adenosine triphosphate, ATP, is a high-energy molecule that plays a central role for a host of fundamental cellular processes. Maintaining adequate ATP availability is paramount for cell survival during both normal physiological states and in response to stress or injury. Mitochondria are fundamental in maintaining the dynamic, yet persistent, demand for ATP by coupling the electrochemical gradient generated by complexes I, II, and III of the electron transport chain (ETC) to the reduction of molecular oxygen (O2) by ATP synthase (complex V). Understanding the role and regulation of this high-energy cellular “currency” has defined the field of mitochondrial bioenergetics since early last century (for review, see ref. 4). More recent advances have highlighted the observed variability in the coupling between ETC flux/O2 consumption and ATP synthesis, or oxidative phosphorylation (OXPHOS) efficiency. Uncoupling proteins (UCPs) are mitochondrial proton transporters present in the inner membrane that act as a shunt between ETC complexes and ATP synthase (7). Activation of the uncoupling process results in a futile cycle of O2 consumption without ATP synthesis, with dissipation of oxidation energy as heat. In developing endotherms where heat loss secondary to a higher surface area-to-volume ratio is substantial, UCPs play a critical role in maintaining normothermia. OXPHOS efficiency remains relevant in the adult as flux through the ETC and ATP availability independently coordinate intracellular Ca2+ handling, initiation of apoptosis, and the regulation of oxidant production, processes that determine cellular fate during both normal physiologic functioning and in response to stress (5).
Traditional methods to assess OXPHOS efficiency have been technically limited in resolution: direct assessment of O2 consumption and [ATP] require separate, independent measurements, introducing intersample variability, while assessing O2 consumption at a known level of [ADP] as substrate for OXPHOS is limited in dynamic range and does not account for other sources of ADP rephosphorylation (e.g., from phosphocreatine/creatine kinase). However, advances in fluorescent imaging techniques have dramatically improved the ability to simultaneously observe intracellular biochemical processes in real time. Recently, Gouspillou and colleagues (2) described an enzymatically coupled approach to measure OXPHOS affinity for ADP in isolated mitochondria. By enzymatically coupling ATP as substrate for the reducing equivalent nicotinamide adenine dinucleotide phosphate (NADP+), and simultaneously assessing reduced NADP+ (NADPH) bioluminescence and polarographic O2 consumption (Fig. 1), Gouspillou et al. assessed OXPHOS affinity for ADP directly during physiologic steady-state conditions, and at a range of substrate concentrations. This approach, in which [ADP] and [ATP] were effectively clamped, served to increase the quantitative resolution of OXPHOS affinity for ADP by: 1) reducing intersample variability via simultaneous measurements of O2 consumption and NADPH; and, 2) permitting repeated measures within a single experiment thereby increasing the fidelity of measurement.
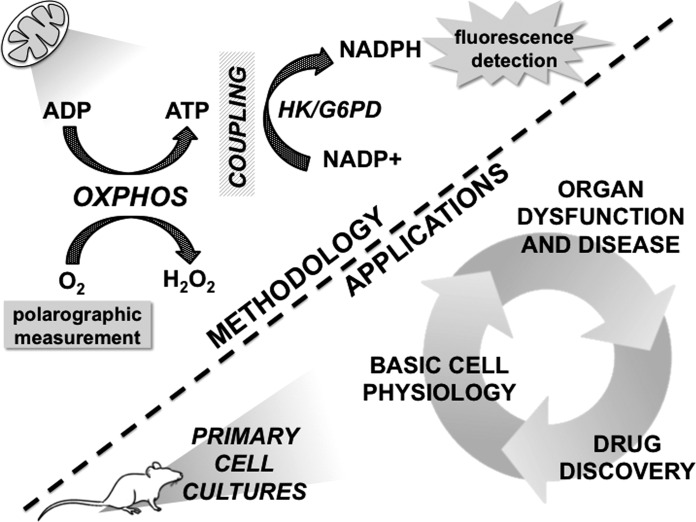
Fig. 1.
The technique employed by Lark et al. for high-resolution measurement of oxidative phosphorylation (OXPHOS) efficiency in permeabilized skeletal muscle fiber bundles (top), and potential applications in primary cell cultures from other organ systems (bottom). OXPHOS efficiency is defined as the molar ratio of ATP produced to O2 consumed. Enzymatic coupling of ATP as substrate for NADH+ reduction to NADPH allows real-time quantification of ATP via NADPH fluorescence, while O2 consumption is simultaneously measured polarographically. ATP, adenosine triphosphate; ADP, adenosine diphosphate; G6PD, glucose-6-phosphate dehydrogenase; H2O2, hydrogen peroxide; HK, hexokinase; NADP+, nicotinamide adenine dinucleotide phosphate; NADPH, reduced nicotinamide adenine dinucleotide phosphate; O2, molecular oxygen.
In the current issue of American Journal of Physiology-Cell Physiology, Lark and colleagues (3) for the first time apply a similar high-resolution approach to assess OXPHOS efficiency in intact, living cells. By utilizing the technique in permeabilized skeletal muscle fiber bundles (PmFBs), which retain a functional mitochondrial reticulum and intracellular energy transfer systems, the authors assessed OXPHOS efficiency in a system that more closely approximates the in vivo intracellular milieu. Using this approach, Lark et al. compared OXPHOS efficiency in PmFBs and isolated skeletal muscle mitochondria at lower levels of [ADP] and observed that OXPHOS efficiency was substantially decreased in PmFBs relative to isolated mitochondria (∼23% vs. 98%, respectively), which more closely approximated in vivo estimates (∼50%). Moreover, Lark et al. observed that OXPHOS efficiency in PmFBs improved as a function of [ADP], to as high as 84%. This finding is relevant for: 1) the capacity of mitochondria to meet large, acute changes in ATP demand, such as the transition from rest to high-intensity exercise; and, 2) previous observations that treatments which improve OXPHOS efficiency can augment both exercise efficiency and the capacity for sustained exercise (1). However, this observation raises several basic physiological questions. For example, what are the cellular mechanisms that regulate alterations in OXPHOS efficiency with increased ATP demand, and more broadly, what is the relevance of mitochondrial uncoupling in the adult, where total body heat loss is less substantial with a lower body surface area-to-volume ratio? Application of the model developed by Lark et al. (Fig. 1) may provide answers to these and other fundamental physiological questions, as well as provide a platform for testing of pharmacological agents with the potential to alter OXPHOS efficiency in skeletal muscle.
The capability for real-time, high-resolution measurements of OXPHOS efficiency in living cells also holds relevance in other organ systems that have high tissue-specific O2/ATP requirements, such as in the heart and brain. Even transient disruptions in O2 delivery to these critical organs can reduce cellular ATP availability, inducing cell death and dysfunction and resulting in a high potential for death or disability. However, the physiologic relevance of OXPHOS efficiency in normal cellular function and in the response to ischemic injury in these organ systems remains largely undetermined. With advances in live-cell permeabilization techniques (6), real-time assessment of OXPHOS efficiency may be possible in primary cultures from these and other organ systems, providing a platform for cell-specific drug discovery. Moreover, innovative imaging techniques, such as two-photon intravital microscopy, or optical O2 measurement in tissues via phosphorescence decay, may enhance the resolution of measurement, further increasing the ability to quantify and understand the fundamental mechanics of mitochondrial bioenergetics in living systems. Advancing the development and application of this technique to other types of living cells may identify universal, cell- and organ-specific pathways regulating OXPHOS efficiency, further clarifying the role mitochondria play in normal physiological function, and in organ dysfunction and disease.
GRANTS
This was work was supported, in part, by American Heart Association Grant FTF19970029.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
C.M.S. drafted manuscript.
REFERENCES
- 1.Conley KE. Mitochondria to motion: optimizing oxidative phosphorylation to improve exercise performance. J Exp Biol 219: 243–249, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gouspillou G, Rouland R, Calmettes G, Deschodt-Arsac V, Franconi JM, Bourdel-Marchasson I, Diolez P. Accurate determination of the oxidative phosphorylation affinity for ADP in isolated mitochondria. PLoS One 6: e20709, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lark DS, Torres MJ, Lin C, Ryan TE, Anderson EJ, Neufer PD. Direct real-time quantification of mitochondrial oxidative phosphorylation efficiency in permeabilized skeletal muscle myofibers. Am J Physiol Cell Physiol (June 22, 2016). doi: 10.1152/ajpcell.00124.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madeira VM. Overview of mitochondrial bioenergetics. Methods Mol Biol 810: 1–6, 2012. [DOI] [PubMed] [Google Scholar]
- 5.Markham A, Bains R, Franklin P, Spedding M. Changes in mitochondrial function are pivotal in neurodegenerative and psychiatric disorders: how important is BDNF? Br J Pharmacol 171: 2206–2229, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Medepalli K, Alphenaar BW, Keynton RS, Sethu P. A new technique for reversible permeabilization of live cells for intracellular delivery of quantum dots. Nanotechnology 24: 205101, 2013. [DOI] [PubMed] [Google Scholar]
- 7.Rousset S, Alves-Guerra MC, Mozo J, Miroux B, Cassard-Doulcier AM, Bouillaud F, Ricquier D. The biology of mitochondrial uncoupling proteins. Diabetes 53 Suppl 1: S130–S135, 2004. [DOI] [PubMed] [Google Scholar]