ABSTRACT
Arl2 and Arl3 are Arf-like small GTP-binding proteins of the Arf subfamily of the Ras superfamily. Despite their structural similarity and sharing of many interacting partners, Arl2 and Arl3 have different biochemical properties and biological functions. Growing evidence suggest that Arl2 and Arl3 play a fundamental role as regulators of trafficking of lipid modified proteins between different compartments. Here we highlight the similarities and differences between these 2 homologous proteins and discuss the sorting mechanism of lipidated cargo into the ciliary compartment through the carriers PDE6δ and Unc119 and the release factors Arl2 and Arl3.
KEYWORDS: Arl2, Arl3, Arls, cargo release, cilia, myristoylation, PDE6б prenyl transferases, prenylation, small GTPases and their effector proteins, sorting signal, structural biology and protein-protein interactions, structure/function studies, Unc119
The similarity between Arl2 and Arl3
The Ras superfamily of proteins comprises 20-25 kDa small GTP-binding proteins categorized in several subfamilies such as the Arf subfamily. They cycle between GDP- and GTP-bound conformational states and are regulated by Guanine Nucleoide Exchange Factors (GEFs) and GTPase Activating Proteins (GAPs). The existence of Arl2 and Arl3 genes was revealed by PCR amplification using degenerate primers in a search for new members of the Ras superfamily.1 Although they are classified as Arf-like GTP-binding proteins they have no ADP ribosylation factor activity.2 Unlike most Arfs, they are not N-terminally myristoylated even though the glycine residue at position 2 is highly conserved between organisms, but the serine at position 6 important for myristoylation is missing.3 However they do fit into the Arf subfamily of the Ras superfamily because the structural analysis has shown that both Arl2 and Arl3 display the very dramatic conformational change between the GDP- and the GTP-bound conformations, whereby the first 2 strands of the β-sheet, also called the interswitch toggle, move by 2 residues along the rest of the sheet.4 Both proteins also have an N-terminal amphiphatic helix characteristic for the Arf subfamily.5
A strong argument for an apparently similar biological function of Arl2/3 comes from the fact that they interact with the same set of effectors. According to their interaction mode Arl2/3 effectors can be divided into 2 distinct types (Fig. 1). The first to be identified type1 effectors was BART (Binder of Arl Two) which despite its name binds to both Arl2 and Arl3.6,7 A homolog of BART, originally identified as CCDC104 (coiled-coiled domain containing 104), now called BARTL1, is much longer than BART but has a domain very similar to the latter.8 Both BART and BARTL1 bind to the switch regions and also to the amphiphatic helix of the proteins in a similar way.8,9 The second type of effectors (type2) which bind to both Arl2 and Arl3 are the carrier proteins PDE6δ10 and HRG4/Unc119a11 and Unc119b.12 These proteins have a β-sandwich fold and bind only to the switch regions of Arl2 and Arl3. The binding of type 1 and type 2 effectors is nevertheless mutually exclusive.8,9,13-15
Figure 1.
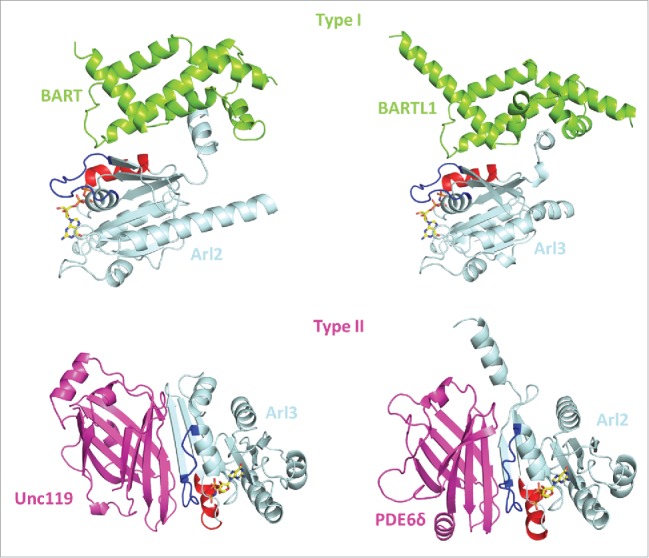
Type 1 and type 2 effectors of Arl2 and Arl3. Crystal structures of Arl2 and Arl3 in complex with type 1 effectors (BART and BARTL1; PDB codes: 3DOI and 4ZI2), which interact with the switch regions and the N-terminal helix and type 2 effectors (PDE6δ and Unc119a; PDB codes: 1KSG and 4GOJ), which interacts with the switch regions only. Arl2/3 (light blue), type 1 effectors (green), type 2 effectors (pink), Switch I (blue), Switch II (red), GppNHp (a non-hydrolysable GTP analog; yellow).
Functional differences between Arl2 and Arl3
Differences in the properties of Arl2 and Arl3 have gradually emerged and it is now established that they have different biochemical and biological functions. One of the biochemical differences is the affinity to nucleotides. While Arl3 is a typical member of the Ras superfamily proteins, with affinities to GDP and GTP in the nano- or subnanomolar range, the affinity of Arl2 toward nucleotides falls into the micromolar range.16,17 Thus the nucleotide dissociation rate for Arl3 is 2-3 order of magnitudes slower than that of Arl2. As a consequence Arl3 definitively requires a GEF for its activation while this does not seem necessary for Arl2.
RP2, a protein mutated in X-linked Retinitis pigmentosa, is an Arl3–specific GAP and does not act on Arl2.18 It is classic GAP with an arginine finger pointing into the active site.16 A number of RP2 patient mutations have been described. They cluster at the RP2-Arl3 interaction interface and have been shown to display much lower GAP activity for Arl3.18 A family of ELMO domain containing proteins (ELMODs) has been identified as GAPs for Arl2. Their specificity is very relaxed, as they act on both Arl2 and Arl3 and also on some other Arf proteins. They also have an arginine residue required for their activity, but no mechanistic or structural details of the interaction have been reported sofar.19-21 Another major difference between Arl2 and Arl3 concerns the involvement of Arl2 in tubulin folding.22 After tubulin monomers exit from the TRiC/CCT chaperonin system, the formation of αβ-tubulin heterodimers requires the activities of the tubulin cofactors TBCs. Arl2 has been shown to bind to the tubulin-specific chaperone cofactor TBCD. A recent study in yeast (which however has only a single Arl2 homolog) Arl2 was found to form a high molecular weight complex with a cage-like structure involving Arl2 and tubulin cofactors TBCC, TBCD, TBCE.23 In this complex, TBCC, the homolog of RP2 which has a similar β-helix domain structure, seems to be responsible for the GTP hydrolysis of Arl2. An additional difference between Arl2 and Arl3 touches their interaction with membranes. Both proteins bind into the liquid-disordered domain, but in contrast to Arl2 the binding of Arl3 is strongly dependent on nucleotide loading.24
One more indication of a different biological function came from cellular studies by Zhou et al. which showed that the knockdown of Arl2 and Arl3 or the overexpression of a GTPase negative mutant have entirely different (or no apparent) effects on cell function.25 The latter study revealed that Arl3 localizes in cilia, which we could confirm using a kidney cell line stably transfected with GFP-labeled Arl2 and Arl3, showing Arl3 to be localized inside (and outside) cilia, whereas Arl2 is excluded from cilia.8 The mechanism that allows Arl3 but not Arl2 ciliary entry (or retention) is not resolved considering that the proteins are small enough to be able to cross the barrier of cilia.26 Arl2 has recently been implicated in the function of mitochondria.27
While an Arl2 knockout mouse has not been described, its downregulation in cell culture by RNAi has drastic consequences on cell function28 in particular the localization of Ras. A germline Arl3 knockout causes a syndromic ciliopathy, with ciliary dysfunction in kidney, liver and pancreas.29 Recently Arl3 was shown to regulate trafficking of prenylated proteins in photoreceptors and a photoreceptor specific knockout of Arl3 causes severe defects in photoreceptor function and structure, in line with the recent identification of an Arl3 mutant in retinitis pigmentosa.30-32
The cargo carriers PDE6δ and Unc119
The structure of the complex between Arl2 and PDE6δ was solved many years ago.13 The high homology with RhoGDI and the presence of a hydrophobic pocket suggested that PDE6δ might also bind prenyl groups. This was indeed verified by a number of biochemical and cellular studies which suggested that PDE6δ is a general prenyl binding protein (therefore also called PrBP) without apparent selectivity.33-39 In contrast to RhoGDI and RabGDI which are specific for the GDP-bound conformation of prenylated Rho or Rab proteins,39,40 PDE6δ binds Ras and Rheb independent of their nucleotide-bound conformation.34,41 The structure of the Arl2-PDE6δ complex shows however that the hydrophobic pocket is partially occupied suggesting a mutually exclusive binding to prenylated cargo and/or Arl2. Biochemical studies with farnesylated peptides showed that both Arl2 and Arl3 in the GTP-bound conformation can allosterically release Ras or Rheb from PDE6δ41 and that Arl2/3 or cargo binding are indeed mutually exclusive.
When the structures between PDE6δ and farnesylated Rheb or certain farnesylated peptides were determined, the mutual exclusive binding could be defined in more detail (Fig. 2). The structures showed how the binding of Arl2 (or Arl3) induces the hydrophobic pocket to narrow such that the C-terminal farnesylated end of the protein gets squeezed out of the pocket.41 A structure of PDE6δ and a geranylgeranylated peptide (with 4 isoprenyl groups rather than 3 compared to farnesylated peptides) from the α' subunit of PDE6 could also be solved. It shows that the C20 modification can bind into the same pocket such that the extra 5 residues of the geranylgeranyl group extend the pocket by 5 C atoms.42 Since Ras requires farnesylation to be bound to membranes and since membrane binding is required for Ras function it was argued that PDE6δ might be a target for anti Ras drugs. This was indeed verified and a number of compounds such as Deltarasin and Deltazinone were identified which inhibited Ras function in cells and in Xenograft mouse models.43,44
Figure 2.
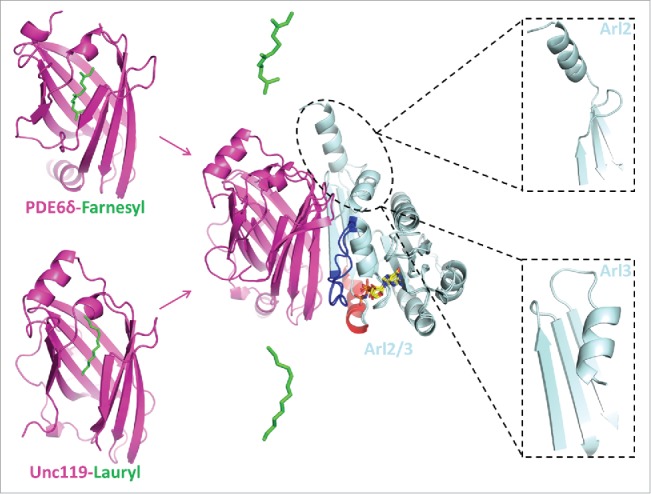
The cargo release by Arl2 and Arl3. Left panel; crystal structures of PDE6δ in complex with farnesyl peptide (PDB code: 3T5I) and Unc119 in complex with laurylated peptide (PDB code: 3RBQ). Middle panel; the binding of Arl2/3 to the carrier proteins PDE6δ and Unc119 result in the release of the lipid moiety. Right panel; the N-terminal helix of Arl3 and Arl2 display different conformation in the complex with the carrier proteins.
In a proteomic study Unc119a and Unc119b were identified as proteins binding to nephrocystin 3 (NPHP3) and the binding was found to be dependent on the N-terminal myristoyl group of NPHP3.12 Furthermore Unc119b was found to be required for the import of NPHP3 into cilia. The structure of a complex between Unc119a and Arl3 showed that Arl2/3 binding to Unc119 might be unfavorable for the binding of myristoylated cargo. This was deduced from a superimposition with the structure between a lauroylated peptide from GNAT1 (the α subunit of transducin)15 and Unc119a (Fig. 2). We have recently shown that both Unc119a and Unc119b bind with similar affinity to a variety of myristoylated peptides.45
Cargo release from PDE6δ by Arl2/3
We and others had established that PDE6δ is a carrier for prenylated cargo proteins, while Unc119 binds myristoylated cargo. Based on studies with Ras or Rheb, 2 prenylated proteins that reside in cytoplasmic membranes, it was assumed that the nature of cargo does not influence the interaction with PDE6δ.41 It was also shown that the affinity is dictated by only the last few C-terminal residues of these proteins, since C-terminal peptides of 6-7 residues bind with the same affinity as the full-length protein.34 However, investigation of the interaction of the ciliary protein inositol 5′ phosphatase (INPP5E) with PDE6δ revealed a difference in the binding affinity when compared to the non-ciliary proteins Ras and Rheb. INPP5E showed an approximately 100 fold higher binding affinity to PDE6δ with a dissociation constant in the nanomolar range as compared to submicromolar affinities for Ras and Rheb.46 Stimulated by the findings that only Arl3 can release high affinity ciliary cargo from Unc11912,15 and from PDE6δ,38 we could confirm that under identical concentrations only Arl3 is able to release a C-terminal prenylated INPP5E peptide from its complex with PDE6δ, while Arl2 did not.46 Kinetic studies showed that the stimulated release of farnesylated INPP5E peptide by Arl3•GTP is 600fold faster than by Arl2•GTP.46 Structural and biochemical analysis of Arl2/3 complexes with PDE6δ and Unc119 showed that the major determinant for this difference is the structure and dynamics of the N-terminal amphiphatic helix which is absolutely required for the release activity of Arl3. The N-terminal helix of Arl3 but not Arl2 folds into a hydrophobic pocket on the surface of the protein and contributes to the release of the bound lipidated cargo15,24,46 (Fig. 2).
Manipulation of the affinity between carrier and cargo and cargo sorting by Arl2/3
Analysis of the structures of PDE6δ in complex with INPP5E peptide or Rheb peptide (or full-length Rheb protein) revealed that the farnesyl groups in both structures superimpose very well and that the first 3 residues N-terminal to the F-Cys-OMe (the farnesylated and methylated cysteine) are also located in the binding pocket. Differences in the interaction pattern due to the difference in sequence could be observed here.46 The structures suggested that the residues -1 and -3 relative to the farnesylated cysteine are responsible for the difference in affinity (Fig. 3). We thus created chimeric peptides where the residues at -1 and -3 positions were swapped, by changing Ser and Ile in INPP5E to Lys and Ser, and Lys and Ser in Rheb to Ser and Ile. Binding experiments with INPP5E(KS) and Rheb(SI) showed that we could reverse the binding affinities of these peptides to PDE6δ.46
Figure 3.
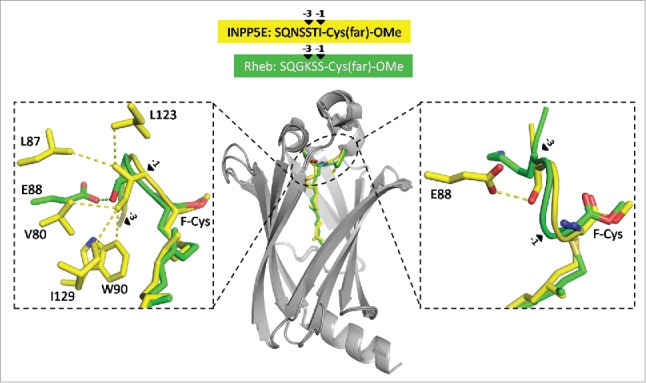
The sorting signal of farnesylated cargo. Superimposition of PDE6δ structures in complex with farnesylated INPP5E peptide (PDB code: 5F2U) and Rheb protein (PDB code: 3T5G). The residues at the -1 and -3 positions (arrows) relative to the farnesylated cysteine (F-Cys) from Rheb (green) and INPP5E (yellow) display different interaction patterns with the surrounding residues from PDE6δ (gray).
That the difference in affinity of the cargo-carrier complex is the major factor for ciliary entry was demonstrated by expressing the mutated proteins INPP5E(KS) and Rheb(SI) as GFP fusion proteins from a stable promoter in IMCD3 cells. This resulted in a dramatic turnaround in the specificity of localization of the proteins.46 INPP5E is no longer exclusive in the cilium and Rheb is now able to be localized inside the organelle. The fact that the localization of the mutated proteins is not exclusive for cilia or cytoplasm argues for a retention mechanism of lipidated ciliary proteins which keeps them inside once they have entered the compartment. Incidentally, the experiments also show that the low affinity mutant can be released by Arl2 in the cytoplasm.46
The combination of these data with the different release activities and localization pattern of Arl2/3 suggested a sorting mechanism of fanesylated proteins between cilia and other compartments (Fig. 4). The first step is the binding of high or low affinity farnesylated cargo to PDE6δ. In the second step high-affinity cargo is released by Arl3 inside cilia and low-affinity cargo by Arl2 in the rest of the cell. The last step is mediated by the specific retention of the farnesylated cargo at its destination. In line with this mechanism we showed that knockdown of Arl3 results in the redistribution of high affinity cargo (INPP5E) over the entire cell46 and a knockdown of Arl2 was shown to induce mis-localization of low affinity cargo such as Ras.28 We assume that a similar sorting mechanism applies also for the carrier protein Unc119 and its myristoylated cargo. This assumption is supported by the findings that Unc119 binds with different affinities to myristoylated cargo,12,15,45,47 where the high affinity cargo is exclusively released by Arl3.12,15 A further support for such a mechanism comes from our recent study which showed that manipulating the affinity between Unc119 and its cargo results in changing its cellular distribution.45
Figure 4.
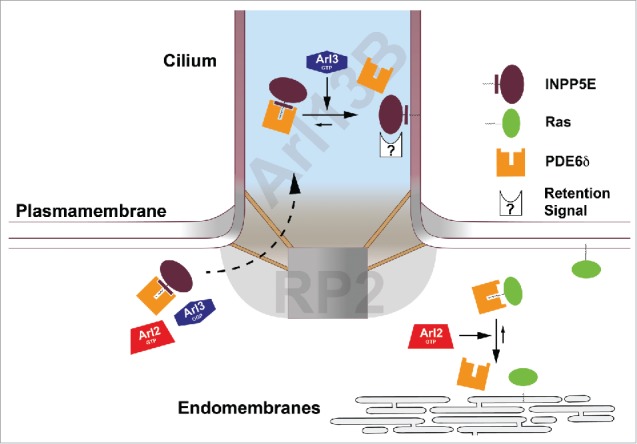
Sorting of farnesylated cargo by the Arl2/Arl3 system. The localization of Arl13B (Arl3GEF) inside the cilia and RP2 (Arl3GAP) at the ciliary exit creates an Arl3•GTP domain inside the cilium (the Arl13B domain is shaded blue and the RP2 domain is shaded gray). Farnesylated cargo such as INPP5E which binds to PDE6δ with high affinity cannot be released in the cytosol by Arl2•GTP or the inactive Arl3•GDP. Arl3•GTP releases INPP5E from its complex with PDE6δ inside the cilium. In contrast, Ras which binds to PDE6δ with low affinity can be released by Arl2•GTP in the cytosol. Mislocalization of Ras is observed after knockdown of both PDE6δ and Arl2.28,43 After release from PDE6δ, INPP5E is retained inside the cilia by the interaction with the ciliary membrane or specific interacting partners while Ras is retained outside the cilia by the interaction with plasma membrane and endomembranes.
The cilium as an Arl3•GTPcompartment
If lipidated ciliary cargo, due to its high affinity to the carrier proteins PDE6δ or Unc119, is supposed to be released by Arl3 only inside the cilium and not outside, the presence of an Arl3-specific guanine exchange factor exclusively localized inside the organelle is required to spatially activate Arl3. Such an Arl3-GEF was indeed identified as Arl13B.48 Arl13B is a GTP-binding protein itself, which in addition to the G domain contains a coiled-coil and a proline-rich domain and localizes exclusively inside the cilium.49 Surprisingly the G domain and a part of the CC domain are responsible for the GEF activity. The structure shows that Arl13B contacts Arl3 via its switch regions, which explains the finding that the GEF activity of Arl13B is dependent on its nucleotide state. Although the structure could not delineate the exact mechanism of how the nucleotide is kicked out, biochemical experiments suggest that an extended helical end (not visible in the structure) could directly reach into and interfere with the nucleotide binding site of Arl3,48 as demonstrated previously for many other GEFs.
The exclusive release of high affinity ciliary cargo by active Arl3 inside the cilia requires the additional condition, that active Arl3 is prevented from escaping the cilium. We have shown earlier that RP2 is an Arl3-specific GAP.18 In line with the proposal that the cilium is a compartment enriched in activated Arl3 (i.e. Arl3•GTP), localization studies using a GFP-RP2 stably expressed in IMCD3 cells show that RP2 is excluded from cilia. It is localized to the rest of the cell and is enriched at the base of the cilium.50,51 Taken together, one would postulate that Arl3, which is small enough to cross the barrier of the cilium, would get activated by Arl13B only inside the cilium and Arl3-bound GTP would be hydrolyzed by RP2 when exiting this compartment (Fig. 4). Furthermore the available evidence would suggest that these findings are not confined to IMCD3 cells but are applicable to most if not all ciliated cells, as all proteins involved in this mechanism (PDE6δ, Unc119, Arl2, Arl3, Arl13B and RP2) are conserved in evolution of high eukaryotics and ubiquitously expressed in cells and tissues. Moreover, RP2 and Arl13B are mutated in Retinitis Pigmentosa52 and Joubert syndrome (JBTS8),53 respectively, suggesting that both an increased concentration of Arl3•GTP (when RP2 activity is missing) or a decreased concentration of Arl3•GTP (when the GEF activity is low) lead to human diseases commonly called ciliopathies. Incidentally mutations in PDE6δ (JBTS22) and INPP5E (JBTS1) also lead to ciliopathies.38
Conclusion
We have summarized here the finding that the cilium is a compartment enriched in Arl3•GTP. This gradient of activated Arl3 across the barrier that separates the cilium from the rest of the cell is required and considered the driving force for the sorting of lipidated cargo into this structure. This situation is very much reminiscent of the nucleus where the action of a Ran•GTP gradient regulates nuclear transport across the nuclear pore complex (NPC) which is the barrier between nucleus and cytoplasm.54 The Ran-specific guanine nucleotide exchange factor RCC1 is located inside the nucleus because it binds to chromosomes.55,56 Ran-GAP is found in the cytoplasm but is concentrated at the exit site of the NPC because Ran-GAP is bound to a component of the NPC.57 The hydrolysis of Ran•GTP outside the nucleus leads to the release of the Ran•GTP-cargo-exportin complex, whereas the active Ran•GTP inside the nucleus dissociates the importin cargo complex by allosteric regulation just as Arl3•GTP allosterically releases cargo from PDE6d and Unc119.58
Localized activation should be a more general principle of the function of small G proteins, and is for example described for the Rab proteins, where the localization of GEF is responsible for the localized activation of a specific Rab and the initiation of specific transport processes.59 The same is true for the activation of Rho proteins which require a distinct localization of a particular Rho-GEF and a halo of a particular Rho-GAP around the area of activation, in order to induce locally defined and directed membrane deformations such as neurite protrusions or filopodia.60 A good example of temporal hierarchical regulation of the GTPase cycle has been demonstrated for the entry of Salmonella typhimurium into cells. The bacterium first injects, via the type III secretion system the Rho-GEF SopE into cell to activate actin remodeling and later injects the Rho-GAP SptP to finish the uptake.61 One can conclude that the separation of GEF and GAP activity in space and/or time is a general principle for small (and large) GTP-binding proteins which should be in place to avoid futile cycles of GTP hydrolysis.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was funded by the European Research Council (ERC Grant 268782) and Sonderforschungsbereich-DFG (SFB 642).
References
- [1].Clark J, Moore L, Krasinskas A, Way J, Battey J, Tamkun J, Kahn RA. Selective amplification of additional members of the ADP-ribosylation factor (ARF) family: cloning of additional human and Drosophila ARF-like genes. Proc Natl Acad Sci U S A 1993; 90:8952-6; PMID:8415637; http://dx.doi.org/ 10.1073/pnas.90.19.8952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gillingham AK, Munro S. The small G proteins of the Arf family and their regulators. Ann Rev Cell Dev Biol 2007; 23:579-611; PMID:17506703; http://dx.doi.org/ 10.1146/annurev.cellbio.23.090506.123209 [DOI] [PubMed] [Google Scholar]
- [3].Bologna G, Yvon C, Duvaud S, Veuthey AL. N-Terminal myristoylation predictions by ensembles of neural networks. Proteomics 2004; 4:1626-32; PMID:15174132; http://dx.doi.org/ 10.1002/pmic.200300783 [DOI] [PubMed] [Google Scholar]
- [4].Pasqualato S, Renault L, Cherfils J. Arf, Arl, Arp and Sar proteins: a family of GTP-binding proteins with a structural device for 'front-back' communication. EMBO Reports 2002; 3:1035-41; PMID:12429613; http://dx.doi.org/ 10.1093/embo-reports/kvf221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cherfils J, Zeghouf M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol Rev 2013; 93:269-309; PMID:23303910; http://dx.doi.org/ 10.1152/physrev.00003.2012 [DOI] [PubMed] [Google Scholar]
- [6].Sharer JD, Kahn RA. The ARF-like 2 (ARL2)-binding protein, BART. Purification, cloning, and initial characterization. J Biol Chem 1999; 274:27553-61; PMID:10488091; http://dx.doi.org/ 10.1074/jbc.274.39.27553 [DOI] [PubMed] [Google Scholar]
- [7].Sharer JD, Shern JF, Van Valkenburgh H, Wallace DC, Kahn RA. ARL2 and BART enter mitochondria and bind the adenine nucleotide transporter. Mol Biol Cell 2002; 13:71-83; PMID:11809823; http://dx.doi.org/ 10.1091/mbc.01-05-0245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lokaj M, Kosling SK, Koerner C, Lange SM, van Beersum SE, van Reeuwijk J, Roepman R, Horn N, Ueffing M, Boldt K, et al.. The Interaction of CCDC104/BARTL1 with Arl3 and implications for ciliary function. Structure 2015; 23:2122-32; PMID:26455799; http://dx.doi.org/ 10.1016/j.str.2015.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhang T, Li S, Zhang Y, Zhong C, Lai Z, Ding J. Crystal structure of the ARL2-GTP-BART complex reveals a novel recognition and binding mode of small GTPase with effector. Structure 2009; 17:602-10; PMID:19368893; http://dx.doi.org/ 10.1016/j.str.2009.01.014 [DOI] [PubMed] [Google Scholar]
- [10].Linari M, Hanzal-Bayer M, Becker J. The delta subunit of rod specific cyclic GMP phosphodiesterase, PDE delta, interacts with the Arf-like protein Arl3 in a GTP specific manner. FEBS Letters 1999; 458:55-9; PMID:10518933; http://dx.doi.org/ 10.1016/S0014-5793(99)01117-5 [DOI] [PubMed] [Google Scholar]
- [11].Kobayashi A, Kubota S, Mori N, McLaren MJ, Inana G. Photoreceptor synaptic protein HRG4 (UNC119) interacts with ARL2 via a putative conserved domain. FEBS Letters 2003; 534:26-32; PMID:12527357; http://dx.doi.org/ 10.1016/S0014-5793(02)03766-3 [DOI] [PubMed] [Google Scholar]
- [12].Wright KJ, Baye LM, Olivier-Mason A, Mukhopadhyay S, Sang L, Kwong M, Wang W, Pretorius PR, Sheffield VC, Sengupta P, et al.. An ARL3-UNC119-RP2 GTPase cycle targets myristoylated NPHP3 to the primary cilium. Genes Dev 2011; 25:2347-60; PMID:22085962; http://dx.doi.org/ 10.1101/gad.173443.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hanzal-Bayer M, Renault L, Roversi P, Wittinghofer A, Hillig RC. The complex of Arl2-GTP and PDE delta: from structure to function. EMBO J 2002; 21:2095-106; PMID:11980706; http://dx.doi.org/ 10.1093/emboj/21.9.2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhang H, Constantine R, Vorobiev S, Chen Y, Seetharaman J, Huang YJ, Xiao R, Montelione GT, Gerstner CD, Davis MW, et al.. UNC119 is required for G protein trafficking in sensory neurons. Nat Neurosci 2011; 14:874-80; PMID:21642972; http://dx.doi.org/ 10.1038/nn.2835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ismail SA, Chen YX, Miertzschke M, Vetter IR, Koerner C, Wittinghofer A. Structural basis for Arl3-specific release of myristoylated ciliary cargo from UNC119. EMBO J 2012; 31:4085-94; PMID:22960633; http://dx.doi.org/ 10.1038/emboj.2012.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Veltel S, Kravchenko A, Ismail S, Wittinghofer A. Specificity of Arl2/Arl3 signaling is mediated by a ternary Arl3-effector-GAP complex. FEBS Letters 2008; 582:2501-7; PMID:18588884; http://dx.doi.org/ 10.1016/j.febslet.2008.05.053 [DOI] [PubMed] [Google Scholar]
- [17].Hanzal-Bayer M, Linari M, Wittinghofer A. Properties of the interaction of Arf-like protein 2 with PDEdelta. J Mol Biol 2005; 350:1074-82; PMID:15979089; http://dx.doi.org/ 10.1016/j.jmb.2005.05.036 [DOI] [PubMed] [Google Scholar]
- [18].Veltel S, Gasper R, Eisenacher E, Wittinghofer A. The retinitis pigmentosa 2 gene product is a GTPase-activating protein for Arf-like 3. Nat Struct Mol Biol 2008; 15:373-80; PMID:18376416; http://dx.doi.org/ 10.1038/nsmb.1396 [DOI] [PubMed] [Google Scholar]
- [19].Jaworek TJ, Richard EM, Ivanova AA, Giese AP, Choo DI, Khan SN, Riazuddin S. An alteration in ELMOD3, an Arl2 GTPase-activating protein, is associated with hearing impairment in humans. PLoS Genetics 2013; 9:e1003774; PMID:24039609; http://dx.doi.org/ 10.1371/journal.pgen.1003774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].East MP, Bowzard JB, Dacks JB, Kahn RA. ELMO domains, evolutionary and functional characterization of a novel GTPase-activating protein (GAP) domain for Arf protein family GTPases. J Biol Chem 2012; 287:39538-53; PMID:23014990; http://dx.doi.org/ 10.1074/jbc.M112.417477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ivanova AA, East MP, Yi SL, Kahn RA. Characterization of recombinant ELMOD (cell engulfment and motility domain) proteins as GTPase-activating proteins (GAPs) for ARF family GTPases. J Biol Chem 2014; 289:11111-21; PMID:24616099; http://dx.doi.org/ 10.1074/jbc.M114.548529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bhamidipati A, Lewis SA, Cowan NJ. ADP ribosylation factor-like protein 2 (Arl2) regulates the interaction of tubulin-folding cofactor D with native tubulin. J Cell Biol 2000; 149:1087-96; PMID:10831612; http://dx.doi.org/ 10.1083/jcb.149.5.1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Nithianantham S, Le S, Seto E, Jia W, Leary J, Corbett KD, Moore JK, Al-Bassam J. Tubulin cofactors and Arl2 are cage-like chaperones that regulate the soluble alphabeta-tubulin pool for microtubule dynamics. Elife 2015; 4; PMID:26208336; http://dx.doi.org/ 10.7554/eLife.08811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kapoor S, Fansa EK, Mobitz S, Ismail SA, Winter R, Wittinghofer A, Weise K. Effect of the N-Terminal helix and nucleotide loading on the membrane and effector binding of Arl2/3. Biophysical J 2015; 109:1619-29; PMID:26488653; http://dx.doi.org/ 10.1016/j.bpj.2015.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhou C, Cunningham L, Marcus AI, Li Y, Kahn RA. Arl2 and Arl3 regulate different microtubule-dependent processes. Mol Biol Cell 2006; 17:2476-87; PMID:16525022; http://dx.doi.org/ 10.1091/mbc.E05-10-0929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Breslow DK, Koslover EF, Seydel F, Spakowitz AJ, Nachury MV. An in vitro assay for entry into cilia reveals unique properties of the soluble diffusion barrier. J Cell Biol 2013; 203:129-47; PMID:24100294; http://dx.doi.org/ 10.1083/jcb.201212024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Newman LE, Zhou CJ, Mudigonda S, Mattheyses AL, Paradies E, Marobbio CM, Kahn RA. The ARL2 GTPase is required for mitochondrial morphology, motility, and maintenance of ATP levels. PloS One 2014; 9:e99270; PMID:24911211; http://dx.doi.org/ 10.1371/journal.pone.0099270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Schmick M, Vartak N, Papke B, Kovacevic M, Truxius DC, Rossmannek L, Bastiaens PI. KRas localizes to the plasma membrane by spatial cycles of solubilization, trapping and vesicular transport. Cell 2014; 157:459-71; PMID:24725411; http://dx.doi.org/ 10.1016/j.cell.2014.02.051 [DOI] [PubMed] [Google Scholar]
- [29].Schrick JJ, Vogel P, Abuin A, Hampton B, Rice DS. ADP-ribosylation factor-like 3 is involved in kidney and photoreceptor development. Am J Pathol 2006; 168:1288-98; PMID:16565502; http://dx.doi.org/ 10.2353/ajpath.2006.050941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Strom SP, Clark MJ, Martinez A, Garcia S, Abelazeem AA, Matynia A, Parikh S, Sullivan LS, Bowne SJ, Daiger SP, et al.. De novo occurrence of a variant in ARL3 and apparent autosomal dominant transmission of retinitis pigmentosa. PloS One 2016; 11:e0150944; PMID:26964041; http://dx.doi.org/ 10.1371/journal.pone.0150944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wright ZC, Singh RK, Alpino R, Goldberg AF, Sokolov M, Ramamurthy V. ARL3 regulates trafficking of prenylated phototransduction proteins to the rod outer segment. Hum Mol Genetics 2016; PMID:26936825; http://dx.doi.org/26814127 10.1093/hmg/ddw077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hanke-Gogokhia C, Wu Z, Gerstner CD, Frederick JM, Zhang H, Baehr W. Arf-like Protein 3 (ARL3) regulates protein trafficking and ciliogenesis in mouse photoreceptors. J Biol Chem 2016; 291:7142-55; PMID:26814127; http://dx.doi.org/ 10.1074/jbc.M115.710954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhang H, Liu XH, Zhang K, Chen CK, Frederick JM, Prestwich GD, Baehr W. Photoreceptor cGMP phosphodiesterase delta subunit (PDEdelta) functions as a prenyl-binding protein. J Biol Chem 2004; 279:407-13; PMID:14561760; http://dx.doi.org/ 10.1074/jbc.M306559200 [DOI] [PubMed] [Google Scholar]
- [34].Chen YX, Koch S, Uhlenbrock K, Weise K, Das D, Gremer L, Brunsveld L, Wittinghofer A, Winter R, Triola G, et al.. Synthesis of the Rheb and K-Ras4B GTPases. Angewandte Chemie 2010; 49:6090-5; PMID:20652921; http://dx.doi.org/ 10.1002/anie.201001884 [DOI] [PubMed] [Google Scholar]
- [35].Florio SK, Prusti RK, Beavo JA. Solubilization of membrane-bound rod phosphodiesterase by the rod phosphodiesterase recombinant delta subunit. J Biol Chem 1996; 271:24036-47; PMID:8798640; http://dx.doi.org/ 10.1074/jbc.271.39.24036 [DOI] [PubMed] [Google Scholar]
- [36].Humbert MC, Weihbrecht K, Searby CC, Li Y, Pope RM, Sheffield VC, Seo S. ARL13B, PDE6D, and CEP164 form a functional network for INPP5E ciliary targeting. Proc Natl Acad Sci U S A 2012; 109:19691-6; PMID:23150559; http://dx.doi.org/ 10.1073/pnas.1210916109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Chandra A, Grecco HE, Pisupati V, Perera D, Cassidy L, Skoulidis F, Ismail SA, Hedberg C, Hanzal-Bayer M, Venkitaraman AR, et al.. The GDI-like solubilizing factor PDEdelta sustains the spatial organization and signalling of Ras family proteins. Nat Cell Biol 2012; 14:148-58; http://dx.doi.org/ 10.1038/ncb2394 [DOI] [PubMed] [Google Scholar]
- [38].Thomas S, Wright KJ, Le Corre S, Micalizzi A, Romani M, Abhyankar A, Saada J, Perrault I, Amiel J, Litzler J, et al.. A homozygous PDE6D mutation in Joubert syndrome impairs targeting of farnesylated INPP5E protein to the primary cilium. Hum Mutation 2014; 35:137-46; PMID:24166846; http://dx.doi.org/ 10.1002/humu.22470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hoffman GR, Nassar N, Cerione RA. Structure of the Rho family GTP-binding protein Cdc42 in complex with the multifunctional regulator RhoGDI. Cell 2000; 100:345-56; PMID:10676816; http://dx.doi.org/ 10.1016/S0092-8674(00)80670-4 [DOI] [PubMed] [Google Scholar]
- [40].Rak A, Pylypenko O, Durek T, Watzke A, Kushnir S, Brunsveld L, Waldmann H, Goody RS, Alexandrov K. Structure of Rab GDP-dissociation inhibitor in complex with prenylated YPT1 GTPase. Science 2003; 302:646-50; PMID:14576435; http://dx.doi.org/ 10.1126/science.1087761 [DOI] [PubMed] [Google Scholar]
- [41].Ismail SA, Chen YX, Rusinova A, Chandra A, Bierbaum M, Gremer L, Triola G, Waldmann H, Bastiaens PI, Wittinghofer A. Arl2-GTP and Arl3-GTP regulate a GDI-like transport system for farnesylated cargo. Nat Chem Biol 2011; 7:942-9; PMID:22002721; http://dx.doi.org/ 10.1038/nchembio.686 [DOI] [PubMed] [Google Scholar]
- [42].Fansa EK, O'Reilly NJ, Ismail S, Wittinghofer A. The N- and C-terminal ends of RPGR can bind to PDE6delta. EMBO Reports 2015; 16:1583-5; PMID:26553937; http://dx.doi.org/ 10.15252/embr.201541404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zimmermann G, Papke B, Ismail S, Vartak N, Chandra A, Hoffmann M, Hahn SA, Triola G, Wittinghofer A, Bastiaens PI, et al.. Small molecule inhibition of the KRAS-PDEdelta interaction impairs oncogenic KRAS signalling. Nature 2013; 497:638-42; PMID:23698361; http://dx.doi.org/ 10.1038/nature12205 [DOI] [PubMed] [Google Scholar]
- [44].Papke B, Murarka S, Vogel HA, Martin-Gago P, Kovacevic M, Truxius DC, Fansa EK, Ismail S, Zimmermann G, Heinelt K, et al.. Identification of pyrazolopyridazinones as PDEdelta inhibitors. Nat Commun 2016; 7:11360; PMID:27094677; http://dx.doi.org/ 10.1038/ncomms11360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Jaiswal M, Fansa EK, Kosling SK, Mejuch T, Waldmann H, Wittinghofer A. Novel biochemical and structural insights into the interaction of myristoylated cargo with Unc119 and their release by Arl2/3. J Biol Chem 2016; PMID:27481943; http://dx.doi.org/27063844 10.1074/jbc.M116.741827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Fansa EK, Kosling SK, Zent E, Wittinghofer A, Ismail S. PDE6delta-mediated sorting of INPP5E into the cilium is determined by cargo-carrier affinity. Nat Commun 2016; 7:11366; PMID:27063844; http://dx.doi.org/ 10.1038/ncomms11366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Mejuch T, van Hattum H, Triola G, Jaiswal M, Waldmann H. Specificity of lipoprotein chaperones for the characteristic lipidated structural motifs of their cognate lipoproteins. Chem Bio Chem 2015; 16:2460-5; PMID:26503308; http://dx.doi.org/ 10.1002/cbic.201500355 [DOI] [PubMed] [Google Scholar]
- [48].Gotthardt K, Lokaj M, Koerner C, Falk N, Giessl A, Wittinghofer A. A G-protein activation cascade from Arl13B to Arl3 and implications for ciliary targeting of lipidated proteins. Elife 2015; 4:e11859; http://dx.doi.org/ 10.7554/eLife.11859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Miertzschke M, Koerner C, Spoerner M, Wittinghofer A. Structural insights into the small G-protein Arl13B and implications for Joubert syndrome. Biochem J 2014; 457:301-11; PMID:24168557; http://dx.doi.org/ 10.1042/BJ20131097 [DOI] [PubMed] [Google Scholar]
- [50].Evans RJ, Schwarz N, Nagel-Wolfrum K, Wolfrum U, Hardcastle AJ, Cheetham ME. The retinitis pigmentosa protein RP2 links pericentriolar vesicle transport between the Golgi and the primary cilium. Hum Mol Genetics 2010; 19:1358-67; PMID:20106869; http://dx.doi.org/ 10.1093/hmg/ddq012 [DOI] [PubMed] [Google Scholar]
- [51].Hurd T, Zhou W, Jenkins P, Liu CJ, Swaroop A, Khanna H, Martens J, Hildebrandt F, Margolis B. The retinitis pigmentosa protein RP2 interacts with polycystin 2 and regulates cilia-mediated vertebrate development. Hum Mol Genetics 2010; 19:4330-44; PMID:20729296; http://dx.doi.org/ 10.1093/hmg/ddq355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Heckenlively JR, Yoser SL, Friedman LH, Oversier JJ. Clinical findings and common symptoms in retinitis pigmentosa. Am J Ophthalmol 1988; 105:504-11; PMID:3259404; http://dx.doi.org/ 10.1016/0002-9394(88)90242-5 [DOI] [PubMed] [Google Scholar]
- [53].Cantagrel V, Silhavy JL, Bielas SL, Swistun D, Marsh SE, Bertrand JY, Audollent S, Attié-Bitach T, Holden KR, Dobyns WB, et al.. Mutations in the cilia gene ARL13B lead to the classical form of Joubert syndrome. Am J Hum Genetics 2008; 83:170-9; PMID:18674751; http://dx.doi.org/ 10.1016/j.ajhg.2008.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kalab P, Pralle A, Isacoff EY, Heald R, Weis K. Analysis of a RanGTP-regulated gradient in mitotic somatic cells. Nature 2006; 440:697-701; PMID:16572176; http://dx.doi.org/ 10.1038/nature04589 [DOI] [PubMed] [Google Scholar]
- [55].Ohtsubo M, Kai R, Furuno N, Sekiguchi T, Sekiguchi M, Hayashida H, Kuma K, Miyata T, Fukushige S, Murotsu T, et al.. Isolation and characterization of the active cDNA of the human cell cycle gene (RCC1) involved in the regulation of onset of chromosome condensation. Genes Dev 1987; 1:585-93; PMID:3678831; http://dx.doi.org/ 10.1101/gad.1.6.585 [DOI] [PubMed] [Google Scholar]
- [56].Renault L, Kuhlmann J, Henkel A, Wittinghofer A. Structural basis for guanine nucleotide exchange on Ran by the regulator of chromosome condensation (RCC1). Cell 2001; 105:245-55; PMID:11336674; http://dx.doi.org/ 10.1016/S0092-8674(01)00315-4 [DOI] [PubMed] [Google Scholar]
- [57].Seewald MJ, Korner C, Wittinghofer A, Vetter IR. RanGAP mediates GTP hydrolysis without an arginine finger. Nature 2002; 415:662-6; PMID:11832950; http://dx.doi.org/ 10.1038/415662a [DOI] [PubMed] [Google Scholar]
- [58].Stewart M. Molecular mechanism of the nuclear protein import cycle. Nat Rev Mol Cell Biol 2007; 8:195-208; PMID:17287812; http://dx.doi.org/ 10.1038/nrm2114 [DOI] [PubMed] [Google Scholar]
- [59].Blumer J, Rey J, Dehmelt L, Mazel T, Wu YW, Bastiaens P, Goody RS, Itzen A. RabGEFs are a major determinant for specific Rab membrane targeting. J Cell Biol 2013; 200:287-300; PMID:23382462; http://dx.doi.org/ 10.1083/jcb.201209113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Braun AC, Olayioye MA. Rho regulation: DLC proteins in space and time. Cell Signal 2015; 27:1643-51; PMID:25889896; http://dx.doi.org/ 10.1016/j.cellsig.2015.04.003 [DOI] [PubMed] [Google Scholar]
- [61].Zhou D, Galan J. Salmonella entry into host cells: the work in concert of type III secreted effector proteins. Microbes Infect 2001; 3:1293-8; PMID:11755417; http://dx.doi.org/ 10.1016/S1286-4579(01)01489-7 [DOI] [PubMed] [Google Scholar]


