Abstract
The behavior of adult-born cells can be easily monitored in cell culture or in lower model organisms, but longitudinal observation of individual mammalian adult-born cells in their native microenvironment still proves to be a challenge. Here we have established an approach named optical cell positioning system for long-term in vivo single-cell tracking, which integrates red-green-blue cell labeling with repeated angiography. By combining this approach with in vivo two-photon imaging technique, we characterized the in vivo migration patterns of adult-born neurons in the olfactory bulb. In contrast to the traditional view of mere radial migration of adult-born cells within the bulb, we found that juxtaglomerular cells switch from radial migration to long distance lateral migration upon arrival in their destination layer. This unique long-distance lateral migration has characteristic temporal (stop-and-go) and spatial (migratory, unidirectional or multidirectional) patterns, with a clear cell age-dependent decrease in the migration speed. The active migration of adult-born cells coincides with the time period of initial fate determination and is likely to impact on the integration sites of adult-born cells, their odor responsiveness, as well as their survival rate.
Keywords: adult neurogenesis, migration, single-cell tracking, RGB marking, two-photon microscopy
Introduction
It has been more than two decades since the discovery of the long-distance migration of mammalian adult-born neuroblasts from the subventricular zone (SVZ) to the olfactory bulb (OB) through the rostral migratory stream (RMS)1,2. Dividing neuroblasts undergo tangential migration in the RMS, and then radial migration up into the OB, where they differentiate into two groups of functional interneurons: granule cells (GCs) and juxtaglomerular neurons (JGNs)3,4,5,6,7,8,9,10. Whereas the dynamics of SVZ cell migration as well as the tangential migration of neuroblasts in, and their detachment from the RMS, have been studied in much detail10,11,12,13,14,15,16, little is known about their migratory behavior within the OB.
Most studies of the migratory behavior of adult-born cells within the OB used immunohistochemistry, providing only static views of this dynamic process17,18. Monitoring migration of eGFP-positive neuroblasts in acute brain slices of adult and juvenile mice, Nam et al.15 have shown that in contrast to the majority of neuroblasts in the SVZ and along caudal RMS, which are migratory, more rostral neuroblasts slow down or stop at the edge of the RMS. They concluded that “migration is minimal when cells reach the periglomerular layer (GL) of the OB”. In an elegant in vivo study, Mizrahi19 described the dynamic changes of dendrite morphology in adult-born cells, and hypothesized that adult-born JGNs may migrate along the GL, but could not test his hypothesis due to the lack of a robust approach for single-cell tracking. In our previous work, we also observed a displacement of cell bodies of JGNs during a 4-h-long observation time window (e.g., Figure 2a in ref. 20), but were unable to perform long-term single-cell tracking in acute in vivo experiments.
There are two main challenges for long-term single-cell tracking in the OB. One is to mark individual cells with unique tags; the other is the lack of a stable landmark that can be visualized repetitively and conveniently.
In this study, we have overcome these obstacles by introducing a new approach, “optical cell positioning system” (oCPS), allowing an accurate monitoring of the positions of many individual cells over days and weeks of their migration within the OB. In contrast to what was assumed previously (see above), the long-term single-cell tracking reveals, for the first time, the dynamic radial migration of the GCs and JGNs, and a unique switch of migration patterns in adult-born JGNs: from radial to long-range lateral migration. Thus, our findings shed new light on the behavior of adult-born neurons before their integration in the pre-existing neural network.
Results
The use of oCPS for long-term in vivo tracking of individual cells
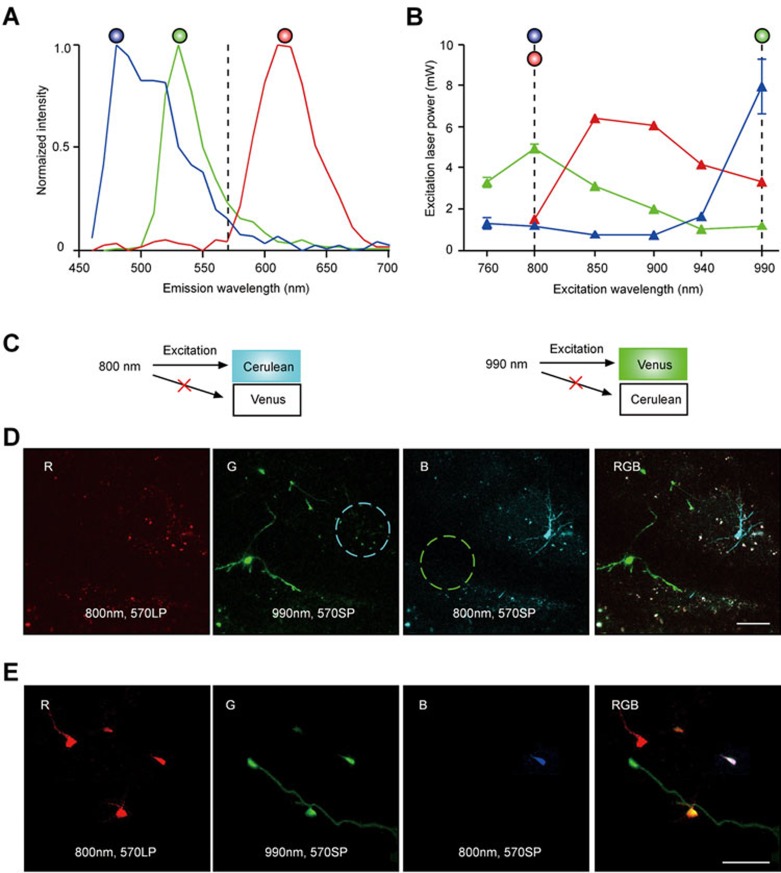
Specific multicolor labeling of individual adult-born neuroblasts was achieved using red-green-blue (RGB) cell-marking approach, utilizing simultaneous, viral vector-mediated expression of genes encoding fluorescent proteins (FPs) in the three basic colors mCherry (red), Venus (green) and Cerulean (blue)21. To enable monitoring of RGB-marked cells by means of in vivo two-photon imaging, we examined the excitation/emission spectra of each fluorophore. Because of an overlap between the emission spectra of Venus and Cerulean (Figure 1A), excitation splitting was used to differentiate between these two dyes (Figure 1B) so that the sequential scanning of the specimen with 800- and 990-nm excitation light allowed the acquisition of non-overlapping fluorescence signals from each of the three FPs (Figure 1C). We first tested this strategy in vitro in HEK-293 cells (Supplementary information, Figure S1A) and then in vivo after retroviral labeling of the adult-born cells in the RMS (Figure 1D and 1E). Under our setting (emission: short pass 570 nm; excitation: either 800 nm (Cerulean) or 990 nm(Venus)), there was a clear distinction between fluorescence signals from Cerulean and Venus (Figure 1D). Adding the red channel (emission: long pass 570 nm; excitation: 800 nm), we were able to perform signal collection from the three RGB fluorophores in a time-efficient way (Figure 1E).
Figure 1.
Separation of fluorescence signals emitted by mCherry, Venus and Cerulean into red, green and blue channels, respectively, by means of two-photon microscopy. (A) Emission spectra of the three RGB fluorophores measured in HEK-293 cells expressing one of the three fluorophores. The spectra of mCherry, Venus and Cerulean are plotted in red, green and blue, respectively. Dashed line at 570 nm shows where the dichroic mirror splits emission light. (B) Excitation spectra of RGB fluorophores. Venus, mCherry and Cerulean were expressed separately in HEK-293 cells and the laser power that causes a given fluorescence intensity (fixed at 1 400 arbitrary units) of each FP was measured and plotted against the excitation wavelength. Color dots indicate the excitation wavelengths selected for acquisition of fluorescent signals from different FPs in this study. (C) Schematic illustration of the findings in D: 800 nm efficiently excites Cerulean but not Venus, whereas 990 nm efficiently excites Venus but not Cerulean. This provides the basis for splitting the fluorescence of the two fluorophores based on their excitation wavelength (see an example in D). (D) In vivo maximal intensity projection (MIP) images (54-82 μm, step 2 μm) showing two adult-born JGNs in the same field of view (FOV) expressing Cerulean and Venus, respectively. The red channel is empty (left most image), because there is no cell expressing mCherry in this FOV. The right most image (RGB) is a merge of the other three images. Scale bar, 50 μm. (E) MIP images (16-68 μm, step 2 μm) illustrating the fluorescent signals from a few RGB-marked adult-born JGNs acquired as described in D. Scale bar, 50 μm.
Next, we addressed the issue of landmarks in the brain. Blood vessels have been routinely used for this purpose, but traditional angiography involves the intravenous injection of fluorescent dyes22,23, which cannot be performed on a daily basis easily for long-term cell tracking. We thus used the intraperitoneal (i.p.) route for administration of fluorescent dyes20,24. Examination of a few fluorescent dyes led us to choose sulforhodamine B, which can be effectively excited by two-photon laser and can be cleared from the body in a few hours, allowing enough acquisition time20 while minimizing the potential side effects, if there are any. To make angiography compatible with RGB marking, we chose 900 nm for simultaneous excitation of sulforhodamine B, Venus and Cerulean (Supplementary information, Figure S1B-S1D). As adult-born cells positive only for mCherry constitute only 7.2 ± 9.9% of the whole labeled cell population (see below), the position information for the majority of RGB-marked cells could still be obtained. Thus, the sequential scanning of the OB with 800, 990 and then 900 nm light (after i.p. sulforhodamine B injection) generates sufficient spectral and positional information for long-term single-cell tracking, constituting the oCPS.
oCPS reveals an active migration of adult-born neurons in the OB
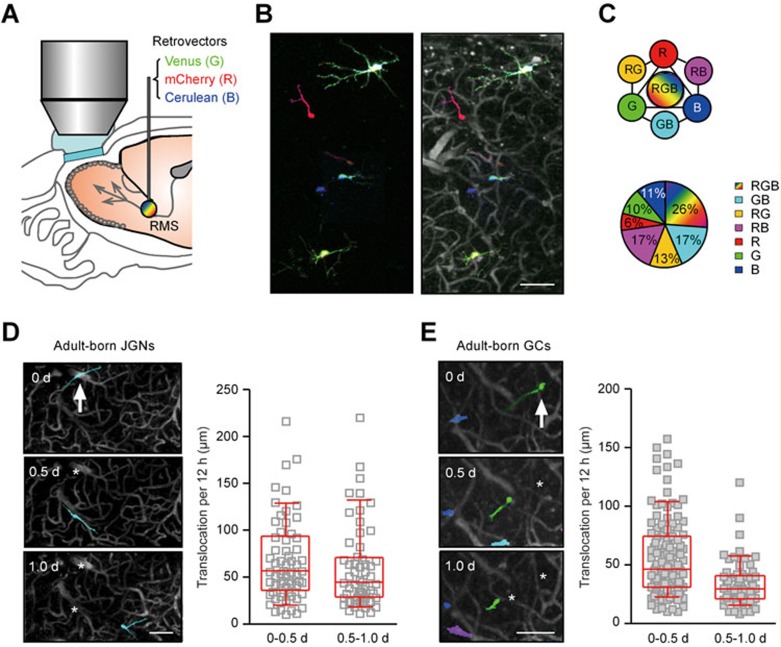
To evaluate the effectiveness of oCPS for tracking of adult-born cells in the OB, the mixture of the RGB retroviral vectors was injected into the RMS (Figure 2A), where adult-born cells are actively dividing18,20,25. Stochastic transduction of cells by the three retroviruses labeled them in one of seven easily distinguishable colors (three basic colors: red, green blue; four mixed colors: yellow, cyan, violet and white; Figure 2B and 2C), whereas angiography allowed us to identify the position of each RGB-marked cells (Figure 2B, right). The relative fraction of cells labeled with a given color depends on many factors including viral titer, diffusion properties after injection, cell cycle status, expression of anti-viral restriction factors, etc.26,27. Data collected from 6 mice (34-136 cells per mouse) indicated that under our experimental conditions, around a quarter of FP-positive JGNs were triple-labeled (23.7 ± 3.6%, mean ± SEM), 6-19% double-labeled (GB 18.9 ± 15.2%; RB 6.5 ± 11.3%; RG 12.2 ± 6.6%) and 7-16% were labeled by a single color (G 9.0 ± 7.5%; B 16.2 ± 15.3%; R 7.2 ± 9.9%; Figure 2C). The sparse nature of the intra-RMS infection of adult-born cells with retroviruses (e.g., Figure 2) further improved the fidelity of cell tracking. In addition to the spectral information (color ID), a few other clues were used for recognition of the same cells in consecutive imaging sessions, such as the soma size, morphology of processes and the positions of stationary cells in the neighborhood. Thus, through sequential collection of spectral and positional information for each RGB-marked cell, each cell received a color hue as well as coordinates in 3D in relation to its surrounding landmarks.
Figure 2.
Visualization of the migratory behavior of adult-born neurons by oCPS. (A) Schematic drawing of the experimental setup. The mixture of concentrated retroviruses encoding the three fluorescent proteins (Venus as green, mCherry as red and Cerulean as blue) at the ratio of 1:1:1 in terms of infectious titer was injected into the mouse RMS for transduction of adult-born neurons in mice with chronic cranial windows. Transduced cells were imaged every 12 h starting from DPI 6 by means of two-photon microscopy. (B) Left: a MIP image (40-110 μm depth, step 2 μm) of RGB-labeled cells was generated by overlaying three images of basic colors red, green and blue acquired with the pre-determined settings (see Materials and Methods). Right: blood vessels were labeled with sulforhodamine B (i.p. injection) and the image of RGB-marked cells (left) was overlaid with blood vessel pattern to obtain a landmark reference for each cell. (C) Upper: schematic drawing illustrating the seven colors used for marking individual cells in this study. Lower: an example pie chart showing the fraction of adult-born neurons labeled by these colors (out of 111 cells from one of the 6 RGB-marked mice). (D) Left: a MIP image (70-120 μm depth, step 2 μm) of an adult-born JGN (arrow) migrating in the GL of the OB. Day 0 is the day of cell's arrival at the GL. Here and in E: asterisks mark previous locations of the cell. Right: plots showing the distance between the two consecutive positions of the cells during their first 12 h (0-0.5 d) or second 12 h (0.5-1.0 d) in the GL (translocation per 12 h; n = 65 cells, 5 mice). Here and in E: medians ± interquartile ranges are shown in red. (E) Left: a MIP image (216-336 μm depth, step 2 μm) of an adult-born GC (arrow) migrating in the GCL of the OB. Day 0 is the first day when adult-born GC was observed in the GCL. Right: the same graph as in D, but for data obtained from adult-born GCs (n = 141 cells, 5 mice). Scale bars, 100 μm.
Using oCPS, the RGB-labeled cells were imaged every 12 h starting from 6 days post injection of the virus mixture (DPI 6). We measured a net displacement of the cell's soma in 12 h (i.e., the migration speed) and found that during the first few days after arrival at their destination layers, the migration speed of adult-born cells was high. Adult-born JGNs, for example, migrated 54.8 ± 57.8 μm and 43.7 ± 42.9 μm during 0-0.5 and 0.5-1.0 day in the layer, respectively (Figure 2D, n = 65 cells, 5 mice). Under similar recording conditions, adult-born GCs traversed 45.6 ± 43.2 and 28.4 ± 19.0 μm during 0-0.5 and 0.5-1.0 day, respectively (n = 141 cells, 5 mice; Figure 2E). It should be noted, however, that due to the limited depth penetration of our imaging system, adult-born GCs were first visualized in the middle of the granule cell layer (GCL; ∼350 μm below the dura). Together, oCPS proved to be a reliable single-cell tracking platform, and has revealed the active migration of adult-born cells in the OB upon arrival in their target layers.
Adult-born JGNs switch from radial to lateral migration in the GL
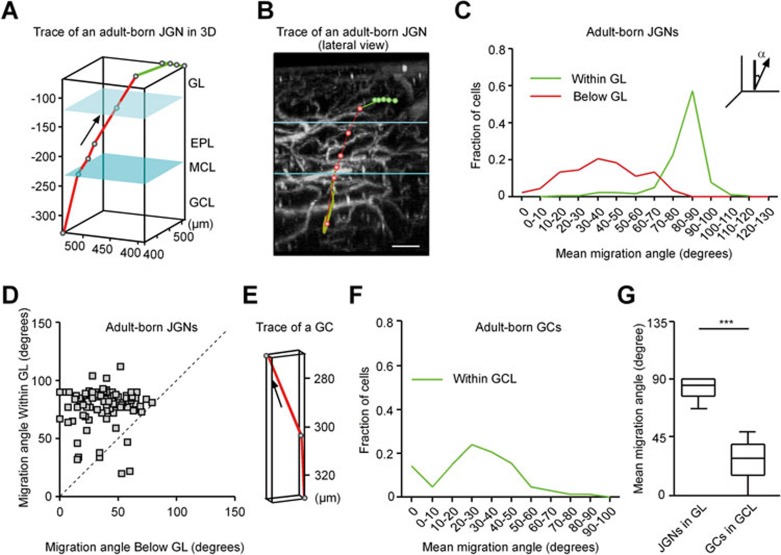
To characterize migration features of individual cells in more detail, we plotted the migration trajectory of adult-born cells with dots representing the cell's positions during consecutive imaging sessions (Figure 3A, 3B and 3E, and Supplementary information, Figure S2). When plotting the mean migration angle (defined in Figure 3C) for each JGN, we found that in the GL 97.3% of adult-born JGNs examined (n = 225 cells) migrate at mean angles between 50° and 130° with a clear peak at 80°-90°. These data indicate that lateral migration is the preferred migration mode of adult-born cells within the GL. Below the GL, JGNs (n = 92 cells) migrated at much sharper angles (Figure 3A-3C). Paired comparison of mean migration angles of the individual JGNs below and within the GL showed that the mean migration angle within the GL was significantly higher compared with the mean migration angle below GL (Figure 3D; P < 0.001, Wilcoxon matched pairs test), suggesting that adult-born JGNs switch from radial to lateral migration after entering the GL.
Figure 3.
Migration angles of adult-born cells in the OB. (A, B) An example migration trajectory of an adult-born JGN. The green line indicates the trace in the GL and the red line indicates the trace below the GL or upon entering the GL. EPL, external plexiform layer; GCL, granule cell layer; GL, glomerular layer; MCL, mitral cell layer. MCL is in cyan and the boundary between GL and EPL is in light cyan. The cell was false colored in grass-green color. Dots represent the cell's positions during consecutive imaging sessions. Scale bar, 100 μm. (C) Distributions of mean migration angles (α) of adult-born JGNs, as they migrate within the GL or below the GL (including entry steps into the GL); n = 225 cells for within the GL and 92 cells for below the GL, 5 mice. Migration angle is defined as the angle between vertical line and the migration vector in space (see inset). Mean migration angle for each cell is calculated based on all the migration steps of the cell within the given layer. (D) Paired comparison of mean migration angles of the individual JGNs below and within the GL (n = 92 cells, 5 mice). Each data point represents one cell with complete migration history. The dashed line is the identity line (x = y). (E) An example migration trajectory of an adult-born GC. (F) Distribution of mean migration angles of adult-born GCs migrating in the GCL (n = 144 cells, 5 mice). (G) Box plot comparing mean migration angles of JGNs in the GL and GCs in the GCL (values are shown as median ± interquartile range; n = 225 JGNs, 144 GCs from 5 mice; ***P < 0.001, Mann-Whitney test).
In contrast, adult-born GCs underwent only radial migration (Figure 3E). The majority (91.7%) of adult-born GCs migrated at mean angles between 0 and 60°, with a distribution of migration angles centered at 20°-30° (Figure 3F). Comparison of migration angles between JGNs migrating in the GL and GCs migrating in the GCL confirmed that the former population migrated at almost right angles (85.0 ± 13.0°, n = 225 cells, 5 mice) and the latter at sharp angles (28.7 ± 24.3°, n = 144 cells, 5 mice), with a significant difference between the two (Figure 3G; P < 0.001, Mann-Whitney test).
Together, these results clearly show that adult-born JGNs undergo a switch in the pattern of their migration: from radial migration below GL to lateral migration within GL, whereas adult-born GCs only migrate radially up to their target locations in the GCL.
Lateral migration patterns of adult-born JGNs in the GL of the OB
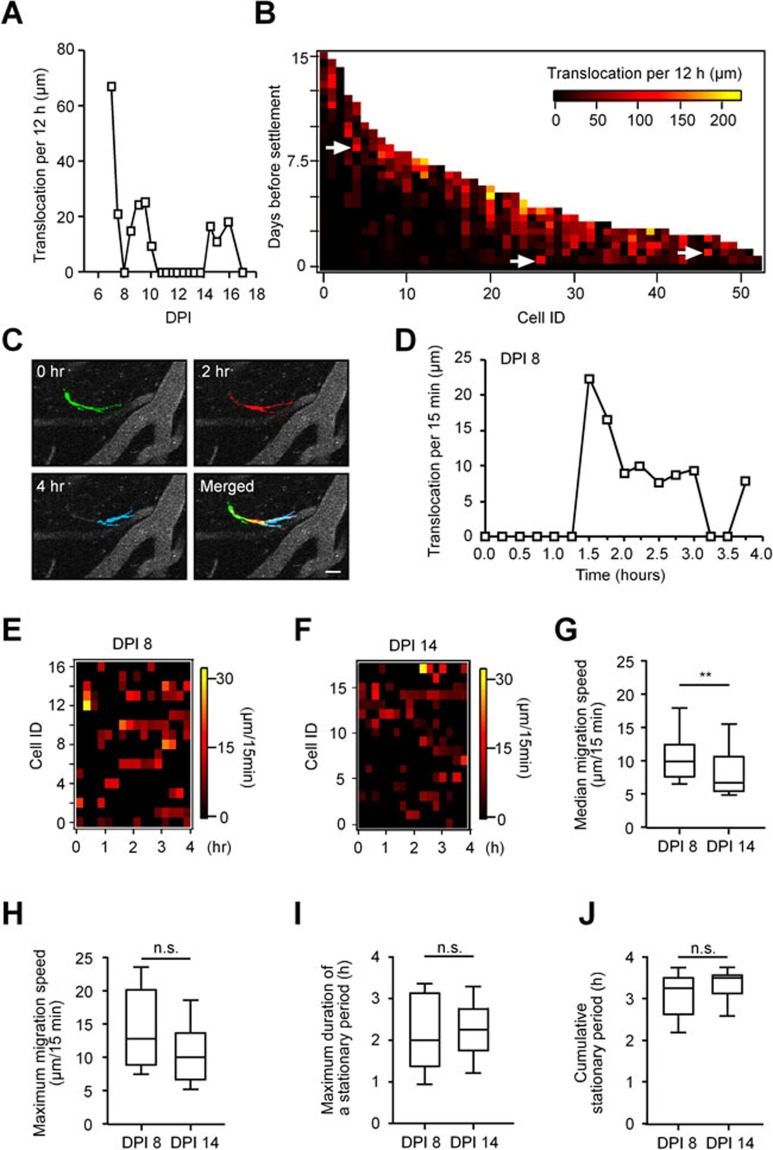
Next, we examined the lateral migration pattern of adult-born JGNs in more detail. Out of 213 cells within the fields of view (FOVs) between DPI 6 and 60 (data set acquired ≥ every 12 h), 51.3 ± 5.1% (mean ± SEM, n = 5 mice) displayed saltatory migration patterns, defined as alternating fast moving and stationary periods (Figure 4A and 4B10). The remaining cells always changed their position between the imaging sessions. Sixty-two out of 213 cells stayed within the FOVs during the entire recording period, whereas the others did not (likely moved out of the FOV or died). Of these 62 cells (Figure 3B), 64.2 ± 13.0% showed saltatory migration.
Figure 4.
Temporal migration pattern of adult-born JGNs. (A) Migration speed (translocation per 12 h) of an example adult-born JGN plotted against time (DPI). Note the stop-and-go behavior of the cell. (B) 2D plot of the migration speed for adult-born JGNs, which settled down within the FOVs (n = 62 cells, 5 mice), on different days before their settlement. Arrows point to the high speed phase of a few JGNs. (C) False-colored single-frame images of an adult-born JGN and surrounding blood vessels (DPI 8, 30 μm below the dura) taken at different time points as indicated (see timestamp, relative time). Scale bar, 20 μm. (D) Distance traversed by the cell shown in C during consecutive 15-min-long time intervals plotted as a function of time. (E, F) 2D plots of the migration speed (translocation per 15 min) of adult-born JGNs on DPI 8 (E, n = 17 cells, 5 mice) and on DPI 14 (F, n = 18 cells, 4 mice). (G, H) Box plots showing the median migration speed (G) and maximum migration speed (H) of cells shown in E and F. Median migration speed was significantly different between the two age groups (Mann-Whitney test, **P ≤ 0.01). No significant difference was found between the two groups in terms of maximum migration speed (P = 0.1). (I) Box plot showing the maximum duration of a stationary period during the period of 4 h. No significant difference was found between DPI 8 and DPI 14 (Mann-Whitney test, P = 0.8). (J) Box plot showing the cumulative stationary period for each cell during the 4-h-long observation time window. No significant difference was found between DPI 8 and DPI 14 (Mann-Whitney test, P = 0.2). In G-J, all values are shown as median ± interquartile range. Data shown in E-J belong to the same data set.
To get a closer insight into the temporal migration pattern of JGNs, we imaged them every 15 min over 4 h on 8, 14, 20 and 30 DPI in another set of experiments (Figure 4C-4J). Out of 49 cells imaged on DPI 8, only 34.5 ± 17.5% of cells (n = 5 mice) changed their position during the 4-h-long recording period, consistent with the notion that cells can remain still for hours and days (Figure 4A and 4B). On DPI 14, these were only 14.0 ± 4.1% of cells and very few cells were moving at later time points (DPI 20-30, see below). Of note, both at DPI 8 and 14, all migrating cells (n = 17 and 18, respectively) showed saltatory migration pattern, characterized by alternating stop-and-go phases (Figure 4D-4F). The migration speed of individual cells varied between 4.8 and 28.8 μm/15 min, with median (Figure 4G, P ≤ 0.01, Mann-Whitney test), but not maximum (Figure 4H, P = 0.1, Mann-Whitney test) migration speed decreasing significantly between DPI 8 and DPI 14. At the same time, there was no significant difference in the maximum duration of individual stationary periods (Figure 4I) as well as the cumulative duration of a stationary phase within the 4-h observation window (Figure 4J) between the two age groups (P = 0.83 and 0.24, respectively, Mann-Whitney test). Note, however, that cells of both age groups spent > 80% of their time at rest (DPI 8: 81 ± 22%, n = 17 cells, 5 mice; DPI 14: 88 ± 11%, n = 18 cells, 5 mice). In the remaining time, the cells were migrating at a median speed of 9.9 ± 4.8 μm/15 min (DPI 8) and 6.7 ± 5.2 μm/15 min (DPI 14, Figure 4G). Taking into account the long resting intervals of migrating JGNs and the fact that 100% of cells migrating during the 4-h-long recording period showed saltatory migration pattern, we concluded that saltatory migration is the main temporal migration pattern of adult-born JGNs within the GL.
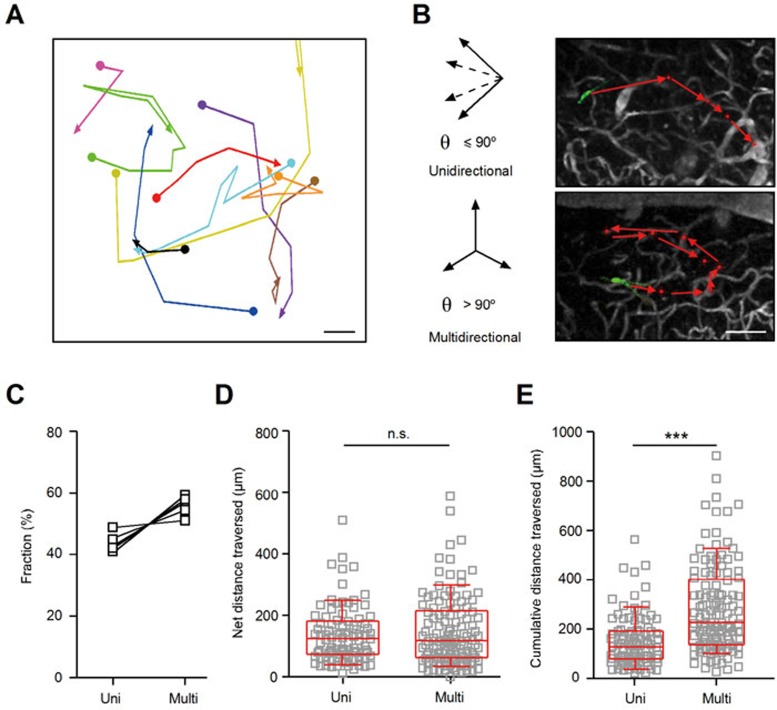
The spatial migration patterns of individual adult-born JGNs were highly variable (Figure 5A and Supplementary information, Figures S2, S3A-S3C). For quantification, we used two different procedures: first, quantifying the maximal angle (θ) between the two immediate migration steps and second, quantifying the ratio between the net (the distance between the cell's entry point in the GL and its final location) and total distances traversed by the cell. For the first method, if θ was ≤ 90°, the migration of the cell was categorized as unidirectional (Figure 5B, upper panel); otherwise, as multidirectional migration (Figure 5B, lower panel). The unidirectional group included cells showing little change in the direction of their movement, whereas the multidirectional cells underwent abrupt changes in the direction of the migration. On average, 43.7 ± 1.5% (mean ± SEM, n = 93/213 cells in 5 mice) of migrating cells were unidirectional and the remaining 56.3 ± 1.5% were multidirectional (Figure 5C). Of these, saltatorily migrating cells constituted 60.8 ± 4.8% and 41.6 ± 4.4% of multidirectionally and unidirectionally migrating groups, respectively, pointing towards preferred saltatory behavior of multidirectionally migrating cells (Student's t-test, P < 0.05, n = 5). Among the cells that remained within the FOVs for the entire recording period (62 cells, 5 mice), 55.5 ± 5.8% and 44.5 ± 5.8% were multidirectionally and unidirectionally migrating cells, respectively. These numbers are similar to the ones obtained for general population (paired t-test, P = 0.9 for either migratory behavior).
Figure 5.
Spatial migration pattern of adult-born JGNs. (A) Migration history of 10 JGNs in an example FOV (top view). Each trace is shown in a different color. Scale bar, 50 μm. (B) Examples of two different types of migration trajectories of adult-born JGNs. Upper: unidirectional migration, the maximal migration angle (θ) between the two immediately adjacent migration steps is ≤ 90°. Lower: multidirectional migration, θ > 90°. Scale bar, 100 μm. (C) Fraction of uni- or multidirectionally migrating cells (n = 5 mice, 15-69 cells per mouse). Solid lines connect the data obtained in the same animal. (D) Comparison of net migration distance (distance between the entry sites into the GL and the final cell's position) between unidirectional and multidirectional JGNs (n = 93 and 120 cells, respectively, 5 mice). No significant difference was found by Mann-Whitney test (P = 0.99). (E) Comparison of total migration distance (the cumulative distance traveled in GL) between unidirectional and multidirectional cells (n = 93 and 120 cells, respectively, 5 mice). Significantly longer cumulative distances were found for cells with multidirectional migration; ***P < 0.001, Mann-Whitney test. All values are shown as median ± interquartile range.
In the second categorizing method (established by Nam et al.15), cells were subdivided into “migratory”, “exploratory” and “intermediate” according to the ratio of the net to the total distance traversed (migratory: 0.6-1.0; exploratory: 0.0-0.4; intermediate: 0.4-0.6). Out of 213 recorded cells (n = 5 mice), 62.7 ± 3.2% were “migratory”, 16.5 ± 3.5% “intermediate” and 20.5 ± 2.4% “exploratory” cells (mean ± SEM, Supplementary information, Figure S3D). We found that there was no significant difference in the net migration distance between unidirectional (Figure 5D, 120.0 ± 106.5 μm, n = 93 cells, 5 mice) and multidirectional cells (114.0 ± 149.5 μm, n = 120 cells, 5 mice, P = 1.0, Mann-Whitney test). However, the cumulative (total) distance (summation of all migration steps in the GL) in multidirectional group (225.0 ± 263.5 μm, n = 120 cells, 5 mice) was significantly larger than that in unidirectional group (Figure 5E, 133.0 ± 112.0 μm, n = 93 cells, 5 mice, P < 0.001, Mann-Whitney test).
When comparing the two categorizing methods, we found that virtually all cells (97.2 ± 2.0%) in unidirectional migration group were “migratory”, with a small fraction (2.8 ± 2.0%) going to the “intermediate” group (Supplementary information, Figure S3E). None of them were “exploratory” cells. In contrast, cells in multidirectional migration group were scattered among “migratory”, “intermediate” and “exploratory” groups (35.2 ± 4.7%, 27.7 ± 4.9% and 37.1 ± 4.4%, respectively; n = 5 mice).
Taken together, these results show that adult-born JGNs undergo stereotyped migration patterns during their lateral movement in the GL: saltatory migration on the temporal scale, and unidirectional or multidirectional migration on the spatial scale. Although the total distances traversed amounted to 900 μm, the cells usually settled down about 1-2 glomeruli apart from the point where they initially entered the GL.
Odor deprivation does not affect the migration of adult-born JGNs
Having characterized the lateral migration pattern of adult-born JGNs in the GL, we next asked whether any of the migration parameters (speed, pattern and distance) are sensitive to global changes in the sensory input. To answer this question, we used unilateral naris closure on DPI 6 and compared the migration properties of adult-born JGNs in the odor-deprived (OD) side with the non-OD side as control. The OD group included only those bulbs (n = 4), in which naris occlusion resulted in a significant decrease in the expression of tyrosine hydroxylase (TH), confirmed immunohistochemically (Supplementary information, Figure S4A and S4B). Despite a clear reduction in TH expression in OD bulbs, a known consequence of the naris closure28,29, there was no significant difference in migration speed between the cells in control and OD groups (Supplementary information, Figure S4C). Similarly, we did not observe any difference in mean migration angles (Supplementary information, Figure S4D), the fraction of cells with uni- or multidirectional migration patterns (Supplementary information, Figure S4E), and net or cumulative distances that cells traveled in the GL (Supplementary information, Figure S4F). Taken together, these data suggest that the pattern of lateral migration of adult-born JGNs is not sensitive to an overall decrease in the strength of sensory input.
Layer-specific migratory behavior of adult-born neurons
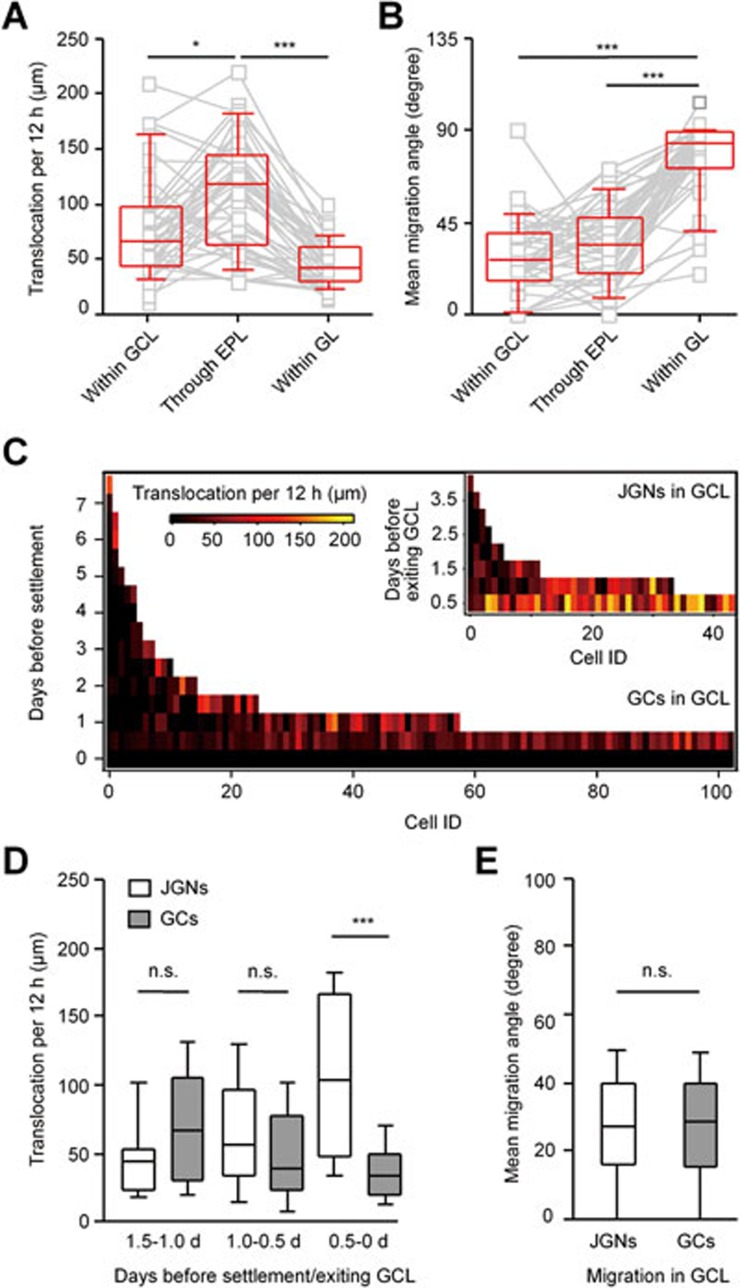
We next focused on the migration of adult-born neurons below GL: in the external plexiform layer (EPL) and in the GCL (Figure 6). We analyzed cells that were first identified when migrating inside the GCL and then monitored until they took up their final position in either the GCL or the GL (e.g., Supplementary information, Figure S2). First, we compared the mean migration speed of cells, destined to become JGNs, in the three different layers of the OB: GCL, EPL and GL. Neuroblasts migrating towards GL moved significantly faster in the EPL than in the two other layers (median migration speed through EPL: 118.0 ± 82.0 μm/12 h, within GCL: 66.0 ± 53.0 μm/12 h and within GL 41.0 ± 30.0 μm/12 h; Figure 6A). As for the migration angle, there was no significant difference between GCL and EPL, in which cells migrated rather vertically (within GCL: 27.0 ± 24.0°, through EPL: 34.9 ± 27°, P = 0.1, Kruskal-Wallis test followed by Dunn's multiple comparison test; Figure 6B). However, the migration angle in the GL (84.0 ± 18.0°) was significantly different from the former two (P < 0.001, Kruskal-Wallis test followed by Dunn's multiple comparison test, n = 43 cells with paired data, 4 mice), consistent with the lateral migration of adult-born cells in the GL (see also Figure 3C for non-paired data sample).
Figure 6.
Adult-born neurons show different migration speed across layers of the OB. (A) Comparison of median migration speed of the same JGNs in different layers of the OB. Paired data were obtained from the same cells migrating Within GCL, through EPL (between MCL and GL) or within GL (n = 43 cells with recorded trace inside GCL, 4 mice, **P < 0.05, ***P < 0.001, Kruskal-Wallis test followed by Dunn's multiple comparison test). Here and in B for cells, which made more than one migration step within the layer, the entries represent the mean values. (B) Comparison of mean migration angles measured as cells migrated through different layers of the OB. Same data set as in A; ***P < 0.001, Kruskal-Wallis test followed by Dunn's multiple comparison test. (C) 2D plots of the migration speed (translocation per 12 h) of JGNs and GCs in the GCL on different days before settlement (for GCs) or before leaving GCL (n = 43 JGNs, 4 mice, and 103 GCs, 5 mice). (D) Comparison of migration speed of JGNs and GCs on different time points before exiting GCL (for JGNs) or settlement (for GCs). Same data set as in C; P = 0.3 (Mann-Whitney test) for comparison between the JGNs and GCs on 1.5-1.0 d; P = 0.1 on 1.0-0.5 d and ***P < 0.001 on 0.5-0 d. (E) Migration angles of JGNs and GCs in GCL; P = 1.0, Mann-Whitney test; for JGNs, n = 43 from 4 mice; for GCs, n = 144 from 5 mice. Values are shown as median ± interquartile range.
Next we asked whether the cells going to become either adult-born GCs or JGNs display different migratory behavior when migrating in the GCL. To answer this question, we compared the speed and migration angles of the two cell types. For adult-born GCs, the day they arrived in their final position was defined as day 0, whereas for adult-born JGNs, day 0 was the day they exited GCL. It turned out that 1.5-0.5 days before settlement or exiting GCL both cells population had very similar migrating speed (Figure 6C and 6D). However, on day 0.5-0 GCs slowed down, whereas JGNs speeded up to exit the GCL. This opposing trend resulted in a significant difference in the migration speed between the two cell populations (Figure 6D, P < 0.001, Mann-Whitney test). As for mean migration angle, no difference was found between JGNs and GCs when they were migrating in the GCL (Figure 6E).
These results suggest that future granule and juxtaglomerular cells show similar migration properties in the GCL. The differences are seen only during the last 12 h, when the decision to settle down and become a GC or to speed up and leave the GCL becomes obvious.
Gradual settlement of adult-born neurons in the OB
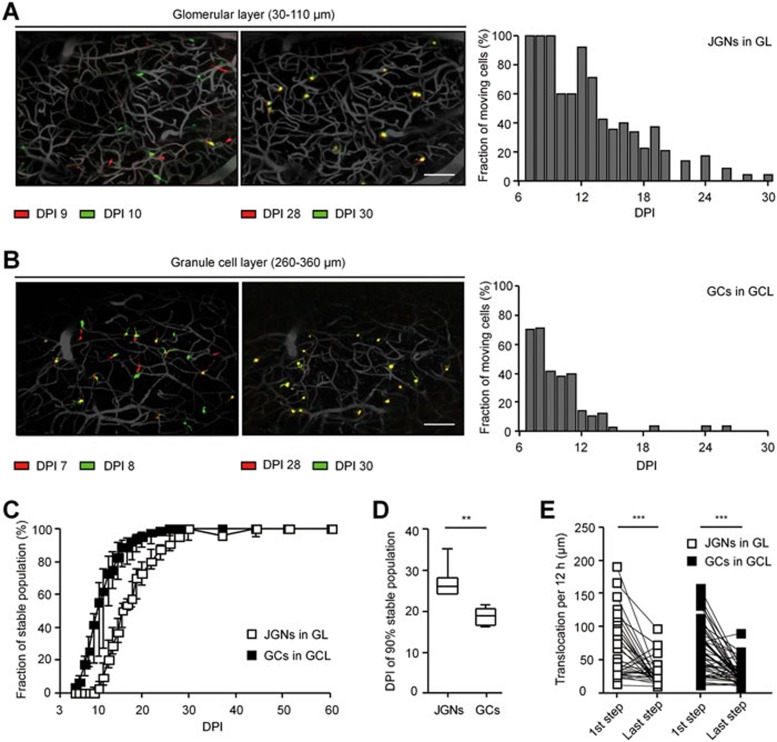
Next, we examined the general motility of the population of adult-born neurons over time. Soon after arrival of the cells at their destination layer (Figure 7A and 7B; left panels) the majority of adult-born cells were mobile, changing their position between the imaging sessions. Plotting the fraction of moving cells over time clearly showed a gradual decrease in the motility of both adult-born JGNs (Figure 7A, right panel) and GCs (Figure 7B, right panel). As the fraction of mobile cells was < 5% from DPI 30 onwards, we defined cell populations at DPI 30+ as stable populations and plotted the fraction of stable population as a function of time (Figure 7C). We observed a gradual accumulation of stable population both for JGNs and GCs. However, the adult-born GCs seemed to settle down significantly earlier than JGNs (Kolmogorov-Smirnov test, P ≤ 0.05). The time required to achieve 90% of stable population was significantly shorter for GCs compared with JGNs (Figure 7D, DPI 18.0 ± 5.75 days for GCs versus DPI 26.0 ± 4.0 days for JGNs, Mann-Whitney test, P < 0.01).
Figure 7.
Motility of adult-born cells gradually decreases with time spent in the destination layer. (A) Overlay of adult-born JGN positions recorded at DPI 9 and DPI 10 (left), as well as at DPI 28 and DPI 30 (middle). MIP images from 30 to 110 μm, step 2 μm. Scale bar, 100 μm. Right: a graph showing the fractions of moving cells at different DPIs. (B) Similar data as in A but obtained for adult-born GCs. MIP images from 260 to 360 μm, step 2 μm. Scale bar, 100 μm. (C) The fraction of stable cells plotted as the function of time (DPI). Median with one interquartile range is shown on each time point (n = 6 FOVs from 4 mice for GCs and n = 11 FOVs from 4 mice for JGNs). (D) Box plot illustrates the day when accumulation of 90% of the stable population is achieved. All values are shown as median ± interquartile range. Same data set as C, **P < 0.01, Mann-Whitney test. (E) Paired comparison of the first and the last migration steps of individual adult-born JGNs (n = 32 cells, 5 mice) and GCs (n = 48 cells, 5 mice) in their destination layers; ***P < 0.001, Wilcoxon matched pairs test. The median size of the first and the last step was also compared between JGNs and GCs. Mann-Whitney test, P = 0.9 and 0.8, respectively.
At the level of individual adult-born cells, we compared the length of the first migration step within the destination layer with the length of the last migration step before settlement and found significant differences between the two for both JGNs and GCs (Figure 7E). No significant difference was found between the length of either first or last step when comparing JGNs with GCs (Mann-Whitney test, P = 0.92 and 0.76, respectively).
Taken together, our data show that adult-born neurons gradually decrease their migration speed as they mature and settle down to form a stable population. In mice, this process lasts ∼4 weeks for JGNs and ∼3 weeks for GCs.
Discussion
A significant challenge for long-term in vivo cell tracking is to follow the same cells at different time points (identity), and to precisely trace the cell's position (location). The oCPS introduced here addresses both issues, enabling monitoring of the behavior of individual cells in their native environment over prolonged periods of time (weeks to months). To quantify the fidelity of the RGB marking with seven easily distinguishable colors, we used JGNs as an example. First, we examined the distribution of the distances between the two consecutive positions of each juxtaglomerular cell (step distance) for all imaging sessions (Supplementary information, Figure S5). The median distance was 41.95 ± 49.95 μm (interquartile range, IQR), with only 10% of step distances being larger than 109 μm. Next, we determined the fraction of cells having the same color code (out of 7 colors mentioned above) and located at the same DPI in the same FOV at the distance smaller than 109 μm. Out of the total 225 cells analyzed in this study, we found 16 cases (16 pairs of cells, i.e., 32 cells), amounting to 14.2% of the total population. We thus conclude that for the vast majority of cases (85.8%) the RGB marking with seven distinct colors allows precise tracking of individual cells. In fact, the deceleration of the cells in the course of migration (Figures 4A and 7E) and existence of a number of additional criteria (e.g., the soma size, the direction of the leading process as indicator of migration direction, the ratio of the fluorescence recorded in different channels (proportional to the number of incorporated virus copies and the strength of FP expression) and the existence of stationary cells in the neighborhood) reduce the ambiguity in the system even further. It has to be noticed that the RGB-marking approach is not restricted to seven colors and, as shown previously21,26, can provide a large number of different color hues. The precise readout of these hues, however, requires more sophisticated imaging and color-identification approaches, which seem unnecessary within the framework of the present study. Importantly, the oCPS is not restricted to RGB marking, it is possible to adapt this strategy to other labeling methods for identification of individual cells, such as Brainbow30 and MAGIC31. Furthermore, the oCPS has the full potential to be applied to other regions of the brain, and the body, making it a promising approach for long-term tracking of cells of other lineages.
Our novel experimental approach enabled us to identify migrating cells deep within the OB and to follow their migration until arrival at their final destination within the GCL or the GL. Our data (graphically summarized in Figure 8) suggest that immediately after exiting RMS, all adult-born neurons are very similar in their migratory behavior. They migrate radially towards the surface of the bulb with future GCs and JGNs migrating at similar speeds. The difference in the migratory behavior of the two cell types first becomes evident when adult-born JGNs are about to leave the GCL: JGNs considerably speed up, whereas the adult-born GCs slow down and eventually settle within the GCL. The JGNs traverse the EPL at the highest speed observed during the entire migration period monitored in this study, slow down when entering GL, and switch to lateral migration. Although the lateral migration of the adult-born JGNs within the GL is similar to tangential migration of immature GABAergic interneurons during embryonic development10, we use the word lateral to avoid misunderstanding, because the word tangential is widely used for migration of neuronal precursor cells in the RMS4.
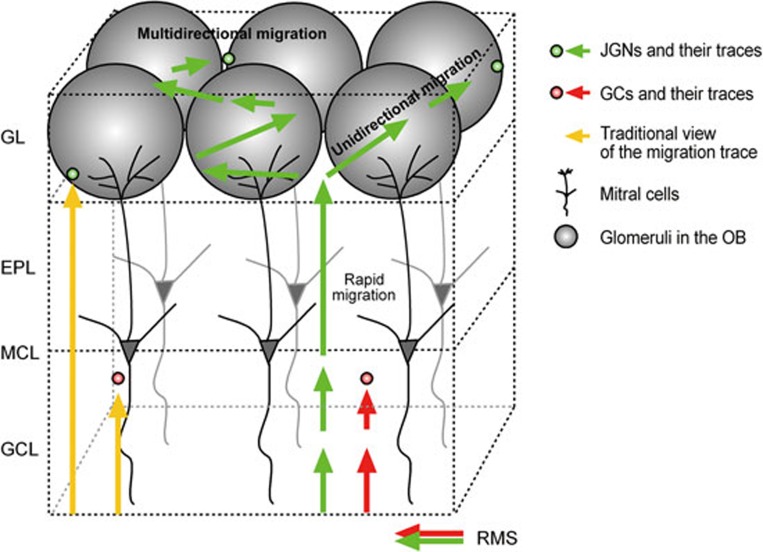
Figure 8.
An updated model of adult-born cell migration in the olfactory bulb. Adult-born cells within the bulb were previously thought to undergo radial migration only (yellow arrows). The migration speed was unknown and therefore assumed to be homogenous. In this study, we found that pure radial migration is seen only for adult-born granule cells (GCs). Adult-born JGNs first migrate radially together with GCs in the GCL (red arrows for GCs, green arrows for JGNs). Whereas GCs slow down to integrate into the GCL, the JGNs speed up to quickly traverse EPL. When reaching the GL adult-born JGNs switch to lateral migration, moving either unidirectionally or multidirectionally. They gradually slow down, integrating some 1-2 glomeruli away from their initial entry site.
Our direct, in vivo observation of adult-born neuroblasts' behavior uncovered a switch from radial to lateral migration for adult-born JGNs and the long-range lateral migration thereafter. This differs from what can be inferred from the literature, which postulates that adult-born cells (JGNs as well as GCs) either undergo radial migration only3,4,9 or first migrate predominantly to the GL before populating the GCL18. In contrast to what could be inferred from the in vitro data15, our results demonstrate a surprisingly vigorous migration of the adult-born JGNs in the GL. Around 40% of adult-born JGNs undergo unidirectional migration without abrupt changes in migration direction, ending up somewhere one-to-two glomeruli away from the initial entry points. Unidirectional migration is similar to the directed migratory behavior of neuroblasts in the RMS of juvenile/adult mice15, albeit happening at a slower speed (see below). It is therefore not surprising that 97.3 ± 2.0% of unidirectional cells showed migratory behavior, when analyzed according to the criteria used by Nam et al.15. The remaining 60% of adult-born JGNs underwent multidirectional migration, traveling up to 900 μm in cumulative distance, before they also settled down one-to-two glomeruli away from the initial entry points (Figure 8). The multidirectional migration mode is reminiscent of the “multipolar” migration mode described for the embryonic cerebral cortex32. In both cases, the cells migrate in various directions and change their direction frequently, but generally move away from their original location. This differs from the exploratory behavior of neuroblasts in the neonatal, juvenile and adult SVZ11,15, where the frequent direction change results in large cumulative but rather small net displacement of the cell body. Consistently, only 20.5% of JGNs were exploratory under our experimental conditions. The multidirectional migration mode seems to be a hallmark of adult-born JGNs, as we have never observed multidirectional migration in adult-born GCs. The latter migrated radially without direction change (unidirectional migration) and gradually settled down in the GCL.
In the temporal dimension, both adult-born JGNs and GCs exhibited saltatory stop-and-go movement, typical for many types of migrating cells10,33. In previous in vitro studies, the intermittent stops during the migration of individual cells were rather brief, giving an impression of a continuous movement11,15,34,35, but many JGNs analyzed here stopped for very long time periods (from 12 h to a few days) before resuming the movement. Continuous imaging of the cells at high temporal resolution, i.e., every 15 min, revealed that cells were migrating at median speeds of 0.4-0.8 μm/min, or 24-48 μm/h, which are comparable to the values obtained in organotypic slices from perinatal mice for cells detaching from the RMS and starting radial migration into the bulb12, or for SVZ neuroblasts migrating in exploratory/intermediate manner in acute OB slices from juvenile/adult mice15. At the same time, these values are lower than the ones obtained in different studies of RMS migration (from 40-60 μm/h to 70-150 μm/h12,15,16,36). In between the migration steps, the cells remained stationary for prolonged periods of time (with individual stationary periods lasting > 80% of the total observation time). This finding together with the skewed migration trajectory of the cells explains why within 12 h cells translocated much less than can be expected from a simple scaling up of their migration speed. Interestingly, the median migration speed decreased significantly as the time passed by, paralleled by a decrease in the size of migration step per 12 h and in the fraction of migrating cells. Taken together, these data suggest that general motility of adult-born JGNs is age-dependent and decreases as the cells mature.
Our population analysis has pinpointed a time window during which adult-born cells are actively migrating, possibly seeking integration targets. This period of time, which we named “pre-integration phase”, is characterized by a vigorous migratory behavior of adult-born cells, long-distance lateral migration of JGNs with characteristic temporal (stop and go) and spatial (migratory, unidirectional or multidirectional) patterns and odor responsiveness20 of migrating cells. Lasting for 3-4 weeks, pre-integration phase is the time period of initial fate determination of adult-born cells, critically impacting on the settlement location of cells, their odor responsiveness (for JGNs predetermined by the glomeruli in which they integrate37) and likely also their survival rate. Several lines of evidence suggest that the pre-integration phase has an important role in defining the functional properties and the fate of adult-born neurons. During the early pre-integration phase, the migratory behavior of adult-born cells is stereotypic and is likely to be driven by the molecular clues18,38. Only a few days later, however, migrating JGNs (and likely also GCs) become responsive to sensory stimuli20. From this point forward, the migration of adult-born JGNs is likely to be driven both by extrinsic sensory stimuli and by intrinsic molecular clues, some of which are influenced by sensory stimuli18,39. On the other hand, global migration parameters of JGNs (e.g., migration angle and speed, net and cumulative distance traversed) were similar in the presence and the absence of the extrinsic sensory input. As the radial migration of adult-born GCs was not affected by odor deprivation either17, it is likely that the overall strength of olfactory input does not affect the long-range migration within the OB. However, considering preferential incorporation of adult-born neurons into active neural circuits in the dentate gyrus40 and activity-dependent expression of tenascin-R, a molecular marker controlling the migration of neuroblasts in the OB18, we postulate that local activity patterns, rather than overall strength of the signal, might influence migration properties of adult-born cells. Together, the extrinsic stimuli and intrinsic factors might govern the adult-born JGNs to active glomeruli and orchestrate their survival/integration therein.
Taken together, our data have overthrown the classical migration model of adult-born cells in the OB, revealing significant radial migration of GCs and a unique radial-to-lateral switch in migration patterns of adult-born JGNs. In addition, it introduces the pre-integration phase that overlaps with the critical period for maturation of adult-born cells (around 10-25 days after birth), when sensory activity critically impacts on their survival39,41,42. Our study has opened new fields of investigation for (i) understanding the mechanisms underlying lateral migration and its implication for adult neurogenesis (ii) applying this new knowledge in developing/testing new strategies in stem cell therapy.
Materials and Methods
Ethics statement
All experimental procedures involving the handling and use of mice were performed in accordance with Institutional Animal Welfare Guidelines and were approved by the state government of Baden-Württemberg, Germany.
Animals and cranial window for optical imaging
A chronic cranial window was made on male 3-6-month-old C57/BL6 mice as described earlier20. Briefly, mice were anesthetized by i.p. injections of ketamine/xylazine (80/4 μg/g body weight (BW); ketamine, Fagron, Barsbuettel, Germany, and xylazine, Sigma-Aldrich, St. Louis, MO, USA). Anesthetic depth was monitored by toe pinch throughout the surgery and additional ketamine/xylazine (40/2 μg/g of BW) was injected when necessary. Dexamethasone (2 μg/g BW, Sigma-Aldrich, St. Louis, MO, USA) was administered i.p. before the surgery and lidocaine (2%, AstraZeneca, Wedel, Germany) was applied subcutaneously before removing the scalp over the OB. A circular groove (3 mm in diameter) was made with a microdrill over the two OB hemispheres by repeated drilling. Then, the skull over each side of the OB was gently removed with tweezers. Extreme care was taken not to damage the blood vessels at the caudal part of the OB. Standard extracellular solution (125 mM NaCl, 4.5 mM KCl, 26 mM NaHCO3, 1.25 mM NaH2PO4, 2 mM CaCl2, 1 mM MgCl2 and 20 mM glucose, pH 7.4, bubbled continuously with 95% O2 and 5% CO2) was used to rinse the opening in the skull, which was then covered with a 3-mm glass coverslip (Warner Instruments, Hamden, CT, USA). The gap between the edge of the coverslip and the skull was filled with cyanoacrylate glue, and then strengthened by dental cement. During the surgery, and until full recovery, the mouse was kept on a heated plate with temperature of 38 °C. Postoperative care included an analgesic dose of carprofen (5 μg/g BW, Pfizer, Berlin, Germany) for 3 days subcutaneously and the antibiotic baytril (1:100 v/v, Bayer, Leverkusen, Germany) in drinking water for 10 days. Mice were allowed to recover for at least 3 weeks and were subsequently examined for window clarity and new bone growth. Those mice that passed the quality control were injected with retrovirus into the RMS.
Retroviral vector production
Design, production and application of retroviral vectors are described in detail in ref.27. Briefly, the viral vectors were derived from the γ-retroviral vector RSF91.GFP.pre* 43, whose marker gene (eGFP) has been replaced by one of the three FPs (Cerulean, Venus and mCherry), driven by an enhancer/promoter from the spleen focus-forming virus within the long terminal repeats. Vector maps and sequence data for all vectors are available upon request; more information can be found at http://www.LentiGO-Vectors.de. Cell-free supernatants containing viral particles were produced by transient transfection of HEK-293T-packaging cells as described previously26,43. Retroviral vectors have been packaged using pcDNA3.MLVgp and phCMV-VSV-G. In some sets of experiments, retrovector encoding eGFP driven by CAG44 was utilized and packaged by 1F8 cells derived from 293GPG cell line45. Supernatants containing retroviral particles were concentrated about 100-fold by centrifugation at 8 000× g and 6 °C for 8-12 h. Concentrated supernatants were titrated26 on 293T cells. For titration, 293T cells were incubated at 5 × 104 cells in 0.5-ml medium in each well of a 24-well plate in the presence of 8 μg/ml polybrene. After addition of particle-containing supernatant, the plate was centrifuged at 1 000× g for 1 h at 25 °C. Initial gene transfer rates were analyzed 48-72 h after transduction by FACS. To acquire FACS data, the cytometer LSRFortessa (405/488/561/640-nm lasers) was used (Becton Dickinson, Heidelberg, Germany). Titers of about 8 × 109 virus particles per ml concentrated supernatant were obtained for the vectors used (range: 6.6-9.8 × 109 virus particles per ml).
Retroviral vector injection into the RMS
Animals with a chronic cranial window over the OB were anesthetized as above and fixed to a stereotaxic device (Stoelting, Wood Dale, IL, USA) by ear bars, and 2% lidocaine was applied subcutaneously to the skin overlying the left and the right injection sites. The dental cement covering the injection sites was removed by drilling. Premixed retrovectors (1.5 μl, 1:1:1 for retrovectors encoding the three FPs with different colors) or the retrovector encoding eGFP were stereotactically injected into the RMS at the following coordinates: anteroposterior, +3.0 mm from bregma; mediolateral, ±0.82, and −2.9 mm from pial surface20. Then, a metal bar for head fixation was fixed to the caudal part of the skull with dental acrylic and cement. The other exposed parts of the skull were also covered with dental cement. The mice were returned to the home cage, and carprofen (5 μg/g BW) was injected subcutaneously for 3 days.
Odor deprivation and two-photon imaging of OD mice
In mice with RGB-marked adult-born neurons, unilateral naris closure was performed on DPI 6 using well-established technique29. The nasal septum separates the left and the right nasal cavities and olfactory sensory neurons project exclusively to the ipsilateral OB. Therefore, unilateral naris closure blocks the sensory inputs to the ipsilateral OB29. Animals were anesthetized by ketamine/xylazine as described above. Nose plugs were constructed out of small bits of polyethylene tubing (1.5 mm in diameter) that were closed at one end by heating before insertion, and inserted into the right or left external naris of mice. After insertion of the plug, the animals were returned to their home cages. From DPI 6 (first day of occlusion) to DPI 16, the OBs from either side of the brain were imaged according to procedures described below to track the migration of the RGB-marked adult-born neurons. Three to five FOVs were acquired from each side of the OB and in each FOV, 5-20 cells were tracked. At end points, mice were transcardially perfused with 4% paraformaldehyde and brains were cryoprotected in 25% sucrose overnight. Coronal slices (40 μm) were obtained by cryosectioning the OB. The expression level of TH in either side of the OB was examined through immunostaining with antibody against TH (Millipore, Billerica, MA, USA).
In vivo imaging and OBPs setup
Mice with chronic cranial windows were anesthetized with isofluorane (2.5% for induction, 0.8-1.5% for maintenance). Breathing rate and body temperature were monitored continuously using the anesthesia monitoring system from AD Instruments (Sydney, Australia). The head of the mouse was fixed through the metal bar to the X-Y table, ensuring consistent positioning through imaging sessions. In vivo two-photon imaging was performed using a customized microscope based on the Olympus FV1000 system (Olympus, Tokyo, Japan) and MaiTai Deep See Laser (Spectra Physics, Mountain View, CA, USA), with a Zeiss × 20 water-immersion objective lens (NA 1.00). The adult-born cells were imaged starting from DPI 6 according to the following protocol: every 12 h (DPI 6-DPI 15), every 24 h (DPI 15-DPI 20), every 2 days (DPI 20-DPI 30) and once a week (DPI 30-DPI 60). Each imaging session lasted ∼2 h. The oCPS approach combined two complementary techniques: multicolor single-cell imaging and blood vessel visualization. We used following setting to split fluorescence emitted by FP-labeled adult-born cells: red, excitation, 800 nm and emission, long-pass (LP) channel of 570-nm dichroic mirror (DM570); green, excitation, 990 nm and emission, short-pass (SP) channel of DM570; blue, excitation, 800 nm and emission, SP channel of DM570. To create landmarks for single-cell tracking, we visualized blood vessels in the OB through i.p. injection of sulforhodamine B (0.1 ml/10 g BW, 1 mM in PBS, Sigma-Aldrich, St. Louis, MO, USA). One single injection enabled visualization of blood vessels for around 2 h. The acquisition setting for sulforhodamine B was excitation, 900 nm and emission, LP channel of DM570. In practice, each FOV (636 × 636 μm, from dura to 350 μm) was sequentially scanned with 800 and 990 nm. Then, sulforhodamine B was injected and the same FOV was scanned again with 900 nm. As shown previously (Supplementary information, Figure S2 in ref. 20) blood vessel pattern is very stable over prolonged periods of time, providing a reliable framework for long-term tracking of individual cells.
Data analysis
The combined three-dimensional blood vessels/cells image stacks (e.g., Supplementary information, Figure S3A-S3C) acquired during the consecutive imaging sessions were aligned offline using the blood vessel pattern as anatomic landmarks (see Supplementary information, Figure S2 in ref. 20). Due to the stability of the landmarks throughout the imaging series, the alignment errors (estimated by comparing images of stable cells at DPI 28 and 30) did not exceed 1.25-2.5 μm in X-Y direction and 2-4 μm in Z direction. Cells located up to a depth of 120 μm under the dura were regarded as adult-born JGNs, whereas the cells below mitral cell layer were taken as adult-born GCs (unless they were retrospectively found to be migrating JGNs). Each cell was annotated with an identity number. The color hue of each cell was determined as described above. Cells from the consecutive time points were tracked according to their RGB spectrum, location, and size throughout the 33 time points from DPI 6 to DPI 60, generating a series of annotated stacks. Then, according to annotated stacks, the history and trace of each single cell was sorted out. The center of cell's soma in each annotated stack was marked as a position point, and then the cell's trajectory was reconstructed based on its position points in all annotated stacks of the same FOV. Next, the X-Y coordinates for each position point of a cell were read using the Fluoview 3.0 Viewer (Olympus). For Z axis coordinates, the depth of cells (Zac, actual depth of a cell) was the relative depth from the dura: Zac = Zreading−Zdura. With 3D coordinates for each track point, migration distance (D) between two points in space was calculated by the following formula:
where (X1, Y1, Z1) and (X0, Y0, Z0) were coordinates of the target cell position measured at current or previous temporally consecutive stacks, respectively. Unless otherwise indicated, migration speed was defined as the translocation of cell's soma between the two consecutive sessions divided by the respective time period. Taking into account the 2-μm step size of the acquired stacks, a cell was considered moving if the translocation of cell's soma between the two consecutive sessions was > 4 μm.
The migration angle (α) between migration direction and Z axis was calculated by the following formula: α = arccos ((Z1−Z0)/D). In our experiments, Z axis (the optical axis of the objective) deviated slightly (by γ = 4.36 ± 4.11° median, IQR (see Supplementary information, Data S1 for the description of γ estimation procedure)) from the radial axis of the bulb (n = 206 image stacks). This deviation, however, was too small to affect the quality of data analysis (not shown).
To categorize the migration modes of JGNs in the GL, two methods were used. One method15 binned cells into migratory, exploratory and intermediate cells according to the ratio of the net distance a cell has traversed to the total distance it has traversed: 0.6-1.0 as migratory, 0.0-0.4 as exploratory, and 0.4-0.6 as intermediate. The second method defined two types of migration modes based on the maximal migration angle (θ) between the two immediately adjacent migration steps. If θ was ≤ 90°, then the migration of the cell was categorized as unidirectional; otherwise, as multidirectional. For plots of the fraction of moving cells at a certain time point, the proportion of cells, whose positions differed from that recorded at immediately preceding time point, was calculated in relation to the total number of cells in that stack. 3D cell trajectory and 2D plot of cell migration speed were generated by IGOR Pro (WaveMetrics, Portland, OR, USA). Projected images from stacks were processed by ImageJ software (http://rsb.info.nih.gov/ij/).
Statistics
Statistical analysis consisted of the following: Shapiro-Wilk (n ≥ 7) or Kolmogorov-Smirnov (n ≥ 5) normality tests. For normally distributed data, mean ± SEM was calculated and Student's t-test was used for comparison of two groups. For data that were not normally distributed, median ± 1 IQR is presented as box plot, and 10-90 percentiles are shown as whiskers. In the text, all data are presented as median ± IQR, unless otherwise indicated. Nonparametric tests were used for comparisons between not normally distributed data sets. Mann-Whitney test was used for comparison between the two groups and Kruskal-Wallis nonparametric test was used for comparison between more than two groups followed by the Dunn's multiple comparison test. Wilcoxon matched pairs test was used for nonparametric comparison of two paired samples. All the statistical tests were two sided. Significance level was set to P ≤ 0.05.
Author Contributions
YL and OG designed the experiments, YL and KL performed the experiments and analyzed the data with contributions from AM and YK KR, BF and DGN provided essential reagents. YL and OG wrote the manuscript. All authors discussed and revised the manuscript.
Competing Financial Interests
The authors declare no competing financial interests.
Acknowledgments
We thank E Zirdum, A Weible, G Heck and K Schoentag for technical assistance; F Gage for the retroviral vector backbone; J Nabekura for his advice on naris closure protocols, and V H Perry and J Sheng for valuable comments on the manuscript. This work was funded by fortüne-Programm of Tübingen University, Nr. 2175-0-0. KR and BF have been supported by SFB841.
Footnotes
(Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary Information
Building up the oCPS.
A three-dimensional view on the migration traces of olfactory bulb interneurons within a sample field of view
An example migration trace of an adult-born JGN and comparison of methods categorizing the migration mode.
The effect of naris closure on migration properties of adultborn JGNs in the glomerular layer.
Distribution of migration steps of juxtaglomerular neurons.
Supplementary methods.
References
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science 1994; 264:1145–1148. [DOI] [PubMed] [Google Scholar]
- Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science 1996; 271:978–981. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci 2002; 22:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci 2006; 7:179–193. [DOI] [PubMed] [Google Scholar]
- Lepousez G, Nissant A, Lledo PM. Adult neurogenesis and the future of the rejuvenating brain circuits. Neuron 2015; 86:387–401. [DOI] [PubMed] [Google Scholar]
- Whitman MC, Greer CA. Adult neurogenesis and the olfactory system. Prog Neurobiol 2009; 89:162–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 1999; 97:703–716. [DOI] [PubMed] [Google Scholar]
- Garcia AD, Doan NB, Imura T, Bush TG, Sofroniew MV. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci 2004; 7:1233–1241. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 2011; 70:687–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei HT, Lai C, Anton ES. Neuronal migration in the adult brain: are we there yet? Nat Rev Neurosci 2007; 8:141–151. [DOI] [PubMed] [Google Scholar]
- Kakita A, Goldman JE. Patterns and dynamics of SVZ cell migration in the postnatal forebrain: monitoring living progenitors in slice preparations. Neuron 1999; 23:461–472. [DOI] [PubMed] [Google Scholar]
- De Marchis S, Fasolo A, Shipley M, Puche A. Unique neuronal tracers show migration and differentiation of SVZ progenitors in organotypic slices. J Neurobiol 2001; 49:326–338. [DOI] [PubMed] [Google Scholar]
- Suzuki SO, Goldman JE. Multiple cell populations in the early postnatal subventricular zone take distinct migratory pathways: a dynamic study of glial and neuronal progenitor migration. J Neurosci 2003; 23:4240–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RL, Chopp M, Gregg SR, et al. Patterns and dynamics of subventricular zone neuroblast migration in the ischemic striatum of the adult mouse. J Cereb Blood Flow Metab 2009; 29:1240–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam SC, Kim Y, Dryanovski D, et al. Dynamic features of postnatal subventricular zone cell motility: a two-photon time-lapse study. J Comp Neurol 2007; 505:190–208. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Garcia-Verdugo JM, Alvarez-Buylla A. Direct evidence for homotypic, glia-independent neuronal migration. Neuron 1997; 18:779–791. [DOI] [PubMed] [Google Scholar]
- Petreanu L, Alvarez-Buylla A. Maturation and death of adult-born olfactory bulb granule neurons: role of olfaction. J Neurosci 2002; 22:6106–6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghatelyan A, de Chevigny A, Schachner M, Lledo PM. Tenascin-R mediates activity-dependent recruitment of neuroblasts in the adult mouse forebrain. Nat Neurosci 2004; 7:347–356. [DOI] [PubMed] [Google Scholar]
- Mizrahi A. Dendritic development and plasticity of adult-born neurons in the mouse olfactory bulb. Nat Neurosci 2007; 10:444–452. [DOI] [PubMed] [Google Scholar]
- Kovalchuk Y, Homma R, Liang Y, et al. In vivo odourant response properties of migrating adult-born neurons in the mouse olfactory bulb. Nat Commun 2015; 6:6349. [DOI] [PubMed] [Google Scholar]
- Weber K, Thomaschewski M, Warlich M, et al. RGB marking facilitates multicolor clonal cell tracking. Nat Med 2011; 17:504–509. [DOI] [PubMed] [Google Scholar]
- Christie RH, Bacskai BJ, Zipfel WR, et al. Growth arrest of individual senile plaques in a model of Alzheimer's disease observed by in vivo multiphoton microscopy. J Neurosci 2001; 21:858–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolmont T, Haiss F, Eicke D, et al. Dynamics of the microglial/amyloid interaction indicate a role in plaque maintenance. J Neurosci 2008; 28:4283–4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masamoto K, Tomita Y, Toriumi H, et al. Repeated longitudinal in vivo imaging of neuro-glio-vascular unit at the peripheral boundary of ischemia in mouse cerebral cortex. Neuroscience 2012; 212:190–200. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci 1997; 17:5046–5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K, Thomaschewski M, Benten D, Fehse B. RGB marking with lentiviral vectors for multicolor clonal cell tracking. Nat Protoc 2012; 7:839–849. [DOI] [PubMed] [Google Scholar]
- Gomez-Nicola D, Riecken K, Fehse B, Perry VH. In-vivo RGB marking and multicolour single-cell tracking in the adult brain. Sci Rep 2014; 4:7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker H, Morel K, Stone DM, Maruniak JA. Adult naris closure profoundly reduces tyrosine hydroxylase expression in mouse olfactory bulb. Brain Res 1993; 614:109–116. [DOI] [PubMed] [Google Scholar]
- Cummings DM, Brunjes PC. The effects of variable periods of functional deprivation on olfactory bulb development in rats. Exp Neurol 1997; 148:360–366. [DOI] [PubMed] [Google Scholar]
- Livet J, Weissman TA, Kang H, et al. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature 2007; 450:56–62. [DOI] [PubMed] [Google Scholar]
- Loulier K, Barry R, Mahou P, et al. Multiplex cell and lineage tracking with combinatorial labels. Neuron 2014; 81:505–520. [DOI] [PubMed] [Google Scholar]
- Tabata H, Nakajima K. Multipolar migration: the third mode of radial neuronal migration in the developing cerebral cortex. J Neurosci 2003; 23:9996–10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson JC, Hatten ME. Glial-guided granule neuron migration in vitro: a high-resolution time-lapse video microscopic study. J Neurosci 1987; 7:1928–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro H, Yacubova E, Rakic P. Mode and tempo of tangential cell migration in the cerebellar external granular layer. J Neurosci 2001; 21:527–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaar BT, McConnell SK. Cytoskeletal coordination during neuronal migration. Proc Natl Acad Sci USA 2005; 102:13652–13657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenne M, Custody C, Charneau P, Lledo PM. In vivo imaging of migrating neurons in the mammalian forebrain. Chem Senses 2005; 30 Suppl 1:i115–i116. [DOI] [PubMed] [Google Scholar]
- Homma R, Kovalchuk Y, Konnerth A, Cohen LB, Garaschuk O. In vivo functional properties of juxtaglomerular neurons in the mouse olfactory bulb. Front Neural Circuits 2013; 7:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack I, Bancila M, Loulier K, Carroll P, Cremer H. Reelin is a detachment signal in tangential chain-migration during postnatal neurogenesis. Nat Neurosci 2002; 5:939–945. [DOI] [PubMed] [Google Scholar]
- Khodosevich K, Lazarini F, von Engelhardt J, Kaneko H, Lledo PM, Monyer H. Connective tissue growth factor regulates interneuron survival and information processing in the olfactory bulb. Neuron 2013; 79:1136–1151. [DOI] [PubMed] [Google Scholar]
- Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci 2007; 10:355–362. [DOI] [PubMed] [Google Scholar]
- Mouret A, Gheusi G, Gabellec MM, de Chaumont F, Olivo-Marin JC, Lledo PM. Learning and survival of newly generated neurons: when time matters. J Neurosci 2008; 28:11511–11516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M, Mori K. Critical period for sensory experience-dependent survival of newly generated granule cells in the adult mouse olfactory bulb. Proc Natl Acad Sci USA 2005; 102:9697–9702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schambach A, Mueller D, Galla M, et al. Overcoming promoter competition in packaging cells improves production of self-inactivating retroviral vectors. Gene Ther 2006; 13:1524–1533. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Zhao C, Gage FH. Retrovirus-mediated single-cell gene knockout technique in adult newborn neurons in vivo. Nat Protoc 2006; 1:3049–3055. [DOI] [PubMed] [Google Scholar]
- Ory DS, Neugeboren BA, Mulligan RC. A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc Natl Acad Sci USA 1996; 93:11400–11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Building up the oCPS.
A three-dimensional view on the migration traces of olfactory bulb interneurons within a sample field of view
An example migration trace of an adult-born JGN and comparison of methods categorizing the migration mode.
The effect of naris closure on migration properties of adultborn JGNs in the glomerular layer.
Distribution of migration steps of juxtaglomerular neurons.
Supplementary methods.