CD47, CD36, VEGF-R1, VEGF-R2, PDGF-Rα, PDGF-Rβ, ICAM-1, ICAM-2, and VCAM-1 by FACS analysis. The shaded area shows control IgG staining. Please note the increased level of bestrophin, CD47, CD36, and VEGF-R1 in high glucose and osmotic stress conditions, whereas increased ICAM-1, ICAM-2, and PDGF-Rβ expression levels only occurred in high glucose compared with control. The expression of VEGF-R2 and PDGF-Rα was low and did not change in different glucose conditions. In contrast, the level of VCAM-1 was significantly decreased in high glucose and osmotic stress. A decrease in PDGF-Rβ level was also noted in osmotic stress. The mean fluorescence intensities are shown for each sample. These experiments were repeated with two different isolations of RPE cells with similar results.
PDGF-Rβ signaling plays a crucial role in RPE cell proliferation and migration (12, 47). We observed a modest increase in the level PDGF-Rβ in high glucose compared with normal glucose. This was also consistent with no significant changes in the level of phosphorylated PDGF-Rβ (not shown), suggesting that the increased migratory phenotype of RPE cells under high glucose conditions was not associated with increased levels and/or activity of PDGF-Rβ. This is further supported by a dramatic decrease in PDGF-Rβ of RPE cells in osmotic stress, which are also more migratory (Fig. 1). We also observed enhanced migration of RPE cells in response to PDGF-BB regardless of the glucose or PEDF status (Fig. 7D). However, the reason for the modest increase in the level of PDGF-Rβ in high glucose may be attributed to a dramatic decrease of its levels in response to the impact of osmotic stress diminishing any direct impact of high glucose on its level. The level of PDGF-Rα expressions in RPE cells was not affected under different glucose conditions (Fig. 10). VCAM-1 also plays a major role in modulation of RPE cell migration and activation (18). However, high glucose conditions decreased VCAM-1 expression compared with normal glucose (Fig. 10).
High glucose conditions may induce the activation of STAT3 signaling in RPE cells (49). We did not observe any significant changes in the level of phospho-STAT3 and STAT3 under high glucose conditions (not shown). Hyperglycemia and hypoxia induce the proapoptotic transcription factor CHOP/GADD153 in RPE cells (55). We did not observe any changes in CHOP expression in RPE cells cultured under high glucose conditions. Previous studies reported that production of angiopoietin-like 4 (ANGPTL4) by RPE cells is involved in retinal angiogenesis under high glucose conditions (85). Here high glucose conditions did not result in increased levels of ANGPTL4 in RPE cells (not shown). The expression of aquaporin (AQP) 1 by RPE cells is involved in efficient water transport across the RPE layer and contributes to maintaining retinal attachment and prevention of subretinal edema (71). Altered expression of AQPs have been linked to the outer blood-retinal barrier breakdown and retinal edema (56). We did not observe any significant changes in the level of AQP1 in RPE cells under high glucose conditions (not shown).
RPE cell phagocytosis.
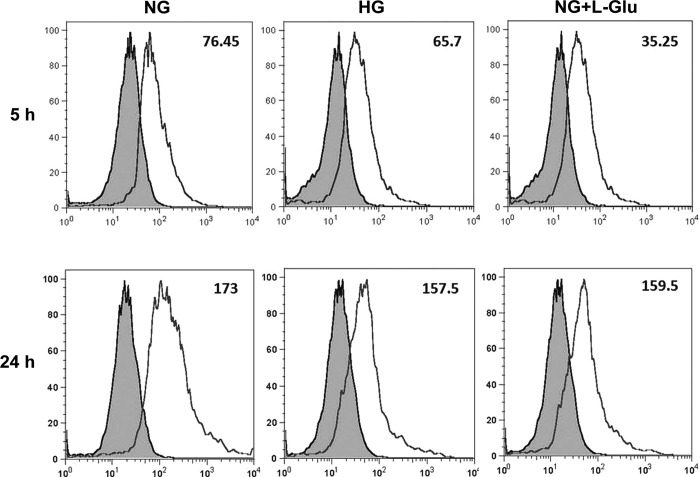
The phagocytic function of RPE cells plays an essential role in the removal of toxic metabolites and RPE cell survival. Abnormal photoreceptor phagocytosis is associated with severe retinal pathologies (9, 24, 39). Altered expression of integrins and receptors involved in phagocytic activity, including CD47 and CD36, suggested changes in the phagocytic function of RPE cells exposed to high glucose conditions. We next determined the RPE cell phagocytic activity. We observed a decrease in the intracellular level of particles taken up by RPE cells under high glucose conditions compared with normal glucose. This was still evident after 24 h (Fig. 11). Thus high glucose conditions may impact phagocytic activity of RPE cells and contribute to retinal changes during diabetes.
Fig. 11.
Altered phagocytic activity of RPE cells. The RPE cells in different glucose conditions were incubated with medium containing pHrodo TM green Escherichia coli BioParticles conjugates for different times (5 and 24 h). The cellular fluorescence intensities were determined by FACS analysis. Please note the decreased phagocytic activity of RPE cells in high glucose and osmotic stress conditions compared with normal glucose. The mean fluorescence intensities are shown for each sample. These experiments were repeated with two different isolations of RPE cells with similar results.
Enhanced capillary morphogenesis of choroidal endothelial cells incubated with CM from RPE cells cultured under different glucose conditions.
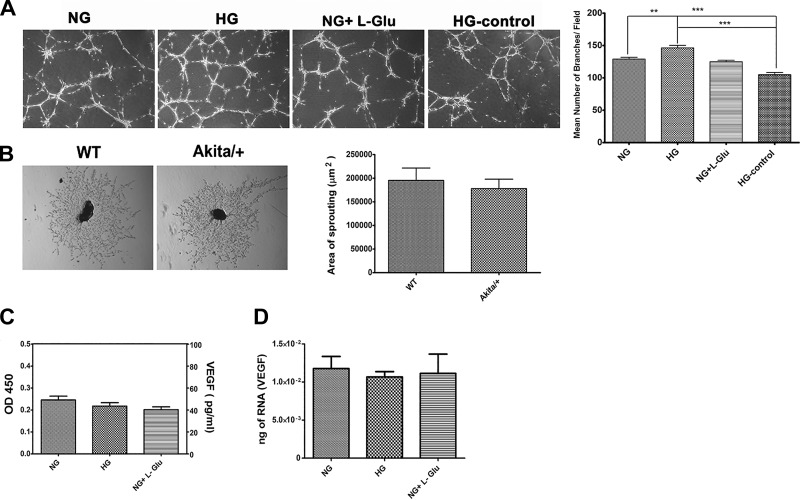
The proliferative stage of diabetic retinopathy is associated with the growth of new blood vessels, which are fragile and leaky, compromising vision (72). We next determined the effects of the CM collected from RPE cells cultured under different glucose conditions on capillary morphogenesis of choroidal endothelial cells (ChEC) and ex vivo sprouting of choroid-RPE complexes from wild-type and Akita/+ diabetic mice. Most endothelial cells (ECs) have the ability to form a capillary-like network when plated on Matrigel resembling the late stages of angiogenesis. The CM prepared from RPE cells under high glucose enhanced the capillary morphogenesis of ChEC compared with normal glucose and osmolarity control. Representative images of capillary morphogenesis are shown in Fig. 12A. High glucose medium by itself did not affect capillary morphogenesis of ChEC supporting the production factor(s) by RPE cells that promote capillary morphogenesis.
Fig. 12.
The capillary morphogenesis of choroidal EC and sprouting of RPE-choroidal explants. A: the capillary morphogenesis of choroidal EC incubated with conditioned medium prepared from RPE cells in different glucose conditions. Unconditioned high glucose medium was used as a control. Please note a significant increase in branching morphogenesis of choroidal EC incubated with conditioned medium prepared from RPE cells cultured in high glucose (**P < 0.01 and ***P < 0.001, n = 3). B: sprouting of choroid-RPE tissues prepared from 3-mo-old wild-type and Akita/+ mice. Images shown here represent results obtained from five different mice with the desired genotype (×40). Please note no significant difference was observed in the degree of choroidal sprouting in Akita/+ mice compared with wild-type mice (P > 0.05, n = 5). C: the level of secreted VEGF protein was determined in conditioned medium collected from RPE cells in different glucose conditions by using an ELISA. Please note there was no significant difference in the amount of VEGF produced by RPE cells in different glucose conditions (P > 0.05, n = 3). D: the level of VEGF mRNA was determined by qPCR using RNA prepared from RPE cells in different glucose conditions. Please note there was no significant difference in the amount of VEGF mRNA detected in RPE cells in different glucose conditions (P > 0.05, n = 3).
We also examined the ex vivo sprouting of the choroid-RPE complex prepared from wild-type and diabetic Akita/+ mice. We observed no significant differences in degree of sprouting angiogenesis in Akita/+ mice with 3 mo of diabetes compared with nondiabetic wild-type mice (Fig. 12B). We also did not observe any significant changes in the protein or mRNA levels of VEGF in RPE cells under different glucose conditions (Fig. 12, C and D).
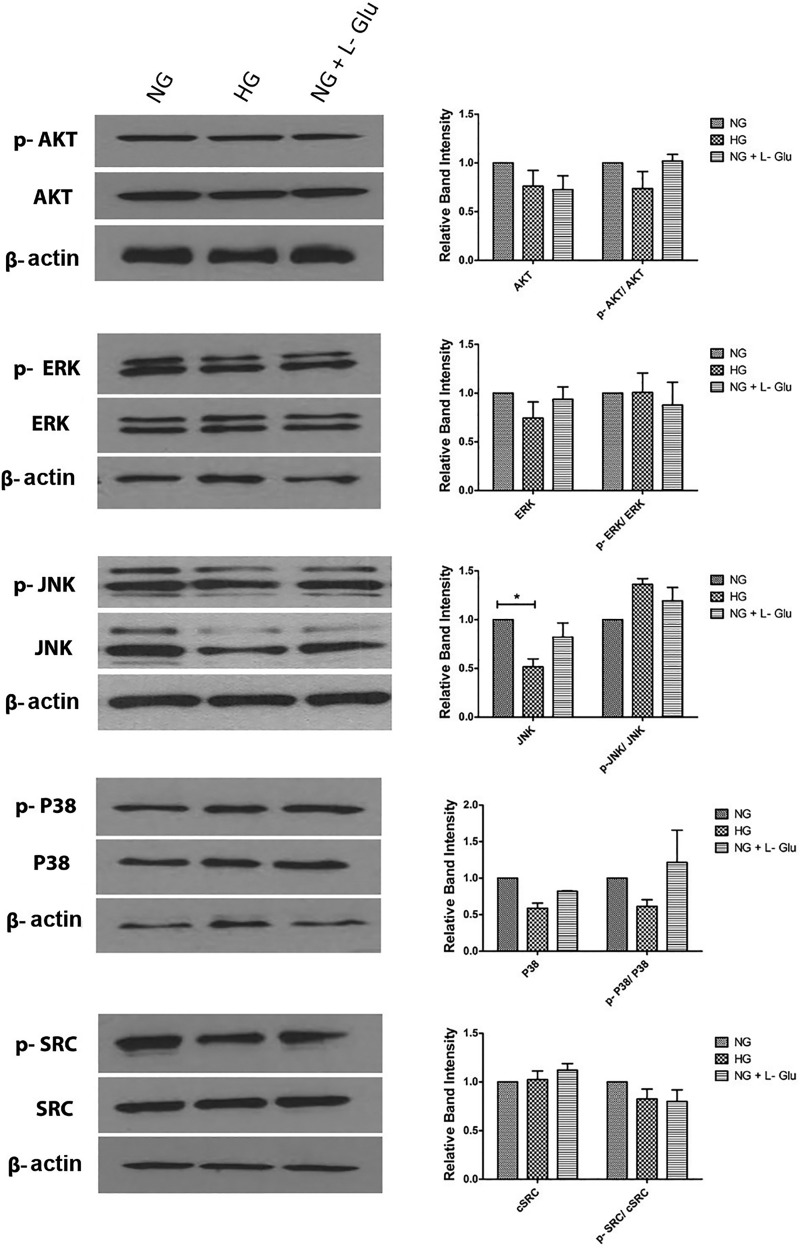
The role of MAPK pathways in oxidative stress signaling is controversial. Akt has a crucial role in cell survival and proliferation (11, 81). Here we did not observe any changes in Akt expression and activation under various glucose conditions (Fig. 13). This is consistent with no alterations in the proliferation and apoptosis of RPE cells exposed to high glucose. The ERK1/2 has been reported with different controversial roles, including cell proliferation, differentiation, and/or apoptotic response (21, 28). High glucose did not impact ERK1/2 expression and activation (Fig. 13). MAP kinases JNK and P38 are protective, proapoptotic, or not involved in apoptosis (86). Although an increasing trend in the level of phospho-JNK was observed under high glucose conditions, it did not reach significance. High glucose was also associated with lower level of total JNK (Fig. 13). We did not observe significant changes in the total level of P38 in all cells. The family of Src kinases also plays a key role in EC permeability. The activation of Src kinase is associated with VEGF and IL-1β-induced permeability, which has been suggested as one of the underlying mechanism involved in many eye diseases, including diabetic retinopathy (66). The expression and activation of Src was not affected in RPE cells under different glucose conditions (Fig. 13). Our results suggest a minimal role for Akt, Src, and MAPK pathways in RPE cell adverse responses under high glucose conditions.
Fig. 13.
High glucose conditions minimally affected the Src, Akt, and MAPK signaling pathways. The steady state levels and activation status of Src kinase, MAPK, and Akt pathways were evaluated by Western blot analysis of lysates prepared from RPE cells in different glucose conditions by using specific antibodies as detailed in materials and methods. Please note no significant changes in these pathways were detected in different glucose conditions (P > 0.05, n = 3). Please also note decreased levels of JNK in RPE cells in high glucose compared with normal glucose or osmotic stress (*P < 0.05, n = 3). These experiments were repeated with two different isolations of RPE cells with similar results.
DISCUSSION
Macular edema is a major complication of diabetes when the outer retinal barrier becomes compromised. This barrier is established by RPE cells through formation of a specialized junctions that controls the exchange of materials between the outer retina and choriocapillaris. How hyperglycemia contributes to this barrier dysfunction and more specifically what defects in RPE cells contribute to diabetes-mediated retinal vascular dysfunction and macular edema needs further elucidation. Although a number of studies have examined the impact of high glucose conditions on human RPE cell function, the results of these studies are contradictory. This may be attributed to use of various human RPE cells, primary vs. established cell lines. In addition, such studies have not been previously reported in mouse RPE cells. Here we show that high glucose conditions resulted in enhanced migration of RPE cells without a significant impact on their adhesion to various matrix proteins and rates of proliferation and apoptosis. However, a significant change in expression of integrins and ECM proteins occurred. We observed an increase in the levels of cell-associated tenascin-C and TSP1, but the level of fibronectin and collagen IV were not affected. These changes could contribute to enhanced migration of RPE cells and altered outer retinal barrier/leakage under high glucose conditions. Although the junctional organization of RPE cells appeared unaffected under high glucose conditions, a significant increase in ZO-1 nuclear localization and a decrease in the total level of β-catenin were observed in high glucose. High glucose also resulted in increased oxidative stress in both mouse and human RPE cells, as previously demonstrated. The increase in migration of RPE cells was consistent with increased levels of oxidative stress and PEDF in high glucose, which were reversed in the presence of the antioxidant NAC.
The decrease in the total level of β-catenin in high glucose suggests the attenuation of Wnt signaling pathway and enhanced NF-κB signaling and inflammation by IL-1β-induced NF-κB target genes. These results are also consistent with the increased levels of PEDF observed in high glucose and attenuation of Wnt/β-catenin signaling recently reported by PEDF (61). We also observed an increase in the level of ICAM-1, a target of NF-κB, in RPE cells with high glucose, which may contribute to recruitment of blood mononuclear cells and inflammation (79). In contrast, the level of VCAM-1, another target of NF-κB, was decreased (51). The interaction of PEDF with caveolin-1 attenuates caveolin-1-mediated proinflammatory activity of EC through downregulation of inflammatory mediators including, MCP-1, VCAM-1, and plasminogen activator inhibitor-1 (PAI-1) (54). Whether such interactions occur in RPE cells remains the subject of future studies. We also did not observe a significant change in the expression of inflammatory mediators, including IL-18, IL-6, TNF-α, MCP-1, and IL-1β and activation of MAPK pathways in RPE cells under high glucose conditions. Thus high glucose conditions are not sufficient to elicit a full inflammatory phenotype of RPE cells, and may be further influenced by ischemic conditions as RPE cells become dysfunctional with age and/or accumulation of oxidative and vascular damage with longer duration of diabetes in vivo.
High glucose conditions are shown to exacerbate choroidal neovascularization in diabetic mice through increased production VEGF by RPE cells (49). We observed a similar level of VEGF produced by RPE cells under various glucose conditions. In addition, the increase in the VEGF-R1 level may suggest a decrease in VEGF signaling. We observed an increase in the level of VEGF-R1, without a significant effect on VEGF-R2 levels, in RPE cells under high glucose conditions. These results are also consistent with the lack of proinflammatory changes indicated above. In addition, a recent study showed that PEDF binds VEGF-R1 and VEGF-R2 and inhibits angiogenesis, perhaps by promoting their turnover in EC (37). However, the mechanism by which increased PEDF expression in RPE cells under high glucose conditions contribute to changes in VEGF receptor expression and/or activity needs further evaluation.
Increased oxidative stress results in activation of unfolded protein response and endoplasmic stress in RPE cells, perhaps through altered intracellular proteolytic and phagocytic activities (83). Phagocytosis is a major function of RPE cells removing excess photoreceptor outer segments. High glucose conditions resulted in decreased phagocytic activity of RPE cells. This is consistent with increased oxidative stress observed under high glucose conditions and perhaps increased ER stress. However, we observed no changes in the level of CHOP (a proapoptotic transcription factor) or Bcl-2 in response to high glucose conditions in RPE cells. These results are consistent with lack of changes in the rate of RPE cell apoptosis under high glucose conditions. However, these results do not rule out the possibility that RPE cells may undergo autophagy under high glucose conditions. Inhibition of autophagy in ARPE-19 cells cultured under high glucose conditions resulted in reduced viability, NLRP3 inflammasome activation, and release of IL-1β (67). Thus it is possible that autophagy is responsible for lack of reduced viability and anti-inflammatory activity in mouse RPE cells under high glucose conditions.
CHOP has been previously shown to be induced in RPE cells exposed to high glucose and hypoxia (55). We observed increased oxidative stress in primary human and mouse RPE cells in high glucose as well as in diabetic Akita/+ mice. Although osmotic stress also resulted in an increase in oxidative stress, it did not reach significant levels. This may be mediated, in part, through uptake of d-glucose, but not l-glucose, by RPE cells and enhanced aldose reductase activity (13). However, the increased oxidative stress in RPE cells was not associated with changes in oxidative response genes, including NRF2 and its downstream effectors HO-1 and Prdx2. Uruno et al. (77) recently demonstrated that nuclear localization of Nrf2 regulates glycogen metabolism in skeletal muscle and the liver to reduce blood glucose levels and protect against diabetes development and progression. Thus the increased nuclear localization of Nrf2 in RPE cells in high glucose, and not osmotic stress, may act as a feedback mechanism to compensate for increased levels of extracellular glucose, which deserves further exploration.
We also observed no significant changes in expression of other inflammatory-related genes, including MFG-E8, PDI, COX-1, COX-2, and PAR-3. These observations are consistent with the high oxidative capacity of RPE cells and utilization of pentose phosphate pathway and CO2 production in high glucose, resulting in increased oxidative stress. This may explain the lack of significant increase in oxidative stress observed in osmotic stress. However, the pathological manifestation of these changes may require additional insults, including hypoxia/ischemia (14).
Yao et al. (84) reported an increase in VEGF and a decrease in PEDF levels in human RPE cells cultured under high glucose conditions. Here we observed no change in the VEGF level, but the PEDF level was increased. This is consistent with our in vivo studies where we observed an increase in PEDF levels in diabetic mouse retina (69). Our data is consistent with Ohno-Matsui et al. (58) who demonstrated that an increase in VEGF/VEGF-R1 signaling increased PEDF levels. Although we saw no changes in VEGF level, the level of VEGF-R1 was increased in high glucose, and to a greater extent in osmotic stress, and may contribute to an increased level of PEDF, as observed here. Retinoic acid treatment of RPE cells inhibits their adhesion and migration through increased production of PEDF and TSP1 (75). We showed that incubation under high glucose conditions resulted in increased levels of PEDF and TSP1 with a significant increase in the migration of RPE cells. Thus PEDF does not appear to inhibit migration of RPE cells. However, PEDF can disrupt ZO-1-based junctional complexes and rearrange actin organization in EC and increase paracellular permeability through Rho A activation (31). RhoA and its downstream effectors, ROCKs, have been implicated in complications of diabetes (68). We observed an increase in expression of ROCK2, but not ROCK1, in RPE cells under high glucose conditions compared with normal glucose (not shown). We also observed no significant changes in total ZO-1 levels, but its increased nuclear localization was observed in high glucose. Others have also shown that high glucose increases transepithelial resistance without changes in occludin and ZO-1 levels, but increased claudin-1 expression. The expression of claudin-1 was not affected in the RPE cells cultured under different glucose conditions here (not shown). How nuclear localization of ZO-1 may contribute to altered migratory activity of RPE cells in high glucose, and whether Rho A activity plays a role, awaits future investigation.
A potential limitation of these studies is the use of immortalized mouse RPE cells generated here. These cells have an enhanced proliferative capacity and may not closely resemble the characterization of primary RPE cells at confluence. For the majority of studies performed here, we have used cells which are nearly confluent or confluent. In addition, where relevant we have confirmed our cell culture observation in RPE/sclera samples freshly prepared from nondiabetic and diabetic animals. Furthermore, our observation using the cells we generated here are consistent with studies reported by others as discussed above.
In summary, the exposure of RPE cells to high glucose conditions resulted in a significant increase in oxidative stress and expression of PEDF, with osmotic stress playing a modest role. The increased oxidative stress and expression of PEDF derived the enhanced migratory phenotype of RPE cells in high glucose. This was reversed in the presence of the antioxidant NAC. The lack of inflammatory changes in RPE cells under high glucose conditions could be attributed to their inherent antioxidant and protective functions, which become further compromised under hypoxic/ischemic conditions associated with chronic diabetes conditions, and/or ability of RPE cells to reduce the impact of high glucose through Nrf2 nuclear localization and modulation of extracellular levels of glucose.
GRANTS
Supported for this study was provided by National Institutes of Health Grants EY-022883, P30 EY-016665, and P30 CA-014520; Environmental Protection Agency Grant 83573701; and an unrestricted departmental award from Research to Prevent Blindness. N. Sheibaniis a recipient of the Alice R. McPherson Retina Research Foundation Chair.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.F., C.H., C.M.S., and N.S. conception and design of research; M.F., C.H., C.S., and B.R.P. performed experiments; M.F., C.H., C.S., and N.S. analyzed data; M.F., C.H., C.M.S., and N.S. interpreted results of experiments; M.F. prepared figures; M.F., C.M.S., and N.S. drafted manuscript; M.F., C.H., C.S., B.R.P., C.M.S., and N.S. edited and revised manuscript; M.F., C.H., C.S., B.R.P., C.M.S., and N.S. approved final version of manuscript.
REFERENCES
- 1.Abcouwer SF, Antonetti DA. A role for systemic inflammation in diabetic retinopathy. Invest Ophthalmol Vis Sci 54: 2384, 2013. [DOI] [PubMed] [Google Scholar]
- 2.Ablonczy Z, Crosson CE. VEGF modulation of retinal pigment epithelium resistance. Exp Eye Res 85: 762–771, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ablonczy Z, Crosson CE. VEGF modulation of retinal pigment epithelium resistance. Exp Eye Res 85: 762–771, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aiello LP. Angiogenic pathways in diabetic retinopathy. N Engl J Med 353: 839–841, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Al-Shabrawey M, Mussell R, Kahook K, Tawfik A, Eladl M, Sarthy V, Nussbaum J, El-Marakby A, Park SY, Gurel Z, Sheibani N, Maddipati KR. Increased expression and activity of 12-lipoxygenase in oxygen-induced ischemic retinopathy and proliferative diabetic retinopathy: implications in retinal neovascularization. Diabetes 60: 614–624, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antonetti DA, Klein R, Gardner TW. Diabetic Retinopathy. N Engl J Med 366: 1227–1239, 2012. [DOI] [PubMed] [Google Scholar]
- 7.Bailey TA, Kanuga N, Romero IA, Greenwood J, Luthert PJ, Cheetham ME. Oxidative stress affects the junctional integrity of retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 45: 675–684, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Berman ER. Biochemistry of the Eye. New York: Plenum Press, 1991, p. 309–467. [Google Scholar]
- 9.Bhutto I, Lutty G. Understanding age-related macular degeneration (AMD): relationships between the photoreceptor/retinal pigment epithelium/Bruch's membrane/choriocapillaris complex. Mol Aspects Med 33: 295–317, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Busik JV, Mohr S, Grant MB. Hyperglycemia-induced reactive oxygen species toxicity to endothelial cells is dependent on paracrine mediators. Diabetes 57: 1952–1965, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byeon SH, Lee SC, Choi SH, Lee HK, Lee JH, Chu YK, Kwon OW. Vascular endothelial growth factor as an autocrine survival factor for retinal pigment epithelial cells under oxidative stress via the VEGF-R2/PI3K/Akt. Invest Ophthalmol Vis Sci 51: 1190–1197, 2010. [DOI] [PubMed] [Google Scholar]
- 12.Chan CM, Chang HH, Wang VC, Huang CL, Hung CF. Inhibitory effects of resveratrol on PDGF-BB-induced retinal pigment epithelial cell migration via PDGFRβ, PI3K/Akt and MAPK pathways. PLoS One 8: e56819, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang KC, Snow A, LaBarbera DV, Petrash JM. Aldose reductase inhibition alleviates hyperglycemic effects on human retinal pigment epithelial cells. Chem Biol Interact 234: 254–260, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coffe V, Carbajal R, Salceda R. Glucose metabolism in rat retinal pigment epithelium. Neurochem Res 31: 103–108, 2006. [DOI] [PubMed] [Google Scholar]
- 15.D'Andrea MR, Saban MR, Nguyen NB, Andrade-Gordon P, Saban R. Expression of protease-activated receptor-1, -2, -3, and -4 in control and experimentally inflamed mouse bladder. Am J Pathol 162: 907–923, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Decanini A, Karunadharma PR, Nordgaard CL, Feng X, Olsen TW, Ferrington DA. Human retinal pigment epithelium proteome changes in early diabetes. Diabetologia 51: 1051–1061, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deroide N, Li X, Lerouet D, Van V, xE, Emily Baker L, Harrison J, Poittevin M, Masters L, Nih L, Margaill I, Iwakura Y, Ryffel B, Pocard M, Tedgui A, Kubis N, Mallat Z. MFGE8 inhibits inflammasome-induced IL-1β production and limits postischemic cerebral injury. J Clin Invest 123: 1176–1181, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dewispelaere R, Lipski D, Foucart V, Bruyns C, Frère A, Caspers L, Willermain F. ICAM-1 and VCAM-1 are differentially expressed on blood-retinal barrier cells during experimental autoimmune uveitis. Exp Eye Res 137: 94–102, 2015. [DOI] [PubMed] [Google Scholar]
- 19.DiMaio TA, Sheibani N. PECAM-1 isoform-specific functions in PECAM-1-deficient brain microvascular endothelial cells. Microvasc Res 75: 188–201, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong A, Xie B, Shen J, Yoshida T, Yokoi K, Hackett SF, Campochiaro PA. Oxidative stress promotes ocular neovascularization. J Cell Physiol 219: 544–552, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dridi S, Hirano Y, Tarallo V, Kim Y, Fowler BJ, Ambati BK, Bogdanovich S, Chiodo VA, Hauswirth WW, Kugel JF, Goodrich JA, Ponicsan SL, Hinton DR, Kleinman ME, Baffi JZ, Gelfand BD, Ambati J. ERK1/2 activation is a therapeutic target in age-related macular degeneration. Proc Natl Acad Sci U S A 109: 13781–13786, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan J, Xu G, Jiang T, Qin Y. Pharmacologic induction of heme oxygenase-1 plays a protective role in diabetic retinopathy in rats. Invest Ophthalmol Vis Sci 53: 6541–6556, 2012. [DOI] [PubMed] [Google Scholar]
- 23.Farnoodian M, Kinter JB, Yadranji Aghdam S, Zaitoun I, Sorenson CM, Sheibani N. Expression of pigment epithelium-derived factor and thrombospondin-1 regulate proliferation and migration of retinal pigment epithelial cells. Physiol Rep 3: e12266, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finnemann SC, Bonilha VL, Marmorstein AD, Rodriguez-Boulan E. Phagocytosis of rod outer segments by retinal pigment epithelial cells requires αvβ5 integrin for binding but not for internalization. Proc Natl Acad Sci U S A 94: 12932–12937, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frank RN. Diabetic Retinopathy. N Engl J Med 350: 48–58, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Georgiadis A, Tschernutter M, Bainbridge JWB, Balaggan KS, Mowat F, West EL, Munro PMG, Thrasher AJ, Matter K, Balda MS, Ali RR. The tight junction associated signalling proteins ZO-1 and ZONAB regulate retinal pigment epithelium homeostasis in mice. PLoS One 5: e15730, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilley R, March HN, Cook SJ. ERK1/2, but not ERK5, is necessary and sufficient for phosphorylation and activation of c-Fos. Cell Signal 21: 969–977, 2009. [DOI] [PubMed] [Google Scholar]
- 29.Gómez N, Tamm E, Strauβ O. Role of bestrophin-1 in store-operated calcium entry in retinal pigment epithelium. Pflugers Arch Eur J Physiol 465: 481–495, 2013. [DOI] [PubMed] [Google Scholar]
- 30.Hahm E, Li J, Kim K, Huh S, Rogelj S, Cho J. Extracellular protein disulfide isomerase regulates ligand-binding activity of αMβ2 integrin and neutrophil recruitment during vascular inflammation. Blood 121: 3789–3800, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He T, Hu J, Yan G, Li L, Zhang D, Zhang Q, Chen B, Huang Y. Pigment epithelium-derived factor regulates microvascular permeability through adipose triglyceride lipase in sepsis. Clin Sci 129: 49–61, 2015. [DOI] [PubMed] [Google Scholar]
- 32.Ho CCM, Guo N, Sockolosky JT, Ring AM, Weiskopf K, Özkan E, Mori Y, Weissman IL, Garcia KC. “Velcro” engineering of high affinity CD47 ectodomain as signal regulatory protein α (SIRPα) antagonists that enhance antibody-dependent cellular phagocytosis. J Biol Chem 290: 12650–12663, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Houssier M, Raoul W, Lavalette S, Keller N, Guillonneau X, Baragatti B, Jonet L, Jeanny JC, Behar-Cohen F, Coceani F, Scherman D, Lachapelle P, Ong H, Chemtob S, Sennlaub F. CD36 deficiency leads to choroidal involution via COX2 down-regulation in rodents. PLoS Med 5: e39, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang Q, Sheibani N. High glucose promotes retinal endothelial cell migration through activation of Src, PI3K/Akt1/eNOS, and ERKs. Am J Physiol Cell Physiol 295: C1647–C1657, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hudspeth AJ, Yee AG. The intercellular junctional complexes of retinal pigment epithelia. Invest Ophthalmol Vis Sci 12: 354–365, 1973. [PubMed] [Google Scholar]
- 36.Jiang Y, He SK. [The effect of oxidative stress on retinal pigment epithelial cell migration.] Zhonghua Yan Ke Za Zhi 41: 100–105, 2005. [PubMed] [Google Scholar]
- 37.Johnston EK, Francis MK, Knepper JE. Recombinant pigment epithelium-derived factor PEDF binds vascular endothelial growth factor receptors 1 and 2. In Vitro Cell Dev Biol Anim 51: 730–738, 2015. [DOI] [PubMed] [Google Scholar]
- 38.Kang Dong H, Lee Doo J, Lee Kyung W, Park Yoon S, Lee Joo Y, Lee SH, Koh Young J, Koh GY, Choi C, Yu DY, Kim J,Kang Sang W. Peroxiredoxin II is an essential antioxidant enzyme that prevents the oxidative inactivation of VEGF receptor-2 in vascular endothelial cells. Molecular Cell 44: 545–558, 2011. [DOI] [PubMed] [Google Scholar]
- 39.Kevany BM, Palczewski K. Phagocytosis of retinal rod and cone photoreceptors. Physiology 25: 8–15, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim DI, Park MJ, Lim SK, Choi JH, Kim JC, Han HJ, Kundu TK, Park JI, Yoon KC, Park SW, Park JS, Heo YR, Park SH. High-glucose-induced CARM1 expression regulates apoptosis of human retinal pigment epithelial cells via histone 3 arginine 17 dimethylation: role in diabetic retinopathy. Arch Biochem Biophys 560: 36–43, 2014. [DOI] [PubMed] [Google Scholar]
- 41.Klettner A. Oxidative stress induced cellular signaling in RPE cells. Front Biosci 4: 392–411, 2012. [DOI] [PubMed] [Google Scholar]
- 42.Kobayashi S, Fukuta M, Kontani H, Yanagita S, Kimura I. A quantitative assay for angiogenesis of cultured choroidal tissues in streptozotocin-diabetic Wistar and spontaneously diabetic GK rats. Jpn J Pharmacol 78: 471–478, 1998. [DOI] [PubMed] [Google Scholar]
- 43.Kociok N, Joussen A. Varied expression of functionally important genes of RPE and choroid in the macula and in the periphery of normal human eyes. Graefes Arch Clin Exp Ophthalmol 245: 101–113, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Korte GE, Reppucci V, Henkind P. RPE destruction causes choriocapillary atrophy. Invest Ophthalmol Vis Sci 25: 1135–1145, 1984. [PubMed] [Google Scholar]
- 45.Lavine JA, Sang Y, Wang S, Ip MS, Sheibani N. Attenuation of choroidal neovascularization by beta2-adrenoreceptor antagonism. JAMA Ophthalmol 131: 376–382, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee IT, Liu SW, Chi PL, Lin CC, Hsiao LD, Yang CM. TNF-α Mediates PKCδ/JNK1/2/c-Jun-dependent monocyte adhesion via ICAM-1 induction in human retinal pigment epithelial cells. PLoS One 10: e0117911, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lei H, Rheaume MA, Kazlauskas A. Recent developments in our understanding of how platelet-derived growth factor (PDGF) and its receptors contribute to proliferative vitreoretinopathy. Exp Eye Res 90: 376–381, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li W, Febbraio M, Reddy SP, Yu DY, Yamamoto M, Silverstein RL. CD36 participates in a signaling pathway that regulates ROS formation in murine VSMCs. J Clin Invest 120: 3996–4006, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li X, Cai Y, Wang YS, Shi YY, Hou W, Xu CS, Wang HY, Ye Z, Yao LB, Zhang J. Hyperglycaemia exacerbates choroidal neovascularisation in mice via the oxidative stress-induced activation of STAT3 signalling in RPE cells. PLoS One 7: e47600, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lim SK, Park MJ, Lim JC, Kim JC, Han HJ, Kim GY, Cravatt BF, Woo CH, Ma SJ, Yoon KC, Park SH. Hyperglycemia induces apoptosis via CB1 activation through the decrease of FAAH 1 in retinal pigment epithelial cells. J Cell Physiol 227: 569–577, 2012. [DOI] [PubMed] [Google Scholar]
- 51.Ma B, Fey M, Hottiger MO. WNT/β-catenin signaling inhibits CBP-mediated RelA acetylation and expression of proinflammatory NF-κB target genes. J Cell Sci 128: 2430–2436, 2015. [DOI] [PubMed] [Google Scholar]
- 52.Malfait M, Gomez P, van Veen TA, Parys JB, De Smedt H, Vereecke J, Himpens B. Effects of hyperglycemia and protein kinase C on connexin43 expression in cultured rat retinal pigment epithelial cells. J Membr Biol 181: 31–40, 2001. [DOI] [PubMed] [Google Scholar]
- 53.Martín F, Pastor JC, de la Rúa E, Mayo-Iscar A, García-Arumí J, Martínez V, Fernández N, Saornil MA. Proliferative vitreoretinopathy: cytologic findings in vitreous samples. Ophthalmic Res 35: 232–238, 2003. [DOI] [PubMed] [Google Scholar]
- 54.Matsui T, Higashimoto Y, Taira J, Yamagishi S. Pigment epithelium-derived factor (PEDF) binds to caveolin-1 and inhibits the pro-inflammatory effects of caveolin-1 in endothelial cells. Biochem Biophys Res Commun 441: 405–410, 2013. [DOI] [PubMed] [Google Scholar]
- 55.Miranda S, González-Rodríguez Á, García-Ramírez M, Revuelta-Cervantes J, Hernández C, Simó R, Valverde ÁM. Beneficial effects of fenofibrate in retinal pigment epithelium by the modulation of stress and survival signaling under diabetic conditions. J Cell Physiol 227: 2352–2362, 2012. [DOI] [PubMed] [Google Scholar]
- 56.Motulsky E, Koch P, Janssens S, Liénart M, Vanbellinghen AM, Bolaky N, Chan CC, Caspers L, Martin-Martinez MD, Xu H, Delporte C, Willermain F. Aquaporin expression in blood-retinal barrier cells during experimental autoimmune uveitis. Mol Vis 16: 602–610, 2010. [PMC free article] [PubMed] [Google Scholar]
- 57.Nandrot EF, Anand M, Almeida D, Atabai K, Sheppard D, Finnemann SC. Essential role for MFG-E8 as ligand for αvβ5 integrin in diurnal retinal phagocytosis. Proc Natl Acad Sci U S A 104: 12005–12010, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ohno-Matsui K, Morita I, Tombran-Tink J, Mrazek D, Onodera M, Uetama T, Hayano M, Murota Si, Mochizuki M. Novel mechanism for age-related macular degeneration: An equilibrium shift between the angiogenesis factors VEGF and PEDF. J Cell Physiol 189: 323–333, 2001. [DOI] [PubMed] [Google Scholar]
- 59.Park S, DiMaio TA, Scheef EA, Sorenson CM, Sheibani N. PECAM-1 regulates proangiogenic properties of endothelial cells through modulation of cell-cell and cell-matrix interactions. Am J Physiol Cell Physiol 299: C1468–C1484, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Picard E, Houssier M, Bujold K, Sapieha P, Lubell W, Dorfman A, Racine J, Hardy P, Febbraio M, Lachapelle P, Ong H, Sennlaub F, Chemtob S. CD36 plays an important role in the clearance of oxLDL and associated age-dependent sub-retinal deposits. Aging 2: 981–989, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qi W, Yang C, Dai Z, Che D, Feng J, Mao Y, Cheng R, Wang Z, He X, Zhou T, Gu X, Yan L, Yang X, Ma JX, Gao G. High levels of pigment epithelium-derived factor in diabetes impair wound healing through suppression of Wnt signaling. Diabetes 64: 1407–1419, 2015. [DOI] [PubMed] [Google Scholar]
- 62.Rizzolo LJ. Barrier properties of cultured retinal pigment epithelium. Exp Eye Res 126: 16–26, 2014. [DOI] [PubMed] [Google Scholar]
- 63.Rizzolo LJ, Peng S, Luo Y, Xiao W. Integration of tight junctions and claudins with the barrier functions of the retinal pigment epithelium. Prog Retin Eye Res 30: 296–323, 2011. [DOI] [PubMed] [Google Scholar]
- 64.Senanayake P, Calabro A, Hu JG, Bonilha VL, Darr A, Bok D, Hollyfield JG. Glucose utilization by the retinal pigment epithelium: evidence for rapid uptake and storage in glycogen, followed by glycogen utilization. Exp Eye Res 83: 235–246, 2006. [DOI] [PubMed] [Google Scholar]
- 65.Senanayake Pd Calabro A, Hu JG, Bonilha VL, Darr A, Bok D, Hollyfield JG. Glucose utilization by the retinal pigment epithelium: Evidence for rapid uptake and storage in glycogen, followed by glycogen utilization. Exp Eye Res 83: 235–246, 2006. [DOI] [PubMed] [Google Scholar]
- 66.Sheikpranbabu S, Kalishwaralal K, Venkataraman D, Eom SH, Park J, Gurunathan S. Silver nanoparticles inhibit VEGF- and IL-1β-induced vascular permeability via Src dependent pathway in porcine retinal endothelial cells. J Nanobiotechnol 7: 8–8, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shi H, Zhang Z, Wang X, Li R, Hou W, Bi W, Zhang X. Inhibition of autophagy induces IL-1beta release from ARPE-19 cells via ROS mediated NLRP3 inflammasome activation under high glucose stress. Biochem Biophys Res Commun 463: 1071–1076, 2015. [DOI] [PubMed] [Google Scholar]
- 68.Shin ES, Huang Q, Gurel Z, Palenski TL, Zaitoun I, Sorenson CM, Sheibani N. STAT1-mediated Bim expression promotes the apoptosis of retinal pericytes under high glucose conditions. Cell Death Dis 5: e986, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sorenson CM, Wang S, Gendron R, Paradis H, Sheibani N. Thrombospondin-1 deficiency exacerbates the pathogenesis of diabetic retinopathy. J Diabetes Metab Suppl 12: 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sparrow JRHD, Hamel CP. The retinal pigment epithelium in health and disease. Curr Mol Med 10: 802–823, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stamer WD, Bok D, Hu J, Jaffe GJ, McKay BS. Aquaporin-1 channels in human retinal pigment epithelium: role in transepithelial water movement. Invest Ophthalmol Vis Sci 44: 2803–2808, 2003. [DOI] [PubMed] [Google Scholar]
- 72.Stitt AWLN, Medina RJ, Adamson P, Curtis TM. Advances in our understanding of diabetic retinopathy. Clin Sci 125: 1–17, 2013. [DOI] [PubMed] [Google Scholar]
- 73.Strauss O. The retinal pigment epithelium in visual function. Physiol Rev 85: 845–881, 2005. [DOI] [PubMed] [Google Scholar]
- 74.Tamiya S, Liu L, Kaplan HJ. Epithelial-mesenchymal transition and proliferation of retinal pigment epithelial cells initiated upon loss of cell-cell contact. Invest Ophthalmol Vis Sci 51: 2755–2763, 2010. [DOI] [PubMed] [Google Scholar]
- 75.Uchida H, Hayashi H, Kuroki M, Uno K, Yamada H, Yamashita Y, Tombran-Tink J, Kuroki M, Oshima K. Vitamin A up-regulates the expression of thrombospondin-1 and pigment epithelium-derived factor in retinal pigment epithelial cells. Exp Eye Res 80: 23–30, 2005. [DOI] [PubMed] [Google Scholar]
- 76.Umazume K, Tsukahara R, Liu L, Fernandez de Castro JP, McDonald K, Kaplan HJ, Tamiya S. Role of retinal pigment epithelial cell β-catenin signaling in experimental proliferative vitreoretinopathy. Am J Pathol 184: 1419–1428, 2014. [DOI] [PubMed] [Google Scholar]
- 77.Uruno A, Yagishita Y, Katsuoka F, Kitajima Y, Nunomiya A, Nagatomi R, Pi J, Biswal SS, Yamamoto M. Nrf2-mediated regulation of skeletal muscle glycogen metabolism. Mol Cell Biol 36: 1655–1672, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Villarroel M, García-Ramírez M, Corraliza L, Hernandez C, Simo R. Effects of high glucose concentration on the barrier function and the expression of tight junction proteins in human retinal pigment epithelial cells. Exp Eye Res 89: 913–920, 2009. [DOI] [PubMed] [Google Scholar]
- 79.Xie M, Hu A, Luo Y, Sun W, Hu X, Tang S. Interleukin-4 and melatonin ameliorate high glucose and interleukin-1β stimulated inflammatory reaction in human retinal endothelial cells and retinal pigment epithelial cells. Mol Vis 20: 921–928, 2014. [PMC free article] [PubMed] [Google Scholar]
- 80.Yamamoto N, Ueda-Wakagi M, Sato T, Kawasaki K, Sawada K, Kawabata K, Akagawa M, Ashida H. Measurement of glucose uptake in cultured cells. Curr Protoc Pharmacol 71: 1–26, 2015. [DOI] [PubMed] [Google Scholar]
- 81.Yang P, Peairs JJ, Tano R, Jaffe GJ. Oxidant-mediated Akt activation in human RPE cells. Invest Ophthalmol Vis Sci 47: 4598–4606, 2006. [DOI] [PubMed] [Google Scholar]
- 82.Yang YS, Sharma R, Sharma A, Awasthi S, Awasthi YC. Lipid peroxidation and cell cycle signaling: 4-hydroxynonenal, a key molecule in stress mediated signaling. Acta Biochim Pol 50: 319–336, 2003. [PubMed] [Google Scholar]
- 83.Yao J, Tao ZF, Li CP, Li XM, Cao GF, Jiang Q, Yan B. Regulation of autophagy by high glucose in human retinal pigment epithelium. Cell Physiol Biochem 33: 107–116, 2014. [DOI] [PubMed] [Google Scholar]
- 84.Yao Y, Guan M, Zhao XQ, Huang YF. [Downregulation of the pigment epithelium derived factor by hypoxia and elevated glucose concentration in cultured human retinal pigment epithelial cells.] Zhonghua Yi Xue Za Zhi 83: 1989–1992, 2003. [PubMed] [Google Scholar]
- 85.Yokouchi H, Eto K, Nishimura W, Takeda N, Kaburagi Y, Yamamoto S, Yasuda K. Angiopoietin-like protein 4 (ANGPTL4) is induced by high glucose in retinal pigment epithelial cells and exhibits potent angiogenic activity on retinal endothelial cells. Acta Ophthalmol 91: e289–e297, 2013. [DOI] [PubMed] [Google Scholar]
- 86.Yuan Z, Feng W, Hong J, Zheng Q, Shuai J, Ge Y. p38MAPK and ERK promote nitric oxide production in cultured human retinal pigmented epithelial cells induced by high concentration glucose. Nitric Oxide 20: 9–15, 2009. [DOI] [PubMed] [Google Scholar]