Abstract
Purpose
Linking multifocal electroretinography (mfERG) and optical coherence tomography (OCT) findings with visual acuity in retinitis pigmentosa (RP) patients.
Design
Prospective, cross-sectional, nonintervention study.
Subjects
Patients with typical RP and age-matched controls, who underwent SD-OCT (spectral domain OCT) and mfERG, were included.
Methods
MfERG responses were averaged in three zones (zone 1 (0°–3°), zone 2 (3°–8°), and zone 3 (8°–15°)). Baseline-to-trough- (N1) and trough-to-peak amplitudes (N1P1) of the mfERG were compared with corresponding areas of the OCT. The papillomacular area (PMA) was analyzed separately. Correlations between best-corrected visual acuity (BCVA, logMAR) and each parameter were determined.
Main outcome measures
Comparing structural (OCT) and functional (mfERG) measures with the BCVA.
Results
In RP patients, the N1 and N1P1 responses showed positive association with the central retinal thickness outside zone 1 (P≤0.002), while the central N1 and the N1P1 responses in zones 1, 2, and 3—with the BCVA (P≤0.007). The integrity of the IS/OS line on OCT showed also a positive association with the BCVA (P<0.001). Isolated analysis of the PMA strengthened further the structure–function association with the BCVA (P≤0.037). Interactions between the BCVA and the OCT, respectively, the mfERG parameters were more pronounced in the RP subgroup without macular edema (P≤0.020).
Conclusion
In RP patients, preserved structure–function of PMA, measured by mfERG amplitude and OCT retinal thickness, correlated well with the remaining BCVA. The subgroup analyses revealed stronger links between the examined parameters, in the RP subgroup without appearance of macular edema.
Introduction
Retinitis pigmentosa (RP) constitutes a heterogeneous group of inherited retinal diseases, marked by progressive photoreceptor cell degeneration, affecting primarily rod photoreceptors, and later on-cone photoreceptors. With the disease progression, only a central island of functioning receptors remains, at which stage the RP patient is left with tunnel vision.1, 2, 3
The macula and the papillomacular area (PMA) are the points of great interest in RP patients, first due to their importance for assessing the remaining visual function, and second because of their particular anatomical structure and role. In RP, the degenerative process initially occurs in the rod photoreceptors, later-in the cone photoreceptors, and with tissue remodeling in the ganglion cells and their axons.4, 5, 6, 7
A ‘standard' diagnostic tool for clinical diagnosis of inherited retinal diseases, the full-field electroretinography8, 9, 10 has a long history of use. Assessment of the retinal pigment epithelium (RPE) function, supplemented by electrooculogram (EOG), shows in advanced stages of RP disease reduced Arden ratio (AR).11 However, only the introduction of multifocal electroretinogram (mfERG)12, 13 allowed measuring cone-mediated responses in the regions with remaining photoreceptors function in RP patients.14, 15, 16, 17 The relationship between local mfERGs responses and other measurements of visual function in patients with RP has been examined by a number of studies.14, 16, 18, 19, 20, 21 The amplitude of the central segment of mfERGs has shown significant correlation to the visual acuity.22
Recently, retinal imaging, in particular optical coherence tomography (OCT) gained acceptance for evaluating the retinal structure in vivo.23 Noticeably, the ganglion cell inner plexiform layer of the macula, which contains the cell bodies and dendrites of the retinal ganglion cells, has been found to be significantly thicker in the nasal, than in the temporal region.24 Later, this finding has been linked to the normal metabolic layout: lower oxygen saturation levels have been measured in the temporal peripapillary disc area (respectively in the nasal macular area), supposedly due to the higher oxygen extraction.25
Since the macula and the PMA have an abundance of cone photoreceptors and ganglion cells, it seems likely that a disease progression in this island will produce central vision deterioration. In RP patients, several OCT studies have identified a correlation between detected OCT structural changes of the macula and the visual function, in particular at the junction between the inner and outer segments, the so called inner/outer segment junction (IS/OS) line.26, 27, 28
Although the visual acuity is generally preserved until the late stages of RP, cases of decreased visual acuity in the early stages have also been reported.29, 30 It is, therefore, to note, that the quality of life of RP patients is greatly dependent on the structural and functional integrity of the papillomacular region of the retina. Consequently, we aimed at evaluating to what extent the mfERG findings in the central and in the PMA of RP patients correlate with the retinal integrity, assessed through OCT, and with the best-corrected visual acuity (BCVA).
Subjects and methods
Subjects
Demographic data of our RP patients and controls are given in Table 1a. We included 31 consecutive patients (62 eyes) with RP and recruited 25 age-matched controls (49 eyes). The RP patients' age range was 14–60 years (mean: 40.19±12.01 years), while the controls' age range was 19–51 years (mean: 35.67±8.42 years), (P=0.118, Table 1a).
Table 1a. Demographic data of our RP patients and controls.
| Demographic characteristics |
Retinitis pigmentosa patients |
Controls | ||
|---|---|---|---|---|
| Total | No-ME-RP subgroup | ME-RP subgroup | ||
| Number of patients (eyes) | 31 (62) | 24 (44) | 11 (18) | 25 (49) |
| Age range (years) | 14–60 | 14–60 | 21–54 | 19–51 |
| mean±SD | 40.19±12.01 | 40.18±13.31 | 40.22±8.34 | 35.67±8.42 |
| Sex: ♀/♂ | 17/14 | 12/12 | 5/6 | 18/7 |
Table 1a represents the demographic data of RP patients and control subjects. Depending on the appearance of macular edema, the RP group is divided further in two subgroups: without macular edema (the no-ME-RP subgroup) and with macular edema (the ME-RP subgroup). Four patients were part of both subgroups, having each one eye with ME.
All subjects were examined at the diagnostic unit of the Department of Ophthalmology at the University of Basel. Each subject gave written informed consent after being given full explanation of the tests and procedures. The tenets of the Declaration of Helsinki were followed throughout the study.
All controls and RP patients underwent standard ophthalmologic examination, including BCVA (Snellen charts), Goldmann applanation tonometry measurement, biomicroscopy, and fundoscopy. The clinical phenotype of RP patients was characterized following clinical and electrophysiological assessment.
Our inclusion criteria for RP patients were: Caucasian origin, characteristic fundoscopic findings of RP, reduced or non-detectable a- and b-wave amplitudes of the scotopic full-field ERG (ffERG; Table 1b). Respectively, our inclusion criteria for controls were: Caucasian origin, best-corrected Snellen visual acuity at distance≥0.7.
Table 1b. Descriptive statistics for the BCVA, full-field ERG and EOG parameters evaluated in our RP patients' group and in the control group.
| Parameters mean±SD |
Retinitis pigmentosa patients
mean±SD |
Controls mean±SD | P-values (univariate ANOVA) | ||
|---|---|---|---|---|---|
| RP patients total | No-ME-RP subgroup | ME-RP subgroup | |||
| BCVA | |||||
| logMAR | 0.391±0.432 | 0.411±0.483 | 0.341±0.273 | 0.003±0.022 | <0.001* |
| Full-field ERG | |||||
| Dark-adapted 3.0 ERG | |||||
| A-wave amplitude (μV) | −43.02±60.99 | −54.81±67.70 | −14.20±22.72 | −237.17±75.36 | <0.001* |
| B-wave amplitude (μV) | 78.27±91.10 | 100.45±97.29 | 24.06±38.14 | 405.97±146.95 | <0.001* |
| Light-adapted 3.0 ERG | |||||
| A-wave amplitude (μV) | −13.58±15.71 | −16.24±17.55 | −7.09±6.64 | −46.07±30.92 | <0.001* |
| B-wave amplitude (μV) | 41.88±39.86 | 41.89±41.49 | 41.8638±36.71 | 201.42±64.43 | <0.001* |
| EOG | |||||
| Arden ratio | 1.64±0.74 | 1.79±0.84 | 1.32±0.31 | 2.46±0.38 | <0.001* |
| Dark-trough amplitude (μV) | 239.79±110.25 | 242.83±111.71 | 233.55±110.10 | 360.54±109.81 | <0.001* |
| Light-peak amplitude (μV) | 367.80±174.03 | 403.92±185.69 | 293.55±120.45 | 861.97±238.91 | <0.001* |
P-values of <0.05 are considered statistically significant and are marked with asterisk. Within the RP subgroups, ME-RP patients tend to have lower a- and b-wave amplitudes of the scotopic full-field ERG (P=0.030 and P=0.016, respectively), as well as lower Arden ratio (P=0.013) of the EOG when compared with no-ME-RP patients.
Exclusion from participation, for RP patients and controls, was implemented in cases of: history of surgical treatment (including cataract surgery) on the examined eye, clinical signs of macular pathology other than RP, unstable fixation, systemic diseases (such as diabetes mellitus, hypertension, or other metabolic and neurodegenerative diseases, potentially affecting the mfERG recording), and history of antidepressant, alcohol, or drug consumption.
For each patient, BCVA was measured with a standard decimal Snellen visual acuity chart. For our statistical analyses, to plot and compare the standard BCVA, recorded as fraction values, to other linear values (ERG, OCT data), we converted the fraction BCVA units into linear units calculating a logarithm of the minimum angle of resolution (logMAR).
Full-field ERG
FfERG recordings were performed on Retiscan ERG device (Roland Consult System, GmbH, Brandenburg, Germany). In the course of the test preparation, the pupils were maximally dilated with tropicamide 0.5% and phenylephrine 1% (Hospital Pharmacy, University of Basel, Switzerland). Patients were adapted to scotopic light conditions (30 min) before starting the recording session. Electrical responses were recorded binocularly via single-use microfiber electrode (DTL Plus Electrode, Diagnosys LLC, Lowell, MA, USA). The DTL electrode was placed on the surface of the cornea, the negative electrode-on the temporal orbital rim, and the ground electrode-on the forehead. For each eye, ffERG was recorded only once. Both eyes were tested simultaneously for each stimulus condition. The stimuli were generated according the test order provided by Retiscan software (Roland Consult Systems, GmbH), pre-programmed according to the International Society for Clinical Electrophysiology of Vision (ISCEV) standard protocol.31 A- and b-wave amplitudes of the dark-adapted 3.0 ERG (maximal or standard combined 3.0 cd s/m2 rod–cone response) and of the light-adapted 3.0 ERG (single-flash 3.0 cd s/m2 cone response) were analyzed.
Electrooculogram
EOG recordings were performed on Retiscan equipment (Roland Consult Systems, GmbH). The background illumination was 100 cd/m2. Fast oscillations were set at 1.5 s, six cycles with a total cycle duration of 75 s. The dark phase of the recording included a pre-adaptation of 6 min followed by alternate fixation with 4-min duration and 20-s pause. The light phase (total duration: 14 min) included 4-min adaptation to light, followed by alternate fixation with 10-min duration and fixation-rest of 20 s.32 Amplifier was bandpass filtered between 0.1 and 50 Hz. We analyzed the dark-trough amplitude, following the offset of retinal illumination, and the light-peak amplitude, following the re-illumination. The resulting dark-trough/light-peak ratio, known as AR, was analyzed right after.
Multifocal electroretinogram
We used VERIS 6.0. (Electro-Diagnostic Imaging, San Mateo, CA, USA) for the mfERG recording.33 Before the recording session, the patients were adapted to ambient room light for 30 min. Recording was performed on FMS III stimulator (Electro-Diagnostic Imaging). During the recording session hexagons flickered between black and white according to m-sequence of 215 (frame rate: 75 Hz). The stimulus array consisted of 103 hexagons (Figures 1a and b). The central retina (50° around the center) was stimulated. During the light phase, the maximal luminance of the hexagons (Lmax) was 200 cd/m2 and during the dark phase (Lmin) <1 cd/m2. The retinal signals were bandpass-filtered at 10–200 Hz. The responses were recorded monocularly with a single-use microfiber DTL electrode, placed on the surface of the cornea. The negative electrode was placed on the temporal orbital rim and the ground electrode-on the forehead. If a refractive error was present, corrections were done using the FMS III fundus stimulator (EDI, San Mateo, CA, USA). The subjects' fixation was continually monitored during the mfERG recording session with an infrared camera incorporated in the device.
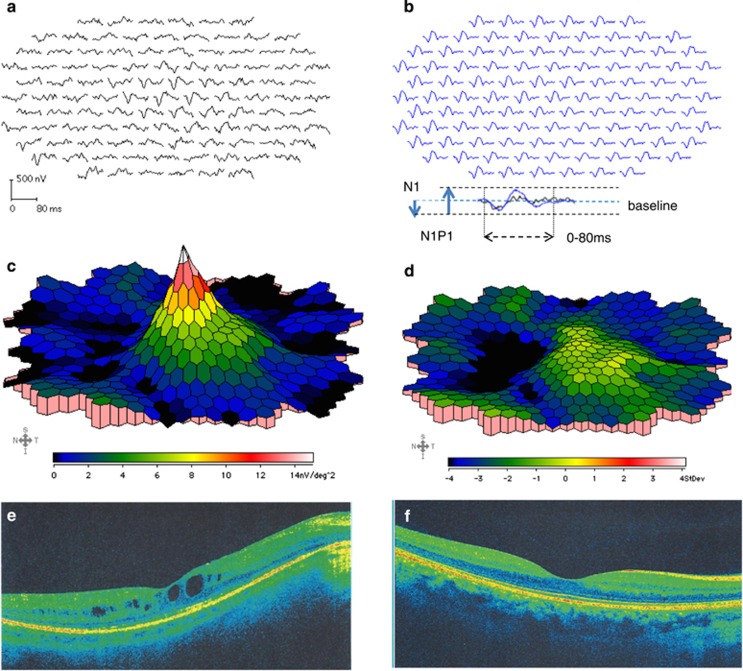
Figure 1.
Multifocal electroretinogram (mfERG) and optical coherence tomography (OCT) of a RP patient and a control. (a, b) depict mfERG tracings from the right eye of a representative RP patient (a, tracings in black) and an age-matched control subject (b, tracings in blue). The mfERGs were recorded on VERIS 6.0. The stimulus array consisted of 103 hexagons, where the central 50° of the retina were stimulated. A 3D waveform of the response density of an RP patient. Panel c represents a normal response in the center and severely attenuated responses outside the fovea. The SD of the patients' responses against those of age-matched controls is plotted in d, showing preserved responses within the PMA for RP patients. Panels e, f represent optical coherence tomography images (OCTs) from the right eye of a representative RP patient (left) and a control subject (right). The OCTs were performed on Cirrus OCT.
We measured the N1 response amplitude of the mfERG from the baseline to the trough of the first negative wave (N1), while we estimated the N1P1 response amplitude from the trough of the first negative wave (N1) to the peak of the first positive wave (P1) (Figure 1b, bottom). The first order response (0–80 ms) of the mfERG was analyzed as response densities (nV/deg2).
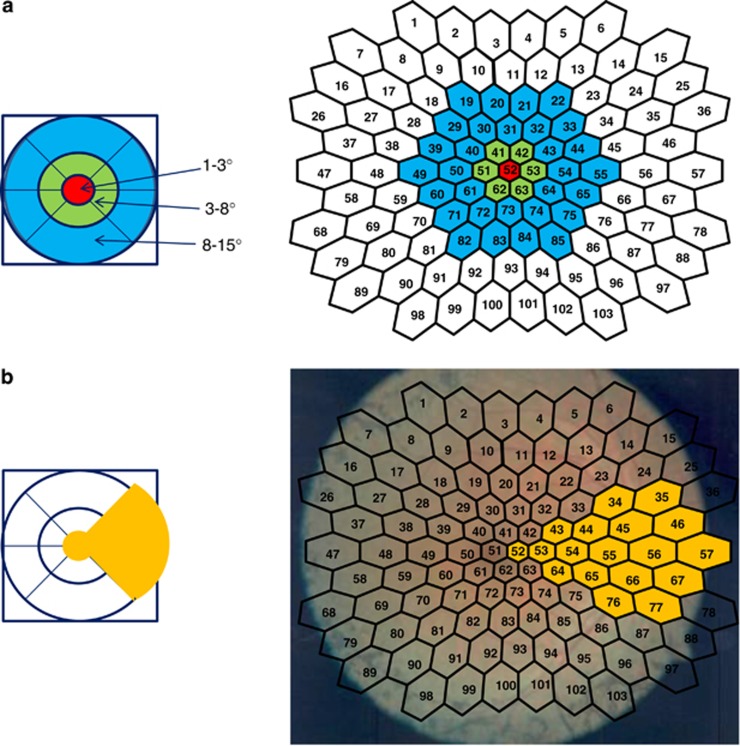
To determine the structure–function relationships, we focused on analyzing the mfERG responses within the (circular) central 15° area. The mfERG responses were averaged in three concentric rings, zones, around the foveola (Figure 2a): foveola or zone 1 (within the central 3°), fovea or zone 2 (between 3° and 8°), and paracentral area, or zone 3 (between 8° and 15°). A separate analysis included the PMA as shown in Figure 2b.
Figure 2.
Structure–function analysis of foveola, fovea, paracentral area and papillomacular area. (a, b) The OCTs were performed on Cirrus OCT. The software of the Cirrus OCT provides a macular thickness map divided into nine subfields. The mfERGs were recorded on VERIS 6.0. The stimulus array consisted of a total of 103 hexagons, 18 of which were in the PMA. Panel a shows on the right-hand side the way the mfERG responses were averaged on a circular-like pattern: within the central 3° (foveola; zone 1), between 3° and 8° (fovea; zone 2), and between 8° and 15° (paracentral area; zone 3). As shown on the left-hand side, corresponding to the mfERG zones, we computed the mean macular thickness into these three areas, as well. b outlines in yellow portions, based on which we performed a separate structure–function analysis of the papillomacular area (PMA).
Optical coherence tomography
For the evaluation of the retinal structure, we performed an OCT using Cirrus OCT (Carl Zeiss Meditec. Dublin, CA, USA). The OCT images were taken by implementing macular thickness protocol (Macular Cube 512 × 128) and high-definition image-protocol (fovea located HD 5 Line Raster (Carl Zeiss Meditec, Dublin, CA, USA), 4 × scanned, 6.0 mm length, 0.25 mm spacing, 0° angle; Figures 1e and f). The software of the Cirrus OCT provided a macular thickness map (μm), divided into nine subfields, where the thickness is calculated from the internal limiting membrane to the RPE.
Response averages were calculated, with the anatomical and physiological structure of the central retina in mind. Corresponding to the mfERG zones, we computed the mean macular thickness for three concentric areas, as well: zone 1 up to 3° from the center, zone 2 between 3° and 8°, and zone 3 between 8° and 15° (Figure 2a). Furthermore, due to its structural specificity, we analyzed the structure–function relationship in the PMA (Figure 2b) separately.
The highly reflective line above the RPE layer indicates the photoreceptor IS/OS. In RP patients, the integrity of the IS/OS line has been strongly associated with better visual function, brighter fundal autofluorescent parafoveolar density and higher retinal sensitivity.28, 34, 35 With the latter in mind, we measured the length of the intact IS/OS line for every eye on HD 5 Line Raster OCT images, using a measurement tool from the Cirrus OCT.
In addition, two observers (KK, MGT) evaluated vertical and horizontal scan OCT images from each RP patient for clinical appearance of macular edema, by the presence of intraretinal spaces situated within the fovea within the PMA zone (Figures 1e and f).
Statistical analysis
IBM SPSS Statistics version 21 (International Business Machines Corp., Armonk, NY, USA) was applied for the statistical analysis of the data. A linear mixed-effects model, allowing for repeated measurements, was performed. In RP patients, the degeneration occurs as a non-uniform process. Therefore, mixed-effects models show their strength, since they are stable in the presence of slightly imbalanced parameters.
Interactions and categories of data sampled from normal distributions fit well into a linear mixed-effects model. The linear model allows us to test whether the association of the evaluated functional and structural parameters (mfERG and OCT) against the BCVA is dependable on the group.
Here, the linear mixed-effects model was performed for each pair of the tested methods, where the BCVA (logMAR) is a dependent variable. Age, gender, location average (zone 1 (0°–3°), zone 2 (3°–8°), zone 3 (8°–15°) and PMA zone), amplitude of the mfERG (nV/deg2), macular thickness (μm) and study group were treated as covariates. Potential interactions between study groups and the other covariates were included in the regression model as well. Subject was treated as a random factor. To exclude any potential influence of a macular edema on the structure–function relationship, its presence, when detected in the RP group, was considered in the regression model.
Our results are presented as regression coefficients (P-values). P-values of <0.05 were considered statistically significant.
Results
We examined a total of 62 eyes of 31 patients with RP and 49 eyes of 25 controls. Demographic characteristics of our patients and controls are represented in Table 1a (univariate ANOVA). The RP group had two subgroups: with (ME-RP) and without appearance of macular edema (no-ME-RP), defined by the presence of intraretinal spaces situated between the foveal center and the PMA region. In the no-ME-RP subgroup, 24 patients had 44 eyes (23 right and 21 left) without macular edema, and in the ME-RP subgroup 11 patients had 18 eyes (8 right and 10 left) with macular edema. Four patients were part of both subgroups, each having only one eye with ME. Both RP subgroups showed different mutations affecting different genes, including simple and syndromic cases with an autosomal-dominant, autosomal-recessive and X-linked manner of inheritance (Supplementary Table 1).
The logMAR BCVA varied between 0.00 and 2.0 (mean: 0.411±0.483) in the no-ME-RP subgroup, it varied between 0.00 and 1.0 (mean: 0.341; ±0.273) in the ME-RP subgroup and was between 0.00 and 0.15 in controls (mean: 0.003; ±0.022) (P<0.001, Table 1b). Our ME-RP patients tended to have slightly higher BCVA than no-ME-RP patients. However, the two RP subgroups did not differ from one another by the mean logMAR BCVA (P=0.386).
In general, the RP group was different from controls (Table 1c): the full-field a- and b-wave amplitudes in dark-adapted 3.0 ERG and light-adapted 3.0 ERG were significantly lower in RP patients than in the control group (P<0.001, Table 1b). Also, our RP group differed significantly from controls when the AR, as well as the dark-trough amplitudes and the light-peak amplitudes were evaluated (P<0.001, Table 1b). Within RP patients, those patients clinically classified as ME-RP patients showed more attenuated a- and b-wave amplitudes of the scotopic ffERGs (P=0.030 and P=0.016), together with heavier reduction of the AR (P=0.013), than the no-ME-RP patients. Within the RP group, the mfERGs showed preserved central retinal function, and simultaneously a severely reduced function outside the central retina (P<0.001).14, 15, 16, 17
Table 1c. Descriptive statistics for the OCT and mfERG parameters evaluated in our RP patients' group and in the control group.
| Parameters mean±SD |
Retinitis pigmentosa patients
mean±SD |
Controls mean±SD | P-values (univariate ANOVA) | ||
|---|---|---|---|---|---|
| Total | No-ME-RP subgroup | ME-RP subgroup | |||
| OCT (μm) | |||||
| Zone 1 | 262.88±90.25 | 231.09±51.37 | 342.50±104.83 | 260.31±26.75 | <0.001* |
| Zone 2 | 298.55±57.70 | 284.20±47.18 | 341.04±57.52 | 317.69±12.13 | <0.001* |
| Zone 3 | 251.30±36.02 | 247.78±36.97 | 272.86±33.97 | 271.30±23.79 | <0.001* |
| PMA | 282.38±63.63 | 262.51±45.01 | 330.94±76.68 | 290.99±12.93 | <0.001* |
| IS/OS line | 2502.77±1794.44 | 2354.26±1988.16 | 2824.56±1269.36 | 5984.89±103.56 | <0.001* |
| mfERG | |||||
| N1 amplitudes (nV/deg2) | |||||
| Zone 1 | −21.11±15.83 | −19.71±16.94 | −24.44±12.59 | −60.95±15.58 | <0.001* |
| Zone 2 | −12.19±9.18 | −11.66±10.13 | −13.46±6.41 | −34.70±7.31 | <0.001* |
| Zone 3 | −5.30±3.88 | −4.79±4.09 | −6.53±3.08 | −17.35±3.82 | <0.001* |
| PMA | −4.46±3.16 | −4.24±3.38 | −4.99±2.56 | −13.57±3.24 | <0.001* |
| N1P1 amplitudes (nV/deg2) | |||||
| Zone 1 | 44.11±26.29 | 42.34±29.29 | 48.36±17.09 | 136.98±32.18 | <0.001* |
| Zone 2 | 21.84±14.68 | 20.79±15.89 | 24.36±11.29 | 74.71±15.70 | <0.001* |
| Zone 3 | 8.64±7.01 | 8.50±7.30 | 8.96±6.46 | 35.73±7.90 | <0.001* |
| PMA | 6.50±4.68 | 6.24±5.13 | 7.14±3.43 | 26.42±6.58 | <0.001* |
Descriptive statistics for the OCT readings (μm) and mfERG amplitudes (nV/deg2), evaluated in zones, presented for our RP patients' group and in the control group separately. For all examined locations, the retinal thickness, measured by OCT, was significantly thicker in the ME-RP subgroup when compared with the no-ME-RP subgroup (P≤0.003). This was, however, not the case for the IS/OS line length (P=0.272). The two RP subgroups did not differ from one another by means of mfERG response amplitudes (P≥0.098).
The OCT imaging, consistent with previous studies on RP patients,34, 35, 36 confirmed loss of photoreceptors (impaired IS/OS line integrity), distortions of the retinal microstructure and/or macular edema.
MfERG vs OCT
Within our RP group, the interaction between mfERG responses and macular thickness, measured with OCT, was determined by the location (linear mixed-effects model, Table 2a). For all RP patients, the structure–functional correlations for the N1 and N1P1 responses of the mfERG against the corresponding OCT area, were significant outside the central 3° (in zone 2 and zone 3; P≤0.002). In the PMA, the structure–functional correlations were significant for both N1 and N1P1 responses against the corresponding OCTs (P=0.015, Table 2a).
Table 2a. Structure–function interactions between the variables: the recorded N1/N1P1 mfERG amplitudes (nV/deg2) vs the OCT thickness (μm) measurements within the RP group and the RP subgroups: P-values.
| Predictors |
OCT (μm) |
|||
|---|---|---|---|---|
| Zone 1 | Zone 2 | Zone 3 | PMA | |
| mfERG (nV/deg2) | ||||
| mfERG, N1 amplitudes (nV/deg2) | ||||
| Zone 1 | ||||
| Within RP group | 0.272 | |||
| No-ME-RP subgroup | 0.012* | |||
| ME-RP subgroup | 0.065 | |||
| Zone 2 | ||||
| Within RP group | 0.001* | |||
| No-ME-RP subgroup | 0.001* | |||
| ME-RP subgroup | 0.218 | |||
| Zone 3 | ||||
| Within RP group | <0.001* | |||
| No-ME-RP subgroup | 0.002* | |||
| ME-RP subgroup | 0.062 | |||
| PMA | ||||
| Within RP group | 0.015* | |||
| No-ME-RP subgroup | 0.065 | |||
| ME-RP subgroup | 0.012* | |||
| mfERG, N1P1 amplitudes (nV/deg2) | ||||
| Zone 1 | ||||
| Within RP group | 0.104 | |||
| No-ME-RP subgroup | 0.001* | |||
| ME-RP subgroup | 0.195 | |||
| Zone 2 | ||||
| Within RP group | <0.001* | |||
| No-ME-RP subgroup | <0.001* | |||
| ME-RP subgroup | 0.236 | |||
| Zone 3 | ||||
| Within RP group | 0.002* | |||
| No-ME-RP subgroup | 0.003* | |||
| ME-RP subgroup | 0.178 | |||
| PMA | ||||
| Within RP group | 0.015* | |||
| No-ME-RP subgroup | 0.119 | |||
| ME-RP subgroup | 0.049* | |||
Statistically significant values (P<0.05) are marked with asterisk.
When the RP subgroups were evaluated separately, the no-ME-RP group showed for all three zones statistically significant structure–functional correlations (P≤0.012). That is, mfERG responses were reduced when the retinal thickness was decreased. The subgroup ME-RP showed for the PMA a statistically significant N1 mfERG/OCT correlation (P=0.012) and N1P1 mfERG/OCT correlation (P=0.049), where the mfERG responses were reduced with increasing retinal thickness.
To assess the visual acuity in regard to the residual retinal function and OCT alterations, the BCVA data were compared to the mfERG- and to the OCT data averaged in zones.
BCVA vs mfERG
The BCVA showed a significant group/location interaction between the groups (controls vs RP group) and the N1 and N1P1 mfERG response amplitudes averaged in zones (P≤0.031; Table 2b). Significant interaction effect was found for the N1P1 mfERG responses in the PMA, as well (P=0.013).
Table 2b. Outcome from BCVA (logMAR) correlation to the amplitudes of mfERG (nV/deg2) recordings by groups and RP subgroups.
| Predictors (P-values) |
mfERG amplitudes (nV/deg2) |
|||||||
|---|---|---|---|---|---|---|---|---|
|
Zone 1 |
Zone 2 |
Zone 3 |
PMA |
|||||
| N1 | N1P1 | N1 | N1P1 | N1 | N1P1 | N1 | N1P1 | |
| BCVA (logMAR) | ||||||||
| Interaction effect group/location | 0.031* | <0.001* | 0.028* | <0.001* | 0.004* | 0.001* | 0.084 | 0.013* |
| RP | ||||||||
| Within the RP group | 0.007* | <0.001* | 0.001* | <0.001* | <0.001* | <0.001* | 0.013* | 0.005* |
| No-ME-RP subgroup | 0.020* | <0.001* | 0.003* | <0.001* | <0.001* | <0.001* | 0.007* | 0.003* |
| ME-RP subgroup | 0.300 | 0.095 | 0.361 | 0.128 | 0.998 | 0.675 | 0.736 | 0.743 |
Predicts standard BCVA outcome (logMAR) from the mfERG-measurements (nV/deg2) based on linear mixed-effects model. P-value of <0.05, considered statistically significant, is marked with asterisk.
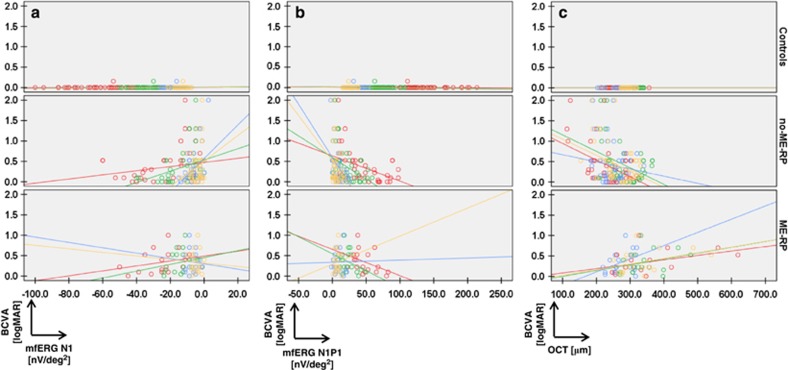
Within the RP group, the relationship between the BCVA and the N1 and the N1P1 mfERG responses was evidenced by statistically significant values. For the N1 amplitude responses, they were the following: zone 1 (P=0.007; slope: −0.504 logMAR/nV/deg2), zone 2 (P=0.001; slope: −0.481 logMAR/nV/deg2) and zone 3 (P<0.001; slope: −0.518 logMAR/nV/deg2; Table 2b,Figure 3a, where the red circles depict the measurements in zone 1, the green—in zone 2, the blue—in zone 3, and the yellow—in the PMA). For the N1P1 amplitude responses, the corresponding interactions were the following: zone 1 (P<0.001; slope: −0.706 logMAR/nV/deg2), zone 2 (P<0.001; slope: −0.638 logMAR/nV/deg2), zone 3 (P<0.001, slope: −0.485 logMAR/nV/deg2; Table 2b, Figure 3b). When the presence of macular edema was taken into account for the purpose of evaluating the relationship between BCVA and mfERG, within the no-ME-RP subgroup, the N1 and N1P1 average responses in all zones showed statistically significant values (P≤0.020), which documented a correlation in which a reduced vision (logMAR BCVA) yielded reduced N1 and N1P1 mfERG responses (nV/deg2). In the PMA, the values also demonstrated significant interactions (P≤0.007; Figure 3a, with measurements by zones presented in colors identical to Figure 3b zone measurements).
Figure 3.
Scatter-plots showing the correlations drawn between the logMAR BCVA and the functional- (mfERG), respectively structural (OCT) measures in controls and RP subgroups. (a) BCVA (logMAR) vs N1 mfERG data (nV/deg2). Scatter-plots presented as individual graphs: on the top for controls, in the middle for no-ME-RP patients (without clinical appearance of macular edema) and on the bottom for ME-RP patients (with macular edema). The BCVA data (logMAR) are plotted on the y-axis and the N1 mfERG data (nV/deg2) are plotted on the x-axis (in red: zone 1, green: zone 2, blue: zone 3 and yellow: PMA). (b) BCVA (logMAR) vs N1P1 mfERG data (nV/deg2). Panel b represents, in analogy to panel a, scatter-plots for the correlations between the BCVA data (logMAR), plotted on the y-axis, and the N1P1 mfERG data (nV/deg2), plotted on the x-axis, for controls (top), no-ME-RP patients (middle), as well as ME-RP patients (bottom). Individual graphs depict the correlations by zones and are presented in colors identical to those in Figure 2a and b. (c) BCVA (logMAR) vs OCT data (macular thickness in μm). (c) Linear mixed-effects model is applied to correlate the BCVA measurements to the OCT thickness measurements, divided in zones, the PMA inclusively. The red circles represent the measurements in zone 1, the green—in zone 2, the blue—in zone 3, and the yellow—in the PMA. The BCVA data (logMAR) are plotted on the y-axis and the OCT data (μm) on the x-axis.
For the ME-RP subgroup, the correlations between the BCVA and the functional measures did not reach statistically significant values either in the first three zones, or in the PMA (P≥0.095).
BCVA vs OCT
The interaction between the group and the location did not show significant values when the BCVA was evaluated against the OCT, averaged in zones (P≥0.384, Table 2c, Figure 3c).
Table 2c. Interactions drawn between logMAR BCVA and OCT (μm) measurements in groups and RP subgroups, by zones and with PMA and inner/outer segment line analyses included (linear mixed-effects model).
| Predictors |
OCT (μm) |
||||
|---|---|---|---|---|---|
| Zone 1 | Zone 2 | Zone 3 | PMA | IS/OS | |
| BCVA (logMAR) | |||||
| Interaction effect group/location | 0.646 | 0.384 | 0.508 | 0.657 | 0.879 |
| RP | |||||
| Within the RP group | 0.076 | 0.003* | 0.064 | 0.037* | <0.001* |
| No-ME-RP subgroup | <0.001* | <0.001* | 0.014* | <0.001* | <0.001* |
| ME-RP subgroup | 0.024* | 0.126 | 0.142 | 0.051 | 0.530 |
All statistically significant values (P<0.05) are marked with asterisk. Potential interactions between study groups and the OCT data averaged in zones, included in the regression model, showed strong correlation for all examined locations, for the PMA, as well as for the length of the IS/OS line in the no-ME-RP subgroup.
Within the RP group, however, the BCVA correlated significantly with the retinal thickness in zone 2 (P=0.003) and in the PMA (P=0.037). This was also the case for the relationship between the residual BCVA and the length of the IS/OS line (P<0.001). That is, shortened and disrupted IS/OS line was associated with reduced BCVA.
When the presence of macular edema was accounted for in the regression model, there was a strong correlation for all examined locations, as well as for the PMA and the length of the IS/OS line in the no-ME-RP subgroup (P≤0.014; Table 2c): the BCVA was reduced with decreasing retinal thickness. For the ME-RP subgroup this was the case in zone 1 (P=0.024), whereas in the PMA the values showed only a significant trend (P=0.051). Here, however, the interaction was inversed. That is, the BCVA was progressively reduced with increasing retinal thickness in the ME-RP subgroup (Figure 3c).
Discussion
The process of photoreceptor degeneration in RP follows a concentric centripetal pattern of progression.1, 2, 3 Given that the quality of life of RP patients is greatly dependent on their remaining central vision, we aimed at comparing the functional (mfERG) and the structural (OCT) alterations to the remaining visual acuity (BCVA) in patients suffering from RP.
The functional measures (mfERGs) usually confirm the preserved central retinal function, opposed to a severely reduced function outside of the central retina.14, 15, 16, 17 The structural measure (OCT) traditionally displays the retinal microstructure and/or macular edema, and IS/OS line distortions.34, 35, 36 In agreement, the RP patients in our study could be differentiated from controls based on the results of functional and morphological assessments.
In relatively non-advanced RP stages, transneuronal degeneration ought to be less prominent in the central and pericentral macular area, than in the peripheral macula, due to the higher cone density in the central retina.37, 38 Also, good numbers of ganglion cells in the pericentral region were still seemingly preserved, despite recorded underlying photoreceptor loss in RP eyes.39
However, recent studies of RP have shown that morphologic changes are to be expected and found in the macular area, and that a macular OCT may also be sensitive in assessing progressive RP retinal damage.26, 40, 41 Furthermore, novel studies using adaptive optics scanning laser en face images in RP patients, have shown reduced cone photoreceptors' density, even with preserved visual acuity. The results have strongly correlated with the retinal eccentricity. Within the macular area, morphologic changes in the outer nuclear layer have proved to be dependent on the reduction of the cone photoreceptors' density.39 Correspondingly, within our RP group, outside zone 1, we found significant structure–functional correlations: the mfERG responses were reduced when the retinal thickness was decreased.
The above cited study39 confirmed also the reduced cone density to be more pronounced in the nasal retina. Deductively, we presumed that the structural and functional integrity of the nasal (papillomacular) area (PMA), which has not been studied yet in details, would logically correspond to the residual visual acuity. To verify this theory, we developed a novel approach, to compare the averaged mfERG responses and averaged retinal thicknesses from the corresponding retinal regions and within the PMA.
As expected, in the PMA a significant structure–function interaction effect was found, well-differentiating controls from RP patients (P=0.015). In RP patients, preserved structure–function of PMA, measured by mfERG amplitude and OCT retinal thickness, correlated markedly with the remaining BCVA. More precisely, in the PMA, the relationship between preserved retinal structure (demonstrated through OCT) and sufficiently pronounced electrical activity (mfERG), as an objective indicator of good visual function, showed also strong interaction (P≤0.037).
Retinal remodeling encompasses different phases, beginning in the rod photoreceptors, involving later cone photoreceptors, and continuing with apoptosis and RPE/inner retina remodeling.4, 5, 6, 7 Some authors have already hypothesized the process of retinal remodeling to be responsible for the thickening of retinal layers despite present or ongoing photoreceptor degeneration.42, 43
Therefore, we proceeded with dividing our RP patients into two subgroups, differentiated one from the other by the presence of macular edema. With increasing severity of rod-photoreceptors retinopathy, a deterioration of a- and b-wave amplitudes of the scotopic ffERG, as well as of the AR of the EOG might be expected.8, 9, 10, 11 Further, dividing our RP patients in two subgroups, based on the presence of macular edema, the ME-RP patients showed more attenuated a- and b-wave amplitudes of the scotopic ffERGs (P≤0.030), together with heavier reduction of the AR (P=0.013), than those RP patients clinically classified in the no-ME-RP subgroup. Thus, ME-RP patients may have, even with preserved central vision, more advanced stage of photoreceptor degeneration. However, even having increased retinal thickness in all evaluated locations, the local functional activity in the ME-RP patients, measured by mfERG, was not significantly impaired when compared with the no-ME-RP subgroup (P≥0.098). This indicates a different relationship between the retinal thickness and the functional parameters for the ME-RP group, compared with the no-ME-RP group. Therefore, we continued studying the interactions between the functional-, as well as structural parameters and the remaining visual acuity within each RP subgroup.
Here, the correlations between BCVA and the structure–function parameters were more pronounced in the no-ME-RP subgroup. For the ME-RP subgroup, neither in the first three zones, nor in the PMA did the correlations between BCVA and the functional measures (mfERG) reach statistically significant values. For the structural (OCT) measure against BCVA, in the ME-RP subgroup, the correlations in zone 1 were significant, whereas in the PMA the values showed only a significant trend. These results are based on the influence of the macular edema (in the ME-RP subgroup) on the assessment of retinal thickening and its significance for a corresponding loss of visual function. For both RP groups altogether, the presence of an intact IS/OS line on OCT translated into better BCVA, association which remains in accordance with previous reports.26, 40, 41 This was, however, not the case, when the subgroup interaction was performed for the IS/OS line length against the BCVA: the integrity of the IS/OS line within the ME-RP subgroup did not relate to the residual BCVA.
In some patients with early-stage RP, changes in refraction, resulting from outer retina thickening due to macular edema, which is producing a hyperopic component, seem to be a plausible explanation for their preserved BCVA findings. In advanced stages, however, a remodeling of the outer and inner retina takes place, and an irreversible loss of photoreceptors is to be accounted for, resulting in further visual deterioration. Thus, a significant vision loss may—or may not be detected, depending on whether the edema is present in an advanced rather than early-stage disease, and whether edema occurs more centrally rather than peripherally.
In our patients with completed genetic analyses, a variety of mutations affecting different genes was found. In some of these cases, a unilateral, instead of bilateral, macular edema was present. Hence, for RP patients, the role of inheritance in the genesis of macular edema is to be further elucidated.
Finally, and as a most encompassing conclusion, we came to the understanding that the correlations between retinal structure and function, found in the present study, point toward the need for a multimodal approach to RP, to record and follow the pathologic processes on a new, higher and more efficient level. The applied structure–function approach may add new tool in evaluating potential therapeutic responses.
Summary
We document the link between PMA integrity and preserved visual acuity. MfERG recordings and OCT measurements of retinal thickness are found to correlate positively with BCVA in RP. A detailed analysis of this correlation is performed for concentric retinal zones, and, separately, for the PMA. The subgroup analyses revealed stronger links between the examined parameters, in the RP subgroup without appearance of macular edema.
| Activity evaluation | ||||
| 1. The activity supported the learning objectives. | ||||
| Strongly disagree | Strongly agree | |||
| 1 | 2 | 3 | 4 | 5 |
| 2. The material was organized clearly for learning to occur. | ||||
| Strongly disagree | Strongly agree | |||
| 1 | 2 | 3 | 4 | 5 |
| 3. The content learned from this activity will impact my practice. | ||||
| Strongly disagree | Strongly agree | |||
| 1 | 2 | 3 | 4 | 5 |
| 4. The activity was presented objectively and free of commercial bias. | ||||
| Strongly disagree | Strongly agree | |||
| 1 | 2 | 3 | 4 | 5 |
Acknowledgments
MGT was partially supported by unrestricted grant from OPOS (Stiftung Ostschweizerische Pleoptik- and Orthoptik-Schule) and by unrestricted grant from LHW (Liechtenstein Stiftung).
Footnotes
Supplementary Information accompanies this paper on Eye website (http://www.nature.com/eye)
MGT and RIB have full access to all the data in the study and hold complete responsibility for the data integrity and the accuracy of the analysis. The authors declare no conflict of interest.
Supplementary Material
References
- Ammann F, Klein D, Franceschetti A. Genetic and epidemiological investigations on pigmentary degeneration of the retina and allied disorders in Switzerland. J Neurol Sci 1965; 2: 183–196. [DOI] [PubMed] [Google Scholar]
- Hamel C. Retinitis pigmentosa. Orphanet J Rare Dis 2006; 1: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartong DT, Berson E, Dryja TP. Retinitis pigmentosa. Lancet 2006; 368: 1795–1809. [DOI] [PubMed] [Google Scholar]
- Milam AH, Li ZY, Fariss RN. Histopathology of the human retina in retinitis pigmentosa. Prog Retin Eye Res 1998; 17: 175–205. [DOI] [PubMed] [Google Scholar]
- Panfoli I, Calzia. D, Bianchini P, Ravera S, Diaspro A, Candiano G et al. Evidence for aerobic metabolism in retinal rod outer segment disks. Int J Biochem Cell Biol 2009; 41: 2555–2565. [DOI] [PubMed] [Google Scholar]
- Marc RE, Jones BW. Retinal remodeling in inherited photoreceptor degenerations. Mol Neurobiol 2003; 28: 139–147. [DOI] [PubMed] [Google Scholar]
- Jones BW, Marc RE. Retinal remodeling during retinal degeneration. Exp Eye Res 2005; 81: 123–137. [DOI] [PubMed] [Google Scholar]
- Fishman GA. Electrophysiology and inherited retinal disorders. Doc Ophthalmol 1985; 60: 107–119. [DOI] [PubMed] [Google Scholar]
- Gouras P, Carr RE. Electrophysiological studies in early retinitis pigmentosa. Arch Ophthalmol 1964; 72: 104–110. [DOI] [PubMed] [Google Scholar]
- Birch DG, Sandberg MA. Dependence of cone b-wave implicit time on rod amplitude in retinitis pigmentosa. Vision Res 1987; 27: 1105–1112. [DOI] [PubMed] [Google Scholar]
- Arden GB, Wolf JE. The electro-oculographic responses to alcohol and light in a series of patients with retinitis pigmentosa. Invest Ophthalmol Vis Sci 2000; 41: 2730–2734. [PubMed] [Google Scholar]
- Hood DC. Assessing retinal function with the multifocal technique. Prog Retin Eye Res 2000; 19: 607–646. [DOI] [PubMed] [Google Scholar]
- Bearse MA Jr, Sutter EE. Imaging localized retinal dysfunction with the multifocal electroretinogram. J Opt Soc Am A 1996; 13: 634–640. [DOI] [PubMed] [Google Scholar]
- Gränse L, Ponjavic V, Andréasson S, Full-field ERG. multifocal ERG and multifocal VEP in patients with retinitis pigmentosa and residual central visual fields. Acta Ophthalmol Scand 2004; 82: 701–706. [DOI] [PubMed] [Google Scholar]
- Hood DC, Zhang X, Multifocal ERG. and VEP responses and visual fields: comparing disease-related changes. Doc Ophthalmol 2000; 100: 115–137. [DOI] [PubMed] [Google Scholar]
- Gerth C, Wright T, Héon E, Westall CA. Assessment of central retinal function in patients with advanced retinitis pigmentosa. Invest Ophthalmol Vis Sci 2007; 48: 1312–1318. [DOI] [PubMed] [Google Scholar]
- Greenstein VC, Holopigian K, Seiple W, Carr RE, Hood DC. Atypical multifocal ERG responses in patients with diseases affecting the photoreceptors. Vis Res 2004; 44: 2867–2874. [DOI] [PubMed] [Google Scholar]
- Sandberg MA, Weigel-DiFranco C, Rosner B, Berson EL. The relationship between visual field size and electroretinogram amplitude in retinitis pigmentosa. Invest Ophthalmol Vis Sci 1996; 37: 1693–1698. [PubMed] [Google Scholar]
- Ma Y, Kawasaki R, Dobson LP, Ruddle JB, Kearns LS, Wong TY et al. Quantitative analysis of retinal vessel attenuation in eyes with retinitis pigmentosa. Invest Ophthalmol Vis Sci 2012; 53: 4306–4314. [DOI] [PubMed] [Google Scholar]
- Nagy D, Schönfisch B, Zrenner E, Jägle H. Long-term follow-up of retinitis pigmentosa patients with multifocal electroretinography. Invest Ophthalmol Vis Sci 2008; 49: 4664–4671. [DOI] [PubMed] [Google Scholar]
- Wen Y, Klein M, Hood DC, Birch DG. Relationships among multifocal electroretinogram amplitude, visual field sensitivity, and SD-OCT receptor layer thicknesses in patients with retinitis pigmentosa. Invest Ophthalmol Vis Sci 2012; 53: 833–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschos MM, Chatziralli IP, Verriopoulos G, Triglianos A, Ladas DS, Brouzas D. Correlation between optical coherence tomography and multifocal electroretinogram findings with visual acuity in retinitis pigmentosa. Clin Ophthalmol 2013; 7: 2073–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata LM, Deleon-Ortega J, Sakata V, Girkin CA. Optical coherence tomography of the retina and optic nerve—a review. Clin Exp Ophthalmol 2009; 37: 90–99. [DOI] [PubMed] [Google Scholar]
- Mwanza JC, Durbin MK, Budenz DL, Girkin CA, Leung CK, Liebmann JM et al. Profile and predictors of normal ganglion cell-inner plexiform layer thickness measured with frequency-domain optical coherence tomography. Invest Ophthalmol Vis Sci 2011; 52: 7872–7879. [DOI] [PubMed] [Google Scholar]
- Jani PD, Mwanza JC, Billow KB, Waters AM, Moyer S, Garg S. Normative values and predictors of retinal oxygen saturation. Retina 2014; 34: 394–401. [DOI] [PubMed] [Google Scholar]
- Aizawa S, Mitamura Y, Baba T, Hagiwara A, Ogata K, Yamamoto S. Correlation between visual function and photoreceptor inner/outer segment junction in patients with retinitis pigmentosa. Eye (Lond) 2009; 23: 304–308. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Joe SG, Lee DH, Lee JY, Kim JG, Yoon YH. Correlations between spectral-domain OCT measurements and visual acuity in cystoid macular edema associated with retinitis pigmentosa. Invest Ophthalmol Vis Sci 2013; 54: 1303–1309. [DOI] [PubMed] [Google Scholar]
- Mitamura Y, Mitamura-Aizawa S, Katome T, Naito T, Hagiwara A, Kumagai K et al. Photoreceptor impairment and restoration on optical coherence tomographic image. J Ophthalmol 2013; 2013: 518170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Chung H, Yu HG. Association of p.P347L in the rhodopsin gene with early-onset cystoid macular edema in patients with retinitis pigmentosa. Ophthalmic Genet 2012; 33: 96–99. [DOI] [PubMed] [Google Scholar]
- Van Huet RA, Collin RW, Siemiatkowska AM, Klaver CC, Hoyng CB, Simonelli F et al. IMPG2-associated retinitis pigmentosa displays relatively early macular involvement. Invest Ophthalmol Vis Sci 2014; 55: 3939–3953. [DOI] [PubMed] [Google Scholar]
- McCulloch DL, Marmor MF, Brigell MG, Hamilton R, Holder GE, Tzekov R et al. ISCEV Standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol 2015; 130: 1–12. [DOI] [PubMed] [Google Scholar]
- Türksever C, Orguel S, Todorova MG. Comparing short-duration electro-oculograms with and without mydriasis in healthy subjects. Klin Monbl Augenheilkd 2015; 232: 471–476. [DOI] [PubMed] [Google Scholar]
- Hood DC, Bach M, Brigell M, Keating D, Kondo M, Lyons JS et al. ISCEV standard for clinical multifocal electroretinography (mfERG) (2011 edition). Doc Ophthalmol 2012; 124: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitamura Y, Mitamura-Aizawa S, Nagasawa T, Katome T, Eguchi H, Naito T. Diagnostic imaging in patients with retinitis pigmentosa. J Med Invest 2012; 59: 1–11. [DOI] [PubMed] [Google Scholar]
- Iriyama A, Yanagi Y. Fundus autofluorescence and retinal structure as determined by spectral domain optical coherence tomography, and retinal function in retinitis pigmentosa. Graefes Arch Clin Exp Ophthalmol 2012; 250: 333–339. [DOI] [PubMed] [Google Scholar]
- Sandberg MA, Brockhurst RJ, Gaudio AR, Berson EL. The association between visual acuity and central retinal thickness in retinitis pigmentosa. Invest Ophthalmol Vis Sci 2005; 46: 3349–3354. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Allen KA, Sloan KR, Lerea CL, Hurley JB, Klock IB et al. Distribution and morphology of human cone photoreceptors stained with anti-blue opsin. J Comp Neurol 1991; 312: 610–624. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Sloan KR Jr, Packer O, Hendrickson AE, Kalina RE. Distribution of Cones in human and monkey retina: Individual variability and radial asymmetry. Science 1987; 236: 579–582. [DOI] [PubMed] [Google Scholar]
- Menghini M, Lujan BJ, Zayit-Soudry S, Syed R, Porco TC, Bayabo K et al. Correlation of outer nuclear layer thickness with cone density values in patients with retinitis pigmentosa and healthy subjects. Invest Ophthalmol Vis Sci 2014; 56: 372–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon CH, Park TK, Ohn YH. Association between multifocal electroretinograms, optical coherence tomography and central visual sensitivity in advanced retinitis pigmentosa. Doc Ophthalmol 2012; 125: 113–122. [DOI] [PubMed] [Google Scholar]
- Yoon CK, Yu HG. The structure-function relationship between macular morphology and visual function analyzed by optical coherence tomography in retinitis pigmentosa. J Ophthalmol 2013; 2013: 821460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZY, Kljavin IJ, Milam AH. Rod photoreceptor neurite sprouting in retinitis pigmentosa. J Neurosci 1995; 15: 5429–5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BW, Watt CB, Frederick JM, Baehr W, Chen CK, Levine EM et al. Retinal remodeling triggered by photoreceptor degenerations. J Comp Neurol 2003; 464: 1–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.