Abstract
Erythropoietin (EPO) is a glycoprotein hormone conventionally thought to be responsible only in producing red blood cells in our body. However, with the discovery of the presence of EPO and EPO receptors in the retinal layers, the EPO seems to have physiological roles in the eye. In this review, we revisit the role of EPO in the eye. We look into the biological role of EPO in the development of the eye and the physiologic roles that it has. Apart from that, we seek to understand the mechanisms and pathways of EPO that contributes to the therapeutic and pathological conditions of the various ocular disorders such as diabetic retinopathy, retinopathy of prematurity, glaucoma, age-related macular degeneration, optic neuritis, and retinal detachment. With these understandings, we discuss the clinical applications of EPO for treatment of ocular disorders, modes of administration, EPO formulations, current clinical trials, and its future directions.
Introduction
Erythropoietin (EPO) is a glycoprotein hormone conventionally thought to be responsible only in producing red blood cells (RBC) in our body. Kidney cells are the major producer of EPO in adults and the secreted protein will travel through the blood stream to reach to the bone marrow to stimulate hematopoietic stem cell differentiation to RBC.1 However, ample studies have evinced the presence of EPO protein and its receptors in extra-hematopoietic tissues including the retina tissue.2, 3
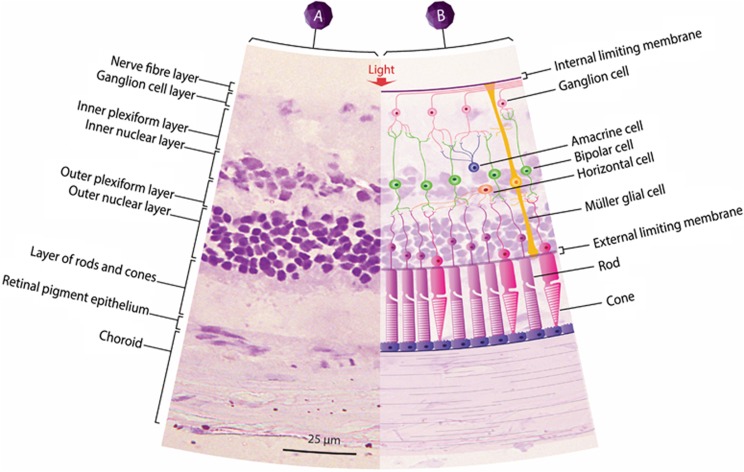
In adults, there is a wide distribution of EPO and EPO receptors (EPOR) reported on the human retinal tissue4, 5, 6, 7 and retinal pigment epithelium (RPE)4 (Figure 1). According to Grimm et al,8 EPOR expression was initially found to localize on the inner segments and synaptic terminals of the photoreceptors. Later, numerous studies showed evidence of EPOR expression in the inner nuclear layer (horizontal, Műller glia, bipolar, and amacrine cells) and the ganglion cell layer.3, 7, 9, 10 Szabo et al11 also demonstrated that the ganglion cells principally produce and secrete EPO, which will then target and bind to the EPOR present mainly on the amacrine, horizontal, and photoreceptor cells. Another study showed that EPO expression was localized mainly in the bipolar and amacrine cells.12
Figure 1.
The basic retinal structure. (A) Retina histological appearance of a Sprague Dawley rat stained with cresyl violet and viewed at magnification × 200. (B) An overlaid diagram showing the general types of cells found in the retina. The retina is arranged in different layers of cells, from the retinal pigment epithelium (RPE), the outer nuclear layer (ONL), the outer plexiform layer (OPL), the inner nuclear layer (INL), the inner plexiform layer (IPL), and the ganglion cell layer. The retinal layers harbor five retinal neurons, such as, the rod and cone photoreceptors, the Műller glia, the horizontal cell, the bipolar cell, the amacrine cell, and the retinal ganglion cell (RGC).
The vast presence of EPO and EPOR in the retinal tissue suggests that EPO has important physiological roles in the eye. Hence, we reviewed on the biological role of EPO in the development of the eye and the physiological roles that it has. We also discussed the mechanisms and pathways of EPO that contribute to the therapeutic and pathogenical conditions of ocular disorders. Finally, we reviewed on the clinical aspects of EPO regarding to the usage, modes of administration, EPO formulations, current clinical trials, and future directions of EPO as a potential therapy in various types of ocular disorders, including diabetic retinopathy (DR), retinopathy of prematurity, glaucoma, optic neuritis, age-related macular degeneration (AMD), and retinal detachment.
The role of EPO in the eye
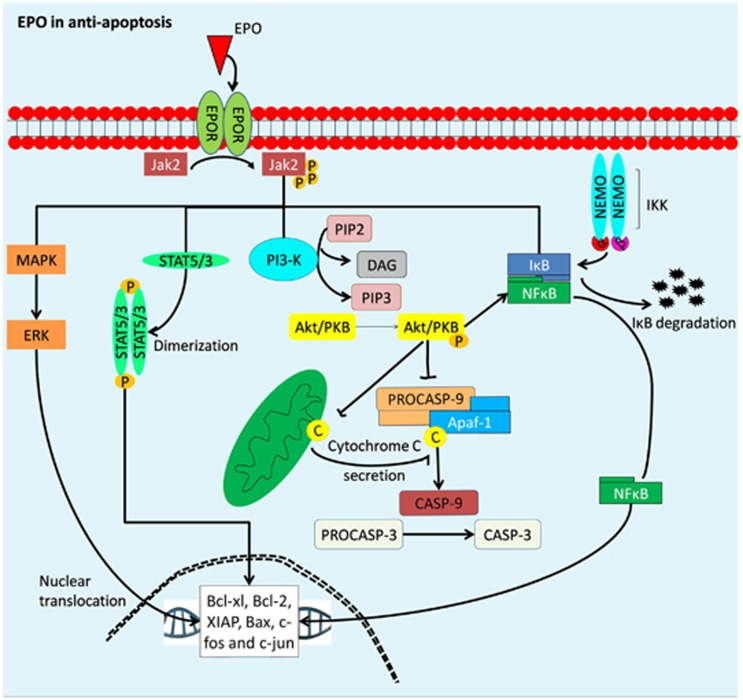
The basic human eye development requires proper coordination of regulatory genes that determines the fate of cells from different origins. EPO has a significant involvement in the development of visual function before birth. In recent years, a study reported that there is a direct correlation of fetal retina EPO mRNA amount in vitreous humor with increasing gestational age.13 This is further supported by Wu et al14 on the involvement of EPO in the development of human eye. During the process of organogenesis in the eye, they discovered the expression of EPO, along with signal transducer activator-of-transcription (STAT)-5, EPOR, and pro-apoptotic protein Bcl-2-associated X (Bax) expressions throughout the lens and retinal development.14 Both studies concluded that the involvement of EPO during the eye development could be contributing to its protective role in preventing cell apoptosis (Figure 2).13, 14 Figure 2 outlines on the schematic model of cell signaling of EPO in guarding the eye development. It begins with binding of EPO to the homodimeric EPOR, present on the ocular progenitor cells. Following binding, Janus kinase-2 (JAK2) activates several tyrosine residues on the receptor and forms attachment sites for several molecules, including mitogen-activated protein kinase (MAPK), nuclear factor-kappa light chain enhancer-of-activated B cells (NF-κB), phosphatidylinositol-3-kinase/Akt (PI3-K/Akt), and STAT. The activation of these molecules leads to the suppression of cell apoptosis.
Figure 2.
The role of erythropoietin in eye development via anti-apoptotic action. Binding of EPO/EPOR induces JAK2 phosphorylation, dimerization, and subsequently, activation of several tyrosine residues forming docking site for STAT, NF-κB, PI3-K/Akt, and MAPK. Phosphory lated MAPK and STAT molecules translocate into the nucleus and activate the expression of anti-apoptotic Bcl-2 and Bcl-xl, an apoptotic regulator of Bcl-2 family, to inhibit cell apoptosis. Whereas activation of PI3-K/Akt undergoes phosphorylation to block caspase activity by preventing cytochrome c leakage from mitochondria, thus attenuates DNA degradation and inflammatory cells activity. Furthermore, phosphorylation of JAK2 mediates NF-κB to recruit and activate cytoplasmic I-κB kinase (IKK) complex to be phosphorylated by I-κB for ubiquitylation and proteasomal degradation. Free NF-κB then translocates into the nucleus and up-regulates expression of anti-apoptotic factor (SOD and IAP).
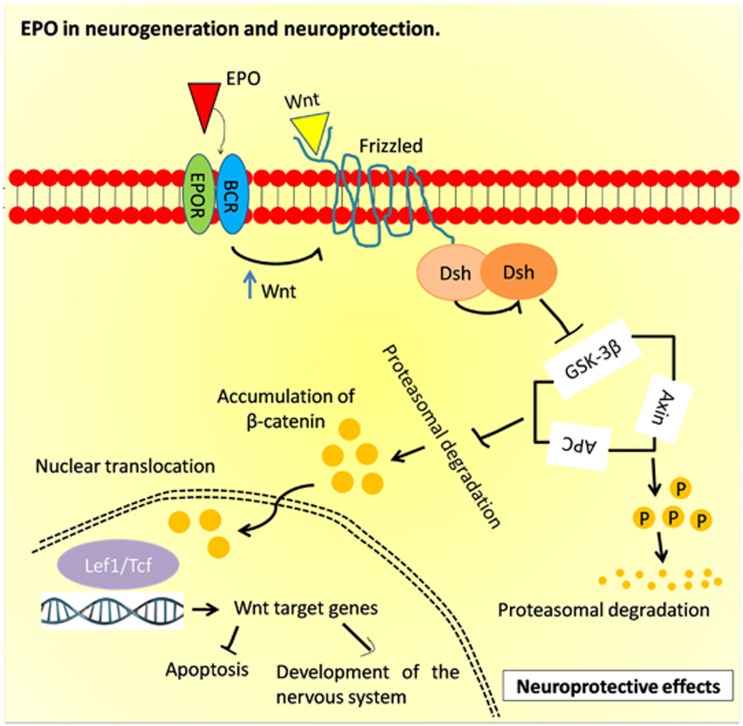
The process of eye development requires a well-controlled differentiation of ocular progenitor cells into specific retinal neuron phenotypes. Following to the previous outcomes,13, 14 several studies further identified EPO in neural retina differentiation. Alternatively, EPO could facilitate the differentiation through binding with a heterodimer complex, consisting of EPOR and interleukin beta-common receptor (βcR; CD131). Recent data indicates that there are expressions of heterodimer complex in the retina spread across the photoreceptor cells, INL and RGC.15 More importantly, expression of βcR is also found on the eye stem cells. This has provided evidence that EPO can interact with heterodimer EPOR/βcR complex, and that binding of the EPO to EPOR/βcR activates Wingless (Wnt) signaling pathway (Figure 3). The Wnt signaling pathway has an important role in the developmental processes of the eye field, lens, and retina.16, 17, 18 As presented in Figure 3, binding of EPO to heterodimeric EPOR/βcR complex, leads to binding of Wnt to the Frizzled transmembrane receptors and subsequent inhibition of the β-catenin phosphorylation by glycogen synthase kinase (GSK)-3β. Activated β-catenin then escapes from proteasomal destruction and translocates into the nucleus through Wnt pathway, triggers transcription and activation of Wnt-responsive genes expression responsible for the proliferation, differentiation, orientation, adhesion, survival and apoptosis.17
Figure 3.
The role of erythropoietin in eye development via neuroprotective action. Binding of EPO to EPOR/βcR activates Wnt signaling and inhibits GSK-3β phosphorylation and proteasomal degradation of β-catenin. Free β-catenin translocates into the nucleus and upregulates expression of lymphoid enhancer-binding factor 1/T-cell factor 1 (Lef1/Tcf) and ultimately promotes neuronal survivability, protection, and differentiation.
EPO in pathogenesis of ocular disorders: a protective or causative role?
Our perspective on EPO no longer confined to the stimulation of RBC production. To date, the presence of EPO and their receptors were well established in the eye (Table 1) and they had various physiological roles as mentioned above.8 Taken together, EPO has now been recognized to act through many mechanisms to offer protection to cells in ocular tissue (Table 2). Given the link between the protective response of EPO during eye development and the presence of EPOR in the retina, preliminary studies have looked into the potential role of EPO in the ocular system. Recent studies subjected to the use of EPO in various types of ocular disorders have reported on its neuroprotective functions such as anti-apoptotic,15, 19, 20, 21 anti-oxidant,22, 23, 24, 25 and anti-inflammatory properties.26, 27, 28
Table 1. Localization of EPO and EPOR in the eye.
| Site of expression | Experimental model | EPO | EPOR | References |
|---|---|---|---|---|
| Retinal pigment epithelium | Human | + | + | 4 |
| Outer nuclear layer (ONL); photoreceptor cells | Mouse, Human | - | + | 7, 8 |
| Inner nuclear layer (INL); amacrine, bipolar and horizontal cells | Mouse, Human | - | + | 3, 10 |
| Ganglion cell layer | Mouse, human | + | + | 7, 9, 11, 21 |
Table 2. Extended biological functions of EPO in ocular tissue.
| Erythropoietin function | Experimental design/research or disease model | Route of introduction/injection | Research outcomes | References |
|---|---|---|---|---|
| Anti-apoptotic | Light-induced retinal degeneration in a mouse model | Intraperitoneal | Administration of EPO to degenerated retina reduced photoreceptor cells apoptosis by direct action of EPO on the retina | 29 |
| Anti-apoptotic | Acute elevated intraocular pressure (IOP) in a rat model | Retrobulbar; 10 U/μl | Administration of EPO, after the onset of the disease, improved RGC survivability, restored mitochondrial structure, and elevated EPO and EPOR expression, through increased Bcl-xl and Bcl-2 expressions, trigger nuclear translocation of NF-κB and regulate membrane potential in the mitochondrial, respectively | 78 |
| Anti-apoptotic | Mouse model of glaucoma | Intraperitoneal; 3000, 6000, and 12 000 U/kg | Administration of EPO reduced RGC death without influencing the IOP, through direct binding of EPO to EPOR on RGC | 69 |
| Anti-apoptotic | Myelin oligodendrocyte glycoprotein (MOG)-induced optic neuritis in a rat model | Intraperitoneal; 5000 U/kg | Administration of EPO delayed onset of disease, reduced disease severity, increased RGC survivability and functionality through direct binding of EPO to EPOR on RGC, increase Bcl-2 expression and activation of Akt and MAPK1/2 signal transductions | 30 |
| Anti-apoptotic | Acute hypoxia-induced mice | Intravenous | Administration of EPO after retinal injury preserved retinal architecture and function, and reduced retinal apoptosis by blocking caspase-1 activation and direct binding of EPO to EPOR on the photoreceptors | 8 |
| Anti-apoptotic | Glutamate and nitric oxide (NO)-induced toxicity in RGC primary cells | In vitro delivery; 1.5 U/ml | Delivery of EPO to damaged RGC decreased RGC apoptosis by the increase of Bcl-2 expression | 138 |
| Anti-apoptotic | Retinal degeneration slow (rds) mouse | Subretinal; 10 U | Administration of EPO to degenerated retina reduced photoreceptor cells apoptosis, with no increase in hematocrit level, and abolished reactivity of Műller cells, by direct action of EPO on the retina | 10 |
| Anti-apoptosis | Oxidative-induced human RPE cells | In vitro delivery; 1 IU/ml | Delivery of EPO to damaged RPE reduced RPE cells apoptosis through activation of PI3-K/Akt signal transduction pathway to inhibit caspase-3-like activities | 22 |
| Anti-apoptotic | RGC-induced axonal degeneration in a rat model | Intravitreal; 10 and 25 U | Administration of EPO improved RGC survivability by inhibiting caspase-3 activation | 139 |
| Anti-apoptotic | Primary adult injured retina following ON lesion | Intraocular | Administration of EPO to injured retina reduced RGC apoptosis in the entire retinal section through activation of PI3-K/Akt signaling pathway and anti-apoptotic Bcl-xl expression, and blocking of caspase-3 activity | 140 |
| Anti-inflammatory | Chronic experimental autoimmune encephalomyelitis (EAE) model induced in mice | Intraperitoneal; 50 μg/kg | Administration of low concentration of EPO before immunization, during and after onset of disease, reduced disease severity, and inflammatory response by reducing secretion of inflammatory cytokines; TNF-α, IL-1β, IL-1 receptor antagonist, and IFN-γ in the spinal cord and peripheral lymphocytes, respectively | 31 |
| Anti-inflammatory | Acute EAE model induced in a rat model | Intraperitoneal; 5000 U/kg | Administration of EPO delayed the progression of disease, attenuated disease severity by inhibiting inflammatory cell secretion of IL-6 in the spinal cord and delaying expression of TNF | 26 |
| Anti-inflammatory | Retinal degeneration in diabetic rats | Intraperitoneal | Administration of EPO peptide to damaged retina showed no increase in hematocrit level, prevented Müller cell gliosis, DNA damage, and RGC apoptosis, through reduction of TNF-α, IL-6 and IL-1β secretion, and increase of IL-10 secretion | 67, 141 |
| Anti-oxidative | Oxidative-induced human RPE cells | In vitro delivery; 1 IU/ml | Delivery of EPO to damaged RPE restored mitochondrial membrane integrity by reducing TNF-α and IL-1β inflammatory cytokines secretion, inhibiting caspase-3 activity and blocking cytochrome c production | 22 |
| Neuroregeneration | RGC-induced axonal degeneration in a rat model | Intravitreal; 10 and 25 U | Administration of EPO-induced RGC axon regeneration within and beyond the peripheral nerve through JAK2/STAT3 pathway | 139 |
| Neuroregeneration | Primary adult injured retina following ON lesion | In vitro delivery and intravitreal; 1000 U/ml | Administration of EPO to injured retina induced RGC axon regeneration into morphological and functional active neurite, at dose-dependent manner via an increase of Bcl-xl expression through activation of JAK2/STAT3 signaling pathway | 140 |
| Inhibition of pre-retinal neovascularization | Retinal degeneration in diabetic rats | Intraperitoneal | Administration of EPO peptide to damaged retina reduced retinal vasculature degeneration and retinal hypoxia by decreased VEGF expression | 22 |
However, their roles in the pathogenesis of ocular disorders were unclear. The use of EPO for the treatment of ocular disorders has raised concerns and doubts as to whether they aggravate pathogenicity or provide protection to counter the insults to the eye. Here, we looked into the literature reviews regarding the possible protective and causative mechanisms of EPO in ocular disorders.
The effects of physical conditions such as oxygen and light are crucial for retinal functions. Several distinguished investigations addressed the properties of the EPO to mediate protection against retinal damage through anti-apoptotic,29, 30 anti-oxidant,22 and anti-inflammatory26, 31 actions in hypoxia-, light-, and genetic-induced models.5, 8, 12, 15 Furthermore, Chung et al20 reported on his finding for the expression of EPO and EPOR were regulated according to a circadian rhythm in the retina, with a higher expression seen before daily light onset. Thus, this may further justify EPO as a form of cellular defense to counter physical insults following intense exposure to bright light and hypoxia condition effects.
Notably, numerous studies have also successfully outlined the molecular properties of EPO in the context of its neuro- and tissue-protective mechanisms. Grimm et al8 found that systemic administration of EPO into a hypoxia-induced model prevented photoreceptor cell deaths. Here, in this article the author clarified on the mode of EPO administration that allows EPO to cross the blood-retinal barrier (BRB), interacts with the EPOR on the damaged photoreceptor cells and terminates cell apoptosis via inhibition of caspase activity.8 Likewise, EPO showed significant preservation of the retinal architecture and function in an ischemia-induced model and Junk et al12 postulated that the action of EPO in NF-κB, MAPK and PI3-K/Akt signal transduction pathways, to stimulate the secretion of anti-apoptotic factors (Bcl-xl and Bcl-2) and neuroprotective gene transcription such as superoxide dismutase and inhibitors of apoptosis.12 Another study by Xie et al32 showed that delivery of exogenous EPO through intravitreal injection upregulated Bcl-xl expression and attenuated caspase-3 activity. These studies clearly explained the protective mechanism of EPO in the eye.
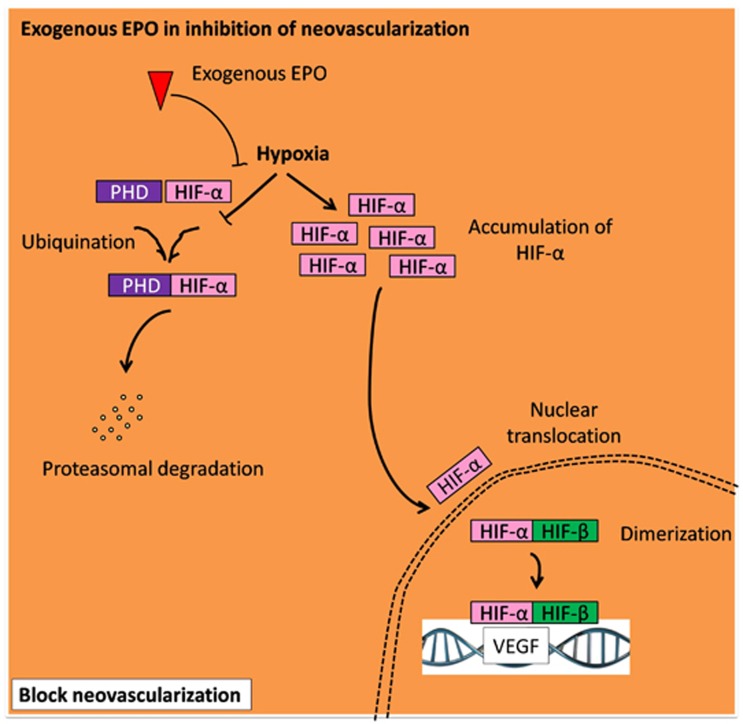
A balanced homeostasis to the eye microenvironment is important and any disturbances, including persistent reduction in oxygenation, will progressively develop angiogenesis, leading to abnormality in the retinal vasculature, disruption of BRB integrity, and finally, retinal neovascularization.33 Figure 4 illustrates the intracellular event that leads to the inhibition of neovascularization by the action of exogenous EPO. In normal condition, hypoxia-inducible factor (HIF)-1α is targeted by an oxygen detector named as prolyl hydroxylase (PHD) for degradation in the cytoplasm. However, during hypoxic conditions, degradation of HIF-1α is inhibited due to the downregulation of PHD, thus, leading to building up of HIF-1α and translocation into the nucleus.34 Translocated HIF-1α associates with HIF-β and induces transcription of vascular endothelial growth factor (VEGF) expression and initiates an angiogenesis mechanism.34 Another study by Zhang et al35 supported how EPO exerted a protective effect by preventing apoptosis on the endothelial cell and preserved tight junction proteins in the retinal vasculature, via crossing the BRB. A similar study showed that the lack of EPO would aggravate progression of retinopathy in the early phase and the administration of EPO has protected retinal vasculature by suppression of retinal vessel loss, thus inhibiting subsequent blood vessel formation and hypoxia.9 The mechanism underlying the protective effect of EPO in the maintenance of retinal vasculature is mediated by JAK2 and PI3-K/Akt pathways.9 A study by Zhang et al36 demonstrated in early DR model that exogenous delivery of EPO through intravitreal injection, could inhibit neovascularization by downregulating protein expressions of HIF-1α and VEGF. Different modes of EPO administration, such as systemic delivery or intravitreal injection have given positive effect for ocular disorders. Likewise, Shen et al37 showed that intraperitoneal delivery of EPO in retinopathy rat repressed vascular leakage in the retina with improvement on the retinal vascular networks and diminished focal vascular lesions.
Figure 4.
The role of erythropoietin in modulating neovascularization. Delivery of EPO, under hypoxic condition, promotes HIF-α hydroxylation via PHD, leading to proteasomal degradation of HIF-α and ultimately inhibits transcription of VEGF expression and angiogenesis mechanism.
The well-known findings of several reports showed that EPO has common binding domain with a few other types of receptors such as interleukin-6 receptor (IL6-R), βcR, and so on. The extracellular domain of EPOR shares a common binding domain with IL6-R.38, 39 IL-6 is a type of immune-regulatory cytokine, in which abnormalities in IL-6 signaling will lead to autoimmune disease40 or increased susceptibility to pathogen infection.41 The complex binding of IL-6 to the receptor requires further association with glycoprotein 130 (gp130) before generating a downstream signal transduction.42 Recently, Leibinger et al43 found that optic nerve injury triggered upregulation of IL-6 and the binding of IL-6 to its receptor on the retina, via activation of JAK/STAT3 transduction pathway, ultimately promoted neurite outgrowth and prolonged survivability of mature retinal ganglion cells in culture. In another study, IL-6 was found to prevent photoreceptors from dying when neuroretina layer was detached from the RPE.44 Hence, the binding of EPO to IL6-R may also confer the positive effects on anti-inflammatory properties of retinal neuron cells via activation of IL-6 downstream signaling pathway.
Apart from its protective role, the challenges of using EPO are also defined by its pathological effect on the regulation of microvascular permeability and ocular angiogenesis. The development of blood vessels is essential to supply sufficient concentration of oxygen to maintain optimal tissue function. In the adult, the process of angiogenesis is predominantly mediated by VEGF stimulation from the initiation of vascular permeability, followed by endothelial cell proliferation and migration.45 Finally, formation of new vascular network through proper maturation and remodeling.45 In adults, angiogenesis is normally quiescent, unless, stimulated by appropriate angiogenic factors, this process can occur during embryogenesis,45 menstruation,45 and wound healing.45 However, angiogenesis has been associated with many pathological diseases, such as ocular retinopathy, arthritis, and tumor formation.
In the eye, high level of VEGF can be caused by ischemia,45, 46 hypoxia,45, 46 or hyperglycemia45, 46 that results in vascular leakage and neovascularization, which is prominently observed in ocular disorders, like DR and AMD.46 Therefore, targeting VEGF for the management of retinal neovascularization has always been the strategy employed to minimize diabetic macular edema45, 46 and choroidal neovascularization45, 46 in patients with diabetic maculopathy and AMD.46 However, studies have described that VEGF alone does not complete angiogenesis, thus search for other angiogenic factors that are equally contributing to angiogenesis has unraveled the involvement of EPO.47, 48
Angiogenesis is caused by the activation of HIF-1α, under hypoxic conditions, promotes expression of VEGF transcript.34 At the same time, regulation of EPO is also mediated by the control of HIF-1α.34, 36 Thus, the binding of EPO/EPOR may have the potential to induce new blood vessel formation. It was observed that EPO stimulates angiogenesis as potently as VEGF, regardless of VEGF induction, through an independent mechanism.49, 50 Interestingly, Kertesz et al51 reported that the expression of VEGF was upregulated, in the absence of HIF-1α transcription factor, but in response to EPO stimulation. Evidence from the study hypothesized that increase in the expression and activation of EPOR can be triggered by VEGF-A, present in the vascular endothelium, upon binding to VEGF receptor-2 (VEGFR-2).34, 36, 51 Phosphorylated VEGFR-2 then interacts with phosphorylated EPOR to enhance angiogenesis via a STAT3 signaling pathway51 and this was equally reported by Nakano et al,52 Sautina et al,53 and Yang et al.54 In conclusion, the EPO also modulates angiogenesis by inducing the secretion of VEGF, which then binds and activates VEGFR-2.51, 52, 53, 54
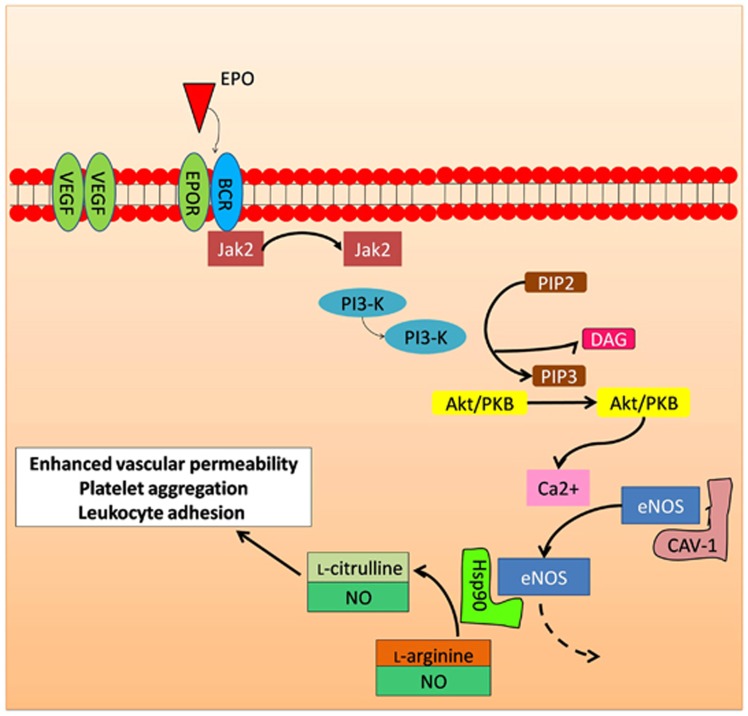
Apart from VEGFR-2, Sautina et al53 also demonstrated that the stimulation of EPO is dependent on the βcR. On the basis of our initial findings, we have discussed the role of the EPO on tissue protection through the interaction of heterodimeric EPOR/βcR.17 The effect of EPO on endothelial progenitor cells (EPC) has been postulated to be secondary to the EPO-induced phosphorylation of Akt, and release of nitric oxide (NO) molecules.53 Figure 5 shows the downstream signaling transduction event upon complex binding and initiation of increased vascular permeability and relaxation further leads to angiogenesis.45
Figure 5.
The role of erythropoietin in regulating the retinal vasculature. Binding of EPO/EPOR induces JAK2 phosphorylation, dimerization, and subsequently, phosphorylation of Akt. Akt activates and translocates endothelial nitric oxide synthase (eNOS) into the nucleus, upregulates nitric oxide production for regulation of leukocyte adhesion, cellular proliferation, vascular tone, and platelet aggregation in endothelial cells. These processes can lead to preservation of ischemic vasculature.
However, the interaction of the two receptors, EPOR/βcR, was reported to participate in the pathological consequences of EPO by stimulating the release of intracellular NO, which leads to the activation of angiogenesis.39, 53 This study proposed that NO stimulation was regulated by the heterotrimeric interaction between EPOR/βcR/VEGF-R2 upon binding of EPO to EPOR/βcR.39, 51, 52, 53, 54, 55
Meanwhile, studies have reported that genetic polymorphism in the EPO gene may be a pathogenic factor for acquiring risk of proliferative DR.56 Katsura et al55 showed that vitreous EPO was present in significantly higher amount in proliferative DR patients than in macular hole patients who had no retinopathy. The authors further showed the high EPO concentration was not induced by systemic anemia.55 This result was in agreement with a later study which showed that vitreous EPO and expression of EPO mRNA was higher in retina layer (RPE and neuroretina cells) and was not mediated by HIF in diabetic patients.57 It is observed that the patients manifested positive detection of single nucleotide polymorphism rs1617640 on the EPO promoter region (1125 bp upstream of the transcriptional start site), where the AP1 enhancer will bind to. The change of genotype (TT in replacement of GG) might enhance the transcription of the EPO gene as EPO was present in higher amount in vitreous fluid, even in samples that were collected from non-diabetic individuals.56
We have illustrated with reasonable evidence on the role, mechanism, and interaction of EPO as a therapeutic and pathological agent under different pre-clinical settings for different models of ocular disorders. These will be the basis for extrapolation to rational strategies for EPO application and administration for ocular disorders.
Current clinical trials
With encouraging results of neuroprotection for optic neuritis in animal studies, recombinant EPO had been tested clinically in a small pilot study in patients with acute autoimmune optic neuritis secondary to multiple sclerosis.58 EPO was well tolerated at 20 000 units (U) daily for 5 days and found to be effective even though one patient developed cerebral sinus venous thrombosis.58 Recombinant EPO was further tested in a phase II clinical trial in patients with autoimmune optic neuritis as an add-on therapy with methylprednisolone (NCT00355095). Recombinant EPO was given intravenously in bolus doses of 33 000 IU per day for 3 consecutive days continuously with methylprednisolone. Promisingly, the results demonstrated that the delivery of EPO reduced the retinal nerve fiber layer thinning, optic nerve atrophy, and improved the visual function without any safety issues.59 On the contrary, a similar study has also been carried out by Shayegannejad et al60 with the same regime and showed no significant improvement in clinical outcome. Regardless of dissimilar outcomes, a phase III clinical trial has commenced (NCT01962571) with a similar regime and is still ongoing.
EPO has also been tested in a pilot study to treat traumatic optic neuropathy.61 Seven patients with indirect traumatic optic neuropathy were given 10 000 units per day of recombinant EPO for 3 days and compared with placebo. The patients showed significant clinical improvement. Further EPO study proceeded to phase I and phase II clinical trials (NCT01783847) looking into EPO treatment for traumatic optic neuropathy. However, in this clinical trial, the dosage was doubled to 20 000 units of recombinant EPO given intravenously for 3 days.
Apart from these two types of optic neuropathy, optic nerve can also be affected by accidental ingestion of methanol causing blindness. The healing property of EPO for methanol-associated optic neuropathy was reported by Pakravan and Sanjari.62 Two patients were sequentially injected intravenously with EPO through a combination of systemic delivery of steroids and B-group vitamins, markedly restored their vision within 3 days to 3 weeks. This successful treatment strategy with EPO for methanol-associated optic neuropathy has encouraged the initiation of phase II clinical trial (NCT02376881) and currently is still ongoing. In this trial, a dosage of 20 000 units of recombinant EPO (alfa) was given intravenously for 3 days and patients were further monitored for the improvement in visual outcome.
Clinical trials involving EPO therapy were not only confined to optic neuropathy, but also extended for the treatment of retinopathy of prematurity. EPO treatment has been shown to be neuroprotective in many pre-clinical studies in both low and high doses. In view of this, a clinical trial (NCT00910234) comparing a low dose (100 U/kg) versus high dose (3000 U/kg) of intravenous EPO was evaluated in very preterm infants for protection against brain injury and retinopathy of prematurity.
Apart from formal clinical trials, EPO therapy has been found promising on several other retinal diseases. Intravitreal delivery of EPO (5 U/50 μl; three doses every 6 weeks) to the patients with chronic macular edema was found to improve visual acuity.63 The patients' visual acuity with an administration of EPO was significantly improved and lasted at least 18 weeks. Similar study was applied in patients with AMD-related geographical atrophy.64 The clinical findings reported that the rate of geographical atrophy enlargement reduced dose dependently when compared to the untreated eyes. EPO therapy was also evaluated in a small group of subjects with non-arteritic anterior ischemic optic neuropathy.65 A single dose of intravitreal injection (2000 U) was given and patients were followed up to 6 months post injection.65 Visual acuity improved in 61% of patients and maintained for up to 3 months before deteriorating back to baseline.65 However, there was no improvement observed in the retina for a group of patients with acute vascular occlusion.66
In human, these clinical trials and case studies will help to establish the safety and effectiveness of EPO with regard to the optimal dosage and regime for the different ocular disorders.58, 62, 64, 65 However, further human studies are needed before the EPO becomes fully established for treatment of ocular disorders.
Application of EPO therapy for ocular disorders
With better understanding of the role of EPO in both the causative and protective mechanisms in the various ocular disorders such as DR, retinopathy of prematurity, AMD and others, the next consideration is the application of this knowledge to the treatment of eye diseases. Here, we also described the ideal timeframe to initiate treatment and in which conditions EPO should be used with caution.
The role of EPO in neural protection by anti-apoptosis makes it an ideal treatment in ocular disorders such as AMD,67 retinitis pigmentosa,10 glaucoma,68, 69 optic neuritis,30 and retinal detachment.32 Besides that, it is able to restore proteins at the tight junction level and protect BRB. This added benefit makes it a favorable treatment in early DR,35, 70 optic neuritis,30, 59 and retinopathy of prematurity.9
However, EPO therapy is a double-edged sword in the treatment of eye disease. Its role in angiogenesis causing neovascularization is a dreaded occurrence in the eye.48 Ischemia induces increased concentration of EPO, which stimulates proliferation,45 migration,45 and angiogenesis47, 48 in endothelial cells that express EPOR.45, 46, 71 Late treatment with EPO has been shown to induce pathological neovascularization in proliferative DR and advanced stage of retinopathy of prematurity.9
Both DR and retinopathy of prematurity are proliferative retinopathies that share similar etiopathogenesis. In the early stages of proliferative retinopathies, EPO is protective as it acts on a different pathway to prevent breakdown in BRB and neuronal cell death.36 However, administration of EPO is detrimental in advanced stage of proliferative retinopathies due to severe hypoxia condition that triggers neovascularization.49, 50 Several studies observed that high level of EPO had been found in proliferative DR72 and advanced retinopathy of prematurity,73 where EPO has a pathological role. Therefore, EPO should be given strictly in the early stage.
For retinal degenerative diseases and optic neuritis, the level of hypoxia is not as high as in proliferative retinopathies and would benefit from EPO treatment with a safe margin without the risk of neovascularization. A study comparing the level of EPO in pathological eyes with macular edema secondary to DR, cataract, and wet AMD showed a significantly high level of EPO in eyes with macular edema secondary to DR and a normal level of EPO in AMD and cataract.74
Several studies reported on the high amount of retinal cell death during retinal detachment and the amount of cell death was found to be accompanied by high EPO and EPOR expressions.75 The upregulation of EPO and EPOR expression were due to the cell protective mechanism to reduce the percentage of apoptosis. Therefore, administration of EPO reduced the cell death prominently in the photoreceptors.32 This knowledge may also be applied clinically during the primary treatment of retinal detachment and before retinal detachment surgery. EPO can be injected in patients with rhegmatogenous retinal detachment while waiting for their retinal detachment surgery to improve the functional outcome by reducing apoptosis of photoreceptor cells. This is an important area for future studies. In exudative retinal detachment, EPO may be injected to reduce the apoptosis before the primary treatment of the underlying disease to take effect.
Administration of EPO therapy for ocular disorders
In order to achieve the desired therapeutic effect of EPO in various ocular disorders, we need to consider the optimal mode of administration. Different ocular disorders require different approaches and mode of administration. Treatment for the eye can be given either locally or administered systemically to the eye. The advantages and disadvantages of both administrations for EPO have to be considered carefully in view of the many effects of EPO in our body.
The eye is ideal for a variety of local administrations. Local treatments that include eye drops, subconjunctival, intracameral, intravitreal, subretinal, subtenon, peribulbar, and retrobulbar injections are usually preferred to avoid systemic side effects. Local administration routes that have been studied for EPO include intravitreal,35 subretinal,76 subconjunctival,77 eye drops administration (patent: EP2590666A1), and retrobulbar injections.78
Among the local administration, subconjunctival approach has been tested pre-clinically for ocular penetration, for retinal neuroprotection, and for potential systemic side effect of hematocrit elevation in rats. They observed a good level of EPO expression in retinal ganglion cell layers without symptoms of hematocrit.77 However, so far there was no report on human studies to evaluate the feasibility of this mode of administration.
Eye drops are mainly reserved for anterior segment disorders. The formulation of eye drops has been used in isolated cases for various corneal disorders on patients such as refractory corneal ulcers, dry eyes, chemical injury, and others (patent: EP2590666A1). However, these were not formally reported. Livny et al79 did a study on topical EPO therapy using cellulose-based gel on corneal epithelial defects healing in rabbits. They found no improvement in the rate of healing with corneal stroma neovascularization as a significant side effect.79 In view of this unwanted side effect of corneal neovascularization, until further extensive study has been done, subconjunctival and eye drops application of EPO have to be used with caution.
Lagreze et al66 has evaluated the feasibility of a single intravitreal injection of 2000 units of EPO in humans in a small pilot study. Serum EPO, hematocrit level and eye parameters such as visual acuity, visual field, intraocular pressure, and electroretinograph were monitored. There was a transient rise in serum EPO not exceeding normal serum level with no toxicity issue reported.66
Intravitreal mode of administration had been widely studied and used both in pre-clinical and clinical trials for EPO therapy.65, 66, 80, 81 In intravitreal injections, the injected formulations will affect the dosage and regime used. If the EPO formulation has a rapid clearance, it would be ideal for repeated injections when needed. Asialoerythropoietin (asialoEPO) is a form of recombinant EPO produced by total enzymatic desialylation, which has a very short half-life. It has been tested pre-clinically for neuroprotection in animal models for central nervous system-associated disorders such as stroke, spinal cord injury, and others.82, 83, 84 This formulation is non-erythropoietic due to its rapid half-life and ideal for use in proliferative retinopathies such as DR. Repeated intravitreal injection of EPO with rapid clearance when indicated clinically would be ideal for proliferative retinopathies in the early stage.
If there is a sustained release form of EPO, it should be used with caution in proliferative retinopathies. This is because these proliferative retinopathies diseases may progress and we do not want a sustained high level of EPO in the eye that would exacerbate the neovascularization in advanced stages. Instead, sustained release formulation would be ideal for retinal degenerative disease such as retinitis pigmentosa, glaucoma, or optic neuropathy. Sustained release can be either an improved formulation or delivery through an absorbable vehicle delivery system.
For retinal degeneration, subretinal route that is nearer to the retina can be considered. Sustained release formulation would be ideal in retinal degenerative disease as ischemia causing neovascularization is not an issue here. EPO gene therapy using adeno-associated viral vector (AAV) for inherited retinal degeneration is an attractive mode of delivery.15 Besides that, the retrobulbar injection of EPO for neuroprotection in glaucoma has been used in animal study and found to be effective.78 From a practical aspect, retrobulbar procedure itself has many undesirable complications such as retrobulbar hemorrhage, globe perforation, and others. However, if the EPO formulation is a stable, sustained release, the patient may need very few injections.
EPO has been shown to be able to cross the blood–brain barrier85 and BRB8 when administered systemically. This allows EPO to be delivered systemically to patients orally, subcutaneously, or intravenously. For ocular disorders, intravenous delivery has been used clinically mainly for optic neuropathy and retinopathy of prematurity.
However, intravenous delivery of EPO may cause unwanted side effects. Systemic administration can cause an increase in hematocrit,29 which carries a risk of thrombosis in patients and cause hypertension.86 Systemic administration still needs to be studied further and used with caution as there is a possible risk of death from stroke, myocardial infarct and venous thromboembolism. Furthermore, in proliferative retinopathies, intravenous EPO therapy seems to increase the risk of development and worsening of retinopathy of prematurity.87 Elevated plasma erythropoietin was found to be a risk factor for proliferative DR.88
Formulation of EPO for the treatment in ocular disorders
Currently, recombinant EPO is used in clinical trials for ocular disorders. Recombinant EPO has a variety of glycosylation patterns and is produced in various forms by different pharmaceutical companies, mainly for improving hematocrit count in anemic patients. Among recombinant EPO, carbamylated EPO (CEPO) does not bind to the classical EPOR, hence, a failure to induce erythropoiesis. In addition to that, CEPO does not induce angiogenesis89 and specifically activates the neuron cells instead.90 CEPO may be suitable for ocular disorders such as DR and retinopathy of prematurity in which blood vessel formation is undesired. Pre-clinical study using CEPO showed retinal neuroprotection effects in early-stage diabetic rats without inducing neovascularization.91 CEPO is well studied for neuroprotection in other central nervous system disorders.92, 93 CEPO has also progressed to clinical trial for patients with acute ischemic stroke (NCT00756249). Although it has not been used in ocular disorders, CEPO can be considered as a good alternative.
In inherited ocular disorders such as retinal degenerative disease, gene therapy to provide a sustained regulated release of EPO for neuroprotection is desired. This will reduce the complications associated with repeated injection locally or systemically. Pre-clinical studies demonstrated that recombinant AAV vectors have been a promising tool to deliver EPO to the retina. Colella and Auricchio94 described a method using AAV viral serotype 2 to deliver EPO subretinally in a light-damage model of induced retinal degeneration and observed successful neuroprotective effect. They used non-erythropoietic EPO derivatives, which did not increase hematocrit level.15
As EPO is a pleiotropic protein, there is a need to regulate expression from AAV vector.95 Auricchio et al96 showed that rapamacyin could control EPO expression from the vector in a dose-dependent manner and the expression was confined to the eye. Recently, Hines-Beard et al97 devised tetracycline inducible promoter system in AAV vector to control delivery of EPO in the subretinal layer. The low dosage of doxycycline used compared with normal anti-bacterial dosage made this method attractive. However, further studies need to be done before application for human use.
Future directions
EPO upholds cell proliferation and differentiation into neuron to maintain optimal tissue function.2, 13, 14, 98, 99 Based on the positive pre-clinical outcomes with various animal models,5, 8, 12, 15 EPO may offer a promising future therapy for treatment of many common ocular disorders plaguing us today such as DR, retinal detachment, glaucoma, retinopathy of prematurity, AMD, and optic neuritis. Current clinical trials are using established recombinant EPO such as epoietin alfa100, 101 and darbepoietin,100, 102 which do not specifically target to correct nerve circuitry activity in the eye. Hence, refining the preparation and administration of recombinant EPO for this purpose would be the major focus of scientists in the near future.
Numerous strategies have been employed to synthesize EPO derivatives, such as asialoEPO and CEPO, either through desialylation of EPO carbohydrate90, 103, 104 or chemically modifications of EPO amino acid. The brief binding of asialoEPO to EPOR and the specificity of CEPO on retinal neurons may suggest suitable indication for AMD and DR.31, 82, 90, 105 We foresee that more clinical trials will be conducted to address on the bio-distribution, safety and efficacy of these new derivatives or peptide (peginesatide)106 before delineating their optimal use in ocular disorders. So far, Hallym University Medical Center is sponsoring a clinical trial (NCT01131533) to determine the intraocular pharmacokinetics following single intravitreal injection of epoietin alfa. Other clinical trials using the recombinant EPO have shown positive effects and have aforementioned. These clinical trials will not only increase the opportunity but also increase the demand and need to produce new targeted EPO derivatives in the near future.
Stem cells are cells capable of self-renewal and differentiation into many different types of functional cells.107 Various stem cells such as embryonic stem cells,108, 109, 110 hematopoietic stem cells,111, 112, 113 mesenchymal stem cells (MSCs),114, 115, 116 induced pluripotent stem cells,117, 118 and EPC119, 120 have been shown to demonstrate the capability to regenerate into retinal neurons,108, 110, 111, 112, 113, 117 retinal pigmented epithelium,109, 121, 122 and improve blood vessels permeability123 in both in vitro and in vivo studies. It is noteworthy that the transplanted cells will need to survive the harsh microenvironment before cell programming can occur for the replacement of damaged tissue. Hence, EPO administration before stem cell transplantation may help to condition the microenvironment for better chance of cell survival20, 35, 124, 125 and maximize tissue repair with smaller number of transplanted cells.
Incorporation of gene-editing technology into stem cells for treatment of disorder due to defective genes has seen several successes.126, 127 MSCs are a type of adult stem cells11 that have been intensively explored by Eliopoulos et al128 for delivery of EPO to correct anemia successfully in mice.129 We have previously transplanted MSCs derived from human Wharton's Jelly into the subretinal layer of the Royal College of Surgeon's rats and found that these cells had the ability to differentiate into retinal cells, which was in concurrance with other study.130, 131 The use of MSCs as a vector to deliver EPO132 through intravenous injection for ocular disorders is feasible in the future as these cells could home into the inflammatory site133, 134 and cross the BRB.7, 31, 33, 34, 35 The presence of EPO in stem cells could also enhance the survivability of transplanted cells128, 135 in local (intraocular) site of injection. Guan et al76 has compared the effect of transplantation of MSCs and EPO gene-modified MSCs subretinally in a rat model of retinal degeneration. They found that the improvement in the retinal morphology and function was greater in the rats transplanted with EPO gene-modified MSCs.76 Thus, incorporating this therapeutic gene with a better controlled regulation and safe system will potentially magnify the beneficial effect of stem cell therapy136, 137 for ocular disorders in the future.
Research associated to EPO therapy for ocular disorders should not stop at the efficiency study for tissue repair. There is a change of complex network of molecule interaction in response to EPO administration in the damaged microenvironment. Thus, researchers could center on transcriptomic approaches such as microarray and next-generation sequencing to deepen our understanding of the expression and function of each gene in the eye following the administration of EPO. These researches might lead to new knowledge on the specific mechanisms of tissue repair in the eye.
Conclusion
EPO has an important role in the eye right from the time of ocular development to the many protective and pathological pathways in ocular disorders. Realizing the potential of harnessing the beneficial effects of EPO for treatment of ocular disorders, many pre-clinical studies have been conducted and are still ongoing to understand the mechanisms and effects of EPO in the various ocular disorders such as DR, retinal detachment, glaucoma, retinopathy of prematurity, AMD, and optic neuritis. Some have embarked on clinical trials, even though it is in its early stage with no strong conclusive results published. In this review, we revisit the role of EPO in the eye, its potential for treatment of ocular disorders, clinical application, and future directions.
Acknowledgments
This research was completely supported by the Fundamental Research Grants Scheme (FRGS), Ministry of Educations, Malaysia, under the grant number 5524401. This work was also supported by the Putra Grant, Universiti Putra Malaysia, Malaysia (9436300).
Author contributions
SL Shirley Ding and SN Leow collected the data, designed figures, and co-wrote the manuscript; R Munisvaradass collected and analyzed the data; EH Koh designed figures; MLC Bastion and KY Then edited and analyzed the data; S Kumar collected the data, edited and analyzed the data, and PL Mok designed this work, collected and analyzed the data, co-wrote the manuscript and edited the manuscript.
The authors declare no conflict of interest.
References
- Chateauvieux S, Grigorakaki C, Morceau F, Dicato M, Diederich M. Erythropoietin, erythropoiesis and beyond. Biochem Pharmacol 2011; 82, pp 1291–1303. [DOI] [PubMed] [Google Scholar]
- Brines M, Grasso G, Fiordaliso F, Sfacteria A, Ghezzi P, Fratelli M et al. Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroreceptor. Proc Natl Acad Sci USA 2004; 101: 14907–14912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprara C, Britschgi C, Samardzija M, Grimm C. The erythropoietin receptor is not required for the development, function, and aging of rods and cells in the retinal periphery. Mol Vis 2014; 20: 307–324. [PMC free article] [PubMed] [Google Scholar]
- García-Ramírez M, Hernández C, Simó R. Expression of erythropoietin and its receptor in the human retina: a comparative study of diabetic and nondiabetic subjects. Diabetes Care 2008; 31: 1189–1194. [DOI] [PubMed] [Google Scholar]
- Rex TS, Allocca M, Domenici L, Surace EM, Maguire AM, Lyubarsky A et al. Systemic but not intraocular Epo gene transfer protects the retina from light-and genetic-induced degeneration. Mol Ther 2004; 10: 855–861. [DOI] [PubMed] [Google Scholar]
- Philbin MK, Ballweg DD, Tsakiri S, Robinson L, Posz N, Gray L et al. Hospital cost savings and physiologic benefit of developmentally supportive care for very low birth weight newborns 222. Pediatr Res 1998; 43: 40–40.9432111 [Google Scholar]
- Shah SS, Tsang SH, Mahajan VB. Erythropoetin receptor expression in the human diabetic retina. BMC Res Notes 2009; 2: 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm C, Wenzel A, Groszer M, Mayser H, Seeliger M, Samardzija M et al. HIF-1-induced erythropoietin in the hypoxic retina protects against light-induced retinal degeneration. Nat Med 2002; 8: 718–724. [DOI] [PubMed] [Google Scholar]
- Chen J, Connor KM, Aderman CM, Smith LEH. Erythropoietin deficiency decreases vascular stability in mice. J Clin Invest 2008; 118: 526–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex TS, Wong Y, Kodali K, Merry S. Neuroprotection of photoreceptors by direct delivery of erythropoietin to the retina of the retinal degeneration slow mouse. Exp Eye Res 2009; 89: 735–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo A, Vegvari D, Deak G, Lukats A, Berta AI, Szel A. The expression of erythropoietin and its receptor in the developing rat retina. Invest Ophthalmol Vis Sci 2008; 49: 5896. [Google Scholar]
- Junk AK, Mammis A, Savitz SI, Singh M, Roth S, Malhotra S et al. Erythropoietin administration protects retinal neurons from acute ischemia-reperfusion injury. Proc Natl Acad Sci USA 2002; 99: 10659–10664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Rowe MJ, Winters SA, Ohls RK. Elevated erythropoietin mRNA and protein concentrations in the developing human eye. Pediatr Res 2008; 63: 394–397. [DOI] [PubMed] [Google Scholar]
- Wu L, Chang S, Chen Y, Xia Q, Forbes M, Tsai JC. Erythropoietin receptor plays a role in the cell differentiation of retina and lens during eye development. Invest Ophthalmol Vis Sci 2008; 49: 3078. [Google Scholar]
- Colella P, Iodice C, Di Vicino U, Annunziata I, Surace EM, Auricchio A. Non-erythropoietic erythropoietin derivatives protect from light-induced and genetic photoreceptor degeneration. Hum Mol Genet 2011; 20: 2251–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond WS, Rex TS. Evidence that erythropoietin modulates neuroinflammation through differential action on neurons, astrocytes, and microglia. Front Immunol 2014; 5: 31–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Sarosi I, Cattley RC, Pretorius J, Asuncion F, Grisanti M et al. Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone 2006; 39: 754–766. [DOI] [PubMed] [Google Scholar]
- De Iongh RU, Abud HE, Hime GR. 2442 WNT/Frizzled signaling in eye development and disease. Front Biosci 2006; 11: 2442–2464. [DOI] [PubMed] [Google Scholar]
- Chang ZY, Yeh MK, Chiang CH, Chen YH, Lu DW. Erythropoietin protects adult retinal ganglion cells against NMDA-, trophic factor withdrawal-, and TNF-α-induced damage. PLoS One 2013; 8: e55291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H, Lee H, Lamoke F, Hrushesky WJM, Wood PA, Jahng WJ. Neuroprotective role of erythropoietin by antiapoptosis in the retina. J Neurosci Res 2009; 87: 2365–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilic U, Kilic E, Soliz J, Bassetti CI, Gassmann M, Hermann DM. Erythropoietin protects from axotomy-induced degeneration of retinal ganglion cells by activating ERK-1/-2. FASEB J 2005; 19: 249–251. [DOI] [PubMed] [Google Scholar]
- Wang Z, Shen L, Tu L, Hu D, Liu G-Y, Zhou Z et al. Erythropoietin protects retinal pigment epithelial cells from oxidative damage. Free Radic Biol Med 2009; 46: 1032–1041. [DOI] [PubMed] [Google Scholar]
- Zhang H, Gong Y, Wu X, Shi Y, Yin L, Qiu Y. Erythropoietin protects the retinal pigment epithelial barrier against non-lethal H 2 O 2 -induced hyperpermeability. African J Biotechnol 2013; 10: 3695–3703. [Google Scholar]
- Li F, Chong ZZ, Maiese K. Microglial integrity is maintained by erythropoietin through integration of Akt and its substrates of glycogen synthase kinase-3beta, beta-catenin, and nuclear factor-kappaB. Curr Neurovasc Res 2006; 3: 187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M-H, Cho G-W, Huh Y-M, Kim SH, Koh S-H, Huh Y-M et al. Transduction of human EPO into human bone marrow mesenchymal stromal cells synergistically enhances cell-protective and migratory effects. Mol Biol (Mosk) 2010; 44: 656–663. [PubMed] [Google Scholar]
- Agnello D, Bigini P, Villa P, Mennini T, Cerami A, Brines ML et al. Erythropoietin exerts an anti-inflammatory effect on the CNS in a model of experimental autoimmune encephalomyelitis. Brain Res 2002; 952: 128–134. [DOI] [PubMed] [Google Scholar]
- Wei X, Li Y, Sun X, Zhu X, Feng Y, Liu J et al. Erythropoietin protects against murine cerebral malaria through actions on host cellular immunity. Infect Immun 2014; 82: 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa P, Bigini P, Mennini T, Agnello D, Laragione T, Cagnotto A et al. Erythropoietin Selectively attenuates cytokine production and inflammation in cerebral ischemia by targeting neuronal apoptosis. J Exp Med 2003; 198: 971–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm C. Constitutive overexpression of human erythropoietin protects the mouse retina against induced but not inherited retinal degeneration. J Neurosci 2004; 24: 5651–5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sättler (née Hobom) MB, Merkler D, Maier K, Stadelmann C, Ehrenreich H, Bähr M et al. Neuroprotective effects and intracellular signaling pathways of erythropoietin in a rat model of multiple sclerosis. Cell Death Differ 2004; 11: S181–S192. [DOI] [PubMed] [Google Scholar]
- Savino C, Pedotti R, Baggi F, Ubiali F, Gallo B, Nava S et al. Delayed administration of erythropoietin and its non-erythropoietic derivatives ameliorates chronic murine autoimmune encephalomyelitis. J Neuroimmunol 2006; 172: 27–37. [DOI] [PubMed] [Google Scholar]
- Xie Z, Chen F, Wu X, Zhuang C, Zhu J, Wang J et al. Safety and efficacy of intravitreal injection of recombinant erythropoietin for protection of photoreceptor cells in a rat model of retinal detachment. Eye (Lond) 2012; 26: 144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arjamaa O, Nikinmaa M. Oxygen-dependent diseases in the retina: role of hypoxia-inducible factors. Exp Eye Res 2006; 83: 473–483. [DOI] [PubMed] [Google Scholar]
- Maes C, Carmeliet G, Schipani E. Hypoxia-driven pathways in bone development, regeneration and disease. Nat Rev Rheumatol 2012; 8: 358–366. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wu Y, Jin Y, Ji F, Sinclair SH, Luo Y et al. Intravitreal injection of erythropoietin protects both retinal vascular and neuronal cells in early diabetes. Invest Ophthalmol Vis Sci 2008; 49: 732–742. [DOI] [PubMed] [Google Scholar]
- Lai NM, Nalliah S. Information-seeking practices of senior medical students: the impact of an evidence-based medicine training programme. Educ Health (Abingdon) 2010; 23: 151. [PubMed] [Google Scholar]
- Shen W, Chung SH, Irhimeh MR, Li S, Lee SR, Gillies MC. Systemic administration of erythropoietin inhibits retinopathy in RCS rats. PLoS One 2014; 9: e104759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan JF. A novel family of growth factor receptors: A common binding domain in the growth hormone, prolactin, erythropoietin and IL-6 receptors, and the p75 IL-2 receptor β-chain. Biochem Biophys Res Commun 1989; 164: 788–795. [DOI] [PubMed] [Google Scholar]
- Su KH, Shyue SK, Kou YR, Ching LC, Chiang AN, Yu YB et al. Common receptor integrates the erythropoietin signaling in activation of endothelial nitric oxide synthase. J Cell Physiol 2011; 226: 3330–3339. [DOI] [PubMed] [Google Scholar]
- Kristiansen OP, Mandrup-Poulsen T. Interleukin-6 and diabetes: The good, the bad, or the indifferent? Diabetes 2005; 54: S114–S124. [DOI] [PubMed] [Google Scholar]
- LeBlanc R a, Pesnicak L, Cabral ES, Godleski M, Straus SE. Lack of interleukin-6 (IL-6) enhances susceptibility to infection but does not alter latency or reactivation of herpes simplex virus type 1 in IL-6 knockout mice. J Virol 1999; 73: 8145–8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M, Hibi M, Nakagawa N, Nakagawa T, Yasukawa K, Yamanishi K et al. IL-6-induced homodimerization of gp130 and associated activation of a tyrosine kinase. Science 1993; 260: 1808–1810. [DOI] [PubMed] [Google Scholar]
- Leibinger M, Müller a, Gobrecht P, Diekmann H, Andreadaki a, Fischer D. Interleukin-6 contributes to CNS axon regeneration upon inflammatory stimulation. Cell Death Dis 2013; 4: e609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong DY, Boehlke CS, Zheng QD, Zhang L, Han Y, Zacks DN. Interleukin-6 as a photoreceptor neuroprotectant in an experimental model of retinal detachment. Invest Ophthalmol Vis Sci 2008; 49: 3193–3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature 2000; 407: 242–248. [DOI] [PubMed] [Google Scholar]
- Witmer AN, GFJM Vrensen, Van Noorden CJF, Schlingemann RO. Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res 2003; 22: 1–29. [DOI] [PubMed] [Google Scholar]
- Brown MS, Barón AE, France EK, Hamman RF. Association between higher cumulative doses of recombinant erythropoietin and risk for retinopathy of prematurity. J AAPOS 2006; 10: 143–149. [DOI] [PubMed] [Google Scholar]
- Watanabe D, Suzuma K, Matsui S, Kurimoto M, Kiryu J, Kita M et al. Erythropoietin as a retinal angiogenic factor in proliferative diabetic retinopathy. N Engl J Med 2005; 353: 782–792. [DOI] [PubMed] [Google Scholar]
- Jaquet K, Krause K, Tawakol-Khodai M, Geidel S, Kuck K-H. Erythropoietin and VEGF exhibit equal angiogenic potential. Microvasc Res 2002; 64: 326–333. [DOI] [PubMed] [Google Scholar]
- Pagonopoulou O, Efthimiadou A, Lambropoulou M, Papadopoulos N, Nikolettos NK. Erythropoietin and growth factors exhibit differential angiogenic potential in mouse heart. In Vivo 2008; 22: 587–592. [PubMed] [Google Scholar]
- Zhu HF, Xu XY, Wan D, Zhang F, Wang HL, Xue LJ. The role of erythropoietin in regulating angiogenesis in brain. Chinese Pharmacol Bull 2011; 27: 451–454. [Google Scholar]
- Nakano M, Satoh K, Fukumoto Y, Ito Y, Kagaya Y, Ishii N et al. Important role of erythropoietin receptor to promote VEGF expression and angiogenesis in peripheral ischemia in mice. Circ Res 2007; 100: 662–669. [DOI] [PubMed] [Google Scholar]
- Sautina L, Sautin Y, Beem E, Zhou Z, Schuler A, Brennan J et al. Induction of nitric oxide by erythropoietin is mediated by the β common receptor and requires interaction with VEGF receptor 2. Blood 2010; 115: 896–905. [DOI] [PubMed] [Google Scholar]
- Yang Z, Wang H, Jiang Y, Hartnett ME. VEGFA activates erythropoietin receptor and enhances VEGFR2-mediated pathological angiogenesis. Am J Pathol 2014; 184: 1230–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsura Y, Okano T, Matsuno K, Osako M, Kure M, Watanabe T et al. Erythropoietin is highly elevated in vitreous fluid of patients with proliferative diabetic retinopathy. Diabetes Care 2005; 28: 2252–2254. [DOI] [PubMed] [Google Scholar]
- Tong Z, Yang Z, Patel S, Chen H, Gibbs D, Yang X et al. Promoter polymorphism of the erythropoietin gene in severe diabetic eye and kidney complications. Proc Natl Acad Sci USA 2008; 105: 6998–7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández C, Fonollosa A, García-Ramírez M, Higuera M, Catalán R, Miralles A et al. Erythropoietin is expressed in the human retina and it is highly elevated in the vitreous fluid of patients with diabetic macular edema. Diabetes Care 2006; 29: 2028–2033. [DOI] [PubMed] [Google Scholar]
- Borhani-Haghighi A, Ghodsi M, Razeghinejad MR, Mardani S, Mardani M, Nikseresht AR et al. Erythropoietin for acute multiple sclerosis in patients with optic neuritis as a first demyelination event. Neurosciences (Riyadh) 2012; 17: 151–155. [PubMed] [Google Scholar]
- Sühs K-W, Hein K, Sättler MB, Görlitz A, Ciupka C, Scholz K et al. A randomized, double-blind, phase 2 study of erythropoietin in optic neuritis. Ann Neurol 2012; 72: 199–210. [DOI] [PubMed] [Google Scholar]
- Shayegannejad V, Shahzamani S, Dehghani A, Dast Borhan Z, Rahimi M, Mirmohammadsadeghi A. A double-blind, placebo-controlled trial of adding erythropoietin to intravenous methylprednisolone for the treatment of unilateral acute optic neuritis of unknown or demyelinative origin. Graefes Arch Clin Exp Ophthalmol 2015; 253: 797–801. [DOI] [PubMed] [Google Scholar]
- Kashkouli MB, Pakdel F, Sanjari MS, Haghighi A, Nojomi M, Homaee MH et al. Erythropoietin: a novel treatment for traumatic optic neuropathy-a pilot study. Graefes Arch Clin Exp Ophthalmol 2011; 249: 731–736. [DOI] [PubMed] [Google Scholar]
- Pakravan M, Sanjari N. Erythropoietin treatment for methanol optic neuropathy. J Neuroophthalmol 2012; 32: 325–328. [DOI] [PubMed] [Google Scholar]
- Li W, Sinclair SH, Xu G-T. Effects of intravitreal erythropoietin therapy for patients with chronic and progressive diabetic macular edema. Ophthalmic Surg Lasers Imaging 2010; 41: 18–25. [DOI] [PubMed] [Google Scholar]
- Sinclair SH, Hurley K, Parvus B, Presti P. Intravitreal Erythropoietin in Eyes with Geographic Atrophy Secondary to Age-related Macular Degeneration. Invest Ophthalmol Vis Sci 2014; 55: 5890–5890.25125601 [Google Scholar]
- Modarres M, Falavarjani KG, Nazari H, Sanjari MS, Aghamohammadi F, Homaii M et al. Intravitreal erythropoietin injection for the treatment of non-arteritic anterior ischaemic optic neuropathy. Br J Ophthalmol 2011; 95: 992–995. [DOI] [PubMed] [Google Scholar]
- Lagrèze WA, Feltgen N, Bach M, Jehle T. Feasibility of intravitreal erythropoietin injections in humans. Br J Ophthalmol 2009; 93: 1667–1671. [DOI] [PubMed] [Google Scholar]
- Luo W, Hu L, Wang F. The protective effect of erythropoietin on the retina. Ophthalmic Res 2015; 53: 74–81. [DOI] [PubMed] [Google Scholar]
- Tsai JC, Song BJ, Wu L, Forbes M. Erythropoietin: a candidate neuroprotective agent in the treatment of glaucoma. J Glaucoma 2007; 16: 567–571. [DOI] [PubMed] [Google Scholar]
- Zhong L, Bradley J, Schubert W, Ahmed E, Adamis AP, Shima DT et al. Erythropoietin promotes survival of retinal ganglion cells in DBA/2J glaucoma mice. Invest Ophthalmol Vis Sci 2007; 48: 1212–1218. [DOI] [PubMed] [Google Scholar]
- Hernández C, Simó R. Erythropoietin produced by the retina: Its role in physiology and diabetic retinopathy. Endocrine 2012; 41: 220–226. [DOI] [PubMed] [Google Scholar]
- Kase S, Saito W, Ohgami K, Yoshida K, Furudate N, Saito A et al. Expression of erythropoietin receptor in human epiretinal membrane of proliferative diabetic retinopathy. Br J Ophthalmol 2007; 91: 1376–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan N, Monickaraj F, Balasubramanyam M, Rema M, Mohan V. Imbalanced levels of angiogenic and angiostatic factors in vitreous, plasma and postmortem retinal tissue of patients with proliferative diabetic retinopathy. J Diabetes Complications 2012; 26: 435–441. [DOI] [PubMed] [Google Scholar]
- Romagnoli C, Tesfagabir MG, Giannantonio C, Papacci P. Erythropoietin and retinopathy of prematurity. Early Hum Dev 2011; 87: S39–S42. [DOI] [PubMed] [Google Scholar]
- Jonas JB, Neumaier M. Erythropoietin levels in aqueous humour in eyes with exudative age-related macular degeneration and diabetic retinopathy. Clin Experiment Ophthalmol 2007; 35: 186–187. [DOI] [PubMed] [Google Scholar]
- Xie Z, Wu X, Qiu Q, Gong Y, Song Y, Gu Q et al. Expression pattern of erythropoietin and erythropoietin receptor in experimental model of retinal detachment. Curr Eye Res 2007; 32: 757–764. [DOI] [PubMed] [Google Scholar]
- Guan Y, Cui L, Qu Z, Lu L, Wang F, Wu Y et al. Subretinal transplantation of rat MSCs and erythropoietin gene modified rat MSCs for protecting and rescuing degenerative retina in rats. Curr Mol Med 2013; 13: 1419–1431. [DOI] [PubMed] [Google Scholar]
- Resende AP, São-Braz B, Delgado E. Alternative route for erythropoietin ocular administration. Graefes Arch Clin Exp Ophthalmol 2013; 251: 2051–2056. [DOI] [PubMed] [Google Scholar]
- Zhong Y-S, Liu X-H, Cheng Y, Min Y-J. Erythropoietin with retrobulbar administration protects retinal ganglion cells from acute elevated intraocular pressure in rats. J Ocul Pharmacol Ther 2008; 24: 453–459. [DOI] [PubMed] [Google Scholar]
- Livny E, Livnat T, Yakimov M, Masoud M, Weinberger D, Bahar I. Effect of erythropoietin on healing of corneal epithelial defects in rabbits. Ophthalmic Res 2013; 50: 129–133. [DOI] [PubMed] [Google Scholar]
- Tsai JC. Safety of intravitreally administered recombinant erythropoietin (an AOS thesis). Trans Am Ophthalmol Soc 2008; 106: 459–472. [PMC free article] [PubMed] [Google Scholar]
- Song BJ, Cai H, Tsai JC, Chang S, Forbes M, Del Priore L V. Intravitreal recombinant human erythropoietin: a safety study in rabbits. Curr Eye Res 2008; 33: 750–760. [DOI] [PubMed] [Google Scholar]
- Erbayraktar S, Grasso G, Sfacteria A, Xie Q, Coleman T, Kreilgaard M et al. Asialoerythropoietin is a nonerythropoietic cytokine with broad neuroprotective activity in vivo. Proc Natl Acad Sci USA 2003; 100: 6741–6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso G, Sfacteria A, Erbayraktar S, Passalacqua M, Meli F, Gokmen N et al. Amelioration of spinal cord compressive injury by pharmacological preconditioning with erythropoietin and a nonerythropoietic erythropoietin derivative. J Neurosurg Spine 2006; 4: 310–318. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhu C, Wang X, Gerwien JG, Schrattenholz A, Sandberg M et al. The nonerythropoietic asialoerythropoietin protects against neonatal hypoxia-ischemia as potently as erythropoietin. J Neurochem 2004; 91: 900–910. [DOI] [PubMed] [Google Scholar]
- Brines ML, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC, Cerami C et al. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci USA 2000; 97: 10526–10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provatopoulou ST, Ziroyiannis PN. Clinical use of erythropoietin in chronic kidney disease: outcomes and future prospects. Hippokratia 2011; 15: 109–115. [PMC free article] [PubMed] [Google Scholar]
- Shah N, Jadav P, Jean-Baptiste D, Weedon J, Cohen LM, Kim MR. The effect of recombinant human erythropoietin on the development of retinopathy of prematurity. Am J Perinatol 2010; 27: 67–71. [DOI] [PubMed] [Google Scholar]
- Gholamhossein Y, Behrouz H, Asghar Z. Diabetic Retinopathy Risk Factors: Plasma Erythropoietin as a Risk Factor for Proliferative Diabetic Retinopathy. Korean J Ophthalmol 2014; 28: 373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez R, Carracedo J, Nogueras S, Buendia P, Merino A, Cañadillas S et al. Carbamylated darbepoetin derivative prevents endothelial progenitor cell damage with no effect on angiogenesis. J Mol Cell Cardiol 2009; 47: 781–788. [DOI] [PubMed] [Google Scholar]
- Carelli S, Marfia G, Di Giulio AM, Ghilardi G, Gorio A. Erythropoietin: Recent developments in the treatment of spinal cord injury. Neurol Res Int 2011; 2011: 453179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhu B, Zou H, Hu D, Gu Q, Liu K et al. Carbamylated erythropoietin mediates retinal neuroprotection in streptozotocin-induced early-stage diabetic rats. Graefes Arch Clin Exp Ophthalmol 2015; 253: 1263–1272. [DOI] [PubMed] [Google Scholar]
- Boesch S, Nachbauer W, Mariotti C, Sacca F, Filla A, Klockgether T et al. Safety and tolerability of carbamylated erythropoietin in Friedreich's ataxia. Mov Disord 2014; 29: 935–939. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Kirkeby A, Zivin JA, Sager TN. Therapeutic window for nonerythropoietic carbamylated-erythropoietin to improve motor function following multiple infarct ischemic strokes in New Zealand white rabbits. Brain Res 2008; 1238: 208–214. [DOI] [PubMed] [Google Scholar]
- Colella P, Auricchio A. Photoreceptor degeneration in mice: Adeno-associated viral vector-mediated delivery of erythropoietin. Methods Mol Biol 2013; 982: 237–263. [DOI] [PubMed] [Google Scholar]
- Bohl D, Heard JM. Delivering erythropoietin through genetically engineered cells. J Am Soc Nephrol 2000; 11(Suppl 1): S159–S162. [PubMed] [Google Scholar]
- Auricchio A, Rivera VM, Clackson T, O'Connor EE, Maguire AM, Tolentino MJ et al. Pharmacological regulation of protein expression from adeno-associated viral vectors in the eye. Mol Ther 2002; 6: 238–242. [DOI] [PubMed] [Google Scholar]
- Hines-Beard J, Desai S, Haag R, Esumi N, D'Surney L, Parker S et al. Identification of a therapeutic dose of continuously delivered erythropoietin in the eye using an inducible promoter system. Curr Gene Ther 2013; 13: 275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang L, Dey S, Alnaeeli M, Suresh S, Rogers H et al. Erythropoietin action in stress response, tissue maintenance and metabolism. Int J Mol Sci 2014; 15: 10296–10333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielyan L, Schäfer R, Schulz A, Ladewig T, Lourhmati A, Buadze M et al. Survival, neuron-like differentiation and functionality of mesenchymal stem cells in neurotoxic environment: the critical role of erythropoietin. Cell Death Differ 2009; 16: 1599–1614. [DOI] [PubMed] [Google Scholar]
- Cersosimo RJ, Jacobson DR. Epoetin alfa versus darbepoetin alfa in chemotherapy-related anemia. Ann Pharmacother 2006; 40: 58–65. [DOI] [PubMed] [Google Scholar]
- Ostrvica E, Mesic E, Ostrvica D, Delic J, Delic-Custendil S, Hukic F. Effectiveness of treating the renal anemia in chronic hemodialyzed patients by epoietin alpha and beta. Med Arh 2010; 64: 4–6. [PubMed] [Google Scholar]
- Patton J, Reeves T, Wallace J. Effectiveness of darbepoetin alfa versus epoetin alfa in patients with chemotherapy-induced anemia treated in clinical practice. Oncologist 2004; 9: 451–458. [DOI] [PubMed] [Google Scholar]
- Sharples EJ, Thiemermann C, Yaqoob MM. Novel applications of recombinant erythropoietin. Curr Opin Pharmacol 2006; 6: 184–189. [DOI] [PubMed] [Google Scholar]
- Torup L, Leist M. Development of non-erythropoietic erythropoietin variants for neuroprotection. In: Höke A (ed). Erythropoietin and the Nervous System. Springer US: Boston, MA, USA, 2006. pp 1–9. [Google Scholar]
- Rabie T, Marti HH. Brain protection by erythropoietin: a manifold task. Physiology (Bethesda) 2008; 23: 263–274. [DOI] [PubMed] [Google Scholar]
- Kaspar AA, Reichert JM. Future directions for peptide therapeutics development. Drug Discov Today 2013; 18: 807–817. [DOI] [PubMed] [Google Scholar]
- Mok PL, Leong CF, Cheong SK. Cellular mechanisms of emerging applications of mesenchymal stem cells. Malays J Pathol 2013; 35: 17–32. [PubMed] [Google Scholar]
- Gonzalez-Cordero A, West EL, Pearson R a, Duran Y, Carvalho LS, Chu CJ et al. Photoreceptor precursors derived from three-dimensional embryonic stem cell cultures integrate and mature within adult degenerate retina. Nat Biotechnol 2013; 31: 741–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idelson M, Alper R, Obolensky A, Ben-Shushan E, Hemo I, Yachimovich-Cohen N et al. Directed differentiation of human embryonic stem cells into functional retinal pigment epithelium cells. Cell Stem Cell 2009; 5: 396–408. [DOI] [PubMed] [Google Scholar]
- Lamba DA, Karl MO, Ware CB, Reh TA. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc Natl Acad Sci USA 2006; 103: 12769–12774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kicic A, Shen W-Y, Wilson AS, Constable IJ, Robertson T, Rakoczy PE. Differentiation of marrow stromal cells into photoreceptors in the rat eye. J Neurosci 2003; 23: 7742–7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao YX, Xu HW, Yin ZQ, Fitzgibbon T. Noggin induces human bone marrow-derived mesenchymal stem cells to differentiate into neural and photoreceptor cells. Indian J Exp Biol 2010; 48: 444–452. [PubMed] [Google Scholar]
- Tomita M, Adachi Y, Yamada H, Takahashi K, Kiuchi K, Oyaizu H et al. Bone marrow-derived stem cells can differentiate into retinal cells in injured rat retina. Stem Cells 2002; 20: 279–283. [DOI] [PubMed] [Google Scholar]
- Mead B, Logan A, Berry M, Leadbeater W, Scheven Ba. Intravitreally transplanted dental pulp stem cells promote neuroprotection and axon regeneration of retinal ganglion cells after optic nerve injury. Invest Ophthalmol Vis Sci 2013; 54: 7544–7556. [DOI] [PubMed] [Google Scholar]
- Mead B, Logan A, Berry M, Leadbeater W, Scheven BA. Paracrine-mediated neuroprotection and neuritogenesis of axotomised retinal ganglion cells by human dental pulp stem cells: Comparison with human bone marrow and adipose-derived mesenchymal stem cells. PLoS One 2014; 9: e109305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanheira P, Torquetti L, Nehemy MB, Goes AM. Retinal incorporation and differentiation of mesenchymal stem cells intravitreally injected in the injured retina of rats. Arq Bras Oftalmol 2008; 71: 644–650. [DOI] [PubMed] [Google Scholar]
- Mellough CB, Sernagor E, Moreno-Gimeno I, Steel DHW, Lako M. Efficient stage-specific differentiation of human pluripotent stem cells toward retinal photoreceptor cells. Stem Cells 2012; 30: 673–686. [DOI] [PubMed] [Google Scholar]
- Osakada F, Ikeda H, Sasai Y, Takahashi M. Stepwise differentiation of pluripotent stem cells into retinal cells. Nat Protoc 2009; 4: 811–824. [DOI] [PubMed] [Google Scholar]
- Klassen HJ, Ng TF, Kurimoto Y, Kirov I, Shatos M, Coffey P et al. Multipotent retinal progenitors express developmental markers, differentiate into retinal neurons, and preserve light-mediated behavior. Invest Ophthalmol Vis Sci 2004; 45: 4167–4173. [DOI] [PubMed] [Google Scholar]
- Qiu G, Seiler MJ, Mui C, Arai S, Aramant RB, De Juan E et al. Photoreceptor differentiation and integration of retinal progenitor cells transplanted into transgenic rats. Exp Eye Res 2005; 80: 515–525. [DOI] [PubMed] [Google Scholar]
- Buchholz DE, Pennington BO, Croze RH, Hinman CR, Coffey PJ, Clegg DO. Rapid and efficient directed differentiation of human pluripotent stem cells into retinal pigmented epithelium. Stem Cells Transl Med 2013; 2: 384–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossmerbaeumer U, Ohnesorge S, Kuehl S, Haapalahti M, Kluter H, Jonas JB et al. Retinal pigment epithelial phenotype induced in human adipose tissue-derived mesenchymal stromal cells. Cytotherapy 2009; 11: 177–188. [DOI] [PubMed] [Google Scholar]
- Hou H-Y, Liang H-L, Wang Y-S, Zhang Z-X, Wang B-R, Shi Y-Y et al. A therapeutic strategy for choroidal neovascularization based on recruitment of mesenchymal stem cells to the sites of lesions. Mol Ther 2010; 18: 1837–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch S, Kannt A, Kolibabka M, Schlotterer A, Wang Q, Lin J et al. Systemic treatment with erythropoietin protects the neurovascular unit in a rat model of retinal neurodegeneration. PLoS One 2014; 9: e102013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Tian J, Cheng J, Zhang J. Effect of erythropoietin on the migration of bone marrow-derived mesenchymal stem cells to the acute kidney injury microenvironment. Exp Cell Res 2013; 319: 2019–2027. [DOI] [PubMed] [Google Scholar]
- Vollrath D, Feng W, Duncan JL, Yasumura D, D'Cruz PM, Chappelow a et al. Correction of the retinal dystrophy phenotype of the RCS rat by viral gene transfer of Mertk. Proc Natl Acad Sci USA 2001; 98: 12584–12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita H, Sugano E, Isago H, Murayama N, Tamai M. Gene therapy for retinitis pigmentosa. InTech: Martin F (ed). Gene Therapy - Tools and Potential Applications. Vol 127 2013, pp 1–21.
- Eliopoulos N, Zhao J, Forner K, Birman E, Young YK, Bouchentouf M. Erythropoietin gene-enhanced marrow mesenchymal stromal cells decrease cisplatin-induced kidney injury and improve survival of allogeneic mice. Mol Ther 2011; 19: 2072–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnhold S, Klein H, Semkova I, Addicks K, Schraermeyer U. Neurally selected embryonic stem cells induce tumor formation after long-term survival following engraftment into the subretinal space. Invest Ophthalmol Vis Sci 2004; 45: 4251–4255. [DOI] [PubMed] [Google Scholar]
- Leow SN, Luu CD, Nizam MHH, Mok PL, Ruhaslizan R, Wong HS et al. Safety and efficacy of human Wharton's Jelly-derived mesenchymal stem cells therapy for retinal degeneration. PLoS One 2015; 10: e0128973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund RD, Wang S, Lu B, Girman S, Holmes T, Sauvé Y et al. Cells isolated from umbilical cord tissue rescue photoreceptors and visual functions in a rodent model of retinal disease. Stem Cells 2007; 25: 602–611. [DOI] [PubMed] [Google Scholar]
- Mok PL, Cheong SK, Leong CF, Chua KH, Ainoon O. Extended and stable gene expression via nucleofection of MIDGE construct into adult human marrow mesenchymal stromal cells. Cytotechnology 2012; 64: 203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan SK. Mesenchymal stem cells home to inflamed ocular surface and suppress allosensitization in corneal transplantation. Invest Ophthalmol Vis Sci 2014; 55: 6631–6638. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Zhang Y, Zhang L, Wang M, Zhang X, Li X. Therapeutic effect of bone marrow mesenchymal stem cells on laser-induced retinal injury in mice. Int J Mol Sci 2014; 15: 9372–9385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daga A, Muraglia A, Quarto R, Cancedda R, Corte G. Enhanced engraftment of EPO-transduced human bone marrow stromal cells transplanted in a 3D matrix in non-conditioned NOD/SCID mice. Gene Ther 2002; 9: 915–921. [DOI] [PubMed] [Google Scholar]
- Mok PL, Cheong SK, Leong CF, Chua KH, Ainoon O. Human mesenchymal stromal cells could deliver erythropoietin and migrate to the basal layer of hair shaft when subcutaneously implanted in a murine model. Tissue Cell 2012; 44: 249–256. [DOI] [PubMed] [Google Scholar]
- Mok P-L, Cheong S-K, Leong C-F, Othman A. In vitro expression of erythropoietin by transfected human mesenchymal stromal cells. Cytotherapy 2008; 10: 116–124. [DOI] [PubMed] [Google Scholar]
- Yamasaki M, Mishima HK, Yamashita H, Kashiwagi K, Murata K, Minamoto A et al. Neuroprotective effects of erythropoietin on glutamate and nitric oxide toxicity in primary cultured retinal ganglion cells. Brain Res 2005; 1050: 15–26. [DOI] [PubMed] [Google Scholar]
- King CE, Rodger J, Bartlett C, Esmaili T, Dunlop SA, Beazley LD. Erythropoietin is both neuroprotective and neuroregenerative following optic nerve transection. Exp Neurol 2007; 205: 48–55. [DOI] [PubMed] [Google Scholar]
- Kretz A, Happold CJ, Marticke JK, Isenmann S. Erythropoietin promotes regeneration of adult CNS neurons via Jak2/Stat3 and PI3K/AKT pathway activation. Mol Cell Neurosci 2005; 29: 569–579. [DOI] [PubMed] [Google Scholar]
- McVicar CM, Hamilton R, Colhoun LM, Gardiner TA, Brines M, Cerami A et al. Intervention with an erythropoietin-derived peptide protects against neuroglial and vascular degeneration during diabetic retinopathy. Diabetes 2011; 60: 2995–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]