Abstract
Three populations of muscle-derived cells (PP1, PP3, and PP6) were isolated from mouse skeletal muscle using modified preplate technique and retrovirally transduced with BMP4/GFP. In vitro, the PP1 cells (fibroblasts) proliferated significantly slower than the PP3 (myoblasts) and PP6 cells (muscle-derived stem cells); the PP1 and PP6 cells showed a superior rate of survival compared with PP3 cells under oxidative stress; and the PP6 cells showed significantly superior chondrogenic capabilities than PP1 and PP3 cells. In vivo, the PP6 cells promoted superior cartilage regeneration compared with the other muscle-derived cell populations. The cartilage defects in the PP6 group had significantly higher histological scores than those of the other muscle-derived cell groups, and GFP detection revealed that the transplanted PP6 cells showed superior in vivo cell survival and chondrogenic capabilities compared with the PP1 and PP3 cells. PP6 cells (muscle-derived stem cells) are superior to other primary muscle-derived cells for use as a cellular vehicle for BMP4-based ex vivo gene therapy to heal full-thickness osteo-chondral defects. The superiority of the PP6/muscle-derived stem cells appears to be attributable to a combination of increased rate of in vivo survival and superior chondrogenic differentiation capacity.
Introduction
Articular cartilage (AC) defects do not spontaneously heal and remain a clinical challenge.1–3 Current therapies for treating AC injuries include abrasion arthroplasty, microfracture, autologous chondrocyte implantation, and meniscal or osteo-chondral allografts.4,5 Cartilage tissue engineering based on cell-mediated gene therapy has emerged as a promising new approach to repair AC.3 This approach is based on the transplantation of genetically modified cells, which may serve the dual role of being a cell population capable of chondrogenesis and act as a reservoir for the production of growth factors that can stimulate the donor and/or intrinsic cells to participate in the AC repair.6
There are ongoing efforts to identify new cell populations with chondrogenic potentials that can be isolated and expanded easily. Muscle tissue represents an abundant, accessible, and renewable source of adult stem cells and the existence of osteo-chondro progenitor cells in the skeletal muscle has been already reported.7–11 Satellite cells, or early muscle progenitor cells, have been found to retain the ability to undergo chondrogenic differentiation in the presence of BMPs and/or Transforming growth factor beta-3 (TGF-beta 3) in vitro.12–14 Myoblasts, when stimulated with BMP or TGF-beta 3, upregulate osteocalcin, alkaline phosphatase,12,13 and Sox9.15 Fibroblast-like cells isolated from rat skeletal muscle fascia also demonstrate chondrogenic potential when stimulated with BMP4.16 However, it is still unclear which muscle cell population represents an optimal cell source for AC repair.
We have previously shown that, using the preplate technique, muscle-derived cells (MDCs) can be separated into different populations based on the cells’ ability to adhere to Type 1 collagen.17–23 We have demonstrated that a population of slowly adherent cells also known muscle-derived stem cells (MDSCs), obtained from the later preplate population, exhibit long-term proliferation capacities, high rates of self-renewal, multipotent differentiation potentials and, consequently, high in vivo regenerative capacity in a variety of musculoskeletal tissues.24–27 The unique ability of these cells to resist to oxidative stress also plays a role in their high regenerative capabilities.26 We have also shown that when stimulated with BMP-4 and/or TGF-beta 1, MDSCs can produce cartilaginous-like tissue in vitro, and when retrovirally transduced to express BMP4 have been shown to differentiate into chondrocytes and enhance AC repair in vivo.28–30 As a result, MDSCs represent a potentially attractive gene delivery vehicle for cartilage regeneration; however, it remains unknown as to whether MDSCs genetically engineered to express chondrogenic growth factors can enhance cartilage formation more than other populations of MDCs.
The purpose of this study was to compare the chondrogenic potentials of BMP4-gene modified subpopulations of MDCs to heal full-thickness osteo-chondral defects in rat models.
Results
Characterization and transduction of subpopulations of MDCs
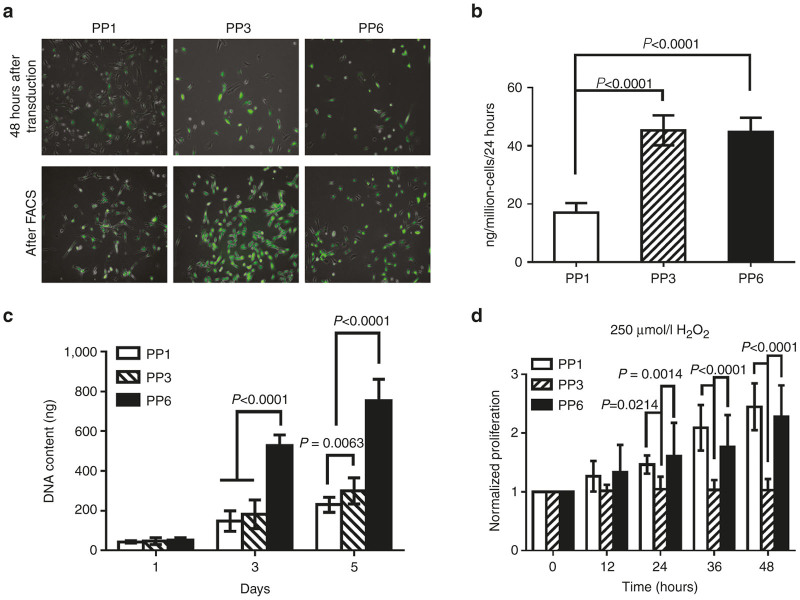
Three subpopulations (PP1, PP3, and PP6 cells) of primary MDCs were isolated from the hind-limb skeletal muscles of three 3-week-old C57/BL10J mice (Jackson Labs, Bar Harbor, ME) by using a modified preplate technique.18,25 Our data and previous studies have shown that the different populations of MDCs separated by the preplate technique consist of a mixture of cells, including myoblasts, fibroblasts, and adipocytes.18,25 The PP1 cells were mostly fibroblastic-like cells and contained nonmyogenic mesenchymal stem cells (MSCs); the PP3 cells consisted mainly of late myogenic progenitor cells (myoblast-like cells); and the PP6 cells consisting mainly of desmin and Sca-1 positive MDSCs18,25,31 (data not shown). The efficiency of retro-BMP4-green florescent protein (GFP) transduction of all three MDC subpopulations was ~80% (Figure 1a, 48 hours after transduction, and Supplementary Figure S1). There were no significant differences among the three cell populations in terms of susceptibility to retro-BMP4-GFP transduction. After purification of GFP positive cells by fluorescence-activated cell sorting, the level of BMP4 secreted by the transduced PP1 cells was significantly lower than the transduced PP3 and PP6 cells (pooled data for three isolations, n = 9, Figure 1b). No significant differences were found in the levels of BMP4 secretion between the transduced PP3 and PP6 cells (Figure 1b).
Figure 1.
RetroBMP4-green florescent protein (GFP) transduction and characterization of transduced muscle-derived cells (MDCs). Primary MDCs were isolated from the hind-limb skeletal muscles of three 3-week-old C57/BL10J mice using a modified preplate technique. The retroviral vectors encoding for BMP4 and the marker gene GFP (retroBMP4-GFP) were used for the transduction. (a) RetroBMP4-GFP transduction of MDCs. The efficiency of retro-BMP4-GFP transduction of all three MDC subpopulations was ~80% (48 hours after transduction, representative images). All populations were purified based on GFP signal by fluorescence-activated cell sorting (FACS) (After FACS, representative images). (b) BMP4 secretion levels of the transduced MDCs after purification by FACS. (n = 9, pooled data for three isolations, n = 3 for each isolation); (c) In vitro proliferation of BMP4-expressing MDCs. (n = 9, pooled data for three isolations, n = 3 for each isolation); (d) Cell survival of BMP4-expressing MDCs under oxidative stress. (n = 9, pooled data for three isolations, n = 3 for each isolation). Data are presented as mean ± SD.
In vitro proliferation of BMP4-expressing MDCs
After retro-BMP4-GFP transduction, three subpopulations of MDCs showed different proliferation kinetics, as determined by DNA content. On day 3 and 5, the DNA content of the PP6 cells was significantly higher than that of both the PP3 and PP1 cells (Figure 1c). The DNA content of the PP3 cells was also significantly higher than that of the PP1 cells on day 5 (Figure 1c).
Cell survival of BMP4-expressing MDCs under oxidative stress
We further tested the responses of the subpopulations of BMP4 expressing MDCs to oxidative stress induced by H2O2. While the proliferation of the PP3 cells was completely halted, the PP6 and PP1 cells could still proliferate and showed a significantly superior survival rate than the PP3 cells; no significant difference in cell survival was observed between the PP6 and PP1 cells. (Figure 1d)
In vitro chondrogenic differentiation of BMP4-expressing MDCs
After chondrogenic induction in monolayer culture for 5 days, reverse transcription-polymerase chain reaction (RT-PCR) results demonstrated that BMP4-transduced PP6 cells underwent chondrogenic differentiation more readily than did the PP1 and PP3 cells. The mRNA expression of aggrecan, Col2A, and Col10A by the PP6 cells was significantly higher than that of PP1 and PP3 cells (Figure 2a). Chondrogenic pellet culture validated the chondrogenic potential of the cells since the PP6 cell pellets stained more intensely with alcian blue than the other MDC populations (Figure 2b). Quantitative analysis of the glycosaminoglycan (GAG) content of the pellets demonstrated that PP6 cell pellets contained significantly more GAG than did the PP1 and PP3 cell pellets. No significant difference in GAG content was found between the PP1 and PP3 cell pellets (Figure 2c).
Figure 2.
In vitro chondrogenic potential of BMP4-expressing muscle-derived cells (MDCs). (a) Reverse transcription-polymerase chain reaction (RT-PCR) gel image (representative images from one isolation); (b) Alcian blue staining of the chondrogenic pellets (representative images from one isolation); (c) Glycosaminoglycan (GAG) content of the chondrogenic pellets. (n = 12, pooled data for three isolations, n = 4 for each isolation). Data are presented as mean ± SD.
In vivo AC repair induced by BMP4-transduced MDCs
Macroscopic examination.
Gross examination of AC defects at 4 and 8 weeks after transplantation revealed glossy white, well-integrated, repaired tissue in the BMP4-transduced PP6 cell group whereas that in the PP1 and PP3 groups appeared patchy, and was only slightly integrated with the surrounding AC (Figure 3). Sixteen weeks after transplantation, the original defects in the BMP4-transduced PP6 group contained glossy white repaired tissue that appeared to be well integrated with the surrounding AC and the PP1 and PP3 groups appeared irregular, and the margin between the regenerated tissue and the native AC was easily distinguishable (Figure 3).
Figure 3.
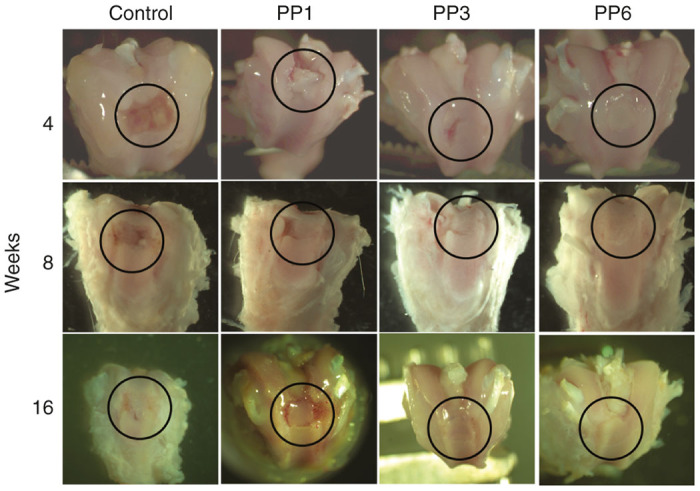
Gross appearances of the defect areas repaired with BMP4-expressing muscle-derived cells (MDCs). Black circles indicate the defect areas. Control: fibrin glue only without cells.
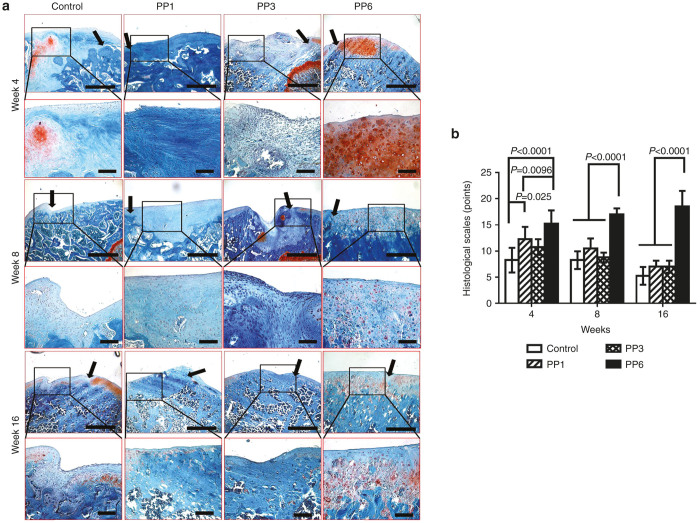
Histologic evaluation.
Four weeks after transplantation, the defects in the PP6 group were filled with repaired tissue that appeared to be well integrated with the surrounding AC and subchondral bone. Most of the cells in the repaired tissue were rounded with a chondrocytic morphology and were positive for Safranin-O staining (Figure 4a, 4 weeks) and most of the regenerated tissues in the PP1 and PP3 groups were poorly integrated with the surrounding tissue and the regenerated tissues appear fibrotic; moreover, the matrix did not stain with Safranin-O (Figure 4a, 4weeks). Eight weeks after transplantation, the regenerated tissue in the PP6 cell group had become thinner and well integrated with the surrounding cartilage. Most of the cells in the repaired tissue displayed chondrocytic morphology and had a reduced level of Safranin-O positive matrix when compared with the week 4 time point (Figure 4a, 8 weeks). In contrast, the regenerated tissue of the PP1 and PP3 groups became thinner, contained mostly fibroblast-like cells, and did not stain positively for Safranin-O (Figure 4a, 8 weeks). Sixteen weeks after transplantation, the repaired tissue in the PP6 group had a smooth surface and the margins were completely integrated with the surrounding cartilage. Morphologically, the regenerated tissue in the defect area looks like normal hyaline cartilage (Figure 4a, 16 weeks). In the other groups, no chondrocyte-like cells were found in the defect areas; instead, bony tissue was found to have grown from the subchondral bone and the adjacent cartilage tissue displayed degenerative changes (Figure 4a, 16 weeks).
Figure 4.
Histological examination of articular cartilage (AC) repair with BMP4-expressing muscle-derived cells (MDCs). (a) Safranin-O staining of the defect areas at week 4, week 8, and week 16 post-transplantation; Black arrows indicate the edge of the defect areas; upper panel (Scale bar = 500 μm, original magnification × 40), lower panel (Scale bar = 200 μm, original magnification × 200). Control: fibrin glue only without cells. (b) Chronological changes of the histological grading score. n = 4, data was presented as mean ± SD.
Chronological changes of histological grading scores are shown in Figure 4b (combined scores), and Supplementary Figure S2 (categorized scores). For combined scores, in the PP6 cell group, histological grading scores improved chronologically until 16 weeks after transplantation; however, the scores in the control group, PP1, and PP3 cell groups deteriorated after 4 weeks postsurgery. Although, the scores in the PP1 and PP3 cell groups were significantly higher than the control group (injured and noninjected) at 4 weeks, there were no significant differences among those groups afterwards. The PP6 cell group showed significantly higher scores when compared with all the other groups at all time-points.
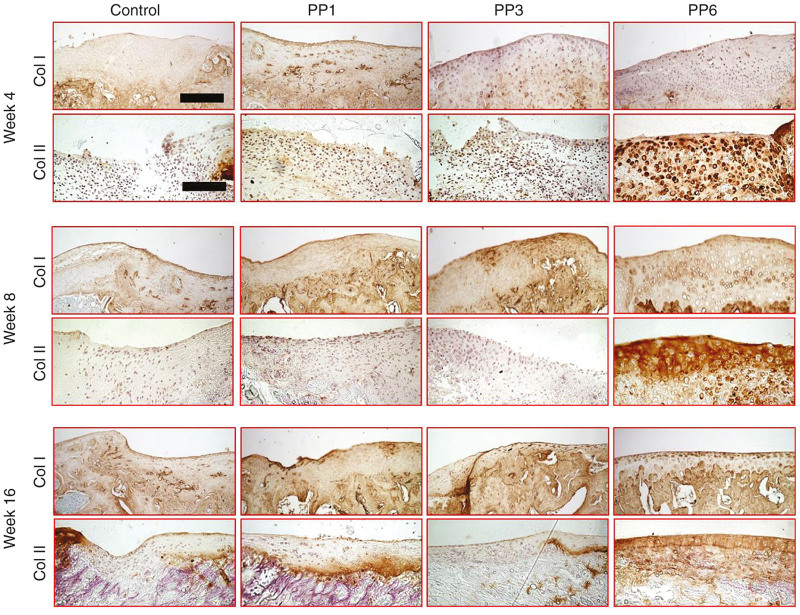
Immunohistochemical staining for Collagen Type 1 and 2 showed that in the PP6 cell group, the transplanted cells expressed more Type 2 collagen and less Type 1 collagen at all time points, when compared with the other groups (Figure 5).
Figure 5.
Immunohistochemical staining of Type 1 and 2 collagens. Upper panel (Scale bar = 200 μm, original magnification × 200), lower panel (Scale bar = 200 μm, original magnification × 200). Control: fibrin glue only without cells.
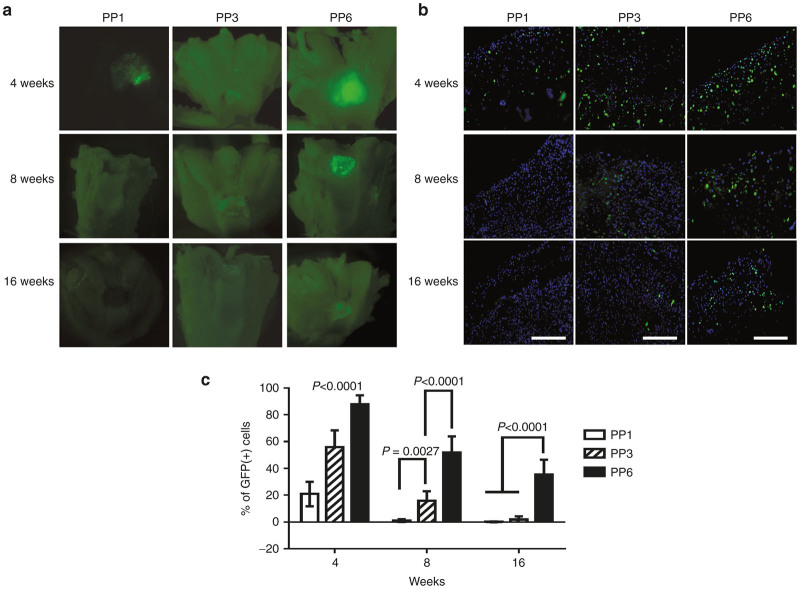
In vivo survival and contribution of the implanted BMP4-expressing MDCs in the repair tissue
Grossly, the GFP signals, which represent the transplanted cells, gradually decreased overtime in all the groups (Figure 6a). In the PP1 cell group, the GFP signal was rapidly lost by 8 weeks after transplantation and no GFP signal was detected at the cartilage surface after that time point. In the PP3 cell group, GFP signals also decreased gradually and only very weak GFP signals could be detected at 16 weeks. Although the number of GFP-positive cells decreased over time in the PP6 cell group, a fair number (~34.8%) of GFP-positive cells could still be detected at 16 weeks after transplantation. The PP6 cell group showed the highest level of GFP signals at all the time-points tested when compared with the PP1 and PP3 cell groups (Figure 6a). Further examination of the defect areas was conducted to quantify the percentage of GFP-positive cells within the defect areas. At week 4, most of the cells within the repaired tissue in the PP6 group were GFP-positive (89.36%); significantly fewer GFP-positive cells were found in the PP1 (21.13%) and PP3 groups (55.57%) (Figure 6b,c). At 8 weeks, quantitative analysis showed that 1.22, 13.34, and 52.9% GFP-positive cells remained in the repaired tissues of the PP1, PP3, and PP6 cell groups respectively (Figure 6b,c). At 16 weeks, very few GFP-positive cells could be found in the repaired tissues of the PP1 and PP3 cells groups; and 34.81% GFP-positive cells still present in the PP6 cell group (Figure 6b,c).
Figure 6.
In vivo survival and contribution of BMP4-expressing muscle-derived cells (MDCs). (a) gross examination of green florescent protein (GFP) signals in the defect areas; (b) GFP signal in the cross-sections of the defect area (Scale bar = 200 μm, original magnification × 200); (c) percentage of GFP positive cells within the defect area. (n = 12). Data was presented as mean ± SD.
Discussion
Transplantation of genetically modified adult stem cells is a promising approach to repair AC defects,3 because the genetically modified cells could serve the dual role of being a cell population capable of chondrogenesis and acting as reservoir for the production of key growth factors that can stimulate AC repair through activation of endogenous cells.6 In this study, a retroviral vector encoding for the BMP4 and GFP genes was used to transduce the MDCs with transduction efficiency of ~80% for all three populations of MDCs; there were no significant differences observed among the groups and no evidence of obvious cellular toxicity. After purification by GFP signal, BMP4 expression by the PP1 cells was significantly lower than that of the PP3 and PP6 cells. We have not yet determined whether the lower level of BMP4 expression by the transduced PP1 cells led to their inferior chondrogenic differentiation capacity; however, the similar inferior chondrogenic potential of the PP3 cells, which had a similar expression level of BMP4 to the PP6 cells, suggests that the difference in chondrogenic potential between the cells tested was not due to a differential level of BMP4 expression. Thus, the inferior chondrogenic differentiation capabilities observed in the PP1 and PP3-BMP4 cells appears to be primarily due to their intrinsic inferior chondrogenic potential and/or the presence of a lower number of chondrogenic progenitor cells within these cell populations.
GFP enabled us to track the transplanted cells in vivo for their survivability and direct participation in the healing of the cartilage defects. PP1, PP3, and PP6 cells from the same isolation were tested for their regenerative potential in vivo. Very few GFP-positive cells were detectable 4 weeks after transplantation in the PP1 group, and no GFP signals could be found at the cartilage surface at 8 weeks. Similarly, only a few PP3 cells could be found after 8 weeks. Adachi et al.32 previously reported that rabbit allogeneic MDCs (equivalent to the PP3 population in this study) and chondrocytes transduced with the LacZ gene were detectable for only up to 4 weeks after transplantation into an osteo-chondral defect. These findings corroborate our current findings that the early adherent cells (PP1 and PP3 cells) have limited engraftment capacity in the osteochondral defect in vivo. On the other hand, significantly more GFP-positive cells could be found in the defect areas transplanted with PP6 cells than the other MDCs groups. Indeed even by 16 weeks, a large number of implanted GFP-positive PP6 cells could still be detected in the defect area, which suggests a superior in vivo survival rate by the PP6 cells compared with the PP1 and PP3 cells. During the AC healing process, increased survival of the transplanted MDCs could lead to significantly enhanced and prolonged BMP4 transgene expression, which in turn could enhance AC healing; however, there is a possibility that excessive local BMP4 expression might result in the occurrence of undesired ossification. In this study, we did not observe any ectopic ossification at any of the time-points in the PP6 cell group.
In pathological conditions, such as osteoarthritis, oxygen tension in the synovial fluid is subject to fluctuation.33 In response to partial oxygen pressure (pO2) variations, mechanical stress, immune-modulatory and inflammatory mediators, chondrocytes respond and interact with abnormal levels of reactive oxygen species.34,35 When imbalances between oxidants and antioxidants are large enough, structural and/or functional cellular and tissue changes can occur. This phenomenon is known as “oxidative stress” which can be observed in many pathological conditions involving AC lesions.36–39 Donor cells that are transplanted into an injured joint will inevitably be exposed to this harsh microenvironment, which in turn can reduce the cells’ ability to survive and function appropriately in the target tissue.40,41 Therefore, choosing or engineering a population of cells that has superior antioxidative properties is critical for cell survival after transplantation.41 As evidenced by the in vitro data, the PP6 and PP1 cells demonstrated superior antioxidative properties than the PP3 cells. These data agree with previous publications which demonstrated that MDSCs display enhanced resistance to oxidative stress after transplantation into injured myocardium when compared with myoblasts.42 These findings support the idea that the superior antioxidative properties of the PP6 cells are responsible, at least in part, for their higher survival rate in the AC defect. Interestingly, the PP1 cells, which also showed superior antioxidative properties compared with the PP6 cells in vitro, did not survive well in vivo, which implies that other mechanisms, such as apoptosis, inflammation, and mechanical stress,43 may also influence the in vivo survival of transplanted cells.
Although all MDCs populations formed chondrogenic pellets under chondrogenic induction, and demonstrated positive proteoglycan deposition, the intensity of proteoglycan was much greater for the PP6 cells. In addition, the PP6 cells expressed all of the chondrogenic related mRNAs including aggrecan, Sox-9, and Collagen Type 2 in contrast to the other MDCs populations. These in vitro evidences indicate that PP6 cells are more chondrogenic than other MDCs populations. Although the exact mechanism by which PP6 cells are more chondrogenic is still not known, they likely represent the most suitable cell population for the treatment of AC defects than other MDC populations that were isolated by the preplate technique. Donor cells that can efficiently differentiate into functional chondrocytes are extremely beneficial since local host chondrocytes have a very limited proliferation capacity and are inadequate in number to support natural healing44,45; hence the reason AC injuries cannot spontaneously heal.
It should be noted that, although the BMP4-expressing PP6 cells regenerated AC tissue that appeared morphologically similar to native cartilage at 16 weeks, we observed a remarkable decrease in the number of donor cells at this time point (more than half of the cells were lost by week 16). The majority of chondrocytes within the defect area were host derived (GFP-negative), indicating that the endogenous host cells were the main cells that participated in the repair of the defect especially at the later stage. This observation emphasizes the fact that the transplanted gene-modified donor cells primarily act as a reservoir of secreted molecules which influence the regenerative activity of host cells. Therefore, it makes sense to speculate that the better persistence of the PP6 cells in the osteochondral defect led to an enhanced or prolonged paracrine effects on host cells. Although beyond of the scope of this study, we believe that the host responses induced by the genetically modified donor cells are critical for achieving long-term clinical results. Additional studies are required to clarify the optimized duration of transgene expression, dosage of the donor cells and the type of chondrogenic factors that should be delivered in order to induce more efficient and effective host responses to form long-lasting and self-renewable hyaline cartilage.
We also noticed that, 4 weeks after transplantation, PP6 cells were able to integrate and to generate a cartilaginous tissue rich in GAG. However, a significant decrease in Safranin-O staining was observed at week 8. These observations indicate the continuous remodeling of the regenerated tissue by host cells, which correlate with the continuous replacement of the GFP positive donor cells by host cells in the defect area. Although our results indicate that the host cells play a major role in the repair process, the origin of these host cells remain unclear but likely involved cells originating from the bone marrow and the synovium.
In conclusion, this study demonstrated that PP6 cells (MDSCs) are superior to other primary MDCs (PP1 and PP3 cells) for use as a cellular vehicle for BMP4-based ex vivo gene therapy for the treatment of osteochondral defects. The unique ability of the PP6 cells to survive longer within the injured tissues as well as retain their ability for chondrogenic differentiation appears to play a role in their enhanced regenerative potential for AC. The regenerative advantage of the MDSCs over other fractions of muscle cells for AC is in agreement with previous findings showing the high regenerative index of MDSCs in various tissues like bone, heart, and skeletal muscle24–27 when compared with more differentiated muscle cells (such as myoblasts). In future studies, the cartilage regenerative potential of MDSCs will be compared with other purified muscle stem cell populations, such as pericytes, myo-endothelial cells, and with other well-characterized nonmuscle derived stem cell populations, such as bone marrow- and adipose-derived MSCs to provide broader and in-depth information for further clinical translation.
Materials and Methods
Isolation and transduction of primary subpopulations of MDCs
Primary MDCs were isolated from the hind-limb skeletal muscles of three 3-week-old C57/BL10J mice (The Jackson Laboratory, Bar Harbor, ME) by using a modified preplate technique.18,25 Briefly, after the muscle was dissociated enzymatically and mechanically, the resultant cell suspension was placed in a Type 1 collagen-coated flask. After 1 hour, the adhered cells were designated as PP1 (pre-plate 1) cells; nonadherent cells were transferred to a fresh collagen-coated flask (PP2) and then 24 hours later those nonadhering cells in the second preplate were transferred to another fresh collagen-coated flask (PP3). This procedure was repeated until PP6 was obtained. Two populations of rapidly adhering cells (PP1 and PP3) and one population of slowly adhering cells (PP6 also known as Muscle Derived Stem Cells, MDSCs18) were utilized for this study.
The retroviral vectors encoding for BMP4 and the marker gene GFP (retroBMP4-GFP) were used for the transduction of the different subpopulations of MDCs.30,46 The transduced cells were sorted for GFP by fluorescence-activated cell sorting (FACSAria; BD Biosciences, San Jose, CA). After purification of the GFP-positive cells, the level of BMP4 expression was determined by enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN).
Proliferation assay
A DNA assay was used to determine cell proliferation.47 Briefly, 2 × 103 retro-BMP4-GFP transduced MDCs per well were seeded on a 48-well plate. At days 1, 3, and 5, the cell lysates were prepared by the addition of 0.1% Triton X-100 (Sigma-Aldrich, St. Louis, MO), followed by three freeze-thaw cycles. Double-stranded DNA content was measured using a Quant-iT dsDNA high-sensitivity assay kit (Invitrogen, Carlsbad, CA).
Cell survival under oxidative stress
Cell survival under oxidative stress was assessed using a live cell imaging system (LCI, Kairos Instruments LLC, Pittsburgh, PA).48 MDCs were plated in a 24-well plate and supplemented with 250 μmol/l H2O2 (Sigma). All wells were also supplemented with PI (propidium iodide, 2 μg/ml; Sigma) in order to identify the dead cells. Fluorescent (red channel) and bright-field images were taken every 10 minutes in three different locations per well for a period of 48 hours. All images were analyzed using ImageViewer software (Karios Instruments). The number of live cells was quantified at successive 12-hour time points and normalized to the cell numbers at time point zero over a 48-hour time period.
In vitro assessment of chondrogenesis
All three isolations of different subpopulations of MDCs were tested for chondrogenic differentiation, micromasses of 2.5 × 105 cells were formed and kept for 4 weeks in chondrogenic medium (Lonza, Basel, Switzerland). Alcian blue staining was performed and GAG content was tested. For alcian blue staining, randomly selected pellets (n = 4 per data point per isolation) were fixed in 4% paraformaldehyde, CMC (NEG50, Richard-Allan Scientific, San Diego, CA) embedded, frozen, and 5 μm serial sections were cut. For GAG analysis, randomly selected pellets (n = 4 per data point per isolation) were digested for 6 hours at 60ºC with 125μg/ml papain in phosphate buffered saline (PBS) buffer (100 mmol/l phosphate, 10 mmol/l ethylenediaminetetraacetate (EDTA), PH 6.5) containing 10 mmol/l cysteine using 100 μl of enzyme per sample. GAG content was measured using dimethylmethylene blue dye and a spectrophotometer (Infinite M200, TECAN, Männedorf, Switzerland) with bovine chondroitin sulfate used as a standard. GAG content of the pellets were normalized to the DNA content per pellet.
mRNA expressions of chondrogenic genes of the pellets were tested by reverse transcription-polymerase chain reaction. The mRNA expression of aggrecan, Sox-9, collagen Type 2 and collagen Type 10 were analyzed by semiquantitative reverse transcription-polymerase chain reaction. Oligonucleotide primers are presented in Supplementary Table S1.
Osteochondral defect model and cell transplantation
All animal experiments were approved by the Animal Research and Care Committee at the University of Pittsburgh. Twenty-four 16-week-old female nude rats (NIH-Whn NIHRNU-M; Taconic, Hudson, NY) were used. The animals were anesthetized by isoflurane. Both knee joints were exposed by medial para-patellar incision, and the trochlear groove was exposed by lateral dislocation of the patella. A customized trephine drill with a 1.5 mm outer diameter and a stopper to control the depth was used to create bilateral osteochondral defects (1.5 × 1.5 mm) in the trochlear groove of the femur.28,30 2 × 105 BMP4-GFP-MDCs (PP1, PP3, and PP6 cells from the same isolation) were mixed with fibrin glue (Tisseel VH; Baxter Healthcare, Deerfield, IL) and applied to the AC defects. The knees were randomly divided into four groups: (i) control group: fibrin glue only; (ii) BMP4-PP1 group: BMP4 transduced PP1 cells with fibrin glue; (iii) BMP4-PP3 group: BMP4 transduced PP3 cells; (iv) BMP4-PP6 group: BMP4 transduced PP6 cells. Rats were killed at 4, 8, and 16 weeks after cell transplantation, and four distal femora in each group were dissected at each time point.
Histology and immunohistochemistry
The distal femur was dissected and the appearance and the integration of regenerated cartilage were examined using a stereo-microscope (SZX16, Olympus, Tokyo, Japan) and GFP signals at the defect areas were imaged. After imaging, the samples were fixed in 4% paraformaldehyde, CMC embedded, cryo-sectioned at 5 μm. Sections were mounted with cryo-film49(Section Lab, Japan) and were used for histological examination and immunohistochemical staining. Slides were stained with Safranin-O/Fast Green. Histologic grading was performed, as described by O’ Driscoll et al.50 to evaluate the quality of the repaired cartilage. GFP signals were also imaged in the defect area with 4’,6-diamidino-2-phenylindole (DAPI) staining to reveal the nuclei. The percentage of GFP-positive cells over the total cells within the defect area were calculated in three randomly chosen sections per sample. Synthesis of Type 1 and Type 2 collagen was verified using immunohistochemical staining. Sections were treated with proteinase K (ab64220, Abcam) for 20 minutes and incubated with Collagen Type 1 (ab34710, Abcam, 1:100) or Type 2 (SC7763, Santa Cruz Biotechnology, 1: 50) primary antibodies at 4°C overnight. Slides were then incubated with biotinylated secondary antibodies, and the signals were visualized using the chromogen substrate diaminobenzidine.
Statistical analysis
Three isolations of MDCs were tested by in vitro assays, and all in vitro data were pooled for statistical analysis. Statistical tests were performed using SPSS 16.0 (SPSS, Armonk, NY) Data was presented as mean ± SD. All data was tested for normality and equal variance before analysis. Statistical differences were calculated using analysis of variance (ANOVA or ANOVA on ranks if equal variance testing failed). Bonferroni post-tests were used to compare different groups.
Acknowledgments
This study was funded by Department of Defense (W81XWH-08-0076), National Institute of Health (1R21AR065669-01A1), and the Henry J Mankin endowed chair at the University of Pittsburgh. We thank James Cummins for his editorial assistance.
There is no conflict of interest to disclose for all authors except that Huard received remuneration as a consultant and royalties from Cook Myocyte.
References
- Buckwalter, JA (1998). Articular cartilage: injuries and potential for healing. J Orthop Sports Phys Ther 28: 192–202. [DOI] [PubMed] [Google Scholar]
- Hunziker, EB (2002). Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage 10: 432–463. [DOI] [PubMed] [Google Scholar]
- Steinert, AF, Nöth, U and Tuan, RS (2008). Concepts in gene therapy for cartilage repair. Injury 39 Suppl 1: S97–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris, EA, Gomoll, AH, Malizos, KN, Hu, JC and Athanasiou, KA (2015). Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol 11: 21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran, CJ, Pascual-Garrido, C, Chubinskaya, S, Potter, HG, Warren, RF, Cole, BJ et al. (2014). Restoration of articular cartilage. J Bone Joint Surg Am 96: 336–344. [DOI] [PubMed] [Google Scholar]
- Madry, H and Cucchiarini, M (2011). Clinical potential and challenges of using genetically modified cells for articular cartilage repair. Croat Med J 52: 245–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Putte, KA, and Urist, MR (1965). Osteogenesis in the interior of intramuscular implants of decalcified bone matrix. Clin Orthop Relat Res 43: 257–270. [DOI] [PubMed] [Google Scholar]
- Volek-Smith, H and Urist, MR (1996). Recombinant human bone morphogenetic protein (rhBMP) induced heterotopic bone development in vivo and in vitro. Proc Soc Exp Biol Med 211: 265–272. [DOI] [PubMed] [Google Scholar]
- Mastrogiacomo, M, Derubeis, AR and Cancedda, R (2005). Bone and cartilage formation by skeletal muscle derived cells. J Cell Physiol 204: 594–603. [DOI] [PubMed] [Google Scholar]
- Levy, MM, Joyner, CJ, Virdi, AS, Reed, A, Triffitt, JT, Simpson, AH et al. (2001). Osteoprogenitor cells of mature human skeletal muscle tissue: an in vitro study. Bone 29: 317–322. [DOI] [PubMed] [Google Scholar]
- Bosch, P, Musgrave, DS, Lee, JY, Cummins, J, Shuler, T, Ghivizzani, TC et al. (2000). Osteoprogenitor cells within skeletal muscle. J Orthop Res 18: 933–944. [DOI] [PubMed] [Google Scholar]
- Tajbakhsh, S, Rocancourt, D and Buckingham, M (1996). Muscle progenitor cells failing to respond to positional cues adopt non-myogenic fates in myf-5 null mice. Nature 384: 266–270. [DOI] [PubMed] [Google Scholar]
- Asakura, A, Komaki, M and Rudnicki, M (2001). Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation 68: 245–253. [DOI] [PubMed] [Google Scholar]
- Cairns, DM, Liu, R, Sen, M, Canner, JP, Schindeler, A, Little, DG et al. (2012). Interplay of Nkx3.2, Sox9 and Pax3 regulates chondrogenic differentiation of muscle progenitor cells. PLoS One 7: e39642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita, T, Matsui, N, Fujioka, H, Kubo, S, Kuroda, R, Kurosaka, M et al. (2004). Expression of transcription factor Sox9 in rat L6 myoblastic cells. Connect Tissue Res 45: 164–173. [DOI] [PubMed] [Google Scholar]
- Li, G, Zheng, B, Meszaros, LB, Vella, JB, Usas, A, Matsumoto, T et al. (2011). Identification and characterization of chondrogenic progenitor cells in the fascia of postnatal skeletal muscle. J Mol Cell Biol 3: 369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deasy, BM, Jankowski, RJ and Huard, J (2001). Muscle-derived stem cells: characterization and potential for cell-mediated therapy. Blood Cells Mol Dis 27: 924–933. [DOI] [PubMed] [Google Scholar]
- Qu-Petersen, Z, Deasy, B, Jankowski, R, Ikezawa, M, Cummins, J, Pruchnic, R et al. (2002). Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol 157: 851–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharaibeh, B, Lu, A, Tebbets, J, Zheng, B, Feduska, J, Crisan, M et al. (2008). Isolation of a slowly adhering cell fraction containing stem cells from murine skeletal muscle by the preplate technique. Nat Protoc 3: 1501–1509. [DOI] [PubMed] [Google Scholar]
- Lee, JY, Qu-Petersen, Z, Cao, B, Kimura, S, Jankowski, R, Cummins, J et al. (2000). Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing. J Cell Biol 150: 1085–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando, TA and Blau, HM (1994). Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol 125: 1275–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang, JH, Yuk, SH, Lee, JH, Lyoo, WS, Ghil, SH, Lee, SS et al. (2004). Isolation of muscle derived stem cells from rat and its smooth muscle differentiation [corrected]. Mol Cells 17: 57–61. [PubMed] [Google Scholar]
- Rouger, K, Fornasari, B, Armengol, V, Jouvion, G, Leroux, I, Dubreil, L et al. (2007). Progenitor cell isolation from muscle-derived cells based on adhesion properties. J Histochem Cytochem 55: 607–618. [DOI] [PubMed] [Google Scholar]
- Ota, S, Uehara, K, Nozaki, M, Kobayashi, T, Terada, S, Tobita, K et al. (2011). Intramuscular transplantation of muscle-derived stem cells accelerates skeletal muscle healing after contusion injury via enhancement of angiogenesis. Am J Sports Med 39: 1912–1922. [DOI] [PubMed] [Google Scholar]
- Qu, Z, Balkir, L, van Deutekom, JC, Robbins, PD, Pruchnic, R and Huard, J (1998). Development of approaches to improve cell survival in myoblast transfer therapy. J Cell Biol 142: 1257–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada, M, Payne, TR, Drowley, L, Jankowski, RJ, Momoi, N, Beckman, S et al. (2012). Human skeletal muscle cells with a slow adhesion rate after isolation and an enhanced stress resistance improve function of ischemic hearts. Mol Ther 20: 138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, H, Usas, A, Gearhart, B, Olshanski, A, Shen, HC and Huard, J (2004). Converse relationship between in vitro osteogenic differentiation and in vivo bone healing elicited by different populations of muscle-derived cells genetically engineered to express BMP4. J Bone Miner Res 19: 630–641. [DOI] [PubMed] [Google Scholar]
- Kuroda, R, Usas, A, Kubo, S, Corsi, K, Peng, H, Rose, T et al. (2006). Cartilage repair using bone morphogenetic protein 4 and muscle-derived stem cells. Arthritis Rheum 54: 433–442. [DOI] [PubMed] [Google Scholar]
- Matsumoto, T, Cooper, GM, Gharaibeh, B, Meszaros, LB, Li, G, Usas, A et al. (2009). Cartilage repair in a rat model of osteoarthritis through intraarticular transplantation of muscle-derived stem cells expressing bone morphogenetic protein 4 and soluble Flt-1. Arthritis Rheum 60: 1390–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto, T, Kubo, S, Meszaros, LB, Corsi, KA, Cooper, GM, Li, G et al. (2008). The influence of sex on the chondrogenic potential of muscle-derived stem cells: implications for cartilage regeneration and repair. Arthritis Rheum 58: 3809–3819. [DOI] [PubMed] [Google Scholar]
- Shen, HC, Peng, H, Usas, A, Gearhart, B, Cummins, J, Fu, FH et al. (2004). Ex vivo gene therapy-induced endochondral bone formation: comparison of muscle-derived stem cells and different subpopulations of primary muscle-derived cells. Bone 34: 982–992. [DOI] [PubMed] [Google Scholar]
- Adachi, N, Sato, K, Usas, A, Fu, FH, Ochi, M, Han, CW et al. (2002). Muscle derived, cell based ex vivo gene therapy for treatment of full thickness articular cartilage defects. J Rheumatol 29: 1920–1930. [PubMed] [Google Scholar]
- Blake, DR, Merry, P, Unsworth, J, Kidd, BL, Outhwaite, JM, Ballard, R et al. (1989). Hypoxic-reperfusion injury in the inflamed human joint. Lancet 1: 289–293. [DOI] [PubMed] [Google Scholar]
- Häuselmann, HJ, Stefanovic-Racic, M, Michel, BA and Evans, CH (1998). Differences in nitric oxide production by superficial and deep human articular chondrocytes: implications for proteoglycan turnover in inflammatory joint diseases. J Immunol 160: 1444–1448. [PubMed] [Google Scholar]
- Fermor, B, Weinberg, JB, Pisetsky, DS, Misukonis, MA, Banes, AJ and Guilak, F (2001). The effects of static and intermittent compression on nitric oxide production in articular cartilage explants. J Orthop Res 19: 729–737. [DOI] [PubMed] [Google Scholar]
- Henrotin, Y, Kurz, B and Aigner, T (2005). Oxygen and reactive oxygen species in cartilage degradation: friends or foes? Osteoarthritis Cartilage 13: 643–654. [DOI] [PubMed] [Google Scholar]
- Henrotin, YE, Bruckner, P and Pujol, JP (2003). The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthritis Cartilage 11: 747–755. [DOI] [PubMed] [Google Scholar]
- Schiller, J, Fuchs, B, Arnhold, J and Arnold, K (2003). Contribution of reactive oxygen species to cartilage degradation in rheumatic diseases: molecular pathways, diagnosis and potential therapeutic strategies. Curr Med Chem 10: 2123–2145. [DOI] [PubMed] [Google Scholar]
- Yudoh, K, Nguyen, vT, Nakamura, H, Hongo-Masuko, K, Kato, T and Nishioka, K (2005). Potential involvement of oxidative stress in cartilage senescence and development of osteoarthritis: oxidative stress induces chondrocyte telomere instability and downregulation of chondrocyte function. Arthritis Res Ther 7: R380–R391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey, TE, Saiget, MK, Reinecke, H and Murry, CE (2008). Systems approaches to preventing transplanted cell death in cardiac repair. J Mol Cell Cardiol 45: 567–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, W, Song, BW, Moon, JY, Cha, MJ, Ham, O, Lee, SY et al. (2013). Anti-death strategies against oxidative stress in grafted mesenchymal stem cells. Histol Histopathol 28: 1529–1536. [DOI] [PubMed] [Google Scholar]
- Oshima, H, Payne, TR, Urish, KL, Sakai, T, Ling, Y, Gharaibeh, B et al. (2005). Differential myocardial infarct repair with muscle stem cells compared to myoblasts. Mol Ther 12: 1130–1141. [DOI] [PubMed] [Google Scholar]
- Steinert, AF, Ghivizzani, SC, Rethwilm, A, Tuan, RS, Evans, CH and Nöth, U (2007). Major biological obstacles for persistent cell-based regeneration of articular cartilage. Arthritis Res Ther 9: 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelbaum, BR, Browne, JE, Fu, F, Micheli, L, Mosely, JB Jr, Erggelet, C et al. (1998). Articular cartilage lesions of the knee. Am J Sports Med 26: 853–861. [DOI] [PubMed] [Google Scholar]
- Hunziker, EB (1999). Articular cartilage repair: are the intrinsic biological constraints undermining this process insuperable? Osteoarthritis Cartilage 7: 15–28. [DOI] [PubMed] [Google Scholar]
- Peng, H, Usas, A, Gearhart, B, Young, B, Olshanski, A and Huard, J (2004). Development of a self-inactivating tet-on retroviral vector expressing bone morphogenetic protein 4 to achieve regulated bone formation. Mol Ther 9: 885–894. [DOI] [PubMed] [Google Scholar]
- Li, H, Usas, A, Poddar, M, Chen, CW, Thompson, S, Ahani, B et al. (2013). Platelet-rich plasma promotes the proliferation of human muscle derived progenitor cells and maintains their stemness. PLoS One 8: e64923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drowley, L, Okada, M, Beckman, S, Vella, J, Keller, B, Tobita, K et al. (2010). Cellular antioxidant levels influence muscle stem cell therapy. Mol Ther 18: 1865–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto, T and Shimizu, M (2000). A method for preparing 2- to 50-micron-thick fresh-frozen sections of large samples and undecalcified hard tissues. Histochem Cell Biol 113: 331–339. [DOI] [PubMed] [Google Scholar]
- O’Driscoll, SW, Keeley, FW and Salter, RB (1988). Durability of regenerated articular cartilage produced by free autogenous periosteal grafts in major full-thickness defects in joint surfaces under the influence of continuous passive motion. A follow-up report at one year. J Bone Joint Surg Am 70: 595–606. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.