Synopsis
The development of organ dysfunction (OD) is related to the intensity and balance between trauma-induced simultaneous, opposite inflammatory responses. Early proinflammation via innate immune system activation may cause early OD, while anti-inflammation, via inhibition of the adaptive immune system and apoptosis, may induce immunoparalysis, impaired healing, infections, and late OD. Patients discharged with low level OD may develop the persistent inflammation-immunosuppression catabolism syndrome (PICS). Although the incidence of multiple organ failure (MOF) has decreased over time, it remains morbid, lethal and resource-intensive. Single OD, especially acute lung injury, however, remains frequent. At this time, treatment is limited, and prevention remains the mainstay strategy.
Keywords: organ dysfunction, postinjury inflammation, SIRS, CARS, SARS, PICS
HISTORICAL PERSPECTIVE: EVOLVING CONCEPTS ON THE PATHOGENESIS OF MULTIPLE ORGAN FAILURE
As advances in prehospital and acute hospital care conquered “the golden hour”, multiple organ failure (MOF) emerged as the leading cause of late trauma death.1–3 Eiseman et al. coined the term “multiple organ failure” (MOF) in 1977, with a clinical description of 42 patients with progressive organ dysfunction. 4 By the 1990s, Moore et al. proposed that MOF was a bimodal phenomenon. 5 In the “one-event” model, a massive traumatic insult would induce intense systemic inflammation response syndrome (SIRS) and precipitate organ dysfunction (OD). In the “two-event” model, patients initially resuscitated into moderate SIRS became vulnerable to a second activating event (infections, embolism, transfusions, secondary operations, etc.) during the so-called compensatory anti-inflammatory response syndrome (CARS) and could develop late MOF.
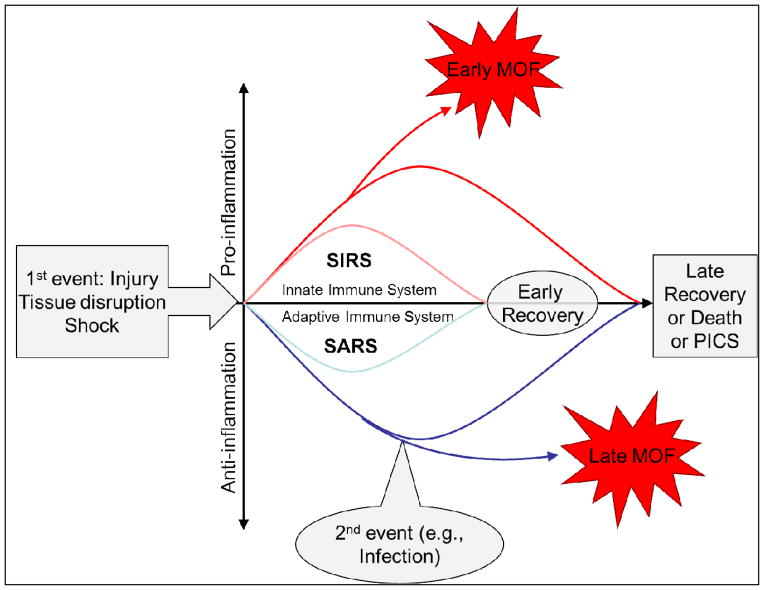
Modern hypotheses propose that injury triggers simultaneous, opposite responses: the proinflammation (SIRS) and the anti-inflammation, previously misnamed compensatory (CARS) (Figure 1).6 OD is related to the intensity and the balance between these opposing trauma-induced inflammatory responses. Severe SIRS, a proinflammation via activation of the innate immune system, causes early OD while early anti-inflammation, via inhibition of the adaptive immune system and apoptosis, limits proinflammation and creates a preconditioned state to protect against second hits and hasten healing. When countering unbalanced proinflammation, persistent anti-inflammation leads to severe systemic anti-inflammatory response syndrome (SARS, a more appropriate term than CARS), setting the stage for immunoparalysis, impaired healing, infections, and late OD.6,7 This was confirmed in a study by the Inflammation and Host Response to Injury Large-Scale Collaborative Research Program (Glue Grant) showing that alterations in the genomic expression of the classical inflammatory and anti-inflammatory responses occurred simultaneously. 8
Figure 1.
Theoretical framework for postinjury multiple organ failure: The synchronous immunoinflammatory model
As MOF began to recede as a result of aggressive prevention, a new OD phenotype emerged among patients discharged after lengthy ICU stays to long term facilities, where they suffer from a persistent inflammation-immunosuppression catabolism syndrome (PICS). 20 Although the phenotypes and epidemiology of postinjury OD have changed considerably over the past 20 years, it remains morbid, lethal and resource-intensive, as described in the next sections.21
DEFINING MOF
The Denver score (Table 1) 9 and the Multiple Organ Dysfunction Score (MODS) 10–12 are among the most common validated definitions in trauma studies. For others, the reader is referred to Baue’s excellent review. 13 The Denver score grades the dysfunction of four systems (pulmonary, renal, hepatic, and cardiovascular), while the MODS grades six organ systems (pulmonary, renal, hepatic, cardiovascular, hematologic and neurologic). Both scores have good predictive performance, but the MODS tends to be more sensitive (high incidence of MOF, low case-fatality rate), while the Denver MOF score tends to be more specific (low incidence, high case-fatality rate).
TABLE 1.
Denver Postinjury Multiple Organ Failure Score
| Organ System | Grade 0 | Grade 1 | Grade 2 | Grade 3 |
|---|---|---|---|---|
| Pulmonary | ||||
| PaO2/FiO2 ratio | >250 | 250–200 | 200–100 | ≤100 |
| Renal | ||||
| Creatinine (mg/dL) | ≤1.8 | 1.9–2.5 | 2.51–5.0 | >5.0 |
| Creatinine (μmol/L) | <159 | 160–221 | 222–442 | >442 |
| Hepatic | ||||
| Bilirubin (mg/dL) | ≤2.0 | 2.0–4.0 | 4.1–8.0 | >8.0 |
| Bilirubin (μmol/L) | <34 | 34–68 | 69–137 | >137 |
| Cardiac | No inotropes | Only one inotrope at a small dosea | Any inotrope at moderate dose or >1 agent, all at small dosesa | Any inotrope at large dose or >2 agents at moderate dosesa |
| Small | Moderate | Large | |
|---|---|---|---|
| Milrinone | <0.3 | 0.4–0.7 | >0.7 |
| Vasopressin | <0.03 | 0.03–0.07 | >0.07 |
| Dopamine | <6 | 6–10 | >10 |
| Dobutamine | <6 | 6–10 | >10 |
| Epinephrine | <0.06 | 0.06–0.15 | >0.15 |
| Norepinephrine | <0.11 | 0.11–0.5 | >0.5 |
| Phenylephrine | <0.6 | 0.6–3 | >3 |
Inotrope doses (in μg/(kg min)):
Need for inotropes more than dopamine .× μg/(kg min).
PAR: pressure-adjusted heart rate. PAR = heart rate × central venous pressure/mean arterial blood pressure.
EPIDEMIOLOGY AND CLINICAL RELEVANCE
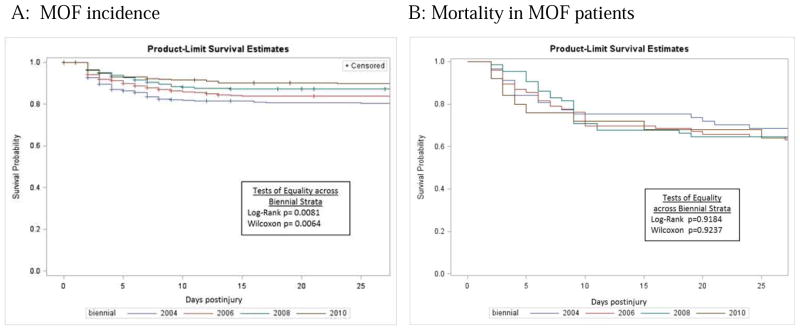
US and Australian studies have shown a steady decline in MOF’s incidence over the past decade. 14–17 Conversely, a large German study reported an increase in MOF incidence. 18 Most studies agree, however, that MOF associated mortality and morbidity remain high. 14–16,18–23 We recently studied MOF temporal trends in the Glue Grant dataset, a prospective study including adults with severe blunt torso injuries and hemorrhagic shock, enrolled from 2003 to 2010 in several US trauma centers sharing standard operating procedures. 15, 24 MOF, defined by the Denver score, was diagnosed in 223 (13.6%) patients, of whom 36% died. Table 2 details the distribution of admission risk factors, fluids, transfusions, complications, and outcomes over time. After adjustment for risk factors, MOF incidence decreased over time while MOF-related mortality remained persistently high (Figure 2). MOF patients continued to demand lengthy ventilator and critical care support. Applying the MODS definition produced similar results.
Table 2.
Population characteristics, admission risk factors, resuscitation fluids and blood transfusions, outcome and complications of 1643 adult, blunt trauma patients admitted to four US trauma centers (Glue Grant Dataset). Data are expressed in Median and Lower Quartile (LQ) and Upper Quartile (UQ) or percentages
| 2003–2004 | 2005–2006 | 2007–2008 | 2009–2010 | p-value * | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N=335 | N=506 | N=546 | N=256 | ||||||||||
| Variable | Median or % | LQ | UQ | Median or % | LQ | UQ | Median or % | LQ | UQ | Median or % | LQ | UQ | |
| DEMOGRAPHIC | |||||||||||||
| Age (years) | 40 | 26 | 54 | 41 | 26 | 54 | 43 | 28 | 57 | 45.5 | 25.5 | 58 | 0.0246 |
| Body Mass Index (kg/m2) | 25.7 | 23.1 | 29.9 | 26.7 | 23.7 | 31.4 | 27.1 | 23.8 | 31.3 | 27.3 | 24.1 | 32.0 | 0.0017 |
| Male sex (%) | 64.5 | 66.6 | 67.2 | 67.6 | 0.3940 | ||||||||
| Comorbidity Index >=2 (%) | 8.4 | 12.9 | 8.4 | 10.2 | 0.8276 | ||||||||
| Anti-platelet therapy (%)** | 6.6 | 7.1 | 10.1 | 8.2 | 0.1414 | ||||||||
| INJURY | |||||||||||||
| Moderate TBI (%)*** | 30.2 | 18.0 | 21.6 | 18.0 | 0.0037 | ||||||||
| Injury severity score | 29 | 22 | 41 | 32 | 22 | 41 | 34 | 24 | 41 | 34 | 22 | 43 | 0.0622 |
| Pre-hospital GCS | 13 | 4 | 15 | 14 | 10 | 15 | 14 | 9 | 15 | 14 | 9 | 15 | <.0001 |
| Pre-hospital SBP (lowest) | 89.0 | 72.5 | 104.5 | 86.0 | 71.0 | 102.0 | 88.0 | 73.0 | 108.0 | 85.0 | 74.0 | 100.0 | 0.8425 |
| Pre-hospital HR (highest) | 116 | 95 | 131 | 118 | 100 | 130 | 115 | 97 | 130 | 118 | 102 | 132 | 0.4810 |
| Admission GCS | 6 | 3 | 15 | 10 | 3 | 15 | 11 | 3 | 15 | 3 | 3 | 15 | 0.1534 |
| Admission SBP (mmHg) | 110 | 93 | 135 | 111 | 90 | 132 | 110 | 90 | 131 | 109.5 | 89 | 128 | 0.0429 |
| Admission SBP<=90mmHg | 22.1 | 25.9 | 26.7 | 29.9 | 0.0350 | ||||||||
| Admission HR (beats/min) | 108 | 86 | 127 | 110 | 90 | 126 | 109 | 91 | 127 | 110 | 92 | 127.5 | 0.2080 |
| FLUIDS/BLOOD | |||||||||||||
| RBC units/12 hours | 5 | 3 | 11 | 6 | 3 | 12 | 5 | 2 | 9 | 5 | 2 | 9 | 0.0739 |
| FFP units/12 hours | 3 | 0 | 8 | 3 | 0 | 8 | 2 | 0 | 6 | 3 | 0 | 7 | 0.0798 |
| 0–6hrs RBC:FFP ratio | 0.6 | 0 | 1.5 | 0.5 | 0 | 1.6 | 0 | 0 | 1.5 | 0.6 | 0 | 1.4 | 0.1224 |
| Platelet units/12 hours | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0.4160 |
| Pre-hospital crystalloids(ml) | 1.6 | 0.6 | 3.0 | 1.8 | 0.7 | 3.3 | 1.4 | 0.5 | 2.9 | 1.8 | 0.7 | 3.1 | 0.3504 |
| Crystalloids (ml)/12 hours | 10.3 | 7.2 | 15.7 | 10.0 | 7.6 | 13.6 | 8.7 | 5.9 | 12.3 | 9.0 | 6.3 | 12.0 | <.0001 |
| LABORATORIAL TESTS | |||||||||||||
| ED Base Excess (mEq/L) | −8.9 | −11.4 | −6 | −8.4 | −11.2 | −6 | −7.6 | −0.8 | −5 | −8 | −11.1 | −5.35 | 0.0012 |
| ED Lactate (mg/dL) | 4.3 | 3 | 5.9 | 3.9 | 2.7 | 5.6 | 3.6 | 2.4 | 5.2 | 4 | 2.4 | 6 | 0.0016 |
| Day 1 Platelet 1,000/mcL | 100 | 79 | 129 | 95 | 76 | 123 | 107 | 87 | 133 | 101 | 84 | 128.5 | 0.0028 |
| PaO2/FiO2 ratio/12 hours | 119 | 66 | 213 | 161 | 86.5 | 256 | 158 | 89 | 269 | 163 | 79 | 285 | 0.0003 |
| ED Hemoglobin g/dL | 10.9 | 9.33 | 13 | 11.3 | 9.5 | 13.1 | 11.9 | 10 | 13.3 | 11.5 | 9.6 | 12.9 | 0.0217 |
| ED INR | 1.2 | 1.1 | 1.5 | 1.3 | 1.1 | 1.5 | 1.21 | 1.1 | 1.5 | 1.3 | 1.1 | 1.5 | 0.3152 |
| COMPLICATIONS | |||||||||||||
| Non-septic complication (%) | 44.5 | 47.2 | 44.1 | 40.4 | 0.2365 | ||||||||
| Surgical site infection (%) | 13.1 | 16.4 | 14.8 | 8.2 | 0.1180 | ||||||||
| VAP (%) | 26.6 | 26.5 | 24.4 | 23.1 | 0.2335 | ||||||||
| OUTCOMES | |||||||||||||
| Multiple organ failure (%) | 17.0 | 15.0 | 11.9 | 9.8 | 0.0033 | ||||||||
| Lung failure (%) | 57.6 | 56.5 | 55.3 | 50.8 | 0.1073 | ||||||||
| Cardiac failure (%) | 20.9 | 17.6 | 16.1 | 12.5 | 0.0064 | ||||||||
| Liver failure (%) | 15.2 | 16.2 | 13.4 | 14.1 | 0.3762 | ||||||||
| Renal failure (%) | 10.1 | 10.7 | 11.9 | 12.5 | 0.2804 | ||||||||
| ICU days | 8 | 4 | 19 | 9 | 4 | 17 | 10 | 5 | 18 | 9 | 5 | 17 | 0.1070 |
| ICU free days | 11 | 0 | 21 | 15 | 4 | 23 | 15 | 3 | 22 | 17 | 8 | 22 | 0.0002 |
| Ventilator days | 6 | 2 | 14 | 5 | 2 | 13 | 7 | 2 | 13 | 6 | 2 | 12 | 0.8414 |
| Ventilator free days | 16 | 0 | 24 | 19 | 7 | 25 | 20 | 8 | 25 | 21 | 12 | 25 | <.0001 |
| Mortality (%) | 23.9 | 15.4 | 12.3 | 10.5 | <.0001 | ||||||||
| MOF RELATED OUTCOMES | |||||||||||||
| Case-fatality (%) | 33.3 | 38.2 | 36.9 | 36.0 | 0.7800 | ||||||||
| ICU days | 22 | 9 | 34 | 17 | 8.5 | 8.0 | 15 | 9 | 27 | 19 | 10 | 24 | 0.1889 |
| ICU free days | 0 | 0 | 4 | 0 | 0 | 8 | 0 | 0 | 10 | 4 | 0 | 7 | 0.2289 |
| Ventilator days | 20 | 9 | 26 | 15 | 6.5 | 27 | 12 | 7 | 21 | 13 | 6 | 19 | 0.0642 |
| Ventilator free days | 0 | 0 | 8 | 0 | 0 | 11.5 | 0 | 0 | 14 | 4 | 0 | 14 | 0.1969 |
| MOF RELATED COMPLICATIONS | |||||||||||||
| Non-septic complication (%) | 77.2 | 75.0 | 83.1 | 80.0 | 0.4481 | ||||||||
| Surgical site infection (%) | 22.8 | 27.6 | 16.9 | 20.0 | 0.4042 | ||||||||
| VAP (%) | 47.3 | 43.4 | 50.8 | 44.0 | 0.8946 | ||||||||
Cochran-Armitage Trend Test for trend was used for categorical variables and the non-parametric Spearman correlation coefficient and test for continuous variables; negative correlation coefficients indicate values decreased over time, while positive coefficients indicate values increased over time ; significance set at p<0.01 to account for large number of comparisons;
: antiplatelet medication previous to injury;
Moderate Traumatic Brain Injury (TBI) : Head Abbreviated Injury Scale(AIS)>3 with Glasgow Coma Scale (GCS) motor component>3; SBP: systolic blood pressure; HR: heart rate; RBC: packed red blood cells; FFP: fresh frozen plasma; INR: international normalized ratio, VAP: ventilator-associated pneumonia; ICU: intensive care unit
Figure 2. Kaplan-Meyer curves for MOF incidence and outcomes across biennial periods from 2003–2010 in 1643 adult, blunt trauma patients admitted to four US trauma centers (Glue Grant Dataset).
*stratum 2004=2003–2004; stratum 2006=2005–2006; stratum 2008=2007–2008; stratum=2009–2010
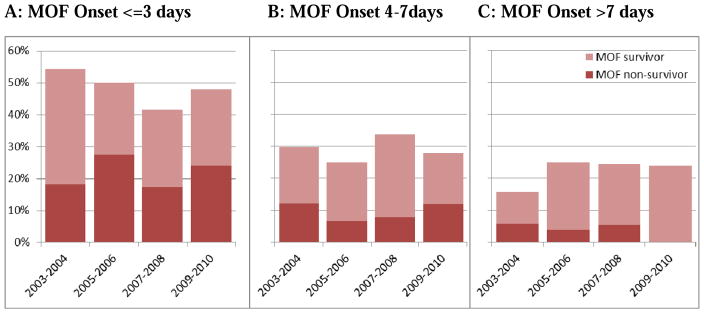
The risk factors for developing MOF included: demographic characteristics (advanced age, male sex, obesity), injury severity, and physiologic derangement upon admission (acidosis, number of transfused units of red blood cells [RBC]/12 hours). MOF-related death was positively associated with female sex, injury severity and RBC units transfused in the first 12 hours postinjury. The time interval between MOF onset and death is usually short, with death ensuing in two days in 58% of the cases. Early MOF (<3 days) carried a higher mortality than later MOF (Figure 3).
Figure 3.
MOF onset and respective case-fatality rates
Lung failure incidence decreased significantly over time, but remained the most common organ failure over the study period, affecting over half of these patients (Table 1). Cardiovascular dysfunction also became significantly less frequent, while renal and liver failures persisted at low, similar levels. The mortality was highest for cardiovascular dysfunction (39%), followed by failure of the kidneys (38%), liver (19%) and lungs (12%). There has been a decrease in the progression from lung dysfunction to MOF over time 25 MOF without lung dysfunction is rare: only 8% of the MOF patients did not have lung involvement.
THE BURDEN OF MOF
In the abovementioned study, Sauaia et al. showed that MOF survivors were responsible for 20% of the total ICU and mechanical ventilation days despite being only 9% of the total population. 15 Based on national estimates of critical care costs 26, the total cost of the critical care delivered to MOF patients in this dataset amounted to $19,990,420, or 22% of the total ICU cost for this population. The estimated median cost per MOF patient was $77,202, compared to the presumed cost of caring for non-MOF patients ($38,442).
PATHOPHYSIOLOGY
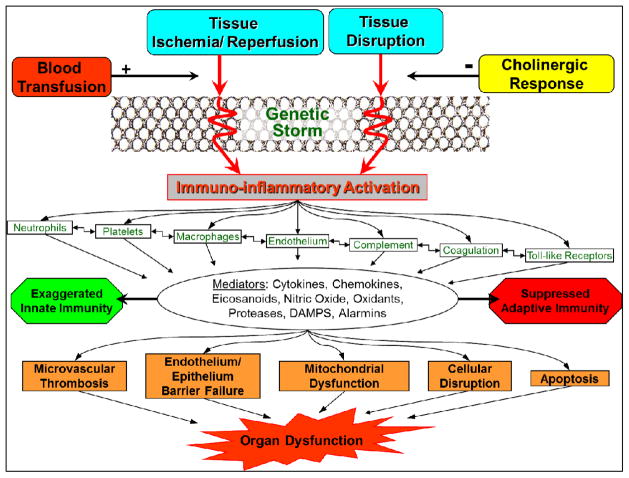
Figure 4 shows a framework for the response to trauma. SIRS is the manifestation of the immuno-inflammatory activation in response to ischemia/reperfusion (I/R) injury and factors released from disrupted tissue, mediated by inherent genetic and environmentally determined host characteristics.
Figure 4.
Response to trauma: hemostatic, inflammatory, endocrine and neurological systems interaction
The 1994 “danger theory” of the inflammatory response following trauma or infection proposed that the immunological system’s role was to protect the body from danger. 27–32 In this model, immunological responses are triggered by specific types of cell death. If a healthy, undamaged cell dies an apoptotic death, it is scavenged without triggering an immune response. Conversely, cell lysis or apoptosis via trauma or infection releases intracellular contents and signals “danger”, triggering both innate and adaptive responses.28,30,33
The injured cell releases endogenous damage-associated molecular patterns (DAMPs), analogous to the microbial pathogen-associated molecular patterns (PAMPs), released in sepsis, both of which activate the innate immunity. 32,34,35 PAMPs are exogenous microbial molecules that alert the organism to pathogens and are recognized by cells of the innate and acquired immunity system, primarily through toll-like receptors (TLRs), and activate several signaling pathways (e.g., NF-κB). 27 DAMPs include HMGB1 (high mobility group box protein-1), heat-shock proteins, uric acid and DNA. HMGB1, a nuclear protein that binds to nucleosomes and promotes DNA bending, has been associated with SIRS and end-organ damage in animals. In humans, it has been shown to be at high levels as early as 1 hour postinjury.27,36,37 Zhang et al. 34 showed that injury releases mitochondrial DAMPs (MTDs) into the circulation, which create a sepsis-like state and may be the key link between trauma, inflammation, and SIRS.
Most proteins identified in the plasma of blunt trauma victims are intracellular molecules that could function as DAMPS/alarmins and trigger pattern recognition receptors. 38 Our laboratory was the first to describe the proteome of human mesenteric lymph collected from critically ill or injured patients using a label-free semi-quantitative mass spectrometry (MS). 39 A total of 477 proteins were identified, including markers of hemolysis, extracellular matrix components, and general tissue damage in addition to the classical serum proteins. Postinjury hemolysis releases hemoglobin to the extracellular environment, where it becomes a redox-reactive DAMP molecule that can bind to PAMPs, trigger toll-like receptor (TLR)-mediated signal transduction and generate reactive oxygen species (ROS) potentially affecting innate immunity. 40 In the mesenteric lymph, we showed several markers of tissue damage and mitochondrial proteins suggestive of lysed mitochondria. 39 Circulating mitochondrial DNA and formyl peptides may mediate OD through PMN activation. 34
Our MS analysis of the plasma metabolome of severely injured patients indicated a hypercatabolic state that could provide carbon and nitrogen sources to compensate for trauma-induced energy consumption and negative nitrogen balance. 41 Our MS analysis also confirmed an altered lipidomic profile, a hallmark of metabolic adaptation to injury, with fatty acid mobilization and lipid breakdown, resulting in accumulation of anionic compounds (e.g., ketone bodies) and acidosis. 41 In addition, we noted elevations in proinflammatory arachidonate metabolites (PGE2, LTB4), which supported the immuno-modulatory effect of diets balancing the ratio of omega-3/omega-6 fatty acids. 42
We also observed significant proteolysis as shown by the accumulation of several aminoacids (alanine, aspartate, cysteine, glutamate, histidine, lysine, and phenylalanine) and cyclic dipeptide cyclo (glu-glu), which stimulates T-lymphocytes. 41 Glutamate and cysteine buildup could fuel new reduced glutathione synthesis, thereby serving as physiologic protection from the increase in trauma-dependent oxidative stress. There was significant nucleoside breakdown as demonstrated by increased levels of purines and pyrimidine catabolites. 41 Increased nicotinamide, a breakdown product of the purine metabolite NAD, may signal exhaustion of NAD+/NADH reservoirs potentially compromising many energy and redox-related processes dependent on these cofactors. Notably, there was no glutamine accumulation, possibly due to enhanced consumption of this amino acid for cellular energy production or fueling transamination reactions. 41 Glutamine supplementation in critically ill patients has been a long-sought therapeutic approach, yet there are no evidence to date demonstrating its benefit.43
Succinate, in particular, has elicited much interest in I/R injury related states. 44–46 This intermediate metabolite, normally produced during cellular respiration, becomes elevated after ischemia due to two potential mechanisms: 1) an interesting activity reversal of the enzyme succinate dehydrogenase (SDH), which, during normal oxygen conditions, breaks down succinate; and 2) macrophage activation by products of tissue ischemia that find to TLRs, leading to glutamine metabolism and succinate production. 46 During reperfusion, succinate is oxidized with the now abundant oxygen, and drives a reversal of the electron transport through complex 1, which produces reactive oxygen species. Succinate has inflammatory signaling capacity, leads to IL-1-beta production and activates immune cells.46,47 Chouchani et al. showed that preventing succinate elevation protected against I/R injury in mouse models of brain and heart ischemia. 44 Binding of succinate to a specific receptor (SUCNR1) in dendritic cells (antigen-presenting cells with an important role in initiating immune responses) appears to enhance the production of pro-inflammatory factors, suggesting that succinate may have a role in alerting the innate system of “danger”. 46,48
Specifically in the lungs, succinate has been shown to mediate stabilization of the hypoxia-inducible factor HIF1-alpha, a transcriptor factor that when stabilized by hypoxia (and also by mechanically stretched lung epithelia) mediates a number of protective actions during low oxygen availability. This provides a direct role of succinate in lung protection during acute lung injury. 49–51
In one of the few clinical studies of ARDS patients, large increases in precursors of uric acid (hypoxanthine, xanthine, guanosine) were observed suggesting that the pathway was activated. (although no uric acid was detected). 52 Uric acid has previously been shown to be a major endogenous danger signal in the lung, activating the NALP3 inflammasome and leading to IL-1β production.53 Bos et al. suggested that metabolomic analyses targeting lung injury focus on exhaled air, namely “breathomics”. 54,55 Indeed, this group used an electronic nose (sNOSE) to detect patterns in volatile organic compounds (VOC) through gas chromatography and mass spectrometry, which discriminated ICU patients with and without ALI with 92% accuracy. These investigators identified three VOC in ARDS patients within the first 24 hours post ICU admission: acetaldehyde (potentially from neutrophil infiltration in the lungs), octane and 3-methylheptane (the latter two possibly related to lipid peroxidation due to oxidative stress). 55
The Glue Grant study suggested that severe blunt trauma produced a “genomic storm” in the expression of over 80% of the leukocyte transcriptome across the first 28 days compared to healthy subjects. 8 The overexpressed genes were related to both the innate and adaptive immunity, while genes related to T-cell function and antigen presentation had decreased expression. The genomic response to blunt injury was remarkably similar to the response observed in burns and endotoxemia. Postinjury complications were associated with greater and prolonged overexpression, although there were no major differences in which genes were invoked. Despite providing compelling evidence, the abovementioned investigation had limitations, including a relatively small sample, inclusion of only blunt torso trauma, and focus on circulating leukocytes. 8 It is conceivable that different tissues have specific, localized inflammation expression patterns.
PICS patients have manageable OD with a long, eventful postinjury clinical course with recurrent inflammatory insults and infections, progressive loss of lean body mass (despite good nutritional support), poor wound healing, and decubitus ulcers. 7 Their labs show persistent neutrophilia and lymphopenia. Discharged to acute long-term care facilities, PICS patients die an indolent death or experience sepsis recidivism and ICU readmission. The elderly with baseline comorbidities and sarcopenia is especially prone to this refractory clinical phenotype. Often, the long-term outcome involves impairment of cognitive and functional status from which recovery is uncertain. 7,56 Clinically, PICS is defined as: long ICU stay (>14 days), persistent inflammation (C-reactive protein concentration >150 μg/dl and retinol binding protein concentrations < 10 μg/dl), immunosuppression (total lymphocyte count < 800/mm3), and a catabolic state (serum albumin < 3.0 mg/dl, creatinine height index < 80%, and weight loss > 10% or BMI<18 kg/m2 during the current hospitalization). Studies are underway to better define the phenotype, its true significance and novel interventions to prevent it or its progression. As the population ages, PICS is likely to be next challenging horizon in surgical critical care.
Role of the Gut
Initially, the dominant hypothesis linking the gut to MOF was related to bacterial translocation. However, inconsistent results led to experiments demonstrating that the mesenteric lymph acted as a bridge between the gut and the systemic circulation, allowing gut-derived inflammatory mediators to reach the systemic circulation. 57–59 Via the thoracic duct, these mediators reach the lungs before other organs, which is consistent with human studies demonstrating that respiratory dysfunction almost always precedes other ODs.60
Role of the PMN and Macrophages
While both MOF patients and non-MOF patients groups develop neutrophilia at 3 hours postinjury, MOF patients show a rapid neutropenia between 6 and 12 hours postinjury suggesting end-organ sequestration.61 PMNs margination in end organs causes direct local cytotoxic cellular effects via degranulation and release of nitric oxide (NO), reactive oxygen species (ROS), and proinflammatory mediators (IL-6, IL-8, TNF-α).62
Following trauma there is an immediate increase in adhesion molecules, including L-selectin and CD18, which allow PMNs to slow and roll along the endothelium and marginate out of circulation. 63 Antibodies directed against the CD11b/CD18 components of the adhesion receptor complex between leukocytes and endothelium significantly attenuate lung injury and prevent the neutropenia associated with tissue sequestration during experimental sepsis. 63 Circulating monocytes and tissue macrophages also become primed after severe injury and the microvascular endothelium has an important role in priming of the innate inflammatory response.62
Role of Platelets
Thrombocytopenia, especially when persistent, is a predictor of postinjury OD. 64,65 Gawaz et al. observed that irreversible degranulation of granule glycoproteins correlated positively with OD severity. 66 Platelet-neutrophil interaction has been shown to be important in models of acute lung injury (ALI) 67 and blocking it reverses ALI in animal models. 68 In a rat model of trauma/hemorrhagic shock, we observed that pretreatment with a platelet P2Y12 receptor antagonist protected from post-injury ALI. 69 Furthermore, isoflurane, an ether that interferes with platelet-granulocyte aggregation, attenuated ALI partially through platelet ADP pathway inhibition.70
Pre-injury antiplatelet therapy has been associated with a decreased risk of ALI, MOF and mortality in transfused blunt trauma patients and in patients with adult-respiratory distress syndrome (ARDS). 71, 72 The multicenter trial LIPS-A (NCT01504867) should provide interesting evidence on the therapeutic use of this anti-platelet agent.
Cytokines
Cytokines can be proinflammatory (TNF-α, MIP, GM-CSF, IFN-γ, IL-1, IL-2, IL-6, IL-8, IL-17, etc.) and anti-inflammatory (IL-4, IL-10, IL-13). 63 Jastrow et al. 73 showed that, compared to non-MOF patients, MOF victims had higher levels of IL-1 receptor antagonist (IL-1Ra), IL-8, eotaxin, granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, inducible protein 10, monocyte chemotactic protein-1, and macrophage inflammatory protein-1. Adams et al.74 demonstrated that IL-8 can activate PMNs via two different receptors, and differential early expression of these receptors explained higher MOF risk.
Although inflammatory mediators’ levels vary greatly according to injury and individual characteristics, most studies agree that the changes start very early postinjury. 75 Indeed, a 2009 German study found that IL-6, IL-8, and IL-10 levels predicted MOF within 90 minutes of injury. 75
Toll-Like Receptors (TLR)
TLRs are transmembranal proteins present in most body cell types, which form the major pattern recognition receptors that transduce signals in response to DAMPs after I/R.76 Innate immune system responses are then initiated, including NF-kappa-B activation and proinflammatory cytokine production. Inhibition of TLR2 or TLR4 seems to be protective for I/R injury in liver, kidneys, brain, and heart, but not in the gut. Because the gut mucosa is continuously exposed to local bacterial endotoxins, local TLRs may be uniquely regulated to prevent inflammation.
Complement
The complement system is a major component of the innate immunity response, enhances the adaptive response and links the immune system with the coagulation system. 29, 77, 78 Complement system activation occurs immediately after trauma, with production of proinflammatory activation products C3a, C3b, and C5a and generation of the terminal C5b–C9 complex (the complement membrane-attack complex) that leads to lysis of the target cells. 29,35 Complement activation also results in the production of oxygen free radicals, arachidonic acid metabolites, and cytokines. However, excessive intravascular C5a may lead to neutrophil function “paralysis”, rendering them incapable to respond to C5a or other chemo-attractants. 79 Complement activation, especially serum C3 and C3a levels as well as C5a, seems to reflect severity and treatment of injury and OD. 80–83
Complement regulatory proteins (CD55, CD46, CD55, CD59), the C5a receptor (CD88) inhibitors of complement, such as C4b-binding protein (C4BP) and factor I, modulate the complement cascade and protect against complement-mediated tissue destruction. Several studies in polytrauma patients indicate that these regulatory factors are significantly altered post-injury suggesting “a trauma-induced complementopathy.” 83–85
Oxidative Stress
Excessive reactive oxygen intermediates (ROIs) cause direct oxidative injury to cellular proteins and nucleic acids, and disrupt cell membranes by inducing lipid peroxidation.76,86 I/R leads to significant disturbances in ROI production. 35,76 ROI secreted from PMNs after I/R injury induces cytokines, chemokines (IL-8), HSP, and adhesion molecules (P-selectin, ICAM-1) leading to cell and tissue damage.35
Under normal conditions, NO production greatly exceeds O2− production in the endothelial cell.87 However, with reperfusion, the balance between NO and O2− shifts in favor of O2, leaving little NO available to reduce arteriolar tone, prevent platelet aggregation, and minimize PMN adhesion to endotellium. 88 In addition, NO seems to upregulate the production of proinflammatory cytokines.87 Thus, altering the cell’s redox state may contribute to the ongoing inflammatory cytokine production and progression to MOF. ROIs also play a role as second messengers in the intracellular signaling pathways of inflammatory cells, in particular activation of NF-κB and activator protein 1 (AP-1), which can be activated by both oxidants and antioxidants depending on the cell type and on intracellular conditions.86
Endogenous antioxidant defenses, including enzymatic (superoxide dismutase, catalase, glutathione peroxidase) and nonenzymatic (vitamins E and C, provitamin A, glutathione, bilirubin, urate) groups, were the focus of interventions to modulate the inflammatory response. However, the REDOX 89–91 and METAPLUS 92 trials demonstrated harm in systemic administration of anti-oxidants. 43 For both glutamine and antioxidants, the greatest potential for harm was renal dysfunction in patients with MOF. 91
Later Risk Factors
Several conditions can serve as secondary stimulus that precipitate OD. Abdominal compartment syndrome (ACS), now in frank decline, leads to high ventilator pressures, decreased cardiac output, and impaired renal function. 93 While ACS physiologic effects usually reverse on decompression, the immunomodulatory effects may persist and trigger MOF.94
Although judicious blood transfusions have contributed to lower MOF incidence, early transfusion remains one of the most powerful independent risk factors for postinjury MOF. 19, 15 Blood products are immunoactive, contain proinflammatory cytokines and lipids, and have an early immunosuppressive effect predisposing to SARS, infection, and late MOF.95 Transfusing stored RBC older than three weeks early postinjury is associated with a higher MOF rates compared to units with shorter storage. 96 Leukodepletion does not remove the potential for blood to act as a second hit, as red blood cells contain proinflammatory mediators. Biologically active lipids, capable of PMN priming, accumulate in stored blood and “passenger leukocytes” have been implicated as pivotal components. Proposed mechanisms include induction of T-cell anergy in the recipient, decreased natural killer cell function, altered ratio of T-helper to T-suppressor cells, and soluble proinflammatory cytokines produced by leukocytes during storage. 95
Other blood-derived products (platelets, plasma, and coagulation factors) are also immunoactive.97 Proteomic analyses of platelet supernatants of healthy donors suggested a storage and sex-dependent impairment of blood coagulation mediators, pro-inflammatory complement components and cytokines, energy and redox metabolic enzymes as well as platelet activation. 98,99
Infections remain important predictors of late MOF. In the late 1970s, intra-abdominal abscess (IAA) was a frequent inciting event 100, but currently nosocomial pneumonia is the principal infection associated with MOF.101 While SARS limits potentially auto-destructive inflammation, it is associated with immunosuppression predisposing the host to infections. 102
The secondary operations can be considered controlled traumatic events.63 While early definitive fracture fixation decreases postinjury morbidity and improves recovery 103, it is not without consequences when performed within the priming window. A 2003 randomized controlled trial (RCT) demonstrated that early external fixation followed by delayed conversion to intramedullary instrumentation was associated with a decreased inflammatory response to the operative fixation.104 The same group compared damage control orthopedics (DC, femoral fracture stabilized with an external fixator) and primary intramedullary nailing (IMN) and showed that, despite more severe injuries, DC patients had less postoperative SIRS compared to IMN. 105
Our group showed that DC was a safer initial approach, significantly decreasing the initial operative exposure and blood loss compared to early total care with IMN for multiple injury patients with femoral shaft fractures. 106 We observed similar beneficial effects regarding pulmonary complications, infections, mechanical ventilation and ICU stay in spine fractures. 107
INTERVENTIONS
Preventing the onset of MOF through therapies directed at modulating the balance of SIRS and SARS offers more practical benefit than efforts to treat MOF once established, when the treatment is largely supportive.15
Protective Resuscitation Techniques
Certain resuscitative strategies protect against distant OD after periods of gut I/R. 57 Resuscitation with isotonic crystalloids in the late 1960s decreased mortality and renal failure, but contributed to the emergence of ARDS. There is still controversy about the use of isotonic crystalloids compared with colloids. The 2004 multicenter, randomized SAFE trial 108 and a 2012 large RCT 109 found similar outcomes, which was confirmed in a 2013 Cochrane systematic review. 110
A 2007 systematic review comparing hypertonic with isotonic crystalloid solutions for trauma/burns resuscitation did not provide enough data to determine a difference in outcomes.111 Hypertonic resuscitation was compared with lactated Ringer’s solution in adult, blunt trauma patients in a 2011 RCT 112, which was stopped at an interim analysis for potential safety concern (increased mortality in the subgroup of non-transfused patients receiving hypertonic saline) and futility. It demonstrated no significant difference in ARDS-free survival (hazard ratio, 1.01; 95% CI: 0.6–1.6). A subsequent analysis of patients in severe shock suggested that hypertonic saline was associated with hyperfibrinolysis. 113
Hypertonicity has an effect on multiple immune response functions, which may translate into improved outcomes when administered locally (as opposed to systemically) or using alternative modes of administration.114 To test this hypothesis, we are conducting a Phase I trial of nebulized hypertonic saline in moderately injured patients (NCT01667666).
Finally, the ALM™ (adenosine, lidocaine, magnesium) resuscitation has shown protective effects in animal models of sepsis and injury as well as in a few clinical trials in cardiac surgery patients. 115,116 Specifically, the combination of these three agents seems to confer cardiovascular protection, improvement of coagulation (presumably by inducing a shift in thrombin substrate specificity from the pro-fibrinolytic protein C pathway to the anti-fibrinolytic TAFI [thrombin-activatable-fibrinolysis-inhibitor] pathway at the endothelial thrombomodulin-thrombin complex level), reduction of proinflammatory factors (e.g., TNF-a) and increase of anti-inflammatory cytokines (e.g., IL-10). However, the mechanisms through which ALM™ exerts the abovementioned beneficial effects remain elusive.
Judicious Use of Blood Transfusions
A 12-year analysis of our Denver trauma dataset suggests that reduction in blood use contributed to the decreased incidence of MOF.19 Current transfusion guidelines support the safety of restrictive transfusion practices in trauma patients.117,118 Other techniques to reduce the deleterious effects of PRBCs are washed PRBCs and prestorage leukoreduction. 95 Washed PRBCs have benefits but it is an unrealistic practice in most settings. Prestorage leukoreduction trials have shown modest improvements in outcomes, except for cardiac surgery patients, among whom mortality was halved. 119,120
Blood substitutes have offered promising results in trauma. Two hemoglobin substitutes have been studied extensively in injured patients: the diaspirin cross-linked hemoglobin (Hemassist ™, Baxter Corporation) and the polymerized, pyridoxylated human hemoglobin (PolyHeme™ Northfield Laboratories).95,121,122
Our experience in the trauma setting with PolyHeme™ suggests it provides an immunologic advantage relative to blood in the injured patient by diminishing PMN priming and decreasing IL-6 and IL-8.123 Although PolyHeme has been associated with outcomes comparable to standard of care and with more adverse events, the benefit-to-risk ratio of PolyHeme was favorable when blood was needed but not available.124 As of 2015, the Food and Drug Administration (FDA) has not approved any blood substitutes, but Hemopure was approved in South Africa for use in acute anemia in 2001 and in Russia for use in the treatment of acute anemia in adults in 2010. 121
Protective Lung Ventilation
Positive pressure ventilation can result in lung injury that is functionally and histologically identical to that seen in ARDS. Areas of low compliance (pulmonary contusion, edema, or infection) force tidal volumes to areas of high compliance resulting in increased alveolar pressures, overdistension, and injury to uninvolved lung tissue. Mechanical injuries to the lung (barotrauma, atelectrauma, volutrauma) initiate a local inflammatory reaction and biotrauma with release of inflammatory mediators from damaged cells and recruitment of PMNs. Mechanical stresses on the living cell are translated into intracellular inflammatory signal transduction and the combination of mechanical damage from positive pressure ventilation and the inflammation increases pulmonary dysfunction.125
The effects of ventilator-induced lung injury extend beyond the lung. Impaired oxygen delivery amplifies post-traumatic I/R injury. Inflammatory cytokines generated in the lung spill over into the systemic circulation and have the capacity to increase the inflammatory state and promote remote OD via direct cell signaling.
The ARDS Network lung-protective ventilation (LPV) trials 126 and a 2013 systematic review convincingly showed that LPV reduced mortality. 127
Adrenal Insufficiency and Cortisol Replacement Therapy
Adrenal insufficiency (AI) occurs frequently in trauma and is associated with mortality.128,129 International guidelines recommend that AI should be suspected in hypotensive patients responding poorly to fluids and vasopressor agents, with laboratorial signs of compromised adrenal function. 130 For patients with vasopressor-dependent septic shock and patients with early severe ARD, the decision to treat should be based mainly on clinical criteria. The corticosteroid dose should be sufficient to downregulate the proinflammatory response without causing immunodeficiency and impaired wound healing. The use of extended course, stress-dose corticosteroids (200–350 mg/dL of hydrocortisone) in critically ill patients has been associated with improved outcomes.
Insulin and Glycemic Control
While glycemic control (≤180 mg/dL) is important, tighter glucose control (81–108 mg/dL) in critical care patients has had conflicting results.131–133 Our Denver MOF showed that, after exclusion of diabetes patients, older patients benefitted more from tight glucose control levels than their younger counterparts. 134 In addition to glucose control and induction of anabolic processes, insulin can attenuate SIRS and modulate the proliferation, apoptosis, differentiation, and functions of monocytes/macrophages, neutrophils, and T cells associated with severe trauma, burn injury, or sepsis.135
Immunonutrition
Two aspects are relevant in immunonutrition: the delivery route (enteral vs. parenteral) and the diet composition. 6 Although controversy exists concerning the safety of feeding the hypoperfused small bowel, evidence supports that early enteral nutrition (EEN) is not only feasible but also associated with decreased incidence of nosocomial infection. EEN effects go far beyond mere nourishment; rather EEN induces a complex immunologic response.135 EEN supports the function of the mucosal-associated lymphoid tissue (MALT) that produces 70% of the body’s secretory IgA.136 Naïve T and B cells target and enter the gut-associated lymphoid tissue (GALT) where they are sensitized and stimulated by antigens sampled from the gut lumen and thereby become more responsive to potential pathogens in the external environment. These stimulated T and B cells then migrate via mesenteric lymph nodes and the thoracic duct and into the vascular tree for distribution to GALT and extraintestinal sites of MALT. Lack of enteral stimulation (i.e., use of TPN) causes a rapid and progressive decrease in T and B cells within GALT and simultaneous decreases in intestinal and respiratory IgA levels. Previously resistant TPN-fed lab animals, when challenged with pathogens via respiratory tree inoculation, succumb to overwhelming infections. These immunologic defects and susceptibility to infection are reversed within 3–5 days after initiating EN.136 Indeed, feeding the gut in critically ill patients has been shown to reverse shock-induced mucosal hypoperfusion and impaired intestinal transit as well as attenuate gut permeability defects and lessen the severity of CARS.6
Regarding content, to be effective, immunonutritional therapies must ameliorate cellular defense, oxidative stress, and mitochondrial function without increasing SIRS. As mentioned in previous sections, rigorous studies on immunomodulating diets, so far, have been disappointing. 43,89,91
TLR-Directed Interventions
Because TLR activation leads to an intense and immediate inflammatory reaction in response to I/R injury, targeting TLRs may be a promising intervention strategy to reduce MOF. Yet, because TLR activation occurs through a variety of mechanisms, generating full antagonists is technically difficult. The development of eritoran, a potent and full antagonist of LPS at TLR4, is a significant advance and offers hope that other TLR-selective antagonists may become available in future years.137
Immunomodulation
In the immunodepression stage of the inflammatory response to injury, it may be useful to enhance immune function to prevent infections that can act as second insults. An analysis of leukocyte gene expression associated with post-trauma Gram-negative bacteremia in the Glue Grant dataset showed that both innate and adaptive immunity appeared to be suppressed by 96 hours postinjury. 138 These findings suggested that immunostimulants, such as interferon-gamma, in patients with decreased immune gene expression, may be a promising therapeutic approach. GM-CSF was tested in small trauma populations and shown to counteract trauma-induced monocyte function depression while IFN-γ had the capacity to enhance HLA-DR on B and T lymphocytes.139,140
Mesenchymal stem or stromal cells (MSC) have been shown to exert protective effects through the release of promitotic, antiapoptotic, antiinflammatory, and immunomodulatory soluble factors.141 Initially, MSC cells were believed to regenerate dysfunctional, damage organs, however, later it became clear that their beneficial effects were mediated by secretion of factors. MSCs tend to migrate toward sites where there is inflammation or injury, thus dysfunctional organs are potential targets. Since most approaches targeting a single pathway or mechanism have not been promising, MSCs are attractive because they tackle several pathways modulating multiple immune cells and the oxidative process, in addition to their antibacterial properties. A few Phase 1 trials have been completed showing an acceptable safety profile. 142,143 At the time of this writing, no Phase 2 trial results are available.
In the 2015 Shock Society Thirty-Eighth Annual Conference on Shock, Sordi and colleagues from Europe presented promising results using artesunate (a medication commonly used to treat falciparum malaria) in a rat model of hemorrhage-induced organ dysfunction. 144 Artesunate treatment was associated with lower levels of creatinine and lung myeloperoxidase activity as well as increased activation of Akt and eNOS, reduced activation of GSK-3 beta and NF-kappa B activation, and attenuated increase in serum TNF-alpha associated with the hemorrhagic shock. These findings suggest that artesunate attenuated organ dysfunction (lungs and kidneys) likely via activation of the Akt-eNOS survival pathway, and/or by reducing inflammation via inhibition of GSK-3 beta and NF-Kappa B. Based on these experiments, the group received funding from the Wellcome Trust for a Phase IIa, single-center, placebo-controlled, randomized, phase II clinical trial of Artesunate for Trauma Organ Protection (TOP-ART) (ISRCTN15731357, available at www.isrctn.com and www.c4ts.qmul.ac.uk/organ-failure--protection/top-art, accessed December 18, 2015).
Key Points.
The development of organ dysfunction (OD) is related to the intensity and balance between trauma-induced simultaneous, opposite inflammatory responses.
Early proinflammation via innate immune system activation may cause early OD while early anti-inflammation, via inhibition of the adaptive immune system and apoptosis, may induce immunoparalysis, impaired healing, infections, and late OD.
Patients discharged with low level OD may develop the persistent inflammation-immunosuppression catabolism syndrome (PICS), which may cause an indolent death.
The incidence of multiple organ failure (MOF) has decreased over time, yet MOF remains morbid, lethal and resource-intensive. Single OD, especially acute lung injury, remains frequent.
At this time, treatment of organ dysfunction is limited, and prevention via adequate resuscitation, ventilation and nutritional support remains the mainstay strategy.
Footnotes
Disclosure: Drs. Angela Sauaia and Ernest E. Moore received funding from the National Institute of General Medical Sciences grant P50 GM049222 and National Heart, Lung and Blood Institute grant UM1 HL120877. Dr. Frederick A. Moore received funding from National Institute of General Medical Sciences grant P50 GM111152. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIGMS, NHLBI or National Institutes of Health. The authors have no commercial or financial conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
ANGELA SAUAIA, Email: Angela.Sauaia@ucdenver.edu, Professor of Public Health and Surgery, University of Colorado Denver, 655 Broadway #365, Denver, Co 80203.
FREDERICK A. MOORE, Email: Frederick.Moore@surgery.ufl.edu, Professor of Surgery, University of Florida, Gainesville, PO BOX 100108, Gainesville, FL 32610.
ERNEST E. MOORE, Email: Ernest.Moore@dhha.org, Professor of Surgery, University of Colorado Denver, Denver Health Medical Center, 655 Broadway #365, Denver, CO 80203.
References
- 1.Baker CC, Oppenheimer L, Stephens B, Lewis FR, Trunkey DD. Epidemiology of trauma deaths. Am J Surg. 1980;140(1):144–150. doi: 10.1016/0002-9610(80)90431-6. [DOI] [PubMed] [Google Scholar]
- 2.Sauaia A, Moore FA, Moore EE, et al. Epidemiology of trauma deaths: a reassessment. The Journal of trauma. 1995;38(2):185–193. doi: 10.1097/00005373-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Pang JM, Civil I, Ng A, Adams D, Koelmeyer T. Is the trimodal pattern of death after trauma a dated concept in the 21st century Trauma deaths in Auckland 2004. Injury. 2008;39(1):102–106. doi: 10.1016/j.injury.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 4.Eiseman B, Beart R, Norton L. Multiple organ failure. Surg Gynecol Obstet. 1977;144(3):323–326. [PubMed] [Google Scholar]
- 5.Moore F, Moore E. Evolving concepts in the pathogenesis of postinjury multiple organ failure. Surg Clin North Am. 1995;75(2):257–277. doi: 10.1016/s0039-6109(16)46587-4. [DOI] [PubMed] [Google Scholar]
- 6.Moore FA, Moore EE. The Evolving Rationale for Early Enteral Nutrition Based on Paradigms of Multiple Organ Failure: A Personal Journey. Nutrition in Clinical Practice. 2009 May 29;24(3):297–304. doi: 10.1177/0884533609336604. [DOI] [PubMed] [Google Scholar]
- 7.Gentile LF, Cuenca AG, Efron PA, et al. Persistent inflammation and immunosuppression: A common syndrome and new horizon for surgical intensive care. The Journal of Trauma and Acute Care Surgery. 2012;72(6):1491–1501. doi: 10.1097/TA.0b013e318256e000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao W, Mindrinos MN, Seok J, et al. A genomic storm in critically injured humans. The Journal of Experimental Medicine. 2011 Dec 19;208(13):2581–2590. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore FA, Sauaia A, Moore EE, Haenel JB, Burch JM, Lezotte DC. Postinjury Multiple Organ Failure. The Journal of Trauma: Injury, Infection, and Critical Care. 1996;40(4):501–512. doi: 10.1097/00005373-199604000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23(10):1638–1652. doi: 10.1097/00003246-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Marshall JC. A scoring system for the multiple organ dysfunction syndrome (MODS) In: Reinhart K, Eyrich K, Sprung C, editors. Sepsis: Current Perspectives in Pathophysiology and Therapy. Berlin: Springer-Verlag; 1994. pp. 38–49. [Google Scholar]
- 12.Sauaia A, Moore EE, Johnson JL, Ciesla DJ, Biffl WL, Banerjee A. Validation of postinjury multiple organ failures scores. Shock (Augusta, Ga) 2009;31(5):438–447. doi: 10.1097/SHK.0b013e31818ba4c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baue AE. MOF, MODS, and SIRS: what is in a name or an acronym? Shock (Augusta, Ga) 2006;26(5):438–449. doi: 10.1097/01.shk.0000228172.32587.7a. [DOI] [PubMed] [Google Scholar]
- 14.Dewar DCMB, Tarrant SMMBB, King KLRNMN, Balogh ZJMDP. Changes in the epidemiology and prediction of multiple-organ failure after injury. Journal of Trauma and Acute Care Surgery. 2013;74(3):774–779. doi: 10.1097/TA.0b013e31827a6e69. [DOI] [PubMed] [Google Scholar]
- 15.Sauaia A, Moore EE, Johnson JL, et al. Temporal trends of postinjury multiple-organ failure: Still resource intensive, morbid, and lethal. The journal of trauma and acute care surgery. 2014;76(3):582–593. doi: 10.1097/TA.0000000000000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benns M, Carr B, Kallan MJ, Sims CA. Benchmarking the incidence of organ failure after injury at trauma centers and nontrauma centers in the United States. J Trauma Acute Care Surg. 2013 Sep;75(3):426–431. doi: 10.1097/TA.0b013e31829cfa19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minei JP, Cuschieri J, Sperry J, et al. The changing pattern and implications of multiple organ failure after blunt injury with hemorrhagic shock. Crit Care Med. 2012 Apr;40(4):1129–1135. doi: 10.1097/CCM.0b013e3182376e9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fröhlich M, Lefering R, Probst C, et al. Epidemiology and risk factors of multiple-organ failure after multiple trauma: An analysis of 31,154 patients from the TraumaRegister DGU. Journal of Trauma and Acute Care Surgery. 2014;76(4):921–928. doi: 10.1097/TA.0000000000000199. [DOI] [PubMed] [Google Scholar]
- 19.Ciesla DJ, Moore EE, Johnson JL, Burch JM, Cothren CC, Sauaia A. A 12-year prospective study of postinjury multiple organ failure: has anything changed? Arch Surg. 2005;140(5):432–438. doi: 10.1001/archsurg.140.5.432. [DOI] [PubMed] [Google Scholar]
- 20.Laudi S, Donaubauer B, Busch T, et al. Low incidence of multiple organ failure after major trauma. Injury. 2007;38(9):1052–1058. doi: 10.1016/j.injury.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 21.Nast-Kolb D, Aufmkolk M, Rucholtz S, Obertacke U, Waydhas C. Multiple organ failure still a major cause of morbidity but not mortality in blunt multiple trauma. The Journal of trauma. 2001;51(5):835–841. doi: 10.1097/00005373-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Durham RM, Moran JJ, Mazuski JE, Shapiro MJ, Baue AE, Flint LM. Multiple organ failure in trauma patients. The Journal of trauma. 2003;55(4):608–616. doi: 10.1097/01.TA.0000092378.10660.D1. [DOI] [PubMed] [Google Scholar]
- 23.Wafaisade A, Lefering R, Bouillon B, et al. Epidemiology and risk factors of sepsis after multiple trauma: an analysis of 29,829 patients from the Trauma Registry of the German Society for Trauma Surgery. Crit Care Med. 2011 Apr;39(4):621–628. doi: 10.1097/CCM.0b013e318206d3df. [DOI] [PubMed] [Google Scholar]
- 24.Cuschieri J, Johnson JL, Sperry J, et al. Benchmarking outcomes in the critically injured trauma patient and the effect of implementing standard operating procedures. Ann Surg. 2012 May;255(5):993–999. doi: 10.1097/SLA.0b013e31824f1ebc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ciesla DJ, Moore EE, Johnson JL, et al. Decreased progression of postinjury lung dysfunction to the acute respiratory distress syndrome and multiple organ failure. Surgery. 2006;140(4):640–647. doi: 10.1016/j.surg.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 26.Dasta JF, McLaughlin TP, Mody SH, Piech CT. Daily cost of an intensive care unit day: the contribution of mechanical ventilation. Crit Care Med. 2005 Jun;33(6):1266–1271. doi: 10.1097/01.ccm.0000164543.14619.00. [DOI] [PubMed] [Google Scholar]
- 27.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. Journal of Leukocyte Biology. 2006 Oct 10;81(1):1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 28.Matzinger P. Tolerance, danger, and the extended family. Annual review of immunology. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 29.Köhl J. Self, Non-Self, and Danger: A Complementary View. In: Lambris J, editor. Current Topics in Complement. Vol. 586. Springer US; 2006. pp. 71–94. [DOI] [PubMed] [Google Scholar]
- 30.Hwang PF, Porterfield N, Pannell D, Davis TA, Elster EA. Trauma is danger. Journal of Translational Medicine. 2011;9:92–92. doi: 10.1186/1479-5876-9-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirsiger S, Simmen H-P, Werner CML, Wanner GA, Rittirsch D. Danger Signals Activating the Immune Response after Trauma. Mediators of Inflammation. 2012;2012:10. doi: 10.1155/2012/315941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lord JM, Midwinter MJ, Chen Y-F, et al. The systemic immune response to trauma: an overview of pathophysiology and treatment. The Lancet. 384(9952):1455–1465. doi: 10.1016/S0140-6736(14)60687-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matzinger P. An innate sense of danger. Seminars in immunology. 1998 Oct;10(5):399–415. doi: 10.1006/smim.1998.0143. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Q, Raoof M, Chen Y, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010 Mar 04;464(7285):104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsukamoto T, Chanthaphavong RS, Pape H-C. Current theories on the pathophysiology of multiple organ failure after trauma. Injury. 2010 Jan;41(1):21–26. doi: 10.1016/j.injury.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 36.Levy RM, Mollen KP, Prince JM, et al. Systemic inflammation and remote organ injury following trauma require HMGB1. AJP: Regulatory, Integrative and Comparative Physiology. 2007 Aug 01;293(4):R1538–R1544. doi: 10.1152/ajpregu.00272.2007. [DOI] [PubMed] [Google Scholar]
- 37.Peltz ED, Moore EE, Eckels PC, et al. HMGB1 IS MARKEDLY ELEVATED WITHIN 6 HOURS OF MECHANICAL TRAUMA IN HUMANS. Shock (Augusta, Ga) 2009;32(1):17–22. doi: 10.1097/shk.0b013e3181997173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu T, Qian W-J, Gritsenko MA, et al. High Dynamic Range Characterization of the Trauma Patient Plasma Proteome. Molecular & cellular proteomics : MCP. 2006;5(10):1899–1913. doi: 10.1074/mcp.M600068-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dzieciatkowska M, Wohlauer MV, Moore EE, et al. Proteomic Analysis of Human Mesenteric Lymph. Shock (Augusta, Ga) 2011;35(4):331–338. doi: 10.1097/SHK.0b013e318206f654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee SK, Ding JL. A Perspective on the Role of Extracellular Hemoglobin on the Innate Immune System. DNA and Cell Biology. 2013;32(2):36–40. doi: 10.1089/dna.2012.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peltz EDDO, D’Alessandro AP, Moore EEMD, et al. Pathologic metabolism: An exploratory study of the plasma metabolome of critical injury. Journal of Trauma and Acute Care Surgery. 2015;78(4):742–751. doi: 10.1097/TA.0000000000000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moore FA, Moore EE, Kudsk KA, et al. Clinical benefits of an immune-enhancing diet for early postinjury enteral feeding. J Trauma. 1994 Oct;37(4):607–615. doi: 10.1097/00005373-199410000-00014. [DOI] [PubMed] [Google Scholar]
- 43.van Zanten AR, Hofman Z, Heyland DK. Consequences of the REDOXS and METAPLUS Trials: The End of an Era of Glutamine and Antioxidant Supplementation for Critically Ill Patients? JPEN Journal of parenteral and enteral nutrition. 2015 Jan 7; doi: 10.1177/0148607114567201. [DOI] [PubMed] [Google Scholar]
- 44.Chouchani ET, Pell VR, Gaude E, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014 Nov 20;515(7527):431–435. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.D’Alessandro A, Slaughter AL, Peltz ED, et al. Trauma/hemorrhagic shock instigates aberrant metabolic flux through glycolytic pathways, as revealed by preliminary (13)C-glucose labeling metabolomics. J Transl Med. 2015;13:253. doi: 10.1186/s12967-015-0612-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Neill LAJ. Biochemistry: Succinate strikes. Nature. 2014;515(7527):350–351. doi: 10.1038/nature13941. [DOI] [PubMed] [Google Scholar]
- 47.Tannahill GM, Curtis AM, Adamik J, et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature. 2013 Apr 11;496(7444):238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rubic T, Lametschwandtner G, Jost S, et al. Triggering the succinate receptor GPR91 on dendritic cells enhances immunity. Nat Immunol. 2008;9(11):1261–1269. doi: 10.1038/ni.1657. [DOI] [PubMed] [Google Scholar]
- 49.Eltzschig HK, Bratton DL, Colgan SP. Targeting hypoxia signalling for the treatment of ischaemic and inflammatory diseases. Nat Rev Drug Discov. 2014;13(11):852–869. doi: 10.1038/nrd4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vohwinkel CU, Hoegl S, Eltzschig HK. Hypoxia signaling during acute lung injury. Journal of applied physiology (Bethesda, Md : 1985) 2015 Nov 15;119(10):1157–1163. doi: 10.1152/japplphysiol.00226.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eckle T, Brodsky K, Bonney M, et al. HIF1A Reduces Acute Lung Injury by Optimizing Carbohydrate Metabolism in the Alveolar Epithelium. PLoS Biology. 2013;11(9):e1001665. doi: 10.1371/journal.pbio.1001665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stringer KA, McKay RT, Karnovsky A, Quémerais B, Lacy P. Metabolomics and Its Application to Acute Lung Diseases. Frontiers in Immunology. 2016;7:44. doi: 10.3389/fimmu.2016.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gasse P, Riteau N, Charron S, et al. Uric Acid Is a Danger Signal Activating NALP3 Inflammasome in Lung Injury Inflammation and Fibrosis. American Journal of Respiratory and Critical Care Medicine. 2009 May 15;179(10):903–913. doi: 10.1164/rccm.200808-1274OC. [DOI] [PubMed] [Google Scholar]
- 54.Bos LDJ, Sterk PJ, Schultz MJ. Measuring Metabolomics in Acute Lung Injury: Choosing the Correct Compartment? American Journal of Respiratory and Critical Care Medicine. 2012 Apr 01;185(7):789–789. doi: 10.1164/ajrccm.185.7.789. [DOI] [PubMed] [Google Scholar]
- 55.Bos LDJ, Weda H, Wang Y, et al. Exhaled breath metabolomics as a noninvasive diagnostic tool for acute respiratory distress syndrome. European Respiratory Journal. 2014 Jul 01;44(1):188–197. doi: 10.1183/09031936.00005614. [DOI] [PubMed] [Google Scholar]
- 56.Ulvik A, Kvåle R, Wentzel-Larsen T, Flaatten H. Multiple organ failure after trauma affects even long-term survival and functional status. Critical Care. 2007;11(5):R95–R95. doi: 10.1186/cc6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Magnotti LJ, Deitch EA. Burns, Bacterial Translocation, Gut Barrier Function, and Failure. Journal of Burn Care & Rehabilitation. 2005;26(5):383–391. doi: 10.1097/01.bcr.0000176878.79267.e8. [DOI] [PubMed] [Google Scholar]
- 58.Magnotti LJ, Upperman JS, Xu DZ, Lu Q, Deitch EA. Gut-derived mesenteric lymph but not portal blood increases endothelial cell permeability and promotes lung injury after hemorrhagic shock. Annals of Surgery. 1998;228(4):518–527. doi: 10.1097/00000658-199810000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moore FA, Moore EE, Poggetti R, et al. Gut bacterial translocation via the portal vein: a clinical perspective with major torso trauma. J Trauma. 1991 May;31(5):629–636. doi: 10.1097/00005373-199105000-00006. discussion 636–628. [DOI] [PubMed] [Google Scholar]
- 60.Ciesla DJ, Moore EE, Johnson JL, Burch JM, Cothren CC, Sauaia A. The role of the lung in postinjury multiple organ failure. Surgery. 2005;138(4):749–757. doi: 10.1016/j.surg.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 61.Botha AJ, Moore FA, Moore EE, Fontes B, Banerjee A, Peterson VM. Postinjury neutrophil priming and activation states: therapeutic challenges. Shock. 1995;3(3):157–166. doi: 10.1097/00024382-199503000-00001. [DOI] [PubMed] [Google Scholar]
- 62.Moore EE, Moore FA, Harken AH, Johnson JL, Ciesla D, Banerjee A. The two-event construct of postinjury multiple organ failure. Shock. 2005;24(Suppl 1):71–74. doi: 10.1097/01.shk.0000191336.01036.fe. [DOI] [PubMed] [Google Scholar]
- 63.Giannoudis PV. Current concepts of the inflammatory response after major trauma: an update. Injury. 2003 Jun;34(6):397–404. doi: 10.1016/s0020-1383(02)00416-3. [DOI] [PubMed] [Google Scholar]
- 64.Sauaia A, Moore FA, Moore EE, Norris JM, Lezotte DC, Hamman RF. Multiple organ failure can be predicted as early as 12 hours after injury. The Journal of trauma. 1998;45(2):291–301. doi: 10.1097/00005373-199808000-00014. [DOI] [PubMed] [Google Scholar]
- 65.Nydam TL, Kashuk JL, Moore EE, et al. Refractory postinjury thrombocytopenia is associated with multiple organ failure and adverse outcomes. J Trauma. 2011 Feb;70(2):401–406. doi: 10.1097/TA.0b013e31820b5c85. discussion 406–407. [DOI] [PubMed] [Google Scholar]
- 66.Gawaz M, Fateh-Moghadam S, Pilz G, Gurland HJ, Werdan K. Severity of multiple organ failure (MOF) but not of sepsis correlates with irreversible platelet degranulation. Infection. 1995 Jan-Feb;23(1):16–23. doi: 10.1007/BF01710051. [DOI] [PubMed] [Google Scholar]
- 67.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. The Journal of Clinical Investigation. 2012;122(8):2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zarbock A, Singbartl K, Ley K. Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J Clin Invest. 2006 Dec;116(12):3211–3219. doi: 10.1172/JCI29499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Harr JN, Moore EE, Wohlauer MV, et al. Activated platelets in heparinized shed blood: the “second hit” of acute lung injury in trauma/hemorrhagic shock models. Shock (Augusta, Ga) 2011 Dec;36(6):595–603. doi: 10.1097/SHK.0b013e318231ee76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harr JN, Moore EE, Stringham J, et al. Isoflurane Prevents Acute Lung Injury Through ADP-Mediated Platelet Inhibition. Surgery. 2012;152(2):270–276. doi: 10.1016/j.surg.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harr JN, Moore EE, Johnson J, et al. Antiplatelet therapy is associated with decreased transfusion-associated risk of lung dysfunction, multiple organ failure, and mortality in trauma patients. Crit Care Med. 2013 Feb;41(2):399–404. doi: 10.1097/CCM.0b013e31826ab38b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boyle A, Di Gangi S, Hamid U, et al. Aspirin therapy in patients with acute respiratory distress syndrome (ARDS) is associated with reduced intensive care unit mortality: a prospective analysis. Critical Care. 2015;19(1):109. doi: 10.1186/s13054-015-0846-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jastrow KM, Gonzalez EA, McGuire MF, et al. Early Cytokine Production Risk Stratifies Trauma Patients for Multiple Organ Failure. Journal of the American College of Surgeons. 2009 Sep;209(3):320–331. doi: 10.1016/j.jamcollsurg.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 74.Adams JM, Hauser CJ, Livingston DH, Lavery RF, Fekete Z, Deitch EA. Early trauma polymorphonuclear neutrophil responses to chemokines are associated with development of sepsis, pneumonia, and organ failure. The Journal of trauma. 2001;51(3):452–456. doi: 10.1097/00005373-200109000-00005. [DOI] [PubMed] [Google Scholar]
- 75.Bogner V, Keil L, Kanz KG, et al. Very early posttraumatic serum alterations are significantly associated to initial massive RBC substitution, injury severity, multiple organ failure and adverse clinical outcome in multiple injured patients. Eur J Med Res. 2009;14(7):284. doi: 10.1186/2047-783X-14-7-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arumugam TV, Okun E, Tang S-C, Thundyil J, Taylor SM, Woodruff TM. TOLL-LIKE RECEPTORS IN ISCHEMIA-REPERFUSION INJURY. Shock (Augusta, Ga) 2009;32(1):4–16. doi: 10.1097/SHK.0b013e318193e333. [DOI] [PubMed] [Google Scholar]
- 77.Carroll MC. The complement system in regulation of adaptive immunity. Nat Immunol. 2004;5(10):981–986. doi: 10.1038/ni1113. [DOI] [PubMed] [Google Scholar]
- 78.Kenawy HI, Boral I, Bevington A. Complement-Coagulation Cross-Talk: A Potential Mediator of the Physiological Activation of Complement by Low pH. Frontiers in Immunology. 2015;6:215. doi: 10.3389/fimmu.2015.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gerard C. Complement C5a in the Sepsis Syndrome — Too Much of a Good Thing? New England Journal of Medicine. 2003;348(2):167–169. doi: 10.1056/NEJMcibr022995. [DOI] [PubMed] [Google Scholar]
- 80.Roumen RM, Redl H, Schlag G, et al. Inflammatory mediators in relation to the development of multiple organ failure in patients after severe blunt trauma. Crit Care Med. 1995 Mar;23(3):474–480. doi: 10.1097/00003246-199503000-00010. [DOI] [PubMed] [Google Scholar]
- 81.Hecke F, Schmidt U, Kola A, Bautsch W, Klos A, Kohl J. Circulating complement proteins in multiple trauma patients--correlation with injury severity, development of sepsis, and outcome. Crit Care Med. 1997 Dec;25(12):2015–2024. doi: 10.1097/00003246-199712000-00019. [DOI] [PubMed] [Google Scholar]
- 82.Donnelly TJ, Meade P, Jagels M, et al. Cytokine, complement, and endotoxin profiles associated with the development of the adult respiratory distress syndrome after severe injury. Crit Care Med. 1994 May;22(5):768–776. doi: 10.1097/00003246-199405000-00010. [DOI] [PubMed] [Google Scholar]
- 83.Huber-Lang M, Kovtun A, Ignatius A. The role of complement in trauma and fracture healing. Seminars in immunology. 2013;25(1):73–78. doi: 10.1016/j.smim.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 84.Amara U, Kalbitz M, Perl M, et al. EARLY EXPRESSION CHANGES OF COMPLEMENT REGULATORY PROTEINS AND C5a RECEPTOR (CD88) ON LEUKOCYTES AFTER MULTIPLE INJURY IN HUMANS. Shock (Augusta, Ga) 2010;33(6):568–575. doi: 10.1097/SHK.0b013e3181c799d4. [DOI] [PubMed] [Google Scholar]
- 85.Burk A-M, Martin M, Flierl MA, et al. Early Complementopathy after Multiple Injuries in Humans. Shock (Augusta, Ga) 2012;37(4):348–354. doi: 10.1097/SHK.0b013e3182471795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bulger EM. Antioxidants in Critical Illness. Archives of surgery (Chicago, Ill : 1960) 2001 Oct 01;136(10):1201. doi: 10.1001/archsurg.136.10.1201. [DOI] [PubMed] [Google Scholar]
- 87.Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxidants & Redox Signaling. 2014;20(7):1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Galkina SI, Dormeneva EV, Bachschmid M, Pushkareva MA, Sud’ina GF, Ullrich V. Endothelium-leukocyte interactions under the influence of the superoxide-nitrogen monoxide system. Medical science monitor : international medical journal of experimental and clinical research. 2004 Sep;10(9):BR307–316. [PubMed] [Google Scholar]
- 89.Heyland DK, Dhaliwal R, Day AG, et al. REducing Deaths due to OXidative Stress (The REDOXS© Study): rationale and study design for a randomized trial of glutamine and antioxidant supplementation in critically-ill patients. Proceedings of the Nutrition Society. 2006 Aug;65(03):250–263. doi: 10.1079/pns2006505. [DOI] [PubMed] [Google Scholar]
- 90.Heyland D, Muscedere J, Wischmeyer PE, et al. A Randomized Trial of Glutamine and Antioxidants in Critically Ill Patients. New England Journal of Medicine. 2013;368(16):1489–1497. doi: 10.1056/NEJMoa1212722. [DOI] [PubMed] [Google Scholar]
- 91.Heyland DK, Elke G, Cook D, et al. Glutamine and antioxidants in the critically ill patient: a post hoc analysis of a large-scale randomized trial. JPEN Journal of parenteral and enteral nutrition. 2015 May;39(4):401–409. doi: 10.1177/0148607114529994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.van Zanten AH, Sztark F, Kaisers UX, et al. High-protein enteral nutrition enriched with immune-modulating nutrients vs standard high-protein enteral nutrition and nosocomial infections in the icu: A randomized clinical trial. JAMA. 2014;312(5):514–524. doi: 10.1001/jama.2014.7698. [DOI] [PubMed] [Google Scholar]
- 93.Madigan MC, Kemp CD, Johnson JC, Cotton BA. Secondary abdominal compartment syndrome after severe extremity injury: are early, aggressive fluid resuscitation strategies to blame? J Trauma. 2008 Feb;64(2):280–285. doi: 10.1097/TA.0b013e3181622bb6. [DOI] [PubMed] [Google Scholar]
- 94.Balogh Z, McKinley BA, Cox CSJ, et al. Abdominal Compartment Syndrome: The Cause or Effect of Postinjury Multiple Organ Failure. Shock (Augusta, Ga) 2003;20(6):483–492. doi: 10.1097/01.shk.0000093346.68755.43. [DOI] [PubMed] [Google Scholar]
- 95.Silliman CC, Moore EE, Johnson JL, Gonzalez RJ, Biffl WL. Transfusion of the injured patient: proceed with caution. Shock (Augusta, Ga) 2004 Apr;21(4):291–299. doi: 10.1097/00024382-200404000-00001. [DOI] [PubMed] [Google Scholar]
- 96.Zallen G, Moore EE, Ciesla DJ, Brown M, Biffl WL, Silliman CC. Stored red blood cells selectively activate human neutrophils to release IL-8 and secretory PLA2. Shock. 2000;13(1):29–33. doi: 10.1097/00024382-200013010-00006. [DOI] [PubMed] [Google Scholar]
- 97.Johnson JL, Moore EE, Kashuk JL, et al. Effect of blood products transfusion on the development of postinjury multiple organ failure. Archives of surgery (Chicago, Ill : 1960) 2010 Oct;145(10):973–977. doi: 10.1001/archsurg.2010.216. [DOI] [PubMed] [Google Scholar]
- 98.Dzieciatkowska M, D’Alessandro A, Burke TA, et al. Proteomics of apheresis platelet supernatants during routine storage: Gender-related differences. Journal of proteomics. 2015 Jan 1;112:190–209. doi: 10.1016/j.jprot.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dzieciatkowska M, D’Alessandro A, Hill RC, Hansen KC. Plasma QconCATs reveal a gender-specific proteomic signature in apheresis platelet plasma supernatants. Journal of proteomics. 2015 Apr 29;120:1–6. doi: 10.1016/j.jprot.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fry DE, Pearlstein L, Fulton RL, Jr, PH Multiple system organ failure. The role of uncontrolled infection. Archives of surgery (Chicago, Ill : 1960) 1980;115(2):136–140. doi: 10.1001/archsurg.1980.01380020006003. [DOI] [PubMed] [Google Scholar]
- 101.Sauaia A, Moore FA, Moore EE, Haenel JB, Read RA. Pneumonia: cause or symptom of postinjury multiple organ failure? Am J Surg. 1993;166(6):606–610. doi: 10.1016/s0002-9610(05)80664-6. [DOI] [PubMed] [Google Scholar]
- 102.EME. Trauma and the immune response: strategies for success. J Trauma. 2007;62(6):S54–S55. doi: 10.1097/TA.0b013e318065aab4. [DOI] [PubMed] [Google Scholar]
- 103.Bone LB, Johnson KD, Weigelt J, Scheinberg R. Early versus delayed stabilization of femoral fractures. A prospective randomized study. J Bone Joint Surg Am. 1989 Mar;71(3):336–340. [PubMed] [Google Scholar]
- 104.Pape HC, Grimme K, Van Griensven M, et al. Impact of intramedullary instrumentation versus damage control for femoral fractures on immunoinflammatory parameters: prospective randomized analysis by the EPOFF Study Group. J Trauma. 2003 Jul;55(1):7–13. doi: 10.1097/01.TA.0000075787.69695.4E. [DOI] [PubMed] [Google Scholar]
- 105.Harwood PJ, Giannoudis PV, van Griensven M, Krettek C, Pape HC. Alterations in the systemic inflammatory response after early total care and damage control procedures for femoral shaft fracture in severely injured patients. J Trauma. 2005 Mar;58(3):446–452. doi: 10.1097/01.ta.0000153942.28015.77. discussion 452–444. [DOI] [PubMed] [Google Scholar]
- 106.Tuttle MS, Smith WR, Williams AE, et al. Safety and efficacy of damage control external fixation versus early definitive stabilization for femoral shaft fractures in the multiple-injured patient. J Trauma. 2009 Sep;67(3):602–605. doi: 10.1097/TA.0b013e3181aa21c0. [DOI] [PubMed] [Google Scholar]
- 107.Stahel PF, VanderHeiden T, Flierl MA, et al. The impact of a standardized “spine damage-control” protocol for unstable thoracic and lumbar spine fractures in severely injured patients: a prospective cohort study. J Trauma Acute Care Surg. 2013 Feb;74(2):590–596. doi: 10.1097/TA.0b013e31827d6054. [DOI] [PubMed] [Google Scholar]
- 108.The SAFE Study Investigators. A Comparison of Albumin and Saline for Fluid Resuscitation in the Intensive Care Unit. New England Journal of Medicine. 2004;350(22):2247–2256. doi: 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]
- 109.Myburgh JA, Finfer S, Bellomo R, et al. Hydroxyethyl Starch or Saline for Fluid Resuscitation in Intensive Care. New England Journal of Medicine. 2012 Nov 15;367(20):1901–1911. doi: 10.1056/NEJMoa1209759. [DOI] [PubMed] [Google Scholar]
- 110.Perel P, Roberts I, Ker K. Colloids versus crystalloids for fluid resuscitation in critically ill patients. The Cochrane database of systematic reviews. 2013;2:CD000567. doi: 10.1002/14651858.CD000567.pub6. [DOI] [PubMed] [Google Scholar]
- 111.Bunn F, Roberts I, Tasker R, Akpa E. Hypertonic versus near isotonic crystalloid for fluid resuscitation in critically ill patients. The Cochrane database of systematic reviews. 2004;(3):CD002045. doi: 10.1002/14651858.CD002045.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bulger EM, May S, Kerby JD, et al. Out-of-hospital hypertonic resuscitation after traumatic hypovolemic shock: a randomized, placebo controlled trial. Ann Surg. 2011 Mar;253(3):431–441. doi: 10.1097/SLA.0b013e3181fcdb22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Delano MJ, Rizoli SB, Rhind SG, et al. Prehospital Resuscitation of Traumatic Hemorrhagic Shock with Hypertonic Solutions Worsens Hypocoagulation and Hyperfibrinolysis. Shock (Augusta, Ga) 2015 Jul;44(1):25–31. doi: 10.1097/SHK.0000000000000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ciesla DJ, Moore EE, Biffl WL, Gonzalez RJ, Moore HB, Silliman CC. Hypertonic saline activation of p38 MAPK primes the PMN respiratory burst. Shock (Augusta, Ga) 2001 Oct;16(4):285–289. doi: 10.1097/00024382-200116040-00009. [DOI] [PubMed] [Google Scholar]
- 115.Dobson GPP, Letson HLM. Adenosine, lidocaine, and Mg2+ (ALM): From cardiac surgery to combat casualty care-Teaching old drugs new tricks. Journal of Trauma and Acute Care Surgery. 2016;80(1):135–145. doi: 10.1097/TA.0000000000000881. [DOI] [PubMed] [Google Scholar]
- 116.Granfeldt A, Letson HL, Dobson GP, Shi W, Vinten-Johansen J, Tonnesen E. Adenosine, lidocaine and Mg2+ improves cardiac and pulmonary function, induces reversible hypotension and exerts anti-inflammatory effects in an endotoxemic porcine model. Critical care (London, England) 2014;18(6):682. doi: 10.1186/s13054-014-0682-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Napolitano LM, Kurek S, Luchette FA, et al. Clinical practice guideline: Red blood cell transfusion in adult trauma and critical care*. Critical Care Medicine. 2009;37(12):3124–3157. doi: 10.1097/CCM.0b013e3181b39f1b. [DOI] [PubMed] [Google Scholar]
- 118.West MA, Shapiro MB, Nathens AB, et al. Inflammation and the Host Response to Injury, a Large-Scale Collaborative Project: Patient-Oriented Research Core???Standard Operating Procedures for Clinical Care. The Journal of Trauma: Injury, Infection, and Critical Care. 2006;61(2):436–439. doi: 10.1097/01.ta.0000232517.83039.c4. [DOI] [PubMed] [Google Scholar]
- 119.Bilgin YM, van de Watering LM, Brand A. Clinical effects of leucoreduction of blood transfusions. The Netherlands journal of medicine. 2011 Oct;69(10):441–450. [PubMed] [Google Scholar]
- 120.Blajchman MA. The clinical benefits of the leukoreduction of blood products. J Trauma. 2006 Jun;60(6 Suppl):S83–90. doi: 10.1097/01.ta.0000199537.09201.7b. [DOI] [PubMed] [Google Scholar]
- 121.Chen J-Y, Scerbo M, Kramer G. A Review of Blood Substitutes: Examining The History, Clinical Trial Results, and Ethics of Hemoglobin-Based Oxygen Carriers. Clinics (Sao Paulo, Brazil) 2009;64(8):803–813. doi: 10.1590/S1807-59322009000800016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lewis CJ, Ross JD. Hemoglobin-based oxygen carriers: an update on their continued potential for military application. J Trauma Acute Care Surg. 2014 Sep;77(3 Suppl 2):S216–221. doi: 10.1097/TA.0000000000000204. [DOI] [PubMed] [Google Scholar]
- 123.Johnson JL, Moore EE, Gonzalez RJ, Fedel N, Partrick DA, Silliman CC. Alteration of the postinjury hyperinflammatory response by means of resuscitation with a red cell substitute. The Journal of trauma. 2003;54(1):133–139. doi: 10.1097/00005373-200301000-00016. [DOI] [PubMed] [Google Scholar]
- 124.Moore EE, Johnson JL, Moore FA, Moore HB. The USA Multicenter Prehosptial Hemoglobin-based Oxygen Carrier Resuscitation Trial: Scientific Rationale, Study Design, and Results. Critical care clinics. 2009;25(2):325. doi: 10.1016/j.ccc.2009.01.002. Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dolinay T. Gene expression profiling of target genes in ventilator-induced lung injury. Physiological Genomics. 2006 Apr 04;26(1):68–75. doi: 10.1152/physiolgenomics.00110.2005. [DOI] [PubMed] [Google Scholar]
- 126.Ventilation with Lower Tidal Volumes as Compared with Traditional Tidal Volumes for Acute Lung Injury and the Acute Respiratory Distress Syndrome. New England Journal of Medicine. 2000 May 04;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 127.Petrucci N, De Feo C. Lung protective ventilation strategy for the acute respiratory distress syndrome. The Cochrane database of systematic reviews. 2013;2:CD003844. doi: 10.1002/14651858.CD003844.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Guillamondegui O, Cotton B, Diaz J, et al. ACUTE ADRENAL INSUFFICIENCY MAY AFFECT OUTCOME IN THE TRAUMA PATIENT. Critical Care Medicine. 2005;33(Supplement):A43. [Google Scholar]
- 129.Offner PJ, Moore EE, Ciesla D. The adrenal response after severe trauma. The American Journal of Surgery. 2002 Dec;184(6):649–653. doi: 10.1016/s0002-9610(02)01101-7. [DOI] [PubMed] [Google Scholar]
- 130.Marik PE, Pastores SM, Annane D, et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: Consensus statements from an international task force by the American College of Critical Care Medicine. Critical Care Medicine. 2008;36(6):1937–1949. doi: 10.1097/CCM.0b013e31817603ba. [DOI] [PubMed] [Google Scholar]
- 131.Wiener RS, Wiener DC, Larson RJ. Benefits and Risks of Tight Glucose Control in Critically Ill Adults: A Meta-analysis. JAMA: The Journal of the American Medical Association. 2008;300(8):933–944. doi: 10.1001/jama.300.8.933. [DOI] [PubMed] [Google Scholar]
- 132.Sperry JL, Frankel HL, Vanek SL, et al. Early hyperglycemia predicts multiple organ failure and mortality but not infection. The Journal of trauma. 2007;63(3):487–493. doi: 10.1097/TA.0b013e31812e51fc. [DOI] [PubMed] [Google Scholar]
- 133.Langley J, Adams G. Insulin-based regimens decrease mortality rates in critically ill patients: a systematic review. Diabetes Metab Res Rev. 2007;23(3):184–192. doi: 10.1002/dmrr.696. [DOI] [PubMed] [Google Scholar]
- 134.Chin TL, Sauaia A, Moore EE, et al. Elderly patients may benefit from tight glucose control. Surgery. 2012;152(3):315–321. doi: 10.1016/j.surg.2012.06.015. doi:310.1016/j.surg.2012.1006.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kudsk KA. Beneficial Effect of Enteral Feeding. Gastrointestinal Endoscopy Clinics of North America. 2007 Oct;17(4):647–662. doi: 10.1016/j.giec.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kang W, Kudsk KA. Is There Evidence That the Gut Contributes to Mucosal Immunity in Humans? Journal of Parenteral and Enteral Nutrition. 2007 May 01;31(3):246–258. doi: 10.1177/0148607107031003246. [DOI] [PubMed] [Google Scholar]
- 137.Korff S, Loughran P, Cai C, Lee YS, Scott M, Billiar TR. Eritoran attenuates tissue damage and inflammation in hemorrhagic shock/trauma. The Journal of surgical research. 2013 Oct;184(2):e17–25. doi: 10.1016/j.jss.2013.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Thompson CM, Park CH, Maier RV, O’Keefe GE. Traumatic Injury, Early Gene Expression, and Gram-Negative Bacteremia. Critical Care Medicine. 2014;42(6):1397–1405. doi: 10.1097/CCM.0000000000000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Flohe S, Lendemans S, Selbach C, et al. Effect of granulocyte-macrophage colony-stimulating factor on the immune response of circulating monocytes after severe trauma. Crit Care Med. 2003;31(10):2462–2469. doi: 10.1097/01.CCM.0000089640.17523.57. [DOI] [PubMed] [Google Scholar]
- 140.Lendemans S, Kreuzfelder E, Waydhas C, Schade FU, Flohé S. Differential immunostimulating effect of Granulocyte-macrophage colony-stimulating factor (GM-CSF), Granulocyte colony-stimulating factor (G-CSF) and Interferon γ (IFNγ) after severe trauma. Inflamm res. 2007 Jan;56(1):38–44. doi: 10.1007/s00011-007-6069-7. [DOI] [PubMed] [Google Scholar]
- 141.Monsel A, Zhu Y-g, Gennai S, Hao Q, Liu J, Lee JW. Cell-based Therapy for Acute Organ InjuryPreclinical Evidence and Ongoing Clinical Trials Using Mesenchymal Stem Cells. Anesthesiology. 2014;121(5):1099–1121. doi: 10.1097/ALN.0000000000000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wilson JG, Liu KD, Zhuo H, et al. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. The Lancet Respiratory medicine. 2015 Jan;3(1):24–32. doi: 10.1016/S2213-2600(14)70291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zheng G, Huang L, Tong H, et al. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respiratory research. 2014;15:39. doi: 10.1186/1465-9921-15-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sordi R, Nandra K, Chiazza F, et al. Abstract P106: Artesunate Protects Against The Organ Injury And Dysfunction Induced By Severe Hemorrhage And Resuscitation. Shock (Augusta, Ga) 2015;43(Suppl 1 6S):76. [Google Scholar]