Abstract
Purpose
Although the length of the average human adult optic nerve (ON) is known, the average length of the normal full-term, newborn ON has never been adequately evaluated, nor has the in vivo growth rate of the human ON been determined. We wanted to identify both the average length of the newborn human ON and its rate of anteroposterior growth.
Patients and methods
Using MRIs from a newly generated set of normal newborn infants rescanned at 1 year, and from different aged groups, we calculated average newborn ON length and growth rate.
Results
The newborn human ON is 25.3±0.3 mm in length from globe to chiasm, and grows by 80% in length after birth, with maximum speed of elongation occurring in the first 3 years of life, attaining full length by 15 years of age.
Conclusion
The human ON grows dramatically in the first 3 years of life, and continues to grow for the first two decades. These data are relevant for pediatric treatments that may impede or alter orbital growth in infants, and maximal susceptibility to oncological procedures in early childhood.
Introduction
The normal human optic nerve (ON) grows from ~25 mm in length at birth to 40–45 mm in length and 3.4 mm in width by adulthood.1, 2 This growth corresponds to the expansion of the normal human skull from birth to the end of puberty.3 To date, no study has ever evaluated the timing and speed of postnatal human ON growth in the living individual. Postnatal mechanisms of ON growth may also lead to an improved understanding of ON repair and regeneration. We therefore evaluated postnatal human ON growth from birth to maturity.
Subjects and methods
Human material
All studies using human material were approved by the University of Maryland-Baltimore institutional review board (IRB) prior to beginning the work.
A ‘standard set' of magnetic resonance images (MRI-T1- and -T2-weighted) from normal full-term infants who were re-imaged at 1 year of age has recently become available for evaluation of growth trajectory through the infant brain imaging study (IBIS) network (www.ibisnetwork.org). Twelve identity-masked individuals from this set (six male and six female) were evaluated at both ages. We also included MRI-T1 and -T2 data from additional groups of masked Caucasian individuals at 3, 5, 10, 15, and 20 years of age. Data from these individuals were obtained from the University of Maryland-Baltimore radiographic center. Individuals were excluded from analysis if they had presented with any ocular or visual complaints. A minimum of 14 individuals were used for each age group. Male to female ratios were equal, except for the 10-year (46.7% n=15) and 15-year (42.9% n=14) groups.
Analysis
We utilized axial sections from good quality MRI scans. The ONs were measured bilaterally from the posterior of the globe to the middle of the optic chiasm on both T1- and T2-weighted images using the Leonardo MR workstation (Siemens Medical Solutions, Malvern, NY, USA) and the results were averaged. Furthermore, ON size was averaged for both sides from all individuals, with mean length in mm +/−SD reported.
Results
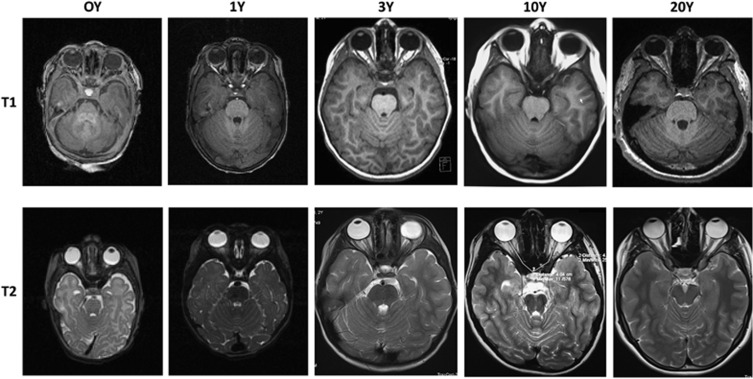
MRI analysis revealed reduced ON myelin signal in full-term newborns, with progressive increase in the relative T1/T2 signal intensity between newborns and 3 y/o (Figure 1; compare 0 and 3Y). T1/T2 signal intensity stabilized by 3 years of age. These changes are consistent with previously reported developmental increases in ON myelin density.4
Figure 1.
T1- and T2-weighted MRI-ON images at 0Y, and at 1, 3, 5, and 20Y. 0, 1Y: Representative IBIS images (1/55 section total through entire brain). 3–20Y: Representative non-IBIS images (1/255 sections). ON measurement analysis is shown in the T2/10Y image. ON length was measured from the distal end of the widest A-P globe diameter, and the line extended using the Siemens Leonardo workstation through multiple scans to the anterior edge of the optic chiasm. Results from T1 and T2 images were averaged for each individual. Measurement from a single T2-weighted image is shown for a 10Y individual.
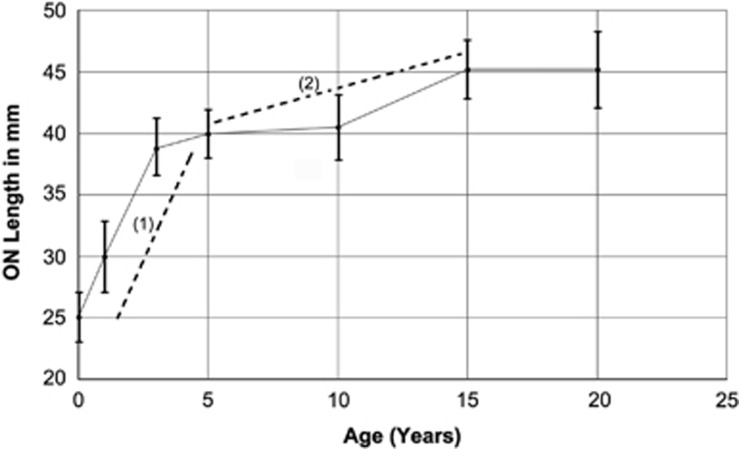
The mean newborn ON is 25.3+/−mm in length, reaching 45.3 (adult) by 20 years of age. Human ON growth is linear in the first 3 years of life, reaching 86% of the total adult length by this time (Figure 2; area indicated by (1): slope y=4.55x+24.214; r2=0.9991). Thereafter, the ON continues to grow slowly, until puberty at approximately 15 y/o (Figure 2; area indicated by (2): slope y=0.52x+36.7; r2=0.8214). ON length is stable through 20 years (Figure 2), indicating cessation of A-P growth.
Figure 2.
Postnatal ON growth analysis. ON length is shown on the ordinate, while age is shown on the abscissa. ON Mean size in mm±SD. ON growth is most rapid in the first 3 years of life, which then slows considerably from 5 to 10 years (compare slope (1) with slope (2)). A modest further increase in ON growth speed occurs between 10 and 15y, with final ON length achieved by 15 years.
Discussion
The current study is the first survey of total ON growth in vivo from birth to maturity. Our data reveal that the human ON grows by 80% after birth, with the greatest linear growth occurring within the first 3 years of postnatal life. ON growth continues at a slower rate from 5 to 15 years and then stops. This growth pattern likely corresponds to the increase in skull size around the age of puberty, and final skull growth.
There are a number of caveats that should be addressed. Ethnic self-reporting may minimize potential differences between races, and different races may have slightly different growth patterns that were not evaluated in the present study. In addition, nearly equivalent numbers of both male and female patients were averaged, minimizing potential sex-associated differences in ON growth that may be present.
The ON early intensive growth period suggests that the axons in the juvenile ON may have enhanced regenerative capacity. Interestingly, it is still unknown whether postnatal ON axonal growth occurs as a diffuse phenomenon, or whether there is a specific focal site (for example, at the junction of the eye and ON).5
Because the majority of ON growth occurs by 3 years of age, this postnatal interval likely represents a period of increased sensitivity and potential damage. Conversely, the factors associated with postnatal growth of the human ON may also increase early resistance and recovery to ON stressors such as elevated IOP, compared with treatment in adulthood. Understanding ON growth is an important step in evaluating diseases likely to affect visual function in the developing infant.
Acknowledgments
This study was funded by NEI grant NEI EY019529 (to SLB) and grant 1S10RR019214 (to RPG). The excellent technical assistance of Ms. Erma Owen is gratefully acknowledged.
Author contributions
SLB conceived of and designed the study, and wrote the majority of the manuscript. MM identified the patients in the University database, performed many of the individual analyses and helped write the manuscript. JZ evaluated the data and helped with the measurement transformations, selected appropriate individual images for analysis. RPG contributed the data from the IBIS dataset, helped interpret the data, suggested the appropriate measurement tools and helped write the manuscript.
The authors declare no conflict of interest.
References
- Scammon RE, Armstrong EL. On the growth of the human eyeball and optic nerve. J Comp Neurol 1925; 38: 165–219. [Google Scholar]
- Miller NR. Embryology of the afferent visual system. In: Walsh and Hoyt's Clinical Neuro-Ophthalmology, Vol 1, 4th edn. Williams and Wilkins: Baltimore, MD, USA, 1982, pp 3–10..
- Morriss-Kay GM, Wilkie AO. Growth of the normal skull vault and its alteration in craniosynostosis: insights from human genetics and experimental studies. J Anat 2005; 207: 637–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng X, Gouttard S, Sharma A, Gu H, Styner M, Lin W et al. Quantitative tract-based white matter development from birth to age 2 years. Neuroimage 2012; 61: 542–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JL. How does an axon grow? Genes Dev 2003; 17: 941–958. [DOI] [PubMed] [Google Scholar]