Abstract
Glucocorticoids strongly influence the mucosal-defense functions performed by the bronchial epithelium, and inhaled corticosteroids are critical in the treatment of patients with inflammatory airway diseases such as asthma, chronic obstructive pulmonary disease, and cystic fibrosis. A common pathology associated with these diseases is reduced mucociliary clearance, a defense mechanism involving the coordinated transport of salt, water, and mucus by the bronchial epithelium, ultimately leading to retention of pathogens and particles in the airways and to further disease progression. In the present study we investigated the role of hydrocortisone (HC) in differentiation and development of the ion transport phenotype of normal human bronchial epithelial cells under air-liquid interface conditions. Normal human bronchial epithelial cells differentiated in the absence of HC (HC0) showed significantly less benzamil-sensitive short-circuit current than controls, as well as a reduced response after stimulation with the selective β2-adrenergic receptor agonist salbutamol. Apical membrane localization of epithelial Na+ channel α-subunits was similarly reduced in HC0 cells compared with controls, supporting a role of HC in the trafficking and density of Na+ channels in the plasma membrane. Additionally, glucocorticoid exposure during differentiation regulated the transcription of cystic fibrosis transmembrane conductance regulator and β2-adrenergic receptor mRNAs and appeared to be necessary for the expression of cystic fibrosis transmembrane conductance regulator-dependent anion secretion in response to β2-agonists. HC had no significant effect on surface cell differentiation but did modulate the expression of mucin mRNAs. These findings indicate that glucocorticoids support mucosal defense by regulating critical transport pathways essential for effective mucociliary clearance.
Nathan Zaidman received the AJP-Cell 2016 Paper of the Year award. Listen to a podcast with Nathan Zaidman and coauthor Scott O'Grady at http://ajpcell.podbean.com/e/ajp-cell-paper-of-the-year-2016-award-podcast/.
Keywords: epithelial ion transport, epithelial sodium channel, cystic fibrosis transmembrane conductance regulator, β2-adrenergic receptors, salbutamol
the airway epithelium establishes a boundary between the internal and the external environment that protects against potential injury and infection caused by inhaled particles, debris, and microbial pathogens (17, 53, 61). To maintain the health of the lungs and surrounding tissues, the epithelium utilizes numerous defense mechanisms that complement the barrier function of the epithelium. These defense mechanisms include the expression of a broad array of innate immune receptors, including Toll-like, nucleotide-binding oligomerization domain-like, and retinoic acid (RA)-inducible gene I receptors, which facilitate the expression and secretion of multiple defense molecules, cytokines, and chemokines essential for recruitment and activation of immune cells (38, 53). Furthermore, the airway epithelium produces antimicrobial agents, such as reactive oxygen species, defensins, iron chelating proteins, and interferons, that effectively prevent infection by inhaled pathogens without activation of an adaptive immune response (38, 53). Chronic inflammation of the airways due to prolonged or repeated exposure to noxious agents can result in epithelial remodeling and fibrosis that leaves the airways more susceptible to infection due to disruption of innate immune processes (54, 66, 70). Loss of epithelial integrity compromises normal mucosal defense, underscoring the important relationship between structure and function of the airway epithelium (30, 55).
The pseudostratified airway epithelium is composed of multiple cell types (57). Multipotent, progenitor basal cells are transit-amplifying cells that reside along the basal lamina of the airway and differentiate into surface cells (29). Basal cells are instrumental in maintaining normal epithelial structure and function, as well as driving orderly regeneration after injury (59, 60). Differentiated surface cells can be divided into two distinct lineages: secretory and ciliated. Secretory cells synthesize and secrete gel-forming polymeric mucins that absorb water to form mucus (1, 41). Ciliated cells propel the mucus gel toward the pharynx and out of the lungs by the directional, synchronized beating of cilia on the cell surface, effectively removing entrapped particles and pathogens from the lungs by a process known as mucociliary clearance (46, 67, 68).
The mucus gel rests atop a thin fluid layer called the airway-surface liquid (ASL). The depth of the ASL is maintained through coordinated regulation of transcellular Na+ absorption and anion secretion pathways within the epithelium, as well as paracellular ion and water fluxes (9, 32). The efficacy of mucociliary clearance depends on the integrity of these and other transport pathways to preserve the height of the ASL, thus facilitating effective ciliary beating (4, 71). Respiratory disorders such as cystic fibrosis (CF), asthma, and chronic obstructive pulmonary disease are associated with defective or ineffective mucociliary clearance that ultimately leads to chronic inflammation, airway remodeling, and permanent loss of lung function (10, 13, 21, 23, 35, 49, 73).
Current treatment strategies for patients with asthma, chronic obstructive pulmonary disease, and other inflammatory airway disorders emphasize a combined therapy involving inhaled corticosteroid (ICS) and long-acting β2-agonists (LABAs) aimed at reducing inflammation and increasing airway caliber (6). LABAs, such as salmeterol and formoterol, are effective long-acting inhaled bronchodilators (7, 62, 72). These drugs stimulate increases in cAMP, which activates protein kinase A-dependent signaling pathways in airway smooth muscle, leading to relaxation and a decrease in airway resistance (8). The therapeutic benefit of ICS treatment is attributed to transrepression of specific genes induced by proinflammatory transcription factors such as NF-κB and activator protein 1, limiting airway hyperresponsiveness (26, 36, 66, 75). Other effects of corticosteroids on airway epithelial cells suggest a role in surface cell diversity by increasing the number of ciliated epithelial cells and decreasing mucus hypersecretion, therefore promoting mucociliary clearance (19, 42).
Although combined therapy is effective at reducing inflammatory immune reactions and airway hyperresponsiveness, its effects on the airway epithelium are not as well characterized. Furthermore, hydrocortisone (HC) is one of the constituents of cell culture media that is commonly used for maintaining normal human bronchial epithelial (NHBE) cells in culture. Therefore, the overall goal of the present study was to investigate the role of glucocorticoids in the development of the ion transport phenotype of bronchial epithelial cells during differentiation. We hypothesized that HC is necessary for in vitro differentiation of airway basal cells into a pseudostratified epithelium with ciliated and secretory surface cells. Additionally, we hypothesized a role for HC in the development of normal transepithelial ion transport pathways essential for mucociliary clearance. The results of the present study demonstrate that early glucocorticoid exposure affects the development and maintenance of specific ion transport pathways required for mucociliary clearance. These findings indicate that ICS treatment supports mucosal defense through direct interactions with the airway epithelium.
MATERIALS AND METHODS
Materials.
RA, benzamil hydrochloride, CFTRinh-172, salbutamol hemisulfate salt, 8-(4-chlorophenylthio)adenosine 3′,5′-cyclic monophosphate sodium salt (8-CPT-cAMP), 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid disodium salt hydrate (DIDS), and uridine triphosphate (UTP) were purchased from Sigma-Aldrich Chemical (St. Louis, MO), and paraformaldehyde, 16% solution, was purchased from VWR (Radnor, PA).
Cell culture.
NHBE cells were purchased from Lonza (Basel, Switzerland) and expanded in bronchial epithelial cell growth medium [BEGM + SingleQuots containing 1.4 μM HC (Lonza)]. Cells were plated at low density on 0.4-μm pore-size Snapwell polyester membranes and maintained under liquid-liquid interface growth conditions with complete BEGM until cells reached confluence (day 0). HC (1.4 μM) was withdrawn from HC0 and HC8 cells at days 0 and 8, respectively. RA (500 nM) was added to BEGM for 48 h to promote differentiation (1). Air-liquid interface (ALI) culture conditions were initiated at day 2 by removal of apical medium, and basolateral growth medium was replaced with differentiation medium (DMEM/Ham's F12 + SingleQuots) containing 100 nM RA. Cells were harvested at days 0, 4, 8, and 24. “Chopstick” electrodes and a voltohmmeter (EVOM, World Precision Instruments, New Haven, CT) were used to measure transepithelial resistance (TER) every-other day for 24 days before media change. All cells were grown at 37°C in a humidified CO2 atmosphere.
Quantitative RT-PCR.
RNA was isolated using the RNeasy Mini Kit (Qiagen, Hilden, Germany). cDNA was produced with the QuantiTect reverse transcription kit with gDNA Wipeout (Qiagen). TaqMan PCR probes were purchased through Life Technologies (Thermo Fisher Scientific, Waltham, MA). Quantitative PCR amplification of 5 ng of cDNA was performed on a real-time PCR system (model 7300, Applied Biosystems). Baseline and threshold values were set according to the manufacturer's instructions. Relative expression was quantified using the comparative cycle threshold (2−ΔCt) method, with GAPDH as the reference gene (65).
Immunofluorescence and Western blot analysis.
Cells were grown on Snapwell polyester membranes as described above and fixed in 4% paraformaldehyde for 20 min. Cell membranes were permeated using 0.3% Triton X-100 and blocked with a PBS + 3% BSA solution for ≥1 h. Cells were incubated with the primary antibody overnight at 4°C diluted in 3% BSA solution. After incubation, cells were rinsed three times in PBS and incubated with the secondary antibody for 1 h at room temperature. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole. Monolayers were excised and mounted on microscope slides using VECATSHIELD HardSet mounting medium (Vector Laboratories, Burlingame, CA). Images were captured using an Olympus FV1000 confocal microscope. The primary antibodies epithelial Na+ channel (ENaC) α-subunit (ENaCα), Na+-K+-ATPase α1-subunit, cystic fibrosis transmembrane conductance regulator (CFTR), β2-adrenergic receptor (β2-AR), and zona occludens 1 (ZO1) were purchased from Abcam (Cambridge, UK) and Prss8/CAP1 from R & D Systems (Minneapolis, MN); the secondary antibodies Alexa Fluor 488, 568, and 647 were purchased from Invitrogen (Carlsbad, CA).
Total protein was collected using Pierce immunoprecipitation lysis buffer and quantified with the Pierce bicinchoninic acid protein assay kit (Thermo Fisher Scientific). Ten or 25 ng of total protein were loaded on NuPAGE 4–12% Bis-Tris gels with Chameleon Duo-prestained protein ladder (Li-Cor Biosciences, Lincoln, NE) and separated using electrophoresis in MOPS SDS running buffer (200 V, 50 min). Proteins were transferred onto activated Immobilon-FL polyvinylidene difluoride membranes (Millipore, Billerica, MA) and blocked in Odyssey blocking buffer overnight at 4°C (Li-Cor Biosciences). Primary antibodies were diluted in Odyssey blocking buffer containing 0.2% Tween 20 and incubated overnight, washed five times in PBS + 0.1% Tween 20, and then incubated with IRDye secondary antibodies diluted in Odyssey blocking buffer for 40 min. Blots were visualized with an Odyssey CLx imager and analyzed using Image Studio Lite (Li-Cor Biosciences). β-Actin primary antibodies (catalog nos. sc-69879 and sc-130656) were purchased from Santa Cruz Biotechnology (Dallas, TX).
Electrophysiology.
Short-circuit current (Isc) was measured on high-resistance (>700 Ω·cm2) monolayers mounted in Ussing chambers bathed on both sides with standard saline solution containing (in mM) 130 NaCl, 6 KCl, 1.5 CaCl2, 1 MgCl2, 20 NaHCO3, 0.3 NaH2PO4, and 1.3 Na2HPO4, pH 7.4, and maintained at 37°C with 95% O2-5% CO2.
Statistics.
Values are means ± SE. For the quantitative RT-PCR experiments (Figs. 2, 6, 7, and 8), significant differences between day 0 and each subsequent day of differentiation were determined by an ANOVA followed by Dunnett's test for comparisons with a common control within each treatment condition. Significant differences between either the HC0 condition or the HC8 condition and the corresponding day of differentiation in the control group were determined by ANOVA followed by a Tukey-Kramer multiple-comparisons test. In Figs. 4 and 5, differences in blocker-sensitive or agonist-sensitive Isc are shown as significant differences between HC0 and control conditions or significant differences between HC0 and HC8 conditions determined by ANOVA followed by a Tukey-Kramer multiple-comparisons test. P < 0.05 was considered significant.
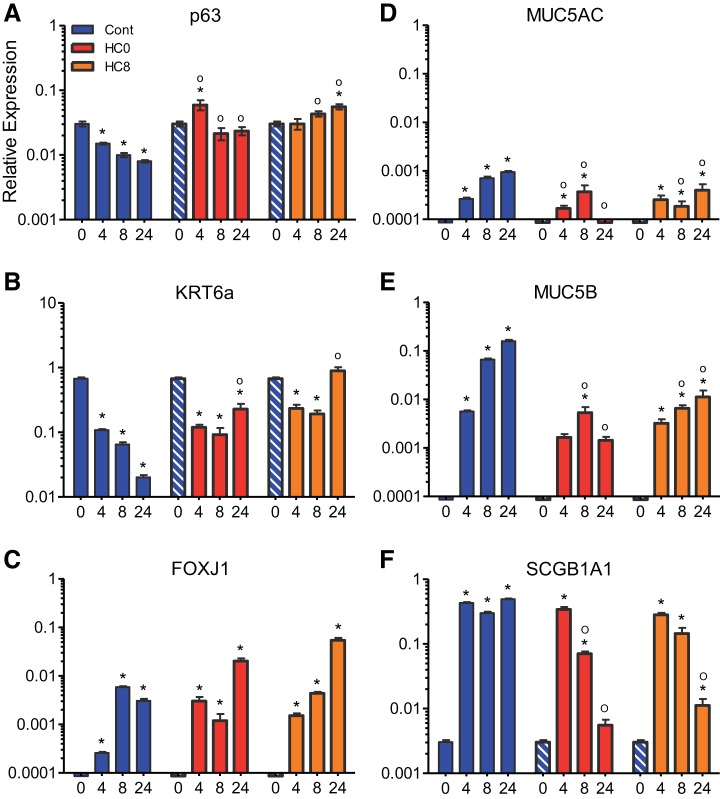
Fig. 2.
Differentiated NHBE cells express mRNAs associated with bronchial basal cells and surface cells. A–C: quantitative RT-PCR analysis showing mRNA expression of consensus bronchial basal cell markers p63, cytokeratin 6a (KRT6a), and the F-box factor FoxJ1 in undifferentiated NHBE cells (day 0) and differentiated NHBE monolayers (n = 6 for each condition). Data were normalized to GAPDH, and cycle threshold (CT) values are 23.1 ± 0.19, 23.1 ± 0.56, and 23.7 ± 0.28 for control, HC0, and HC8, respectively, at day 24. Blue-and-white-striped bars represent undifferentiated control data at day 0 (n = 6 for each bar). D–F: mRNA expression of bronchial surface cell markers was not detected or expressed at low levels in undifferentiated NHBE cells. However, by day 4, mucins (Muc5ac and Muc5b) and secretoglobin protein (SCGB1Aa1) were detected (n = 6 for each bar). *Significant difference between day 0 and each subsequent day of differentiation (by ANOVA followed by Dunnett's test for comparisons with a common control within each treatment condition); °significant difference between HC0 or HC8 and the corresponding day of differentiation in the control group (by ANOVA followed by Tukey-Kramer multiple-comparisons test).
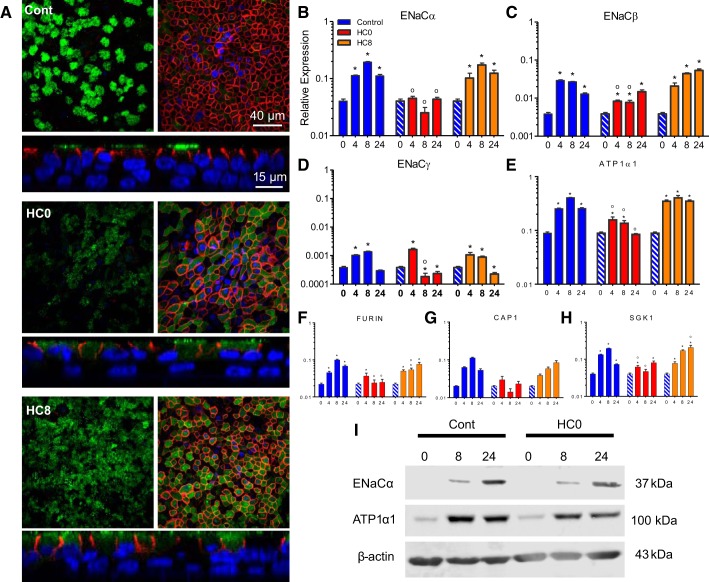
Fig. 6.
HC0 cells express lower ENaC mRNA levels and have fewer apically localized ENaC α-subunits than control and HC8 cells. A: immunocytochemistry comparing control, HC0, and HC8 monolayers at day 24 shows localization of ENaCα (green) and Na+-K+-ATPase α1-subunit (red) at apical and basolateral membranes, respectively. Left: detection of ENaCα at the apical membrane. Right: detection of Na+-K+-ATPase α1-subunit along the lateral boarder just beneath the apical membrane of the same monolayer. Nuclei (blue) were labeled with DAPI. B–E: quantitative RT-PCR analysis showing relative expression of ENaCα, ENaCβ, and ENaCγ, as well as Na+-K+-ATPase α1-subunit (ATP1A1), mRNAs (n = 6 for each bar). F–H: quantitative RT-PCR analysis of mRNAs associated with ENaC proteolytic processing [furin and channel-activating protease 1 (CAP1)] and trafficking [serum/glucocorticoid-regulated kinase 1 (SGK1)] (n = 6 for each bar). Blue-and-white-striped bars represent undifferentiated control data at day 0 (n = 6 for each bar). I: Western blot analysis of cleaved ENaCα and ATP1A1 in control and HC0 cells (10 ng of total protein). The ENaCα antibody recognizes a transmembrane region downstream of the NH2 terminus of full-length ENaCα. *Significant difference between day 0 and each subsequent day of differentiation (by ANOVA followed by Dunnett's test for comparisons with a common control within each treatment condition); °significant difference between HC0 or HC8 and the corresponding day of differentiation in the control group (by ANOVA followed by Tukey-Kramer multiple-comparisons test).
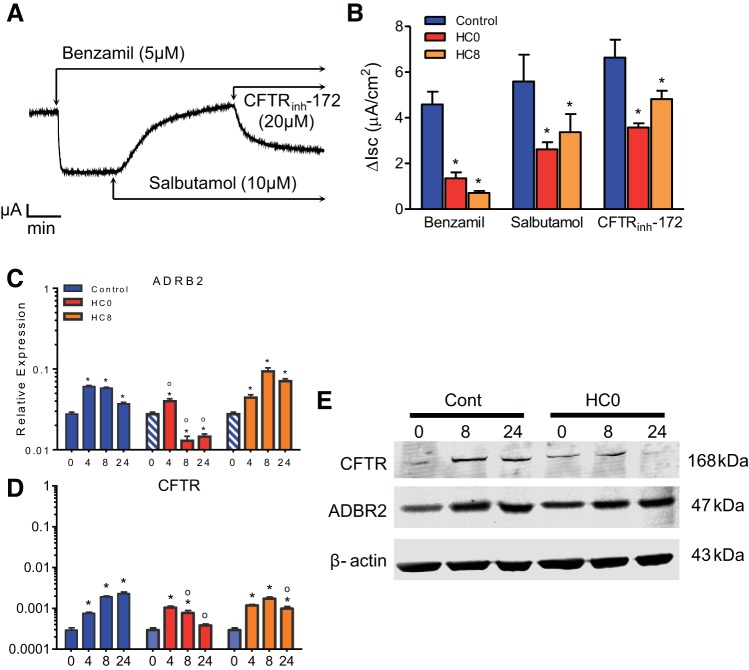
Fig. 7.
Differentiated NHBE monolayers display increased Isc responses after apical treatment with the selective β2-adrenergic receptor (AR) agonist salbutamol. A: representative Isc trace of day 16 monolayers treated apically first with benzamil (5 μM), then with the selective β2-AR agonist salbutamol (10 μM), and finally with CFTRinh-172 (20 μM). Apical addition of salbutamol increased Isc in control HC0 and HC8 monolayers, consistent with stimulated anion secretion. B: summary of benzamil-sensitive, salbutamol-activated, and CFTR-dependent Isc results in A. *Significant differences between treatment and control conditions (n = 4). C–D: quantitative RT-PCR analysis of β2-AR (ADRB2) and CFTR mRNAs (n = 6 for each bar). HC0 monolayers showed reduced ADRB2 and CFTR expression at days 8 and 24. E: Western blot analysis of CFTR and ADBR2 in control and HC0 cells (25 ng of total protein). CFTR protein abundance appeared to be reduced in HC0 cells at days 8 and 24. Blue-and-white-striped bars represent undifferentiated control data at day 0 (n = 6 for each bar). *Significant difference between day 0 and each subsequent day of differentiation (by ANOVA followed by Dunnett's test for comparisons with a common control within each treatment condition); °significant difference between HC0 or HC8 and the corresponding day of differentiation in the control group (by ANOVA followed by Tukey-Kramer multiple-comparisons test).
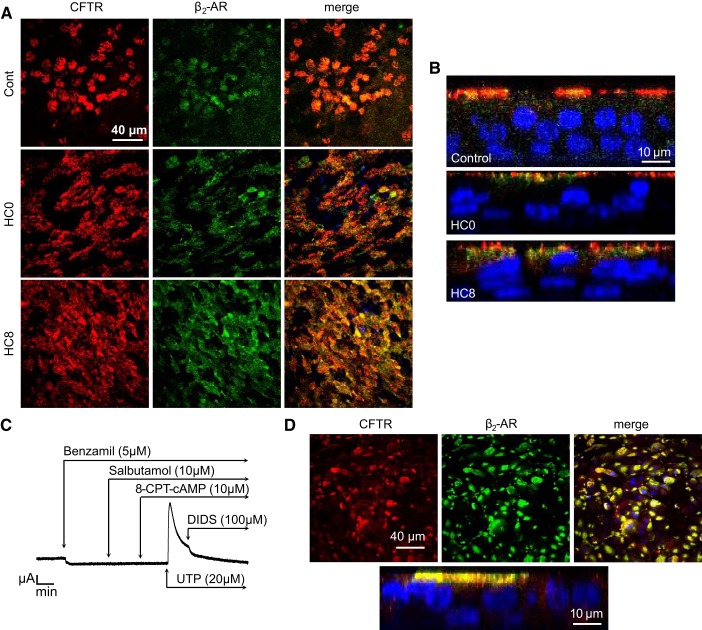
Fig. 8.
CFTR and β2-AR colocalize at the apical membrane of differentiated NHBE monolayers. A: immunocytochemistry shows colocalization of CFTR (red) and β2-AR (green) at the apical membrane in day 24 differentiated control, HC0, and HC8 monolayers. Colocalization of CFTR and β2-AR was detected in cilia-like structures. Nuclei (blue) were labeled with DAPI. B: orthogonal views of day 24 differentiated cells showing apical localization of CFTR and β2-ARs within cilia. C: representative Isc trace of day 16 human Δ508 CF bronchial epithelial (CFBE) cell monolayers treated apically with benzamil (5 μM) and then with the selective β2-AR agonist salbutamol (10 μM). 8-(4-Chlorophenylthio)adenosine 3′,5′-cyclic monophosphate sodium salt (8-CPT-cAMP, 10 μM) was then added to the apical and basolateral compartments, but no change in Isc was detected. Stimulation with UTP (20 μM) produced an increase in Isc that was blocked by the disulfonic stilbene derivative DIDS (100 μM), a Ca2+-activated Cl− channel blocker. D: immunocytochemistry shows localization of CFTR (red) and β2-AR (green) in the perinuclear region below the apical membrane in differentiated ΔF508 CFBE monolayers. Localization of CFTR and β2-AR was not detected in cilia-like structures. Nuclei (blue) were labeled with DAPI.
Fig. 4.
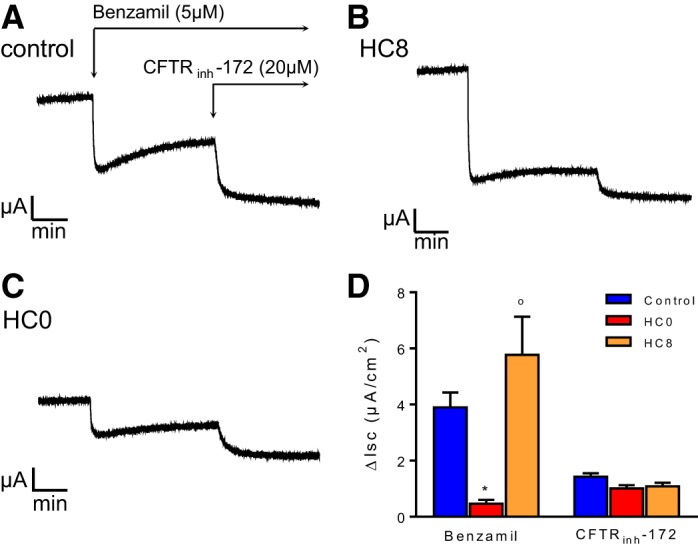
Day 8 HC0 cells have reduced benzamil-sensitive current but normal CFTRinh-172-sensitive current compared with HC-treated controls. A–C: representative short-circuit current (Isc) traces of day 8 differentiated monolayers treated with 5 μM benzamil, a selective blocker of epithelial Na+ channels (ENaC), and 20 μM CFTRinh-172, a selective CFTR blocker. D: summary of Isc results in A–C (n = 6 for each condition). *Significant difference between HC0 and control; °significant difference between HC0 and HC8 (by ANOVA followed by Tukey-Kramer multiple-comparisons test).
Fig. 5.
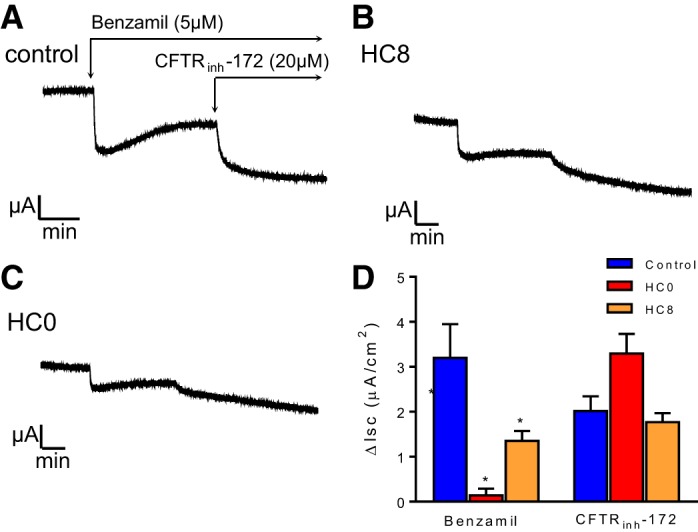
Day 24 HC0 and HC8 cells have reduced total and benzamil-sensitive currents compared with control. A–C: representative Isc traces of day 24 differentiated monolayers treated with 5 μM benzamil and 20 μM CFTRinh-172. D: summary of Isc results in A–C (n = 6 for each condition). *Significant difference between HC0 and control; °significant difference between HC0 and HC8 (by ANOVA followed by Tukey-Kramer multiple-comparisons test).
RESULTS
NHBE cells differentiate into a ciliated-pseudostratified epithelium.
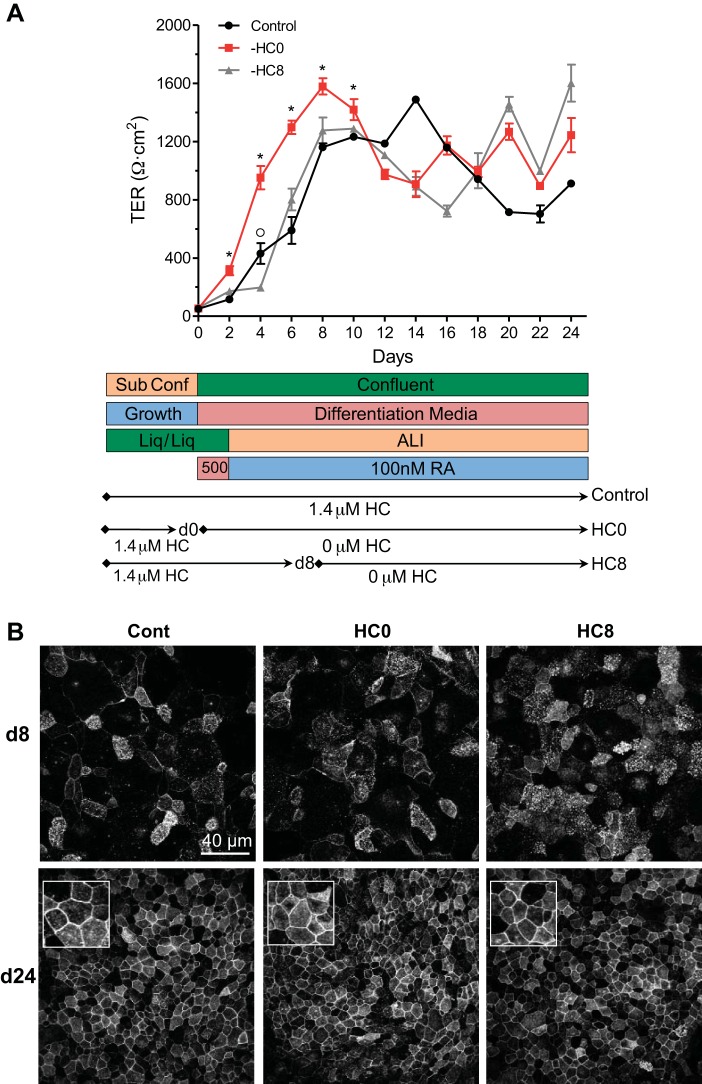
NHBE cells were expanded and then differentiated on polyester Snapwell membranes according to the protocol described in materials and methods. The cells were maintained under liquid-liquid conditions in complete BEGM until they reached confluence (day 0). HC (1.4 μM), supplied in the BEGM SingleQuots, was withdrawn from HC0 and HC8 cells at days 0 and 8, respectively. Apical medium was withdrawn from control, HC0, and HC8 cells at day 2, and cells were maintained under ALI conditions for the duration of the experiment. TER was measured every-other day (Fig. 1A). Increases in TER at days 2–10 were greater in HC0 than control and HC8 cells. Under all three conditions, the mean TER value calculated using measurements from all days after day 8 exceeded 1,000 Ω·cm2: 1,055 ± 85.9, 1,161 ± 79.8, and 1,149 ± 93.5 (SE) Ω·cm2 for control, HC0, and HC8, respectively, from day 8 to day 24. Tight junction formation was visualized by immunocytochemistry targeting the cytoskeletal scaffolding protein ZO1 (74). As shown in Fig. 1B, ZO1 was localized at the lateral borders of control and HC0 cells at day 24, indicating mature tight junction structures.
Fig. 1.
Normal human bronchial epithelial (NHBE) cells develop tight junctions and form electrically tight monolayers during differentiation. A: NHBE cells grown under standard differentiation conditions develop and maintain transepithelial resistances (TERs) over a 24-day period. Top: increases in TERs were greater at earlier time points in cells from which hydrocortisone (HC) was withdrawn at day 0 (HC0 cells) than in control cells and cells from which HC was withdrawn at day 8 (HC8 cells). *Significant difference between HC0 and control/HC8; °significant difference between control and HC8 (by ANOVA followed by a Tukey-Kramer multiple-comparisons test; n ≥ 6 for each time point). Bottom: differentiation conditions. ALI, air-liquid interface; RA, retinoic acid. B: localization of zona occludens 1 (ZO1) immunofluorescence along the basolateral membrane in control (Cont), HC0, and HC8 cells. Insets: 30 × 30 μm images of day 24 monolayers.
We used quantitative RT-PCR to examine changes in expression of specific mRNAs associated with differentiation of bronchial basal cells. The basal cell transcription factor p63 (Fig. 2A) was expressed at the highest level in control cells at day 0 but exhibited a significant reduction in expression at days 4, 8, and 24. More p63 was detected in HC0 and HC8 cells at days 8 and 24 than on the corresponding days in control monolayers, indicating a role for HC in the expression of p63. Transcription of cytokeratin 6a (KRT6a; Fig. 2B), a type II intermediate-filament protein highly expressed in the airway basal cells (27), also showed HC-dependent downregulation in differentiated control cells. However, KRT6a mRNA expression at day 24 was greater in HC0 and HC8 than control cells (CT = 25.4 ± 0.8, 24.3 ± 0.5, and 28.7 ± 0.3, respectively). These results suggest that differentiation in the presence of HC reduced the abundance of basal cell markers within the epithelium but that basal cells are still present within differentiated, pseudostratified monolayers on day 24.
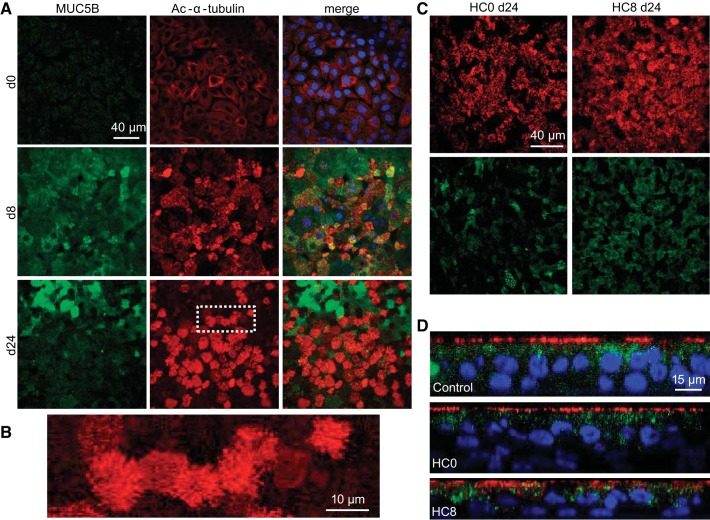
Next, the transcription of genes associated with airway surface cells was investigated. The F-box factor FoxJ1 (Fig. 2C), a master transcription factor of ciliagenesis (76), as well as mucins [Muc5ac (Fig. 2D) and Muc5b (Fig. 2E)], were not detected at day 0 but were observed at days 4, 8, and 24 in control cells, indicating the presence of ciliated and secretory surface cells in differentiated monolayers. This was confirmed by immunocytochemistry (Fig. 3, A and B). Higher levels of FoxJ1 mRNA were expressed in HC0 and HC8 than control monolayers at day 24 (CT = 28.8 ± 0.5, 28.3 ± 0.5, and 31.5 ± 0.3, respectively). Muc5ac was not detected in HC0 cells at day 24. Interestingly, transcription of Muc5b showed HC-dependent upregulation in control cells; however, this does not necessarily indicate increased protein expression or mucus secretion in HC-treated monolayers. HC withdrawal (HC8) cells exhibited reduced expression of Muc5ac and Muc5b at days 8 and 24 compared with control, similar to HC0 cells (Fig. 2E). Moreover, detection of Muc5b by immunocytochemistry at the apical surface of day 24 differentiated HC0 monolayers was reduced compared with control and HC8 cells (Fig. 3, C and D). The secretoglobin protein uteroglobin, or Clara cell 10 protein (SCGB1A1; Fig. 2F), also showed robust HC-dependent expression during differentiation in the presence of HC but significantly reduced expression at days 8 and 24 in HC0 cells and at day 24 in HC8 monolayers. This result suggests an increase in Clara cell abundance within control monolayers during differentiation that required HC for sustained expression.
Fig. 3.
Immunocytochemistry of differentiated NHBE cells reveals a pseudostratified epithelium with ciliated and mucin-containing cells. A: NHBE control monolayers contained surface cells expressing Muc5b (green) and acetylated α-tubulin (red), characteristic of cilia during differentiation. Nuclei (blue) were labeled with 4′,6-diamidino-2-phenylindole (DAPI). B: enlarged image identified in the white box of day 24 acetylated (Ac) α-tubulin-labeled cilia (in A) on the apical surface of differentiated monolayers. C: HC0 and HC8 monolayers contained ciliated and Muc5b-expressing cells. D: orthogonal views of differentiated cells showed pseudostratification and apical localization of acetylated α-tubulin.
HC-sensitive Na+ and anion transport in differentiated NHBE cells.
Differentiated NHBE monolayers were mounted in Ussing chambers for investigation of ion transport function by characterization of ENaC- and CFTR-dependent transport pathways. Total and benzamil-sensitive Isc was statistically greater in day 8 control and HC8 cells than HC0 monolayers, reflecting a greater level of basal Na+ absorption (Fig. 4). HC had no effect on the magnitude of the CFTRinh-172-sensitive Isc, indicating that the level of basal anion secretion was unaffected by the absence of HC. Day 24 HC8 and HC0 monolayers displayed a similar phenotype (Fig. 5). Total and benzamil-sensitive currents were significantly reduced in HC0 and HC8 cells compared with control.
Immunocytochemistry revealed the localization of ENaCα and Na+-K+-ATPase α1-subunit (ATP1A1) at the apical and basolateral membranes, respectively, of differentiated NHBE monolayers (Fig. 6A). ENaCα localized at cilia-like structures on surface cells of control monolayers. Surface ENaCα was reduced in HC0 cells. ENaCα was detected just below the apical membrane in the same focal plane as the Na+-K+-ATPase α1-subunit in HC0 and HC8 cells, implicating a role for HC in trafficking of ENaC to the apical surface and into the cilia through insertion of subapical membrane vesicles. Furthermore, quantitative RT-PCR analysis of ENaCα and ENaCγ mRNAs displayed HC-dependent increases at day 8 (Fig. 6, B and D). HC did not alter the localization of the Na+-K+-ATPase at the basolateral membrane, as shown in Fig. 6A. However, ATP1A1 mRNA was reduced in the absence of HC but showed no change in HC8 cells compared with control (Fig. 6E), indicating that the initial 8-day exposure to HC was sufficient to augment expression of the Na+-K+-ATPase α1-subunit.
Earlier studies demonstrated that ENaCα and ENaCγ are subject to cleavage by proteases that enable complete activation of the channel (33). Therefore, we examined whether glucocorticoid exposure was involved in regulating the expression of known proteases involved in ENaC processing. Our results showed an increase in mRNA expression of furin and channel-activating protease 1 (CAP1) during differentiation in control compared with HC0 monolayers at days 8 and 24, although mRNA for both enzymes was still expressed at high levels in HC0 cells (Fig. 6, F and G). However, similar levels of the 37-kDa NH2-terminal cleavage product of ENaCα was observed in control and HC0 monolayers at days 8 and 24 (Fig. 6H) (43). Serum and glucocorticoid-regulated kinase 1 (SGK1) mRNA, a known modulator of surface ENaC expression, was significantly reduced in HC0 monolayers at days 4 and 8 compared with control cells (Fig. 6H). ATP1A1 protein was observed at days 8 and 24 in HC0 and control cells (Fig. 6H).
Anion secretion increases following β2-AR stimulation.
Signaling pathways coupled to ion transport in airway surface epithelial cells are integral to mucociliary clearance. We used the short-acting selective β2-agonist salbutamol to investigate the role of HC in development of normal β2-AR regulation of CFTR-dependent anion secretion. Previous experiments (Figs. 4 and 5) demonstrated that the transport phenotype of differentiated NHBE monolayers treated with HC was similar at days 8 and 24. Therefore, day 16 monolayers were pretreated with benzamil before stimulation with 10 μM salbutamol. Treatment with CFTRinh-172 after salbutamol stimulation inhibited the Isc in control, HC0, and HC8 monolayers (Fig. 7, A and B). The increase in Isc was less after addition of salbutamol in HC0 and HC8 than control monolayers (Fig. 7B). Quantitative RT-PCR analysis of β2-AR (ADRB2) transcription revealed HC-dependent expression at days 8 and 24 (Fig. 7C). CFTR mRNA expression showed a time-dependent decrease in HC0 cells, while HC8 expression remained similar to control, at day 24 (Fig. 7D). Western blot analysis also revealed a time-dependent decrease in total CFTR abundance in HC0 cells. However, ADBR2 protein abundance did not appear to be reduced in the absence of HC. Immunocytochemistry results in Fig. 8, A and B, show colocalization of the β2-AR and CFTR in cilia-like structures of control monolayers but less distinct cilia localization in HC0 and HC8 monolayers.
To confirm that the salbutamol-activated Isc was dependent on functional CFTR expressed in the apical membrane, human Δ508 CF bronchial epithelial (CFBE) cells were differentiated according to the protocol used for control NHBE cells. CFBE monolayers displayed a reduced benzamil-sensitive current compared with control (1.04 ± 0.16 μA) and were unresponsive to salbutamol as well as 8-CPT-cAMP, a membrane-permeable analog of cAMP known to activate CFTR-dependent anion secretion (Fig. 8C). As a positive control, CFBE cells were subsequently stimulated with a P2Y2 receptor agonist (UTP) known to activate Ca2+-dependent Cl− secretion in airway epithelial cells (45). Although CFTR-dependent anion secretion could not be activated by agents that increase cAMP, CFBE monolayers did exhibit UTP-dependent increases in Isc that could be blocked by DIDS, a disulfonic stilbene compound that inhibits Ca2+-dependent Cl− channels (51). CFBE monolayers also appeared to exhibit colocalization of the β2-AR with CFTR (Fig. 8D), although more extensive coimmunoprecipitation experiments would be needed to confirm this interpretation. However, CFTR expression was not localized in cilia-like structures of ΔF508 monolayers, appearing instead within the perinuclear region below the apical membrane.
DISCUSSION
In vitro differentiation of NHBE cells under ALI conditions provides a unique opportunity to study signaling molecules that regulate the development of a physiologically responsive, pseudostratified epithelium. The technique has been employed by many groups to study the effects of cigarette smoke, pathogenesis of influenza infection, and bronchial wound healing and as a model for airway drug transport (18, 25, 44, 48, 69). The precise culture conditions used to differentiate bronchial basal cells into a monolayer containing secretory and ciliated cells are varied. Addition of RA to culture medium is a common practice that promotes pseudostratification and suppresses keratinizing squamous differentiation (15, 24, 50). Moreover, nearly all NHBE cell culture media contain glucocorticoids, but the rationale for supplementation is poorly understood. Corticosteroids are well-studied modulators of multiple processes across a variety of tissues, but their role in the differentiation of airway basal cells into a tissue capable of transepithelial salt and water transport has not been systematically investigated.
Ciliated and secretory cells were detected at day 24 in both HC0 and HC8 cells, and they did not appear to be different from control. However, HC did reduce transcription of mRNAs associated with secretory cells. Relative expression of Muc5ac, Muc5b, and SCGB1A1 was reduced in the absence of HC. Previous studies have reported suppression of goblet cell abundance in the presence of glucocorticoids (40, 58). Although not necessarily indicative of surface cell-type abundance, our data suggest increased transcription of mucin proteins in the presence of HC. One possible explanation may involve HC-dependent downregulation of basal cells in parallel with an increase in surface cell expression, as indicated by reductions in the levels of p63 and KRT6a and increased expression of surface cell markers. HC0 monolayers expressed greater relative amounts of p63 and KRT6a at later time points than control cells, which may be indicative of basal cell proliferation, potentially leading to squamous metaplasia (3).
We also identified a role for HC in determining the magnitude of basal benzamil-sensitive Na+ absorption in well-differentiated NHBE cells. Reduced Na+ absorption was observed at days 8 and 24 in NHBE cells differentiated in the absence of HC (HC0). Furthermore, surface expression of ENaCα was reduced at day 24, although the α-subunit was detected by immunocytochemistry just below the apical membrane, suggesting localization within subapical membrane vesicles. In contrast, cells differentiated in the presence of HC exhibited ENaCα localization within cilia-like structures, consistent with previous results showing localization of ENaC within motile cilia of native airways, as well as two-pore K+-selective (K2P) channels in cilia of differentiated human bronchial epithelial cells (22, 77). Additionally, withdrawal of HC after 8 days of differentiation (HC8) resulted in a similarly reduced benzamil-sensitive Isc at day 24, although unlike HC0 monolayers, ENaCα expression within the apical membrane was comparable to that in monolayers that were differentiated in the continuous presence of HC. One potential explanation for the reduced levels of Na+ transport associated with HC0 monolayers is an effect of HC on mRNA expression of ENaC subunits. Glucocorticoids have been previously shown to directly regulate ENaC transcription, and in this study, HC-dependent regulation of ENaCα and ENaCγ, but not ENaCβ, mRNA was observed when HC was removed at the start of the differentiation protocol. Earlier studies demonstrated dexamethasone- and HC-dependent upregulation of all three ENaC subunits in human middle ear (39) and human mammary epithelial (11) cells, respectively, as well as dexamethasone, but not aldosterone, regulation of ENaCβ and ENaCγ mRNA expression in bovine mammary epithelial cells (56). Interestingly, dexamethasone did not stimulate an increase in ENaCα or ENaCγ protein expression in CFBE41o− cells, an immortalized human bronchial epithelial cell line stably expressing wild-type CFTR or the ΔF508 CFTR mutation (63).
Furthermore, HC could also regulate ENaC function by altering the expression of enzymes involved in proteolytic processing of the channel. Previous investigations have shown that ENaCα and ENaCγ are subject to proteolytic cleavage, resulting in functionally mature Na+ channels (33). Multiple proteases have been reported to mediate ENaC processing, and we hypothesized a role of HC in regulating the expression of these enzymes based on previous reports showing that aldosterone directly controls the expression of prostasin, also known as CAP1. Our results demonstrate that removal of HC prior to the start of differentiation reduces mRNA expression of two proteases known to regulate ENaC processing: furin, which cleaves the extracellular loop of ENaCα and ENaCγ, and CAP1, which cleaves the extracellular loop of ENaCγ (12). Although we observed a reduction in ENaCα mRNA in the absence of HC, we detected similar total abundance of cleaved ENaCα by Western blotting, indicating that a mechanism other than transcription is responsible for the reduced benzamil-sensitive current in HC0 and HC8 monolayers.
The reduced ENaC protein expression within the apical membrane of HC0 monolayers supports a role for HC in the trafficking of channel subunits to the apical surface. ENaC retrieval from the plasma membrane is known to be regulated by the ubiquitin-protein ligase Nedd4-2. Nedd4-2 activity is controlled by SGK1. SGK1-mediated phosphorylation of Nedd4-2 reduces its interaction with ENaC, leading to an increase in channel density in the apical membrane (20, 37). Therefore, in this study we tested the hypothesis that removal of HC from the medium at the start of differentiation reduces SGK1 mRNA expression, which in turn reduces ENaC surface density. SGK1 mRNA expression was reduced in the absence of HC compared with control and HC8 monolayers, consistent with previous studies on HC stimulation of ENaC surface expression through regulation of the SGK1-Nedd4-2-ENaC axis in middle ear epithelial cells (39).
In addition to regulation of ENaC function, the mineralocorticoid hormone aldosterone has been shown to directly stimulate Na+-K+-ATPase activity and transcription in distal nephron and collecting duct epithelial cells, resulting in increased Na+ absorption and water retention (64). Similarly, dexamethasone has been shown to regulate Na+-K+-ATPase activity in corneal endothelial cells and to increase transcription of the β1-subunit of the enzyme in renal carcinoma cells (31, 34). Therefore, we investigated whether HC was involved in regulating ATP1A1 localization and expression during differentiation of NHBE cells. Our results demonstrate reduced transcription of ATP1A1 mRNA in the absence of HC but no effect on membrane localization of the protein. The reduced expression and protein abundance of the α1-subunit of the pump were consistent with the decrease in ENaC mRNA and surface expression of ENaCα subunits in HC0 monolayers and may have contributed to the lower rate of basal Na+ transport. Interestingly, once differentiation was initiated and HC exposure sustained for 8 days (HC8 monolayers), withdrawal of HC for the next 16 days did not reduce ATP1A1 mRNA expression. A similar effect was observed for ENaCα, ENaCβ, and ENaCγ mRNA expression in HC8 monolayers, supporting continuous expression of ENaC subunits within the apical membrane at day 24. Transcriptional regulation by HC involves histone modifications, which may have longer-lasting effects than the HC exposure and may explain sustained gene transcription of ENaC and ATP1A1 after HC withdrawal (5). Curiously, despite the sustained expression of ENaC and ATP1A1 mRNA subunits and their appropriate membrane localization, basal Na+ transport in HC8 monolayers at day 24 was reduced to a level similar to that observed in HC0 monolayers. At this time, the mechanistic basis for the reduction in basal ENaC-dependent Isc is unknown, but one possible explanation could be a decrease in basolateral K+ conductance, which would reduce the driving force for transepithelial Na+ absorption.
Previous studies have suggested that ICS potentiates LABA efficacy by enhancing the functional response to β2-AR agonists (2, 52). In the present study, quantitative RT-PCR results indicate that HC plays a role in regulating the transcription of CFTR mRNA. Although the basal CFTRinh-172-sensitive Isc was not affected by HC, salbutamol-stimulated currents did show differences between control and HC-deficient conditions at day 16. The increase in Isc following addition of salbutamol was blocked by the addition of CFTRinh-172 and was not observed in ΔF508 CFTR-expressing monolayers, which lacked functional CFTR. Earlier studies showed that CFTR forms a signaling complex with β2-ARs, and here we show colocalization of CFTR and β2-AR in NHBE cells and in ΔF508 CFTR-expressing monolayers. Although this protein-protein interaction between CFTR and the β2-AR appeared to show no dependence on HC, the relative abundance of β2-AR mRNA was HC-dependent, such that, in the absence of HC, mRNA transcription was significantly reduced, although β2-AR protein expression was sustained at levels comparable to control conditions. Glucocorticoid effects on β2-ARs in airway epithelial cells and other tissues have been reported (2, 14, 16). These studies suggest a role for glucocorticoids as a permissive hormone in regulating the expression and density of β2-ARs in plasma membranes, including airway smooth muscle cells (28, 47). Furthermore, glucocorticoids have been reported to protect airway smooth muscle from proasthmatic effects of LABAs through indirect suppression of phosphodiesterase E4 (52). Although basal CFTR currents were not different between control and HC0 conditions, salbutamol-induced CFTR currents were significantly lower in HC0 and HC8 cells than control monolayers. The reduced Isc response in HC0 cells may have been due to the decrease in β2-AR expression; however, mRNA levels in HC8 cells were comparable to those in control monolayers, so other mechanisms are likely to be involved in these cells.
Summary and conclusions.
The key findings of this study that relate to the effects of glucocorticoids during differentiation include 1) enhanced mRNA expression of ENaCα, ENaCβ, and ENaCγ, 2) coordinated upregulation of ENaC-processing (furin and CAP1) enzymes, as well as SGK1, 3) increased expression of CFTR and β2-ARs, 4) colocalization of ENaC, CFTR, and β2-ARs within the cilia of surface cells, 5) increased Na+-K+-ATPase α1-subunit expression, and 6) increased CFTR-dependent anion secretion evoked by stimulation with β2-AR agonists. These results support the conclusion that glucocorticoid treatment facilitates normal differentiation and development of ion transport protein expression and function critical for mucociliary clearance. Furthermore, glucocorticoid exposure during differentiation appears to be necessary for the expression of apical β2-ARs that regulate CFTR-dependent anion secretion in response to inhaled β2-agonists. Thus the combined clinical use of glucocorticoids and LABAs for the treatment of asthma and other inflammatory airway diseases would be expected to promote epithelial restitution and mucociliary clearance necessary for maintaining mucosal barrier function and innate defense against airway pathogens. Inclusion of glucocorticoids in media used for differentiating airway progenitor cells may also be necessary for producing a fully functional pseudostratified airway epithelium for use in the bioengineering of human airways and perhaps, ultimately, intact human lungs.
GRANTS
This work was supported by National Institute of Biomedical Imaging and Bioengineering Grant F31 EB-018707 (N. A. Zaidman) and National Heart, Lung, and Blood Institute Grants R01 HL-108627 (A. Panoskaltsis-Mortari) and R01 HL-110539 (S. M. O'Grady).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.A.Z., A.P.-M., and S.M.O. developed the concept and designed the research; N.A.Z. performed the experiments; N.A.Z. analyzed the data; N.A.Z., A.P.-M., and S.M.O. interpreted the results of the experiments; N.A.Z. prepared the figures; N.A.Z. drafted the manuscript; N.A.Z., A.P.-M., and S.M.O. edited and revised the manuscript; A.P.-M. and S.M.O. approved the final version of the manuscript.
REFERENCES
- 1.Adler KB, Tuvim MJ, Dickey BF. Regulated mucin secretion from airway epithelial cells. Front Endocrinol (Lausanne) 4: 129, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aksoy MO, Mardini IA, Yang Y, Bin W, Zhou S, Kelsen SG. Glucocorticoid effects on the β-adrenergic receptor-adenylyl cyclase system of human airway epithelium. J Allergy Clin Immunol 109: 491–497, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Araya J, Cambier S, Markovics JA, Wolters P, Jablons D, Hill A, Finkbeiner W, Jones K, Broaddus VC, Sheppard D, Barzcak A, Xiao Y, Erle DJ, Nishimura SL. Squamous metaplasia amplifies pathologic epithelial-mesenchymal interactions in COPD patients. J Clin Invest 117: 3551–3562, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Astrand AB, Hemmerling M, Root J, Wingren C, Pesic J, Johansson E, Garland AL, Ghosh A, Tarran R. Linking increased airway hydration, ciliary beating, and mucociliary clearance through ENaC inhibition. Am J Physiol Lung Cell Mol Physiol 308: L22–L32, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes PJ. Corticosteroid effects on cell signalling. Eur Respir J 27: 413–426, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Barnes PJ. Drugs for asthma. Br J Pharmacol 147 Suppl 1: S297–S303, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger WE, Nadel JA. Efficacy and safety of formoterol for the treatment of chronic obstructive pulmonary disease. Respir Med 102: 173–188, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Billington CK, Ojo OO, Penn RB, Ito S. cAMP regulation of airway smooth muscle function. Pulm Pharmacol Ther 26: 112–120, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blouquit-Laye S, Chinet T. Ion and liquid transport across the bronchiolar epithelium. Respir Physiol Neurobiol 159: 278–282, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Boucher RC. Airway surface dehydration in cystic fibrosis: pathogenesis and therapy. Annu Rev Med 58: 157–170, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Boyd C, Naray-Fejes-Toth A. Steroid-mediated regulation of the epithelial sodium channel subunits in mammary epithelial cells. Endocrinology 148: 3958–3967, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Bruns JB, Carattino MD, Sheng S, Maarouf AB, Weisz OA, Pilewski JM, Hughey RP, Kleyman TR. Epithelial Na+ channels are fully activated by furin- and prostasin-dependent release of an inhibitory peptide from the γ-subunit. J Biol Chem 282: 6153–6160, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Camner P, Mossberg B, Philipson K. Tracheobronchial clearance and chronic obstructive lung disease. Scand J Respir Dis 54: 272–281, 1973. [PubMed] [Google Scholar]
- 14.Cheng JB, Goldfien A, Ballard PL, Roberts JM. Glucocorticoids increase pulmonary β-adrenergic receptors in fetal rabbit. Endocrinology 107: 1646–1648, 1980. [DOI] [PubMed] [Google Scholar]
- 15.Choi JY, Cho KN, Yoo KH, Shin JH, Yoon JH. Retinoic acid depletion induces keratinizing squamous differentiation in human middle ear epithelial cell cultures. Acta Otolaryngol (Stockh) 123: 466–470, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Collins S, Caron MG, Lefkowitz RJ. β-Adrenergic receptors in hamster smooth muscle cells are transcriptionally regulated by glucocorticoids. J Biol Chem 263: 9067–9070, 1988. [PubMed] [Google Scholar]
- 17.Davies DE. Epithelial barrier function and immunity in asthma. Ann Am Thorac Soc 11 Suppl 5: S244–S251, 2014. [DOI] [PubMed] [Google Scholar]
- 18.Davis AS, Chertow DS, Moyer JE, Suzich J, Sandouk A, Dorward DW, Logun C, Shelhamer JH, Taubenberger JK. Validation of normal human bronchial epithelial cells as a model for influenza A infections in human distal trachea. J Histochem Cytochem 63: 312–328, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Kluijver J, Schrumpf JA, Evertse CE, Sont JK, Roughley PJ, Rabe KF, Hiemstra PS, Mauad T, Sterk PJ. Bronchial matrix and inflammation respond to inhaled steroids despite ongoing allergen exposure in asthma. Clin Exp Allergy 35: 1361–1369, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Debonneville C, Flores SY, Kamynina E, Plant PJ, Tauxe C, Thomas MA, Munster C, Chraibi A, Pratt JH, Horisberger JD, Pearce D, Loffing J, Staub O. Phosphorylation of Nedd4-2 by Sgk1 regulates epithelial Na+ channel cell surface expression. EMBO J 20: 7052–7059, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ehre C, Ridley C, Thornton DJ. Cystic fibrosis: an inherited disease affecting mucin-producing organs. Int J Biochem Cell Biol 52: 136–145, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Enuka Y, Hanukoglu I, Edelheit O, Vaknine H, Hanukoglu A. Epithelial sodium channels (ENaC) are uniformly distributed on motile cilia in the oviduct and the respiratory airways. Histochem Cell Biol 137: 339–353, 2012. [DOI] [PubMed] [Google Scholar]
- 23.Foster WM, Langenback EG, Bergofsky EH. Disassociation in the mucociliary function of central and peripheral airways of asymptomatic smokers. Am Rev Respir Dis 132: 633–639, 1985. [DOI] [PubMed] [Google Scholar]
- 24.Fulcher ML, Gabriel S, Burns KA, Yankaskas JR, Randell SH. Well-differentiated human airway epithelial cell cultures. Methods Mol Med 107: 183–206, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Ghio AJ, Dailey LA, Soukup JM, Stonehuerner J, Richards JH, Devlin RB. Growth of human bronchial epithelial cells at an air-liquid interface alters the response to particle exposure. Part Fibre Toxicol 10: 25, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gras D, Chanez P, Vachier I, Petit A, Bourdin A. Bronchial epithelium as a target for innovative treatments in asthma. Pharmacol Ther 140: 290–305, 2013. [DOI] [PubMed] [Google Scholar]
- 27.Hackett NR, Shaykhiev R, Walters MS, Wang R, Zwick RK, Ferris B, Witover B, Salit J, Crystal RG. The human airway epithelial basal cell transcriptome. PLos One 6: e18378, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hadcock JR, Malbon CC. Regulation of β-adrenergic receptors by “permissive” hormones: glucocorticoids increase steady-state levels of receptor mRNA. Proc Natl Acad Sci USA 85: 8415–8419, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hajj R, Baranek T, Le Naour R, Lesimple P, Puchelle E, Coraux C. Basal cells of the human adult airway surface epithelium retain transit-amplifying cell properties. Stem Cells 25: 139–148, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Hamid Q. Effects of steroids on inflammation and cytokine gene expression in airway inflammation. J Allergy Clin Immunol 112: 636–638, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Hatou S, Yamada M, Mochizuki H, Shiraishi A, Joko T, Nishida T. The effects of dexamethasone on the Na,K-ATPase activity and pump function of corneal endothelial cells. Curr Eye Res 34: 347–354, 2009. [DOI] [PubMed] [Google Scholar]
- 32.Hollenhorst MI, Richter K, Fronius M. Ion transport by pulmonary epithelia. J Biomed Biotechnol 2011: 174306, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hughey RP, Mueller GM, Bruns JB, Kinlough CL, Poland PA, Harkleroad KL, Carattino MD, Kleyman TR. Maturation of the epithelial Na+ channel involves proteolytic processing of the α- and γ-subunits. J Biol Chem 278: 37073–37082, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Huynh TP, Barwe SP, Lee SJ, McSpadden R, Franco OE, Hayward SW, Damoiseaux R, Grubbs SS, Petrelli NJ, Rajasekaran AK. Glucocorticoids suppress renal cell carcinoma progression by enhancing Na,K-ATPase β1-subunit expression. PLos One 10: e0122442, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ingram JL, Kraft M. IL-13 in asthma and allergic disease: asthma phenotypes and targeted therapies. J Allergy Clin Immunol 130: 829–842, 2012. [DOI] [PubMed] [Google Scholar]
- 36.Kagoshima M, Ito K, Cosio B, Adcock IM. Glucocorticoid suppression of nuclear factor-κB: a role for histone modifications. Biochem Soc Trans 31: 60–65, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Kamynina E, Staub O. Concerted action of ENaC, Nedd4-2, and Sgk1 in transepithelial Na+ transport. Am J Physiol Renal Physiol 283: F377–F387, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Kato A, Schleimer RP. Beyond inflammation: airway epithelial cells are at the interface of innate and adaptive immunity. Curr Opin Immunol 19: 711–720, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim BG, Kim JY, Kim M, Kim CH, Choi JY, Kim SH. Gene regulation by glucocorticoid in ENaC-mediated Na+ transport by middle ear epithelial cells. Laryngoscope 124: E27–E33, 2014. [DOI] [PubMed] [Google Scholar]
- 40.Kim EY, Kim J, Morton JD, Swanson S, Patel A, Castro M, Holtzman MJ. Glucocorticoids suppress goblet cell metaplasia in natural and experimental asthma. J Allergy Clin Immunol 113: S116, 2004. [Google Scholar]
- 41.Kreda SM, Okada SF, van Heusden CA, O'Neal W, Gabriel S, Abdullah L, Davis CW, Boucher RC, Lazarowski ER. Coordinated release of nucleotides and mucin from human airway epithelial Calu-3 cells. J Physiol 584: 245–259, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laitinen A, Altraja A, Kampe M, Linden M, Virtanen I, Laitinen LA. Tenascin is increased in airway basement membrane of asthmatics and decreased by an inhaled steroid. Am J Respir Crit Care Med 156: 951–958, 1997. [DOI] [PubMed] [Google Scholar]
- 43.Lazrak A, Jurkuvenaite A, Chen L, Keeling KM, Collawn JF, Bedwell DM, Matalon S. Enhancement of alveolar epithelial sodium channel activity with decreased cystic fibrosis transmembrane conductance regulator expression in mouse lung. Am J Physiol Lung Cell Mol Physiol 301: L557–L567, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin H, Li H, Cho HJ, Bian S, Roh HJ, Lee MK, Kim JS, Chung SJ, Shim CK, Kim DD. Air-liquid interface (ALI) culture of human bronchial epithelial cell monolayers as an in vitro model for airway drug transport studies. J Pharm Sci 96: 341–350, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Mall M, Gonska T, Thomas J, Schreiber R, Seydewitz HH, Kuehr J, Brandis M, Kunzelmann K. Modulation of Ca2+-activated Cl− secretion by basolateral K+ channels in human normal and cystic fibrosis airway epithelia. Pediatr Res 53: 608–618, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Mall MA. Role of cilia, mucus, and airway surface liquid in mucociliary dysfunction: lessons from mouse models. J Aerosol Med Pulm Drug Deliv 21: 13–24, 2008. [DOI] [PubMed] [Google Scholar]
- 47.Mano K, Akbarzadeh A, Townley RG. Effect of hydrocortisone on β-adrenergic receptors in lung membranes. Life Sci 25: 1925–1930, 1979. [DOI] [PubMed] [Google Scholar]
- 48.Mathis C, Poussin C, Weisensee D, Gebel S, Hengstermann A, Sewer A, Belcastro V, Xiang Y, Ansari S, Wagner S, Hoeng J, Peitsch MC. Human bronchial epithelial cells exposed in vitro to cigarette smoke at the air-liquid interface resemble bronchial epithelium from human smokers. Am J Physiol Lung Cell Mol Physiol 304: L489–L503, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mezey RJ, Cohn MA, Fernandez RJ, Januszkiewicz AJ, Wanner A. Mucociliary transport in allergic patients with antigen-induced bronchospasm. Am Rev Respir Dis 118: 677–684, 1978. [DOI] [PubMed] [Google Scholar]
- 50.Million K, Tournier F, Houcine O, Ancian P, Reichert U, Marano F. Effects of retinoic acid receptor-selective agonists on human nasal epithelial cell differentiation. Am J Respir Cell Mol Biol 25: 744–750, 2001. [DOI] [PubMed] [Google Scholar]
- 51.Mitchell C, Syed NI, Gurney AM, Kennedy C. A Ca2+-dependent chloride current and Ca2+ influx via Cav1.2 ion channels play major roles in P2Y receptor-mediated pulmonary vasoconstriction. Br J Pharmacol 166: 1503–1512, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nino G, Hu A, Grunstein JS, Grunstein MM. Mechanism of glucocorticoid protection of airway smooth muscle from proasthmatic effects of long-acting β2-adrenoceptor agonist exposure. J Allergy Clin Immunol 125: 1020–1027, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parker D, Prince A. Innate immunity in the respiratory epithelium. Am J Respir Cell Mol Biol 45: 189–201, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Postma DS, Kerstjens HA. Characteristics of airway hyperresponsiveness in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 158: S187–S192, 1998. [DOI] [PubMed] [Google Scholar]
- 55.Puchelle E, Zahm JM, Tournier JM, Coraux C. Airway epithelial repair, regeneration, and remodeling after injury in chronic obstructive pulmonary disease. Proc Am Thorac Soc 3: 726–733, 2006. [DOI] [PubMed] [Google Scholar]
- 56.Quesnell RR, Han X, Schultz BD. Glucocorticoids stimulate ENaC upregulation in bovine mammary epithelium. Am J Physiol Cell Physiol 292: C1739–C1745, 2007. [DOI] [PubMed] [Google Scholar]
- 57.Rackley CR, Stripp BR. Building and maintaining the epithelium of the lung. J Clin Invest 122: 2724–2730, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reber LL, Daubeuf F, Plantinga M, De Cauwer L, Gerlo S, Waelput W, Van Calenbergh S, Tavernier J, Haegeman G, Lambrecht BN, Frossard N, De Bosscher K. A dissociated glucocorticoid receptor modulator reduces airway hyperresponsiveness and inflammation in a mouse model of asthma. J Immunol 188: 3478–3487, 2012. [DOI] [PubMed] [Google Scholar]
- 59.Rock JR, Hogan BL. Epithelial progenitor cells in lung development, maintenance, repair, and disease. Annu Rev Cell Dev Biol 27: 493–512, 2011. [DOI] [PubMed] [Google Scholar]
- 60.Rock JR, Randell SH, Hogan BL. Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis Model Mech 3: 545–556, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ross AJ, Dailey LA, Brighton LE, Devlin RB. Transcriptional profiling of mucociliary differentiation in human airway epithelial cells. Am J Respir Cell Mol Biol 37: 169–185, 2007. [DOI] [PubMed] [Google Scholar]
- 62.Rossi A, Khirani S, Cazzola M. Long-acting β2-agonists (LABA) in chronic obstructive pulmonary disease: efficacy and safety. Int J Chron Obstruct Pulmon Dis 3: 521–529, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rubenstein RC, Lockwood SR, Lide E, Bauer R, Suaud L, Grumbach Y. Regulation of endogenous ENaC functional expression by CFTR and ΔF508-CFTR in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 300: L88–L101, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salyer SA, Parks J, Barati MT, Lederer ED, Clark BJ, Klein JD, Khundmiri SJ. Aldosterone regulates Na+,K+ ATPase activity in human renal proximal tubule cells through mineralocorticoid receptor. Biochim Biophys Acta 1833: 2143–2152, 2013. [DOI] [PubMed] [Google Scholar]
- 65.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3: 1101–1108, 2008. [DOI] [PubMed] [Google Scholar]
- 66.Schuliga M. NF-κB signaling in chronic inflammatory airway disease. Biomolecules 5: 1266–1283, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sears PR, Yin WN, Ostrowski LE. Continuous mucociliary transport by primary human airway epithelial cells in vitro. Am J Physiol Lung Cell Mol Physiol 309: L99–L108, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stannard W, O'Callaghan C. Ciliary function and the role of cilia in clearance. J Aerosol Med 19: 110–115, 2006. [DOI] [PubMed] [Google Scholar]
- 69.Stewart CE, Torr EE, Mohd Jamili NH, Bosquillon C, Sayers I. Evaluation of differentiated human bronchial epithelial cell culture systems for asthma research. J Allergy (Cairo) 2012: 943982, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tagaya E, Tamaoki J. Mechanisms of airway remodeling in asthma. Allergol Int 56: 331–340, 2007. [DOI] [PubMed] [Google Scholar]
- 71.Tarran R, Trout L, Donaldson SH, Boucher RC. Soluble mediators, not cilia, determine airway surface liquid volume in normal and cystic fibrosis superficial airway epithelia. J Gen Physiol 127: 591–604, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tashkin DP, Rennard SI, Martin P, Ramachandran S, Martin UJ, Silkoff PE, Goldman M. Efficacy and safety of budesonide and formoterol in one pressurized metered-dose inhaler in patients with moderate to very severe chronic obstructive pulmonary disease: results of a 6-month randomized clinical trial. Drugs 68: 1975–2000, 2008. [DOI] [PubMed] [Google Scholar]
- 73.Tilley AE, Walters MS, Shaykhiev R, Crystal RG. Cilia dysfunction in lung disease. Annu Rev Physiol 77: 379–406, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wan H, Winton HL, Soeller C, Stewart GA, Thompson PJ, Gruenert DC, Cannell MB, Garrod DR, Robinson C. Tight junction properties of the immortalized human bronchial epithelial cell lines Calu-3 and 16HBE14o. Eur Respir J 15: 1058–1068, 2000. [DOI] [PubMed] [Google Scholar]
- 75.Westergaard CG, Porsbjerg C, Backer V. Emerging corticosteroid agonists for the treatment of asthma. Expert Opin Emerg Drugs 20: 653–662, 2015. [DOI] [PubMed] [Google Scholar]
- 76.You Y, Huang T, Richer EJ, Schmidt JE, Zabner J, Borok Z, Brody SL. Role of f-box factor foxj1 in differentiation of ciliated airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 286: L650–L657, 2004. [DOI] [PubMed] [Google Scholar]
- 77.Zhao KQ, Xiong G, Wilber M, Cohen NA, Kreindler JL. A role for two-pore K+ channels in modulating Na+ absorption and Cl− secretion in normal human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 302: L4–L12, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]