Abstract
Background
Altered reward circuitry function is observed in individuals with bipolar disorder (BD) and their unaffected offspring (OBP). While OBP are at elevated risk for BD, modifiable risk factors that may exacerbate neural vulnerabilities in OBP remain under-characterized. As sleep loss is strongly linked to mania in BD, this study tested associations between sleep duration, reward circuitry function, and mood dysregulation in OBP.
Methods
Two groups of youth unaffected with BD (9-17yr) completed a number-guessing fMRI reward paradigm: 25 OBP and 21 age-sex-IQ-matched offspring of control parents with non-BD psychopathology (OCP), to differentiate risk for BD from risk for psychopathology more broadly. Regressions tested effects of group status, self-reported past-week sleep duration, and their interaction on neural activity and bilateral ventral striatum(VS) functional connectivity to win>control. Correlations with parent-reported mood dysregulation were assessed.
Results
Group effects were observed for right posterior insula activity (OCP>OBP) and VS-left posterior insula connectivity (OBP>OCP). Group*sleep duration interactions were observed for left dorsal anterior-mid-cingulate(daMCC) activity and VS-left anterior insula/ventrolateral prefrontal cortex(VLPFC) connectivity. Specifically, sleep duration and daMCC activity were positively related in OBP, but negatively related in OCP and sleep duration and VS-left anterior insula/VLPFC connectivity were negatively related in OBP, but positively in OCP. Additionally, increased VS-left posterior insula connectivity and VS-left anterior insula/VLPFC connectivity were associated with greater mood dysregulation in OBP only.
Limitations
Cross-sectional design and small sample size.
Conclusions
Altered reward-related VS-insula connectivity could represent a neural pathway underpinning mood dysregulation in OBP, and may be modulated by shortened sleep duration.
Keywords: Bipolar disorder risk, sleep, reward processing, functional MRI
Introduction
Characterizing the neurobehavioral processes that predispose youth to bipolar disorder (BD) is vital for preventing the disorder and improving treatment outcome. Neuroimaging studies have identified functional abnormalities within neural circuitry supporting information processing domains known to be disturbed in BD, such as reward processing (Phillips and Swartz, 2014). As parental history of BD is one of the most robust risk factors for developing BD (Birmaher et al., 2009), recent neuroimaging studies have focused on examining reward-related neural circuitry in offspring of bipolar parents (OBP), and have yielded valuable insights into neural processes that may predispose to BD (Manelis et al., 2016, Singh et al., 2014). Yet, while OBP are at elevated risk for BD, many do not develop the disorder. Thus, it is necessary to begin characterizing potentially modifiable risk factors that may interact with familial risk to amplify neural vulnerability for BD. The present study focuses on sleep duration as one such risk factor, as sleep loss has been linked to both the development of mania (Plante and Winkelman, 2008) and altered reward circuitry function (Hasler et al., 2015).
Reward processing in humans is supported by a complex prefrontal-subcortical neural network (Haber and Knutson, 2010; Liu et al., 2011), which includes the ventral striatum (VS), orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), medial prefrontal cortex (mPFC), ventrolateral prefrontal cortex (VLPFC), and insula. Neuroimaging studies in BD patients have observed functional abnormalities during reward processing relative to healthy controls, namely elevated neural activity within the ventromedial PFC (vmPFC), OFC, VLPFC, and VS (for review see Nusslock et al. 2014) and reduced negative VS-VLPFC functional connectivity (Trost et al., 2014). Two recent fMRI studies have also observed functional abnormalities during reward tasks in OBP. In one report, elevated negative bilateral VS-right VLPFC connectivity to win and loss trials (vs. control trials) distinguished OBP from both offspring of healthy parents and offspring of parents with non-BD disorders (Manelis et al., 2016). Another study observed elevated left VLPFC activation and reduced pregenual ACC-VLPFC connectivity during reward processing in healthy OBP relative to healthy control youths (Singh et al., 2014). VS and insula activation to reward receipt were also positively related to impulsivity in OBP (Singh et al., 2014). Together, these data indicate that both BD patients and OBP exhibit elevated reward-related neural activity and altered functional connectivity among ventral prefrontal cortical-striatal regions (vmPFC, OFC, VLPFC, VS) and insula.
A separate line of work has demonstrated a key role of sleep disruption in BD (Plante and Winkelman, 2008). Disturbed sleep is a predictor of BD onset in at-risk samples (Levenson et al., 2015; Ritter et al., 2011), it is prospectively linked to worsening symptoms of mania and depression in youth with BD (Lunsford-Avery et al., 2012), and it is the top prodromal symptom of mania in adult BD (Jackson et al., 2003). Sleep loss, or short sleep duration, may have particular importance to the emergence of mood dysregulation characteristic of BD. Reduced sleep need is a unique (American Psychiatric Association, 2001) and highly prevalent (for review see Harvey, 2008) feature of mania, experimental sleep deprivation can trigger (hypo)mania in BD patients (e.g., Barbini et al., 1998; Colombo et al., 1999), and sleep loss predicts subsequent manic symptoms (Bauer et al., 2006). Links between shortened sleep duration and mood disturbance have also been observed in community samples and across affective disorders more broadly (e.g., Barnes and Meldrum, 2015; Nixon et al., 2008; Raniti et al., 2016; Sivertsen et al., 2014; Zohar et al., 2005).
Disturbed sleep, and sleep loss in particular, has also been tied to altered reward circuitry function in healthy samples. In healthy adolescents, correlational studies of sleep and neural response to monetary rewards have linked shorter habitual sleep duration with decreased VS activity (Holm et al., 2009); more variable sleep timing with reduced dorsal mPFC (dmPFC)/ACC and VS activity (Hasler et al., 2012); and poorer sleep quality with increased insula activity and decreased lateral PFC-insula connectivity (Telzer et al., 2013). Adult studies using acute sleep deprivation paradigms have reported increased VS, vmPFC, and OFC activity (Mullin et al., 2013; Venkatraman et al., 2007; Venkatraman et al., 2011a), and reduced dmPFC/ACC deactivation (Mullin et al., 2013), in response to monetary reward. A study examining the rewarding effects of pleasure-evoking images observed that acute sleep deprivation biased young adults toward more positive appraisals of the images, and led to elevated activity (striatum, amygdala, insula) and altered connectivity (e.g., amygdala, insula, and lateral PFC) within reward-related regions (Gujar et al., 2011). Overall, studies have associated sleep loss (or disrupted sleep) with increased reward-related neural activity and altered connectivity among ventral prefrontal cortical-striatal regions (vmPFC, OFC, VS) and insula, along with deactivation within dorsal frontal regions (dmPFC, ACC).
While there is evidence for separate effects of family history of BD and sleep loss on reward processing, research has not yet examined the interaction of these risk factors. In OBP who have not yet developed BD, sleep loss may exacerbate existing functional abnormalities in reward-related ventral prefrontal cortical-striatal regions and reduce activation within dorsal frontal regions. However, in examining this question in OBP, it is important to differentiate risk for BD from risk for psychopathology more broadly. OBP are at heightened risk of developing a range of non-BD psychiatric conditions in addition to BD (Birmaher et al., 2009). Parents with BD also have high rates of non-BD comorbid disorders (Merikangas et al., 2007). Thus, it is necessary to account for both the impact of risk for non-BD psychopathology and environmental effects of living with parents with non-BD psychopathology when selecting a comparison offspring group. To control for these effects, we included offspring of control parents with non-BD psychiatric disorders (OCP), which included lifetime diagnoses of depression, anxiety, behavioral, and substance use disorders.
The goal of this study was thus to examine associations between sleep duration, reward circuitry function, and mood dysregulation in youth at high- and low-familial risk for BD (as a function of parental history of BD). We used a well-validated number-guessing paradigm (win, loss, and control blocks) (Bebko et al., 2014; Forbes et al., 2009) to examine reward-related neural circuitry. Sleep duration was assessed using a validated self-report questionnaire. Symptoms of mood dysregulation (e.g., mood lability, positive mood/energy dysregulation) were assessed via parent report. Two aims were evaluated. Aim 1 was to test the association between self-reported sleep duration and reward circuitry function, and the moderating effect of high- or low-familial risk for BD (as a function of parental history of BD). We hypothesized that, across all youth, shorter sleep duration would be associated with (a) reduced activity in dorsal prefrontal cortical regions implicated in reward processing (dACC, dmPFC), (b) elevated activity in ventral prefrontal cortical-striatal regions implicated in reward processing (vmPFC, OFC, VLPFC, VS) and insula, and (c) altered VS connectivity with ventral prefrontal cortical regions (VLPFC, OFC, vmPFC) and insula to win-control. Given previous findings showing similar patterns of altered reward circuitry function to those described above in OBP versus OCP (Manelis et al., 2016), we also predicted that group status would moderate sleep-reward circuitry function relationships, such that the above associations between sleep duration and reward circuitry function would be stronger in OBP than OCP. Aim 2 was to examine whether group- or sleep-related alterations in reward circuitry function were associated with mood dysregulation symptoms (e.g., mood lability, positive mood/energy dysregulation) in OBP and OCP. We hypothesized that, within OBP, reward-related activation and connectivity patterns related to group status, sleep duration, or their interaction would be associated with elevated symptoms on these measures.
Methods
Participants
Two groups of participants (9–17 years old) who were not affected with BD were included in this study: (1) 25 offspring of parents with bipolar disorder type I or II (OBP) and (2) 21 age-, sex-, and IQ-matched children of parents with non-BD psychopathology (offspring of control parents; OCP). Several participants were taking psychotropic medication (OBP N=2; OCP N=3) and had non-BD psychopathology (OBP N=9; OCP N=10). Participants were recruited from the Pittsburgh Bipolar Offspring study (BIOS), an ongoing longitudinal study on the psychopathology and functioning of offspring of individuals diagnosed with BD (Birmaher et al., 2009). This study was approved by the Institutional Review Board of the University of Pittsburgh (Pittsburgh, PA, USA) and all participants provided written informed assent/consent as appropriate. Exclusion criteria for all parents included: lifetime diagnoses of schizophrenia, mental retardation, and mood disorders secondary to substance abuse, medical conditions, or medications. OCP parents were also excluded if they had first-degree relatives with BD. Offspring exclusion criteria included: systemic medical illness, neurological disorders, history of head trauma, alcohol or illicit substance use, presence of metal objects in their body, claustrophobia, IQ<70 as assessed by the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999), unable to read and write in English, corrected visual acuity worse than 20/40 on the Snellen visual acuity test, and missing sleep data. The total sample included 70 offspring (OBP N=35; OCP N=35). Of these, participants were excluded due to: not meeting study inclusion criteria [lack of parental Axis I pathology in OCP (N=5); pervasive developmental disorder diagnosis (OCP N=1)]; an inability to complete the functional tasks in the fMRI scanner either due to scheduling limitations, participant cooperation, or scanner malfunction (OBP N=6; OCP N=5); excessive motion (>4mm) during the fMRI task (OBP N=3; OCP N=1); and/or missing sleep data (OBP N=1; OCP N=2). A comparison between the 46 included participants and the 17 participants excluded due to fMRI or sleep data loss can be found in Supplemental Table 1.
Assessment Procedures
Comprehensive clinical evaluations were conducted prior to a scanning visit. Participants and their parents were interviewed. Parental Axis I psychopathology was assessed using the Structural Clinical Interview for DSM-IV (SCID-I; First et al., 1997) and the Family History Screen (Weissman et al., 2000). Participant (offspring) Axis I psychopathology was assessed using The Schedule for Affective Disorders and Schizophrenia for School Aged Children – Present and Lifetime Version (KSADS-PL; Kaufman et al., 1997). Interviewers were blind to participant status.
Before the scan, participants completed clinical and demographic questionnaires. All participants documented psychotropic medication use, and completed drug/alcohol/pregnancy screens, the Edinburgh Handedness Inventory (EHI; Oldfield, 1971), the Snellen visual acuity test, the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999), and a modified version of the self-report Pittsburgh Sleep Quality Index (Buysse et al., 1989) to assess sleep patterns and quality in the prior week. Self-reported sleep was preferred for youth, as youth are more reliable reporters of their sleep than their parents (Short et al., 2013). Item responses pertaining to sleep patterns on the modified one-week PSQI version (m-PSQI) are consistent with gold-standard daily sleep diary ratings (Broderick et al., 2013). Average sleep duration in the week prior to scan was derived from the m-PSQI. Pubertal status was assessed using a self-report questionnaire (Petersen et al., 1988). Parents completed the Mood and Feelings Questionnaire to assess depressive symptoms in the past two weeks in their children (MFQ-P; Angold et al., 1995), the Self-report for Childhood Anxiety Related Emotional Disorders to assess their anxiety symptoms in the past two weeks in their children (SCARED-P; Birmaher et al. 1997), and the Hollingshead scale to assess socioeconomic status (SES; Hollingshead, 1975). To evaluate aspects of mood dysregulation in their children, parents also completed the Parent General Behavior Inventory-10 Item Mania Scale to assess positive mood and energy dysregulation the past six months (PGBI-10M; Youngstrom et al., 2008) and the Child Affective Lability Scale - Parent Report to assess severity of mood lability (CALS-P; Gerson et al., 1996). Elevated scores on these mood dysregulation measures (PGBI-10M, CALS-P) have been linked to a BD diagnosis in youth (Birmaher et al., 2013; Findling et al., 2010).
Neuroimaging
Reward Paradigm
A validated block-design functional magnetic resonance imaging paradigm (Bebko et al., 2014; Forbes et al., 2009; Manelis et al., 2016, In Press) was used to probe reward-related neural circuitry. Participants played a card number-guessing game that contained win, loss, and control guessing trials. The task was approximately 6 minutes. Participants had 3000 msec to guess via button press whether the value of a visually presented card was higher or lower than 5, with possible card values ranging from 1 to 9. For win and loss trials, after a choice was made the actual numerical value of the card (500 msec) and then outcome feedback (500msec; Win: green upward-facing arrow; Loss: red downward-facing arrow) were visually presented. For control trials, participants pressed a button to the letter “X” (3000 msec), then viewed an asterisk (500 msec), followed by a yellow circle (500 msec). After each trial ended, participants viewed a fixation cross (3000 msec inter-trial interval). The paradigm contained 9 blocks: 3 win (each comprising 80% win, 20% loss trials), 3 loss (each comprising 80% loss, 20% win trials), and 3 control (no change in earnings) blocks. Guessing blocks (Win and Loss) had 5 trials with predetermined outcome order (Win block: win, win, win, loss, win; Loss block: loss, loss, win, loss, loss). Control blocks had 6 control trials. Participants were told that their performance on the card game would determine a monetary reward to be received at the end of the game; however outcome was fixed at $10 for all participants. Prior studies with this task have shown that participants were unaware of the fixed outcomes and believed their performance was due to chance (Forbes et al., 2009). Participants practiced the task and minimizing head movement in an fMRI simulator before scanning. Throughout practice and scanning, participants were verbally encouraged to perform to the best of their abilities and to minimize movement.
fMRI Data Acquisition and Preprocessing
Neuroimaging data were acquired using a Siemens MAGNETOM TrioTim 3T MR system. A high-resolution structural image (1×1×1mm) was acquired using MPRAGE (TR=2300 msec, TE=3.93 msec, FOV-256, FA=9 degrees, 192 slices). Blood oxygen-level dependent (BOLD) functional data were collected using a gradient-echo, echo-planar sequence (voxel size: 3.2×3.2×3.1mm, TR=2000 msec, TE=28 msec, FOV=205, FA=90°, 38 slices). These data comprised 178 volumes (TRs). A PC with E-prime software (Psychology Software Tools (PST), Pittsburgh, PA) controlled stimulus display.
Statistical Parametric Mapping software (SPM8; http://www.fil.ion.ucl.ac.uk/spm) was used to preprocess and analyze fMRI data. Preprocessing involved realignment, coregistration, segmentation, normalization into a standard stereotactic space (Montreal Neurologic Institute, MNI; http://www.bic.mni.mcgill.ca), and spatially smoothing using a Gaussian kernel (FWHM: 8mm).
fMRI Data Analyses: Activity
Neuroimaging data were analyzed using SPM8. At the first level analysis, individual whole brain statistical maps were computed to evaluate the contrasts of interest (win-control and loss-control). Our main focus was on examining relationships among group, sleep duration and neural activity to reward, thus the stimulus contrast of interest was the win-control contrast. We report findings for the loss-control contrast in supplemental materials (Supplemental Table 3). Movement parameters obtained from the realignment stage of preprocessing served as covariates of no interest.
Second level random-effects analyses were conducted in a ROI mask comprising key regions in reward circuitry: the bilateral ACC (BA24/32), mPFC (BA10), OFC (BA11), VLPFC (BA47), insula, and ventral striatum (VS; bilateral spheres centered on −9, 9, −8 and 9, 9, −8; radius=8mm; see Postuma and Dagher, 2006, Di Martino et al., 2008 for meta-analyses). The mask was created using the WFU PickAtlas (Maldjian et al., 2003). These neural regions are consistently implicated in reward processing in healthy and psychiatric samples (Caseras et al., 2013; Liu et al., 2011; Nusslock et al., 2012). Voxelwise regression analyses in SPM8 tested the effects of group status, sleep duration, and group*sleep duration interaction on BOLD response to win-control within the bilateral ROI mask. Group status was dummy-coded (OBP=0.5, OCP=−0.5). Sleep duration was mean-centered across both groups. A voxelwise threshold of p<0.01, with a 3dClustSim cluster-level correction threshold of p<0.05 (k=40) to correct for family-wise error, was used for ROI analyses.
We extracted parameter estimates from the clusters displaying a significant effect of group, sleep duration and group*sleep interaction on brain activation using the eigenvariate tool in SPM8. These parameter estimates were imported into SPSS to compute R2 and for post hoc simple slope analyses of significant group*sleep duration interaction effects. Simple slopes were computed using the SPSS PROCESS macro (Hayes, 2013).
fMRI Data Analysis: Psychophysiological Interactions (PPI)
A psychophysiological interaction (PPI) analysis was conducted in SPM8 to examine connectivity between a ventral striatum (VS) seed region and bilateral prefrontal-cingulo-insular ROI target regions that included bilateral ACC (BA24/32), mPFC (BA10), OFC (BA11), VLPFC (BA47), and insula. PPI provides information about the modulatory effects of the seed region in the context of an experimental condition on selected targets (Friston et al., 1997). We first created a PPI vector by multiplying mean time series from the bilateral VS seed region by the task condition vectors (win-control and loss-control). Single-subject first-level analyses were run using the PPI vector, bilateral VS time-course vector, and condition vector (win-control) as regressors. The resultant interaction terms were positively weighted in subsequent second level regression analyses given that our main hypotheses focused on coupling between the VS and target regions. As for the activity analyses above, the stimulus contrast of interest was win-control.
Group-level analyses for connectivity between the VS seed region and ROI target mask paralleled the BOLD activation analyses described above. A voxelwise threshold of p<0.01, with a 3dClustSim cluster-level correction threshold of p<0.05 (k=52) to correct for family-wise error, was used for ROI analyses.
Correlations with Mood Dysregulation
Correlation analyses were conducted within each group to examine the extent to which extracted measures of BOLD activation and functional connectivity were associated with mood dysregulation measures. Analyses focused on regions displaying a significant group, sleep duration, or group*sleep duration interaction effects. Positive mood/energy dysregulation (PGBI-10M) and mood lability (CALS-P) were assessed. Statistical significance was set at p=0.025 (Bonferroni-corrected p<0.05/2). Symptom measure total scores were log transformed to better approximate a normal distribution. Due to the high frequency of zero scores on the PGBI-10M in OCP participants, correlation analyses were performed in OBP only for that measure. Exploratory correlations with depression (MFQ-P) and anxiety (SCARED-P) are included in the Supplemental Analyses.
Exploratory Analyses
Psychiatric medication use (e.g., Phillips et al., 2008) and current psychopathology (e.g., Whitton et al., 2014) have been shown to affect neuroimaging findings. Thus, we followed a prior method (Manelis et al., 2016; Manelis et al., 2015) to test the effects of medication use and current diagnosis on the BOLD activity and functional connectivity identified in the primary analysis. Using the extracted data previously described, we conducted regressions paralleling the main analyses on 1) participants without psychopathology and 2) unmedicated participants.
Lastly, we conducted exploratory whole-brain activity and VS connectivity to win-control to examine the extent to which patterns of whole-brain activity to this stimulus contrast were similar to activity and connectivity pattern in our a priori ROI masks (voxelwise threshold of p < 0.005, cluster-level threshold of k=20) (Lieberman and Cunningham, 2009). As in the main analyses, we conducted voxelwise regressions in SPM to examine the effects of group (OBP vs. OCP), sleep duration, and group*sleep duration interaction on BOLD activity and VS connectivity to win-control.
Results
Demographic and Clinical Data
The two groups did not significantly differ with regard to sociodemographic features, including age, sex, SES, IQ, pubertal status and handedness (Table 1). Groups also did not differ on the majority of clinical characteristics, including Axis I disorders, depressive symptoms (MFQ-P), anxiety symptoms (SCARED-P), and sleep parameters (m-PSQI) (Table 1). However, relative to OCP, OBP exhibited greater symptoms of positive mood/energy dysregulation (PGBI-10M) and mood lability (CALS-P) (all p-values<.05). Across all participants, sleep duration was negatively correlated with age (r=−0.45, p=0.002) and pubertal status (r=−0.36, p=0.013), but not other demographic characteristics. Including age or pubertal status as covariates in fMRI analyses did not alter the pattern of neuroimaging findings. In OBP, sleep duration was negatively correlated with PGBI-10M (r=−0.43, p=0.031) and CALS-P (r=−0.40, p=.049). In OCP, sleep duration was not correlated with CALS-P and there was not a sufficient range of PGBI-10M scores within this group to perform a correlation. Rates of parental non-BD psychopathology did not differ between groups (Table 2).
Table 1.
Demographic, clinical, and sleep characteristics in offspring of bipolar parents (OBP) and offspring of control parents (OCP).
| OBP (N=25) | OCP (N=21) | |||
|---|---|---|---|---|
| Demographic & Clinical | Mean (SD) or N (%) | Mean (SD) or N (%) | Statistic | p |
| Age (years) | 14.20 (2.25) | 13.95 (2.24) | t44= 0.38 | 0.703 |
| Sex (% female) | 11 (44.0%) | 11 (52.4%) | X2 = 0.32 | 0.571 |
| IQ (WASI) | 100.40 (15.63) | 101.62 (11.84) | t44=−0.29 | 0.771 |
| Pubertal Status (% Late/Post) | 15 (60.0%) | 14 (66.7%) | X2 = 0.22 | 0.641 |
| SES (Hollingshead) | 2.68 (1.37) | 3.43 (1.57) | t44=−1.73 | 0.092 |
| EHI Handedness (right) | 22 (88.0%) | 19 (90.5%) | X2 = 2.22 | 0.329 |
| MFQ-P Score | 7.08 (9.51) | 4.10 (3.52) | t44= −0.77 | 0.449 |
| SCARED-P Score | 10.00 (6.30) | 12.35 (12.48) | t43= 1.42 | 0.165 |
| PGBI-10M Score | 2.40 (3.15) | 0.81 (1.40) | t44= 2.27 | 0.029 |
| CALS-P Score | 9.76 (11.11) | 3.90 (4.59) | t43= 2.39 | 0.022 |
| Any Axis 1 Disorder | 9 (36.0%) | 10 (47.6%) | X2= 0.64 | 0.425 |
| Depressive Disorders | 4 (16.0%) | 3 (14.3%) | -- | 1.000 |
| Anxiety Disorders | 3 (12.0%) | 5 (23.8%) | -- | 0.439 |
| ADHD | 4 (16.0%) | 3 (14.3%) | -- | 1.000 |
| Disruptive Behavior | ||||
| Disorder | 1 (4.0%) | 2 (9.5%) | -- | 0.585 |
| Eating Disorder | 2 (8.0%) | 0 (0.0%) | -- | 0.493 |
| >1 Axis 1 Disorders | 4 (16.0%) | 5 (23.8%) | -- | 0.711 |
| Psychotropic Medication | 2 (8.0%) | 3 (14.3%) | -- | 0.648 |
| Stimulant | 1 (4.0%) | 2 (9.5%) | -- | 0.585 |
| Antidepressant | 0 (0.0%) | 1 (4.8%) | -- | 0.457 |
| Antipsychotic | 1 (4.0%) | 0 (0.0%) | -- | 1.000 |
| Past-Week Sleep (m-PSQI) | ||||
| Sleep Duration (hours) | 8.00 (1.97) | 7.58 (1.86) | t44= 0.73 | 0.469 |
| Bedtime | 23:00 (2:52) | 23:07 (1:40) | t44=−0.15 | 0.878 |
| Risetime | 7:56 (2:22) | 7:35 (1:49) | t44= 0.59 | 0.557 |
| Sleep Onset Latency (minute) | 19.24 (24.64) | 23.19 (21.55) | t44=−0.57 | 0.569 |
| Sleep Efficiency (%) | 92.05 (12.21) | 90.86 (10.01) | t43= −0.35 | 0.726 |
| m-PSQI Sleep Quality Score | 3.64 (2.92) | 4.95 (4.21) | t43=1.23 | 0.226 |
Note. SD = Standard Deviation; -- = Fisher's Exact Test; t(df); WASI = Wechsler Abbreviated Scale of Intelligence; SES = socioeconomic status; EHI = Edinburgh Handedness Inventory; MFQ-P = Mood and Feelings Questionnaire–Parent Version; SCARED-P = Self-report for Childhood Anxiety Related Emotional Disorders–Parent Version; PGBI-10M = Parent General Behavior Inventory 10-item Mania Scale; CALS-P = Child Affective Lability Scale–Parent Version; ADHD = Attention-Deficit Hyperactivity Disorder; m-PSQI = Past-Week Pittsburgh Sleep Quality Index.
Table 2.
Lifetime psychopathology for parents of OBP and OCP.
| Parents with BD (N=25) | Parents with non-BD psychopathology (N=21) | |||
|---|---|---|---|---|
| N (%) | N (%) | Statistic | p | |
| Bipolar Disorder | X2= 46.0 | <.001 | ||
| Bipolar Disorder, type 1 | 18 (72%) | 0 (0.0%) | ||
| Bipolar Disorder, type 2 | 7 (28%) | 0 (0.0%) | ||
| Depressive Disorders | 0 (0.0%) | 11 (5.4%) | -- | <.001 |
| Anxiety Disorders | 21 (76.2%) | 16 (84.0%) | -- | 0.711 |
| ADHD | 4 (16.0%) | 2 (9.5%) | -- | 1.000 |
| Disruptive Behavior Disorder | 4 (16.0%) | 1 (4.8%) | -- | 0.357 |
| Substance Use Disorder | 17 (68.0%) | 9 (42.9%) | X2= 2.94 | 0.087 |
| Eating Disorders | 3 (12.0%) | 1 (4.9%) | -- | 0.614 |
| Any non-BD Axis I pathology | 23 (92.0%) | 21 (100%) | -- | 0.493 |
Note. OBP = offspring of bipolar parents; OCP = offspring of control parents; ADHD= attention deficit hyperactivity disorder; BD=bipolar disorder; -- = Fisher's Exact Test.
fMRI: BOLD Activity
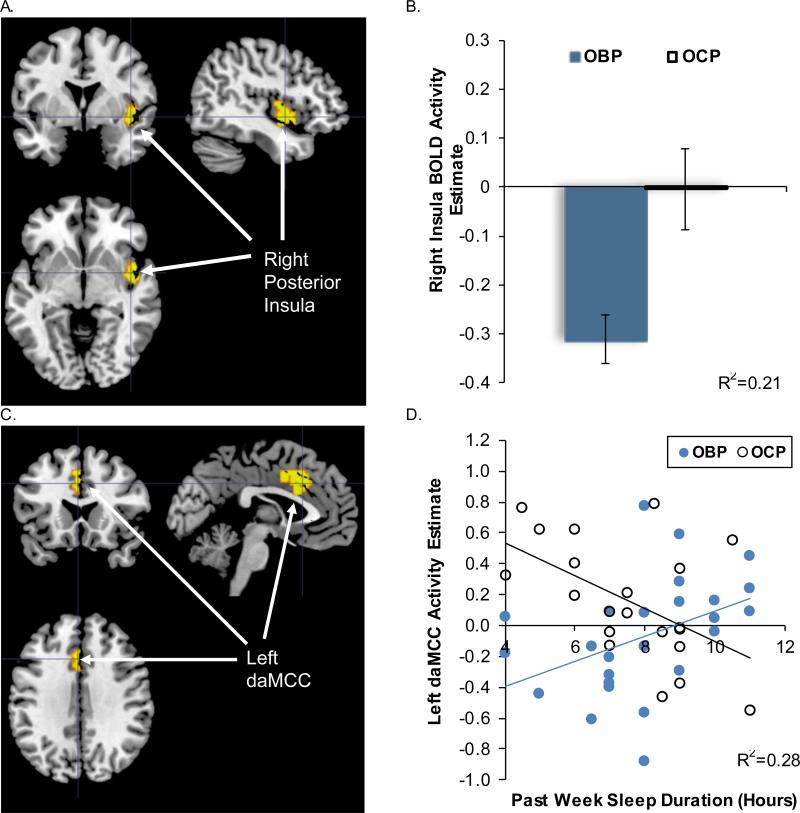
There was a significant effect of group on activation in the right posterior insula (peak voxel MNI xyz= [45, −1, −5], t42=3.52, p=.001, corrected), with greater deactivation to win-control trials in OBP relative to OCP (Table 3, Fig. 1A, 1B). There was no significant effect of sleep duration on BOLD activity. In support of our Aim 1a hypothesis that shortened sleep duration would be more strongly associated with reduced dorsal prefrontal activity in OBP relative to OCP, a significant group*sleep duration interaction effect was observed in left dorsal anterior mid-cingulate cortex (daMCC; peak voxel MNI xyz= [−3, 17, 37], t42=3.57, p<.001, corrected) (Table 3, Fig. 1C, 1D). Post hoc analysis of the simple slopes revealed a positive association between sleep duration and daMCC activity in OBP (b=0.08[0.04], p=.029), and a negative association in OCP (b=−0.11[0.04], p=.013). Contrary to our Aim 1b hypothesis, we did not observe any group*sleep duration interactions for BOLD activity within ventral prefrontal cortical-striatal regions or insula.
Table 3.
ROI regression analyses of group status, sleep duration, and group*sleep duration interaction effects on BOLD activity and PPI functional connectivity to win>control.
| MNI Coordinates | Statistic | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Comparison | Region | Slope | BA | Side | k | x | y | z | t(42) | p |
| BOLD Activation | ||||||||||
| Group Effect | Posterior insula | - | 13 | R | 57 | 45 | −1 | −5 | 3.52 | .001 |
| Sleep Duration Effect | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Group*Sleep Duration Effect | daMCC | + | 24,32 | L | 41 | −3 | 17 | 37 | 3.22 | <.001 |
| PPI functional connectivity with ventral striatum seed | ||||||||||
| Group Effect | Posterior Insula | + | 13 | L | 59 | −42 | −4 | −5 | 3.77 | <.001 |
| Sleep Duration Effect | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Group*Sleep Duration Effect | Anterior insula/VLPFC | - | 13,47 | L | 89 | −36 | 8 | −11 | 3.32 | .001 |
Note. ROI=region of interest; BOLD=blood oxygen level dependent; PPI=psychophysiological interaction; BA= Brodmann Area; -- indicates data not applicable; L = left; R = right; daMCC = dorsal anterior mid-cingulate cortex; VLPFC = ventrolateral prefrontal cortex; Group status was dummy coded OBP=0.5 and OCP=−0.5; k=cluster size in voxels; t(df).
Figure 1.
SPM regression analysis testing group, sleep duration, and group*sleep duration interaction as predictors of BOLD activity to win-control within the bilateral prefrontal-striatal ROI mask. (A) Significant group effect on BOLD activity in the right posterior insula [k=57, peak voxel MNI xyz= [45, −1, −5], t42=3.52, p=.001]. (B) Extracted right insula peak BOLD activity estimates plotted by group; means and standard error bars are presented, R2 represents variance explained for the overall regression. (C) Significant group*sleep duration interaction on left daMCC BOLD activity [k=41, peak voxel MNI xyz= [−3, 17, 37], t42=3.57, p<.001]. (D) Extracted left daMCC BOLD activity estimates plotted versus sleep duration for group*sleep duration interaction, trend lines indicate simple slopes for each group, R2 represents variance explained for the overall regression. OBP = offspring of bipolar parents; OCP = offspring of control parents; daMCC=dorsal anterior mid-cingulate cortex.
fMRI: PPI
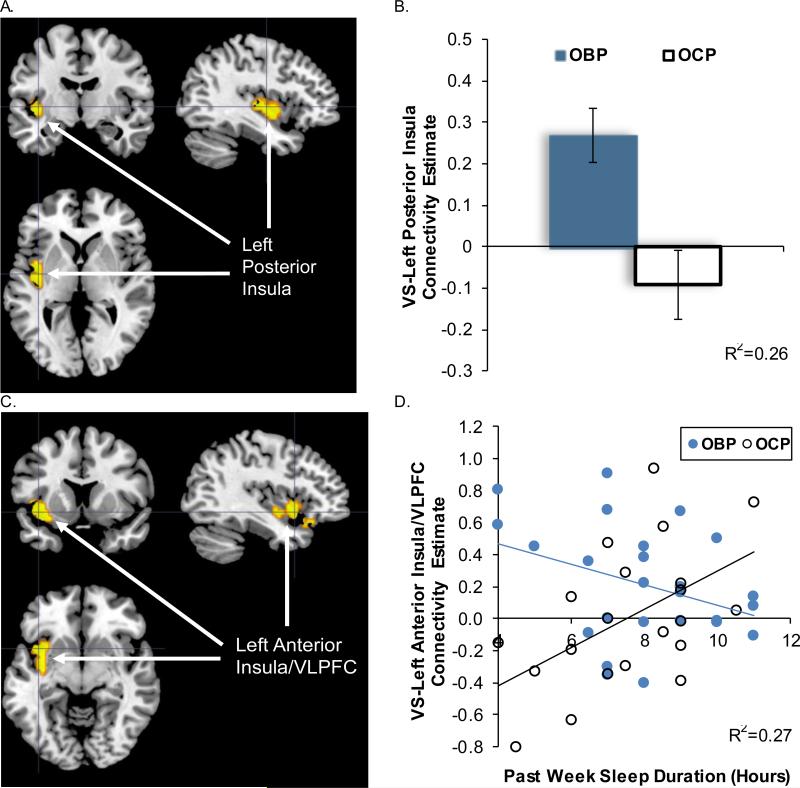
There was a significant effect of group on VS-left posterior insula functional connectivity (peak voxel MNI xyz= [−42, −4, −5], t42=3.77, p<.001, corrected), with greater connectivity in OBP relative to OCP for win-control trials (Table 3, Fig. 2A, 2B). There was no significant effect of sleep duration on VS functional connectivity. However, in support of our Aim 1c hypothesis that shortened sleep duration would be more strongly associated with altered VS connectivity with ventral frontal cortical regions in OBP relative to OCP, there was a significant sleep duration*group interaction for VS-left anterior insula/VLPFC connectivity (peak voxel MNI xyz= [−36, 8, −11], t42=3.11, p<.001, corrected) (Table 3, Fig. 2C, 2D). Post hoc analysis of simple slopes revealed a negative association between sleep duration and VS-left anterior insula/VLPFC connectivity in OBP (b= −0.09[0.04], p=.028), and a positive association in OCP (b= 0.12[0.04], p=.009).
Figure 2.
SPM regression analysis testing group, sleep duration, and group* sleep duration interaction as predictors of VS connectivity to win-control within the bilateral prefrontal cingulo-insular ROI mask. (A) Significant effect of group status on VS connectivity with the left posterior insula: k=59, peak voxel MNI xyz= [−42, −4, −5], t42=3.77, p<.001. (B) Extracted VS-Left posterior insula connectivity estimates displaying a significant difference between groups; means and standard error bars are presented, R2 represents variance explained for the overall regression. (C) Significant group*sleep duration interaction for VS connectivity with the left anterior insula/VLPFC: k=89, peak voxel MNI xyz= [−36, 8, −11], t42=3.11, p=.001. (D) Extracted VS-Left anterior insula/VLPFC connectivity estimates plotted versus sleep duration for group*sleep duration interaction, trend lines indicate simple slopes for each group, R2 represents variance explained for the overall regression. OBP=offspring of bipolar parents; OCP=offspring of control parents; VS=ventral striatum; VLPFC=ventrolateral prefrontal cortex.
Correlations between fMRI Measures and Mood Dysregulation
Our Aim 2 hypothesis that reward-related activation and connectivity patterns associated with group status, sleep duration, or their interaction would correlate with elevated mood dysregulation symptoms in OBP was partially supported. BOLD activation within the right posterior insula and left daMCC was not related to mood dysregulation symptom measures. VS-left posterior insula connectivity was positively correlated with CALS-P (r=0.50, p=0.011) and PGBI-10M (r=0.52, p=0.007) in OBP, but not significantly related to mood dysregulation measures in OCP. VS-left anterior insula/VLPFC functional connectivity was positively correlated with CALS-P (r=0.47, p=0.016) and PGBI-10M (r=0.57, p=0.003) in OBP, but showed no relationship with mood dysregulation measures in OCP.
Exploratory Subgroup Analyses
Regression analyses in the subgroups of unmedicated participants (OBP N=23, OCP N=18) and participants without psychopathology (OBP N=16, OCP N=11) largely paralleled the pattern observed in main analyses. For BOLD activity analyses, OBP without psychopathology showed the same decreased pattern of right posterior insula activity as the larger group (OBP<OCP, b=−0.27[0.11], p=.027), although unmedicated OBP did not show this pattern of right posterior insula difference (b=−0.37[0.42], p=.375). There was a group*sleep duration interaction effect in the left daMCC (unmedicated participants: b=0.21[0.06], p=.001; participants without psychopathology: b=0.28[0.07], p=.001). Post hoc simple slope analyses were consistent with the main analyses for OBP (unmedicated OBP: b=0.10[0.04], p=.024; OBP without psychopathology: b=0.11[0.05], p=.009) and OCP (unmedicated OCP: b=−0.11[0.04], p=.016; OCP without psychopathology: b=−0.17[0.06], p=.020).
For the PPI analyses, there was a group effect for VS-left posterior insula functional connectivity (OBP>OCP; unmedicated participants: b=1.24[0.46], p=.011; participants without psychopathology: b=0.30[0.10], p=.027). There was a group*sleep duration interaction effect for VS-left anterior insula/VLPFC functional connectivity (unmedicated participants: b=−0.21[0.06], p<.001; participants without psychopathology (b=−0.21[0.08], p=.018). Post hoc simple slope analyses were also generally consistent for OBP (unmedicated OBP: b=−0.09[0.04], p=.004; although not significant for OBP without psychopathology: b=−0.01[0.05], p=.814) and OCP (unmedicated OCP: b=0.13[0.04], p=.040; OCP without psychopathology: b=0.20[0.07], p=.006).
Exploratory Whole-Brain Analyses
Exploratory whole-brain regressions revealed patterns of activity and VS connectivity similar to those reported in the ROI analyses, as well as additional findings (See Supplemental Table 2).
Discussion
To improve our understanding of neurobehavioral mechanisms involved in the development of BD, the goal of this preliminary study was to examine associations between sleep duration, reward circuitry function, and symptoms of mood dysregulation in youth at high- and low-familial risk for BD (as a function of parental history of BD). In OBP without established BD, we sought to assess whether reduced sleep duration may represent a factor exacerbating functional abnormalities within reward-related neural circuitry, namely activity and connectivity among ventral prefrontal cortical-striatal and dorsal frontal regions. We used self-reported sleep duration, neuroimaging measures of reward processing circuitry, and parent-reported measures of mood dysregulation in non-BD OBP and OCP. Associations between sleep duration and reward-related neural activity and connectivity, and the moderating effect of parental history of BD, were examined. We also tested whether group-and sleep-related neural activity and connectivity patterns were associated with mood dysregulation.
In support of our Aim 1a hypothesis that shortened sleep duration would be more strongly associated with reduced dorsal prefrontal activity in OBP relative to OCP, we observed a relationship between sleep duration and daMCC activity that differed between groups. Shorter sleep duration was related to greater daMCC activity in OCP and decreased daMCC activity in OBP. The daMCC is proposed to play a key role in attentional/cognitive control (Bush et al., 2000). In healthy individuals, frontal neural regions supporting attentional control are highly susceptible to the effects of sleep loss (Ma et al., 2015; Muzur et al., 2002), but can display compensatory increases in activity depending on task characteristics (Chee and Choo, 2004; Drummond et al., 2005). Thus, the present pattern of findings may reflect a lack of compensatory response in OBP in the context of shorter sleep duration, while this compensatory response may have remained intact in OCP. Contrary to our Aim 1b hypothesis, and findings from total sleep deprivation studies (Gujar et al., 2011; Mullin et al., 2013; Venkatraman et al., 2007; Venkatraman et al., 2011b), associations between sleep duration and activity within ventral prefrontal-striatal regions (vmPFC, OFC, insula, VS) to win-control were not detected here. This could be attributable to our focus on naturalistic sleep duration, rather than experimental sleep deprivation: increased activation has predominantly been observed in these regions under conditions of total sleep deprivation. We also observed that the right posterior insula (Chang et al., 2013) was more deactivated in OBP than OCP. Posterior insula activation was not associated with affective symptoms in either group. This region has been implicated in interoception and physiological reactivity during reward processing (Chang et al., 2013; Menon and Uddin, 2010). However, the group effect in the right posterior insula may have been influenced by current psychotropic medication use, as unmedicated participants did not display a significant group effect in this region. The differences in functioning of the daMCC and right posterior insula, while not related to mood dysregulation, may reflect alternative risk processes, associated with disrupted cognitive control and interoceptive processing, linked to parental history of BD.
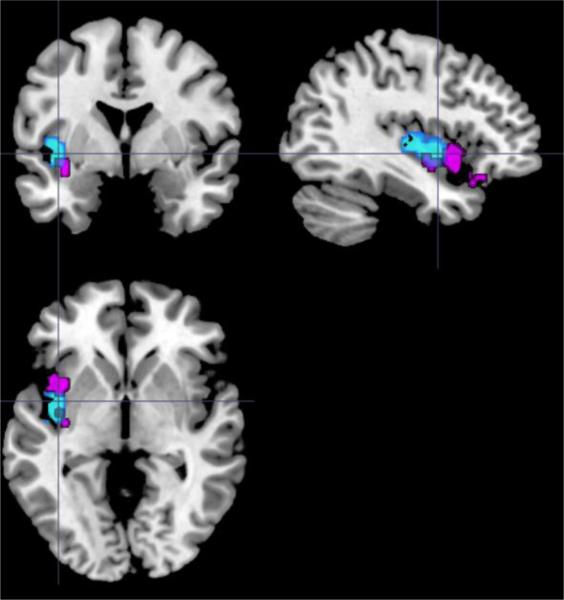
In support of our Aim 1c hypothesis that shortened sleep duration would be more strongly associated with altered VS connectivity with ventral frontal cortical regions in OBP relative to OCP, PPI analyses showed group and group*sleep duration interaction effects in key reward-related functional connectivity. OBP displayed increased VS-left posterior insula connectivity relative to OCP for win-control. A group*sleep duration interaction was observed for VS-left anterior insula/VLPFC connectivity, with shorter sleep duration associated with greater connectivity in OBP, but lower connectivity in OCP. Interestingly, the left posterior insula and left anterior insula/VLPFC regions partially overlapped (Figure 3). Together, these findings could indicate that OBP exhibit increased VS-left insula connectivity overall, with the ventroanterior subregion (Chang et al., 2013) and adjacent left VLPFC affected by sleep duration. The VS supports reward evaluation and anticipation (Knutson et al., 2001; O'Doherty, 2004), the anterior and posterior insula interact to modulate physiological reactivity to salient stimuli (Menon and Uddin, 2010), and the VLPFC is proposed to encode arousal during reward processing (e.g., Dolcos et al., 2004). Both the insula and VLPFC likely have excitatory afferent connections with the VS in humans, as indicated by homologs in animal models (Chikama et al., 1997; Sesack and Grace, 2010). The observed VS connectivity patterns thus suggest that OBP may encode reward cues as more salient/arousing than OCP, and that shorter sleep duration may exacerbate this phenomenon in OBP. Greater VS connectivity with both the left posterior insula and left anterior insula/VLPFC were also associated with elevated symptoms of mood dysregulation (e.g., mood lability, positive mood/energy dysregulation). By contrast, VS connectivity patterns in OCP were not significantly correlated with these symptoms. These findings point to divergent neural mechanisms underlying mood dysregulation in OBP and OCP, but suggest that elevated VS-left insula/VLPFC connectivity to reward is a potential neural mechanism underlying risk for mood dysregulation in OBP.
Figure 3.
Overlap in group (blue – left posterior insula) and group*sleep duration interaction (violet – left anterior insula/ventrolateral prefrontal cortex) effects in PPI analysis of VS connectivity to win-control within the bilateral prefrontal-cingulo-insular ROI target mask. PPI = psychophysiological interaction; VS = ventral striatum; ROI = region of interest.
The left lateralization of the observed striatal connectivity patterns is intriguing, given a growing number of studies reporting increased left VLPFC activation during reward processing in adult BD (for review see Phillips & Swartz, 2014; Nusslock et al., 2014), and are consistent with theories proposing a role of the left prefrontal cortex in encoding approach-related emotions (Pizzagalli et al., 2005). However, the present findings stand in contrast to prior work in OBP observing altered right VLPFC connectivity with pregenual cingulate gyrus (Singh et al., 2014) and bilateral VS (Manelis et al., 2016) during reward processing. The right VLPFC has been interpreted as serving an emotion regulatory function during reward processing (Singh et al., 2014; Manelis et al., 2016). Herein, given that sleep duration was differentially related to VS connectivity with the left anterior insula/VLPFC region, this could reflect a role of sleep duration in the modulation of encoding salient, approach-related emotions during reward processing.
Several limitations should be considered. The relatively small sample size affects statistical power, and may have limited our ability to detect weaker interaction effects. There were also a large number of analyses. Findings should be replicated in a larger sample. There was also no comparison group of healthy offspring of healthy parents, as our focus was on examining the impact of sleep duration on reward circuitry function in at-risk youth. Sleep-reward associations observed herein may not generalize to healthy youth. A very small number of participants were taking medications, which can influence neuroimaging measures (Phillips et al., 2008). Though, we note that the pattern of findings remained largely intact in the sub-group of unmedicated participants. The study design was also cross-sectional in nature, thus causal associations cannot be inferred. Sleep duration was measured via self-report. Some evidence suggests that psychiatric samples can provide less reliable self-report estimates of sleep in comparison with objective estimates (Gonzalez et al., 2013). Our measure of sleep duration from the m-PSQI has been shown to be consistent with gold-standard daily sleep diary ratings, however (Broderick et al., 2013). Mood assessments included in the present study were parent-reported, which can be affected by parental mood at the time of rating (e.g., Birmaher et al., 2013). Clinician-rated mood assessments were not conducted on scan day. Future studies would benefit from incorporating clinician-rated mood assessments with parent- and self-report.
Future research designed to establish causal relationships among sleep, reward circuitry function, and mood dysregulation in at-risk youth is recommended. While reciprocal relationships among these phenomena likely exist, understanding the neurobehavioral pathways linking sleep to the initial emergence of mood dysregulation could provide opportunities for preventative sleep-focused treatments, and identify neural markers to serve as clinical outcomes. Longitudinal research is necessary to establish the hypothesized temporal patterns, particularly whether neuroimaging markers mediate relationships between sleep and mood dysregulation. Experimental manipulations of sleep would also hold great utility for establishing causal links. For example, sleep extension paradigms could be used to ascertain whether brain function and mood dysregulation are improved in high-risk youth with insufficient sleep.
While the focus in the present study was on sleep duration, it is also possible that other sleep parameters are relevant to the onset and course of BD. For example, instability of sleep-wake patterns is also common in BD (e.g., Ng et al., 2014), and a target of existing psychosocial interventions such as Interpersonal and Social Rhythm Therapy. Thus, future studies would benefit from a more comprehensive sleep assessment, examining a wider set of sleep parameters using both objective and subjective measures.
This study is the first to our knowledge to establish a relationship between sleep duration and reward circuitry function in youth at high risk for BD. Parental history of BD moderated the association between sleep duration and reward-related left daMCC activity and VS-left anterior insula/VLPFC connectivity, while VS-left posterior insula connectivity was elevated in OBP relative to OCP. Elevated VS connectivity with both the left posterior insula and left anterior insula/VLPFC were correlated with greater mood dysregulation in OBP. This pattern of findings suggests that elevated VS-left insula connectivity during reward processing may represent a neural mechanism underpinning mood dysregulation in OBP. These results also indicate that reduced sleep duration could be one factor associated with increased of VS-left insula connectivity, shedding light on a potential neurobehavioral risk pathway for mood dysregulation in OBP. These findings, along with future longitudinal and experimental work, could provide neurobehavioral targets to guide preventative treatments in OBP.
Supplementary Material
Highlights.
Offspring of bipolar parents (OBP) exhibit altered reward circuitry function.
Sleep duration predicts altered activity and striatal connectivity to wins in OBP.
Sleep duration is related to mood dysregulation in OBP.
Striatal connectivity is related to mood dysregulation in OBP.
Altered striatal connectivity may link sleep loss to mood dysregulation in OBP.
Acknowledgments
The authors express thanks the families participating in this research study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders (4th edition, text revision) American Psychiatric Association; Washington, D.C.: 2001. [Google Scholar]
- Barbini B, Colombo C, Benedetti F, Campori E, Bellodi L, Smeraldi E. The unipolar-bipolar dichotomy and the response to sleep deprivation. Psychiatry Research. 1998;79:43–50. doi: 10.1016/s0165-1781(98)00020-1. [DOI] [PubMed] [Google Scholar]
- Barnes JC, Meldrum RC. The impact of sleep duration on adolescent development: a genetically informed analysis of identical twin pairs. J Youth Adolesc. 2015;44:489–506. doi: 10.1007/s10964-014-0137-4. [DOI] [PubMed] [Google Scholar]
- Bauer M, Grof P, Rasgon N, Bschor T, Glenn T, Whybrow PC. Temporal relation between sleep and mood in patients with bipolar disorder. Bipolar Disorders. 2006;8:160–167. doi: 10.1111/j.1399-5618.2006.00294.x. [DOI] [PubMed] [Google Scholar]
- Bebko G, Bertocci MA, Fournier JC, Hinze AK, Bonar L, Almeida JR, Perlman SB, Versace A, Schirda C, Travis M, Gill MK, Demeter C, Diwadkar VA, Ciuffetelli G, Rodriguez E, Olino T, Forbes E, Sunshine JL, Holland SK, Kowatch RA, Birmaher B, Axelson D, Horwitz SM, Arnold LE, Fristad MA, Youngstrom EA, Findling RL, Phillips ML. Parsing dimensional vs diagnostic category-related patterns of reward circuitry function in behaviorally and emotionally dysregulated youth in the Longitudinal Assessment of Manic Symptoms study. JAMA Psychiatry. 2014;71:71–80. doi: 10.1001/jamapsychiatry.2013.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Axelson D, Monk K, Kalas C, Goldstein B, Hickey MB, Obreja M, Ehmann M, Iyengar S, Shamseddeen W, Kupfer D, Brent D. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring study. Arch Gen Psychiatry. 2009;66:287–296. doi: 10.1001/archgenpsychiatry.2008.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Goldstein BI, Axelson DA, Monk K, Hickey MB, Fan J, Iyengar S, Ha W, Diler RS, Goldstein T, Brent D, Ladouceur CD, Sakolsky D, Kupfer DJ. Mood lability among offspring of parents with bipolar disorder and community controls. Bipolar Disord. 2013;15:253–263. doi: 10.1111/bdi.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliwise DL, Friedman L, Yesavage JA. Depression as a confounding variable in the estimation of habitual sleep time. Journal of Clinical Psychology. 1993;49:471–477. doi: 10.1002/1097-4679(199307)49:4<471::aid-jclp2270490403>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Broderick JE, Junghaenel DU, Schneider S, Pilosi JJ, Stone AA. Pittsburgh and Epworth sleep scale items: accuracy of ratings across different reporting periods. Behav Sleep Med. 2013;11:173–188. doi: 10.1080/15402002.2012.654549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds C.F.d., Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Caseras X, Lawrence NS, Murphy K, Wise RG, Phillips ML. Ventral striatum activity in response to reward: differences between bipolar I and II disorders. Am J Psychiatry. 2013;170:533–541. doi: 10.1176/appi.ajp.2012.12020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LJ, Yarkoni T, Khaw MW, Sanfey AG. Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cereb Cortex. 2013;23:739–749. doi: 10.1093/cercor/bhs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MW, Choo WC. Functional imaging of working memory after 24 hr of total sleep deprivation. J Neurosci. 2004;24:4560–4567. doi: 10.1523/JNEUROSCI.0007-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikama M, McFarland NR, Amaral DG, Haber SN. Insular cortical projections to functional regions of the striatum correlate with cortical cytoarchitectonic organization in the primate. J Neurosci. 1997;17:9686–9705. doi: 10.1523/JNEUROSCI.17-24-09686.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo C, Benedetti F, Barbini B, Campori E, Smeraldi E. Rate of switch from depression into mania after therapeutic sleep deprivation in bipolar depression. Psychiatry Research. 1999;86:267–270. doi: 10.1016/s0165-1781(99)00036-0. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP. Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Dissociable effects of arousal and valence on prefrontal activity indexing emotional evaluation and subsequent memory: an event-related fMRI study. Neuroimage. 2004;23:64–74. doi: 10.1016/j.neuroimage.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Drummond SP, Meloy MJ, Yanagi MA, Orff HJ, Brown GG. Compensatory recruitment after sleep deprivation and the relationship with performance. Psychiatry Res. 2005;140:211–223. doi: 10.1016/j.pscychresns.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Findling RL, Youngstrom EA, Fristad MA, Birmaher B, Kowatch RA, Arnold LE, Frazier TW, Axelson D, Ryan N, Demeter CA, Gill MK, Fields B, Depew J, Kennedy SM, Marsh L, Rowles BM, Horwitz SM. Characteristics of children with elevated symptoms of mania: the Longitudinal Assessment of Manic Symptoms (LAMS) study. J Clin Psychiatry. 2010;71:1664–1672. doi: 10.4088/JCP.09m05859yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbons M, Williams J. Structured Clinical Interview for DSM IV Axis 1 Disorders: clinician version. American Psychiatric Press; Washington, DC.: 1997. [Google Scholar]
- Forbes EE, Brown SM, Kimak M, Ferrell RE, Manuck SB, Hariri AR. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Mol Psychiatry. 2009;14:60–70. doi: 10.1038/sj.mp.4002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Gerson AC, Gerring JP, Freund L, Joshi PT, Capozzoli J, Brady K, Denckla MB. The Children's Affective Lability Scale: a psychometric evaluation of reliability. Psychiatry Res. 1996;65:189–198. doi: 10.1016/s0165-1781(96)02851-x. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Tamminga C, Tohen M, Suppes T. Comparison of objective and subjective assessments of sleep time in subjects with bipolar disorder. J Affective Disorders. 2013;149:363–366. doi: 10.1016/j.jad.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gujar N, Yoo SS, Hu P, Walker MP. Sleep deprivation amplifies reactivity of brain reward networks, biasing the appraisal of positive emotional experiences. J Neurosci. 2011;31:4466–4474. doi: 10.1523/JNEUROSCI.3220-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AG. Sleep and circadian rhythms in bipolar disorder: seeking synchrony, harmony, and regulation. Am J Psychiatry. 2008;165:820–829. doi: 10.1176/appi.ajp.2008.08010098. [DOI] [PubMed] [Google Scholar]
- Hasler BP, Dahl RE, Holm SM, Jakubcak JL, Ryan ND, Silk JS, Phillips ML, Forbes EE. Weekend-weekday advances in sleep timing are associated with altered reward-related brain function in healthy adolescents. Biol Psychol. 2012;91:334–341. doi: 10.1016/j.biopsycho.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Soehner AM, Clark DB. Sleep and circadian contributions to adolescent alcohol use disorder. Alcohol. 2015;49:377–387. doi: 10.1016/j.alcohol.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. Guildford Press; New York, NY.: 2013. [Google Scholar]
- Hollingshead A. Four-factor index of social status. Yale University, Department of Sociology; New Haven CT.: 1975. [Google Scholar]
- Holm SM, Forbes EE, Ryan ND, Phillips ML, Tarr JA, Dahl RE. Reward-related brain function and sleep in pre/early pubertal and mid/late pubertal adolescents. J Adolesc Health. 2009;45:326–334. doi: 10.1016/j.jadohealth.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A, Cavanagh J, Scott J. A systematic review of manic and depressive prodromes. Journal of Affective Disorders. 2003;74:209–217. doi: 10.1016/s0165-0327(02)00266-5. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson JC, Axelson DA, Merranko J, Angulo M, Goldstein TR, Goldstein BI, Brent DA, Diler R, Hickey MB, Monk K, Sakolsky D, Kupfer DJ, Birmaher B. Differences in sleep disturbances among offspring of parents with and without bipolar disorder: association with conversion to bipolar disorder. Bipolar Disord. 2015;17:836–848. doi: 10.1111/bdi.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Soc Cogn Affect Neurosci. 2009;4:423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, Fan J. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2011;35:1219–1236. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunsford-Avery JR, Judd CM, Axelson DA, Miklowitz DJ. Sleep impairment, mood symptoms, and psychosocial functioning in adolescent bipolar disorder. Psychiatry Res. 2012;200:265–271. doi: 10.1016/j.psychres.2012.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N, Dinges DF, Basner M, Rao H. How acute total sleep loss affects the attending brain: a meta-analysis of neuroimaging studies. Sleep. 2015;38:233–240. doi: 10.5665/sleep.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Manelis A, Ladouceur CD, Graur S, Monk K, Bonar LK, Hickey MB, Dwojak AC, Axelson D, Goldstein BI, Goldstein TR, Bebko G, Bertocci MA, Gill MK, Birmaher B, Phillips ML. Altered functioning of reward circuitry in youth offspring of parents with bipolar disorder. Psychol Med. 2016;46:197–208. doi: 10.1017/S003329171500166X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manelis A, Ladouceur CD, Graur S, Monk K, Bonar LK, Hickey MB, Dwojak AC, Axelson D, Goldstein BI, Goldstein TR, Bebko G, Bertocci MA, Gill MK, Birmaher B, Phillips ML. In Press. Altered Functioning of Reward Circuitry in Youth Offspring of Parents with Bipolar Disorder. Psychological Medicine. doi: 10.1017/S003329171500166X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manelis A, Ladouceur CD, Graur S, Monk K, Bonar LK, Hickey MB, Dwojak AC, Axelson D, Goldstein BI, Goldstein TR, Bebko G, Bertocci MA, Hafeman DM, Gill MK, Birmaher B, Phillips ML. Altered amygdala-prefrontal response to facial emotion in offspring of parents with bipolar disorder. Brain. 2015 doi: 10.1093/brain/awv176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain structure & function. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RM, Petukhova M, Kessler RC. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64:543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullin BC, Phillips ML, Siegle GJ, Buysse DJ, Forbes EE, Franzen PL. Sleep deprivation amplifies striatal activation to monetary reward. Psychol Med. 2013;43:2215–2225. doi: 10.1017/S0033291712002875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzur A, Pace-Schott EF, Hobson JA. The prefrontal cortex in sleep. Trends Cogn Sci. 2002;6:475–481. doi: 10.1016/s1364-6613(02)01992-7. [DOI] [PubMed] [Google Scholar]
- Nixon GM, Thompson JM, Han DY, Becroft DM, Clark PM, Robinson E, Waldie KE, Wild CJ, Black PN, Mitchell EA. Short sleep duration in middle childhood: risk factors and consequences. Sleep. 2008;31:71–78. doi: 10.1093/sleep/31.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Almeida JR, Forbes EE, Versace A, Frank E, Labarbara EJ, Klein CR, Phillips ML. Waiting to win: elevated striatal and orbitofrontal cortical activity during reward anticipation in euthymic bipolar disorder adults. Bipolar Disord. 2012;14:249–260. doi: 10.1111/j.1399-5618.2012.01012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Young CB, Damme K. Elevated reward-related neural activation as a unique biological marker of bipolar disorder: Assessment and treatment implications. Behavioral Research and Therapy . 2014;62:74–87. doi: 10.1016/j.brat.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr Opin Neurobiol. 2004;14:769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. J Youth Adolesc. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Swartz HA. A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. Am J Psychiatry. 2014;171:829–843. doi: 10.1176/appi.ajp.2014.13081008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Travis MJ, Fagiolini A, Kupfer DJ. Medication effects in neuroimaging studies of bipolar disorder. Am J Psychiatry. 2008;165:313–320. doi: 10.1176/appi.ajp.2007.07071066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Sherwood RJ, Henriques JB, Davidson RJ. Frontal brain asymmetry and reward responsiveness: a source-localization study. Psychol Sci. 2005;16:805–813. doi: 10.1111/j.1467-9280.2005.01618.x. [DOI] [PubMed] [Google Scholar]
- Plante DT, Winkelman JW. Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry. 2008;165:830–843. doi: 10.1176/appi.ajp.2008.08010077. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb Cortex. 2006;16:1508–1521. doi: 10.1093/cercor/bhj088. [DOI] [PubMed] [Google Scholar]
- Raniti MB, Allen NB, Schwartz O, Waloszek JM, Byrne ML, Woods MJ, Bei B, Nicholas CL, Trinder J. Sleep Duration and Sleep Quality: Associations with Depressive Symptoms Across Adolescence. Behav Sleep Med. 2016:1–18. doi: 10.1080/15402002.2015.1120198. [DOI] [PubMed] [Google Scholar]
- Ritter PS, Marx C, Bauer M, Leopold K, Pfennig A. The role of disturbed sleep in the early recognition of bipolar disorder: a systematic review. Bipolar Disord. 2011;13:227–237. doi: 10.1111/j.1399-5618.2011.00917.x. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Grace AA. Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short MA, Gradisar M, Lack LC, Wright HR, Chatburn A. Estimating adolescent sleep patterns: parent reports versus adolescent self-report surveys, sleep diaries, and actigraphy. Nature and science of sleep. 2013;5:23–26. doi: 10.2147/NSS.S38369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MK, Kelley RG, Howe ME, Reiss AL, Gotlib IH, Chang KD. Reward processing in healthy offspring of parents with bipolar disorder. JAMA Psychiatry. 2014;71:1148–1156. doi: 10.1001/jamapsychiatry.2014.1031. [DOI] [PubMed] [Google Scholar]
- Sivertsen B, Harvey AG, Lundervold AJ, Hysing M. Sleep problems and depression in adolescence: results from a large population-based study of Norwegian adolescents aged 16-18 years. Eur Child Adolesc Psychiatry. 2014;23:681–689. doi: 10.1007/s00787-013-0502-y. [DOI] [PubMed] [Google Scholar]
- Telzer EH, Fuligni AJ, Lieberman MD, Galvan A. The effects of poor quality sleep on brain function and risk taking in adolescence. Neuroimage. 2013;71:275–283. doi: 10.1016/j.neuroimage.2013.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trost S, Diekhof EK, Zvonik K, Lewandowski M, Usher J, Keil M, Zilles D, Falkai P, Dechent P, Gruber O. Disturbed anterior prefrontal control of the mesolimbic reward system and increased impulsivity in bipolar disorder. Neuropsychopharmacology. 2014;39:1914–1923. doi: 10.1038/npp.2014.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatraman V, Chuah YM, Huettel SA, Chee MW. Sleep deprivation elevates expectation of gains and attenuates response to losses following risky decisions. Sleep. 2007;30:603–609. doi: 10.1093/sleep/30.5.603. [DOI] [PubMed] [Google Scholar]
- Venkatraman V, Huettel SA, Chuah LY, Payne JW, Chee MW. Sleep deprivation biases the neural mechanisms underlying economic preferences. J. Neurosci. 2011a;31:3712–3718. doi: 10.1523/JNEUROSCI.4407-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatraman V, Huettel SA, Chuah LY, Payne JW, Chee MW. Sleep deprivation biases the neural mechanisms underlying economic preferences. J Neurosci. 2011b;31:3712–3718. doi: 10.1523/JNEUROSCI.4407-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. The Psychological Corporation; San Antonio, TX.: 1999. [Google Scholar]
- Weissman MM, Wickramaratne P, Adams P, Wolk S, Verdeli H, Olfson M. Brief screening for family psychiatric history: the family history screen. Arch Gen Psychiatry. 2000;57:675–682. doi: 10.1001/archpsyc.57.7.675. [DOI] [PubMed] [Google Scholar]
- Youngstrom EA, Frazier TW, Demeter C, Calabrese JR, Findling RL. Developing a 10-item mania scale from the Parent General Behavior Inventory for children and adolescents. J Clin Psychiatry. 2008;69:831–839. doi: 10.4088/jcp.v69n0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohar D, Tzischinsky O, Epstein R, Lavie P. The effects of sleep loss on medical residents' emotional reactions to work events: a cognitive-energy model. Sleep. 2005;28:47–54. doi: 10.1093/sleep/28.1.47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.