Abstract
While overwhelmingly behavior is similar in males and females, and correspondingly the brains are similar, sex differences permeate both brain and behavioral measures and these differences have been the focus of increasing scrutiny by neuroscientists. Here we describe milestones of over three decades of research in brain and behavior. This research was necessarily bound by available methodology, and we began by indirect behavioral indicators of brain function such as handedness. We proceeded to using neuropsychological batteries and then to structural and functional neuroimaging that provided the foundations of a cognitive neuroscience based computerized neurocognitive battery. Sex differences were apparent and consistent in neurocognitive measures, with females performing better on memory and social cognition tasks and males on spatial processing and motor speed. Sex differences were also prominent on all major brain parameters, including higher rates of cerebral blood flow, higher percent of gray matter tissue and higher inter-hemispheric connectivity in females compared to higher percent of white matter and greater intra-hemispheric connectivity, as well as higher glucose metabolism in limbic regions in males. Many of these differences are present in childhood but they become more prominent with adolescence, perhaps linked to puberty. Together they indicate complementarity between the sexes that would result in higher adaptive diversity.
Keywords: Brain, Neuroimaging, Cognitive Development, Brain Development
Introduction
There is a vast literature on sex differences in humans that encompasses multiple domains pertinent to health and disease. The study of brain and behavior is a topic that has been investigated with diverse measures that reflect on the progress in tools and technology. As researchers who have examined sex differences over the decades, there are two major themes that we have observed in relation to the presentation of our findings on sex differences. First, the findings were consistent across methods applied. In sufficiently powered samples sex differences are observed in multiple parameters of behavior and brain function. The second theme concerns the reactions generated at times from some in the field and the public questioning the existence of sex differences or even the merit of studying them. In this review we will highlight our major findings on sex differences over the years, offer a synthesis and outline a perspective on challenges and future directions.
Laterality and Behavioral Measures
Investigators in the 1970s already recognized that understanding brain function is a pathway towards developing a science of behavior. The tools available at the time, however, were limited. Most knowledge in the budding field of neuropsychology was gained from lesion studies in humans and animals, in which behavioral sequelae of disruption of brain systems were documented. Such studies were limited in sample sizes and were not very informative on how individual differences in brain organization affect normal everyday functioning.
A salient factor that emerged from human clinical studies was laterality. Shortly following Broca’s discovery of left hemispheric dominance for language, clinicians noted that left handers do not always show impaired language following left hemispheric lesions, and a link has been postulated between the hemisphere controlling the writing hand and that controlling language, the left dominant hemisphere. However, the clinical literature also suggested individual differences in the degree of laterality associated with varied response to unilateral brain damage and recovery. For example, it was observed that females and left-handers show less deficits and faster recovery.
For neuropsychologists such findings offered a means to investigate relations among these parameters of brain organization and establish how they affect cognition. Gur and Gur (1977) first examined handedness and eye dominance, as measures of brain laterality in healthy men and women and reported a stronger association between eye dominance and handedness in females and a nearly double proportion of left handedness in males compared to females. They interpreted these results to support greater vulnerability in males to neurodevelopmental events affecting laterality. More detailed behavioral measures indicative of brain function were made available with the advent of neuropsychological test batteries.
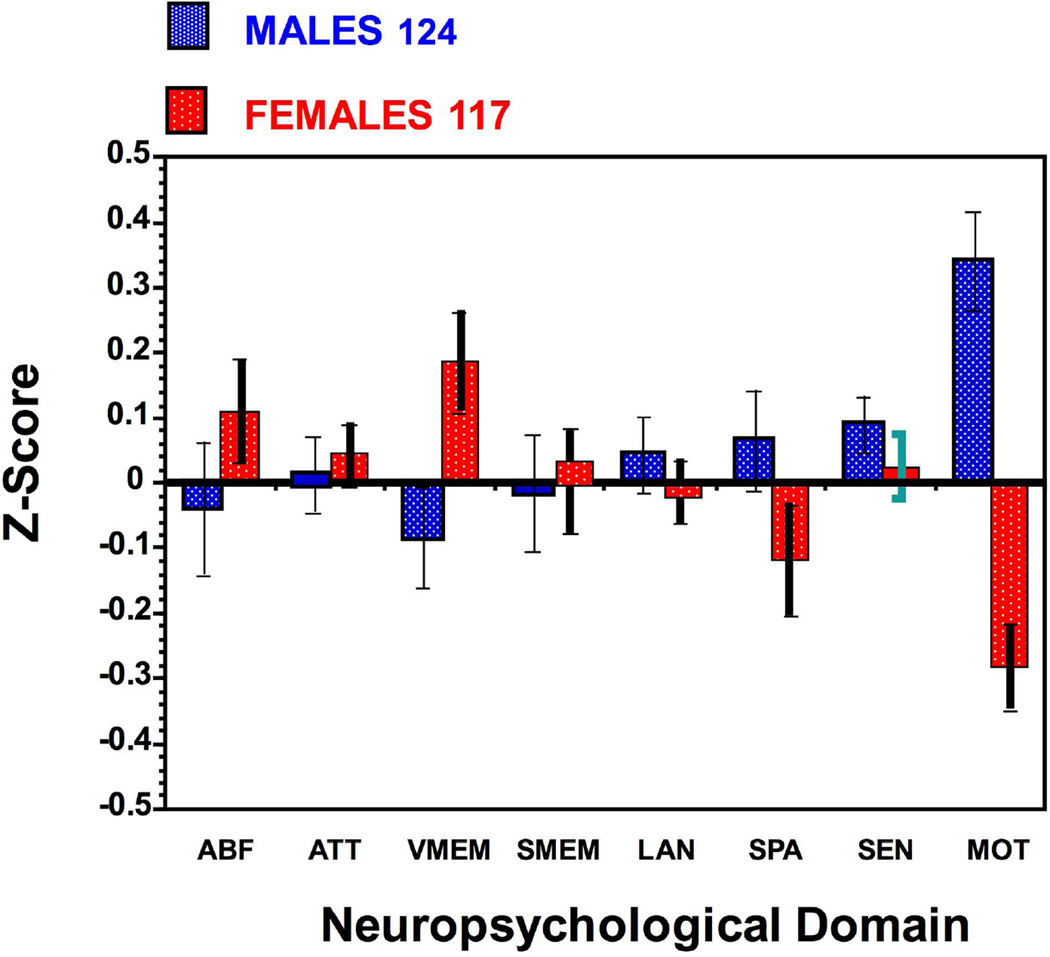
Neuropsychological batteries were designed to evaluate the nature and extant of brain dysfunction in clinical populations. Saykin and colleague (1995) applied such a battery to a normative sample of healthy men (n=76) and women (n=55) age range 18–49. Sex differences were prominent in verbal memory where females outperformed males and in spatial processing and motor speed, where males outperformed females. This sample was expanded to 124 men and 117 women and the results were sustained (Figure 1).
Figure 1.
Sex differences in neuropsychological performance. Means (±SEM) are shown for males (blue) and females (red) on ABF=Abstraction and Mental Flexibility; ATT=Attention; VME=Verbal Memory; FME=Face Memory; SME=Spatial Memory; LAN= Language Reasoning; SPA=Spatial Processing; SEN=Sensory function; MOT=Motor Speed.
The traditional printed neuropsychological batteries have contributed to the literature but have several shortcomings in research. They are lengthy, require expert administrators and professional scorers, and are prone to data handling errors. Gur and colleague (2001) developed a brief computerized neurocognitive "scan" consisting of tasks that were used in functional neuroimaging and that assesses similar domains to traditional batteries with adequate reliability. This short computerized neurocognitive battery (CNB) and a traditional battery were administered to a sample of 92 healthy individuals, 44 men and 48 women, in a counterbalanced order. Both approaches showed a significant "sex-typical" gradient, with women outperforming men in verbal memory relative to spatial tasks. Both methods also yielded similar profiles of sex differences, with the additional computerized measure of face memory showing better performance in women. Age effects were evident for both methods, but the computerized scan isolated the effects to speed rather than accuracy. This study indicated that the CNB has favorable reliability and construct validity and can be applied efficiently to study healthy variability related to age and gender. Gur and colleagues (2010) have upgraded and augmented the CNB adding social cognition measures and validating the new version.
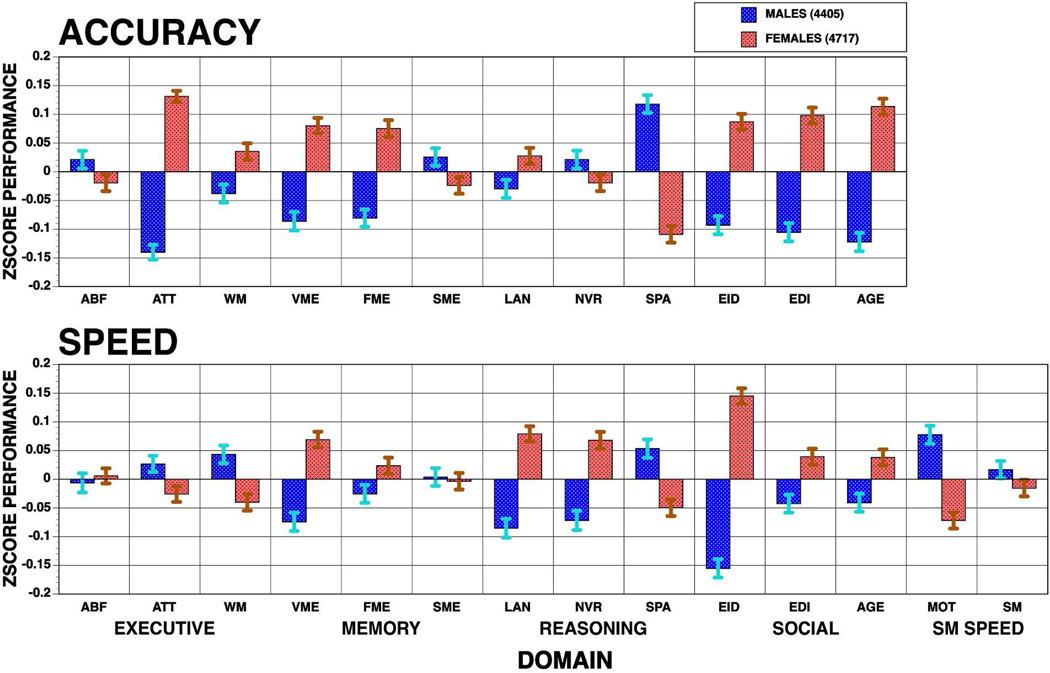
The computerized format of the CNB and its availability on the web resulted in multiple studies in normative and clinical populations. Some of these have examined sex differences and reported consistent findings. Gur and colleagues (2012) evaluated age group effects and sex differences by applying a 1-hour CNB in youths from the Philadelphia Neurodevelopmental Cohort (PNC). This CNB provides measures of performance accuracy and response time for executive-control, episodic memory, complex cognition, social cognition, and sensorimotor speed domains. The first phase of this population-based sample included 3,500 (46.3% males, 53.7% females) genotyped youths ages 8–21 years (Figure 2).
Figure 2.
Sex difference in the computerized neurocognitive battery administered to the Philadelphia Neurodevelopmental Cohort. Means (±SEM) of z-scores for accuracy (top panel) and speed (bottom panel) for females (red bars) and males (blue bars) across the sample on each behavioral domain. ABF=Abstraction and Mental Flexibility; ATT=Attention; WM=Working Memory; VME=Verbal Memory; FME=Face Memory; SME=Spatial Memory; LAN= Language Reasoning; NVR=Nonverbal Reasoning; SPA=Spatial Processing; EMI= Emotion Identification; EMD=Emotion Differentiation; AGD=Age Differentiation; SM=Sensorimotor Speed; MOT=Motor Speed. Stars indicate significant (p<.05) sex differences, dark stars indicate better performance in females, gray stars better performance in males.
Substantial improvement with age was evident for both accuracy and speed, but the rates varied by domain. The most pronounced improvement was noted in executive control functions, specifically attention, and in motor speed, with some effect sizes exceeding 1.8 standard deviation units. The least pronounced age group effect was in memory, where only face memory showed a large effect size on improved accuracy. Sex differences had smaller effect sizes but were nonetheless notable, with females outperforming males on attention, word and face memory, reasoning speed, and all social cognition tests and males outperforming females in spatial processing and motor speed. These sex differences in most domains were seen already at the youngest age groups, and age group × sex interactions indicated divergence at the oldest groups with females becoming faster but less accurate than males. Thus, performance was sexually modulated and most sex differences were apparent by early adolescence.
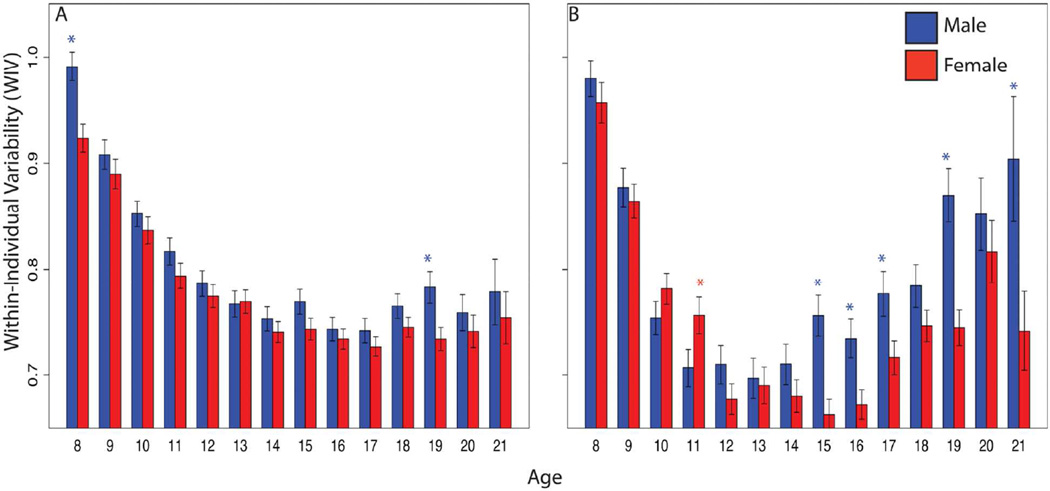
As developmental studies focus on average performance in single domains, they ignore consistency of performance across domains. Within-individual variability (WIV) provides an index of evenness in performance and is a potential marker of development. Developmental psychologists have described initially uneven performance in childhood that becomes more even across domains with maturation, and studies of aging describe increase in WIV associated with age associated cognitive decline. Roalf and colleagues (2014) examined WIV in the Penn CNB results from the entire PNC sample of 9138 youths (4685 females, 4325 males). As expected, performance improved with age, with both accuracy and speed peaking in young adulthood. WIV, however, showed a U-shaped course: highest in childhood, declining yearly into mid-adolescence, and increasing again into adulthood. Young females outperformed and were less variable than males, but by early adulthood male performance matched that of females despite being more variable (Figure 3). Notably, WIV is higher in males than females across age cohorts indicating that males are more likely to be cognitive specialists while females have more even performance across domains and can thus be characterized, on average, as cognitive generalists.
Figure 3.
Within-individual variability (WIV) in performance of males (blue) and females (red) in the Philadelphia Neurodevelopmental Cohort on the Computerized Neurocognitive Battery domains for Accuracy (a) and Speed (b). (From Roalf DR, et al. Neuropsychology. 2014;28:506–518).
In summary, behavioral measures linked to brain function indicate significant sex differences in performance that emerge early in development with domain variability that relates to brain maturation. Notably, our findings are consistent with a robust literature documenting sex difference in laterality and behavior (e.g., Halpern, et al., 2007; Hines, 2010; Linn & Petersen, 1985; Moreno-Briseño, et al., 2010; Thomas & French, 1985; Voyer, Voyer & Bryden, 1995; Williams et al., 2008). These findings support the notion that males and females have complementary neurocognitive abilities with females being more generalists and outperforming males in memory and social cognition tasks and males being more specialists and performing better than females on spatial and motor tasks.
Neuroimaging
The advent of neuroimaging has offered the field an increasing number of rigorous tools for studying brain parameters directly, and strengthening the links between them and behavioral measures. Neuroscientists began to incorporate neuroimaging in the study of brain and behavior in health and disease. The brain could be studied like any other organ with measures of its structure and function that could be linked to its product – behavior. Here we will review our most salient findings on sex differences in brain structure and function.
Structural Neuroimaging
The first discovery of sex differences in brain structure was obtained from a method primarily designed to measure cerebral blood flow, Xenon-133 inhalation, but which nonetheless yielded an anatomic brain parameter related to the percent of tissue with fast perfusion characteristic, presumably gray matter (GM). Gur and colleagues (1980) found with this method greater percent GM in language areas of the left hemisphere, in a male sample. At the time, investigators were admonished to apply new techniques only to males. In a subsequent study of 62 healthy individuals, of both sexes, Gur and colleagues (1982) replicated the hemispheric asymmetry effect but also found that females had a higher percent of GM across brain regions sampled.
Magnetic resonance imaging (MRI) has become the most widely applied technology in the study of neuroanatomy enabling evaluation of GM and white matter (WM) parameters. Earlier samples were relatively small and there were limited image processing tools. Yet, sex differences were evident. Gur and colleagues (1991) evaluated a prospective sample of 69 healthy adults, 34 men, 35 women, aged 18–80 years. Volumes of the entire cranium were obtained by a segmentation algorithm that used proton density and T2 pixel values to correct for field inhomogeneities. Men had 91 ml higher brain and 20 ml higher CSF volume than women. Age was negatively correlated with brain volume and positively correlated with CSF volume. The slope of the regression line with age for CSF was steeper for men than women. The greatest amount of atrophy in elderly men was in the left hemisphere, whereas in women age effects were symmetric. The findings may point to neuroanatomic substrates of hemispheric specialization and sex differences in age-related changes in brain function. They suggest that women are less vulnerable to age-related changes in cognitive abilities, whereas men are particularly susceptible to aging effects on left hemispheric functions.
Subsequently, Cowell and colleagues (1994) investigated effects of age and sex on regional brain structure focusing on the frontal and temporal lobes. Hemispheric volumes were obtained from MRIs of 96 young (53 men, 43 women; aged 18–40 years) and 34 older (17 men, 17 women; aged 41–80) healthy volunteers. The results indicated that age-related reductions in brain volume were lateralized, region specific and different between males and females. Greater decrements in brain volume occurred with age in the frontal lobe than in the temporal lobe. Age-related reductions in both regions were greater in men than in women, demonstrating that sex differences in human neuroanatomy are not fixed, but continue to change throughout adulthood. The possibility that gonadal hormones play a role in the promotion or prevention of neural atrophy with aging was raised.
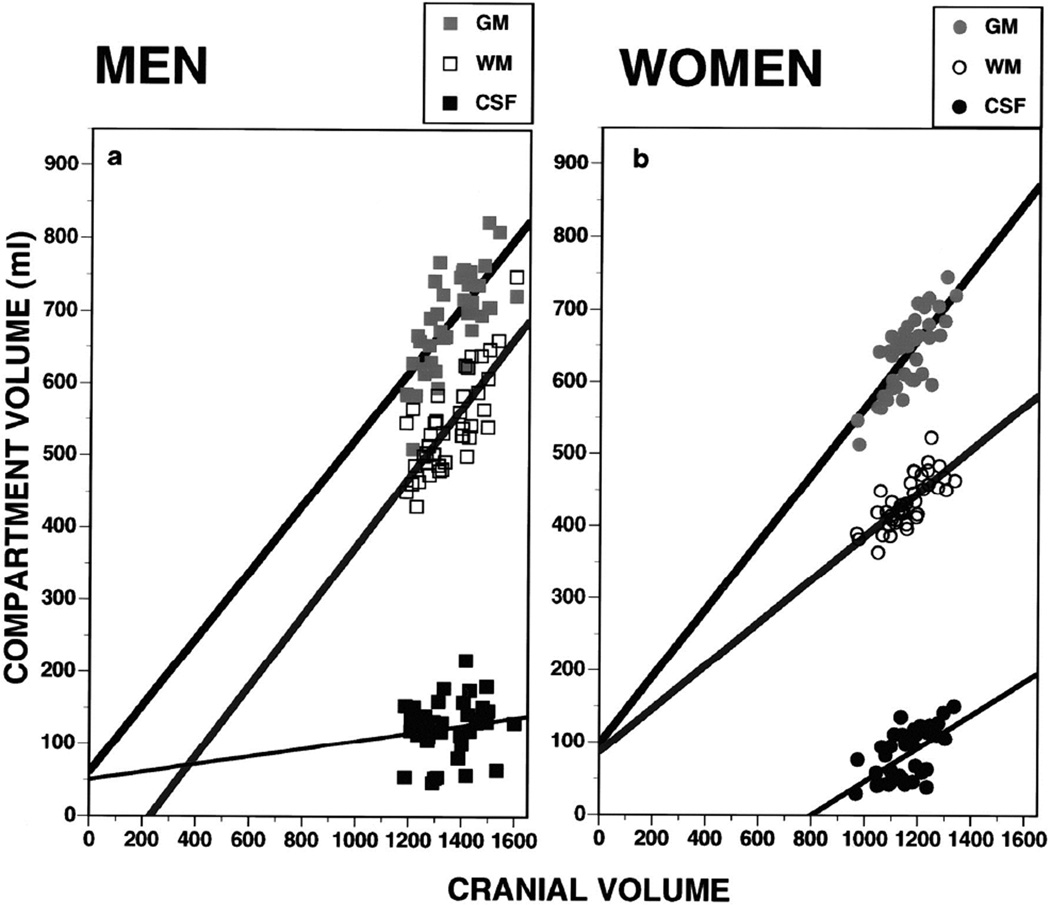
Notably, at this stage of research, differences in volumes of the major cranial compartments have not been examined for the entire brain in association with cognitive performance. Thus, Gur and colleagues (1999) used volumetric segmentation of MRI scans in healthy volunteers (40 men, 40 women) age 18–45. Supertentorial volume was segmented into GM, WM, and CSF. We confirmed that women have a higher percentage of GM, whereas men have a higher percentage of WM and of CSF. These differences sustained a correction for total intracranial volume. In men the slope of the relation between cranial volume and GM paralleled that for WM, whereas in women the increase in WM as a function of cranial volume was at a lower rate (Figure 4). In men the percentage of GM was higher in the left hemisphere, the percentage of WM was symmetric, and the percentage of CSF was higher in the right hemisphere. Women showed no asymmetries. Both GM and WM volumes correlated moderately with global, verbal, and spatial performance across groups. However, the regression of cognitive performance and WM volume was significantly steeper in women. Because GM consists of the somatodendritic tissue of neurons whereas WM comprises myelinated connecting axons, the higher percentage of GM makes more tissue available for computation relative to transfer across distant regions. This could compensate for smaller intracranial space in women. Thus, sex difference in the percentage and asymmetry of the principal cranial tissue volumes may contribute to differences in cognitive functioning.
Figure 4.
Scatterplots and regression lines for gray matter (GM), white matter (WM), and CSF against cranial volumes in men (left, squares) and women (right, circles). (From Ruben C. Gur et al. J. Neurosci. 1999;19:4065–4072. ©1999 by Society for Neuroscience)
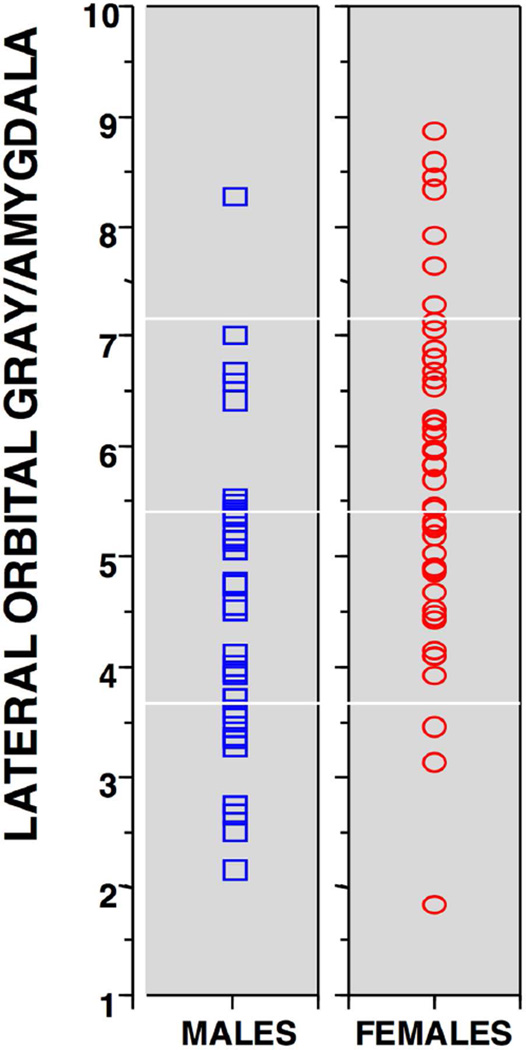
Men and women differ in emotion processing, including perception, experience and expression, most notably reflected in greater male aggression. Gur and colleagues (2002) examined temporo-limbic and prefrontal structures volumetrically in a well-characterized sample of healthy adults, applying morphometric methods across cerebral regions that regulate emotions. Quantitative MRI was performed in 116 healthy adults, 57 men and 59 women, age range 18–49 years. We used reliable methods of region of interest identification to examine sex differences in volume of temporo-limbic and frontal regions. An automated tissue segmentation procedure was used to obtain separate measurements for GM and WM. After correcting for cranial volume, men and women had identical volumes of amygdala and hippocampus, as well as dorsal prefrontal cortex. However, women had larger orbital frontal cortices than men, resulting in highly significant difference in the ratio of orbital GM to amygdala volume (Figure 5). The larger volume of cortex devoted to emotional modulation may relate to behavioral evidence for sex differences in emotion processing.
Figure 5.
Scatterplots showing the distribution of the orbitofrontal to amygdala volume ratios in males (blue squares) and females (red circles). (From Ruben C. Gur et al. Cereb. Cortex 2002;12:998–1003, © Oxford University Press)
The PNC included a subsample of about 1600 youths who also received multimodal imaging, providing an opportunity to examine sex difference in brain development. Satterthwaite and colleagues (2014) examined the effects of puberty on the morphology of the hippocampus and amygdala in males and females. T1-weighted structural MRIs were obtained in a sample of 524 pre- and post-pubertal individuals ages 10 – 22 years. Hippocampal and amygdala volume and shape were quantified and scaled by intracranial volume. Pre-pubertal males and females had similar hippocampal volumes, whereas post-pubertal females had significantly larger bilateral hippocampi, resulting in a significant puberty-by-sex interaction even when controlling for age effects and age-by-sex interaction. This effect was regionally specific and was not apparent in the amygdala. Vertex analysis revealed that post-pubertal differences were most prominent in the lateral aspect of the hippocampus bilaterally, corresponding to the CA1 subfield. Thus, regionally specific sex differences in the effect of puberty on the hippocampus may relate to better performance of females on memory tests.
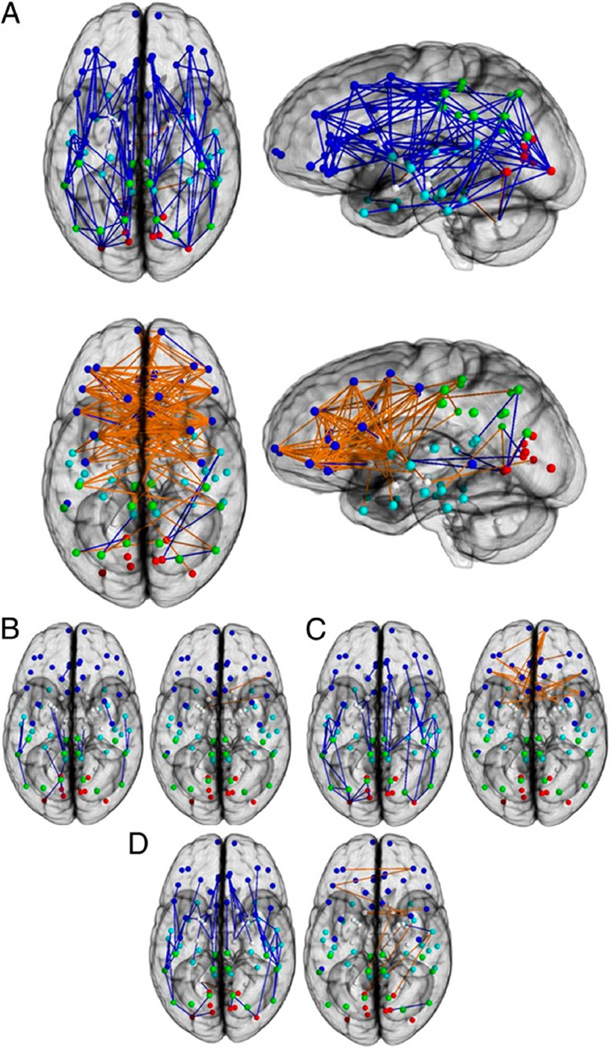
Gur and colleagues (1999) noted that the higher percent of WM volume in males did not include the corpus callosum, where there is evidence that females have similar or even larger volumes. We proposed the hypothesis that female brains are optimal for inter-hemispheric communication whereas male brains are structured for better within hemisphere signaling. Ingalhalikar and colleague (2014) modeled the structural connectome using diffusion tensor imaging in a sample of 949 youths of the PNC, 428 males and 521 females, age 8–22 years, and discovered unique sex differences in brain connectivity during the course of development. Connection-wise statistical analysis, as well as analysis of regional and global network measures, yielded a comprehensive description of network characteristics. In all supratentorial regions, males had greater within-hemispheric connectivity, as well as enhanced modularity and transitivity, whereas between-hemispheric connectivity and cross-module participation predominated in females. However, this effect was reversed in the cerebellar connections, which are notably ipsilateral. Analysis of age related effects demonstrated differences in trajectory between males and females mainly in adolescence and in young adulthood. Overall, the results suggest that male brains are structured to facilitate connectivity between perception and coordinated action, whereas female brains are designed to facilitate communication between analytical and intuitive processing modes (Figure 6).
Figure 6.
Connection-wise analysis. (A) Brain networks show increased connectivity in males (Upper) and females (Lower). Analysis on the child (B), adolescent (C), and young adult (D) groups is shown. Intrahemispheric connections are shown in blue, and interhemispheric connections are shown in orange. The depicted edges are those that survived permutation testing at P = 0.05. Node color representations are as follows: light blue, frontal; cyan, temporal; green, parietal; red, occipital; white, subcortical. GM, gray matter. (From Ingalhalikar M et al. Proc Natl Acad Sci U S A. 2014;111(2):823–8).
In a subsequent analysis, Tunc and colleagues (2016) further examined brain networks in the PNC in relation to sex differences. When using subnetworks that are defined over functional and behavioral domains, increased structural connectivity was observed in males related to the motor, sensory and executive function subnetworks. In females, subnetworks associated with social motivation, attention and memory tasks had higher connectivity. Males showed higher modularity compared to females, with females having higher inter-modular connectivity. Applying multivariate analysis, an increasing separation between males and females in the course of development was noted, not only in behavioral patterns but also in brain structure. The behavioral and structural patterns correlated with each other, establishing a reliable link between brain and behavior.
Neuroanatomic findings in MRI studies indicate sex differences across methods for measuring gray matter and white matter volume as well as white matter tracts. Such neural differences are related to behavioral measures where sex differences are evident. The findings of sex differences and their developmental course, including age-related effects on GM and WM are consistent across laboratories, corroborating our reports highlighted here (e.g., Blakemore, Burnett & Dahl, 2010; De Bellis et al., 2001; Clayden et al., 2012; Dennison et al., 2013; Giedd et al. 1999; Herting et al., 2012; Hsu et al., 2008; Lenroot et al. 2007; Paus, 2005; Sowell et al., 2003; Vijayakumar et al., 2016).
Functional Neuroimaging
Cerebral Blood flow
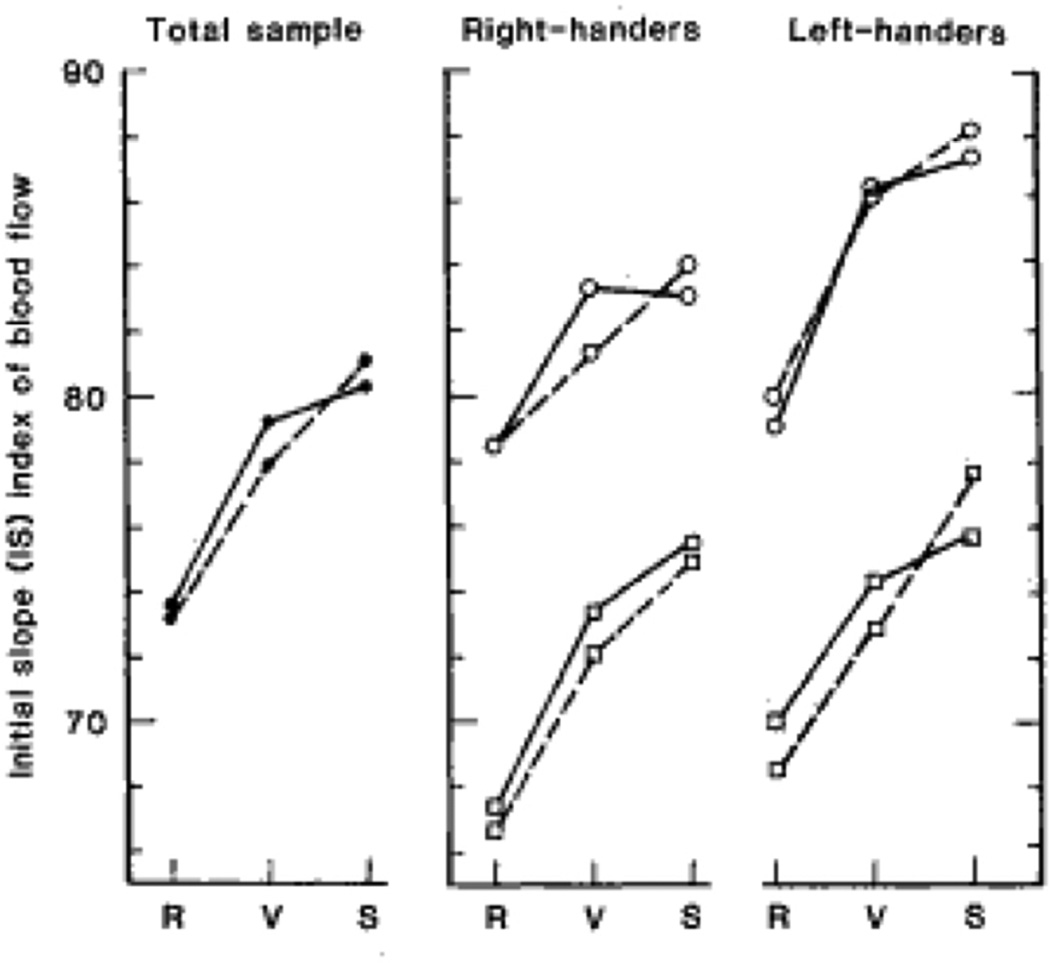
Using an early method, for measuring cerebral blood flow (CBF), the Xenon-133 inhalation, Gur and colleague (1982) showed that cognitive activity resulted in increased flow of blood to the cerebral hemispheres. The increase was greater to the left hemisphere for a verbal task and greater to the right hemisphere for a spatial task. The direction and degree of hemispheric flow asymmetry were influenced by sex and handedness, with females having a higher rate of blood flow per unit weight of brain (Figure 7). That finding, which was the first to show sex differences in rate of CBF, created a stir in the news media and a heated debate in which the very legitimacy of probing for sex differences in brain parameters was questioned.
Figure 7.
Initial slope (lS) index of cerebral gray matter blood flow to the left (solid lines) and right (dashed line) hemispheres for the total sample (left panel) and for right- and left-handed females (circles) and right- and left-handed males (squares) during resting baseline (R) and performance of verbal (V), and spatial tasks (S). (from Gur et al. Science. 1982;217:659–661.1982, Figure 1) (Permission not required per publisher website)
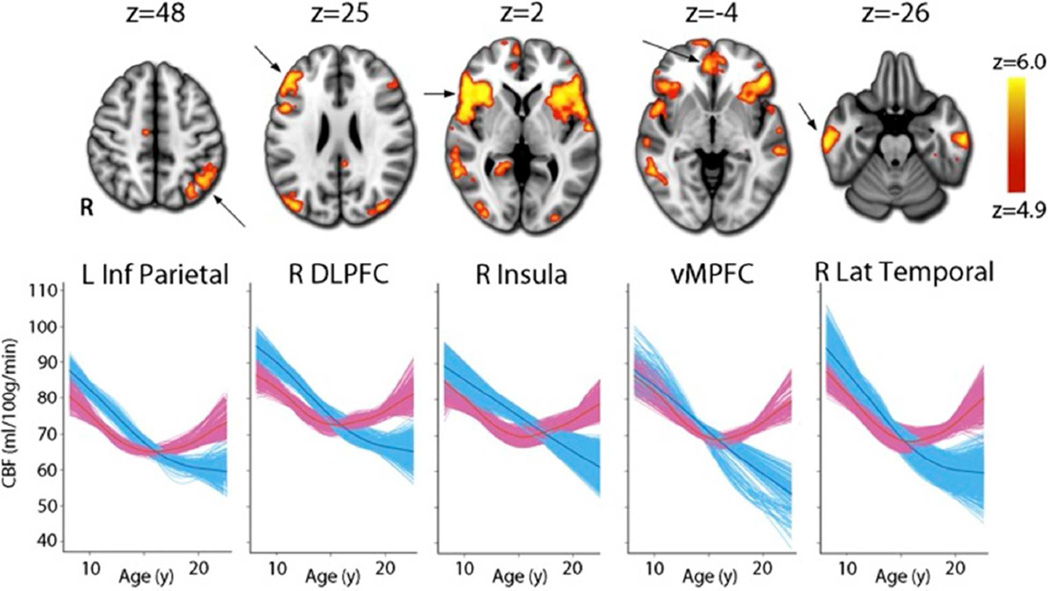
A limitation of isotopic methods for measuring cerebral physiology is that they necessitate exposure to ionizing radiation and hence are not applicable in children. Arterial spin labeling (ASL) using MRI permits noninvasive quantification of cerebral perfusion. Using ASL, Satterthwaite and colleagues (2014) examined the effects of puberty on CBF in the PNC. The sample included 922 youths, 518 females and 404 males, ages 8–22 years. In general, CBF shows decline from childhood onward but males and females had divergent nonlinear trajectories in CBF evolution with development. Seventeen brain regions, including hubs of the executive and default mode networks, showed a robust nonlinear age-by-sex interaction. Notably, within these regions the decline in CBF was similar between males and females in early puberty and only diverged in mid-puberty, with CBF continuing to decline in males but increasing in females (Figure 8). These results delineate sex-specific growth curves for CBF during youth and for the first time link such differential patterns of development to the effects of puberty. They also support the finding of higher CBF in adult females reported by Gur and colleagues (1982) with Xenon-133 clearance and by Ragland and colleagues (2000) with positron emission tomography (PET) using oxygen-15 labeled water.
Figure 8.
A voxelwise generalized additive model (GAM) reveals that the developmental pattern of CBF differs between males (blue) and females (pink) in multiple regions within heteromodal association cortex. Whereas CBF values decline in males until late adolescence, CBF in females declines until mid-adolescence but increases thereafter. Images thresholded at z > 4.9 (Bonferroni corrected P < 0.05), k > 100; age plots in bottom row depict GAM fit for each voxel in a specified cluster, stratified by sex and adjusted for model covariates. (From Satterthwaite TD et al. Proc Natl Acad Sci U S A. 2014;111(23):8643–8).
Cerebral glucose metabolism
PET was used by Gur and colleagues (1995) to evaluate the regional distribution of cerebral glucose metabolism using the fluorine-18 fluoro-deoxy-glucose (FDG) method in 61 healthy adults at rest. Although the absolute rate of glucose metabolism was identical in men and women and the profile of metabolic activity was similar for all cortical regions, sex differences were prominent in components of the limbic system. Men had relatively higher metabolism than women in temporal-limbic regions and cerebellum while women had higher metabolism in cingulate regions. In both sexes, metabolism was relatively higher in left association cortices and the cingulate region and in right ventro-temporal limbic regions and their projections. Thus, at a resting state males had greater metabolic activity in the “older” regions of the emotional brain while females had greater activity in the “newer” more refined limbic components. These results are consistent with the hypothesis that sex differences in emotional processing have biological substrates.
Receptors
The growing interests in distinguishing the biological bases of sex differences in behavior including cognitive abilities, particularly memory, suggest a role for the dopaminergic system. Mozley and colleagues (2001) investigated the relationship between cognition and dopamine transporter availability in healthy men and women. Dopamine transporter levels were measured with a technetium-99m radiolabeled analog of cocaine, TRODAT-1, in 66 healthy volunteers (30 men and 36 women). A neuropsychological battery designed to target functions associated with dopaminergic activity was administered during the uptake interval between the radiopharmaceutical injection and image acquisition. Women and younger participants had higher dopamine availability in the caudate nucleus, and these groups also performed better on verbal learning tasks. Furthermore, dopamine transporter availability was correlated with learning performance within groups. Relationships between dopamine availability in the caudate and putamen and executive and motor functioning were observed in women, but not in men. Thus, there are age effects and sex differences in the neuromodulatory influences of dopamine on behavior in humans. Men showed greater decline in dopamine availability with age than did women, which correlated with greater reduction in memory performance.
Functional connectivity
Resting state functional connectivity MRI (rsfc-MRI) can help identify clusters of regions that show synchronized activation during a resting baseline (“default mode”) condition. The rsfc-MRI was evaluated for sex differences in the PNC sample by Satterthwaite and colleagues (2015). In a sample of 674 participants, 362 females and 312 males, ages 9–22, sex differences in cognitive profiles were related to multivariate patterns of rsfc-MRI. As expected based on the extensive neurobehavioral studies summarized above, males outperformed females on motor and spatial processing tasks while females were more efficient in emotion identification and nonverbal reasoning tasks. Sex differences were prominent in the rsfc-MRI data, with males displaying more between-module connectivity with females demonstrating more within-module connectivity. Multivariate pattern analysis using support vector machine learning classified subject sex on the basis of their cognitive profile with 63% accuracy but was more accurate using functional connectivity data (71% accuracy). Moreover, the degree to which a participant's cognitive profile was "male" or "female" was significantly related to the masculinity or femininity of their pattern of brain connectivity.
These results demonstrated for the first time that sex differences in patterns of cognition are in part represented on a neural level through divergent patterns of brain connectivity. Several methods for evaluating brain function with diverse neuroimaging methodologies indicate that there are sex differences in cerebral blood flow and functional connectivity. The literature on early development is limited because the earlier methodologies, which used ionizing radiation, could not be administered to healthy children. The more recent availability of functional MRI has opened the road for studying the development of sex differences in brain function and functional connectivity and the results we report are consistent with the literature, which is more limited for these specific parameters (e.g., Biswal et al., 2010; Taki et al., 2011;Tian et al., 2011; Wang et al., 2012; Wu et al., 2013; Zuo et al., 2010).
Summary
The studies highlighted above survey over three decades of research examining sex differences in healthy people on parameters of behavior and brain structure and function. These results are consistent and converge to indicate that, against a general background of similarity in behavioral and brain measures; there are distinct differences in major parameters that produce a complementarity between the sexes. Behaviorally, males are on average better at tasks requiring spatial processing and motor speed whereas females outperform males on tasks requiring verbal and facial memory and social cognition. Some of these differences can be linked to sex differences in brain parameters. For example, differences in gray and white matter volumes have been related to performance on verbal and spatial tasks, sex differences in hippocampal volume and in dopamine availability have been linked to memory performance, and sex differences in limbic activity and orbitofrontal volume have been associated with differences in emotion regulation. Increasingly, we have capitalized on more complex analyses of connectivity using graph theoretical methods and concepts to understand brain-behavior relations, and these analyses confirmed and extended our knowledge of sex differences in brain-behavior relationships. These studies indicated that structurally male brains are optimized to process information within hemispheres while in females the predominant connections are inter-hemispheric. Functional connectivity analyses likewise reveled sex differences and these have yet to be related to the structural connectivity findings.
The increasing availability of large-scale developmental studies has also enabled the evaluation of the developmental course of sex differences in brain and behavior. These studies indicate that many sex differences exist in childhood and become more pronounced in adolescence, perhaps associated with puberty. At the other end of the life cycle, female brains appear more robust against aging effects with higher cerebral blood flow and less reduction in volume and dopamine availability. This information on healthy people is important for understanding how sex differences affect health and society and is prerequisite for understanding effects of brain disorders across the lifespan.
Challenges and Future Directions
A major challenge facing the investigation of sex differences in brain and behavior is the paucity of studies that permit integration across brain and behavior parameters with hormonal and environmental effects. The studies to date provide demonstrations of somewhat disjointed effects in relatively small samples, and few direct causal links, which bridge from the neural to the behavioral level, have been established. In this context, most of our knowledge on the developmental course of behavioral and brain parameters comes from cross-sectional designs. To appreciate developmental trajectories requires large-scale longitudinal projects that also permit cross modality integration of data.
The scientific study of sex differences is politically contentious due to concerns that the results of this research will harm the hard fought struggle to achieve greater sexual equality. There is a perception that biological differences can justify unequal opportunities for females. This is a misperception. A purpose of civilized society is to achieve equal treatment for all individuals across demographic characteristics including sex and sexual identity. Sex differences are not a competition; one is not better than the other just because they differ. The effect sizes indicate a multitude of exceptions to norms. Understanding how biology and environment interact in shaping sex differences in behavior should lead to greater appreciation of similarities and differences and not be the basis for discriminatory practices. Indeed, unequal treatment of genders would directly contravene what our research would suggest as optimal for civilized societies. Treating differences in group averages as if they apply to each individual is an obvious fallacy, and instead attention should be focused on the behavioral product rather than the sex or other demographic characteristics of the individual producing the behavior. With such an attitude we can marvel at the evolutionary complementarity of sex differences that produces a more diverse and adaptive society.
Significance Statement.
Differences between males and females in behavior have been known for millennia and have been studied from the inception of behavioral sciences. The development of methods for structural and functional neuroimaging enabled identifying differences in brain systems that may account for some of the behavioral differences. Understanding how sex differences in behavior relate to brain structure and function is important for appreciating the evolutionary advantages of complementary differences as they are shaped during the lifespan. Such understanding is needed in order to appreciate sex differences in the prevalence and severity of brain disorders as they evolve during the lifespan.
Acknowledgments
We thank the many colleagues and dedicated staff who made the research we report here possible and the many research participants who offered their time and efforts. Supported by NIMH Grants P50MH096891 and R01 MH107235.
Footnotes
Conflict of Interest Statement
Ruben C. Gur received royalties from the Brain Resource Center and funding from Mindprint through Penn’s Center for Innovation.
Raquel E. Gur participated in an Advisory Board for Otsuka.
Author’s roles
Both authors had full access to all the data reported in the manuscript and take responsibility for the integrity of the data and the accuracy of the data analysis. The authors contributed equally to the concept and design of the manuscript, as well as drafting of the manuscript and revision of the manuscript for important intellectual content.
Literature Cited
- Biswal BB, Mennes M, Zuo X-N, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, et al. Toward discovery science of human brain function. Proc Natl Acad Sci USA. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S-J, Burnett S, Dahl RE. The role of puberty in the developing adolescent brain. Hum Brain Mapp. 2010;31:926–933. doi: 10.1002/hbm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayden JD, Jentschke S, Muñoz M, Cooper JM, Chadwick MJ, Banks T, Clark CA, Vargha-Khadem F. Normative development of white matter tracts: similarities and differences in relation to age, gender, and intelligence. Cereb Cortex. 2012;22:1738–1747. doi: 10.1093/cercor/bhr243. [DOI] [PubMed] [Google Scholar]
- Cowell PE, Turetsky BI, Gur RC, Grossman RI, Shtasel DL, Gur RE. Sex differences in aging of the human frontal and temporal lobes. J Neurosci. 1994;14(8):4748–4755. doi: 10.1523/JNEUROSCI.14-08-04748.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Beers SR, Hall J, Frustaci K, Masalehdan A, et al. Sex differences in brain maturation during childhood and adolescence. Cereb Cortex. 2001;11:552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- Dennison M, Whittle S, Yucel M, Vijayakumar N, Kline A, Simmons J, Allen NB. Mapping subcortical brain maturation during adolescence: evidence of hemisphere- and sex-specific longitudinal changes. Developmental Science. 2013;16:772–791. doi: 10.1111/desc.12057. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Rajapakse JC, Vaituzis AC, Liu H, Castellanos FX. Development of the human corpus callosum during childhood and adolescence: a longitudinal MRI study. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1999;23:571–588. doi: 10.1016/s0278-5846(99)00017-2. [DOI] [PubMed] [Google Scholar]
- Gur RC, Gunning-Dixon F, Bilker WB, Gur RE. Sex differences in temporo-limbic and frontal brain volumes of healthy adults. Cereb Cortex. 2002;12(9):998–1003. doi: 10.1093/cercor/12.9.998. [DOI] [PubMed] [Google Scholar]
- Gur RC, Gur RE, Obrist WD, Hungerbuhler JP, Younkin D, Rosen AD, Skolnick BE, Reivich M. Sex and handedness differences in cerebral blood flow during rest and cognitive activity. Science. 1982;217(4560):659–661. doi: 10.1126/science.7089587. [DOI] [PubMed] [Google Scholar]
- Gur RC, Mozley PD, Resnick SM, Gottlieb GL, Kohn M, Zimmerman R, Herman G, Atlas S, Grossman R, Berretta D, et al. Gender differences in age effect on brain atrophy measured by magnetic resonance imaging. Proc Natl Acad Sci U S A. 1991;88(7):2845–2849. doi: 10.1073/pnas.88.7.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Mozley LH, Mozley PD, Resnick SM, Karp JS, Alavi A, Arnold SE, Gur RE. Sex differences in regional cerebral glucose metabolism during a resting state. Science. 1995;267(5197):528–531. doi: 10.1126/science.7824953. [DOI] [PubMed] [Google Scholar]
- Gur RC, Packer IK, Hungerbuhler JP, Reivich M, Obrist WD, Amarnek WS, Sackeim HA. Differences in the distribution of gray and white matter in human cerebral hemispheres. Science. 1980;207(4436):1226–1228. doi: 10.1126/science.7355287. [DOI] [PubMed] [Google Scholar]
- Gur RC, Ragland JD, Moberg PJ, Turner TH, Bilker WB, Kohler C, Siegel SJ, Gur RE. Computerized neurocognitive scanning: I. Methodology and validation in healthy people. Neuropsychopharmacology. 2001;25(5):766–776. doi: 10.1016/S0893-133X(01)00278-0. [DOI] [PubMed] [Google Scholar]
- Gur RC, Richard J, Hughett P, Calkins ME, Macy L, Bilker WB, Brensinger C, Gur RE. A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: standardization and initial construct validation. J Neurosci Methods. 2010;187(2):254–262. doi: 10.1016/j.jneumeth.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Richard J, Calkins ME, Chiavacci R, Hansen JA, Bilker WB, Loughead J, Connolly JJ, Qiu H, Mentch FD, Abou-Sleiman PM, Hakonarson H, Gur RE. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8–21. Neuropsychology. 2012;26(2):251–265. doi: 10.1037/a0026712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Turetsky BI, Matsui M, Yan M, Bilker W, Hughett P, Gur RE. Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance. J Neurosci. 1999;19(10):4065–4072. doi: 10.1523/JNEUROSCI.19-10-04065.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RE, Gur RC. Sex differences in the relations among handedness, sighting-dominance and eye-acuity. Neuropsychologia. 1977;15(4–5):585–590. doi: 10.1016/0028-3932(77)90063-x. [DOI] [PubMed] [Google Scholar]
- Ingalhalikar M, Smith A, Parker D, Satterthwaite TD, Elliott MA, Ruparel K, Hakonarson H, Gur RE, Gur RC, Verma R. Sex differences in the structural connectome of the human brain. Proc Natl Acad Sci U S A. 2014;111(2):823–828. doi: 10.1073/pnas.1316909110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern DF, Benbow CP, Geary DC, Gur RC, Hyde JS, Gernsbacher MA. The science of sex differences in science and mathematics. Psychological Science in the Public Interest. 2007;8:1–51. doi: 10.1111/j.1529-1006.2007.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Maxwell EC, Irvine C, Nagel BJ. The impact of sex, puberty, and hormones on white matter microstructure in adolescents. Cereb Cortex. 2012;22:1979–1992. doi: 10.1093/cercor/bhr246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines M. Sex-related variation in human behavior and the brain. Trends in Cognitive Science. 2010;14:448–456. doi: 10.1016/j.tics.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu J-L, Leemans A, Bai C-H, Lee C-H, Tsai Y-F, Chiu H-C, Chen W-H. Gender differences and agerelated white matter changes of the human brain: a diffusion tensor imaging study. Neuroimage. 2008;39:566–577. doi: 10.1016/j.neuroimage.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn MC, Petersen AC. Emergence and characterization of sex differences in spatial ability: a meta analysis. Child Development. 1985;56:1479–1498. [PubMed] [Google Scholar]
- Mozley LH, Gur RC, Mozley PD, Gur RE. Striatal dopamine transporters and cognitive functioning in healthy men and women. Am J Psychiatry. 2001;158(9):1492–1499. doi: 10.1176/appi.ajp.158.9.1492. [DOI] [PubMed] [Google Scholar]
- Moreno-Briseno P, Diaz R, Campos-Romo A, Fernandez-Ruiz J. Sex-related differences in motor learning and performance. Behavioral and brain functions. 2010;6:74. doi: 10.1186/1744-9081-6-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Ragland JD, Coleman AR, Gur RC, Glahn DC, Gur RE. Sex differences in brain-behavior relationships between verbal episodic memory and resting regional cerebral blood flow. Neuropsychologia. 2000;38(4):451–461. doi: 10.1016/s0028-3932(99)00086-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roalf DR, Gur RE, Ruparel K, Calkins ME, Satterthwaite TD, Bilker WB, Hakonarson H, Harris LJ, Gur RC. Within-individual variability in neurocognitive performance: age- and sex-related differences in children and youths from ages 8 to 21. Neuropsychology. 2014;28(4):506–518. doi: 10.1037/neu0000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Shinohara RT, Wolf DH, Hopson RD, Elliott MA, Vandekar SN, Ruparel K, Calkins ME, Roalf DR, Gennatas ED, Jackson C, Erus G, Prabhakaran K, Davatzikos C, Detre JA, Hakonarson H, Gur RC, Gur RE. Impact of puberty on the evolution of cerebral perfusion during adolescence. Proc Natl Acad Sci U S A. 2014;111(23):8643–8648. doi: 10.1073/pnas.1400178111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Vandekar S, Wolf DH, Ruparel K, Roalf DR, Jackson C, Elliott MA, Bilker WB, Calkins ME, Prabhakaran K, Davatzikos C, Hakonarson H, Gur RE, Gur RC. Sex differences in the effect of puberty on hippocampal morphology. J Am Acad Child Adolesc Psychiatry. 2014;53(3):341–350. doi: 10.1016/j.jaac.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Roalf DR, Ruparel K, Erus G, Vandekar S, Gennatas ED, Elliott MA, Smith A, Hakonarson H, Verma R, Davatzikos C, Gur RE, Gur RC. Linked Sex Differences in Cognition and Functional Connectivity in Youth. Cereb Cortex. 2015;25(9):2383–2394. doi: 10.1093/cercor/bhu036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saykin AJ, Gur RC, Gur RE, Shtasel DL, Flannery KA, Mozley LH, Malamut BL, Watson B, Mozley PD. Normative neuropsychological test performance: effects of age, education, gender and ethnicity. Appl Neuropsychol. 1995;2(2):79–88. doi: 10.1207/s15324826an0202_5. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Taki Y, Hashizume H, Sassa Y, Takeuchi H, Wu K, Asano M, Asano K, Fukuda H, Kawashima R. Correlation between gray matter density-adjusted brain perfusion and age using brain MR images of 202 healthy children. Hum Brain Mapp. 2011;32:1973–1985. doi: 10.1002/hbm.21163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Wang J, Yan C, He Y. Hemisphere- and gender-related differences in small-world brain networks: a resting-state functional MRI study. Neuroimage. 2011;54:191–202. doi: 10.1016/j.neuroimage.2010.07.066. [DOI] [PubMed] [Google Scholar]
- Thomas JR, French KE. Gender differences across age in motor performance: A meta-analysis. Psychological Bulletin. 1985;98:260–282. [PubMed] [Google Scholar]
- Tunç B, Solmaz B, Parker D, Satterthwaite TD, Elliott MA, Calkins ME, Ruparel K, Gur RE, Gur RC, Verma R. Establishing a link between sex-related differences in the structural connectome and behaviour. Philos Trans R Soc Lond B Biol Sci. 2016;371(1688) doi: 10.1098/rstb.2015.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar N, Allen NB, Youssef G, Dennison M, Yücel M, Simmons JG, Whittle S. Brain development during adolescence: A mixed-longitudinal investigation of cortical thickness, surface area, and volume. Hum Brain Mapp. 2016;37:2027–2038. doi: 10.1002/hbm.23154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voyer D, Voyer S, Bryden MP. Magnitude of sex differences in spatial abilities: a meta-analysis and consideration of critical variables. Psychological Bulletin. 1995;117:250–270. doi: 10.1037/0033-2909.117.2.250. [DOI] [PubMed] [Google Scholar]
- Wang L, Shen H, Tang F, Zang Y, Hu D. Combined structural and resting-state functional MRI analysis of sexual dimorphism in the young adult human brain: an MVPA approach. Neuroimage. 2012;61:931–940. doi: 10.1016/j.neuroimage.2012.03.080. [DOI] [PubMed] [Google Scholar]
- Williams LM, Mathersul D, Palmer DM, Gur RC, Gur RE, Gordon E. Explicit identification and implicit recognition of facial emotions: I. Age effects in males and females across 10 decades. Journal of Clinical and Experimental Neuropsychology. 2008;19:1–21. doi: 10.1080/13803390802255635. [DOI] [PubMed] [Google Scholar]
- Wu K, Taki Y, Sato K, Hashizume H, Sassa Y, Takeuchi H, Thyreau B, He Y, Evans AC, Li X, et al. Topological organization of functional brain networks in healthy children: differences in relation to age, sex, and intelligence. PLoS One. 2013;8:e55347. doi: 10.1371/journal.pone.0055347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo XN, Kelly C, Di Martino A, Mennes M, Margulies DS, Bangaru S, et al. Growing together and growing apart: regional and sex differences in the lifespan developmental trajectories of functional homotopy. J Neurosci. 2010;30:15034–15043. doi: 10.1523/JNEUROSCI.2612-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]