Abstract
Purpose
To compare the short-term treatment outcome of the 577 nm subthreshold micropulse laser (SML) and half-dose photodynamic therapy (PDT) in patients with chronic central serous chorioretinopathy (cCSC) and persistent subretinal fluid (SRF).
Methods
This retrospective study included 100 eyes of 100 consecutive patients who were treated with the 577 nm SML (Supra Scan, Quantel Medical) (n=42) or half-dose PDT (n=58) for cCSC. The treatment was applied at the leakage sites in the fluorescein and indocyanine green angiography. The treatment success was evaluated 6 weeks after treatment using best-corrected visual acuity, central retinal thickness, and resolution of SRF in spectral domain optical coherence tomography.
Results
Patients showed treatment response more often in the SML group compared with the PDT group (treatment response after SML: 33 eyes (79%), PDT: 34 eyes (59%), P=0.036, χ2 test). The CRT decreased significantly after both treatments (mean CRT before SML: 445±153 μm, after SML: 297±95, P<0.001; mean CRT before PDT: 398±88 μm, after PDT: 322±93 μm, P<0.001, Wilcoxon's signed-rank test). The decrease in CRT was statistically significantly higher in the SML group (decrease in CRT after SML: −148±163 μm, after PDT: −76±104 μm, P=0.041, Mann–Whitney U-test).
Conclusions
Both the half-dose PDT and the 577 nm SML are potent treatments for cCSC with persistent SRF. More patients showed treatment response to the SML treatment and SML leads to a greater decrease in CRT.
Introduction
Central serous chorioretinopathy (CSC) is a common retinal disease in middle-aged patients. It is characterized by a serous detachment of the neurosensory retina with consequential vision loss.1 Choroidal dysfunction is an important cause for retinal pigment epithelium (RPE) dysfunction and subretinal fluid (SRF) accumulation in CSC.2, 3 In CSC two manifestation forms can be distinguished, the acute and the chronic form. The acute CSC is characterized by a focal leakage point (‘hot spot') in the RPE and usually resolves without any treatment within a few weeks. Visual acuity recovers to normal in the majority of patients. In contrast, the chronic form of CSC can lead to permanent structural damage and loss of central vision. Imaging in this patients typically show irregular mildly atrophic RPE changes and choroidal abnormalities with more diffuse leakage instead of a single focal ‘hot spot'.4, 5, 6
Different treatment options for CSC had been suggested. The conventional suprathreshold argon laser photocoagulation can be used for extrafoveal leakage. This treatment can accelerate resolution of SRF.5, 7 However, side effects such as choroidal neovascularization and a reduction of contrast sensitivity had been described. Moreover, the treatment cannot be used in patients with diffuse or central leakage as it leads to scotomas.8, 9
Photodynamic therapy (PDT) is a more frequently used treatment because it can be used in juxtafoveal and subfoveal lesions. Is it assumed that the effect of PDT in CSC is due to the action on the structure of the choroidal vasculature, causing alterations in choroidal permeability.10 However, the PDT also has potential side effects such as RPE atrophy, choroidal neovascularisation, choriocapillaris ischemia, or transient reduction of macular function, even when reduced treatment settings are used.11, 12, 13, 14, 15
Another treatment option is subthreshold micropulse laser (SML) treatment without any visible end point. In contrast to conventional suprathreshold argon laser photocoagulation, the laser energy is delivered in short pulses with enough time in between to allow heat dissipation to prevent thermal structural tissue damage. The idea is that retinal damage is not needed to acquire a therapeutic effect.16
Studies with a 810 nm micropulse diode laser17, 18, 19, 20, 21 and a 577nm micropulse laser22, 23 showed efficacy in CSC patients with subfoveal and extrafoveal leakage sites.
Until now it is not known if one treatment is more effective than another. We have now evaluated patients who were treated with either a 577 nm micropulse laser or half-dose PDT for chronic CSC (cCSC) in our clinic between January 2012 and October 2015.
Patients and methods
Clinical data of patients with cCSC who were treated with the Supra Scan 577 nm laser (Quantel Medical, Cedex, France) or half-dose PDT for cCSC was retrospectively analyzed. The treatment was chosen by the surgeons in agreement with the patient. cCSC was diagnosed by funduscopy, spectral domain optical coherence tomography (SD-OCT), fluorescein angiography (FA), and indocyanine green angiography (ICGA) (Spectralis, Heidelberg Engineering, Heidelberg, Germany). For the definition of cCSC we used the currently available literature. All of the following characteristics had to be present: serous SRF on SD-OCT, ≥1 areas of multifocal diffuse leakage on FA, and corresponding hyperfluorescence on ICGA, as described previously.3 Patients with the presence of other relevant retinal diagnoses, such as choroidal neovascularisation or polypoidal choroidal vasculopathy, were excluded. Patients without a follow-up examination within the first 2 months after treatment were also excluded. We included patients with persistent SRF owing to cCSC for at least 6 weeks who were treated with the 577nm SML or half-dose PDT between January 2012 and October 2015. If both eyes were eligible for the study, only one randomly chosen eye was included in the analysis. If one eye received both treatments, only the first treatment was analyzed.
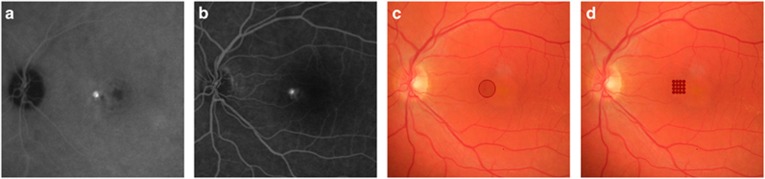
Hyperfluorescent areas on mid-phase ICGA and the corresponding 'hot spots' on mid-phase FA were treated by either SML or half-dose PDT. See Figure 1 for a schematic illustration of the SML and PDT treatment.
Figure 1.
Schematic illustration of the photodynamic therapy (c) and subthreshold micropulse laser (d) on the leakage sites in the fluorescein (a) and indocyanine green angiography (b).
The SML treatment was applied with the Area Centralis contact lens (laser spot magnification x0.94) (Volk Optical Inc., Mentor, OH, USA). We used standardized treatment parameters for all patients. The spot size was 160 μm, the exposure time 0.2 s, and the duty cycle 5%. The confluent laser treatment was performed after the individual power for the patient was titrated at a normal area of the retina, near the affected area. The power titration was performed in the monospot micropulse mode and was started at 700 mW. The power was then increased stepwise until a just visible burn appeared. At this threshold, the power was reduced by 50% for the actual SML treatment.
For the half-dose PDT, the patients were given an intravenous infusion of 3 mg/m2 verteporfin (Visudyne) within 10 min. The PDT treatment was applied with the QuadrAspheric contact lens (laser spot magnification x1.97) (Volk Optical Inc.) 15 min after the start of the infusion at the previously defined area. The standard treatment parameters for the PDT were 50 J/cm2 fluency, a laser wavelength of 689 nm, and a treatment duration of 83 s.
For the treatment outcome, we evaluated best-corrected visual acuity (BCVA), central retinal thickness (CRT), and resolution of SRF 6 weeks after the treatment.
For measuring the CRT, we used the automated segmentation program of the Heidelberg Eye Explorer software (Heidelberg Engineering GmbH, Heidelberg, Germany). We checked all scans for correct segmentation and correct positioning of the scan on the fovea. If necessary we performed a manual adjustment. A treatment response was assumed if the CRT decreased to a minimum of 20 μm after treatment. The presence of SRF was assessed in the volume scans.
Statistical analysis
For the statistical analysis SPSS (IBM SPSS Statistics, IBM Software and Systems, Armonk, NY, USA; version 22) was used. The Wilcoxon's signed-rank test was used to compare the visual acuity and the CRT before and after treatment. The Mann–Whitney U-test was used to look for differences between the SML and half-dose PDT group. The χ2 test was used to compare the treatment response between groups.
Results
One hundred eyes of 100 consecutive patients (71 men (71%) and 29 women (29%)) were included in this study.
The mean age of the included patients was 51 years (SD±9.3, range 32–78 years). The mean duration of disease before therapy was 3.2 years (SD±3.8, range 1.7 month–19 years).
Forty-two eyes of 100 patients received SML treatment (33 men (79%) and 9 woman (21%)). The mean age of the patients was 49 years (SD±8.6, range 32–68 years). The mean duration of disease before therapy was 3.9 years (±4.2, range 1.7 month–19 years). In 12 patients (29%), the duration of disease was <1 year and in 30 patients (71%) >1 year.
Fifty-eight eyes of 100 patients received PDT (38 men (66%) and 20 woman (35%)). The mean age of the patients was 53 years (±9.5, range 37–78 years). The mean duration of disease before therapy was 2.6 years (±3.3, range 2.2 month–18 years). In 26 patients (45%), the duration of disease was <1 year and in 32 patients (55%) >1 year (P=0.098). The duration of disease was shorter in the PDT group (P=0.046)
In the SML group, 24 patients (57%) showed multiple leakage spots, and in the PDT group, 32 patients (55%). There was no statistically significant difference in the number of patients showing multiple leakage spots between the two groups (P=0.845).
There was no statistically significant difference in age (P=0.092) or gender (P=0.156) between the two groups.
Treatment response
Table 1 shows the baseline parameters and the treatment outcome after SML and PDT.
Table 1. Baseline parameters and treatment outcome after subthreshold micropulse laser and photodynamic therapy.
| Treatment response | Complete resolution of SRF | CRT (μm) baseline Mean±SD | CRT (μm) after therapy Mean±SD | P-value | Decrease in CRT (μm) Mean±SD | Increase in BCVA of 1 or more lines | BCVA (LogMAR) baseline Mean±SD | BCVA (LogMAR) after therapy | P-value | Increase in BCVA (LogMAR) Mean±SD | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| All patients (n=100) | 67 of 100 (67%) | 27 of 100 (27%) | 418±121 | 311±94** | P<0.001** Wilcoxon's signed-rank test | -106±136 | 50 of 100 (50%) | 0.37±0.24 | 0.31±0.25** | P=0.001** Wilcoxon's signed-rank test | −0.059±0.168 |
| SML (n=42) | 33 of 42 (79%)* | 15 of 42 (36%) | 445±153 | 297±95** | P<0.001** Wilcoxon's signed-rank test | −148±163* | 23 of 42 (55%) | 0.39±0.24 | 0.31±0.27** | P=0.003** Wilcoxon signed-rank test | −0.081±0.167 |
| PDT (n=58) | 34 of 58 (59%)* | 12 of 58 (21%) | 398±88 | 322±93** | P<0.001** Wilcoxon's signed-rank test | −76±104* | 27 of 58 (47%) | 0.35±0.24 | 0.31±0.24 | P=0.077 Wilcoxon's signed-rank test | −0.043±0.169 |
| P-value* | P=0.036* χ2 test | P=0.095 χ2 test | P=0.242 Mann–Whitney U-test | P=0.041* Mann–Whitney U-Test | P=0.418 χ2 test | P=0.360 Mann–Whitney U-test | P=0.349 Mann–Whitney U-test |
**Statistically significant difference before and after therapy.
*Statistically significant difference between the SML and the PDT group.
Treatment response and disease duration
There was no statistically significant difference in the treatment response of all patients regarding the disease duration (disease duration <1 year: 26 responders (68%), 12 non-responders (32%); disease duration >1 year: 41 responders (66%), 21 non-responders (34%), P=0.813).
A significant higher number of patients with a disease duration of less than one year showed a treatment response in the SML group compared with the PDT group (SML group: 12 patients with disease duration <1 year, 11 responders (92%), 1 non-responder (8%), PDT group: 26 patients with disease duration <1 year, 15 responders (58%), 11 non-responders (42%), P=0.036).
There was no significant difference in the number of patients with a disease duration of >1 year who showed a treatment response between the two treatment groups (SML group: 30 patients with disease duration >1 year, 22 responders (73%), 8 non-responders (27%), PDT group: 32 patients with disease duration >1 year, 19 responders (59%), 13 non-responders (41%), P=0.246).
Non-responder
Thirty-three of the 100 patients (33%) treated with SML or PDT did not respond to therapy. The non-responders showed a statistically significant lower CRT at baseline compared with the responders (CRT at baseline non-responders: 337±81 μm, responders: 442±131 μm, P=0.004). There was no statistically significant difference in age (mean age non-responders: 54±11 years, responders: 50±8 years, P=0.219), duration of disease (mean duration of disease non-responders: 4.0±5.0 years, responders: 2.7±2.9 years, P=0.374), BCVA at baseline (mean LogMAR (logarithm of the minimal angle of resolution) non-responders: 0.36±0.25 responders: 0.37±0.24, P=0.894), or gender (non-responders: 25 men (76%), 8 women (24%), responders: 46 men (67%), 21 woman (31%), P=0.462).
Second treatment
Forty-one of the 100 patients received a second treatment after insufficient success of the first treatment (17 patients in the MP group, 24 patients in the PDT group). Twenty-seven of those patients (12 patients in the MP group, 15 patients in the PDT) group returned for a follow-up visit 6 weeks after the second treatment. Twenty (74%) of the 27 patients showed a treatment response after this second treatment (defined as a decrease in CRT of at least 20 μm compared with the 6-week visit after the first treatment). Ten (37%) of these patients showed complete resolution of SRF after the second treatment.
Safety
Only in the PDT group, one patient developed a CNV after one course of half-dose PDT. Otherwise, no patient showed structural changes in RPE, photoreceptor layer, or inner and outer retinal layers, evaluated by biomicroscopy, SD-OCT, fundus autofluorescence, infrared reflectance image, or FA and ICGA after half-dose PDT or SML. One patient suffered from a moderate allergic reaction during the verteporfin injection (hypotension, tachycardia, dyspnea, and flushing).
Discussion
In our study, we evaluated the efficacy and safety of SML and half-dose PDT in patients with chronic CSC. Both treatments were associated with a significant reduction of CRT and a small increase in BCVA in our cohort. We only included patients with persistent SRF for at least 6 weeks and typical findings for the chronic form of CSC such as irregular mildly atrophic RPE changes and choroidal abnormalities with diffuse leakage in our study. Thus, it could be assumed that the reduction in CRT is caused by the treatment and not a spontaneous resolution of SRF in the majority of cases.
In our study, the morphological response was defined as a complete resolution of SRF 6 weeks after treatment was significantly higher in the SML group. Moreover, the SML treatment led to a significant greater decrease in CRT compared with PDT. There was no statistically significant difference in baseline mean CRT, baseline BCVA, number of patients with multiple leakage sites, and in demographics (gender, age) between the two groups. The duration of disease at baseline was the only parameter that was significantly different between the two groups.
In our study patients, patients with a disease duration of <1 year showed a better treatment response only after SML but not after PDT compared with patients with a disease duration of >1 year. This could indicate that SML should be initated earlier for best treatment outcome. This finding should be verified in a larger cohort.
In contrast to our study, previous studies reported a treatment response after SML in up to 100% of patients and complete resolution of SRF in 33–75%,18, 19, 24, 25, 26 and for PDT a partial resolution of SRF in up to 100% and complete resolution of SRF in 81–100% after PDT.13, 14, 15, 27, 28 This less favorable outcome in our cohort is potentially attributed to the severity of the disease in our patients with often long-standing disease accompanied by chronic retinal and choroidal changes. More than 60% of our patients had a disease duration of >1 year. Moreover, some studies show a slightly higher increase in BCVA after SML17, 23 and PDT13, 15, 29 compared with our results. This could be attributed to the relatively low BCVA in our patients at baseline. The cCSC in our patients could already have led to permanent structural damage and precluded marked visual improvement.
To find an effective treatment for patients without complete resolution of SRF is a big challenge. Some of these patients received the same treatment a second time and the majority benefited from this approach. Another option could be a ‘crossover' treatment where patients who do not respond sufficiently to one therapy are switched to the other treatment option. It could be possible that some patient might respond better to either SML or PDT.
We found a significant lower CRT at baseline in the group of non-responders. This could be due to a more chronic stage of CSC in these patients. The duration of disease did not differ between the responders and non-responders. However, this could be due to the retrospective nature of this study. The duration of disease was obtained from the patient files. It is not replicable if the start of symptoms documented in the files coincides with the actual start of the disease.
One idea is to treat patients as early as possible while they are still in the acute stage and before any permanent structural damage can occur. However, as there is a high chance for spontaneous resolution of SRF in acute CSC, the safety requirements for a therapy performed in patients in this early disease stage have to be high. Owing to known potential side effects of the PDT treatment,11, 12, 13, 14, 15 PDT is normally not performed in acute CSC. So far those side effects are not described after SML, thus SML might be the better option to treat patients with acute CSC.
There are some limitations of our study. Our definition of treatment response (decrease of minimum 20 μm in CRT after treatment) may be arbitrary and may be influenced by methodological inaccuracy, but studies quote a reproducibility of CRT measurements between 1 and 8 μm.30, 31
To evaluate the long-term outcome and safety, much longer follow-up examinations are needed. Further limitations are the lack of randomization, the absence of an untreated control group, and the non-standardized follow-up periods due to the retrospective nature of the study. We are now performing a multicenter randomized controlled treatment trial to compare half-dose PDT with high-density SML as a primary treatment for cCSC (EudraCT number 2012-004555-36). In this trial, the option for a treatment crossover is implemented for patients with persistent SRF after up to two treatments in their designated treatment group.32
In conclusion, SML and half-dose PDT both are effective treatments in cCSC with persistent SRF. In our study, we saw a small advantage of the SML compared with half-dose PDT. Additionally, SML showed no side effects in our cohort, so an early treatment to prevent permanent structural damage and vision loss should be considered. If treatment response was insufficient, a second treatment was efficacious in the majority of patients.

The authors declare no conflict of interest.
References
- Gass J. Pathogenesis of disciform detachment of the neuroepithelium. II. Idiopathic central serous choroidopathy. Am J Ophthalmol 1967; 63: 587–615. [PubMed] [Google Scholar]
- Liew G, Quin G, Gillies M, Fraser-Bell S. Central serous chorioretinopathy: a review of epidemiology and pathophysiology. Clin Experiment Ophthalmol 2013; 41(2):201–214. [DOI] [PubMed] [Google Scholar]
- Gemenetzi M, De Salvo G, Lotery A. Central serous chorioretinopathy: an update on pathogenesis and treatment. Eye 2010; 24(12): 1743–1756. [DOI] [PubMed] [Google Scholar]
- Gilbert CM, Owens SL, Smith PD, Fine SL. Long-term follow-up of central serous chorioretinopathy. Br J Ophthalmol 1984; 68(11): 815–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficker L, Vafidis G, While A, Leaver P. Long-term follow-up of a prospective trial of argon laser photocoagulation in the treatment of central serous retinopathy. Br J Ophthalmol 1988; 72(11):829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fok AC, Chan PP, Lam DS, Lai TY. Risk factors for recurrence of serous macular detachment in untreated patients with central serous chorioretinopathy. Ophthalmic Res 2011; 46(3): 160–163. [DOI] [PubMed] [Google Scholar]
- Leaver P, Williams C. Argon laser photocoagulation in the treatment of central serous retinopathy. Br J Ophthalmol 1979; 63(10): 674–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz H, Yannuzzi LA, Gitter KA. Subretinal neovascularization following argon laser photocoagulation treatment for central serous chorioretinopathy: complication or misdiagnosis? Retina 2012; 32(Suppl 1); OP893–906. [DOI] [PubMed] [Google Scholar]
- Khosla P, Rana S, Tewari H, Azad R, Talwar D. Evaluation of visual function following argon laser photocoagulation in central serous retinopathy. Ophthalmic Surg Lasers 1997; 28(8): 693–697. [PubMed] [Google Scholar]
- Chan W, Lam D, Lai T, Tam B, Liu D, Chan C. Choroidal vascular remodelling in central serous chorioretinopathy after indocyanine green guided photodynamic therapy with verteporfin: a novel treatment at the primary disease level. Br J Ophthalmol 2003; 87(12): 1453–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colucciello M. Choroidal neovascularization complicating photodynamic therapy for central serous retinopathy. Retina 2006; 26(2): 239–242. [DOI] [PubMed] [Google Scholar]
- Lai TY, Chan W-M, Lam DS. Transient reduction in retinal function revealed by multifocal electroretinogram after photodynamic therapy. Am J Ophthalmol 2004; 137(5): 826–833. [DOI] [PubMed] [Google Scholar]
- Fujita K, Imamura Y, Shinoda K, Matsumoto CS, Mizutani Y, Hashizume K et al. One-year outcomes with half-dose verteporfin photodynamic therapy for chronic central serous chorioretinopathy. Ophthalmology 2014; 122: 555–561. [DOI] [PubMed] [Google Scholar]
- Lim JI, Glassman AR, Aiello LP, Chakravarthy U, Flaxel CJ, Spaide RF et al. Collaborative retrospective macula society study of photodynamic therapy for chronic central serous chorioretinopathy. Ophthalmology 2014; 121(5): 1073–1078. [DOI] [PubMed] [Google Scholar]
- Tseng C-C, Chen S-N. Long-term efficacy of half-dose photodynamic therapy on chronic central serous chorioretinopathy. Br J Ophthalmol 2015; 99: 1070–1077. [DOI] [PubMed] [Google Scholar]
- Lanzetta P, Dorin G, Pirracchio A, Bandello F (eds). Theoretical bases of non-ophthalmoscopically visible endpoint photocoagulation. Semin Ophthalmol 2001; 16: 8–11. [DOI] [PubMed] [Google Scholar]
- Chen S-N, Hwang J-F, Tseng L-F, Lin C-J. Subthreshold diode micropulse photocoagulation for the treatment of chronic central serous chorioretinopathy with juxtafoveal leakage. Ophthalmology 2008; 115(12): 2229–2234. [DOI] [PubMed] [Google Scholar]
- Bandello F, Lanzetta P, Furlan F, Polito A. Non visible subthreshold micropulse diode laser treatment of idiopathic central serous chorioretinopathy. A pilot study. Invest Ophthalmol Vis Sci 2003; 44(5): 4858. [DOI] [PubMed] [Google Scholar]
- Gupta B, Elagouz M, McHugh D, Chong V, Sivaprasad S. Micropulse diode laser photocoagulation for central serous chorio-retinopathy. Clin Experiment Ophthalmol 2009; 37(8): 801–805. [DOI] [PubMed] [Google Scholar]
- Lanzetta P, Furlan F, Morgante L, Veritti D, Bandello F. Nonvisible subthreshold micropulse diode laser (810 nm) treatment of central serous chorioretinopathy. A pilot study. Eur J Ophthalmol 2008; 18(6): 934–940. [DOI] [PubMed] [Google Scholar]
- Malik KJ, Sampat KM, Mansouri A, Steiner JN, Glaser BM. Low-intensity/high-density subthreshold micropulse diode laser for chronic central serous chorioretinopathy. Retina 2015; 35(3): 532–536. [DOI] [PubMed] [Google Scholar]
- Scholz P, Ersoy L, Boon CJ, Fauser S. Subthreshold micropulse laser (577 nm) treatment in chronic central serous chorioretinopathy. Ophthalmologica 2015; 234(4): 189–194. [DOI] [PubMed] [Google Scholar]
- Kim JY, Park HS, Kim SY. Short-term efficacy of subthreshold micropulse yellow laser (577-nm) photocoagulation for chronic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol 2015; 253: 2129–2135. [DOI] [PubMed] [Google Scholar]
- Lavinsky D, Palanker D. Nondamaging photothermal therapy for the retina: initial clinical experience with chronic central serous retinopathy. Retina 2015; 35(2): 213–222. [DOI] [PubMed] [Google Scholar]
- Koss M, Beger I, Koch F. Subthreshold diode laser micropulse photocoagulation versus intravitreal injections of bevacizumab in the treatment of central serous chorioretinopathy. Eye 2011; 26(2): 307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav N, Jayadev C, Mohan A, Vijayan P, Battu R, Dabir S et al. Subthreshold micropulse yellow laser (577 nm) in chronic central serous chorioretinopathy: safety profile and treatment outcome. Eye 2015; 29: 258–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolino FC, Eandi CM, Ventre L, de la Longrais RCR, Grignolo FM. Photodynamic therapy for chronic central serous chorioretinopathy. Retina 2003; 23(6): 752–763. [DOI] [PubMed] [Google Scholar]
- Chan W-M, Lai TYY, Lai RYK, Liu DTL, Lam DSC. Half-Dose verteporfin photodynamic therapy for acute central serous chorioretinopathy: one-year results of a randomized controlled trial. Ophthalmology 2008; 115(10): 1756–1765. [DOI] [PubMed] [Google Scholar]
- Reibaldi M, Cardascia N, Longo A, Furino C, Avitabile T, Faro S et al. Standard-fluence versus low-fluence photodynamic therapy in chronic central serous chorioretinopathy: a nonrandomized clinical trial. Am J Ophthalmol 2010; 149(2):307–315. [DOI] [PubMed] [Google Scholar]
- Comyn O, Heng LZ, Ikeji F, Bibi K, Hykin PG, Bainbridge JW et al. Repeatability of spectralis OCT measurements of macular thickness and volume in diabetic macular edema spectralis OCT repeatability in DME. Invest Ophthalmol Vis Sci 2012; 53(12): 7754–7759. [DOI] [PubMed] [Google Scholar]
- Menke MN, Dabov S, Knecht P, Sturm V. Reproducibility of retinal thickness measurements in healthy subjects using spectralis optical coherence tomography. Am J Ophthalmol 2009; 147(3): 467–472. [DOI] [PubMed] [Google Scholar]
- Breukink MB, Downes SM, Querques G, van Dijk EH, den Hollander AI, Blanco-Garavito R et al. Comparing half-dose photodynamic therapy with high-density subthreshold micropulse laser treatment in patients with chronic central serous chorioretinopathy (the PLACE trial): study protocol for a randomized controlled trial. Trials 2015; 16(1): 419. [DOI] [PMC free article] [PubMed] [Google Scholar]