Abstract
Oxidative phosphorylation (OXPHOS) efficiency, defined as the ATP-to-O ratio, is a critical feature of mitochondrial function that has been implicated in health, aging, and disease. To date, however, the methods to measure ATP/O have primarily relied on indirect approaches or entail parallel rather than simultaneous determination of ATP synthesis and O2 consumption rates. The purpose of this project was to develop and validate an approach to determine the ATP/O ratio in permeabilized fiber bundles (PmFBs) from simultaneous measures of ATP synthesis (JATP) and O2 consumption (JO2) rates in real time using a custom-designed apparatus. JO2 was measured via a polarigraphic oxygen sensor and JATP via fluorescence using an enzyme-linked assay system (hexokinase II, glucose-6-phosphate dehydrogenase) linked to NADPH production. Within the dynamic linear range of the assay system, ADP-stimulated increases in steady-state JATP mirrored increases in steady-state JO2 (r2 = 0.91, P < 0.0001, n = 57 data points). ATP/O ratio was less than one under low rates of respiration (15 μM ADP) but increased to more than two at moderate (200 μM ADP) and maximal (2,000 μM ADP) rates of respiration with an interassay coefficient of variation of 24.03, 16.72, and 11.99%, respectively. Absolute and relative (to mechanistic) ATP/O ratios were lower in PmFBs (2.09 ± 0.251, 84%) compared with isolated mitochondria (2.44 ± 0.124, 98%). ATP/O ratios in PmFBs were not affected by the activity of adenylate kinase or creatine kinase. These findings validate an enzyme-linked respiratory clamp system for measuring OXPHOS efficiency in PmFBs and provide evidence that OXPHOS efficiency increases as energy demand increases.
Keywords: adenosine 5′-triphosphate-to-oxygen ratio, adenosine 5′-triphosphate synthesis, permeabilized myofiber, oxidative efficiency
a decline of mitochondrial function, particularly oxidative phosphorylation (OXPHOS), due to stress or aging is thought to contribute to a wide variety of human diseases, including several aspects of the metabolic syndrome (40). Two common methods for measuring OXPHOS function are assessing O2 consumption and ATP production following the addition of respiratory substrates. Together, these outputs yield OXPHOS efficiency, the molar amount of ATP produced per mole of atomic oxygen consumed. Measuring O2 consumption and ATP production, however, requires distinct approaches and equipment, preventing both rates from being obtained simultaneously in the same mitochondrial preparation. To circumvent this challenge, an indirect approach is typically employed by measuring ADP/O, the amount of O2 consumed following the addition of a known amount of ADP. This method has been used extensively since its development in 1955 (11) but is limiting in that it: 1) assumes rather than measures the absolute amount of ATP produced by OXPHOS from a given amount of ADP, 2) does not account for potential ATP/ADP recycling, and 3) only reports OXPHOS efficiency at a single level of demand.
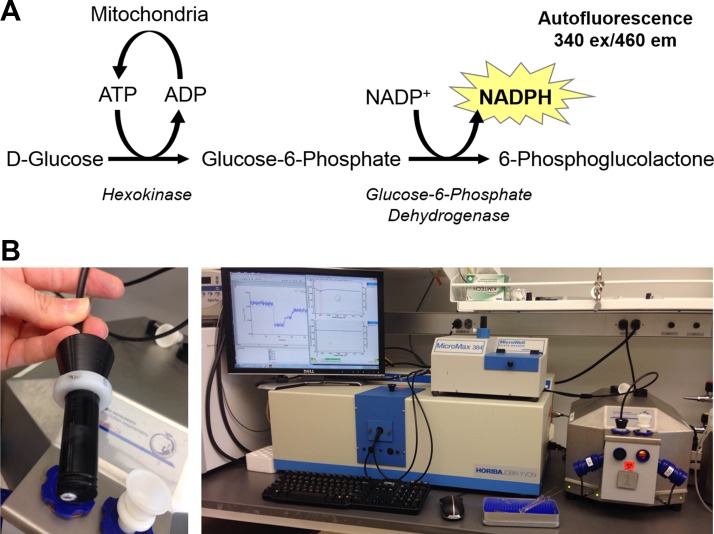
More recently, methods have been developed to measure ATP production and O2 consumption simultaneously by combining respirometry with a spectrophotometric or fluorescent light source and detector. The first uses magnesium-green, a compound that fluoresces when bound to Mg-ATP (20, 33). While this system is a notable improvement over measuring the ADP-to-O ratio, its limitations include: 1) the generation of non-steady-state rates of OXPHOS flux due to continuous accumulation of Mg-ATP, making ADP concentration ([ADP]) nonstatic and 2) the need for multiple standard curves with regressions and numerous assumptions required to quantify ATP production. A recently described alternative system uses hexokinase (HK) and glucose-6-phosphate dehydrogenase (G-6-PDH) to convert ATP to NADPH that is continuously measured spectrophotometrically (21, 22) (Fig. 1A). With the use of this system, ATP production is quantified based on the linear relationship between NADPH and absorbance and therefore does not require further calculations or assumptions. Furthermore, since this enzyme couple hydrolyzes ATP as it leaves the mitochondria, [ADP] and ATP concentration ([ATP]) remain stable (i.e., “clamped”) throughout the experimental period (22), allowing measurement of OXPHOS under steady-state rates. Although this approach has been validated using mitochondria isolated from skeletal muscle, isolated mitochondria lack the functional reticulum and intracellular energy transfer systems normally present in vivo that influence the properties of the respiratory system (i.e., Km for ADP, etc.) (32). Permeabilized skeletal muscle myofiber bundles (PmFBs), on the other hand, retain the native mitochondrial reticulum (34) and energy transfer systems and thus better reflect in vivo energetics (32). The purpose of the present study was to develop and validate a similar enzyme-coupled respiratory-clamped system for use in PmFBs to simultaneously quantify ATP production and oxygen consumption in real time and to determine whether OXPHOS efficiency is affected as a function of metabolic demand.2
Fig. 1.
Assay chemistry and instrumentation. A: ATP production is measured via enzyme-coupled phosphorylation and subsequent oxidation of glucose, consuming ATP and generating NADPH in a 1:1 stoichiometry. NADPH autofluorescence is detected at 340 nm excitation/460 nm emission. B: fluorescence is measured through a randomized fiber optic cable inserted through the airtight stopper of the respirometry chamber (left) and connected back to a spectrofluorometer (right).
MATERIALS AND METHODS
Reagents.
All chemicals and reagents were purchased from Sigma Aldrich (http://www.sigmaaldrich.com).
Preparation of mouse PmFBs.
All aspects of rodent studies were submitted to, and approved by, the East Carolina University Institutional Animal Care and Use Committee. Male C57BL/6NJ mice were purchased from Jackson Laboratories, housed in a temperature (22°C)- and light-controlled room, and given free access to food and water. At the time of experiments, mice were 8–12 wk of age. The PmFB technique used was partially adapted from previous methods (26, 39) and has been described previously (1, 2). Mice were anesthetized by inhalation of isoflurane, after which the red (RG) portion of the gastrocnemius muscle was immediately excised. Muscle samples were placed in ice-cold (4°C) buffer X containing (in mM) 7.23 K2EGTA, 2.77 CaK2EGTA, 20 imidazole, 20 taurine, 5.7 ATP, 14.3 phosphocreatine, 6.56 MgCl2·6H2O, and 50 MES (pH 7.1, 295 mosmol/kgH2O). Under a dissecting microscope, fat and connective tissue were removed, and muscle samples were separated into small bundles of fibers (∼1 mg wet wt/fiber bundle). Fiber bundles were incubated in buffer X supplemented with 40 μg/ml saponin for 30 min as previously described (3). PmFBs were then washed in ice-cold buffer Z containing (in mM) 110 K-MES, 35 KCl, 1 EGTA, 5 K2HPO4, 3 MgCl2·6H2O, and 5 mg/ml bovine serum albumin (BSA, pH 7.4, 295 mosmol/kgH2O) and remained in buffer Z on a rotator at 4°C until analysis (<4 h). To mitigate the effects of contraction on respiratory kinetics, 10 μM blebbistatin was added to the respiration medium (32). At the conclusion of the experiment, PmFBs were removed from the chamber, rinsed in double-distilled H2O, lyophilized (Labconco, Kansas City, MO), and weighed using a precision calibrated scale (Orion Cahn C-35; Thermo Electron, Beverly, MA).
Mitochondrial isolation.
Quadriceps, plantaris, soleus, and whole gastrocnemius muscles were harvested at death and homogenized in mitochondrial isolation medium (MIM: 0.3 M sucrose, 10 mM HEPES, 1 mM EGTA, pH = 7.1) containing 1 mg/ml BSA, on ice (adapted from Ref. 18). Muscle homogenates were subjected to a first centrifugation step (800 g, 10 min, 4°C), and the supernatant was again centrifuged (12,000 g, 10 min, 4°C). The final pellet (isolated mitochondria) was resuspended in 150 μl MIM, and protein quantification was assessed using the BCA Protein Assay Kit (Life Technologies). Thirteen micrograms of isolated mitochondria were used for experiments, giving a final concentration of 5 μg/ml in the assay chamber. Mitochondria isolated using the procedure described regularly displayed a respiratory control ratio more than four (data not shown) and were therefore considered viable (19).
Simultaneous measurement of mitochondrial O2 consumption and ATP production.
ATP was measured by coupling glucose-dependent, HK-catalyzed ATP hydrolysis to G-6-PDH-catalyzed reduction of NADP+ to NADPH in a 1:1 stoichiometry (31). O2-equilibrated buffer Z was supplemented with 1 U/ml HK, 2.5 U/ml G-6-PDH, 2.5 mM d-glucose, and 2.5 mM NADP+. To measure ATP production, autofluorescence of NADPH (340/460 excitation/emission) was measured continuously at 30°C simultaneously with O2 consumption using a customized system integrating monochromatic fluorescence (FluoroMax-3; Horiba Jobin Yvon, Edison, NJ) via a fiber optic cable (Fiberguide Industries) with high-resolution respirometry (Oroboros Oxygraph-2k, Innsbruck, Austria) (Fig. 1B). Respiration was supported by pyruvate (5 mM), malate (0.5 mM), and succinate (5 mM). Rates of ATP synthesis (JATP) were quantified by applying a standard curve generated from ATP titrations in the presence of the enzyme-coupled system and the respiratory substrates. Steady-state OXPHOS flux rates were measured in isolated mitochondria after a single addition of ADP (15 μM) or in PmFB after successive additions of ADP (final concentrations 15, 200, and 2,000 μM). In some experiments, 200 μM P1,P5-di(adenosine-5′)pentaphosphate (Ap5A) was added to inhibit adenylate kinase, a potential alternative source of ATP synthesis (22, 25). Preliminary testing revealed Ap5A induced an oligomycin-sensitive very low level of flux through the HK-G-6-PDH respiratory clamp system (equivalent to ∼0.75 μM ATP; data not shown), presumably due to contaminating ATP or hydrolysis of Ap5A itself. Creatine monohydrate has also been shown to improve ATP exchange in PmFBs (26) and, as such, was included in some experiments (20 mM) to determine the potential influence on OXPHOS efficiency. Creatine did not induce any background effect in the system.
Data analysis and calculations.
For each step of the experimental protocol, the rate of O2 consumption (JO2) or JATP were obtained from identical time points and are reported as the mean of >20 s of steady-state data (>10 individual data points). Instrumental background rates (before any substrate additions) were subtracted from all subsequent values for JO2 and JATP, and data were normalized to fiber dry weight (for PmFB studies) or milligrams of mitochondrial protein (for isolated mitochondria studies). ATP/O ratio was calculated by dividing the rate of ATP synthesis by the rate of atomic oxygen consumed using the following formula:
Statistics.
Data are presented as means ± SE unless otherwise noted. Statistical analyses were performed using one- or two-way analysis of variance with Tukey's post hoc test or Student's two-way unpaired t-test to determine significance between groups where appropriate. The level of significance was set at P < 0.05. Sample sizes (n) refer to individual mice studied.
RESULTS AND DISCUSSION
Boundaries and validation of the assay system.
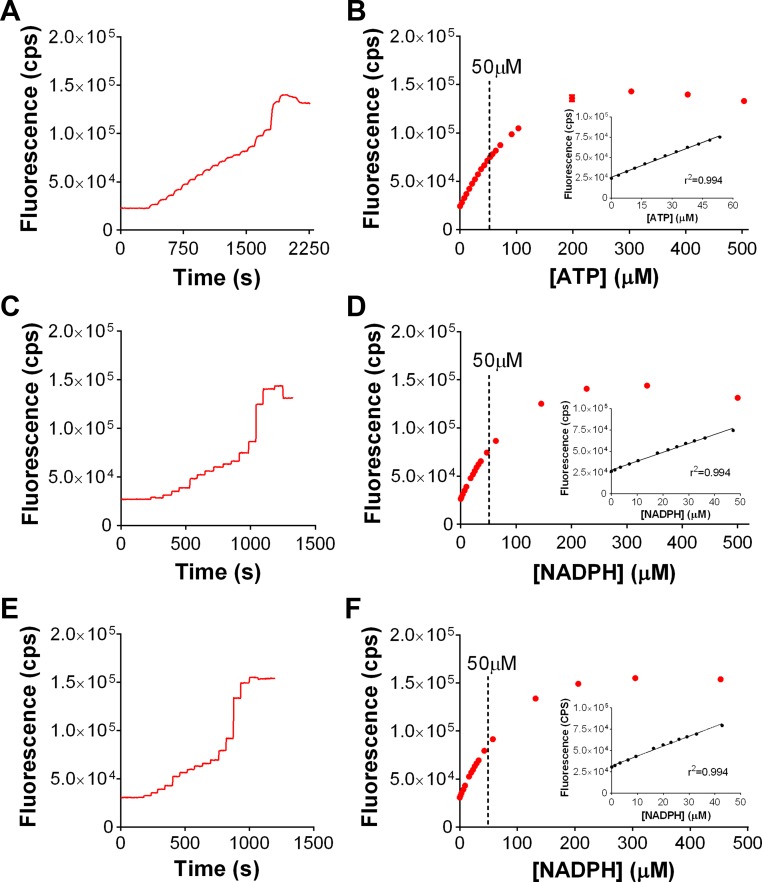
To establish the linear range of the assay system in the absence of mitochondria, NADPH autofluorescence was determined during titration of ATP and found to be linear up to ∼50 μM ATP (Fig. 2, A and B), identical to the linear range of autofluorescence of NADPH (i.e., 50 μM; Fig. 2, C and D), confirming the one-to-one ATP-NADPH stoichiometry of the system. An identical slope was obtained in the presence of a PmFB under uncoupled conditions [+carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone] (Fig. 2, E and F), indicating NADPH produced by the enzyme-coupled system is not subject to oxidation by the PmFB. Fluorescence values equating to 50 μM NADPH accumulation were set as the upper limit for accurately detecting linear rates of NADPH production in all subsequent experiments.
Fig. 2.
Establishment of boundaries of the assay system. Titration of ATP (A and B) or NADPH (C and D) in the presence of 2.5 mM glucose, 1 U/ml hexokinase (HK), 2.5 U/ml glucose-6-phosphate dehydrogenase (G-6-PDH), 2.5 mM NADP+, and substrates (5 mM pyruvate/0.5 mM malate/5 mM succinate) yields a linear increase in fluorescence up to 50 μM ATP, or 50 μM NADPH, respectively. Titration curves up to 50 μM are insets of B and D. E and F: titration of NADPH as performed in A and C, in the presence of permeabilized myofiber bundle (PmFB) and the chemical uncoupler carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP), without respiratory substrates. Inset in F shows linearity also until 50 μM NADPH. Data are means ± SD of 2 independent experiments. R2 values from the respective linear regression are shown.
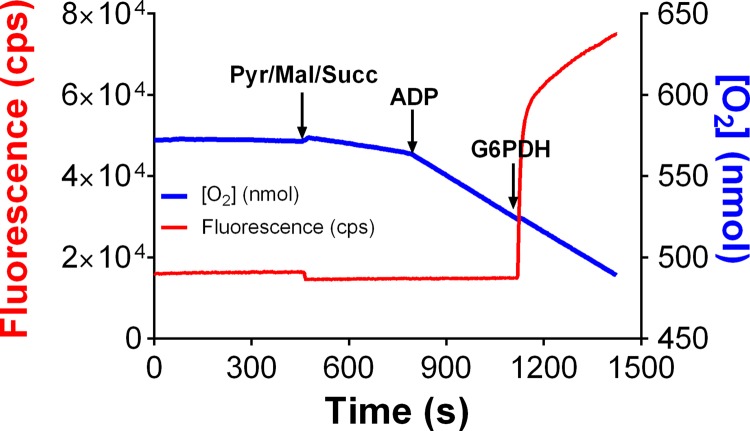
To test the validity of the assay system for measuring ATP production and O2 consumption simultaneously, PmFBs were initially placed in OXPHOS assay buffer without G-6-PDH, resulting in an incomplete enzyme couple where NADPH cannot be produced from glucose 6-phosphate (Fig. 3). Steady-state oxygen consumption was initiated by addition of respiratory substrates followed by ADP. NADPH formation, measured simultaneously, was not detectable until G-6-PDH was added to complete the system. Fluorescence increased rapidly, reflecting the buildup of glucose 6-phosphate before the addition of G-6-PDH, followed by a steady-state rate of increase reflecting the clamped rate of ATP production.
Fig. 3.
Mitochondrial ATP production measured using enzyme-linked NADPH autofluorescence. O2 concentration ([O2], blue) and NADPH autofluorescence (red) were continuously measured in respiration buffer supplemented with 2.5 mM d-glucose, 1 U/ml hexokinase, and 2.5 mM NADP+, but not G-6-PDH. PmFBs were energized with the complex I-linked substrates 5 mM pyruvate, 0.5 mM malate, and 5 mM succinate. ADP (2 mM) was added to stimulate ATP production. The addition of 2.5 U/ml G-6-PDH completes the enzyme system, resulting in an NADPH production rate corresponding to the rate of ATP production.
OXPHOS efficiency is not affected by adenylate kinase or creatine kinase in PmFBs.
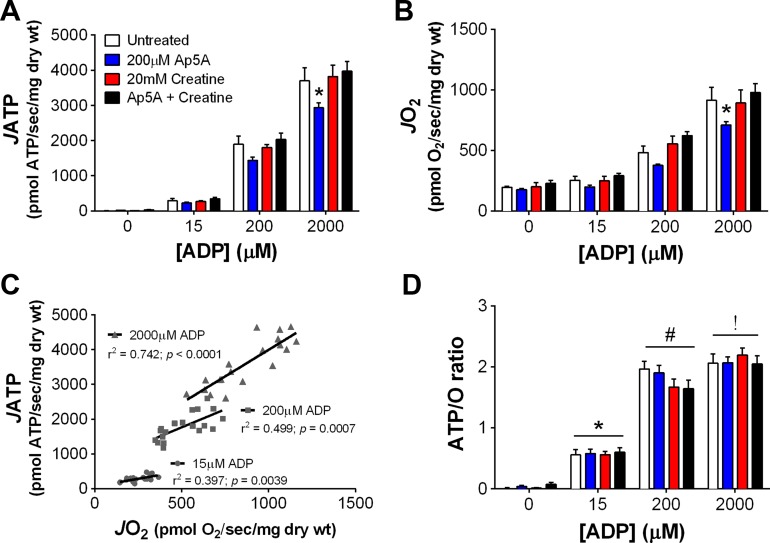
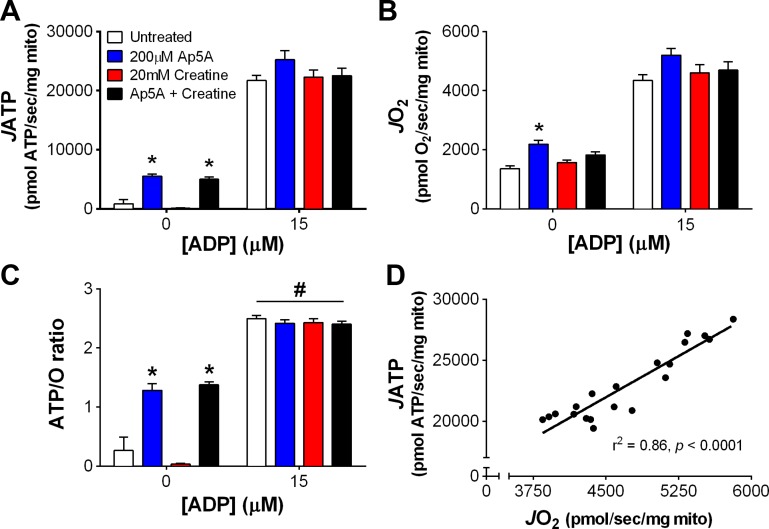
A potential challenge to measuring ATP production in PmFBs is the presence of additional enzymes capable of producing or hydrolyzing ATP. For example, adenylate kinase (AK) is a bidirectional enzyme that catalyzes the formation or hydrolysis of ATP and has been detected in skeletal muscle PmFBs (13). AK activity could synthesize ATP from ADP or, conversely, hydrolyze ATP, either of which could lead to incorrect ATP/O measurements. Creatine kinase (CK) is also endogenously present in PmFBs (37) and performs reversible hydrolysis of ATP to form phosphocreatine. To test whether AK or CK impact JATP, JO2, or ATP/O, RG PmFBs were studied in the presence of an AK inhibitor (200 μM Ap5A), substrate for CK (20 mM creatine monohydrate), or both. JATP (Fig. 4A) and JO2 (Fig. 4B) increased as a function of [ADP] in all four conditions. Addition of Ap5A decreased JATP and JO2 similarly at the highest [ADP], suggesting that the AK reaction may help to support the flux of adenine nucleotides between OXPHOS in the matrix and the HK reaction outside the mitochondria. Creatine alone did not affect either JATP or JO2, but, when combined with Ap5A, negated the decrease observed with Ap5A alone. Independent of treatment, JATP significantly correlated with JO2 at every [ADP] tested (overall r2 = 0.91, P < 0.0001, n = 57 data points; Fig. 4C). ATP/O increased as a function of [ADP] and was not affected by AK inhibition or CK activation (Fig. 4D). To our knowledge, these data are the first to demonstrate a “demand-driven” increase in OXPHOS efficiency in PmFBs. This is noteworthy because it suggests that the relationship between noncomplex V- and complex V-mediated proton conductance changes based on cellular energy state. A caveat to this interpretation is that these experiments were requisitely performed in an environment where [ATP] was very low since the enzyme detection system operates under nonequilibrium conditions to hydrolyze ATP. This is in stark contrast to the ∼8 mM equilibrium concentration of ATP that is maintained in skeletal muscle at rest in vivo (24). Whether and to what extent [ATP] or the ATP/ADP ratio per se may impact OXPHOS efficiency remains to be determined experimentally.
Fig. 4.
Oxidative phosphorylation (OXPHOS) efficiency increases as a function of ADP concentration ([ADP]) in PmFBs. Experiments were performed with red gastrocnemius (RG) PmFBs in the absence (white bars) or presence of 200 μM P1,P5-di(adenosine-5′)pentaphosphate (Ap5A, blue), 20 mM creatine monohydrate (red), or both Ap5A and creatine (black) (n = 4–5/condition). Rates of ATP production (A) and O2 consumption (B) were used to calculate Pearson correlation coefficients (C) for rate of ATP production (JATP) as a function of rate of O2 consumption (JO2) after pooling all samples (n = 19). Data from A and B were used to determine the ATP-to-O ratio (D). P < 0.05 compared with 0 μM ADP (*), 15 μM ADP (#), and 200 μM ADP (!). Values are reported as means ± SE.
OXPHOS efficiency in isolated skeletal muscle mitochondria.
PmFBs and isolated mitochondria differ in both structure and function (26, 34), so we next measured JATP, JO2, and ATP/O in isolated skeletal muscle mitochondria under the same conditions tested in PmFBs. Before the addition of ADP, the presence of Ap5A elicited a slight increase in JATP (Fig. 5A), JO2 (Fig. 5B), and ATP/O (Fig. 5C), presumably due to contaminating ATP in the Ap5A itself (referred to in materials and methods). Consistent with data in PmFBs (Fig. 4C), ADP-stimulated JATP and JO2 were positively correlated (r2 = 0.86, P < 0.0001; Fig. 5D). These data demonstrate that this system, while designed for PmFBs, can also be used in isolated mitochondria.
Fig. 5.
Simultaneous measurement of ATP production and O2 consumption in isolated skeletal muscle mitochondria. JATP (A), JO2 (B), and resulting ATP/O ratio (C) in isolated skeletal muscle mitochondria (n = 5/condition). Experiments were performed in the absence (white bars) or presence of 200 μM Ap5A (blue), 20 mM creatine monohydrate (red), or both Ap5A and creatine (black). D: Pearson correlation coefficient for JATP as a function of JO2 in the presence of 15 μM ADP (n = 20). P < 0.05 compared with the untreated group (*) and no ADP (#). Values are reported as means ± SE.
Mechanistic vs. experimental ATP/O ratios in PmFBs and isolated mitochondria.
The theoretical maximum, or “mechanistic,” ATP/O ratio is based on a fixed stoichiometry for H+ and/or ATP/ADP generated or consumed at various steps in OXPHOS. A current mechanistic ATP/O ratio for pyruvate/malate/succinate-supported OXPHOS can be generated based on the following calculations: 4 H+ are pumped from complex I (23), 2 H+ from complex III (17), and 4 H+ from complex IV (12) per NADH oxidized, yielding 10 H+ per NADH. Because FADH2 from succinate oxidation enters beyond complex I, six H+ are pumped per FADH2 oxidized. The stoichiometry of NADH/FADH2 production from oxidation of pyruvate/malate/succinate is 4:1; thus, an average of 9.2 H+ are pumped during active respiration. Pyruvate import is electroneutral because, although pyruvate carries a net negative charge, it is accompanied by a H+ when entering the mitochondrial matrix (9, 27). Import of succinate, which carries a −2 charge, is also electroneutral since it is exchanged for P−2 (29). Based on the crystal structure of mammalian ATP synthase, 2.7 H+ are thought to be required to synthesize ATP (41). Finally, the electrogenic exchange of ADP + Pi for ATP across the inner membrane via adenine nucleotide translocase (ANT) adds an additional H+ to the bioenergetic cost of making ATP. These values yield an overall H+-to-ATP ratio of 9.2:3.7 for pyruvate/malate/succinate-supported ATP synthesis, and therefore, a mechanistic ATP/O ratio for OXPHOS of 2.48.
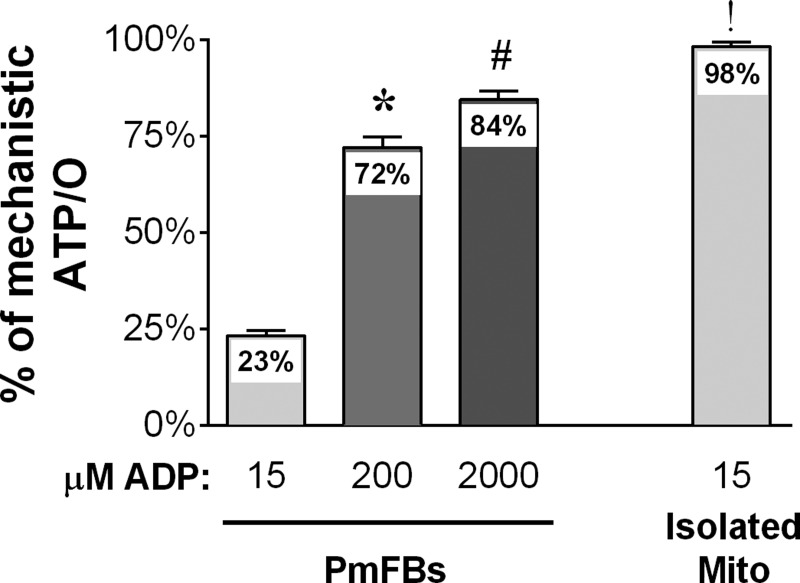
The findings from the present study suggest that PmFBs operate at 23, 72, and 84% of maximal mechanistic ATP/O efficiency at 15, 200, and 2,000 μM ADP, respectively (Fig. 6). [ADP] in resting human skeletal muscle is estimated to be 10–20 μM (35). Assuming this is similar in mouse muscle, these data indicate that ∼75% of resting O2 consumption in PmFBs under the conditions tested is from proton conductance not coupled to ATP synthesis. By contrast, isolated mitochondria operate at a significantly greater (98%) OXPHOS efficiency at 15 μM steady-state ADP concentration (Fig. 6), suggesting that, at rest, only ∼2% of O2 consumption is accounted for by proton leak in isolated mitochondria. This assertion is inconsistent with in vivo physiological studies indicating that ∼50% of whole body energy expenditure is accounted for by proton leak (36). Compared with PmFBs, mitochondria isolated from skeletal muscle are also characterized by heightened sensitivity to Ca2+-induced opening of the permeability transition pore, greater H2O2 emitting potential, and lower ATP synthase-to-complex I stoichiometry (34). Thus, these findings suggest that PmFBs better reflect in vivo OXPHOS efficiency kinetics and function compared with isolated mitochondria from skeletal muscle.
Fig. 6.
Isolated mitochondria have greater maximal OXPHOS efficiency than permeabilized myofibers. Pooled ATP/O measurements from PmFBs (from Fig. 4D) at 15, 200, and 2,000 μM ADP and isolated mitochondria at 15 μM ADP (Fig. 5C), normalized to a mechanistic ATP/O ratio of 2.48 (n = 19–20/condition). P < 0.05 compared with 0 μM ADP (*), 15 μM ADP (#), and 2,000 μM ADP in PmFBs (!). Values are reported as means ± SE.
It is unclear at this time how the ATP/O ratio increases as a function of metabolic demand. Potential effectors include proton leak, proton slip, and/or redox circuit flux. Sources of proton leak include ANT (5, 30) and possibly uncoupling proteins (UCPs), namely UCP3 in heart and skeletal muscle (4, 6, 10). UCP3 is activated by fatty acids (4, 10) and mitochondrial oxidants (15). BSA (present at 5 mg/ml in the present studies) chelates free fatty acids, so it is unlikely the fatty acid-mediating uncoupling occurred under the conditions tested. However, the combination of pyruvate/malate/succinate under state 4 conditions could potentially induce sufficient superoxide to activate UCP3, which would cease upon transition to state 3 respiration and thereby contribute to an increase in ATP/O. Alternatively, ANT is thought to account for ∼50% of basal proton conductance (8) in skeletal muscle mitochondria. In the presence of ADP, ANT adopts a “closed” conformation that conserves proton-motive force and prevents opening of the mitochondrial permeability transition pore (28). Therefore, it is possible that ANT-mediated proton conductance is greatest when in an “open” conformation but conserves the energy associated with proton leak to exchange ATP and ADP across the inner membrane while in the closed conformation. Proton slip remains controversial and possibly diminutive (7) but cannot be ruled out as a potential contributor. Finally, the continuous cycling of redox circuits drawing flux through the energy consuming mitochondrial nicotinamide nucleotide transhydrogenase could also account for a substantial portion of basal proton conductance, which would also be expected to be minimized during the transition to state 3 respiration (16). Future experiments are needed to address these possibilities.
Interassay variation when measuring ATP/O in PmFBs and isolated mitochondria.
To determine the interassay coefficient of variation (%CV) when measuring ATP/O, we generated individual %CV values (%CV = SD/mean) for each of the four conditions tested and at each [ADP] (e.g., 15, 200, and 2,000 μM) for both PmFBs and isolated mitochondria. Individual %CV values were then averaged based on the [ADP] tested. The individual and average %CV values are shown in Table 1. It is worth noting that PmFBs displayed a greater %CV compared with isolated mitochondria at 15 μM ADP. There are at least two explanations for this observation. First, it may be related to the lower affinity for ADP observed in PmFBs compared with isolated mitochondria. Second, this may be a reflection of limited sensitivity of the O2 sensor itself at very low rates of O2 flux. Nonetheless, the degree of interassay variability observed in PmFBs was less than that reported for ATP production measured using a luciferase-based assay (14).
Table 1.
Interassay coefficient of variation (%) for ATP/O measurements
| [ADP] Tested, μM: |
||||
|---|---|---|---|---|
| 15 | 200 | 2,000 | n | |
| Permeabilized Fibers | ||||
| Untreated | 31.3 | 12.9 | 14.9 | 4 |
| Ap5A (200 μM) | 25.9 | 14.3 | 10.0 | 5 |
| Creatine (20 mM) | 19.5 | 17.7 | 11.6 | 5 |
| Ap5A + Creatine | 27.5 | 19.2 | 14.6 | 5 |
| Average: | 26.1 | 16.0 | 12.8 | |
| Isolated Mitochondria | ||||
| Untreated | 4.5 | 5 | ||
| Ap5A (200 μM) | 5.1 | 5 | ||
| Creatine (20 mM) | 6.3 | 5 | ||
| Ap5A + Creatine | 5.0 | 5 | ||
| Average: | 5.2 | |||
Units are %; n, no. of mice. [ADP], ADP concentration; Ap5A, P1, P5-di(adenosine-5′)pentaphosphate.
In conclusion, this study determined the efficacy of measuring OXPHOS efficiency in real time using an enzyme-coupled detection system. This detection system measures ATP production and O2 consumption simultaneously and in real time, permitting determination of OXPHOS efficiency at multiple steady-state rates of metabolic demand. The assay has been designed, validated, and optimized for PmFBs but is also amenable for use with isolated mitochondria. Although not tested here, this method is also likely to be amenable to permeabilized cells grown in culture. OXPHOS efficiency was shown to increase as a function of [ADP] in PmFBs, a finding that could have broader implications for understanding cellular metabolism. This method will be particularly useful for human and mouse studies that require a simple, sensitive, multiplexed assay to assess OXPHOS efficiency and function in health and disease.
GRANTS
Support for this project came from National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-096907 to P. D. Neufer.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.S.L., M.J.T., E.J.A., and P.D.N. conception and design of research; D.S.L., M.J.T., C.-T.L., and T.E.R. performed experiments; D.S.L., M.J.T., C.-T.L., and T.E.R. analyzed data; D.S.L., M.J.T., C.-T.L., T.E.R., E.J.A., and P.D.N. interpreted results of experiments; D.S.L. and M.J.T. prepared figures; D.S.L. and M.J.T. drafted manuscript; D.S.L., M.J.T., and P.D.N. edited and revised manuscript; D.S.L., M.J.T., E.J.A., and P.D.N. approved final version of manuscript.
Glossary
- AK
Adenylate kinase
- ANOVA
Analysis of variance
- ANT
Adenine nucleotide translocase
- Ap5A
P1,P5-di(adenosine-5′) pentaphosphate
- BSA
Bovine serum albumin
- CK
Creatine kinase
- FCCP
Carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone
- G6PDH
Glucose-6-phosphate dehydrogenase
- HK
Hexokinase
- JATP
Rate of ATP production
- JO2
Rate of O2 consumption
- OXPHOS
Oxidative phosphorylation
- Km
Michaelis-Menten constant
- Mg-Green
Magnesium Green
- MIM
Mitochondrial isolation medium
- PmFB
Permeabilized Myofiber Bundle
- RG
Red Gastrocnemius
- UCP
Uncoupling protein
Footnotes
This article is the topic of an Editorial Focus by Creed M. Stary (38).
REFERENCES
- 1.Anderson EJ, Kypson AP, Rodriguez E, Anderson CA, Lehr EJ, Neufer PD. Substrate-specific derangements in mitochondrial metabolism and redox balance in the atrium of the type 2 diabetic human heart. J Am Coll Cardiol 54: 1891–1898, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 119: 573–581, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson EJ, Neufer PD. Type II skeletal myofibers possess unique properties that potentiate mitochondrial H2O2 generation. Am J Physiol Cell Physiol 290: C844–C851, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Anderson EJ, Yamazaki H, Neufer PD. Induction of endogenous uncoupling protein 3 suppresses mitochondrial oxidant emission during fatty acid-supported respiration. J Biol Chem 282: 31257–31266, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Bevilacqua L, Seifert EL, Estey C, Gerrits MF, Harper ME. Absence of uncoupling protein-3 leads to greater activation of an adenine nucleotide translocase-mediated proton conductance in skeletal muscle mitochondria from calorie restricted mice. Biochim Biophys Acta 1797: 1389–1397, 2010. [DOI] [PubMed] [Google Scholar]
- 6.Boudina S, Han YH, Pei S, Tidwell TJ, Henrie B, Tuinei J, Olsen C, Sena S, Abel ED. UCP3 regulates cardiac efficiency and mitochondrial coupling in high fat-fed mice but not in leptin-deficient mice. Diabetes 61: 3260–3269, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem J 435: 297–312, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brand MD, Pakay JL, Ocloo A, Kokoszka J, Wallace DC, Brookes PS, Cornwall EJ. The basal proton conductance of mitochondria depends on adenine nucleotide translocase content. Biochem J 392: 353–362, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byrne E, Hayes DJ, Shoubridge EA, Morgan-Hughes JA, Clark JB. Experimentally induced defects of mitochondrial metabolism in rat skeletal muscle. Biological effects of the mitochondrial uncoupling agent 2,4-dinitrophenol. Biochem J 229: 101–108, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cadenas S, Echtay KS, Harper JA, Jekabsons MB, Buckingham JA, Grau E, Abuin A, Chapman H, Clapham JC, Brand MD. The basal proton conductance of skeletal muscle mitochondria from transgenic mice overexpressing or lacking uncoupling protein-3. J Biol Chem 277: 2773–2778, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Chance B, Williams GR. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem 217: 383–393, 1955. [PubMed] [Google Scholar]
- 12.Colberg SR, Albright AL, Blissmer BJ, Braun B, Chasan-Taber L, Fernhall B, Regensteiner JG, Rubin RR, Sigal RJ. Exercise and type 2 diabetes: American College of Sports Medicine and the American Diabetes Association: joint position statement. Exercise and type 2 diabetes. Med Sci Sports Exerc 42: 2282–2303. [DOI] [PubMed] [Google Scholar]
- 13.De Sousa E, Veksler V, Bigard X, Mateo P, Ventura-Clapier R. Heart failure affects mitochondrial but not myofibrillar intrinsic properties of skeletal muscle. Circulation 102: 1847–1853, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Drew B, Phaneuf S, Dirks A, Selman C, Gredilla R, Lezza A, Barja G, Leeuwenburgh C. Effects of aging and caloric restriction on mitochondrial energy production in gastrocnemius muscle and heart. Am J Physiol Regul Integr Comp Physiol 284: R474–R480, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, Stuart JA, Harper JA, Roebuck SJ, Morrison A, Pickering S, Clapham JC, Brand MD. Superoxide activates mitochondrial uncoupling proteins. Nature 415: 96–99, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Fisher-Wellman KH, Lin CT, Ryan TE, Reese LR, Gilliam LA, Cathey BL, Lark DS, Smith CD, Muoio DM, Neufer PD. Pyruvate dehydrogenase complex and nicotinamide nucleotide transhydrogenase constitute an energy-consuming redox circuit. Biochem J 467: 271–280, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Francesconi R, Hubbard R, Matthew C, Durkot M, Bosselaers M, Leva N. Exercise in the heat: effects of dinitrophenol administration. J Therm Biol 13: 189–195, 1988. [Google Scholar]
- 18.Frezza C, Cipolat S, Scorrano L. Organelle isolation: functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat Protoc 2: 287–295, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Cazarin ML, Snider NN, Andrade FH. Mitochondrial isolation from skeletal muscle. J Vis Exp 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goo S, Pham T, Han JC, Nielsen P, Taberner A, Hickey A, Loiselle D. Multiscale measurement of cardiac energetics. Clin Exp Pharmacol Physiol 40: 671–681, 2013. [DOI] [PubMed] [Google Scholar]
- 21.Gouspillou G, Bourdel-Marchasson I, Rouland R, Calm ettes G, Biran M, Deschodt-Arsac V, Miraux S, Thiaudiere E, Pasdois P, Detaille D, Franconi JM, Babot M, Trezeguet V, Arsac L, Diolez P. Mitochondrial energetics is impaired in vivo in aged skeletal muscle. Aging Cell 13: 39–48, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gouspillou G, Rouland R, Calmettes G, Deschodt-Arsac V, Franconi JM, Bourdel-Marchasson I, Diolez P. Accurate determination of the oxidative phosphorylation affinity for ADP in isolated mitochondria. PLoS One 6: e20709, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herlein JA, Fink BD, Henry DM, Yorek MA, Teesch LM, Sivitz WI. Mitochondrial superoxide and coenzyme Q in insulin-deficient rats: increased electron leak. Am J Physiol Regul Integr Comp Physiol 301: R1616–R1624, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kemp GJ, Meyerspeer M, Moser E. Absolute quantification of phosphorus metabolite concentrations in human muscle in vivo by 31P MRS: a quantitative review. NMR Biomed 20: 555–565, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Kurebayashi N, Kodama T, Ogawa Y. P1,P5-Di(adenosine-5′)pentaphosphate(Ap5A) as an inhibitor of adenylate kinase in studies of fragmented sarcoplasmic reticulum from bullfrog skeletal muscle. J Biochem 88: 871–876, 1980. [DOI] [PubMed] [Google Scholar]
- 26.Kuznetsov AV, Toomas T, Peeter S, Tuuli K, Laurence K, Zoya D, Andr ÈR, Lumme K, Nadya P, Enn S, Valdur AS. Striking differences between the kinetics of regulation of respiration by ADP in slow-twitch and fast-twitch muscles in vivo. Eur J Biochem 241: 909–915, 1996. [DOI] [PubMed] [Google Scholar]
- 27.Melmed C, Karpati G, Carpenter S. Experimental mitochondrial myopathy produced by in vivo uncoupling of oxidative phosphorylation. J Neurol Sci 26: 305–318, 1975. [DOI] [PubMed] [Google Scholar]
- 28.Novgorodov SA, Gudz TI, Brierley GP, Pfeiffer DR. Magnesium ion modulates the sensitivity of the mitochondrial permeability transition pore to cyclosporin A and ADP. Arch Biochem Biophys 311: 219–228, 1994. [DOI] [PubMed] [Google Scholar]
- 29.Palmieri L, Vozza A, Honlinger A, Dietmeier K, Palmisano A, Zara V, Palmieri F. The mitochondrial dicarboxylate carrier is essential for the growth of Saccharomyces cerevisiae on ethanol or acetate as the sole carbon source. Mol Microbiol 31: 569–577, 1999. [DOI] [PubMed] [Google Scholar]
- 30.Parker N, Affourtit C, Vidal-Puig A, Brand MD. Energization-dependent endogenous activation of proton conductance in skeletal muscle mitochondria. Biochem J 412: 131–139, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Passarella S, Ostuni A, Atlante A, Quagliariello E. Increase in the ADP/ATP exchange in rat liver mitochondria irradiated in vitro by helium-neon laser. Biochem Biophys Res Commun 156: 978–986, 1988. [DOI] [PubMed] [Google Scholar]
- 32.Perry CG, Kane DA, Lin CT, Kozy R, Cathey BL, Lark DS, Kane CL, Brophy PM, Gavin TP, Anderson EJ, Neufer PD. Inhibiting myosin-ATPase reveals a dynamic range of mitochondrial respiratory control in skeletal muscle. Biochem J 437: 215–222, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pham T, Loiselle D, Power A, Hickey AJ. Mitochondrial inefficiencies and anoxic ATP hydrolysis capacities in diabetic rat heart. Am J Physiol Cell Physiol 307: C499–C507, 2014. [DOI] [PubMed] [Google Scholar]
- 34.Picard M, Taivassalo T, Ritchie D, Wright KJ, Thomas MM, Romestaing C, Hepple RT. Mitochondrial structure and function are disrupted by standard isolation methods. PLoS One 6: e18317, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prompers JJ, Jeneson JA, Drost MR, Oomens CC, Strijkers GJ, Nicolay K. Dynamic MRS and MRI of skeletal muscle function and biomechanics. NMR Biomed 19: 927–953, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Rolfe DF, Brand MD. Contribution of mitochondrial proton leak to skeletal muscle respiration and to standard metabolic rate. Am J Physiol Cell Physiol 271: C1380–C1389, 1996. [DOI] [PubMed] [Google Scholar]
- 37.Saks VA, Kuznetsov AV, Khuchua ZA, Vasilyeva EV, Belikova JO, Kesvatera T, Tiivel T. Control of cellular respiration in vivo by mitochondrial outer membrane and by creatine kinase. A new speculative hypothesis: possible involvement of mitochondrial-cytoskeleton interactions. J Mol Cell Cardiol 27: 625–645, 1995. [DOI] [PubMed] [Google Scholar]
- 38.Stary CM. A high-resolution method for assessing cellular oxidative phosphorylation efficiency: bringing mitochondrial bioenergetics into focus. Focus on “Direct real-time quantification of mitochondrial oxidative phosphorylation efficiency in permeabilized skeletal muscle myofibers. Am J Physiol Cell Physiol (July 13, 2016). doi: 10.1152/ajpcell.00203.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tonkonogi M, Fernstrom M, Walsh B, Ji LL, Rooyackers O, Hammarqvist F, Wernerman J, Sahlin K. Reduced oxidative power but unchanged antioxidative capacity in skeletal muscle from aged humans. Pflügers Arch 446: 261–269, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Wallace DC. Bioenergetic origins of complexity and disease. Cold Spring Harbor Symp Quant Biol 76: 1–16, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watt IN, Montgomery MG, Runswick MJ, Leslie AG, Walker JE. Bioenergetic cost of making an adenosine triphosphate molecule in animal mitochondria. Proc Natl Acad Sci USA 107: 16823–16827, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]