ABSTRACT
The dynamics of membrane fusion, fission, cargo sorting and organelle maturation in endosomal membrane systems is regulated by Rab and Arf small G proteins. Defining the regulators, effectors and sites of action for each Rab and Arf will enhance our understanding of how endocytic membrane traffic is orchestrated and functions in differentiated cell types. Recent work has also shown how Rab35 and Arf6 might serve as input sensors for 2 forms of endocytosis to balance membrane trafficking to preserve cell surface homeostasis.
KEYWORDS: Arf6, cargo sorting, cell polarity, clathrin-independent endocytosis, clathrin-mediated endocytosis, GTPase, Rab35, T cell
Endocytosis is a fundamental cellular process whereby extracellular nutrients, ligands, and plasma membrane proteins and lipids are taken up into the cell interior. Clathrin-mediated endocytosis (CME) is the best-characterized form of endocytosis and involves selective and concentrative uptake of plasma membrane (PM) proteins that contain cytoplasmic sorting sequences that bind to clathrin adaptor proteins to facilitate their internalization. CME is dependent on dynamin for vesicle scission and is an efficient means of removal of proteins from the cell surface. Hence, it is most effective at removing signaling receptors, including growth factor and G protein-coupled receptors (GPCR) after activation. Clathrin-independent endocytosis (CIE) has been less studied but recently various reports have described an array of potential CIE mechanisms, depending on the cell type and cargo studied. Most CIE is dynamin-independent and dependent on free cholesterol at the PM.1 Since cargo entering cells by CIE lack specific endocytic sorting sequences, CIE is often described as a bulk endocytic process. Despite differing entry mechanisms, once internalized, endocytic cargo proteins join a complex network of endosomal membrane compartments and from there are sorted and directed to different destinations (Fig. 1). Trafficking of cargo through these compartments is regulated by Rab and Arf guanine nucleotide-binding proteins (G proteins).
Figure 1.
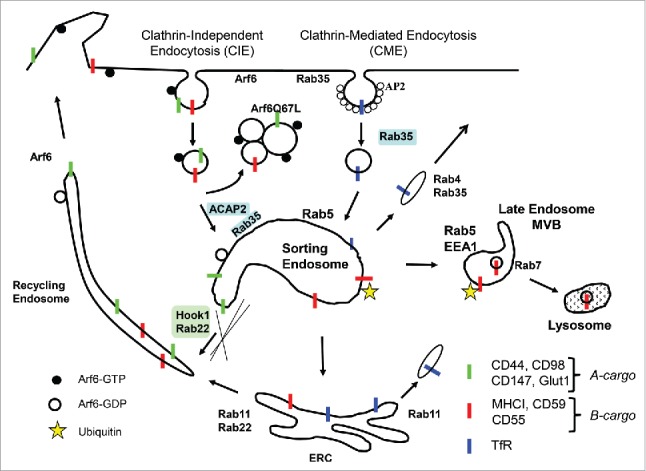
Model of Endosomal Trafficking. Clathrin-mediated endocytosis (CME) and clathrin-independent endocytosis (CIE) entry points are shown. Transferrin receptor (TfR) is a prototypical CME cargo protein. The two types of CIE cargo proteins include MHCI, CD59 and CD55 as B-cargo (for bulk pathway) and CD44, CD98, CD147 and the glucose transporter Glut1 as A-cargo (for alternative pathway). Arf6 and Rab35 are present at the PM. Arf6-GTP (filled circles) and Arf6-GDP (open circles) associate with CIE membranes. Inactivation of Arf6 occurs shortly after endocytosis; expression of the constitutively active Arf6Q67L leads to accumulation of CIE endosomes that trap CIE cargo. Hook1 and Rab22 are involved in sorting of A-cargo proteins away from the Rab5 endosome and into tubular recycling endosomes. Rab35, entering the cell by CME, recruits ACAP2 on the sorting endosome to inactivate Arf6 to allow proper sorting of A-cargo away from B-cargo, some of which goes on to late endosomes and lysosomes for degradation. Disruption of CME leads to elevated Arf6-GTP and altered trafficking of A-cargo proteins to lysosomes for degradation. Other Rabs involved in trafficking steps are shown.
Similar to what is observed in the secretory pathway,2 Rab proteins help define endocytic compartment identity. The central, sorting endosome compartment to which incoming endocytic vesicles fuse is distinguished in large part by the presence of Rab5.3 The Rab5 endosome was originally described as a PI3P- and EEA1-positive endosome that was poised for homotypic endosome fusion. More recently, with the identification of many more effector proteins it is recognized that there are distinct Rab5-positive early endosomes, for example, those associated with APPL1/2 and those associated with EEA1. In many cell types, the Rab5/EEA1 endosomes generally mark progression to late endosomes and lysosomes.4 Indeed, it is thought that Rab5 endosomes mature or progress into later compartments, marked by Rab4 or Rab11 for recycling5 and those marked by Rab7 for conversion into multi-vesicular bodies (MVBs) and late endosome (see Fig. 1). Rab5, 4, 7 and 11 make up the core endosomal Rabs but there are related Rabs (8, 10, 13, 14, 15, 21, 22, 23, 25, 31, 35) that function in parallel or in differentiated cell types. A recent example of this is the finding that Rab8, Rab10 and Rab11 all function in neurite outgrowth in PC12 cells through association with different populations of recycling endosomes (Rab8 on one population and Rab10 and 11 on another).6
Understanding how distinct Rab proteins control the identity of endosomal compartments and facilitate the maturation of the endosomal network is a goal of many researchers. Some of the regulators of Rabs, the guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs) that mediate activation and inactivation, have been identified. We now need to determine where each GEF and GAP is functioning and on which Rab. One interesting model for endosomal maturation involves the sequential conversion through the GTPase cycle of one Rab for the next one; this could be accomplished by the first Rab recruiting a GEF for the next Rab, which could in turn recruit a GAP to inactivate the prior Rab.7 This mutual conversion or inactivation need not be restricted to Rabs. Arf proteins are capable of promoting an Arf cascade; Arf6-GTP recruits ARNO GEFs to the cell surface as “effectors,” which in turn can further stimulate Arf6 activation or that of Arf1.8 Rab and Arf activites can also converge in endosomal systems that include the input into the endosomal system by CIE.
Our lab has focused on a form of CIE that is associated with Arf6. Arf6 is active at the cell surface where it promotes the production of phosphatidylinosoitol 4,5-bisphosphate and regulates the cortical actin cytoskeleton. Although Arf6 is localized to this pathway, in most cells Arf6 does not contribute to the internalization stages of CIE, but its inactivationby GTP hydrolysis is required for the sorting of cargo proteins soon after entry. By contrast, activation of Arf6 is required for the recycling of CIE cargo (see Fig. 1). We have focused on understanding the types of cargo proteins that enter cells via CIE and have defined 2 types of cargo proteins that have different itineraries within the cell. One type of cargo protein, exemplified by the major histocompatibility complex Class I (MHCI) and by the GPI-anchored protein CD59, travels along a bulk route (B-cargo) that directs some cargo for recycling and some cargo toward lysosomes for degradation. Another type of cargo, which includes the amino acid transporter CD98 and other transporters, travels an alternative route (A-cargo) in which nearly all of these cargo proteins directly enter recycling tubules after internalization and do not enter late endosomes and lysosomes (Fig. 1). As a consequence, CD98 and other A-cargo proteins are long-lived. We have identified part of the machinery responsible for sorting of A-cargo proteins after endocytosis. We previously showed that Rab11, Rab22 9 and Arf610 are required for recycling of CIE cargo proteins, and have now demonstrated that Rab22 along with the Hook1 scaffold protein are responsible for sorting, in a microtubule-dependent manner, A-cargo proteins into the recycling tubule system.11
CME and CIE cross talk and role of G proteins in balancing membrane flow
We recently discovered that CME and CIE are carefully balanced and coordinately regulated by 2 G proteins, Rab35 and Arf6. When CME is impaired, through siRNA depletion of clathrin adaptors, CIE still occurs but the trafficking of CIE cargo proteins is altered. In particular, the A-cargo proteins like CD98, now traffic to EEA1 compartments, to lysosomes and are degraded.12 This alteration of CD98 trafficking was more pronounced than what was observed with depletion of Hook1 or Rab22, which mainly delayed their recycling back to the PM.11 Here, the half-life of CD98 and CD147 was significantly shortened. We found that this altered trafficking, with more membrane cargo going to lysosomes, was due to alteration in the balance of activities of Arf6 and Rab35.12
Studies in worms and in human cells have shown previously the coordination of Arf6 and Rab35 in regulating endosomal trafficking.13 Rab35 and Arf6 are both present at the cell surface and they work by mutual antagonism in that they turn each other off by recruitment of GAPs. Arf6 recruits the Rab35 GAP TBC1D10 leading to Rab35 inactivation and Rab35 recruits the Arf6 GAP ACAP2 (and to a lesser extent ACAP1) that inactivates Arf6. In cells overexpressing the active mutant of Arf6 (Q67L), CIE cargo proteins enter the cells and accumulate in enlarged actin coated vacuoles enriched in PIP2.14 Inactivation of Arf6 is required for subsequent sorting and trafficking on the sorting endosome. Our study demonstrates that when CME is blocked, Arf6-GTP levels are elevated, although not to the level or extent of Arf6Q67L-expressing cells. Expression of Rab35, a Rab associated with clathrin-coated vesicles, or ACAP2 rescues the altered trafficking of CIE cargo proteins in the absence of CME.12 Depletion of Rab35 also mimics the CME-block phenotype on CIE cargo traffic. This study suggests that Rab35 input from CME is critical for inactivating Arf6 on the sorting endosomes by recruitment of ACAP2. Upon inactivation of Arf6 the cargoes reach their destination where further sorting occurs that allows A-cargo proteins to reach the tubules and recycle to the plasma membrane. Thus Arf6 from CIE and Rab35 from CME work in harmony to define and balance the 2 arms of the endocytic pathway, re-balancing membrane traffic when one form of endocytosis is impaired to maintain cell surface size and composition.
Other studies have suggested that the interplay between Arf6 and Rab35 may be important for basic cellular functions. Both proteins have been implicated in the final steps of cytokinesis,13 in neurite outgrowth,15 and they have opposite effects in the regulation of cell migration and adhesion. Arf6 promotes the recycling of integrins, migration and is generally upregulated in many cancers.16 Rab35, by contrast, is involved in the recycling of cadherins, promotes cell-cell adhesion and is downregulated in many cancers.16 Recently Mrozowska and Fukuda found that Rab35 is required for the trafficking of podocalyxin to the apical cell surface during lumen formation in MDCK cells grown in 3-D matrix and that this requirement also involves ACAP2, and not other Rab35 effectors.17 Thus, regulation of Rab35 and Arf6 activities are important for establishment of cell polarity and differentiation.
Endosomal Rabs and Arfs in formation of the immunological synapse
Another system that requires endosomal trafficking to establish proper cell polarity is the interaction between T cells and antigen presenting cells during the adaptive immune response. It is theorized that T cells use endosomal trafficking of key signaling molecules to control and fine-tune the immune response.18 The role of small G proteins, especially Rabs and Arfs, are beginning to be elucidated within these cell types.
Antigen presenting cells continually endocytose and display foreign antigens to T cells. T cells use the T cell receptor (TCR) to recognize cognate antigens on the antigen presenting cell.19 After recognition, T cells undergo activation and polarization leading to their many effector functions. Polarization establishes a unique plasma membrane domain adjacent to the antigen presenting cell. This area of the cell membrane has been termed the immunological synapse.20 Endosomal trafficking to the immunological synapse occurs using myosin motors that are linked to the actin cytoskeleton to properly place proteins at the plasma membrane.21
Early evidence of the importance of Rabs in T cell function came from studies in which a dominant negative form of Rab5 (Rab5N133I) was expressed in T lymphocytes of transgenic mice.22 Total numbers of T lymphocytes were reduced as was ligand-induced TCR down-modulation. The delay in TCR down-modulation resulted in enhanced TCR signaling suggesting that Rab5 is an important regulatory component of T cell development and signaling.
Rab11 has also been shown to be important for the delivery of lymphocyte-specific protein tyrosine kinase (Lck), a molecule required for proper TCR signaling at the immunological synapse.23 More recently, Rab11 and its effector FIP3 were shown to be critical for the endosomal trafficking and delivery of Rac1 to the immunological synapse during T cell activation.24
The small G protein Rab35 has also been implicated in trafficking of the TCR. Rab35 co-localizes with the TCR and transferrin in the endosomal recycling compartment.25 Expression of dominant-negative Rab35 or the Rab35 GAP EPI64C reduces transferrin recycling in a resting Jurkat T cell. Upon stimulation with an antigen presenting cell the presence of inactive Rab35 or EPI64C reduces TCR accumulation at the synapse and stable conjugate formation.25 Given the finding that Rab35 and Arf6 act as mutual antagonists, with each recruiting the other's GAP, it will be interesting to determine whether expression of Arf6Q67L, predicted to cause inactivation of Rab35, would have an impact on T cell conjugate formation.
G proteins are critical for trafficking of T cell molecules that are required for proper signaling. Further analysis should be directed at understanding the roles of specialized Rabs in the highly evolved adaptive immune response. It is likely that Rabs establish the proper T cell plasma membrane composition prior to activation and control signaling during activation. This deeper analysis will provide us a method for controlling the T cell response and possibly fine-tuning these cells for immunotherapies.
Abbreviations
- CIE
clathrin-independent endocytosis
- CME
clathrin-mediated endocytosis
- GAP
GTPase activating protein
- GEF
guanine nucleotide exchange factor
- GPCR
G protein-coupled receptor
- MHCI
major histocompatibility complex Class I
- MVB
multi-vesicular body
- PM
plasma membrane
- TCR
T cell receptor
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Mayor S, Parton RG, Donaldson JG. Clathrin-independent pathways of endocytosis. Cold Spring Harb Perspect Biol 2014; 6(6):93-112; http://dx.doi.org/ 10.1101/cshperspect.a016758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zhen Y, Stenmark H. Cellular functions of Rab GTPases at a glance. J Cell Sci 2015; 128(17):3171-6; PMID:26272922; http://dx.doi.org/ 10.1242/jcs.166074 [DOI] [PubMed] [Google Scholar]
- [3].Wandinger-Ness A, Zerial M. Rab proteins and the compartmentalization of the endosomal system. Cold Spring Harb Perspect Biol 2014; 6(11):a022616; PMID:25341920; http://dx.doi.org/ 10.1101/cshperspect.a022616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Leonard D, Hayakawa A, Lawe D, Lambright D, Bellve KD, Standley C, Lifshitz LM, Fogarty KE, Corvera S. Sorting of EGF and transferrin at the plasma membrane and by cargo-specific signaling to EEA1-enriched endosomes. J Cell Sci 2008; 121(Pt 20):3445-58; PMID:18827013; http://dx.doi.org/ 10.1242/jcs.031484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sonnichsen B, De Renzis S, Nielsen E, Rietdorf J, Zerial M. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5 and Rab11. J Cell Biol 2000; 149(4):901-913; PMID:10811830; http://dx.doi.org/ 10.1083/jcb.149.4.901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Homma Y, Fukuda M. Rabin8 regulates neurite outgrowth in both GEF activity-dependent and -independent manners. Mol Biol Cell 2016; 27(13):2107-18; PMID:27170183; http://dx.doi.org/ 10.1091/mbc.E16-02-0091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Segev N. Coordination of intracellular transport steps by GTPases. Semin Cell Dev Biol 2011; 22(1):33-8; PMID:21130177; http://dx.doi.org/ 10.1016/j.semcdb.2010.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cohen LA, Honda A, Varnai P, Brown FD, Balla T, Donaldson JG. Active Arf6 recruits ARNO/cytohesin GEFs to the PM by binding their PH domains. Mol Biol Cell 2007; 18(6):2244-53; PMID:17409355; http://dx.doi.org/ 10.1091/mbc.E06-11-0998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Weigert R, Yeung AC, Li J, Donaldson JG. Rab22a regulates the recycling of membrane proteins internalized independently of clathrin. Mol Biol Cell 2004; 15(8):3758-70; PMID:15181155; http://dx.doi.org/ 10.1091/mbc.E04-04-0342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Radhakrishna H, Donaldson JG. ADP-ribosylation factor 6 regulates a novel plasma membrane recycling pathway. J Cell Biol 1997; 139(1):49-61; PMID:9314528; http://dx.doi.org/ 10.1083/jcb.139.1.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Maldonado-Baez L, Cole NB, Krämer H, Donaldson JG. Microtubule-dependent endosomal sorting of clathrin-independent cargo by Hook1. J Cell Biol 2013; 201(2):233-47; PMID:23589492; http://dx.doi.org/ 10.1083/jcb.201208172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Dutta D, Donaldson JG. Sorting of clathrin-independent cargo proteins depends on Rab35 delivered by clathrin-mediated endocytosis. Traffic 2015; 16:994-1009; PMID:25988331; http://dx.doi.org/23905989 10.1111/tra.12302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chaineau M, Ioannou MS, McPherson PS. Rab35: GEFs, GAPs and effectors. Traffic 2013; 14(11):1109-17; PMID:23905989 [DOI] [PubMed] [Google Scholar]
- [14].Brown FD, Rozelle AL, Yin HL, Balla T, Donaldson JG. Phosphatidylinositol 4,5-bisphosphate and Arf6-regulated membrane traffic. J Cell Biol 2001; 154(5):1007-17; PMID:11535619; http://dx.doi.org/ 10.1083/jcb.200103107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kobayashi H, Fukuda M. Rab35 regulates Arf6 activity through centaurin-beta2 (ACAP2) during neurite outgrowth. J Cell Sci 2012; 125(Pt 9):2235-43; PMID:22344257; http://dx.doi.org/ 10.1242/jcs.098657 [DOI] [PubMed] [Google Scholar]
- [16].Allaire PD, Seyed Sadr M, Chaineau M, Seyed Sadr E, Konefal S, Fotouhi M, Maret D, Ritter B, Del Maestro RF, McPherson PS. Interplay between Rab35 and Arf6 controls cargo recycling to coordinate cell adhesion and migration. J Cell Sci 2013; 126(Pt 3):722-31; PMID:23264734; http://dx.doi.org/ 10.1242/jcs.112375 [DOI] [PubMed] [Google Scholar]
- [17].Mrozowska PS, Fukuda M. Regulation of podocalyxin trafficking by Rab small GTPases in 2D and 3D epithelial cell cultures. J Cell Biol 2016; 213(3):355-69; PMID:27138252; http://dx.doi.org/ 10.1083/jcb.201512024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gallegos AM, Xiong H, Leiner IM, Sušac B, Glickman MS, Pamer EG, van Heijst JW. Control of T cell antigen reactivity via programmed TCR downregulation. Nat Immunol 2016; 17(4):379-86; PMID:26901151; http://dx.doi.org/ 10.1038/ni.3386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wucherpfennig KW, Gagnon E, Call MJ, Huseby ES, Call ME. Structural biology of the T-cell receptor: insights into receptor assembly, ligand recognition, and initiation of signaling. Cold Spring Harb Perspect Biol 2010; 2(4):a005140; PMID:20452950; http://dx.doi.org/ 10.1101/cshperspect.a005140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Huppa JB, Davis MM. T-cell-antigen recognition and the immunological synapse. Nat Rev Immunol 2003; 3(12):973-83; PMID:14647479; http://dx.doi.org/ 10.1038/nri1245 [DOI] [PubMed] [Google Scholar]
- [21].Wulfing C, Davis MM. A receptor/cytoskeletal movement triggered by costimulation during T cell activation. Science 1998; 282(5397):2266-9; PMID:9856952; http://dx.doi.org/ 10.1126/science.282.5397.2266 [DOI] [PubMed] [Google Scholar]
- [22].Andre P, Boretto J, Hueber AO, Régnier-Vigouroux A, Gorvel JP, Ferrier P, Chavrier P. A dominant-negative mutant of the Rab5 GTPase enhances T cell signaling by interfering with TCR down-modulation in transgenic mice. J Immunol 1997; 159(11):5253-63; PMID:9548464 [PubMed] [Google Scholar]
- [23].Gorska MM, Liang Q, Karim Z, Alam R. Uncoordinated 119 protein controls trafficking of Lck via the Rab11 endosome and is critical for immunological synapse formation. J Immunol 2009; 183(3):1675-84; PMID:19592652; http://dx.doi.org/ 10.4049/jimmunol.0900792 [DOI] [PubMed] [Google Scholar]
- [24].Bouchet J, Del Río-Iñiguez I, Lasserre R, Agüera-Gonzalez S, Cuche C, Danckaert A, McCaffrey MW, Di Bartolo V, Alcover A. Rac1-Rab11-FIP3 regulatory hub coordinates vesicle traffic with actin remodeling and T-cell activation. EMBO J 2016; 35(11):1160-74; PMID:27154205; http://dx.doi.org/ 10.15252/embj.201593274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Patino-Lopez G, Dong X, Ben-Aissa K, Bernot KM, Itoh T, Fukuda M, Kruhlak MJ, Samelson LE, Shaw S. Rab35 and its GAP EPI64C in T cells regulate receptor recycling and immunological synapse formation. J Biol Chem 2008; 283(26):18323-30; PMID:18450757; http://dx.doi.org/ 10.1074/jbc.M800056200 [DOI] [PMC free article] [PubMed] [Google Scholar]


