Abstract
Introduction:
The objective of this study was to assess bias in the body mass index (BMI) measure in the Canadian Maternity Experiences Survey (MES) and possible implications of bias on the relationship between BMI and selected pregnancy outcomes.
Methods:
We assessed BMI classification based on self-reported versus measured values. We used a random sample of 6175 women from the MES, which derived BMI from self-reported height and weight, and a random sample of 259 women who had previously given birth from the Canadian Health Measures Survey (CHMS), which derived BMI from self-reported and measured height and weight. Two correction equations were applied to self-reported based BMI, and the impact of these corrections on associations between BMI and caesarean section, small-for-gestational age (SGA) and large-for-gestational age (LGA) births was studied.
Results:
Overall, 86.9% of the CHMS subsample was classified into the same BMI category based on self-reported versus measured data. However, misclassification had a substantial effect on the proportion of women in underweight and obese BMI categories. For example, 14.5% versus 20.8% of women were classified as obese based on self-reported data versus measured data. Corrections improved estimates of obesity prevalence, but overand underestimated other BMI categories. Corrections had nonsignificant effects on the associations between BMI and SGA, LGA, and caesarean section.
Conclusion:
While there was high concordance in BMI classification based on selfreported versus measured height and weight, bias in self-reported based measures may slightly over- or underestimate the risks associated with a particular BMI class. However, the general trend in associations is unaffected.
Keywords: body mass index, self-reported prepregnancy weight, self-reported height, reproductive outcomes, validity
Highlights
Bias in measurement of body mass index (BMI) may have implications on the association between BMI and some pregnancy outcomes, such as caesarean, small-for-gestational age and large-for-gestational age births.
The authors assessed BMI classification based on self-reported versus measured values using random samples of women from the Maternity Experiences Survey and from the Canadian Health Measures Survey.
Discrepancies in the proportion of women in BMI categories were highest for women classified as being underweight or obese based on self-reported height and weight, but overall, there was high concordance between BMI classes based on self-reported versus measured data.
BMI derived from self-reported data appears to be a justifiable and reasonable way to identify overall trends in the association between prepregnancy BMI and pregnancy outcomes.
Introduction
Maternal prepregnancy body mass index (BMI) is an important predictor of certain pregnancy outcomes. Both high and low BMI are associated with increased risks of adverse outcomes for the mother and child, such as caesarean section, small-for-gestational age (SGA) and large-for-gestational age (LGA) births.1-3
Population-level BMI information is often derived from self-reports of height and weight. Past research has demonstrated that such self-reported data tend to overestimate height and underestimate weight, resulting in an underestimation of overweight and obesity (BMI ≥ 25.0 kg/m2).4-6 Directly measured data provide more accurate information but are expensive to collect. Consequently, self-reported data will continue to be a source of prepregnancy BMI information. This makes it important to understand the magnitude and impact of any bias in these data.
The Maternity Experiences Survey (MES), conducted in 2006–2007, gathered information from a nationally representative sample of women who had given birth in Canada in 2005–2006.7 These data included self-reported height and prepregnancy weight; these were used to derive prepregnancy BMI in this population and study its associations with adverse pregnancy outcomes. In 2007–2009, the Canadian Health Measures Survey (CHMS) collected self-reported and measured height and weight for a national representative sample of Canadians, including females of reproductive age.8 Although CHMS data cannot be directly linked to the MES, their availability for a similar period as MES data provides an opportunity to examine a subset of the population comparable to that from which the MES was drawn, to estimate the degree of bias in MES BMI data and determine possible implications of this bias on well-established relationships between prepregnancy BMI and selected pregnancy outcomes.
Methods
Data
The 2006–2007 MES was a cross-sectional survey of a stratified random sample of 6421 women who had a singleton live birth in Canada in 2005–2006 (5–13 months prior to data collection).7 We focussed on a subset of 6175 respondents aged 18 to 44 years whose prepregnancy BMI data, derived from self-reported height and weight, were available. Each MES record was weighted, so this subset represents a population of 74 000 women.
The 2007–2009 CHMS was the first cycle of a national survey of physical health measures collected through in-person interviews and direct measurement. It captured data on height and weight, along with many other determinants of health. It included 259 women who were aged 18 to 44 years old, had ever had a live birth, had complete data on two measures of BMI, one based on self-reported height and weight and the other on measured height and weight, were not currently pregnant and had a child younger than 5 years old in their household.
Like the MES, each CHMS record was weighted, representing a population of 1 386 500 women. The sampling weights in both the MES and the CHMS took into consideration the sample design and non-response; they were calculated within weighting classes, which generally corresponded to the strata used to draw the sample. Detailed information on the development, methodology (including sample design and weighting) and content of both surveys has been reported elsewhere.7,8
Analysis
The MES and CHMS samples were compared across variables common to both datasets and identified in other studies as associated with BMI bias, namely maternal BMI based on self-reported height and weight, age and education.9,10 Ethnicity was categorized differently in the two surveys and could not be compared. BMI was categorized according to the World Health Organization standard as underweight (BMI < 18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2) or obese (BMI ≥ 30.0 kg/m2).11 Using our CHMS subsample, we assessed the magnitude and direction of bias by cross-tabulating the BMI values based on self-reported and measured height and weight.
We applied two correction equations (Box 1) to the BMI based on self-reported values to derive corrected BMI distributions. The first equation was derived by Connor Gorber et al.9 based on data for adult women in the 2005 Canadian Community Health Survey (CCHS). This “reduced model” equation is a simple linear regression of BMI derived from measured data on BMI derived from self-reported data as more complex models (i.e. models with covariates or nonlinear models) provided no predictive advantage.* Although Connor Gorber et al.9 derived correction equations separately for men and women, only an overall sample size for both sexes was reported (n = 2029), suggesting that the reduced model was derived using a subsample of approximately 1000 women.
The value of using the Connor Gorber et al.9 correction equation is that it had been validated against more complex equations and found to have the same corrective value. In addition, the 2005 period for the CCHS was similar to the prepregnancy time period for MES women whose babies were born in 2005–2006.7 However, because this equation was for all adult women regardless of whether they had ever given birth, we derived a second correction equation using the same methods but based on the CHMS (described earlier) subsample of 259 women who had been selected as most similar to the MES population, namely women aged 18 to 44 years who had ever had a live birth and had a child younger than 5 years in the household (see Box 1).
Finally, using rate ratios (RRs), the associations between prepregnancy BMI and three adverse pregnancy outcomes—caesarean delivery, SGA and LGA—were compared for uncorrected and corrected BMI distributions in the MES. SGA was defined as weight below the 10th percentile for gestational age and LGA as weight above the 90th percentile for gestational age.12
All analyses were carried out using sampling weights. We computed results from unrounded weighted components; however, weighted sample sizes were rounded to the nearest hundred, according to Statistics Canada reporting guidelines, as unrounded estimates overstate precision. With the exception of the overall subsample sizes, unweighted counts are not reported to be consistent with Statistics Canada’s disclosure control standards. We calculated 95% confidence intervals using the bootstrap method, which accounts for the variability introduced by the sample design and weighting adjustments.13 In addition, because the CHMS subsample was small and its survey design complex, using a normal approximation to the binomial distribution was not appropriate, particularly for small proportions. As a result, we applied a logit transformation to all CHMS-based analysis.14 The logit transformation interval was obtained by constructing a t-distribution-based Wald interval for the logit transformation of the proportion (p), and transforming the limits back to the original scale. It is based on the assumption that log (p^/(1−p^)) is approximately normal. Logit transformation was not needed for MES-based analyses because of the larger size of the MES sample and simpler sample design. All analyses were carried out using SAS Enterprise Guide version 5.1 (SAS Institute, Cary, NC, USA).
Box 1. Correction equations for BMI derived from self-reported data
Correction 1. Regression coefficients calculated from the 2005 CCHS for a subsample of adult women (≥ 18 years), regardless of birth history (unweighted n ≈ 1000).9
Corrected BMI = −0.12 + 1.05 × BMI derived from self-reported height and weight
Correction 2. Regression coefficients calculated from the 2007–2009 CHMS subsample of women (18–44 years) who had ever had a live birth, with a child younger than 5 years in the household (unweighted n = 259 women)
Corrected BMI = −0.44 + 1.05 × BMI derived from self-reported height and weight
Results
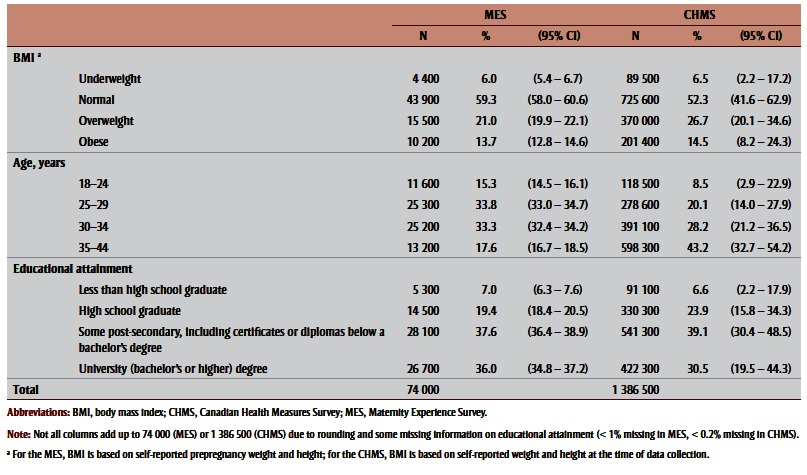
Except when noted, all results are weighted estimates. Table 1 shows maternal BMI derived from self-reported height and weight, age and education distributions in the MES and CHMS subsamples. Only the age distribution varied substantially: the CHMS sample was older, with 43.2% aged 35 to 44 years old compared to 17.6% of the MES sample. A higher proportion of CHMS women reported being overweight or obese and a smaller proportion reported university education; however, these differences were more moderate.
Table 1. Distribution of body mass index, maternal age and educational attainment among mothers aged 18–44 years, in the 2006–2007 Maternity Experience Survey, and women aged 18–44 years with a child aged less than 5 years in the household in the 2007–2009 Canadian Health Measures Survey.
 |
When BMIs based on self-reported and measured values were compared in the CHMS sample, 14.5% of the self-reported sample was classified as obese versus 20.8% of the measured sample (Table 2, columns A and B). Most of this misclassification was because 23.6% of the women classified as overweight based on selfreported data were classified as obese based on measured data (Table 3). A similar degree of misclassification was observed among women classified as underweight based on self-reported data, with 24.5% of these women actually having normal BMIs. The degree of misclassification was lower among women whose BMI corresponded with normal weight or obese BMI. Overall, 86.9% of the CHMS subsample was classified into the same BMI category based on self-reported versus measured data.
Table 2. Distribution of body mass index derived from measured and self-reported height and weight, and after applying two correction equations, 2007–2009 Canadian Health Measures Survey, subsample of women aged 18–44 years with a child less than 5 years in the household.
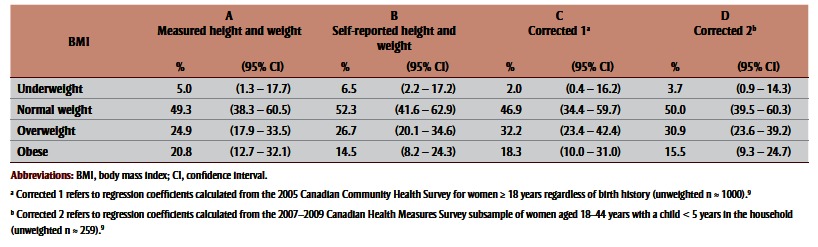 |
Table 3. Classification of body mass index (BMI) derived from self-reported versus measured height and weight, 2007–2009 Canadian Health Measures Survey, subsample of women aged 18–44 years with a child less than 5 years in the household.
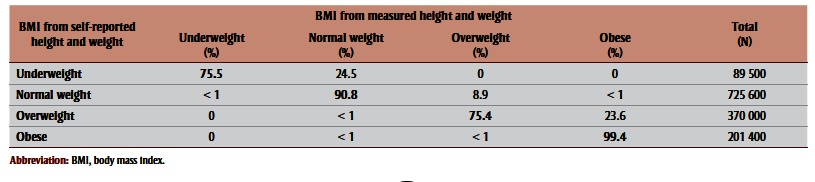 |
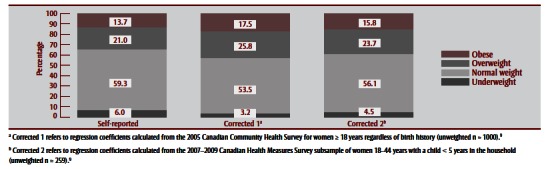
Table 2 (columns C and D) and Table 4 show corrected BMI distributions after applying the two correction equations (Box 1) to BMI derived from self-reported height and weight in the CHMS and MES. Comparing BMI values based on measured data to corrected values in the CHMS subsample indicated that Correction 1 (derived from the CCHS subsample of adult women) was better at correcting for the underestimation of obesity than Correction 2 (derived from the CHMS subsample of women aged 18–44 years who had ever had a live birth, with a child younger than 5 years in the household). However, Correction 2 was better at correcting the prevalence of other BMI categories. Though both corrected BMI distributions improved estimates of obesity prevalence, both resulted in overestimation of overweight and underestimation of underweight prevalence. Applying correction equations to MES data had a similar effect on the BMI distribution in that subsample (Table 4). Figure 1 and Figure 2 illustrate BMI distributions before and after corrections were applied to the CHMS and MES subsamples, respectively.
Table 4. Distribution of prepregnancy body mass index based on self-reported height and weight and after applying two correction equations, 2006–2007 Maternity Experience Survey, subsample of women aged 18–44 years.
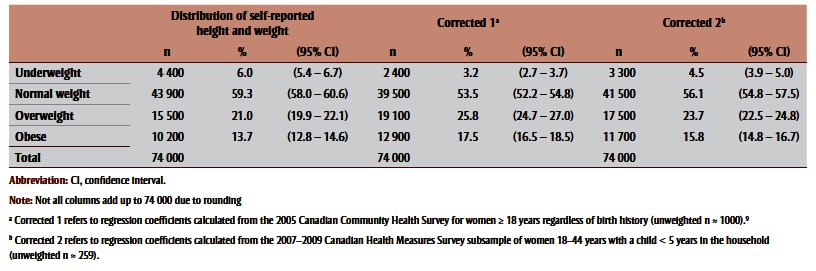 |
FIGURE 1. Body mass index distribution derived from measured height and weight, self-reported height and weight and after applying two correction equations, 2007–2009 Canadian Health Measures Survey, subsample of women aged 18–44 years with a child less than 5 years in the household.
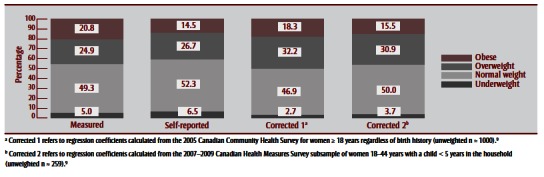
FIGURE 2. Prepregnancy body mass index distribution derived from self-reported height and prepregnancy weight and after applying two correction equations, 2006–2007 Maternity Experiences Survey, subsample of women aged 18–44 years.
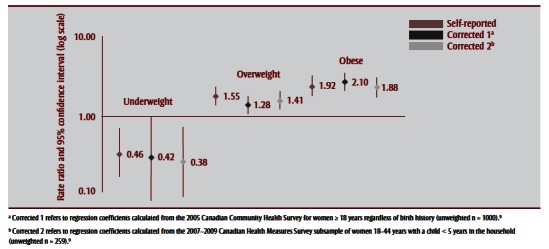
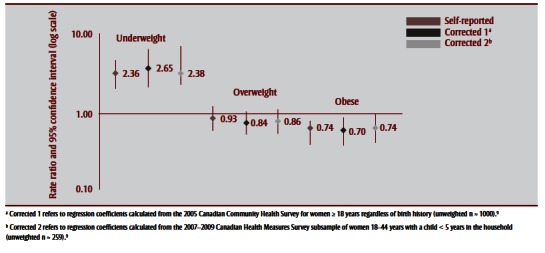
Observed rates and corresponding RRs of uncorrected and corrected BMI distributions to caesarean, SGA and LGA births are shown in Table 5 and Figure 3a –Figure 3c . Corrections had a negligible effect on the association between prepregnancy BMI and caesarean birth; nonsignificant increases were observed for associations with SGA and nonsignificant decreases for associations with LGA. For SGA, both corrections increased its association with being underweight, from an RR of 2.36 (95% CI: 1.67–3.34) to 2.65 (95% CI: 1.74–4.01) for Correction 1 and 2.83 (95% CI: 1.94–4.11) for Correction 2. For LGA, corrections generally decreased the association with being overweight or obese, for overweight prepregnancy BMI from an RR of 1.55 (95% CI: 1.27–1.89) to 1.28 (95% CI:1.05–1.56) following Correction 1 and 1.41 (95% CI:1.16–1.72) following Correction 2, and for obese prepregnancy BMI from an RR of 1.92 (95% CI: 1.54– 2.39) to 2.10 (95% CI: 1.72–2.58) following Correction 1 and 1.88 (95% CI: 1.52–2.31) following Correction 2.
Table 5. Association between adverse outcomes and prepregnancy body mass index based on self-reported height and weight and after applying two correction equations, 2006–2007 Maternity Experiences Survey, subsample of women aged 18–44 years.
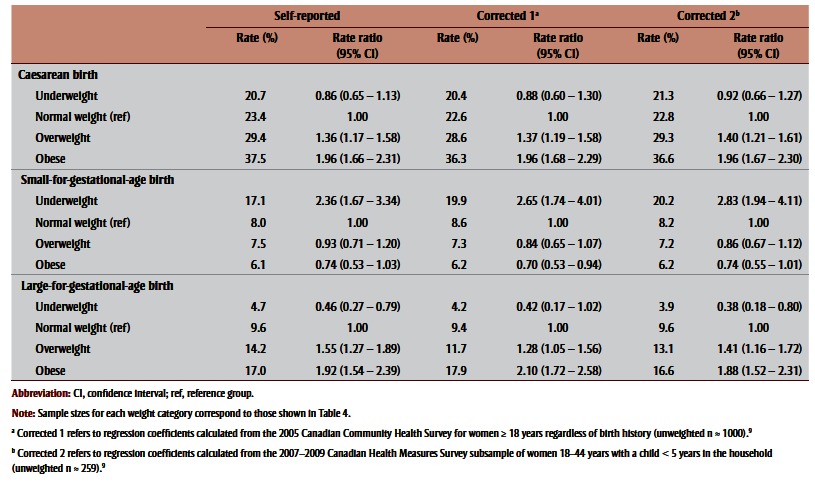 |
FIGURE 3a. Association between caesarean births and prepregnancy body mass index based on self-reported height and prepregnancy weight and after applying two correction equations, 2006–2007 Maternity Experiences Survey, subsample of women aged 18–44 years.
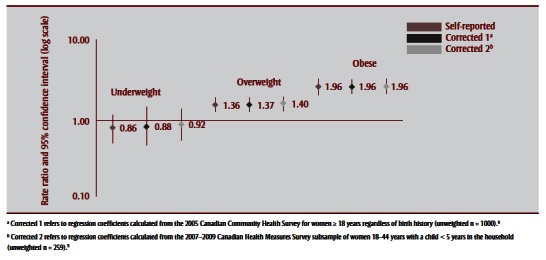
FIGURE 3c. Association between large-for-gestational age births and prepregnancy body mass index based on self-reported height and weight and after applying two correction equations, 2006–2007 Maternity Experiences Survey, subsample of women aged 18–44 years.
Discussion
It is well established that low and high prepregnancy BMI are associated with adverse pregnancy outcomes.1-3 However, the potential impact of biased BMI measures on these associations has not been studied in Canada.
We found overall high concordance between BMI classification based on selfreported and measured data in the CHMS subsample, though misclassification had a substantial effect on the proportion of women in underweight and obese BMI categories. Transformations to correct for possible misclassification in data on BMI in the MES resulted in variable nonsignificant changes in the associations between prepregnancy BMI and pregnancy outcomes.
The high concordance between BMI classes based on self-reported and measured values in our CHMS sample is consistent with results from other studies of adult women.10,15,16 Although we cannot ascribe these findings to the MES, the Connor Gorber et al.9 study found that the age of Canadian women did not significantly influence bias in BMI measures, but that lower education and self-reporting a height and weight combination that indicated overweight was associated with underestimating BMI.9 In comparison with the CHMS, women in the MES were younger and appeared more educated and less overweight. This suggests that the tendency to underestimate BMI due to erroneous self-reported height and weight may be lower in the MES than in the CHMS. Younger age and lower weight among MES participants compared to CHMS women was expected, as MES data refer to the prepregnancy period while CHMS data refer to postpregnancy; women will be younger and generally weigh less prepregnancy than postpregnancy.17
In reproductive health, the extremes of the BMI distribution pose the greatest risk of adverse pregnancy outcomes. We observed that associations based on both reported and corrected prepregnancy BMI showed well-established dose-response patterns of underweight BMI increasing the risk for SGA birth, and overweight and obese BMI increasing the risk for LGA and caesarean birth.1-3 However, there were no statistically significant changes in these patterns after corrections were applied to the MES data. This suggests that selfreported height and weight measures can be reliably used to study patterns of association between prepregnancy BMI and certain pregnancy outcomes.
The lack of significant impact of bias on these associations is encouraging as it suggests that collecting self-reported height and prepregnancy weight when direct measurement is not feasible is justifiable. Not only could such data capture the health effects of prepregnancy BMI, they could also be used to assess the effectiveness of public health programs that promote healthy prepregnancy weight. Accurate monitoring and evaluation of population weights and intervention outcomes is an essential component in tackling the complex issue of unhealthy population weights.18
Although we observed no significant changes in the pattern of associations after correcting the MES data, the impact of bias on the BMI–outcome association could vary depending on the direction of the bias and the nature of the BMI–outcome relationship. Consequently, associations could be under- or overestimated. The nonsignificant increase in association between the underweight category of BMI and SGA after correction likely resulted from those in the underweight category being the most underweight and therefore at most risk of an SGA birth. The tendency towards a decrease in the association between overweight and obese and LGA likely resulted from women in these categories having lower BMIs than those classified as overweight or obese based on self-reported height and weight. These women were thus at lower risk of having an LGA birth. Note that even if BMI based on self-reported height and weight overestimates the obese BMI-LGA association, the population burden of LGA due to obesity may still be underestimated because obesity derived from self-reported height and weight is underestimated.
A study on the impact of bias in selfreported gestational weight gain on low and high birthweight had findings similar to ours;19 another study on the impact of bias in prepregnancy BMI on five pregnancy outcomes (including SGA and LGA)20 found that associations were not significantly impacted, though reporting error attenuated associations. Studies of BMI bias and other outcomes, such as weight-related chronic diseases (e.g. diabetes and high blood pressure) also found that associations can be underestimated21 or overestimated.22 The negligible effect of adjustment on the BMI-caesarean section pattern suggests that other risks for caesarean are more dominant and independent of prepregnancy BMI.
Strengths and limitations
Our study has several limitations. Due to the absence of measured height and weight in the MES, we used data from a CHMS subsample of women aged 18 to 44 years who had ever had a live birth and had a child younger than 5 years in their household, to estimate BMI bias in the MES. Other studies have shown that the validity of such transportability is increased when equations are derived from the same population and in a similar time period.23-25 We used available parameters in the CHMS (e.g. age and history of a live birth) to obtain the most suitable comparison group. Nevertheless, our populations were not exactly the same. Therefore, bias in the two populations may not be exactly the same, though the nature of BMI bias has been found to be similar in prepregnant women and the general population of adult women.26,27
We applied the same correction across BMI categories despite evidence of differential bias in categories. Though this differential bias suggests that category-specific corrections may be more appropriate, more complex correction models that take the BMI category and other covariates into consideration (e.g. models based on polynomial or spline regression) have not shown that they produced corrections in reporting error significantly better than this simpler approach.9
In addition, analysis of bias by BMI categories does not allow for an unrestricted assessment of the relationship between bias and BMI derived from self-reported data, and the consequent impact of this bias on the BMI–outcome association. However, the BMI categorization we used is well established and has public health and clinical relevance.
FIGURE 3b. Association between small-for-gestational age births and prepregnancy body mass index based on self-reported height and prepregnancy weight and after applying two correction equations, 2006–2007 Maternity Experiences Survey, subsample of women aged 18–44 years.
Conclusion
While the level of concordance between BMI classification derived from selfreported and measured data among women of reproductive age is high, possible bias in BMI derived from self-reported data may slightly overor underestimate BMI associations, depending on the pregnancy outcome. Nonetheless, BMI derived from self-reported data appears to be a justifiable and reasonable way to identify overall trends in the association between prepregnancy BMI and certain pregnancy outcomes.
Footnotes
References
- Marshall NE, Spong CY. Obesity, pregnancy complications, and birth outcomes. Semin Reprod Med. 2012;30((6)):465–71. doi: 10.1055/s-0032-1328874. http://dx.doi.org/10.1055/s-0032-1328874. [DOI] [PubMed] [Google Scholar]
- O’Reilly, Reynolds RM. The risk of maternal obesity to the long-term health of the offspring. Clin Endocrinol (Oxf) 2013;78((1)):9–16. doi: 10.1111/cen.12055. http://dx.doi.org/10.1111/cen.12055. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan U, Grant F, Goldenberg T, Zongrone A, Martorell R. Effect of women’s nutrition before and during early pregnancy on maternal and infant outcomes: a systematic review. Paediatr Perinat Epidemiol. 2012;26((Suppl 1)):285–301. doi: 10.1111/j.1365-3016.2012.01281.x. http://dx.doi.org/10.1111/j.1365-3016.2012.01281.x. [DOI] [PubMed] [Google Scholar]
- Shields M, Connor Gorber S, Janssen I, Tremblay MS. Bias in self-reported estimates of obesity in Canadian health surveys: an update on correction equations for adults. Health Rep. 2011;22((3)):1–11. [PubMed] [Google Scholar]
- Merrill RM, Richardson JS. Validity of self-reported height, weight, and body mass index: findings from the National Health and Nutrition Examination Survey, 2001-2006. Prev Chronic Dis. 2009;6((4)):A121. [PMC free article] [PubMed] [Google Scholar]
- Elgar FJ, Stewart JM. Validity of self-report screening of overweight and obesity evidence from the Canadian Community Health Survey. Can J Public Health. 2008;99((5)):423–7. doi: 10.1007/BF03405254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzakpasu S, Kaczorowski J, Chalmers B, et al. The Canadian Maternity Experiences Survey: design and methods. J Obstet Gynaecol Can. 2008;30((3)):207–16. doi: 10.1016/S1701-2163(16)32757-8. http://dx.doi.org/10.1016/s1701-2163(16)32757-8. [DOI] [PubMed] [Google Scholar]
- Statistics Canada. Ottawa (ON):: [updated 2014; cited 2015 Mar 3]. Canadian Health Measures Survey (CHMS) [Internet]. . Available from: http://www23.statcan.gc.ca/imdb/p2SV.pl?Function=getSurvey&SDDS=5071. [Google Scholar]
- Connor Gorber SC, Shields M, Tremblay MS, McDowell I. The feasibility of establishing correction factors to adjust self-reported estimates of obesity. Health Rep. 2008;19((3)):71–82. [PubMed] [Google Scholar]
- Bruner Huber LR. Validity of self-reported height and weight in women of reproductive age. Matern Child Health J. 2007;11((2)):137–44. doi: 10.1007/s10995-006-0157-0. http://dx.doi.org/10.1007/s10995-006-0157-0. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Copenhagen (DE):: [cited 2015 Nov 16]. Body mass index – BMI [Internet]. . Available from: http://www. eur o.who.int/en/health-topics/ disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi. [Google Scholar]
- Kramer MS, Platt RW, Wen SW, et al. A new and improved population-based Canadian reference for birth weight for gestational age. Pediatrics. 2001;108((2)):e35. doi: 10.1542/peds.108.2.e35. http://dx.doi.org/10.1542/peds.108.2.e35. [DOI] [PubMed] [Google Scholar]
- Rao JN, Wu CF, Yue K. Some recent work on resampling methods for complex surveys. Surv Methodol. 1992;18:209–17. [Google Scholar]
- Korn EL, Graubard BI. Confidence intervals for proportions with small expected number of positive counts estimated from survey data. Surv Methodol. 1998;24:193–201. [Google Scholar]
- Spencer EA, Appleby PN, Davey GK, Key TJ. Validity of self-reported height and weight in 4808 EPIC-Oxford participants. Public Health Nutr. 2002;5((4)):561–5. doi: 10.1079/PHN2001322. http://dx.doi.org/10.1079/PHN2001322. [DOI] [PubMed] [Google Scholar]
- Bolton-Smith C, Woodward M, Tunstall- Pedoe H, Morrison C. Accuracy of the estimated prevalence of obesity from self-reported height and weight in an adult Scottish population. J Epidemiol Community Health. 2000;54((2)):143–8. doi: 10.1136/jech.54.2.143. http://dx.doi.org/10.1136/jech.54.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomew S, Heaman M. Public Health Agency of Canada; Ottawa (ON): 2009. Maternal body mass index and weight gain during pregnancy. In: What mothers say: the Canadian Maternity Experiences Survey. pp. 74–8. [Google Scholar]
- The Lancet. Urgently needed: a framework convention for obesity control. Lancet. 2011;378((9793)):741. doi: 10.1016/S0140-6736(11)61356-1. http://dx.doi.org/10.1016/S0140-6736(11)61356-1. [DOI] [PubMed] [Google Scholar]
- Schieve LA, Perry GS, Cogswell ME, et al. Validity of self-reported pregnancy delivery weight: an analysis of the 1988 National Maternal and Infant Health Survey. Am J Epidemiol. 1999;150((9)):947–56. doi: 10.1093/oxfordjournals.aje.a010103. [DOI] [PubMed] [Google Scholar]
- Bodnar LM, Siega-Riz AM, Simhan HN, Diesel JC, Abrams B. The impact of exposure misclassification on associations between prepregnancy BMI and adverse pregnancy outcomes. Obesity. 2010;18:2184–90. doi: 10.1038/oby.2010.25. http://dx.doi.org/10.1038/oby.2010.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith SW, Fontaine KR, Pajewski NM, Mehta T, Allison DB. Use of self-reported height and weight biases the body mass index-mortality association. Int J Obes. 2011;35((3)):401–8. doi: 10.1038/ijo.2010.148. http://dx.doi.org/10.1038/ijo.2010.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields M, Gorber SC, Tremblay MS. Effects of measurement on obesity and morbidity. Health Rep. 2008;19((2)):77–84. [PubMed] [Google Scholar]
- Ellert U, Brettschneider AM, Wiegand S, Kurth BM. Applying a correction procedure to the prevalence estimates of overweight and obesity in the German part of the HBSC study. BMC Res Notes. 2014;7:181. doi: 10.1186/1756-0500-7-181. http://dx.doi.org/10.1186/1756-0500-7-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visscher TL, Viet AL, Kroesbergen IH, Seidell JC. Underreporting of BMI in adults and its effect on obesity prevalence estimations in the period 1998 to 2001. Obesity. 2006;14((11)):2054–63. doi: 10.1038/oby.2006.240. http://dx.doi.org/10.1038/oby.2006.240. [DOI] [PubMed] [Google Scholar]
- Ezzati M, Martin H, Skjold S, Vander Hoorn S, Murray CJ. Trends in national and state-level obesity in the USA after correction for self-report bias: analysis of health surveys. J R Soc Med. 2006;99((5)):250–7. doi: 10.1258/jrsm.99.5.250. http://dx.doi.org/10.1258/jrsm.99.5.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SM, Nagey DA. Validity of self-reported pregravid weight. Ann Epidemiol. 1992;2((5)):715–21. doi: 10.1016/1047-2797(92)90016-j. http://dx.doi.org/10.1016/1047-2797(92)90016-j. [DOI] [PubMed] [Google Scholar]
- Shields M, Connor Gorber SC, Tremblay MS. Estimates of obesity based on self-report versus direct measures. Health Reports. 2008;19((2)):61–76. [PubMed] [Google Scholar]


