Abstract
The water-soluble biotin (vitamin B7) is indispensable for normal human health. The vitamin acts as a cofactor for five carboxylases that are critical for fatty acid, glucose, and amino acid metabolism. Biotin deficiency is associated with various diseases, and mice deficient in this vitamin display enhanced inflammation. Previous studies have shown that biotin affects the functions of adaptive immune T and NK cells, but its effect(s) on innate immune cells is not known. Because of that and because vitamins such as vitamins A and D have a profound effect on dendritic cell (DC) function, we investigated the effect of biotin levels on the functions of human monocyte-derived DCs. Culture of DCs in a biotin-deficient medium (BDM) and subsequent activation with LPS resulted in enhanced secretion of the proinflammatory cytokines TNF-α, IL-12p40, IL-23, and IL-1β compared with LPS-activated DCs cultured in biotin-sufficient (control) and biotin-oversupplemented media. Furthermore, LPS-activated DCs cultured in BDM displayed a significantly higher induction of IFN-γ and IL-17 indicating Th1/Th17 bias in T cells compared with cells maintained in biotin control or biotin-oversupplemented media. Investigations into the mechanisms suggested that impaired activation of AMP kinase in DCs cultured in BDM may be responsible for the observed increase in inflammatory responses. In summary, these results demonstrate for the first time that biotin deficiency enhances the inflammatory responses of DCs. This may therefore be one of the mechanism(s) that mediates the observed inflammation that occurs in biotin deficiency.
Keywords: human, dendritic cells, biotin, inflammation
biotin, a member of the family of water-soluble vitamins (also known as vitamin B7), is an indispensable micronutrient for normal human health due to its essentiality for cellular metabolism, proliferation, and survival. Marginal and severe degrees of biotin deficiency lead to a variety of clinical abnormalities that include neurological disorders and dermal abnormalities (40, 45). Such deficiency/suboptimal levels occur in a variety of conditions including inflammatory bowel disease (IBD) (1, 12), inborn errors in biotin metabolism (multiple carboxylase deficiency) (10), and chronic alcoholism (6) among others. At the metabolic level, biotin acts as a cofactor for five carboxylases that are critical for fatty acid, glucose, and amino acid metabolism (27, 40, 45). Important roles for this vitamin in cellular energy metabolism (i.e., ATP production) and in regulation of cellular oxidative stress (24), as well as in gene expression (where expression of over 2,000 human genes appears to be affected by biotin status; Refs. 36, 38, 40) have also been reported recently. Emerging evidence has also been accumulating showing a role for biotin in the functions of immune cells (20). In reference to the latter, biotin was shown to be important for the activity of human natural killer (NK) lymphocytes (32), for the generation of cytotoxic T lymphocytes (CTLs) (19), and for the maturation and responsiveness of immune cells (4). Defects in T-cell and B-cell immunity have been reported in patients with multiple carboxylase deficiency, a condition associated with biotin deficiency (10). An increase in the levels of proinflammatory cytokines TNF-α and interleukin-1β (IL-1β) has also been observed in biotin deficiency (20–22). Our recent studies in mice with a conditional (intestinal-specific) knockout (KO) of the biotin transporter SMVT (product of the SLC5A6 gene) have shown that these animals also develop chronic spontaneous intestinal inflammation, especially in the cecum (13), presumably in response to the moderate degree of biotin deficiency uniformly induced by defective biotin transport. From the above, we infer that biotin deficiency leads to significant metabolic disturbances and to immune dysfunction.
The majority of the previous studies have examined the effect of biotin deficiency on the functions of adaptive immune T, B, and NK cells (14, 37). Virtually nothing is known about the effect of biotin deficiency on innate immune cells such as dendritic cell (DCs). DCs are the primary antigen-presenting cells and key to initiating and regulating an immune response (5). DCs are distributed throughout the body including below the epithelial cells lining the gut and are among the primary responders to infections (5, 29). DCs sense and capture pathogens via various pathogen recognition receptors (PRRs). Subsequently, DCs become activated by upregulating the expression of costimulatory and antigen-presenting molecules as well as secreting proinflammatory cytokines. During activation, DCs migrate to the draining lymph nodes to prime and activate naïve T cells. The activation molecules and cytokines secreted by DCs have a major influence on T-cell responses (16, 25). Exposure of DCs to ligands of all these PRRs results in production of cytokines that modulate the type of T-cell response and functions. Upon interaction with DCs, CD4+ T cells can differentiate into a variety of effector and regulatory subsets, including classical Th1 cells and Th2 cells, follicular helper T cells, induced regulatory T cells, and the more recently defined Th17 cells (16, 17, 25). The nature of the cytokines produced by DCs in response to various ligands dictates the type of Th-cell responses. For example, IL-12p70 secretion by DCs polarizes towards Th1 cells while the production of IL-23 along with IL-1β from DCs leads to the generation of Th17 cells (2, 3, 23). The cytokines secreted by DCs thus have a major influence on downstream inflammatory responses. Aberrant inflammatory cytokine secretion by DCs has been observed in many diseases including Crohn's disease and rheumatoid arthritis (26, 44) among others. Accordingly, we speculate that understanding the factors that can modulate the DC responses is likely to important in understanding the immune dysregulation in biotin deficiency.
Vitamins have a profound effect on DC responses. For example, vitamin A metabolite, retinoic acid, as well as vitamin D have been reported to induce tolerance in DCs (9, 35). Almost all studies have investigated the effect of fat-soluble vitamins on DC functions and there is a scarcity of information regarding the effect of water-soluble vitamins like biotin on DC function. Here we examined the effect of biotin status on DC functions.
MATERIALS AND METHODS
Blood donors.
Blood samples were obtained from healthy volunteers via Institute for Clinical and Translational Science (ICTS), University of California. This study was approved by the Institutional Review Board of the University of California (Irvine, CA) and volunteers gave written and informed consent.
Preparation of biotin-deficient medium.
DMEM deficient in B vitamins was obtained from Sigma. The media were supplemented with all the B vitamins except biotin. Fetal bovine serum (FBS; obtained from Hyclone), treated with streptavidin beads to remove any traces of biotin, was then added to the culture medium at a concentration of 5%. Finally, biotin-deficient, -sufficient (control), and -oversupplemented culture media were then prepared by adding 0, 10 and 100 μM biotin, respectively.
Culture and stimulation of human monocyte-derived DCs.
Monocyte derived DCs were prepared as described before by culturing the purified monocytes with granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-4. DCs (CD14−HLA-DR+CD11c+ cells) were collected after 6 days (3). The purity of the DCs was >95% as determined by the expression of CD14, CD11c, and HLA-DR. DCs collected were washed and cultured in biotin-deficient, control, and biotin-oversupplemented media for 72 h. For the last 24 h, the cells were stimulated with LPS (100 ng/ml) and supernatant was collected and stored at −70°C until analyzed. Multiplex cytokine/chemokine detection was performed using Magpix kit (eBioscience) as per the manufacturer's protocol.
Control and LPS-stimulated DCs were stained for the expression of CD80, CD86, and HLADR (BD PharMingen) using specific antibodies (3). Analysis was performed with Flow jo (Treestar).
DC-T-cell cocultures.
LPS-stimulated and unstimulated DCs were cultured with magnetic bead purified (StemCell, Vancouver, Canada), allogeneic CD4 T cells at a ratio of 1:10. After 6 days of incubation, the supernatant was collected and the secretion of IFN-γ, IL-10, IL-17, and IL-22 was assessed using multiplex (eBioscience). IL-22 was assayed using ELISA (R&D systems).
Phospho AMPK and total AMPK detection.
DCs cultured in biotin-deficient, control, and biotin-oversupplemented media for 72 h were stimulated with an AMP-activated protein kinase (AMPK) activator, 5-aminoimidazole-4-carboxamide 1-β-d-ribofuranoside, acadesine, N1-(β-d-ribofuranosyl)-5-aminoimidazole-4-carboxamide (AICAR; 1 mM) for 45 min. Subsequently the cells were lysed. Phospho AMPK and total AMPK in the lysates was determined using specific ELISA kit as per the manufacturer's instructions (R&D Systems).
Statistical analysis.
Statistical analysis was performed using Graph Pad Prism. Within group differences between unstimulated and stimulated conditions were tested using paired t-tests. P < 0.05 was considered significant.
RESULTS
Biotin deficiency has no significant effect on DC phenotype.
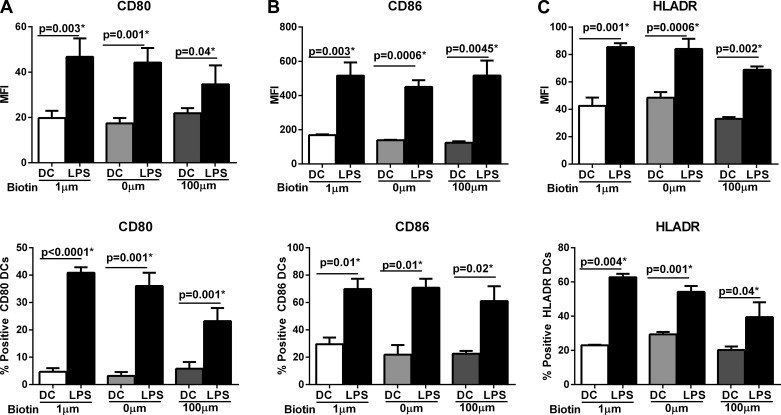
Biotin deficiency may alter the activation of DCs. Therefore, we investigated whether the upregulation of DC activation markers in response to LPS were altered in biotin-deficient (0 μM) or biotin-oversupplemented (100 μM) DCs compared with DCs cultured in control biotin (10 μM) media. Stimulation with the LPS resulted in substantial activation DCs cultured in all biotin media (Fig. 1). DCs cultured in all three media displayed significantly enhanced (P < 0.05) expression of costimulatory marker CD80 (Fig. 1A), CD86 (Fig. 1B), and HLADR (Fig. 1C) in response to LPS compared with un-stimulated DCs. However, the expression of CD80, CD86, and HLADR was comparable between DCs cultured at various concentrations of biotin both at the level of mean fluorescence intensity (MFI) as well as percent positive cells (Fig. 1, A–C). These data suggest that biotin levels (deficiency or oversupplementation) have no significant effect on DC phenotype.
Fig. 1.
Biotin deficiency has no significant effect on dendritic cell (DC) phenotype. DCs were cultured in biotin-deficient (0 μM), control (10 μM), and biotin-oversupplemented (100 μM) media for 48 and subsequently stimulated with LPS for another 24 h. Bar graphs depict the mean fluorescence intensity (MFI) and %positive DCs of the expression of activation molecules on LPS-stimulated aged and young DCs. A: CD80; B: CD86; C: HLADR. Data are mean ± SE of 3 experiments.
Biotin deficiency enhances proinflammatory cytokine secretion from LPS-stimulated DCs.
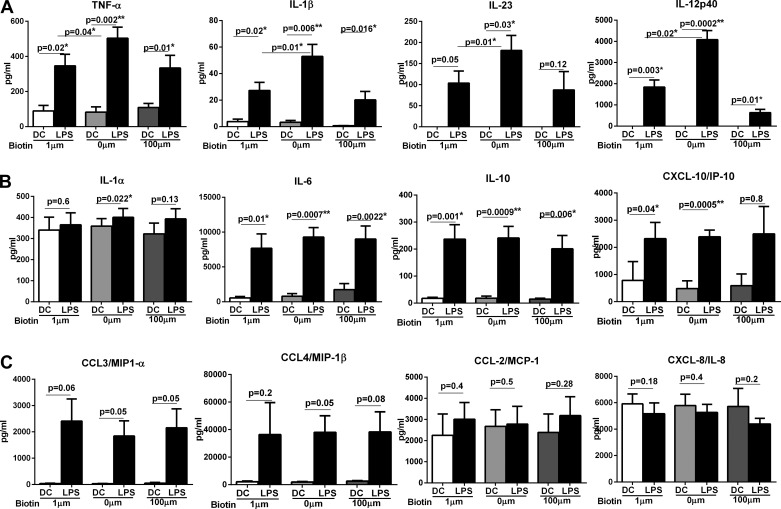
Next, we investigated the cytokines secreted by stimulated DCs. After stimulation with LPS for 24 h, supernatants were collected and assayed with multiplex to quantify cytokine secretion. In keeping with activation markers, stimulation of DCs with LPS resulted in the production of significantly enhanced (P < 0.05) levels of several proinflammatory cytokines including IL-6, TNF-α, IL-1β, IL-1α, IL-23, IL-12, IL-10, and chemokines such as CXCL-10, CCL-3, and CCL-4 (Fig. 2) in all groups. However, the level of the proinflammatory mediators was substantially different between LPS-stimulated DCs cultured in BDM compared with control medium. Compared with DCs cultured in control medium DCs cultured in BDM secreted significantly (P < 0.05) increased levels of TNF-α (BDM ∼500 pg/ml vs. control ∼345 pg/ml), IL-1β biotin deficient (∼53 pg/ml vs. control ∼27 pg/ml), IL-23 (BDM ∼181 pg/ml vs. control ∼100 pg/ml), and IL-12p40 (BDM ∼4,080 pg/ml vs. control ∼1,842 pg/ml) after stimulation with LPS (Fig. 2A). LPS-stimulated DCs cultured in biotin-oversupplemented medium displayed comparable level of these cytokines to controls except for IL-12p40, which was significantly reduced in this group (P = 0.02). IL-23 secretion was also reduced although not to a significant level (P = 0.7).
Fig. 2.
Biotin deficiency enhances proinflammatory cytokine secretion from LPS-stimulated DCs. Bar graphs depict the levels of cytokine and chemokines secreted by LPS-stimulated biotin-deficient, control, and biotin-oversupplemented DCs. A: TNF-α, IL-1β, IL-23, and IL-12p40; B: IL-1α, IL-6, IL-10, and CXCL-10; C: CCL-3, CCL-4, CCL-2, and CXCL-8. Data are mean ± SE of 8 experiments.
In addition to the above cytokines, LPS-stimulated DCs cultured in BDM also secreted significantly (P < 0.05) higher levels of IL-1α, IL-6, CXCL-10, and IL-10 (Fig. 2B) compared with unstimulated DCs. Although there was no significant difference in the level of these cytokines between DCs cultured in BDM vs. the control medium, nevertheless, DCs cultured in BDM displayed higher secretion and increased significance levels for all these cytokines compared with control and biotin-oversupplemented DCs. For example, IL-1α levels were significantly (P < 0.022) increased after LPS stimulation only in DCs cultured in BDM and not in control or biotin-oversupplemented DCs (Fig. 2B). CXCL-10 secretion was also significant (P < 0.005) in LPS-stimulated biotin-deficient DCs vs. biotin-oversupplemented DCs. The secretion of chemokines CCL-3 and CCL-4 was comparable between the three groups (Fig. 2C). Chemokines, CCL-2, and CXCL-8 were not induced at significant levels (P > 0.05) after LPS stimulation in all groups (Fig. 2C). In summary, these data demonstrate that biotin deficiency enhances the capacity of LPS-stimulated DCs to secrete proinflammatory and Th1 and Th17-promoting cytokines and chemokines.
Biotin-deficient DCs bias the Th-cell response towards Th1/Th17.
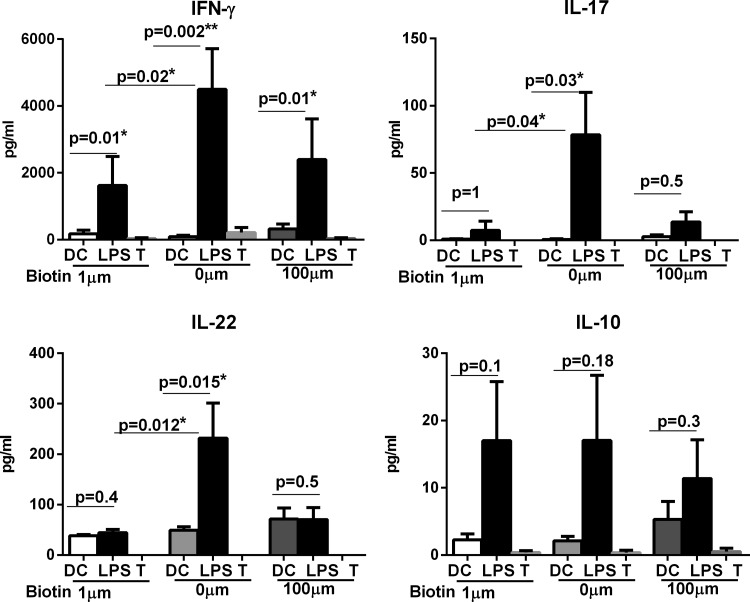
Our own studies (3) as well as evidence from the literature indicate a key role for the type of cytokine secreted by DCs in controlling the polarization of Th cell responses towards Th1, Th2, Treg, or Th17. High IL-23 and IL-1β favor IL-17 production from Th cells, while high IL-12p70 favors IFN-γ production (16, 25). Therefore, given the distinct profile of cytokine secretion by biotin-deficient DCs, we explored its effect on Th cell responses. DCs were cultured in medium with various concentrations of biotin and stimulated with LPS as described in Fig. 1. Subsequently, the DCs were washed and cultured together with purified, CD4 T cells for 5 days to allow differentiation of T cells towards Th17 or Th1. The results showed (Fig. 3) that LPS-stimulated biotin-deficient DCs induced significantly higher (P < 0.05) levels of IFN-γ, IL-17, and IL-22 from CD4 T cells compared with biotin control DCs. Biotin-oversupplemented DCs were comparable to control DCs. The secretion of IL-10 was also comparable among the three groups. Altogether, these data demonstrate that biotin deficiency enhances the secretion of TNF-α, IL-1β, IL-23, and IL-12p40 from DCs, which biases the Th cell responses towards Th1/Th17. Biotin deficiency thus favors inflammation since these are all highly proinflammatory responses.
Fig. 3.
Biotin-deficient DCs bias the Th-cell response towards Th1/Th17. Bar graphs depict the level of cytokines secreted by T cells after 5 days of coculture with LPS-stimulated biotin-deficient, control, and biotin-oversupplemented DCs. IFN-γ, IL-17, IL-22, and IL-10. Data are mean ± SE of 8 experiments.
Biotin deficiency impairs the activation of AMPK signaling pathway in DCs.
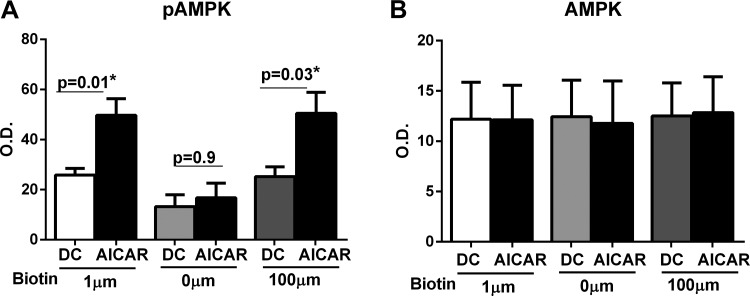
The maintenance of cellular defense systems and removal of pathogens is an energetically demanding process that requires integration of multiple checkpoints to maintain immune cell energy homeostasis (28). AMPK has emerged as an important regulator of inflammatory responses in immune cells including DCs (31). Given that biotin-deficient DCs display increased secretion of inflammatory cytokines, we compared the phosphorylation of AMPK-α in unstimulated and AICAR stimulated DCs from the three different biotin level groups using ELISA. AICAR is an activator of AMPK and was used as a positive control. As evident from the results shown in Fig. 4A, phospho AMPK levels were significantly reduced (P = 0.025) in biotin-deficient DCs compared with control DCs before activation with AICAR. Furthermore, activation with AICAR enhanced the phospho AMPK levels significantly in (P < 0.05) in both control and biotin-oversupplemented DCs but had no significant effect on biotin-deficient DCs (Fig. 4A). The levels of total AMPK were comparable in all three groups both before and after activation AICAR. These results suggest that biotin deficiency impairs the activation of AMPK in DCs, which in turn enhances inflammation.
Fig. 4.
Biotin deficiency impairs the activation of AMPK signaling pathway in DCs. phospho (p)AMPK and total AMPK levels were determined in biotin-deficient, control, and biotin-oversupplemented DCs before and after AICAR stimulation by ELISA. Bar graphs depict the levels of pAMPK (A) and AMPK (B) in DCs. Data are mean ± SE of 6 experiments.
DISCUSSION
Previous studies have shown that biotin deficiency impacts the functions of immune function particularly those of NK and T cells (20). Our investigations into the effect of biotin on DC functions revealed that deficiency of this vitamin results in enhanced proinflammatory cytokine secretion from DCs. The DCs produce significantly high levels of TNF-α, IL-1β, IL-23, and IL-12p40, which prime the Th-cell responses towards IFN-γ and IL-17 producing Th1/Th17 inflammatory cells (Figs. 1 and 2). This is in keeping with previous studies in which an enhanced secretion of TNF-α was also observed in murine macrophages cultured in biotin deficient (21). Moreover, biotin starvation is also reported to enhance the production of reactive oxygen species (24). Our own results with SMVT KO mice (all of which develop biotin deficiency) (13) and with mice made biotin deficient via dietary means (39) also demonstrate the association between biotin deficiency and intestinal inflammation. Furthermore, both the IL-12/Th1 as well as IL-23/Th17 responses although essential for generating immunity against pathogens have also been shown to play a major role in numerous inflammatory diseases. For example, excessive Th1 responses are associated with multiple sclerosis, Crohn's disease, rheumatoid arthritis, and crescentic glomerulonephritis (15). A distinctive positive clinical response to very high-dose biotin supplementation has been reported in multiple sclerosis (34, 41). This reversal of clinical impairment has not been achieved with any other therapy to date.
Similarly, increased levels of IL-23/Th17 have been demonstrated to be of pathogenic relevance in a growing number of chronic inflammatory diseases (7). Genome-wide association studies (GWAS) studies in humans suggest that Th17 cells have a major role in inflammatory diseases of the mucosal tissues including the gut, lung, and skin (33). In this regard increased activity of IL-23/Th17 axis has been implicated in Crohn's disease, ulcerative colitis, and colon cancer in the gut (42, 46). Asthma, chronic obstructive pulmonary disease (COPD), and autoimmune diseases of the lung also display enhanced activation of the Th17 pathway. IL-23/Th17 pathway is also considered a major perpetuator of skin disorders such as psoriasis and atopic dermatitis (46). Recent studies also suggest that in each of the Th17-associated chronic inflammatory diseases both Th17 and Th1-like cells are found in the involved tissues (11). Thus the enhanced induction of Th1/Th17 cells by biotin-deficient DCs may be one of the mechanisms of increased inflammation associated with biotin deficiency.
Biotin has a major role in cellular energy homeostasis because it functions as a key cofactor in various carboxylases that are essential for the mitochondrial metabolism of glucose, fatty acids, and amino acids (19, 24, 27, 40). A recent study in yeasts has also shown that biotin starvation alters cellular respiration. Emerging evidence indicates a major role of AMPK as a metabolic and energy sensor of DC activation (8, 31). AMPK is a serine/threonine kinase composed of three subunits, α, β, and γ, where the α-subunit is the one involved in phosphorylation. It phosphorylates targets that switch off ATP-depleting processes and turns on ATP-generating pathways (43). Recent reports suggest an important role for AMPK in modulating inflammatory responses in DCs (8, 18). Antigen-presenting cells from mice lacking AMPK-α1 promote proinflammatory cytokine production in response to LPS while the presence of AMPK-α1 attenuated these responses (8). Furthermore, activation of AMPK has been shown to reduce NF-κB activation via sirtuin 1 (SIRT1)-mediated deacetylation of p65 at Lys310 in macrophages (47). AMPK becomes activated when the ATP levels in the cells decrease. AMPK activation enhances mitochondrial respiration and fatty acid synthesis (43). The process of activation of DCs depletes the energy reserves of the cell to synthesize/process proteins required for the response. This creates a state of starvation in DCs and instead of obtaining energy from mitochondrial respiration and activating AMPK, DCs shift to glycolysis to meet their demands of the energy (30). Therefore, in DCs decreased AMPK activation is associated with increased Toll-like receptor-induced activation (18, 30). Our results suggest that the enhanced inflammatory responses of biotin-deficient DCs are a consequence of decreased AMPK activation (Fig. 4) are in keeping with the role of AMPK in DC inflammatory responses.
In conclusion, these data demonstrate for the first time that biotin deficiency can enhance the proinflammatory cytokine responses of DCs. The increased production of proinflammatory cytokines, TNF-α, IL-12, IL-23, and IL-1β by DCs in turn leads to the induction of proinflammatory Th1/Th17 responses. We also find that the activation of AMPK, a major regulator of inflammation, is reduced in biotin-deficient DCs. Our studies thus highlight a possible mechanism of inflammation induced by biotin deficiency.
GRANTS
This work was supported by National Institutes of Health Grants AG-045216 (to A. Agrawal) and DK-58057 (to H. M. Said), a grant from the Department of Veterans Affairs (to H. M. Said), and Grant UL1 TR000153 to National Center for Research Resources and the National Center for Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.A. and A.A. performed experiments; S.A. and A.A. analyzed data; S.A. and A.A. prepared figures; S.A., A.A., and H.M.S. approved final version of manuscript; A.A. and H.M.S. conception and design of research; A.A. and H.M.S. interpreted results of experiments; A.A. drafted manuscript; A.A. and H.M.S. edited and revised manuscript.
REFERENCES
- 1.Abad-Lacruz A, Fernandez-Banares F, Cabre E, Gil A, Esteve M, Gonzalez-Huix F, Xiol X, Gassull MA. The effect of total enteral tube feeding on the vitamin status of malnourished patients with inflammatory bowel disease. Int J Vitam Nutr Res 58: 428–435, 1988. [PubMed] [Google Scholar]
- 2.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol 8: 942–949, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Agrawal S, Gupta S, Agrawal A. Human dendritic cells activated via dectin-1 are efficient at priming Th17, cytotoxic CD8 T and B cell responses. PLoS One 5: e13418, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baez-Saldana A, Diaz G, Espinoza B, Ortega E. Biotin deficiency induces changes in subpopulations of spleen lymphocytes in mice. Am J Clin Nutr 67: 431–437, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 392: 245–252, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Bonjour JP. Vitamins and alcoholism. V riboflavin, VI niacin, VII pantothenic acid, and VIII biotin. Int J Vitam Nutr Res 50: 425–440, 1980. [PubMed] [Google Scholar]
- 7.Brand S. Crohn's disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn's disease. Gut 58: 1152–1167, 2009. [DOI] [PubMed] [Google Scholar]
- 8.Carroll KC, Viollet B, Suttles J. AMPKalpha1 deficiency amplifies proinflammatory myeloid APC activity and CD40 signaling. J Leukoc Biol 94: 1113–1121, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med 204: 1757–1764, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cowan MJ, Wara DW, Packman S, Ammann AJ, Yoshino M, Sweetman L, Nyhan W. Multiple biotin-dependent carboxylase deficiencies associated with defects in T-cell and B-cell immunity. Lancet 2: 115–118, 1979. [DOI] [PubMed] [Google Scholar]
- 11.Damsker JM, Hansen AM, Caspi RR. Th1 and Th17 cells: adversaries and collaborators. Ann NY Acad Sci 1183: 211–221, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez-Banares F, Abad-Lacruz A, Xiol X, Gine JJ, Dolz C, Cabre E, Esteve M, Gonzalez-Huix F, Gassull MA. Vitamin status in patients with inflammatory bowel disease. Am J Gastroenterol 84: 744–748, 1989. [PubMed] [Google Scholar]
- 13.Ghosal A, Lambrecht N, Subramanya SB, Kapadia R, Said HM. Conditional knockout of the Slc5a6 gene in mouse intestine impairs biotin absorption. Am J Physiol Gastrointest Liver Physiol 304: G64–G71, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ginsburg CH, Dambrauskas JT, Ault KA, Falchuk ZM. Impaired natural killer cell activity in patients with inflammatory bowel disease: evidence for a qualitative defect. Gastroenterology 85: 846–851, 1983. [PubMed] [Google Scholar]
- 15.Hirahara K, Nakayama T. CD4+ T-cell subsets in inflammatory diseases: beyond the Th1/Th2 paradigm. Int Immunol 28: 163–171, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science 327: 291–295, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol 3: 984–993, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Krawczyk CM, Holowka T, Sun J, Blagih J, Amiel E, DeBerardinis RJ, Cross JR, Jung E, Thompson CB, Jones RG, Pearce EJ. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood 115: 4742–4749, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kung JT, Mackenzie CG, Talmage DW. The requirement for biotin and fatty acids in the cytotoxic T-cell response. Cell Immunol 48: 100–110, 1979. [DOI] [PubMed] [Google Scholar]
- 20.Kuroishi T. Regulation of immunological and inflammatory functions by biotin. Can J Physiol Pharmacol 93: 1091–1096, 2015. [DOI] [PubMed] [Google Scholar]
- 21.Kuroishi T, Endo Y, Muramoto K, Sugawara S. Biotin deficiency up-regulates TNF-alpha production in murine macrophages. J Leukoc Biol 83: 912–920, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Kuroishi T, Kinbara M, Sato N, Tanaka Y, Nagai Y, Iwakura Y, Endo Y, Sugawara S. Biotin status affects nickel allergy via regulation of interleukin-1beta production in mice. J Nutr 139: 1031–1036, 2009. [DOI] [PubMed] [Google Scholar]
- 23.Lyakh L, Trinchieri G, Provezza L, Carra G, Gerosa F. Regulation of interleukin-12/interleukin-23 production and the T-helper 17 response in humans. Immunol Rev 226: 112–131, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madsen CT, Sylvestersen KB, Young C, Larsen SC, Poulsen JW, Andersen MA, Palmqvist EA, Hey-Mogensen M, Jensen PB, Treebak JT, Lisby M, Nielsen ML. Biotin starvation causes mitochondrial protein hyperacetylation and partial rescue by the SIRT3-like deacetylase Hst4p. Nat Commun 6: 7726, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manicassamy S, Pulendran B. Modulation of adaptive immunity with Toll-like receptors. Semin Immunol 21: 185–193, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGeachy MJ, Cua DJ. The link between IL-23 and Th17 cell-mediated immune pathologies. Semin Immunol 19: 372–376, 2007. [DOI] [PubMed] [Google Scholar]
- 27.McMahon RJ. Biotin in metabolism and molecular biology. Annu Rev Nutr 22: 221–239, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell 140: 771–776, 2010. [DOI] [PubMed] [Google Scholar]
- 29.Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Ann Rev Immunol 31: 563–604, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Neill LA. Glycolytic reprogramming by TLRs in dendritic cells. Nat Immunol 15: 314–315, 2014. [DOI] [PubMed] [Google Scholar]
- 31.O'Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature 493: 346–355, 2013. [DOI] [PubMed] [Google Scholar]
- 32.Okabe N, Urabe K, Fujita K, Yamamoto T, Yao T, Doi S. Biotin effects in Crohn's disease. Dig Dis Sci 33: 1495–1496, 1988. [DOI] [PubMed] [Google Scholar]
- 33.Pappu R, Rutz S, Ouyang W. Regulation of epithelial immunity by IL-17 family cytokines. Trends Immunol 33: 343–349, 2012. [DOI] [PubMed] [Google Scholar]
- 34.Peyro Saint Paul L, Debruyne D, Bernard D., Mock DM, Defer GL. Pharmacokinetics and pharmacodynamics of MD1003 (high-dose biotin) in the treatment of progressive multiple sclerosis. Expert Opin Drug Metab Toxicol 12: 327–344, 2016. [DOI] [PubMed] [Google Scholar]
- 35.Piemonti L, Monti P, Sironi M, Fraticelli P, Leone BE, Dal Cin E, Allavena P, Di Carlo V. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J Immunol 164: 4443–4451, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez-Melendez R, Griffin JB, Zempleni J. The expression of genes encoding ribosomal subunits and eukaryotic translation initiation factor 5A depends on biotin and bisnorbiotin in HepG2 cells. J Nutr Biochem 17: 23–30, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez-Melendez R, Schwab LD, Zempleni J. Jurkat cells respond to biotin deficiency with increased nuclear translocation of NF-kappaB, mediating cell survival. Int J Vitam Nutr Res 74: 209–216, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez-Melendez R, Zempleni J. Regulation of gene expression by biotin (review). J Nutr Biochem 14: 680–690, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Sabui S, Bohl Kapadia JA, Cogburn R, Lambrecht K, Ghosal N, Said A, HM. Role of the intestinal biotin uptake system (the sodium-dependent multivitamin transporter, SMVT) in the maintenance of mucosal integrity. Gastroenterology 150: 1, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Said HM. Biotin: biochemical, physiological and clinical aspects. Subcell Biochem 56: 1–19, 2012. [DOI] [PubMed] [Google Scholar]
- 41.Sedel F, Bernard D, Mock DM, Tourbah A. Targeting demyelination and virtual hypoxia with high-dose biotin as a treatment for progressive multiple sclerosis. Neuropharmacology pii: S0028-3908(15)30073-3, 2015. [DOI] [PubMed] [Google Scholar]
- 42.Siakavellas SI, Bamias G. Role of the IL-23/IL-17 axis in Crohn's disease. Discov Med 14: 253–262, 2012. [PubMed] [Google Scholar]
- 43.Steinberg GR, Kemp BE. AMPK in health and disease. Physiol Rev 89: 1025–1078, 2009. [DOI] [PubMed] [Google Scholar]
- 44.Steinman RM. Some active areas of DC research and their medical potential. Eur J Immunol 40: 2085–2088, 2010. [DOI] [PubMed] [Google Scholar]
- 45.Sweetman L, Nyhan WL. Inheritable biotin-treatable disorders and associated phenomena. Annu Rev Nutr 6: 317–343, 1986. [DOI] [PubMed] [Google Scholar]
- 46.Wilke CM, Bishop K, Fox D, Zou W. Deciphering the role of Th17 cells in human disease. Trends Immunol 32: 603–611, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang HS, Wu TC, Sang WW, Ruan Z. MiR-217 is involved in Tat-induced HIV-1 long terminal repeat (LTR) transactivation by down-regulation of SIRT1. Biochim Biophys Acta 1823: 1017–1023, 2012. [DOI] [PubMed] [Google Scholar]