Abstract
Robust genetic evidence in mice and humans indicates that RANK signaling plays a major role in mammary carcinogenesis driven by BRCA1/BRCA2 mutations. These findings may inaugurate a new era of breast cancer prevention, changing the life of millions of women worldwide.
According to current estimates, one in eight women will suffer from breast cancer during her lifetime. Several factors have been associated with an increased risk for breast cancer development, including post-menopausal hormonotherapy, oral contraceptives, obesity and genetic predisposition1. Indeed, as much as 2%-10% of breast cancer cases are associated with germline mutations in BRCA1 and BRCA2, which encode two proteins with a central role in the repair of DNA double-strand breaks. The current standard of care for women who have a familial history of breast cancer and carry BRCA1 or BRCA2 mutations is bilateral radical mastectomy. Such a surgical procedure, which has recently been under the limelight owing to the Angelina Jolie case, has complex psychological repercussions and is not 100% effective1. Moreover, the implementation of population-wide mammography-based screening campaigns failed to decrease the incidence of breast neoplasms that are metastatic at presentation, and might per se increase the risk of breast cancer development2. This implies that mammary carcinogenesis does not proceed according to the model originally proposed by William Stewart Halsted (who hypothesized that tumors form at a single location, grow there and eventually disseminate)2, calling for the development of novel prophylactic tools. Recent work from Josef Penninger's group provides robust genetic and pharmacological evidence in support of the notion that breast cancer developing in the context of BRCA1 or BRCA2 mutations can be prevented by blocking RANKL/RANK signaling3. Since a RANKL-targeting monoclonal antibody (i.e., denosumab) is currently approved by the US Food and Drug Administration (FDA) and equivalent agencies worldwide for the treatment of multiple bone conditions and has an exceptional safety record4,5, these findings may pave the way to a new era of breast cancer prophylaxis, changing the life of millions of women worldwide.
Based on previous findings from their group demonstrating an essential role for RANK in mammary gland development and progestin-driven mammary carcinogenesis6,7, Penninger and collaborators initially set out to determine the impact of RANK signaling in multiple genetic models of mammary carcinogenesis, including: (1) mice bearing homozygous Brca1fl/fl alleles in a Trp53fl/fl context (targeting the master oncosuppressor p53) and expressing the Cre recombinase under the control of the keratin 5 (Krt5) promoter, which is active in multiple epithelia; and (2) Brca1fl/flTrp53fl/fl mice expressing the CreC recombinase under the control of the whey acidic protein (Wap) promoter, which is specifically active in luminal and basal mammary epithelial cells independently of doxycycline and pregnancy. To this aim, they crossed the strains described here above with Tnfrsf11afl/fl mice (in which the RANK-coding sequence is floxed), and monitored not only tumor incidence over time, but also biochemical and pathological parameters of developing neoplasms, including markers of DNA damage, proliferation rate and grade3. Tumors developing in the absence of RANK manifested similar degrees of DNA damage and proliferation rate as tumors developing in a RANK-proficient background. However, the deletion of Tnfrsf11a not only led to reduced tumor grade in both models, but also delayed tumor onset in the WapCreC model (which can be maintained for long periods, at odds with the Krt5Cre model that succumbs to skin tumors at around 4 months of age). Moreover, whereas all mice lacking Brca1 and Trp53 in mammary progenitor cells (as per WapCreC-dependent recombination) developed breast neoplasms by approximately 7 months of age, ∼ 25% of mice lacking Brca1, Trp53 and Tnfrsf11a never developed breast neoplasms3.
To confirm their observations in a clinically relevant model, Penninger and colleagues took advantage of Brca1fl/fl mice expressing Cre under the control of the mouse mammary tumor virus (MMTV) promoter, which is also preferentially active in the breast epithelium. In this setting, mammary carcinogenesis is driven solely by the absence of Brca1, which closely mimics the situation of women with germline BRCA1 or BRCA2 mutations. By the age of 9 months, 33% of these mice spontaneously developed pre-neoplastic lesions as a result of Brca1 loss, which was completely abrogated by the subcutaneous administration of a RANK-targeting antibody fragment. At 15 months of age, as many as 82% mice maintained in control conditions (i.e., receiving an irrelevant antibody fragment) manifested pre-neoplastic breast lesions, while only 7% of mice receiving the RANK-targeting agent did so3. These findings demonstrate that blocking RANKL/RANK limits mammary carcinogenesis driven by BRCA1 mutations, at least in mice.
RANK was also involved in the expansion of murine Lin−CD24+CD49fhi basal mammary progenitor cells in a model of ovariectomy followed by sham treatment or estrogen plus progesterone administration. To confirm the validity of their findings in the human system, Penninger and collaborators isolated mammary progenitor cells from 3 women with germline BRCA1 mutations who underwent prophylactic mastectomy and tested their sensitivity to denosumab in clonogenic assays. Human BRCA1-deficient progenitor cells treated with denosumab had a reduced clonogenic potential as compared to the same cells kept in control conditions3. These data pointed to the involvement of RANK in human mammary carcinogenesis driven by BRCA1 mutations, lending additional support to the possibility that the blockade of RANKL/RANK may constitute a clinically implementable strategy. To corroborate even further this hypothesis, Penninger and colleagues undertook a multipronged analysis of the RANKL/RANK system in clinical settings to draw two major conclusions. First, RANK and RANKL are expressed at high levels only by breast cancers with BRCA1 or BRCA2 mutations, and RANK protein levels exhibit an exquisite correlation with tumor grade in this scenario (in a clinical cohort of ∼250 breast cancer patients). Second, common TNFRSF11A polymorphisms that increase RANK expression levels are associated with an increased risk for breast cancer development in women with BRCA1 or BRCA2 mutations (in a large cohort from the Collaborative Oncological Gene-environment Study cumulatively including 23 000 women with breast cancer)3.
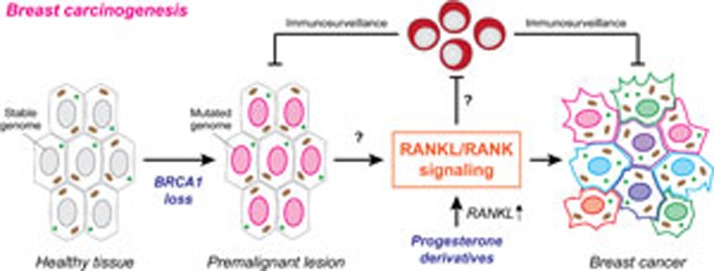
Of note, the ability of BRCA1 mutations to drive mammary transformation has previously been shown to rely on proficient progesterone signaling8, which is known to drive RANKL expression in the mammary epithelium7,9. It will therefore be interesting to fully characterize the molecular mechanisms through which the progesterone system and RANKL/RANK cooperate to support carcinogenesis driven by BRCA1 or BRCA2 mutations. Moreover, it will be important to evaluate whether and how RANK affects natural or therapy-induced immunosurveillance against breast cancer10,11 (Figure 1). Irrespectively, the findings by Penninger and co-authors may inaugurate a new era in which women with BRCA1 or BRCA2 mutations receive denosumab as an efficient prophylaxis instead of undergoing bilateral radical mastectomy. It remains to be seen whether such a prophylactic treatment would also reduce the incidence of ovarian carcinoma driven by BRCA1 or BRCA2 mutations.
Figure 1.
RANKL/RANK signaling in breast cancer. Mammary carcinogenesis driven by BRCA1 mutations relies on autocrine or paracrine RANKL/RANK signaling in mammary progenitor cells. Breast cancers developing as a consequence of BRCA1 mutations also depend on progesterone signaling (knowing that progesterone derivatives also promote mammary carcinogenesis), most likely as a result of progesterone receptor-driven RANKL expression and consequent proliferation of mammary progenitor cells. The mechanisms linking the accumulation of genetic defects to carcinogenesis via the RANKL/RANK system, as well as the possible impact of RANKL/RANK signaling in the mammary epithelium on anticancer immunosurveillance remain to be determined.
References
- Bianchini G, Balko JM, Mayer IA, et al. Nat Rev Clin Oncol 2016. May 17. doi:10.1038/nrclinonc.2016.66 [DOI] [PMC free article] [PubMed]
- Welch HG, Gorski DH, Albertsen PC. N Engl J Med 2015; 373:1685–1687. [DOI] [PubMed]
- Sigl V, Owusu-Boaitey K, Joshi PA, et al. Cell Res 2016; 26:761–774. [DOI] [PMC free article] [PubMed]
- Lacey DL, Boyle WJ, Simonet WS, et al. Nat Rev Drug Discov 2012; 11:401–419. [DOI] [PubMed]
- Vacchelli E, Aranda F, Eggermont A, et al. Oncoimmunology 2014; 3:e 27048.
- Fata JE, Kong YY, Li J, et al. Cell 2000; 103:41–50. [DOI] [PubMed]
- Schramek D, Leibbrandt A, Sigl V, et al. Nature 2010; 468:98–102. [DOI] [PMC free article] [PubMed]
- Poole AJ, Li Y, Kim Y, et al. Science 2006; 314:1467–1470. [DOI] [PubMed]
- Tanos T, Sflomos G, Echeverria PC, et al. Sci Transl Med 2013; 5:182ra155. [DOI] [PubMed]
- Kroemer G, Senovilla L, Galluzzi L, et al. Nat Med 2015; 21:1128–1138. [DOI] [PubMed]
- Galluzzi L, Buque A, Kepp O, et al. Cancer Cell 2015; 28:690–714. [DOI] [PubMed]