ABSTRACT
The target of rapamycin complex 1 (TORC1) plays a central role in controlling eukaryotic cell growth by fine-tuning anabolic and catabolic processes to the nutritional status of organisms and individual cells. Amino acids represent essential and primordial signals that modulate TORC1 activity through the conserved Rag family GTPases. These assemble, as part of larger lysosomal/vacuolar membrane-associated complexes, into heterodimeric sub-complexes, which typically comprise two paralogous Rag GTPases of opposite GTP-/GDP-loading status. The TORC1-stimulating/inhibiting states of these heterodimers are controlled by various guanine nucleotide exchange factor (GEF) and GTPase-activating protein (GAP) complexes, which are remarkably conserved in various eukaryotic model systems. Among the latter, the budding yeast Saccharomyces cerevisiae has been instrumental for the elucidation of basic aspects of Rag GTPase regulation and function. Here, we discuss the current state of the respective research, focusing on the major unsolved issues regarding the architecture, regulation, and function of the Rag GTPase containing complexes in yeast. Decoding these mysteries will undoubtedly further shape our understanding of the conserved and divergent principles of nutrient signaling in eukaryotes.
KEYWORDS: amino acid signaling, EGO complex (EGOC), Lst4-Lst7, Rag GTPases, SEACAT, SEACIT, Target of Rapamycin Complex 1 (TORC1), yeast
Introduction
In eukaryotes, cell growth and proliferation are coordinated through finely tuned signaling networks that sense and respond to diverse signals including growth factors, hormones, and nutrients. One pivotal pathway, which couples such signals to downstream effectors to appropriately and reciprocally control anabolic (e.g., protein translation) and catabolic (e.g., macroautophagy) growth related programs, is the target of rapamycin complex 1 (TORC1) pathway.1,2 Deregulation of this pathway in humans is associated with a number of pathological conditions, including cancer, obesity, type 2 diabetes, and neurodegeneration,2 which underscores the central role of this pathway in eukaryotic growth control. Among the various signals that impinge on TORC1, amino acids represent indispensable and primordial input signals for TORC1 activation that cannot be compensated for by any other stimulus. The underlying mechanism(s) of amino acid-mediated TORC1 activation, however, has long remained obscure. The recent discovery of the Rag family of GTPases and their involvement in amino acid signaling to TORC1 in various model systems, however, has begun to shed light on this essential aspect of TORC1 control.3-5 Accordingly, the Rag family GTPases assemble into heterodimeric sub-complexes consisting of mammalian RagA or RagB and RagC or RagD, or yeast Gtr1 and Gtr2. As part of the lysosomal/vacuolar membrane-associated mammalian Rag-Ragulator or yeast EGO complexes (EGOC), they relay amino acid signals towards TORC1. The TORC1-stimulating state of these heterodimers contains GTP-bound RagA/B/Gtr1 and GDP-bound RagC/D/Gtr2. Due to the rather conserved nature of the respective control mechanisms, studies in simple yeast cells, including for instance the original description of Rag GTPases in heterodimeric complexes,6-8 the first implication of Rag GTPases (as part of the larger EGOC) in TORC1 signaling,9 and the recent discoveries of Rag GTPase regulators,4,10-12 have been valuable in complementing the ones carried out with mammalian and Drosophila cells. Based on our current knowledge on Rag GTPase function and regulation in general,13 we will highlight here some of the outstanding challenges and questions that have been invoked by studies in yeast.
Architecture and localization of Rag GTPases within larger complexes
In higher eukaryotes, the Rag GTPase heterodimers are recruited to lysosomes via the pentameric Ragulator complex that exerts GEF activity towards the Rag GTPases RagA/B.14 The equivalent of the Ragulator complex in yeast, however, only consists of 3 proteins (Ego1/Meh1, Ego2, and Ego3/Slm4). This ternary complex (EGO-TC) mainly acts as a scaffold to tether the Rag GTPases to the vacuolar membrane rather than exerting any GEF activity, which is likely mediated by Vam6 and/or additional associated proteins.13,15-17 Whether these structural and functional differences between Ragulator and EGO-TC are simply due to the fact that additional EGO-TC associated proteins have so far escaped experimental detection remains an important issue to be addressed in yeast (Fig. 1, points 1 and 2).
Figure 1.
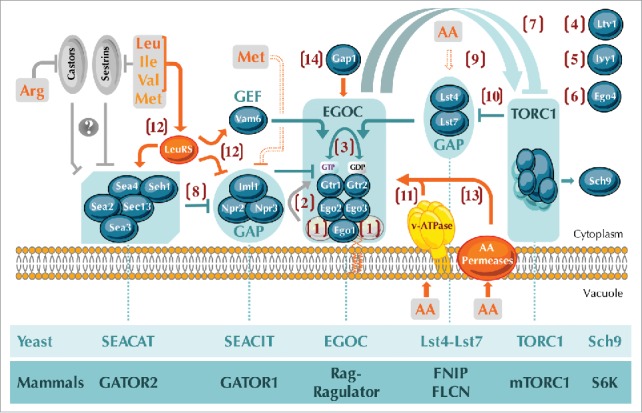
Unsolved questions regarding the amino acid-dependent activation of TORC1 by Rag GTPases and their modulators in yeast. Numbers in brackets refer to the specific questions that remain to be resolved: (1, 2) Is the Gtr1-Gtr2 tethering complex, similar to the mammalian Ragulator, composed of 5 rather than the currently known 3 proteins (Ego1, Ego2, and Ego3) (1), and, if yes, may this potentially pentameric complex exert GEF activity towards Gtr1 (2)? (3) Can the GDP/GTP loading status of Gtr1 impact on the one of Gtr2 and vice versa? (4, 5, 6) What is the role of Ltv1 (4), Ivy1 (5), and Ego4 (6) within the Rag-GTPase-TORC1 network? (7) How do active (i.e. Gtr1GTP-Gtr2GDP) and “inactive” (i.e. Gtr1GDP-Gtr2GTP) Rag GTPases activate and inactivate, respectively, TORC1 in yeast? (8) Does SEACAT negatively regulate SEACIT and, if yes, how? (9) How do amino acids impinge on the Gtr2-GAP complex Lst4-Lst7? (10) Does TORC1 regulate the Lst4-Lst7 complex via a negative feedback loop? (11) Does the v-ATPase mediate vacuolar amino acid signals towards Rag GTPases? (12) By which mechanism does the leucyl-tRNA synthetase signal balanced levels of branched-chain amino acids towards Rag GTPases? (13, 14) Do vacuolar amino acid permeases (13) or the general amino acid permease Gap1 (14) mediate amino acid signals towards Rag GTPases? Yeast SEACAT, SEACIT, EGOC, Lst4-Lst7, and TORC1 complexes have mammalian orthologs coined GATOR2, GATOR1, Rag-Ragulator, FNIP-FLCN, and mTORC1, respectively (lanes below the figure). In addition, the protein kinase Sch9 is the currently best-known bona fide TORC1 target in yeast and is similar to the mammalian TORC1 target S6 kinase (S6K). Color code: grey proteins and arrows/bars refer to mammalian proteins and processes, respectively, while amino acids and focal points that directly sense amino acids are in orange (with the v-ATPase in yellow, as it is currently only known to mediate pH signals towards Rag GTPases). Methionine indirectly prevents the assembly of SEACIT (dashed bar) and specific amino acids directly or indirectly regulate the Lst4-Lst7 complex (dashed arrow). Mammalian Sestrin and Castor proteins sense leucine (and less potently isoleucine, valine, and methionine) and arginine, respectively, and likely (indicated with a question mark) act upstream of the GATOR2-GATOR1 branch. Arrows and bars denote positive and negative interactions, respectively. AA, amino acids; Arg, arginine; Leu, leucine; Ile, isoleucine; Val, valine; Met, methionine; GAP, GTPase activating protein; GEF, guanine nucleotide exchange factor; v-ATPase, vacuolar ATPase. For further details see text.
Within the EGOC, Gtr1 and Gtr2, similar to RagA/B and RagC/D in higher eukaryotes, assemble into asymmetrically loaded, functionally active Gtr1GTP/Gtr2GDP and inactive Gtr1GDP/Gtr2GTP heterodimers that stimulate and inhibit, respectively, TORC1 in vivo. Interestingly, monomeric and homodimeric forms of Gtr1, as well Gtr1/Gtr2 heterodimers that are symmetrically loaded with guanine nucleotides have also been described to occur,6,18-20 but it remains currently unknown whether any of these variants may play a functional role in vivo. In this context, it has been reported that the GTP loading status of Gtr2 likely impacts on the capacity of Gtr1 to hydrolyse GTP,11 which indicates the existence of largely unexplored mechanisms by which the GTP/GDP loading status of Gtr2 may influence the one of Gtr1 and vice versa (Fig. 1, point 3; please also see our recent review for a discussion of structural aspects of the Gtr1-Gtr2 heterodimer that support such mechanisms).13
While the core of the EGOC consists of five proteins (i.e. Gtr1, Gtr2, Ego1, Ego2, and Ego3),15 additional proteins have been found to associate with this complex in yeast. For instance Ltv1, which plays role in the maturation of 40S ribosomal subunits,21 seems to bind the EGOC through its interaction with Gtr1,20 but its functional role within the EGOC-TORC1 pathway, if any, is still obscure (Fig. 1, point 4). Similarly, the I-Bar protein Ivy1, which likely plays its main role in vacuolar membrane homeostasis,22 colocalizes and interacts with the EGOC, but the mechanistic details on how it may be linked to the TORC1 pathway remain to be elucidated (Fig. 1, point 5). Finally, yeast cells express a paralog of Ego2, namely Ego4, which binds the EGOC at least in two-hybrid assays, but seems to play no significant role in TORC1 regulation in exponentially growing cells (Fig. 1, point 6).15 A recent study, however, revealed that the levels of Ego4 are low in exponentially growing cells and strongly increase when cells transit through the diauxic shift.23 Thus, Ego4 may have a more specific role in glucose-starved cells within the EGOC, which would indicate the intriguing possibility that nutritional conditions could shape the composition and structure of the EGOC. Whether such a principle also applies to other EGOC subunits has not yet been addressed experimentally.
In addition to the various open questions regarding the architecture of the EGOC, there is also an important void in our knowledge on the mechanisms that specifically tether the EGOC to both the vacuolar surface and some defined perivacuolar foci of still unknown identity.4,22-25 Key for the proper localization of the entire EGOC are N-terminal lipid modifications of Ego1, which are carried out by the myristoyl-CoA:protein N-myristoyltransferase Nmt1 and the palmitoyltransferases Akr1 and Pfa3.26-28 How the respective modifications target Ego1 to the vacuolar membrane and the perivacuolar foci and whether the underlying process(es) per se may be subjected to nutrient regulation will be interesting issues to be addressed in future studies.
Regulation of TORC1 by Rag GTPases
In higher eukaryotes, amino acid-stimulated Rag GTPases do not directly promote the kinase activity of TORC1, but they recruit TORC1 from the cytoplasm to the lysosomal membrane to enable its activation by Rheb.5 In budding yeast, however, TORC1 is constitutively present at the vacuolar membrane as well as in perivacuolar foci and remains detectable at these sites even in the absence of amino acids (e.g., leucine) or nitrogen.4,24 Moreover, although Rag GTPases seem to shape the relative distribution of TORC1 between vacuolar membranes and perivacuolar foci,25 it remains unclear how this could affect TORC1 activity, since loss of the Rheb ortholog Rhb1 has no measurable effect on the steady state activity of TORC1 in budding yeast.4,29 How Rag GTPases regulate TORC1 in yeast therefore remains one of the central unsolved mysteries in this field (Fig. 1, point 7). Testable assumptions include the possibilities that the Rag GTPases themselves or any of the Rhb1-related GTPases (e.g., Ras1/2), alone or redundantly with Rhb1, directly activate TORC1, as does Rheb in mammalian cells.30 Alternatively, yeast Rag GTPases may be required for local structural adjustments of TORC1 on the vacuolar surface that could critically affect its function. In line with this notion, structural analyses of the Gtr1-Gtr2 heterodimer predict that GTP hydrolysis events induce dynamic G-domain arrangements (within both GTPases) that may control the exposition of a binding surface for the TORC1 subunit Kog1 on the Rag GTPase heterodimer.18,31 This fits also well with the observation that the active Rag GTPase heterodimers, but not the respective inactive ones, strongly bind TORC1 via Kog1 in yeast (like they do with the Kog1-orthologous TORC1 subunit Raptor in mammalian cells).4,5,18,32
Recent, elegant studies in higher eukaryotes have shown that the inactive combination of Rag GTPases recruits the Rheb GAP TSC2 to the lysosome, which is required to completely inactivate TORC1 following amino acid starvation.24,33 Although S. cerevisiae cells do not express any obvious TSC2 ortholog, the inactive Rag GTPase module has also been reported to negatively affect growth, which could be rescued by loss of the TORC1 subunit Tco89.4 These observations indicate that the “inactive” Rag GTPase module in yeast may in fact play an active role in TORC1 downregulation, possibly via a mechanism that involves still elusive proteins (Fig. 1, point 7). Notably, yeast TORC1 can also (reversibly) be inactivated via its disassembly and movement of Kog1 to a single body on the edge of the vacuole. This process is triggered by glucose starvation and mediated via (direct or indirect) AMP kinase (AMPK)/Snf1-dependent phosphorylation of Kog1 within its prion-like motifs.34 Interestingly, organisms that express Kog1/Raptor proteins with prion-like domains (e.g., S. cerevisiae and C. elegans) do not have readily identifiable TSC2-like Rheb GAPs and may therefore utilize Kog1-body formation to inactivate TORC1 following energy-limitation that results from glucose starvation. In contrast, species that do not exhibit such domains in Kog1/Raptor (e.g., S. pombe, Drosophila, and mammals), appear to couple low energy levels to TORC1 via AMPK-mediated activation of the Rheb-GAP TSC2.34 The underlying mechanisms by which TORC1 integrates energy levels may therefore have diverged during early eukaryotic evolution. Since glucose starvation-mediated inhibition of S. cerevisiae TORC1 is largely independent of Rag GTPases,35 Gtr1/2 are likely of marginal importance for Kog1-body formation under these conditions. It remains, however, formally possible that Rag GTPases contribute to the control of Kog1-body formation/dissociation in response to changes in nitrogen or amino acid levels.
Reactivation of TORC1 following re-stimulation of starved yeast cells with amino acids is intriguingly bimodal, involving an early (2–4 min) transient Rag GTPase-dependent and a later (> 30 min) Rag GTPase-independent mechanisms of TORC1 activation.36 These data indicate that Rag GTPases play a particularly prominent role in dynamically adjusting TORC1 activity upon rapid changes in amino acid levels. Interestingly, virtually any amino acid, independently of its quality to serve as nitrogen source, can trigger the early transient activation of TORC1 in cells that are pre-grown on proline as the only nitrogen source.36 This indicates that the underlying mechanism for TORC1 activation may at least in part rely on a direct sensing/signaling event, rather than a more complex mechanism that signals the metabolic value of the respective amino acids (which may in fact be the information required for long-term sustained activation of TORC1).
Regulation of Rag GTPases by GAPs, GEFs, and GDIs
In addition to the open issues regarding the Gtr1 GEF (see above), the currently available data only incompletely explain how amino acids control the different GAP complexes that impinge on Gtr1 and Gtr2. For instance, methionine has been proposed to indirectly promote Ppm1-mediated methylation of the catalytic subunit of the type 2A protein phosphatase (PP2A) to trigger dephosphorylation of Npr2. This prevents the proper assembly of the Npr2/3-Iml1-containing Gtr1 GAP complex coined SEACIT, which inhibits the amino acid sensing pathway (like the orthologous GATOR1 complex in higher eukaryotes).37-39 How amino acids other than methionine regulate SEACIT in yeast is currently not known. Studies in higher eukaryotic cells, however, indicate that Sestrin2 and Castor1 are specific leucine and arginine sensors for the TORC1 pathway, respectively, that likely act upstream of GATOR1. Both proteins do not directly act on GATOR1, but bind the GATOR1-associated GATOR2 complex in the absence of amino acids to inhibit TORC1 by an unknown mechanism.40-44 Of note, whether Sestrin2 plays a role as leucine sensor in organisms other than mammals appears to be subject to controversy.45 In yeast, Sestrin and Castor orthologs are not readily identifiable, but the GATOR2-orthologous complex named SEACAT also mediates amino acid signals to TORC1 via SEACIT.10,11 How this is achieved should be addressed in future studies (Fig. 1, point 8), which may also take into consideration that ubiquitination of Rag GTPases can, as exemplified for the RagA-GATOR1 interaction,46,47 considerably influence the association of Rag GTPases with their regulators.
Recent data described the existence of the Lst4-Lst7 complex that functions (like the FNIP-Folliculin [FLCN] complex for RagC/D)32,48 as a GAP for Gtr2, which, upon refeeding of starved cells with amino acids, transiently binds to and acts on Gtr2 to activate TORC1.12 Interestingly, the Lst4-Lst7 complex clusters at Gtr2-proximal sites on the vacuolar membrane in amino acid starved cells, although interacting only weakly with Gtr2 under these conditions, while being dispersed from these sites upon re-addition of amino acids. Combined, these data can be explained in a model in which the availability of specific amino acids is communicated to the vacuolar membrane-associated pool of Lst4-Lst7 complexes to prompt their binding to and facilitate their GAP function towards Gtr2 (Fig. 1, point 9). This would be followed by TORC1 activation and subsequent release of the Lst4-Lst7 complex from the vacuolar membrane. How the presence of amino acids can be interpreted by the Lst4-Lst7 complex and whether this proposed feedback mechanism contributes to the transient nature of the rapid amino acid-induced, Rag GTPase-dependent activation of TORC1 (see above) will be attractive questions to be tested in the future (Fig. 1, points 9 and 10).
Finally, it remains possible that our compendium of Rag GTPase regulators is still incomplete. For instance, GEFs for Gtr2 (or RagC/D) are currently not known. However, previous biochemical analyses of the on- and off-rates of GDP for Gtr2 and RagC/D indicated that these GTPases have intrinsically high GDP dissociation capacities, suggesting that they may not need specific GEFs.8,14,19 Nevertheless, other regulators, namely guanine nucleotide dissociation inhibitors (GDIs) that block GDP dissociation, might play role in the regulation of GTP binding by Gtr2 (or Gtr1). In this context, it is interesting to note that sestrins, in addition to their role in antagonizing GATOR2,40-42 have also been proposed to act as GDIs for RagA/B.49
Amino acid signaling upstream of Rag GTPase regulators
The amino-acid sensitive events upstream of the Rag GTPase regulators in higher eukaryotes involve lysosomal amino acid sensors such as the vacuolar ATPase (v-ATPase)50 and lysosomal amino acid permeases, which transport and signal arginine and/or glutamine (SLC38A9),51-53 small unbranched amino acids (SLC36A1/PAT1),54 and histidine (SLC15A4) levels.55 They also involve cytoplasmic amino acid sensors such as the leucyl-tRNA synthetase (LeuRS) and Sestrin/Castor proteins.40-44,49,56,57 Orthologous sensory modules that function in Rag GTPase regulation in yeast include so far only the v-ATPase and LeuRS. However, while the yeast v-ATPase has been implicated in signaling cytosolic pH (as a proxy for the quality and quantity of the available carbon source) to Rag GTPases,58 it is currently not known whether it also mediates vacuolar amino acid signals (Fig. 1, point 11). In addition, the means by which LeuRS controls Rag GTPases in yeast appears to be fundamentally different from the one described in mammalian cells. Accordingly, yeast LeuRS (Cdc60) signals balanced levels of the branched-chain amino acids (i.e. leucine, isoleucine, and valine) to promote the GTP-loaded status of Gtr1 via a mechanism that is not yet fully understood (Fig. 1, point 12),56 while mammalian LeuRS has been proposed to act as GAP for RagD when leucine is present,57 although this was not recapitulated in a subsequent study.32 The possibility that mammalian LeuRS regulates Rag GTPases in a manner that is analogous to the one described in yeast has not been addressed yet, but may provide a rationale for the latter divergent results.
As stated above, cytoplasmic amino acid sensors analogous to Sestrin/Castor proteins have not yet been discovered in yeast, which are prototrophic organisms that can synthesize the entire set of amino acids on their own. It will therefore be interesting to study whether Sestrin and Castor proteins may have specifically evolved to sample the levels of (cytoplasmic) amino acids, which have become essential (e.g., leucine) or conditionally essential (e.g., arginine) in higher eukaryotes, and to forward the respective information to the Rag GTPase-TORC1 module. Information on the global levels of amino acids that are stored within the lysosome/vacuole, on the other hand, may be equally important in all eukaryotic systems, which would fit with the observation that the Rag GTPases have so far been found to predominantly associate with the lysosomal/vacuolar membrane in every system studied so far. The vacuolar membrane-resident amino acid permeases in yeast, inlcuding for instance the seven members of the Avt family of amino acid permeases (i.e. Avt1-7) of which Avt2 appears to be most closely related to SLC38A9, could therefore in principle also function as amino acid sensors upstream of the Rag GTPases (Fig. 1, point 13). Interestingly, in this context, recent studies in yeast revealed that the high affinity, broad-specificity general amino acid permease Gap1, which directs uptake of all naturally occurring amino acids, couples amino acid uptake (not metabolism) with signaling events to control the protein kinase A (PKA).59 Because Gap1 also interacts with Gtr2,20 a particularly attractive model posits that amino acid transport through Gap1 is also coupled to the control of the Rag GTPase-TORC1 branch (Fig. 1, point 14), which (possibly in a feedback control loop) then regulates Gap1 sorting.60 This model could therefore perhaps also explain why Rag GTPases have been implicated in controlling Gap1 sorting.20 Of note, unlike the vacuolar membrane-resident amino acid permeases, Gap1 cycles between the internal trans-Golgi network (TGN)/endosomal compartment and the plasma membrane and could in theory signal from the plasma membrane, or sample the environment for amino acids and then signal from an internal compartment following its endocytosis. If this proves to be the case, it will be important to address the questions whether the Rag GTPase-dependent amino acid-sensing (e.g., at the plasma membrane or TGN) and signal transmission (e.g., at the vacuole) events may be spatially and temporally separated or whether a fraction of the pool of EGOC-TORC1 modules may be localized to sites other than the vacuolar/lysosomal membrane.
Conclusions
While many aspects of Rag GTPase-mediated amino acid signaling are conserved from yeast to human, some remain to be experimentally addressed in yeast (or human cells) before final judgment of their evolutionary importance. Our understanding of how this particular nutrient response system has evolved to fulfill the specific life style requirements of higher eukaryotic cells will therefore continue to depend on studies of ancestral model organisms such as yeast. Accordingly, addressing the unsolved mysteries of Rag GTPase signaling in yeast, as highlighted in this commentary, will be helpful in delineating whether the mechanisms by which amino acids impinge on the Rag GTPase-TORC1 module are either conserved or rather idiosyncratic for the respective model organism under study. This information will not only extend our current view of basic mechanisms of amino acid sensing and signaling, but also provide invaluable insight that may be conducive for the development of novel approaches to treat mTORC1-related diseases.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgements
We thank Marie-Pierre Péli-Gulli for critical reading of the manuscript.
Funding
This work was supported by the canton of Fribourg and a grant from the Swiss National Science Foundation to C.D.V.
References
- [1].Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell 2006; 124:471-84; PMID:16469695; http://dx.doi.org/ 10.1016/j.cell.2006.01.016 [DOI] [PubMed] [Google Scholar]
- [2].Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell 2012; 149:274-93; PMID:22500797; http://dx.doi.org/ 10.1016/j.cell.2012.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kim E, Goraksha-Hicks P, Li L, Neufeld TP, Guan KL. Regulation of TORC1 by Rag GTPases in nutrient response. Nat Cell Biol 2008; 10:935-45; PMID:18604198; http://dx.doi.org/ 10.1038/ncb1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Binda M, Péli-Gulli MP, Bonfils G, Panchaud N, Urban J, Sturgill TW, Loewith R, De Virgilio C. The Vam6 GEF controls TORC1 by activating the EGO complex. Mol Cell 2009; 35:563-73; PMID:19748353; http://dx.doi.org/ 10.1016/j.molcel.2009.06.033 [DOI] [PubMed] [Google Scholar]
- [5].Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 2008; 320:1496-501; PMID:18497260; http://dx.doi.org/ 10.1126/science.1157535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nakashima N, Noguchi E, Nishimoto T. Saccharomyces cerevisiae putative G protein, Gtr1p, which forms complexes with itself and a novel protein designated as Gtr2p, negatively regulates the Ran/Gsp1p G protein cycle through Gtr2p. Genetics 1999; 152:853-67; PMID:10388807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wang Y, Nakashima N, Sekiguchi T, Nishimoto T. Saccharomyces cerevisiae GTPase complex: Gtr1p-Gtr2p regulates cell-proliferation through Saccharomyces cerevisiae Ran-binding protein, Yrb2p. Biochem Biophys Res Commun 2005; 336:639-45; PMID:16143306; http://dx.doi.org/ 10.1016/j.bbrc.2005.08.108 [DOI] [PubMed] [Google Scholar]
- [8].Sekiguchi T, Hirose E, Nakashima N, Ii M, Nishimoto T. Novel G proteins, Rag C and Rag D, interact with GTP-binding proteins, Rag A and Rag B. J Biol Chem 2001; 276:7246-57; PMID:11073942; http://dx.doi.org/ 10.1074/jbc.M004389200 [DOI] [PubMed] [Google Scholar]
- [9].Dubouloz F, Deloche O, Wanke V, Cameroni E, De Virgilio C. The TOR and EGO protein complexes orchestrate microautophagy in yeast. Mol Cell 2005; 19:15-26; PMID:15989961; http://dx.doi.org/ 10.1016/j.molcel.2005.05.020 [DOI] [PubMed] [Google Scholar]
- [10].Panchaud N, Péli-Gulli MP, De Virgilio C. SEACing the GAP that nEGOCiates TORC1 activation: evolutionary conservation of Rag GTPase regulation. Cell Cycle 2013; 12:2948-52; PMID:23974112; http://dx.doi.org/ 10.4161/cc.26000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Panchaud N, Péli-Gulli MP, De Virgilio C. Amino acid deprivation inhibits TORC1 through a GTPase-activating protein complex for the Rag family GTPase Gtr1. Sci Signal 2013; 6:ra42; PMID:23716719; http://dx.doi.org/ 10.1126/scisignal.2004112 [DOI] [PubMed] [Google Scholar]
- [12].Péli-Gulli MP, Sardu A, Panchaud N, Raucci S, De Virgilio C. Amino Acids Stimulate TORC1 through Lst4-Lst7, a GTPase-Activating Protein Complex for the Rag Family GTPase Gtr2. Cell Rep 2015; 13:1-7; PMID:26387955; http://dx.doi.org/ 10.1016/j.celrep.2015.08.059 [DOI] [PubMed] [Google Scholar]
- [13].Powis K, De Virgilio C. Conserved regulators of Rag GTPases orchestrate amino acid-dependent TORC1 signaling. Cell Disc 2016; 2:15049; http://dx.doi.org/ 10.1038/celldisc.2015.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bar-Peled L, Schweitzer LD, Zoncu R, Sabatini DM. Ragulator is a GEF for the Rag GTPases that signal amino acid levels to mTORC1. Cell 2012; 150:1196-208; PMID:22980980; http://dx.doi.org/ 10.1016/j.cell.2012.07.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Powis K, Zhang T, Panchaud N, Wang R, De Virgilio C, Ding J. Crystal structure of the Ego1-Ego2-Ego3 complex and its role in promoting Rag GTPase-dependent TORC1 signaling. Cell Res 2015; 25:1043-59; PMID:26206314; http://dx.doi.org/ 10.1038/cr.2015.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Valbuena N, Guan KL, Moreno S. The Vam6 and Gtr1-Gtr2 pathway activates TORC1 in response to amino acids in fission yeast. J Cell Sci 2012; 125:1920-8; PMID:22344254; http://dx.doi.org/ 10.1242/jcs.094219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhang T, Péli-Gulli MP, Yang H, De Virgilio C, Ding J. Ego3 functions as a homodimer to mediate the interaction between Gtr1-Gtr2 and Ego1 in the EGO complex to activate TORC1. Structure 2012; 20:2151-60; PMID:23123112; http://dx.doi.org/ 10.1016/j.str.2012.09.019 [DOI] [PubMed] [Google Scholar]
- [18].Gong R, Li L, Liu Y, Wang P, Yang H, Wang L, et al.. Crystal structure of the Gtr1p-Gtr2p complex reveals new insights into the amino acid-induced TORC1 activation. Genes Dev 2011; 25:1668-73; PMID:21816923; http://dx.doi.org/ 10.1101/gad.16968011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wang Y, Kurihara Y, Sato T, Toh H, Kobayashi H, Sekiguchi T. Gtr1p differentially associates with Gtr2p and Ego1p. Gene 2009; 437:32-8; PMID:19374031; http://dx.doi.org/ 10.1016/j.gene.2009.01.018 [DOI] [PubMed] [Google Scholar]
- [20].Gao M, Kaiser CA. A conserved GTPase-containing complex is required for intracellular sorting of the general amino-acid permease in yeast. Nat Cell Biol 2006; 8:657-67; PMID:16732272; http://dx.doi.org/ 10.1038/ncb1419 [DOI] [PubMed] [Google Scholar]
- [21].Mitterer V, Murat G, Rety S, Blaud M, Delbos L, Stanborough T, Bergler H, Leulliot N, Kressler D, Pertschy B. Sequential domain assembly of ribosomal protein S3 drives 40S subunit maturation. Nat Commun 2016; 7:10336; PMID:26831757; http://dx.doi.org/ 10.1038/ncomms10336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Numrich J, Péli-Gulli MP, Arlt H, Sardu A, Griffith J, Levine T, Engelbrecht-Vandré S, Reggiori F, De Virgilio C, Ungermann C. The I-BAR protein Ivy1 is an effector of the Rab7 GTPase Ypt7 involved in vacuole membrane homeostasis. J Cell Sci 2015; 128:2278-92; PMID:25999476; http://dx.doi.org/ 10.1242/jcs.164905 [DOI] [PubMed] [Google Scholar]
- [23].Murphy JP, Stepanova E, Everley RA, Paulo JA, Gygi SP. Comprehensive temporal protein dynamics during the diauxic shift in Saccharomyces cerevisiae. Mol Cell Proteomics 2015; 14:2454-65; PMID:26077900; http://dx.doi.org/ 10.1074/mcp.M114.045849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kira S, Tabata K, Shirahama-Noda K, Nozoe A, Yoshimori T, Noda T. Reciprocal conversion of Gtr1 and Gtr2 nucleotide-binding states by Npr2-Npr3 inactivates TORC1 and induces autophagy. Autophagy 2014; 10:1565-78; PMID:25046117; http://dx.doi.org/ 10.4161/auto.29397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kira S, Kumano Y, Ukai H, Takeda E, Matsuura A, Noda T. Dynamic relocation of the TORC1-Gtr1/2-Ego1/2/3 complex is regulated by Gtr1 and Gtr2. Mol Biol Cell 2016; 27:382-96; PMID:26609069; http://dx.doi.org/ 10.1091/mbc.E15-07-0470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ashrafi K, Farazi TA, Gordon JI. A role for Saccharomyces cerevisiae fatty acid activation protein 4 in regulating protein N-myristoylation during entry into stationary phase. J Biol Chem 1998; 273:25864-74; PMID:9748261 [DOI] [PubMed] [Google Scholar]
- [27].Nadolski MJ, Linder ME. Molecular recognition of the palmitoylation substrate Vac8 by its palmitoyltransferase Pfa3. J Biol Chem 2009; 284:17720-30; PMID:19416974; http://dx.doi.org/ 10.1074/jbc.M109.005447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Roth AF, Wan J, Bailey AO, Sun B, Kuchar JA, Green WN, Phinney BS, Yates JR 3rd, Davis NG. Global analysis of protein palmitoylation in yeast. Cell 2006; 125:1003-13; PMID:16751107; http://dx.doi.org/ 10.1016/j.cell.2006.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Loewith R, Hall MN. Cell growth: control of cell size. CSHL; 2004. Chapter 5, TOR signaling in yeast: Temporal and spatial control of cell growth; p. 139-65. Available at: https://cshmonographs.org/index.php/monographs/article/view/4831 [Google Scholar]
- [30].Heard JJ, Fong V, Bathaie SZ, Tamanoi F. Recent progress in the study of the Rheb family GTPases. Cellular Signal 2014; 26:1950-7; PMID:24863881; http://dx.doi.org/ 10.1016/j.cellsig.2014.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jeong JH, Lee KH, Kim YM, Kim DH, Oh BH, Kim YG. Crystal structure of the Gtr1p(GTP)-Gtr2p(GDP) protein complex reveals large structural rearrangements triggered by GTP-to-GDP conversion. J Biol Chem 2012; 287:29648-53; PMID:22807443; http://dx.doi.org/ 10.1074/jbc.C112.384420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tsun ZY, Bar-Peled L, Chantranupong L, Zoncu R, Wang T, Kim C, Spooner E, Sabatini DM. The folliculin tumor suppressor is a GAP for the RagC/D GTPases that signal amino acid levels to mTORC1. Mol Cell 2013; 52:495-505; PMID:24095279; http://dx.doi.org/ 10.1016/j.molcel.2013.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Demetriades C, Doumpas N, Teleman AA. Regulation of TORC1 in response to amino acid starvation via lysosomal recruitment of TSC2. Cell 2014; 156:786-99; PMID:24529380; http://dx.doi.org/ 10.1016/j.cell.2014.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hughes Hallett JE, Luo X, Capaldi AP. Snf1/AMPK promotes the formation of Kog1/Raptor-bodies to increase the activation threshold of TORC1 in budding yeast. Elife 2015; 4:e09181; PMID:26439012; http://dx.doi.org/ 10.7554/eLife.09181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hughes Hallett JE, Luo X, Capaldi AP. State transitions in the TORC1 signaling pathway and information processing in Saccharomyces cerevisiae. Genetics 2014; 198:773-86; PMID:25085507; http://dx.doi.org/ 10.1534/genetics.114.168369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Stracka D, Jozefczuk S, Rudroff F, Sauer U, Hall MN. Nitrogen source activates TOR (target of rapamycin) complex 1 via glutamine and independently of Gtr/Rag proteins. J Biol Chem 2014; 289:25010-20; PMID:25063813; http://dx.doi.org/ 10.1074/jbc.M114.574335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bar-Peled L, Chantranupong L, Cherniack AD, Chen WW, Ottina KA, Grabiner BC, Spear ED, Carter SL, Meyerson M, Sabatini DM. A Tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1. Science 2013; 340:1100-6; PMID:23723238; http://dx.doi.org/ 10.1126/science.1232044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wu X, Tu BP. Selective regulation of autophagy by the Iml1-Npr2-Npr3 complex in the absence of nitrogen starvation. Mol Biol Cell 2011; 22:4124-33; PMID:21900499; http://dx.doi.org/ 10.1091/mbc.E11-06-0525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sutter BM, Wu X, Laxman S, Tu BP. Methionine inhibits autophagy and promotes growth by inducing the SAM-responsive methylation of PP2A. Cell 2013; 154:403-15; PMID:23870128; http://dx.doi.org/ 10.1016/j.cell.2013.06.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chantranupong L, Wolfson RL, Orozco JM, Saxton RA, Scaria SM, Bar-Peled L, Spooner E, Isasa M, Gygi SP, Sabatini DM. The Sestrins interact with GATOR2 to negatively regulate the amino-acid-sensing pathway upstream of mTORC1. Cell Rep 2014; 9:1-8; PMID:25263562; http://dx.doi.org/ 10.1016/j.celrep.2014.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kim JS, Ro SH, Kim M, Park HW, Semple IA, Park H, Cho US, Wang W, Guan KL, Karin M, et al.. Sestrin2 inhibits mTORC1 through modulation of GATOR complexes. Sci Rep 2015; 5:9502; PMID:25819761; http://dx.doi.org/ 10.1038/srep09502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Parmigiani A, Nourbakhsh A, Ding B, Wang W, Kim YC, Akopiants K, Guan KL, Karin M, Budanov AV. Sestrins inhibit mTORC1 kinase activation through the GATOR complex. Cell Rep 2014; 9:1281-91; PMID:25457612; http://dx.doi.org/ 10.1016/j.celrep.2014.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Saxton RA, Knockenhauer KE, Wolfson RL, Chantranupong L, Pacold ME, Wang T, Schwartz TU, Sabatini DM. Structural basis for leucine sensing by the Sestrin2-mTORC1 pathway. Science 2016; 351:53-8; PMID:26586190; http://dx.doi.org/ 10.1126/science.aad2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Chantranupong L, Scaria SM, Saxton RA, Gygi MP, Shen K, Wyant GA, Wang T, Harper JW, Gygi SP, Sabatini DM. The CASTOR proteins are arginine sensors for the mTORC1 pathway. Cell 2016; 165:153-64; PMID:26972053; http://dx.doi.org/ 10.1016/j.cell.2016.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lee JH, Cho US, Karin M. Sestrin regulation of TORC1: Is Sestrin a leucine sensor? Sci Signal 2016; 9:re5; PMID:27273098; http://dx.doi.org/ 10.1126/scisignal.aaf2885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Deng L, Jiang C, Chen L, Jin J, Wei J, Zhao L, Chen M, Pan W, Xu Y, Chu H, et al.. The ubiquitination of RagA GTPase by RNF152 negatively regulates mTORC1 activation. Mol Cell 2015; 58:804-18; PMID:25936802; http://dx.doi.org/ 10.1016/j.molcel.2015.03.033 [DOI] [PubMed] [Google Scholar]
- [47].Jin G, Lee SW, Zhang X, Cai Z, Gao Y, Chou PC, Rezaeian AH, Han F, Wang CY, Yao JC, et al.. Skp2-mediated RagA ubiquitination elicits a negative feedback to prevent amino-acid-dependent mTORC1 hyperactivation by recruiting GATOR1. Mol Cell 2015; 58:989-1000; PMID:26051179; http://dx.doi.org/ 10.1016/j.molcel.2015.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Petit CS, Roczniak-Ferguson A, Ferguson SM. Recruitment of folliculin to lysosomes supports the amino acid-dependent activation of Rag GTPases. J Cell Biol 2013; 202:1107-22; PMID:24081491; http://dx.doi.org/ 10.1083/jcb.201307084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Peng M, Yin N, Li MO. Sestrins function as guanine nucleotide dissociation inhibitors for Rag GTPases to control mTORC1 signaling. Cell 2014; 159:122-33; PMID:25259925; http://dx.doi.org/ 10.1016/j.cell.2014.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Zoncu R, Bar-Peled L, Efeyan A, Wang S, Sancak Y, Sabatini DM. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H+-ATPase. Science 2011; 334:678-83; PMID:22053050; http://dx.doi.org/ 10.1126/science.1207056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Rebsamen M, Pochini L, Stasyk T, de Araujo ME, Galluccio M, Kandasamy RK, Kandasamy RK, Snijder B, Fauster A, Rudashevskaya EL, et al.. SLC38A9 is a component of the lysosomal amino acid sensing machinery that controls mTORC1. Nature 2015; 519:477-81; PMID:25561175; http://dx.doi.org/ 10.1038/nature14107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Wang S, Tsun ZY, Wolfson RL, Shen K, Wyant GA, Plovanich ME, Yuan ED, Jones TD, Chantranupong L, Comb W, et al.. Metabolism. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science 2015; 347:188-94; PMID:25567906; http://dx.doi.org/ 10.1126/science.1257132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Jung J, Genau HM, Behrends C. Amino Acid-Dependent mTORC1 Regulation by the Lysosomal Membrane Protein SLC38A9. Mol Cell Biol 2015; 35:2479-94; PMID:25963655; http://dx.doi.org/ 10.1128/MCB.00125-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Ögmundsdóttir MH, Heublein S, Kazi S, Reynolds B, Visvalingam SM, Shaw MK, Goberdhan DC. Proton-assisted amino acid transporter PAT1 complexes with Rag GTPases and activates TORC1 on late endosomal and lysosomal membranes. PLoS One 2012; 7:e36616; PMID:22574197; http://dx.doi.org/ 10.1371/journal.pone.0036616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kobayashi T, Shimabukuro-Demoto S, Yoshida-Sugitani R, Furuyama-Tanaka K, Karyu H, Sugiura Y, Shimizu Y, Hosaka T, Goto M, Kato N, et al.. The histidine transporter SLC15A4 coordinates mTOR-dependent inflammatory responses and pathogenic antibody production. Immunity 2014; 41:375-88; PMID:25238095; http://dx.doi.org/ 10.1016/j.immuni.2014.08.011 [DOI] [PubMed] [Google Scholar]
- [56].Bonfils G, Jaquenoud M, Bontron S, Ostrowicz C, Ungermann C, De Virgilio C. Leucyl-tRNA synthetase controls TORC1 via the EGO complex. Mol Cell 2012; 46:105-10; PMID:22424774; http://dx.doi.org/ 10.1016/j.molcel.2012.02.009 [DOI] [PubMed] [Google Scholar]
- [57].Han JM, Jeong SJ, Park MC, Kim G, Kwon NH, Kim HK, Ha SH, Ryu SH, Kim S. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell 2012; 149:410-24; PMID:22424946; http://dx.doi.org/ 10.1016/j.cell.2012.02.044 [DOI] [PubMed] [Google Scholar]
- [58].Dechant R, Saad S, Ibáñez AJ, Peter M. Cytosolic pH regulates cell growth through distinct GTPases, Arf1 and Gtr1, to promote Ras/PKA and TORC1 activity. Mol Cell 2014; 55:409-21; PMID:25002144; http://dx.doi.org/ 10.1016/j.molcel.2014.06.002 [DOI] [PubMed] [Google Scholar]
- [59].Conrad M, Schothorst J, Kankipati HN, Van Zeebroeck G, Rubio-Texeira M, Thevelein JM. Nutrient sensing and signaling in the yeast Saccharomyces cerevisiae. FEMS Microbiol Rev 2014; 38:254-99; PMID:24483210; http://dx.doi.org/ 10.1111/1574-6976.12065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Merhi A, André B. Internal amino acids promote Gap1 permease ubiquitylation via TORC1/Npr1/14-3-3-dependent control of the Bul arrestin-like adaptors. Mol Cell Biol 2012; 32:4510-22; PMID:22966204; http://dx.doi.org/ 10.1128/MCB.00463-12 [DOI] [PMC free article] [PubMed] [Google Scholar]


